1
Amino acids, peptides and proteins
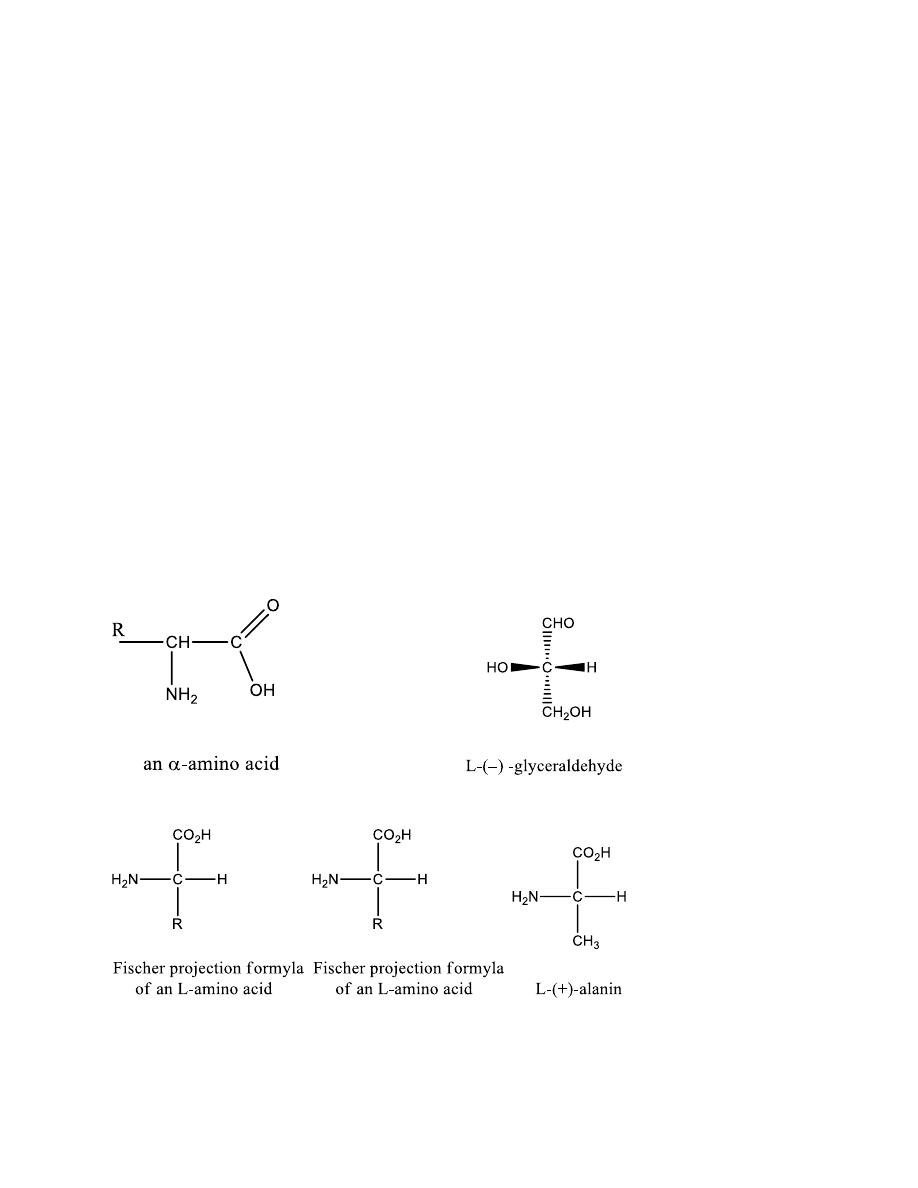
Amino acids: Carboxylic acids with an α–amino group
Peptides: consists of few linked amino acids
Proteins: composed of α–amino acids
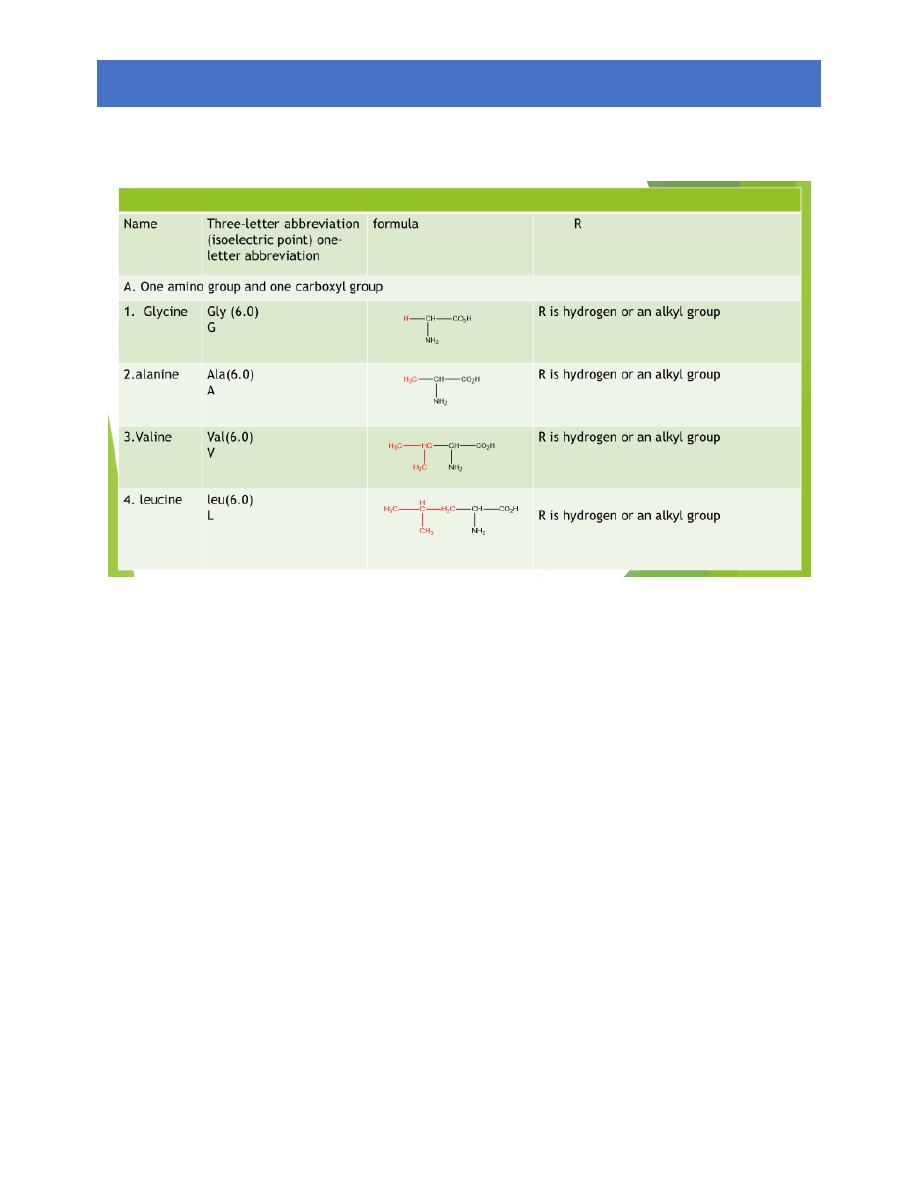
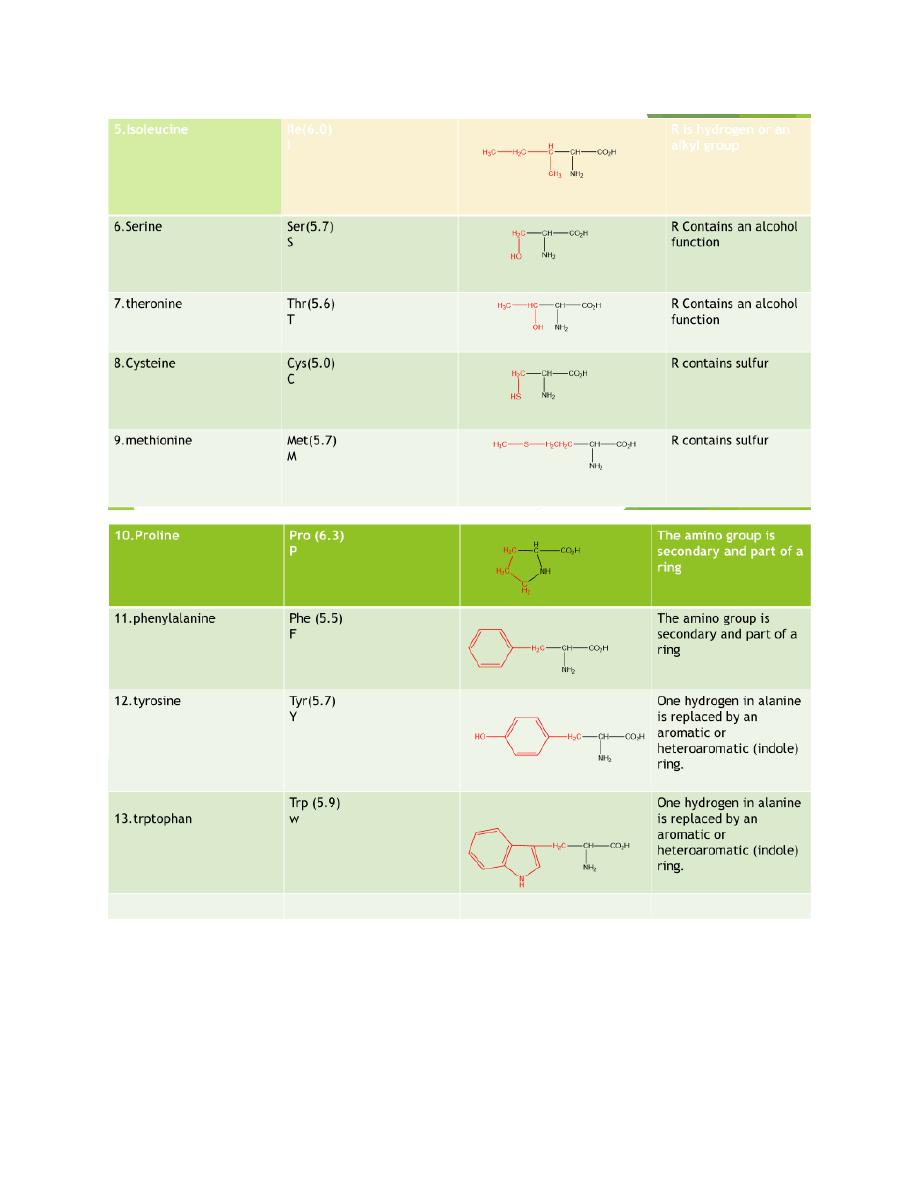
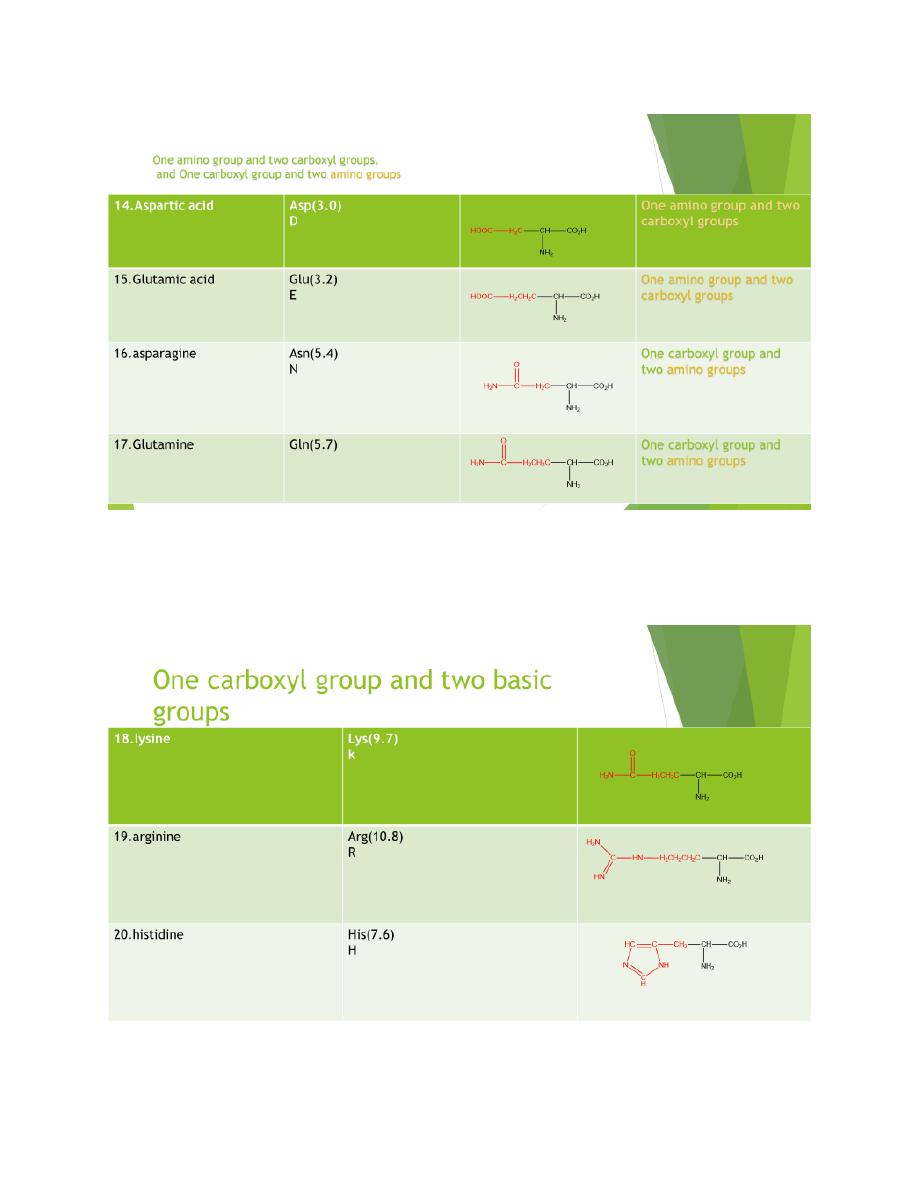
The amino acids obtain from protein hydrolysis are:
