
Biochemistry Lectures: 1st year Lecturer: Hiba A. Alasadi
Lecture No.1
Biochemistry
Science
Science asks how the remarkable properties of living organisms arise
from the thousands of different lifeless biomolecules. When these molecules
are isolated and examined individually, they conform to all the physical and
chemical laws that describe the behavior of inanimate matter—as do all the
processes occurring in living organisms. The study of biochemistry shows how
the collections of inanimate molecules that constitute living organisms
interact to maintain and perpetuate life animated solely by the physical and
chemical laws that govern the nonliving universe.
The chemistry of living organisms is organized around carbon, which
accounts for more than half the dry weight of cells.
Carbon can form single
bonds with hydrogen atoms, and both single and double bonds with oxygen
and nitrogen atoms. Of greatest significance in biology is the ability of carbon
atoms to form very stable carbon–carbon single bonds. Each carbon atom can
form single bonds with up to four other carbon atoms. Two carbon atoms also
can share two (or three) electron pairs, thus forming double (or triple) bonds.
Covalently linked carbon atoms in biomolecules can form linear chains,
branched chains, and cyclic structures. To these carbon skeletons are added
groups of other atoms, called functional groups, which confer specific
chemical properties on the molecule.
Macromolecules
Many biological molecules are macromolecules, polymers of high
molecular weight assembled from relatively simple precursors. Proteins, are
long polymers of amino acids, Nucleic acids, DNA and RNA, are polymers of
nucleotides , Carbohydrates, are polymers of simple sugars such as glucose
and the Lipids, are greasy or oily hydrocarbon derivatives.
ت
سنب
حل
ه ا
م
ً ريا
كث
ى
غط
ق ي
خلل
ء ا
سو
ن
مب أ
ك
ت
يئب
لس
ه ا
م
ً ريا
كث
رت
يس
ق
خلل
ه ا
حس
Biochemistry Lectures: 1st year Lecturer: Hiba A. Alasadi
Lecture No.1
CARBOHYDRATES
Occurrence
Most abundant molecules on earth, most are produced by photo-
