
Upper GI Bleeding&Portal Hypertension
Dr. Ali Jaffer
Background
Bleeding derived from a source proximal to the ligament of Treitz.
Bleeding from the upper
GI tract is approximately 4 times more common than bleeding from the lower GI tract.
Mortality rates from UGIB are 6-10% overall.
Comorbid diseases increase the death rate.
Rebleeding and continued bleeding is a significant factor of mortality.
Aetiology
Causes of upper gastrointestinal bleeding.
Ulcers 60%
Oesophageal 6%
Gastric 21%
Duodenal 33%
Erosions 26%
Oesophageal 13 %
Gastric 9%
Duodenal 4 %
Mallory–Weiss tear 4 %
Oesophageal varices 4 %
Tumour 0.5 %
Vascular lesions, e.g. Dieulafoy’s disease 0.5 %
Others 5%
Prognosis
The following risk factors are associated with an increased mortality, recurrent bleeding, the
need for endoscopic hemostasis, or surgery
:
1. Age older than 60 years
2. Severe comorbidity
3. Active bleeding (eg, witnessed hematemesis, red blood per nasogastric tube, fresh blood per
rectum)
4. Hypotension
5. Red blood cell transfusion greater than or equal to 6 units
6. Inpatient at time of bleed
7. Severe coagulopathy
Patients who present in hemorrhagic shock have a mortality rate of up to 30%
History
Important information
potential comorbid conditions,
medication history, and potential toxic exposures
severity, timing, duration, and volume of the bleeding
Hematemesis, Melena, Hematochezia
Syncope
Dyspepsia, Epigastric pain, Heartburn, Diffuse abdominal pain
Dysphagia, Weight loss
Jaundice
Physical Examination
The goal; to evaluate for shock and blood loss.
Assessing the patient for hemodynamic
instability and clinical signs of poor perfusion is important early in the initial evaluation to
properly triage patients with massive hemorrhage.
Worrisome clinical signs and symptoms of hemodynamic compromise include: Tachycardia
of more than 100 beats per minute (bpm), Systolic blood pressure of less than 90 mm Hg,
Cool extremities, syncope, and other obvious signs of shock, Ongoing brisk hematemesis, The
occurrence of maroon or bright-red stools, which requires rapid blood transfusion.
Pulse and blood pressure should be checked with the patient in supine and upright positions to