α -amino acids
Optically active (except glycine)
Have the L- configuration relative to glyceraldehyde

2
20 amino acids are comely found in proteins
12 can be synthesized in the body
8 (essential amino acids) cannot be synthesized in the
body, and must be obtain from the diet in the form of
proteins
A three letter abbreviation is used when writing the
formulas of peptides
A one letter abbreviation is used to describe the amino
acid sequence in a protein
3
4
5
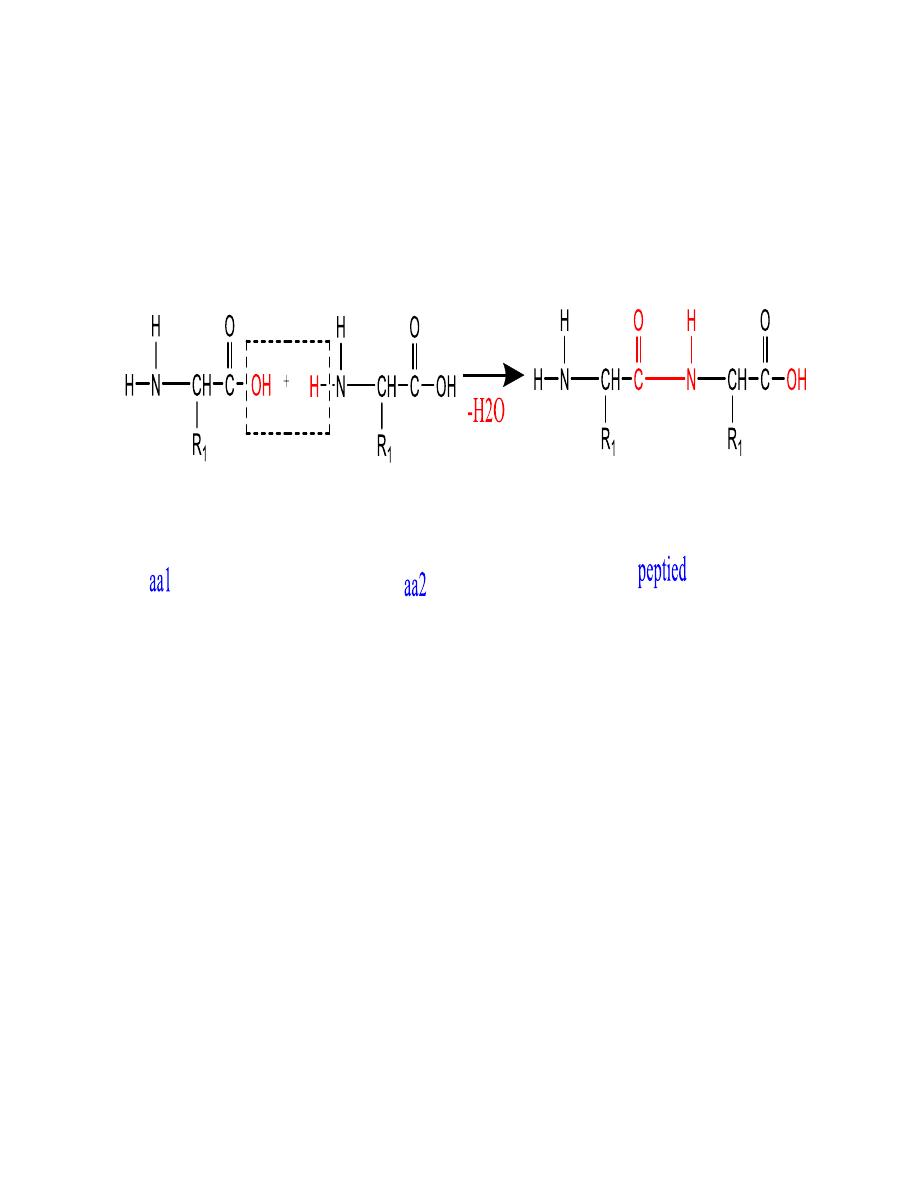
6
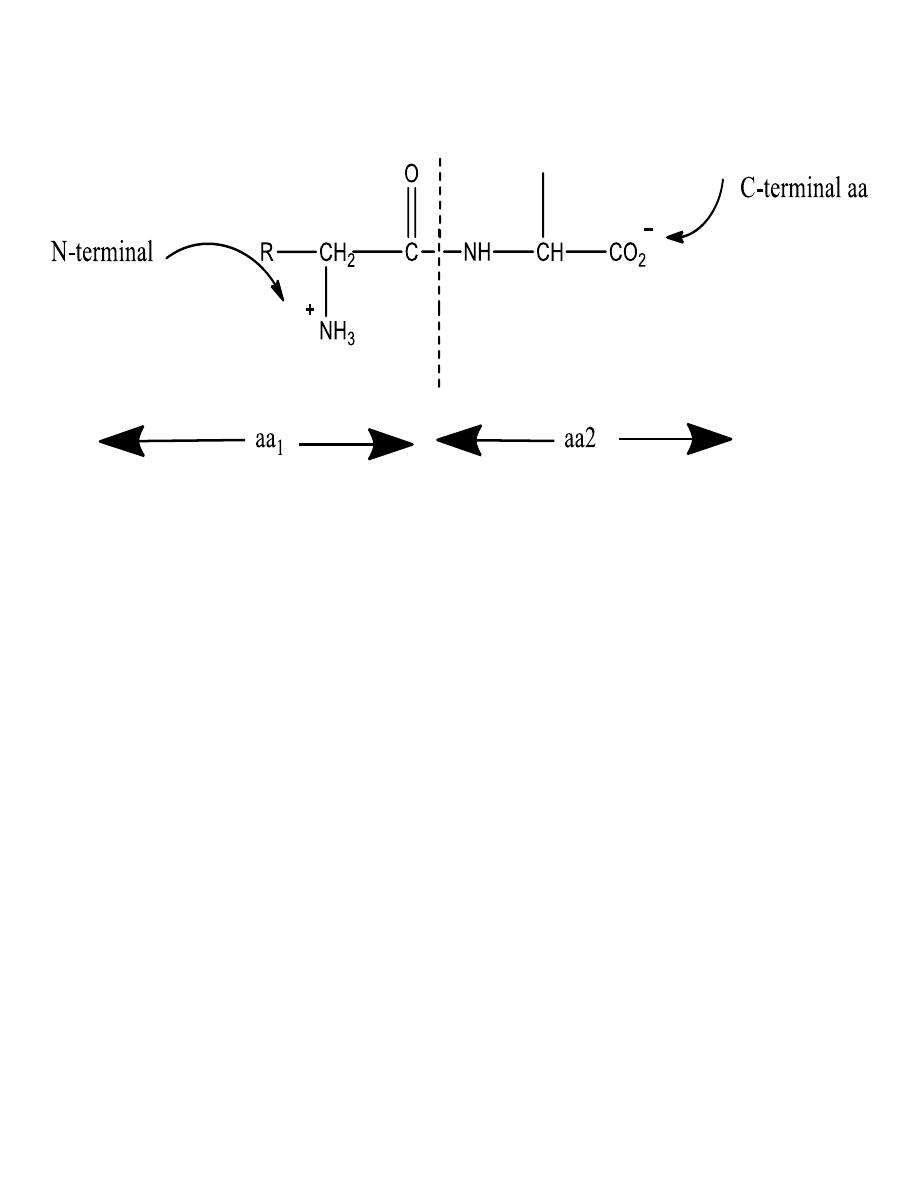
Removal of a water molecule
formation of the peptide
This general formation of
peptides or proteins
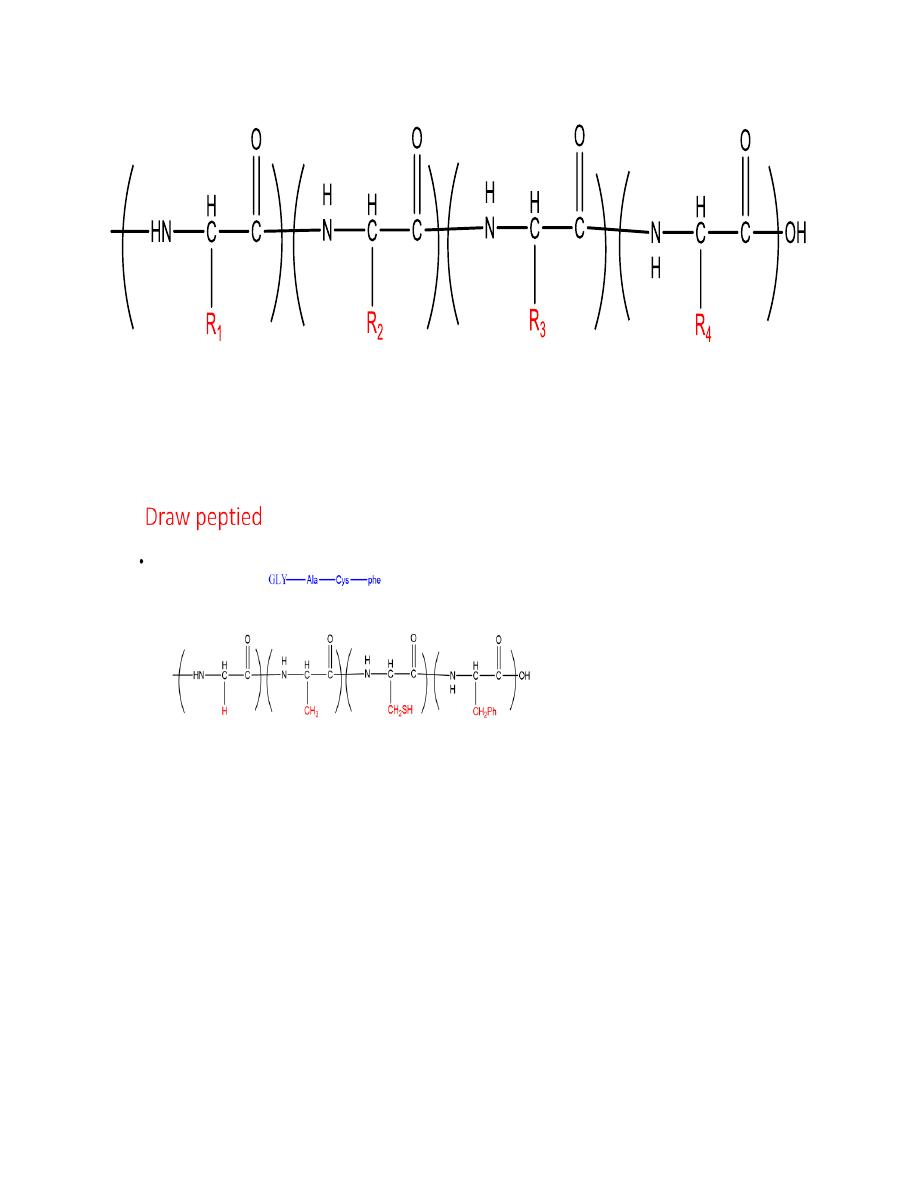
7
The acid base properties
of amino acids