synthesis. They are present in humans, animal tissues, plants and micro-
organisms. Carbohydrates are also present in tissue fluids, blood, milk
secretions and excretions of animals.
Medical And Biological Importance
1. Carbohydrates are the major source of energy for man. For example,
glucose is used in the human body for energy production.
2. Some carbohydrates serves as reserve food material in humans (glycogen)
and in plant (starch).
3. Some of carbohydrates are components of cell membrane and nervous
tissue.
4. Carbohydrates are components of nucleic acids and blood group
substances.
5. Carbohydrates are involved in cell-cell interaction.
6. Derivative of carbohydrates are drugs. For example, streptomycin an
antibiotic is a glycoside.
7. Aminosugar, derivatives of carbohydrates are components of antibiotics
like erythromycin and carbomycin.
8. Ascorbic acid, a derivative is water-soluble vitamin.
9. Bacterial invasion involves hydrolysis of mucopolysaccharides.
Biochemistry Lectures: 1st year Lecturer: Hiba A. Alasadi
Lecture No.1
Chemical Nature Of Carbohydrates
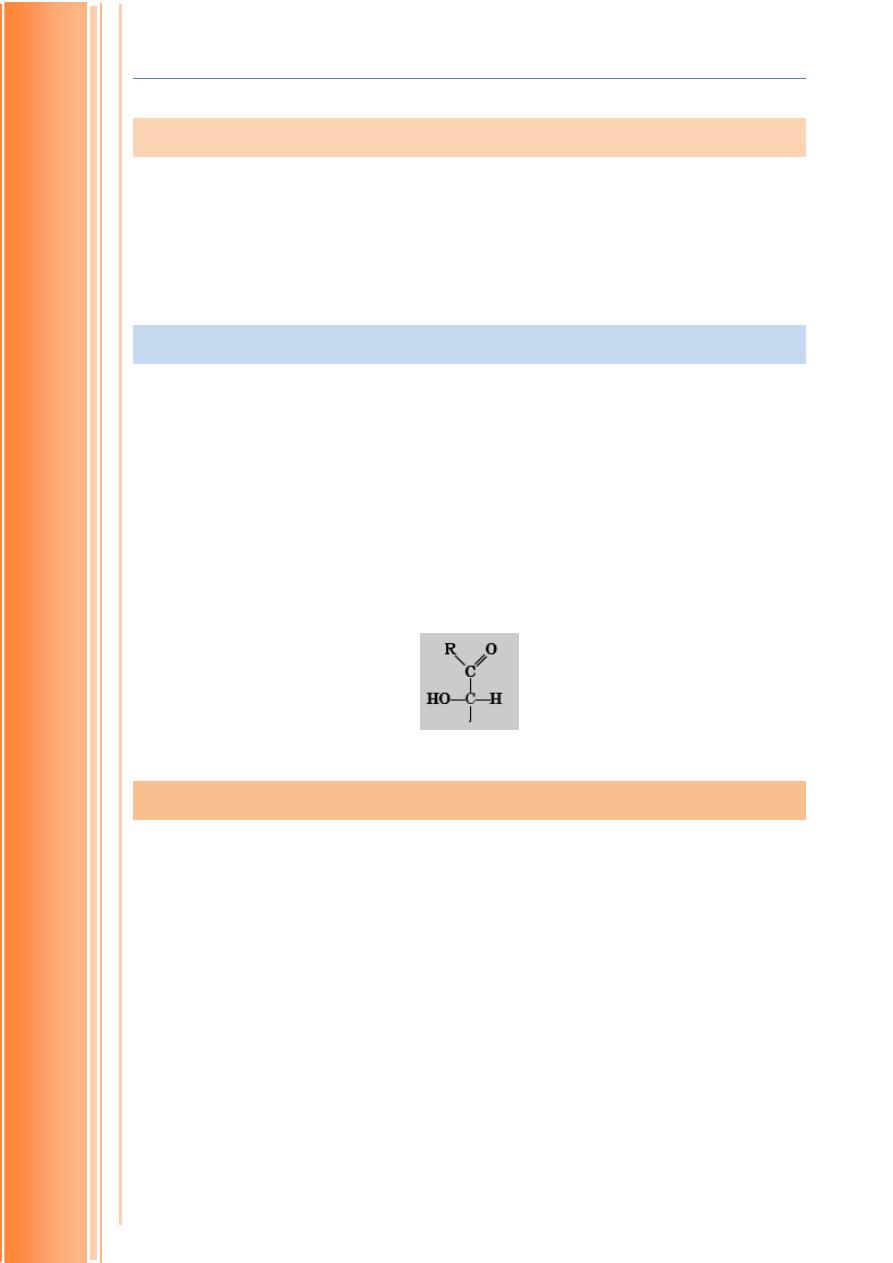
Carbohydrates are polyhydroxy alcohols with a functional aldehyde or
keto group. They are represented with general formula C
n(
H
2
O)
n
. usually the
ratio of carbon and water is one in most of the carbohydrates hence the name
carbohydrate (carbonhydrate).
Classification of carbohydrates
Carbohydrates are classified into three major classes based on number of
carbon chains present. They are:
1. Monosacchrides
2. Oligosacchrides
3. Polysacchrides
All the three classes contain a saccharose group and hence the name
saccharides.
Saccharose group
MONOSACCHARIDES
Monosaccharides are those carbohydrates which cannot be hydrolyzed to
small compounds. Their general formula is C
n
(H
2
O)
n
. They are also called as
simple sugars. Monosaccharides containing three to nine carbon atoms occur
in nature.
Nomenclature
Monosaccharides have common (trivial) names and systematic names.