note the effect of blood loss. Significant changes in vital signs with postural changes indicate
an acute blood loss of approximately 20% or more of the blood volume.
Signs of chronic liver disease should be noted, including spider angiomata, gynecomastia,
splenomegaly, ascites, ....etc. nodular liver, an abdominal mass, and enlarged and firm lymph
nodes.
Work up
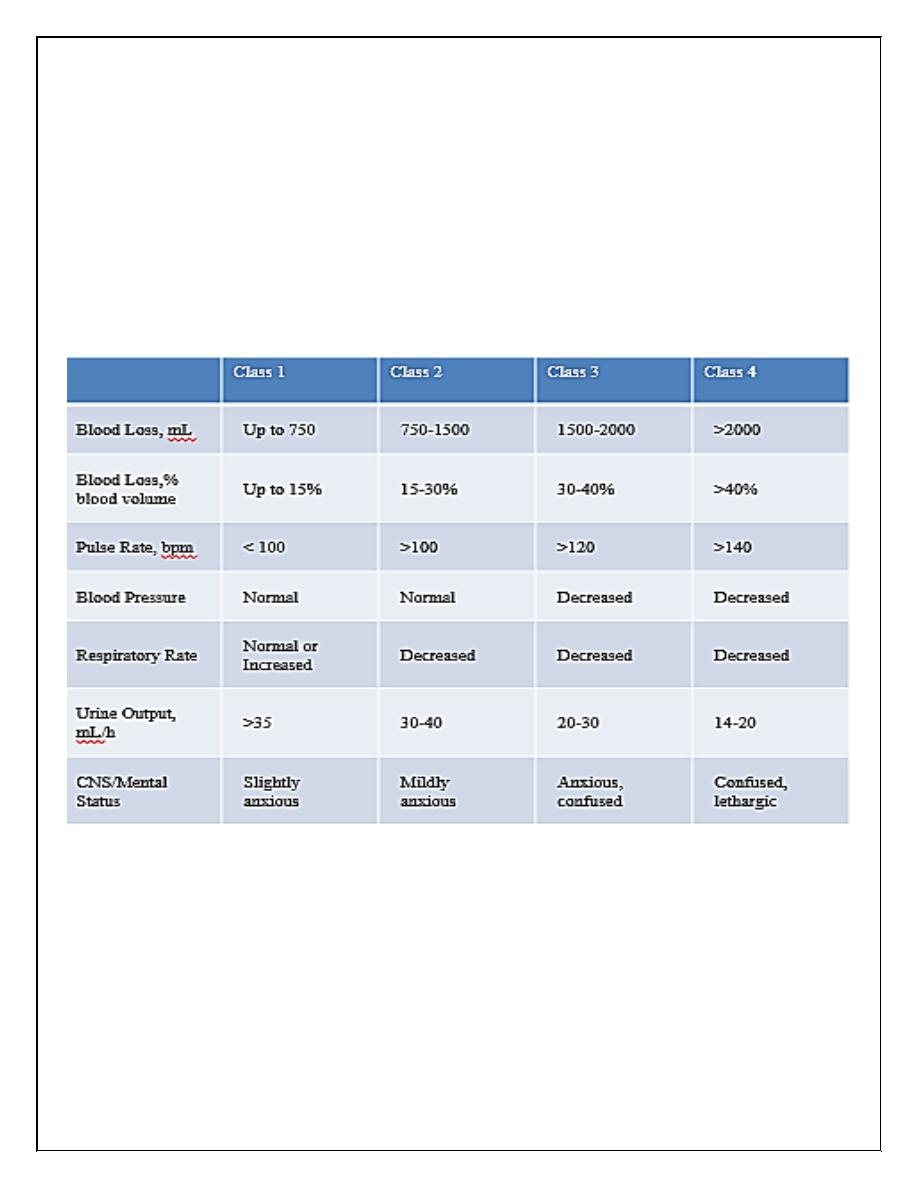
Assessment of hemorrhagic shock
patients who present in hemorrhagic shock have a mortality rate of up to 30%
Estimated Fluid and Blood Losses in Shock:
Hemoglobin Value and Type and Crossmatch Blood
CBC should be checked frequently (4-6h) during the first day. The patient should be
crossmatched for 2-6 units, based on the rate of active bleeding. Patients with significant
comorbid conditions (eg, advanced cardiovascular disease) should receive blood transfusions
to maintain myocardial oxygen delivery to avoid myocardial ischemia.
The more units required, the higher the mortality rate. Operative intervention is indicated once
the blood transfusion number reaches more than 5 units.

Coagulation Profile
The patient's prothrombin time (PT), activated partial thromboplastin time (PTT), and
international normalized ratio (INR) should be checked to document the presence of
coagulopathy. The coagulopathy may be consumptive and associated with a
thrombocytopenia
Endoscopy
Diagnostic&therapeutic. Endoscopy should be performed immediately after endotracheal
intubation (if indicated), hemodynamic stabilization, and adequate monitoring in an intensive
care unit (ICU) setting have been achieved.
Chest Radiography
Computed Tomography Scanning
Liver disease for cirrhosis, pancreatitis with pseudocyst and hemorrhage, aortoenteric fistula,
and other unusual causes of upper GI hemorrhage.
Nuclear Medicine Scanning
Nuclear medicine scans may be useful in determining the area of active hemorrhage.
Angiography
Angiography may be useful if bleeding persists and endoscopy fails to identify a bleeding
site.
Transcatheter arterial embolization (TAE) should be considered for all patients with a
known source of arterial UGIB that does not respond to endoscopic management, with
active bleeding and a negative endoscopy.