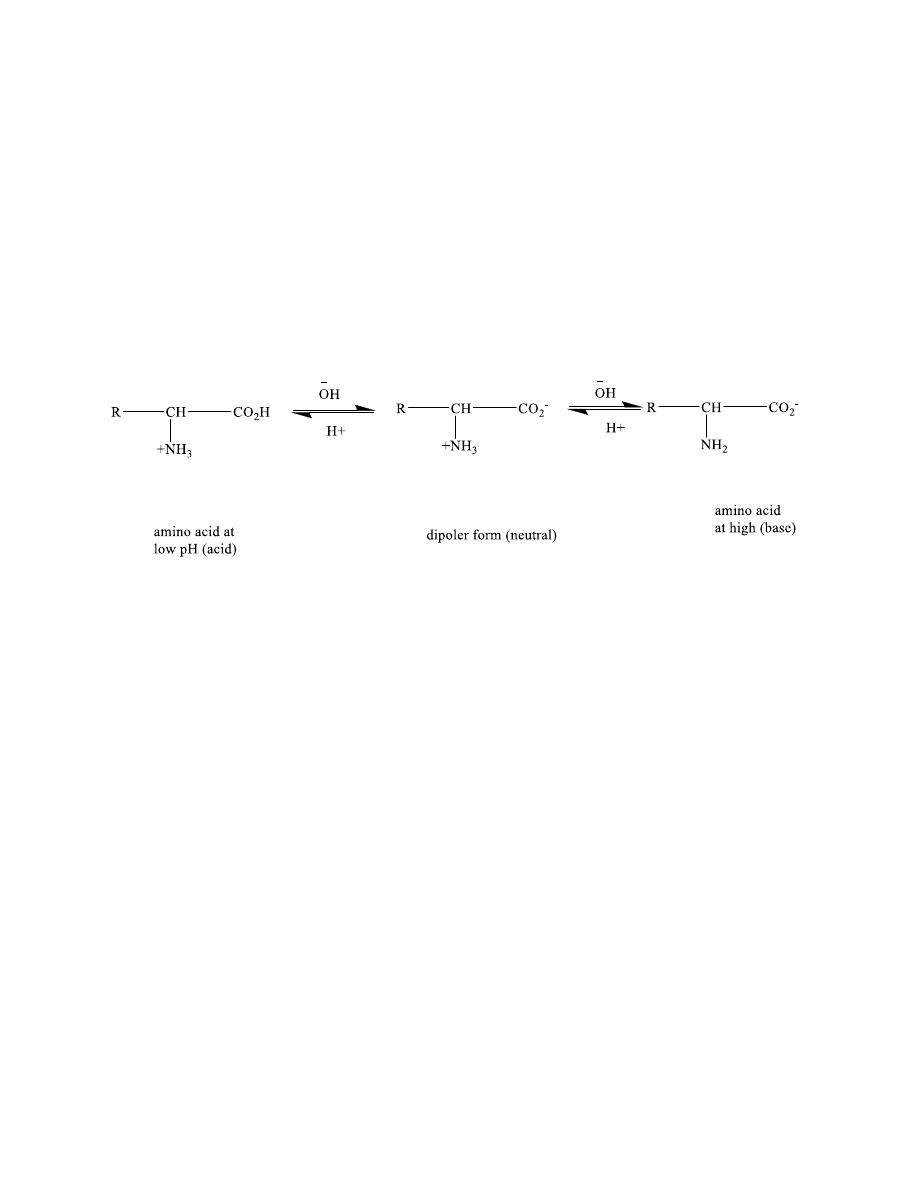
8
COOH (acidic group) NH2(basic group)
Amino acids are better represented by a dipolar ion
structure (Zwitterions)
• Starting with alanine hydrochloride (its structure
at low PH in hydrochloric acid is shown in Figure)
,write equation for its reaction with one equivalent
of sodium hydroxide and then with a second
equivalent of sodium hydroxide.
