organic pharmaceutical chemistry
1-stageLec.1
Dr . Amal H. Anatheil
1
**Introduction
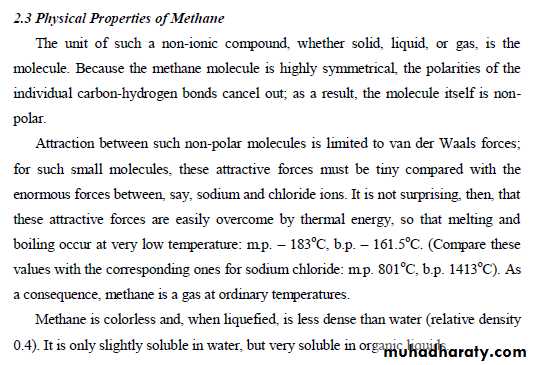
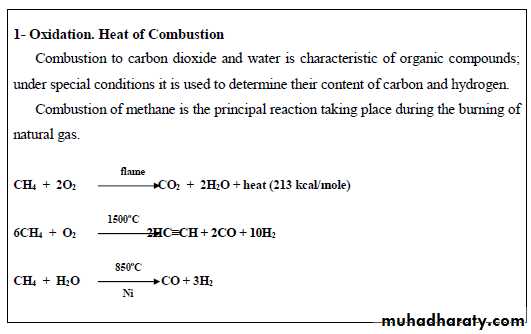
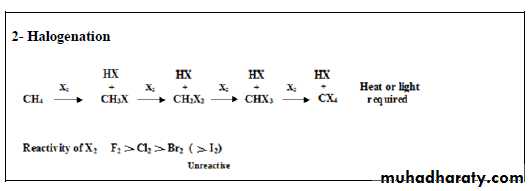
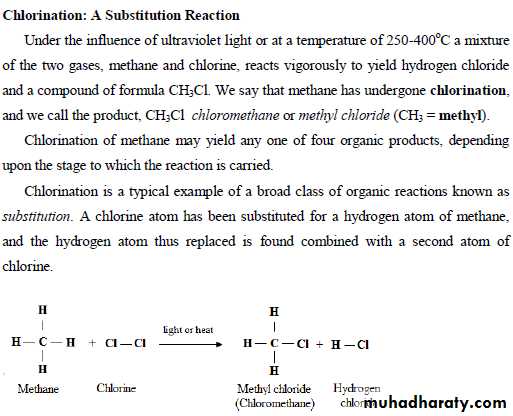
23
Today, although many compounds of carbon are still most conveniently isolated from plant and animal sources, most of them are synthesized. They are sometimes synthesized from inorganic substances like carbonates or cyanides, but more often from other compounds. There are two large reservoirs of organic materials from which simple organic compounds can be obtained; petroleum and coal. Both of these are organic in the old sense, being products of the decay of plants and animals. These simple compounds are used as building blocks from which larger and more complicated compounds can be made.
There are many ways in which these complicated molecules can break apart, or rearrange themselves, to form new molecules; there are many ways in which atoms can be added to these molecules, or new atoms substituted for old ones.
Carbon atoms can attach themselves to one another to an extant not possible for atoms of any other element. Carbon atoms can form chains thousands of atoms long, or rings of all sizes; the chains and rings can have branches and cross-links. To the carbon atoms of these chains and rings there are attached other atoms, chiefly hydrogen, but also fluorine, chlorine, bromine, iodine, oxygen, nitrogen, sulfur, phosphorus, and many others.