Systematic name indicates both the number of carbon atoms present and
aldehyde or ketone group. For example, glyceraldehyde is a simple sugar
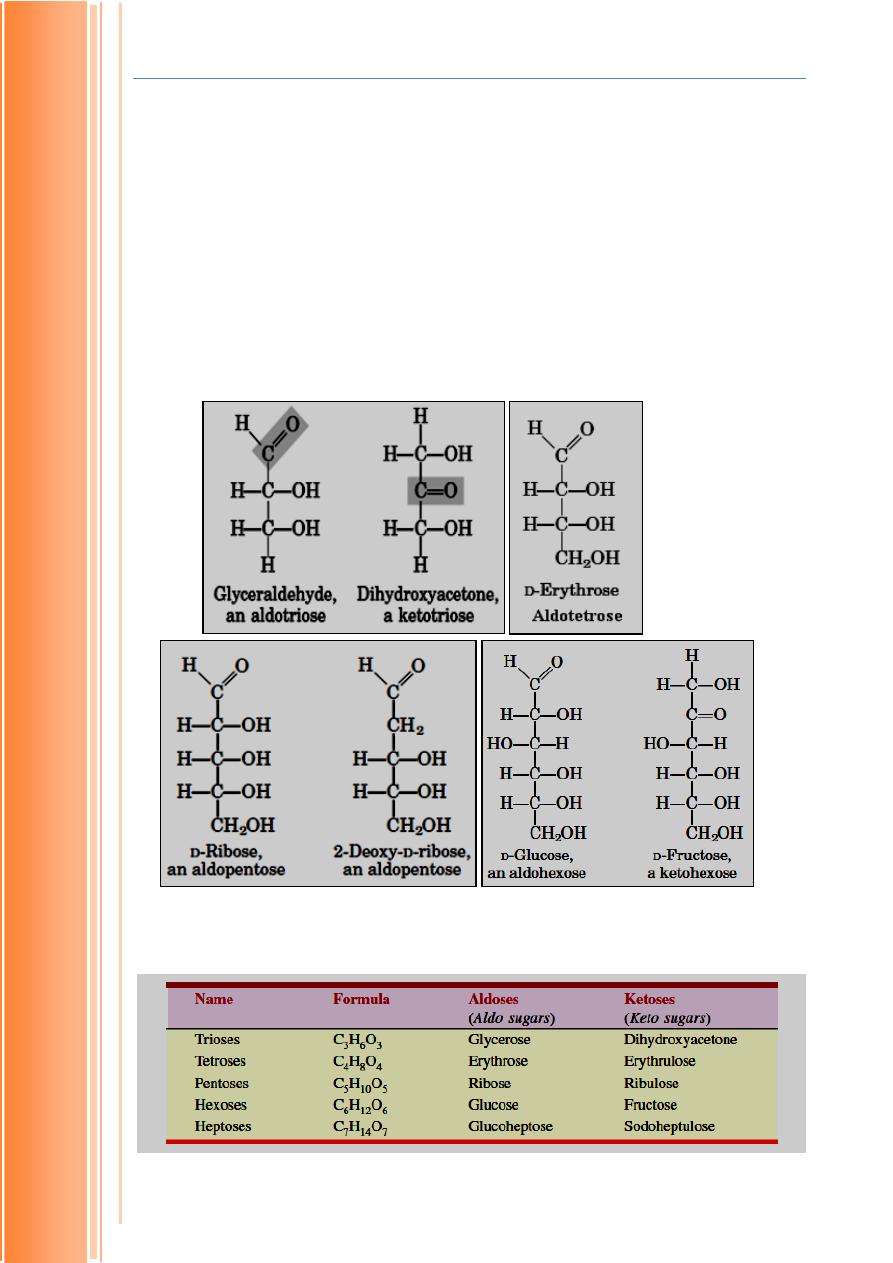
Biochemistry Lectures: 1st year Lecturer: Hiba A. Alasadi
Lecture No.1
containing three carbon atoms and an aldehyde group. Simple sugars
containing three carbon atoms are referred as triose. In addition, sugar
containing aldehyde group or keto group are called as aldoses or ketoses,
respectively. Thus, the systematic name of glyceraldehydes is aldotriose.