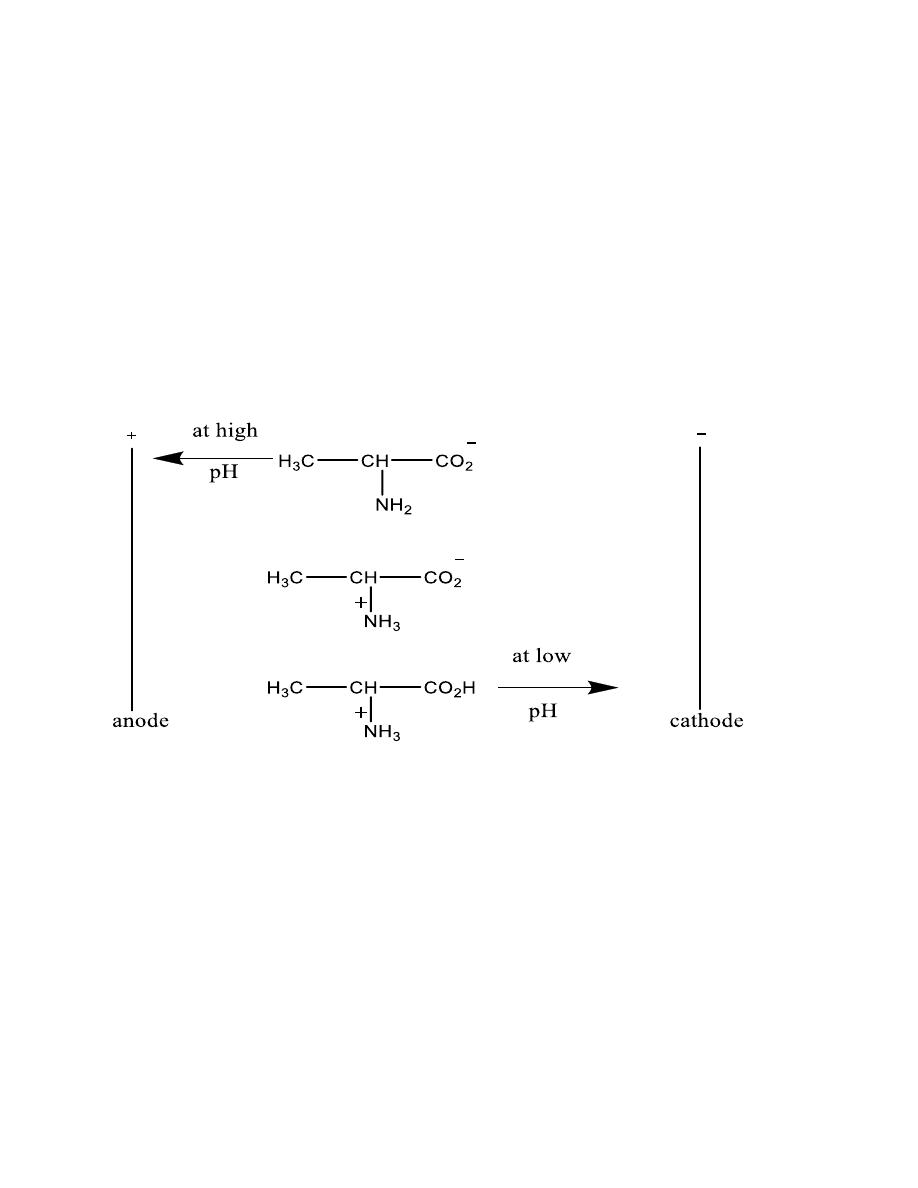
9
Amino acids in electric fields
•
Isoelectric point
: is the pH at which the amino acid
will be dipolar and have a net charge equal to zero.