Nasogastric Lavage, PPIs, Treatment of underlying cause
Portal Hypertension
The portal venous system contributes approximately 75% of the blood and 72% of the oxygen
supplied to the liver. In the average adult, 1000 to 1500 mL/min of portal venous blood is
supplied to the liver. The normal portal venous pressure is 5 to 10 mmHg, and at this pressure,
very little blood is shunted from the portal venous system into the systemic circulation. As
portal venous pressure increases, the collateral communications with the systemic circulation
dilate, and a large amount of blood may be shunted around the liver and into the systemic
circulation.
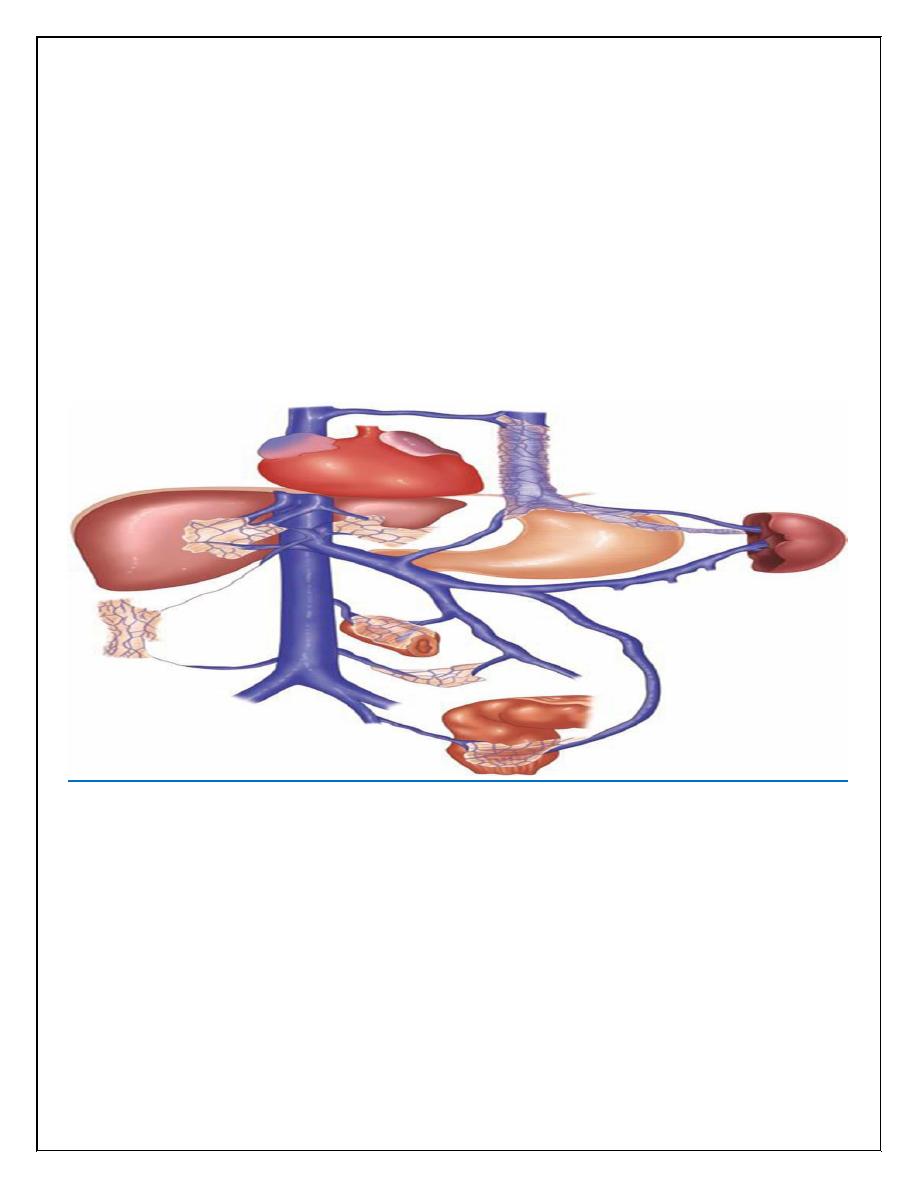
Lower oesophagus; Left gastric veins (portal system) -> lower branches of oesophageal veins
(systemic veins)
Upper part of anal canal; Superior rectal veins (portal) -> inferior and middle rectal veins
(systemic)
Umbilicus; Paraumbilical veins (portal) -> epigastric veins (systemic)
Area of the liver; Intraparenchymal branches of right division of portal vein (portal) ->
retroperitoneal veins (systemic)
Hepatic and splenic flexures; Omental and colonic veins (portal) -> retroperitoneal veins
(systemic)
Aetiology of portal hypertension
1- Presinusoidal
Sinistral/extrahepatic
Splenic vein thrombosis, Splenomegaly, Splenic arteriovenous fistula
Intrahepatic
Schistosomiasis, Congenital hepatic fibrosis, Nodular regenerative hyperplasia, Idiopathic
portal fibrosis, Myeloproliferative disorder, Sarcoid, Graft-versus-host disease

2- Sinusoidal
Intrahepatic: Cirrhosis, Viral infection, Alcohol abuse, Primary biliary cirrhosis, Autoimmune
hepatitis, Primary sclerosing cholangitis, Metabolic abnormality
3- Postsinusoidal
Intrahepatic: Vascular occlusive disease
Posthepatic
Budd-Chiari syndrome, Congestive heart failure, Inferior vena caval web, Constrictive
pericarditis
Portal hypertension per se produces no symptoms, it is usually diagnosed following
presentation with decompensated chronic liver disease and encephalopathy, ascites or variceal
bleeding.
Management of bleeding varices
General resuscitation
Medical emergency, ICU
Two large pore peripheral canulae, Resuscitation, avoid fluid overload (why?)