Similarly, a simple sugar with three carbon atoms and keto group is called
ketotriose. Some monosaccharides along with their common and systematic
names are shown below
Fig.1.1 Some Important Monosaccharides (Systematic Names Are Given Too)
Biochemistry Lectures: 1st year Lecturer: Hiba A. Alasadi
Lecture No.1
PROPERTIES OF MONOSACCHARIDES
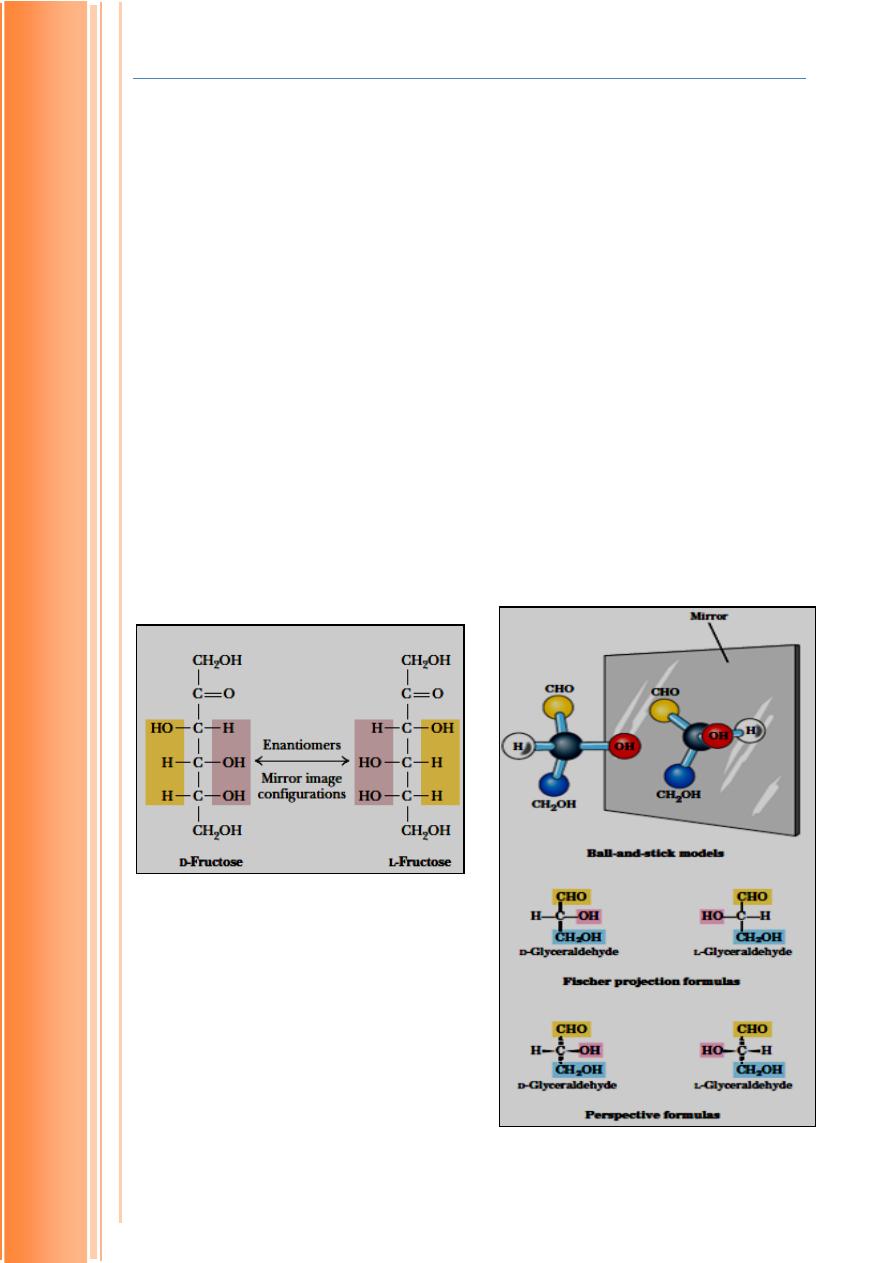
1. Optical Isomers
All the monosaccharides except dihydroxyacetone contain one or more
asymmetric (chiral) carbon atoms and thus occur in optically active isomeric
forms. The simplest aldose, glyceraldehyde, contains one chiral center (the
middle carbon atom) and therefore has two different optical isomers, or
enantiomers (Fig. 1.2). By convention, one of these two forms is designated
the D isomer, the other the L isomer. To represent three-dimensional sugar
structures on paper, we often use Fischer projection formulas. In general, a
molecule with n chiral centers can have 2
n
stereoisomers. Glyceraldehyde has
2
1
= 2; the aldohexoses, with four chiral
center has 2
1
= 2; the aldohexoses,
with four centers, have 2
4
= 16 stereoisomers.
FIGURE 1–2 Three ways to represent the two
stereoisomers of glyceraldehyde. The stereo-
isomers are mirror images of each other. Ball-
and-
stick
models
show
the
actual
configuration of molecules. By convention, in
Fischer projection formulas, horizontal bonds
project out of the plane of the paper, toward
the reader; vertical bonds project behind the
plane of the paper, away from the reader.