It will not move toward either electrode
•
Electrophoresis
Electrophoresis:
is a method for separating amino
acids and proteins based on their charge differences
• Example
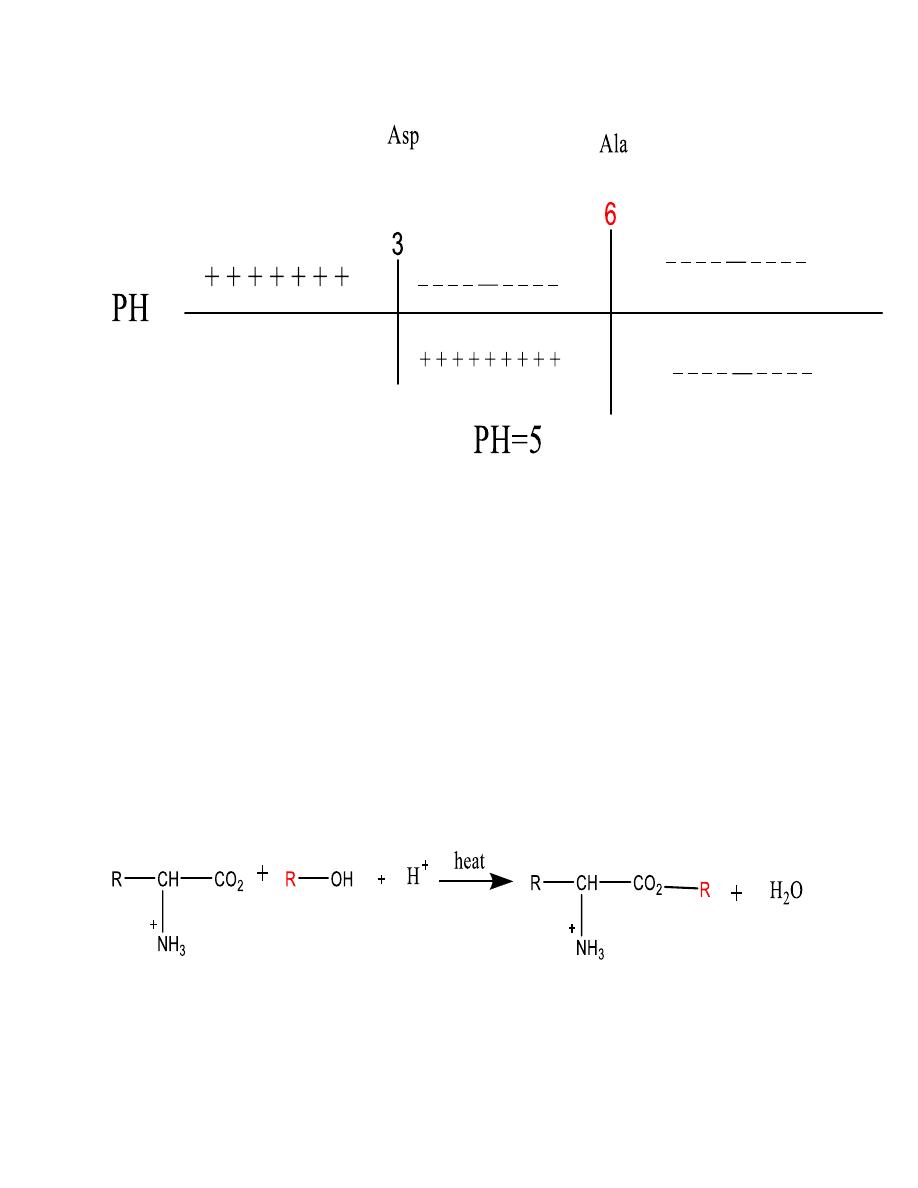
• pI for aspartic acid 3.0
pI for alanine 6.0
•
At pH 5
• Aspartic acid is negative
alanine is positive
10
• So they can be separated
• Problem
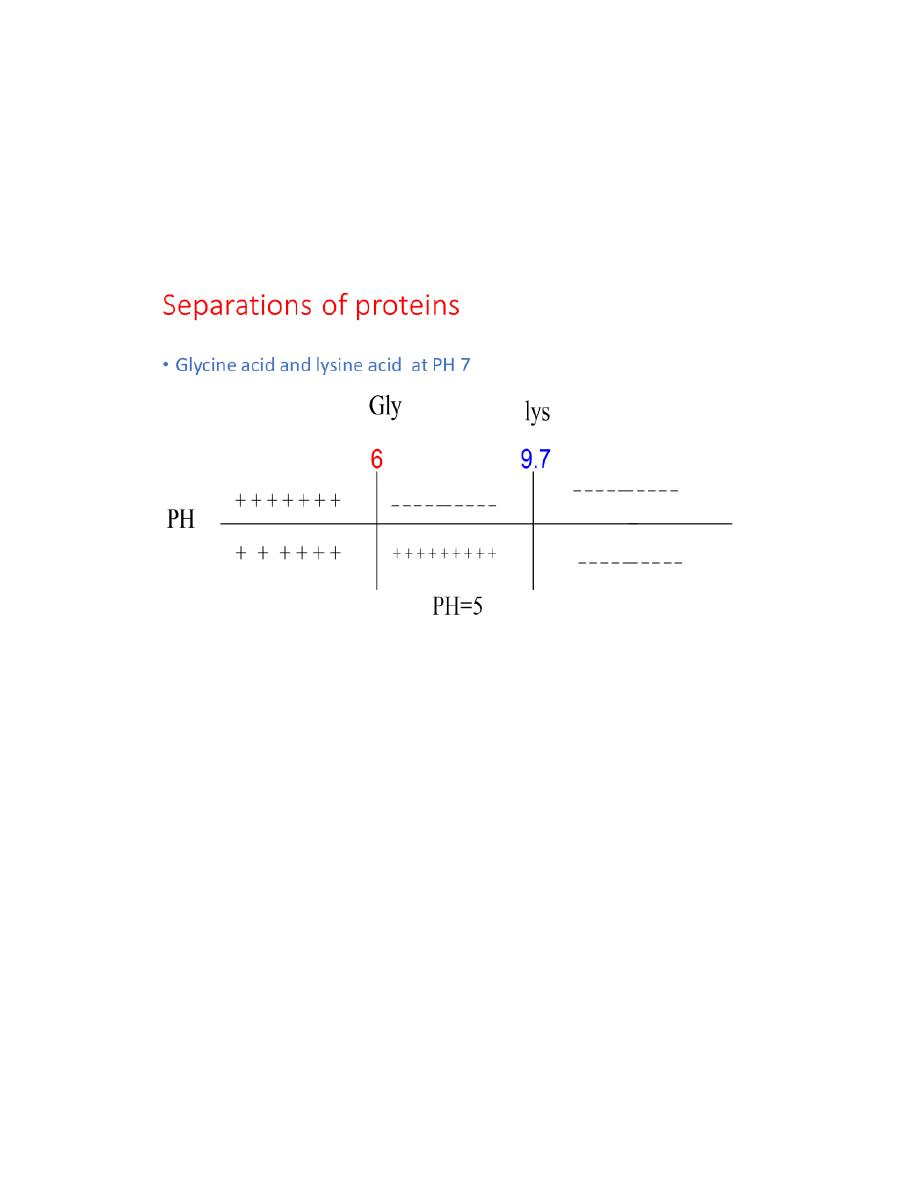
• Glycine and lysine at pH 7