Correction of coagulopathy; Vit K(10mg) i.v., tranexamic acid (1g i.v), FFP, platelet
transfusion
Activation of major blood transfusion protocol
Drug therapy (terlipressin) splanchnic vasoconstriction
Prophylactic antibiotics
Endoscopy ; 50% PHT non variceal bleeding
Sengstaken-Blakemore, temporary control; Once inserted, the gastric balloon is inflated with
300 mL of air and retracted to the gastric fundus, where the varices at the oesophagogastric
junction are tamponaded by the subsequent inflation of the oesophageal balloon to a pressure
of 40 mmHg. The balloons should be temporarily deflated after 12 hours to prevent pressure
necrosis of the oesophagus.
Endoscopic treatment of varices
Endoscopic band ligation, endoscopic sclerotherapy

Transjugular intrahepatic portosystemic stent shunts
the main treatment of variceal haemorrhage that has not responded to drug treatment and
endoscopic therapy.
Complications:
Liver capsule perfuration…. Intraperitoneal hemorrhage, Occlusion resulting
in further variceal bleeding, Post shunt encephalopathy 40% of cases, TIPS stenosis (50%
after one year)
Surgical shunts for variceal haemorrhage
Surgical shunts are an effective method of preventing rebleeding from oesophageal or gastric
varices, as they reduce the pressure in the portal circulation by diverting the blood into the
low-pressure systemic circulation.
Long-term β-blocker therapy and chronic sclerotherapy or
banding are the main alternatives.
Liver transplantation
is the only therapy that will treat both portal hypertension and the
underlying liver disease.
Ascites
Portal vein thrombosis is a common predisposing factor to the development of ascites in
chronic liver disease. In patients without evidence of liver disease, malignancy is a common
cause
Aspiration of the peritoneal fluid allows the measurement of protein content to determine
whether the fluid is an exudate or transudate, an amylase estimation to exclude pancreatic
ascites.
Cytology will determine the presence of malignant cells. Microscopy and culture will
exclude primary bacterial and tuberculous peritonitis.
Treatment of ascites in chronic liver disease
Salt restriction, Diuretics
Abdominal paracentesis
TIPSS
Liver transplantation