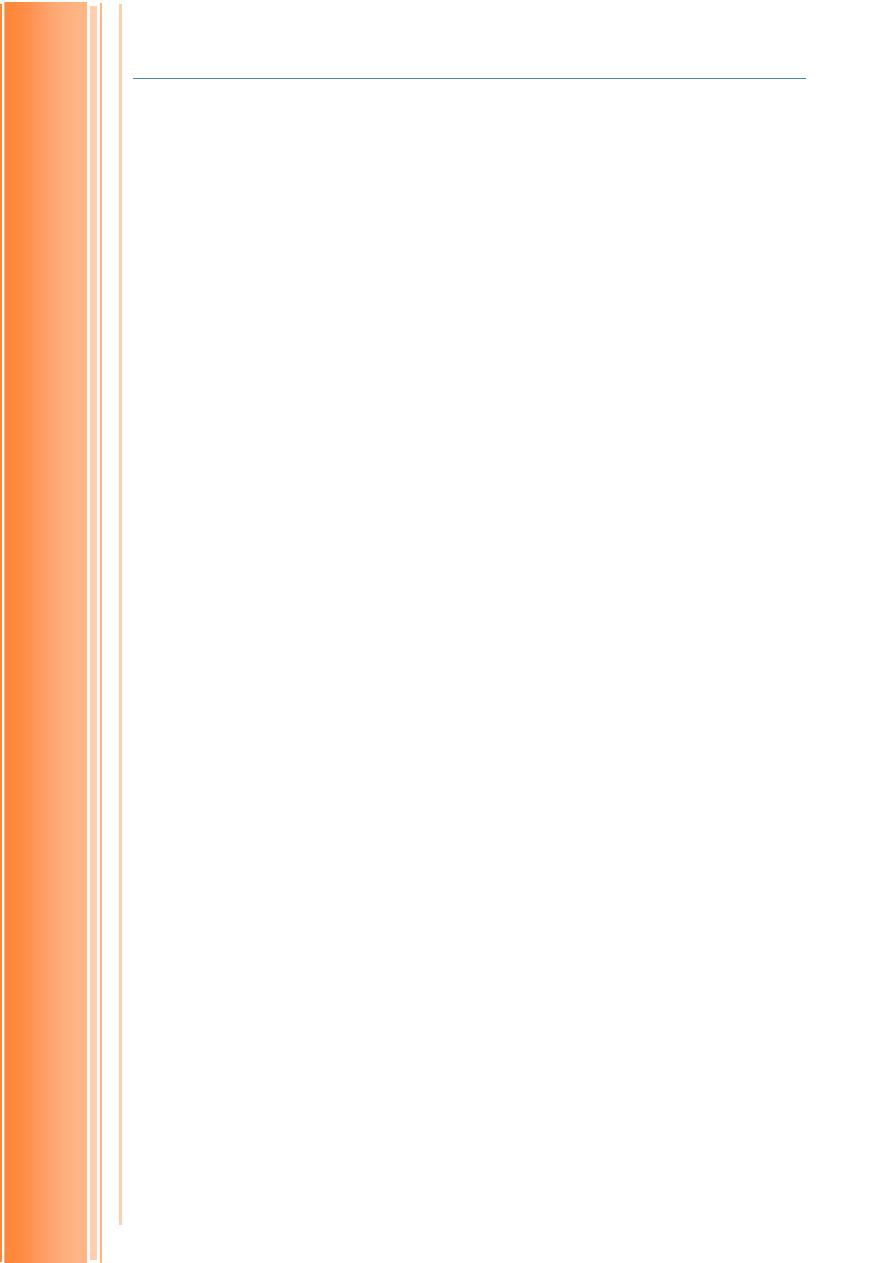
Biochemistry Lectures: 1st year Lecturer: Hiba A. Alasadi
Lecture No.1
The stereoisomers of monosaccharides of each carbon-chain length can be
divided into two groups that differ in the configuration about the chiral center
most distant from the carbonyl carbon. Of the 16 possible aldohexoses, eight
are D forms and eight are L. Most of the hexoses of living organisms are D
isomers.
2. Optical Activity
Monosaccharides except dihydroxy acetone exhibit optical activity because of
the presence of asymmetric carbon atom. If a sugar rotates plane polarized
light to the right then it called as dextrorotatory and if a sugar rotates plane
polarized light to left then it called the levorotatory. Usually '+' sign or 'd'
indicates dextrorotation and ' - ' or ' l ' indicates levorotatory. For example, D-
glucose which is dextrorotatory is designated as D(+) glucose and D-fructose,
which is levorotatory is designated as D(-)fructose.
3. Epimers
Are those monosaccharides that differs in the configuration of -OH group on
2nd, 3rd and 4th carbon atoms. Epimers are also called diasterioisomers.