• Phenylalanine, leucine and proline at pH 6
Separation of proteins
11
Reaction of Amino Acids
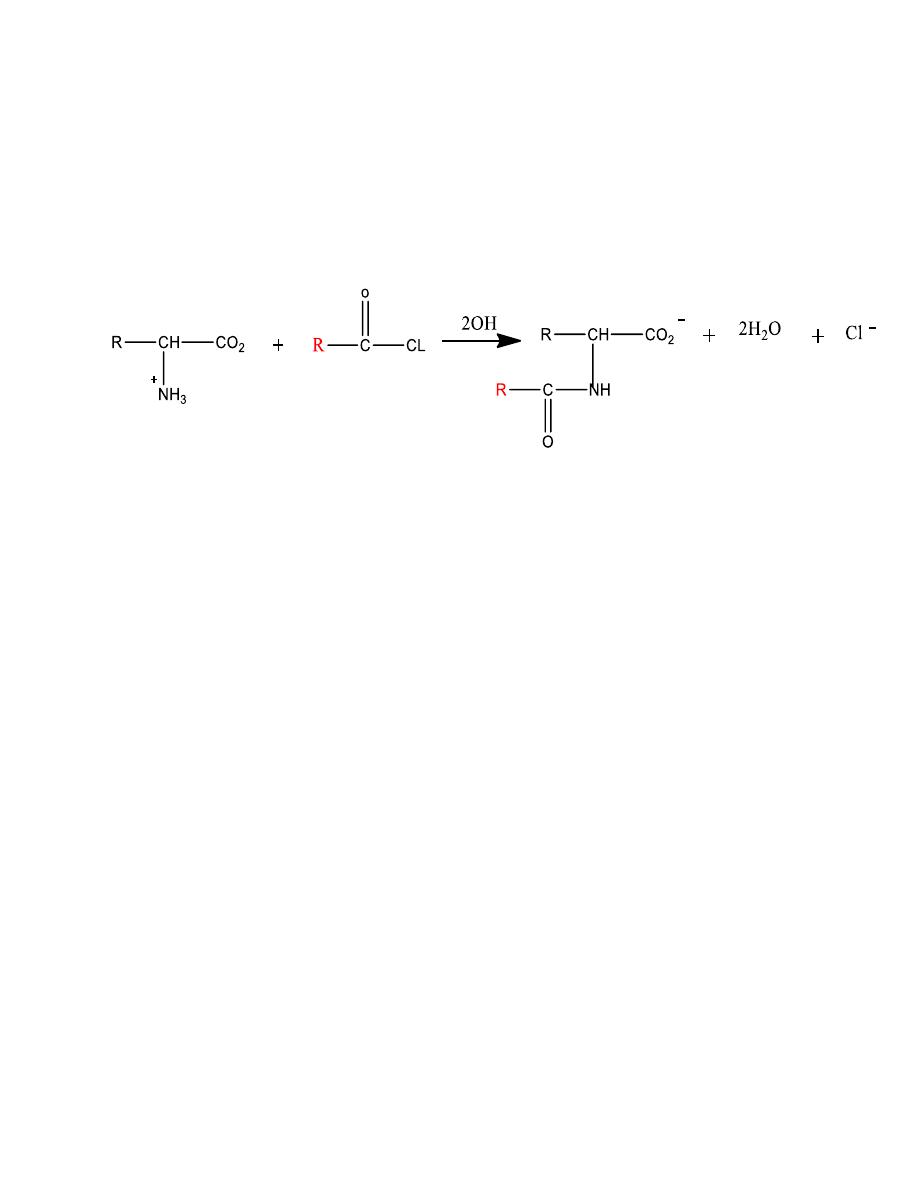
In addition to their acidic and basic behavior amino acids
undergo other reactions typical of carboxylic acids or
amines for example, the carboxyl group can be
esterified:
12
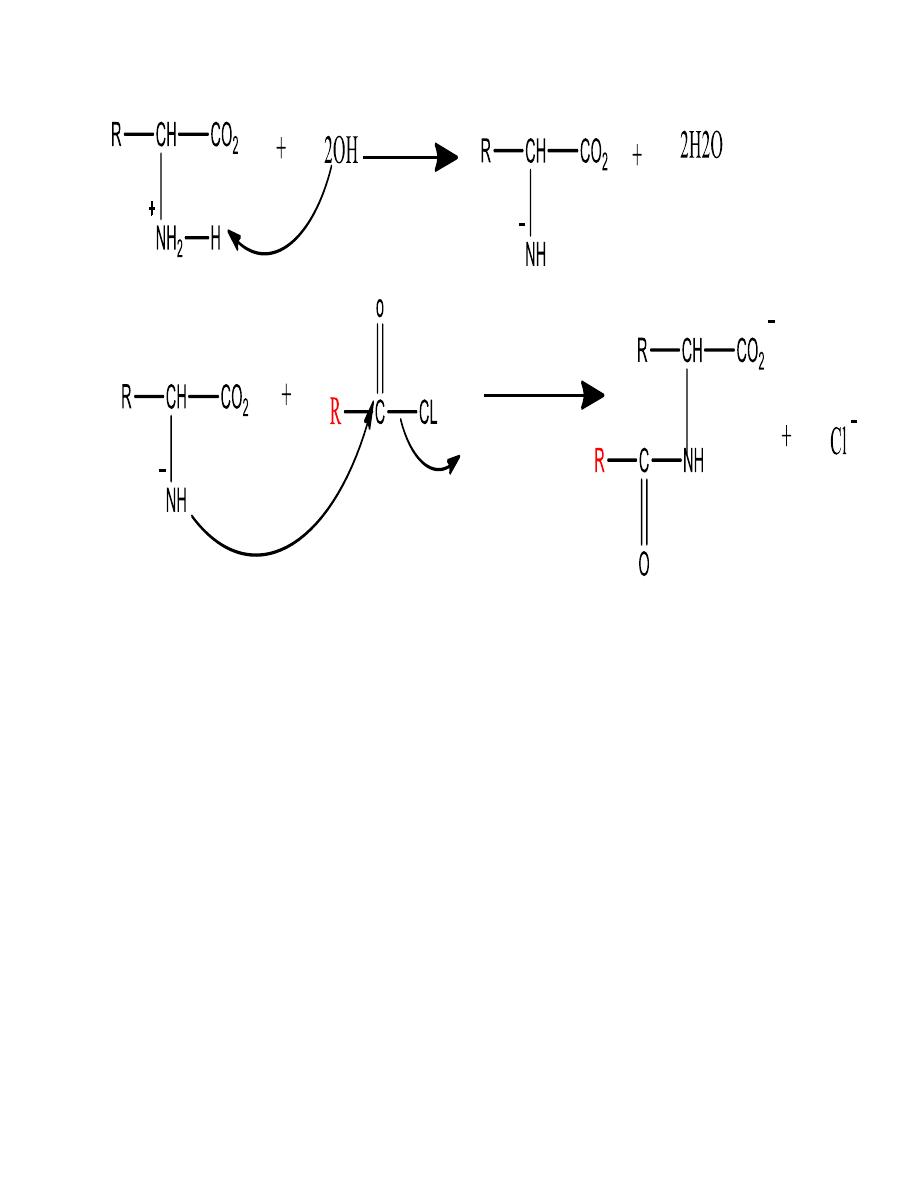
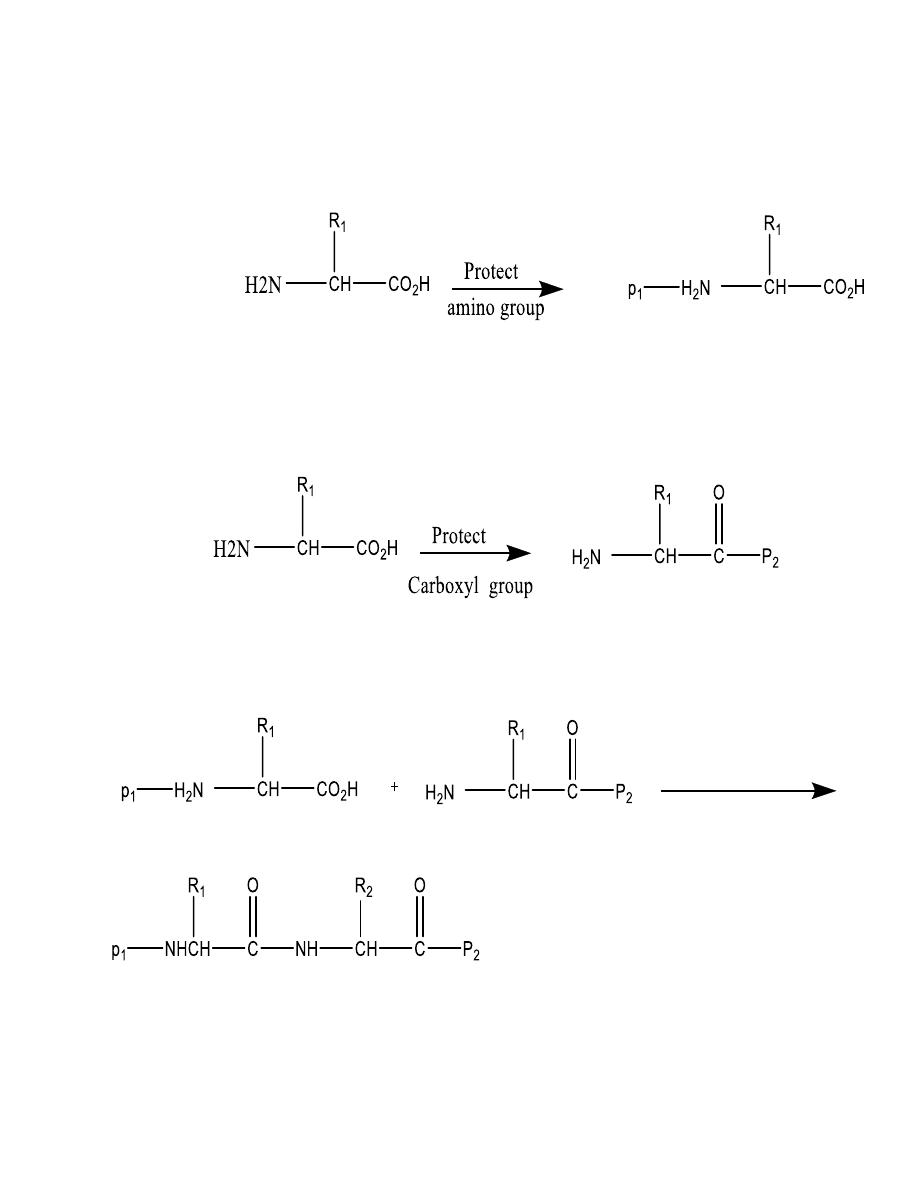
The amino group can be acylated to an amide
Mechanism
13