4
5
6
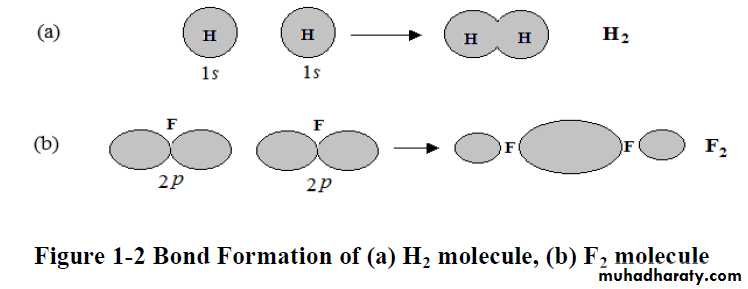
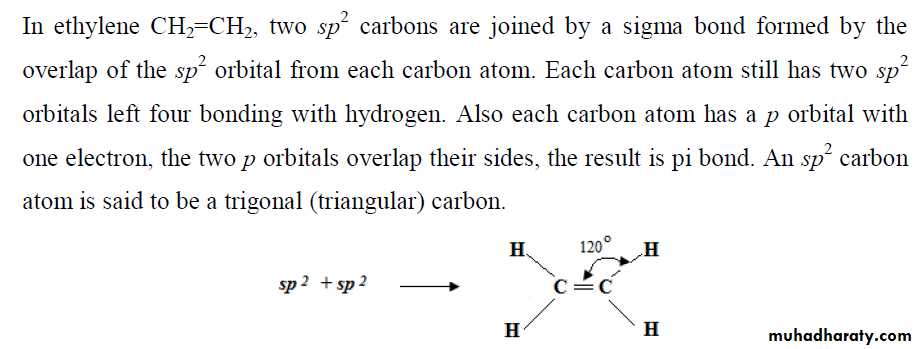
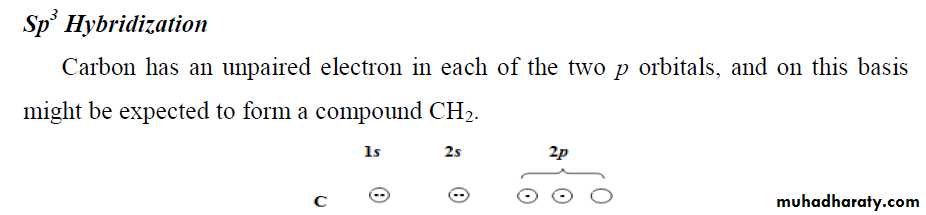
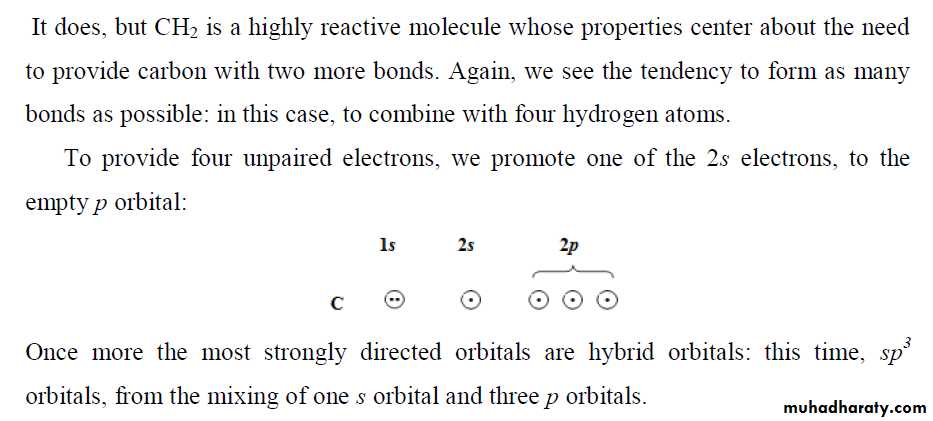
** a covalent bond to form two atoms must be located so that an orbital of one overlaps an orbital of the other; each orbital must contain a single electron. When this happens, the two atomic orbitals merge to form a single bond orbital which is occupied by both electrons (of different spins). This arrangement of electrons and nuclei contains less energy, and it is more stable than the arrangement in the isolated atoms.
** The covalent bond is strong because of the increase in electrostatic attraction. In the isolated atoms, each electron is attracted by, and attracts, one positive nucleus. In the molecule, each electron is attracted by two positive nuclei.
7
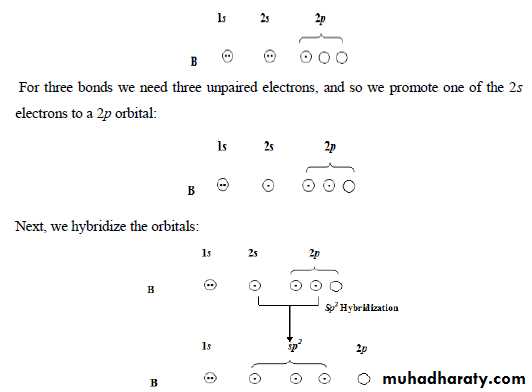
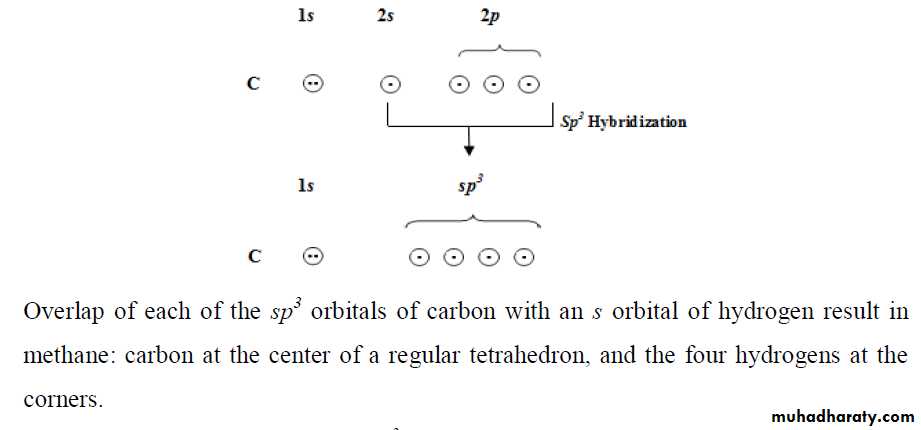
*** Hybrid Orbitals
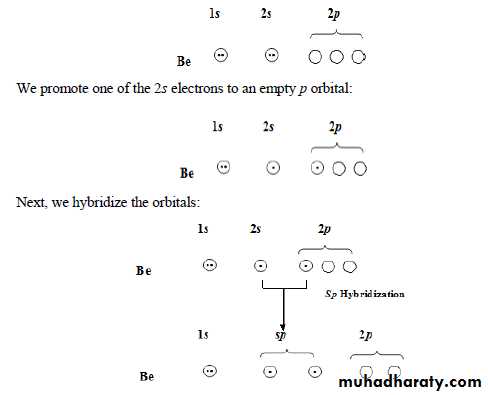
*** sp HybridizationIf we consider beryllium chloride, BeCl2, the electronic configuration of Be is:
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32