Glucose, galactose and mannose are examples for epimers. Galactose is an
epimer of glucose because, configuration of hydroxyl group on 4th carbon
atom of galactose is different from glucose. Similarly, mannose is an epimers
of glucose because configuration of hydroxyl group on 2nd carbon atom of
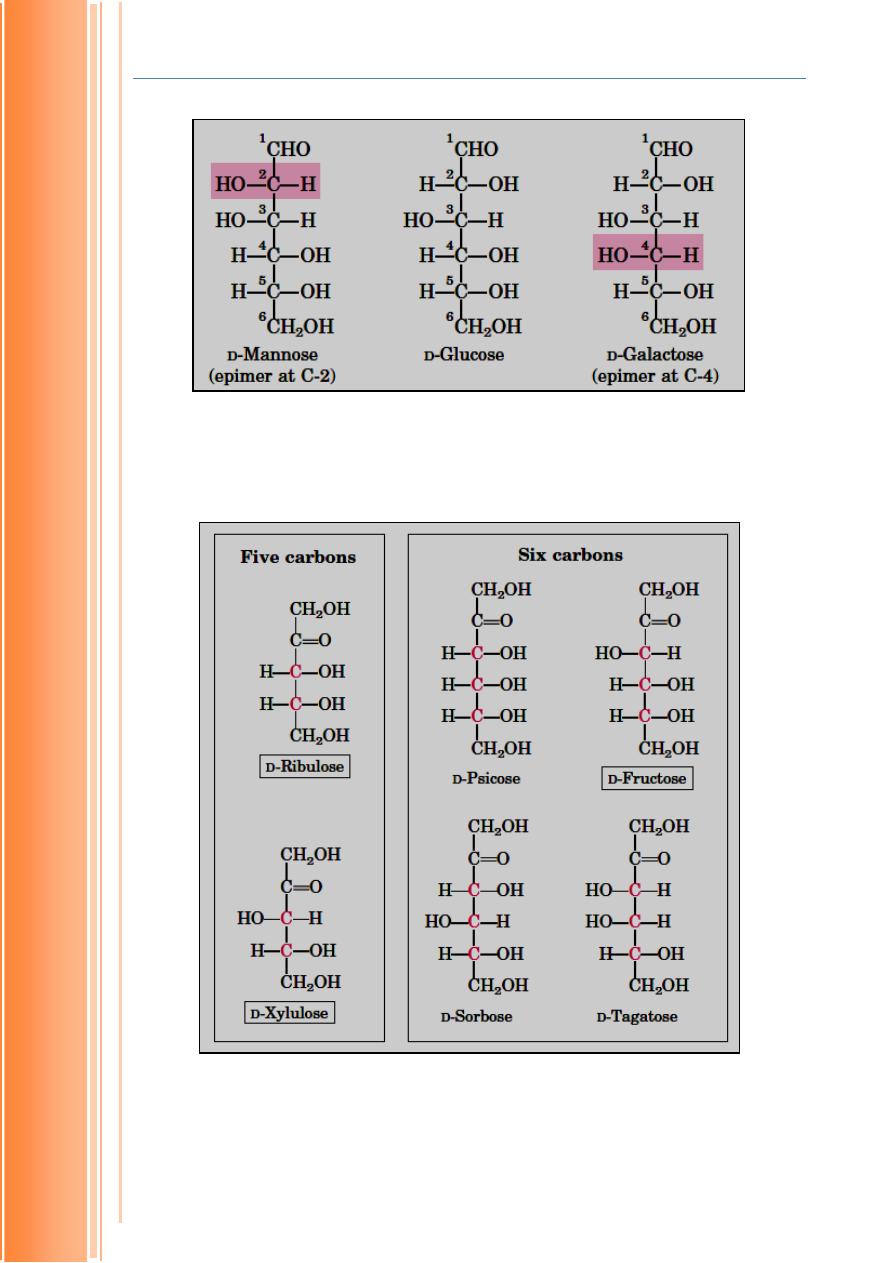
mannose is different from glucose (Fig.1.3). Ribulose and xylulose (both are
ketoses) are also epimers. They are differ in configuration of -OH group on
third carbon atom (Fig. 1.4).
Biochemistry Lectures: 1st year Lecturer: Hiba A. Alasadi
Lecture No.1
FIGURE 1–3 Epimers. D-Glucose and two of its epimers are shown as
projection formulas. Each epimer differs from D-glucose in the configuration
at one chiral center
Figure 1-4 Epimers of some ketoses