These types of reactions are useful in temporarily
modifying or protecting either of the two functional
groups, especially during the controlled linking of amino
acids to form peptides
Type equation here.
14
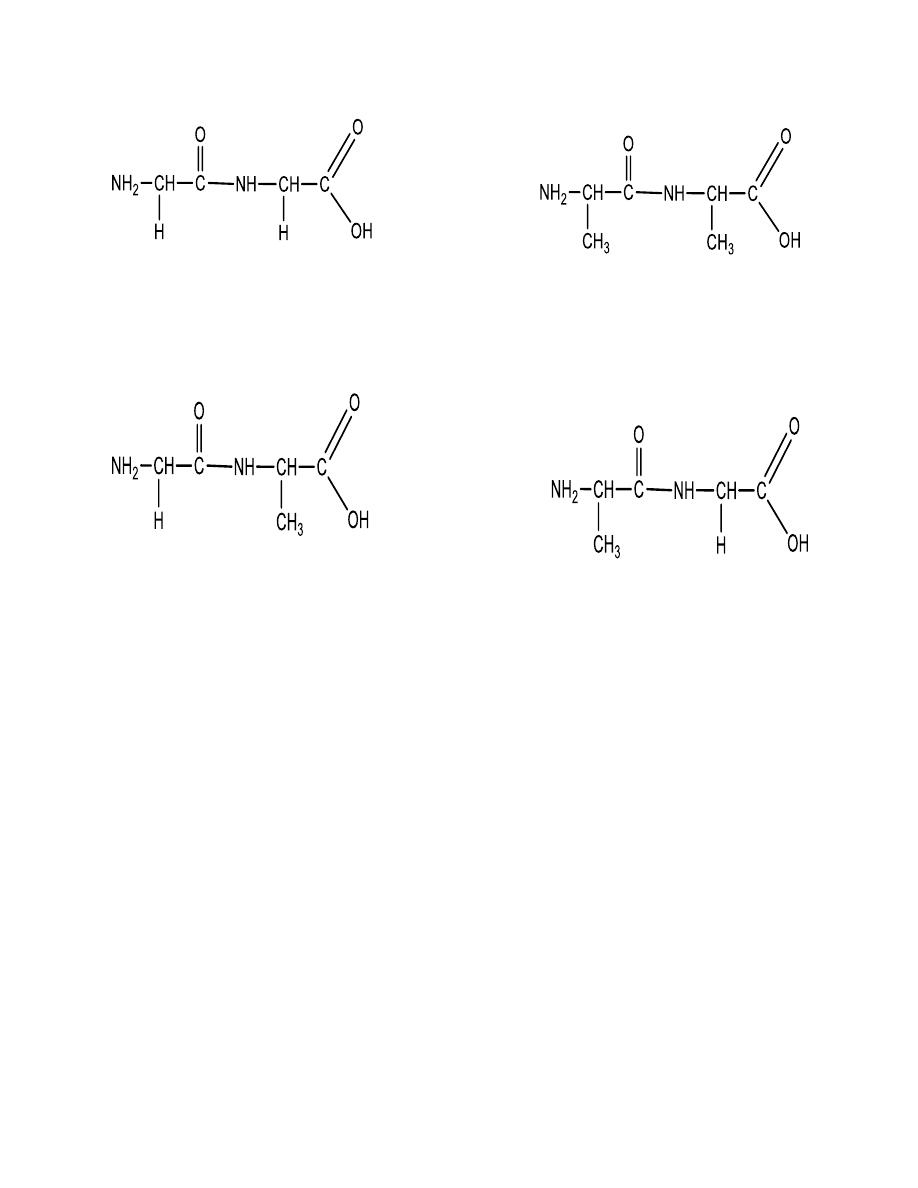
EXAMPLE:
Write the dipeptide structures that can be made by
linking alanine and glycine
SOLUTION There are two possibilities:
15
Peptide synthesis
linking amino acids in a controlled manner
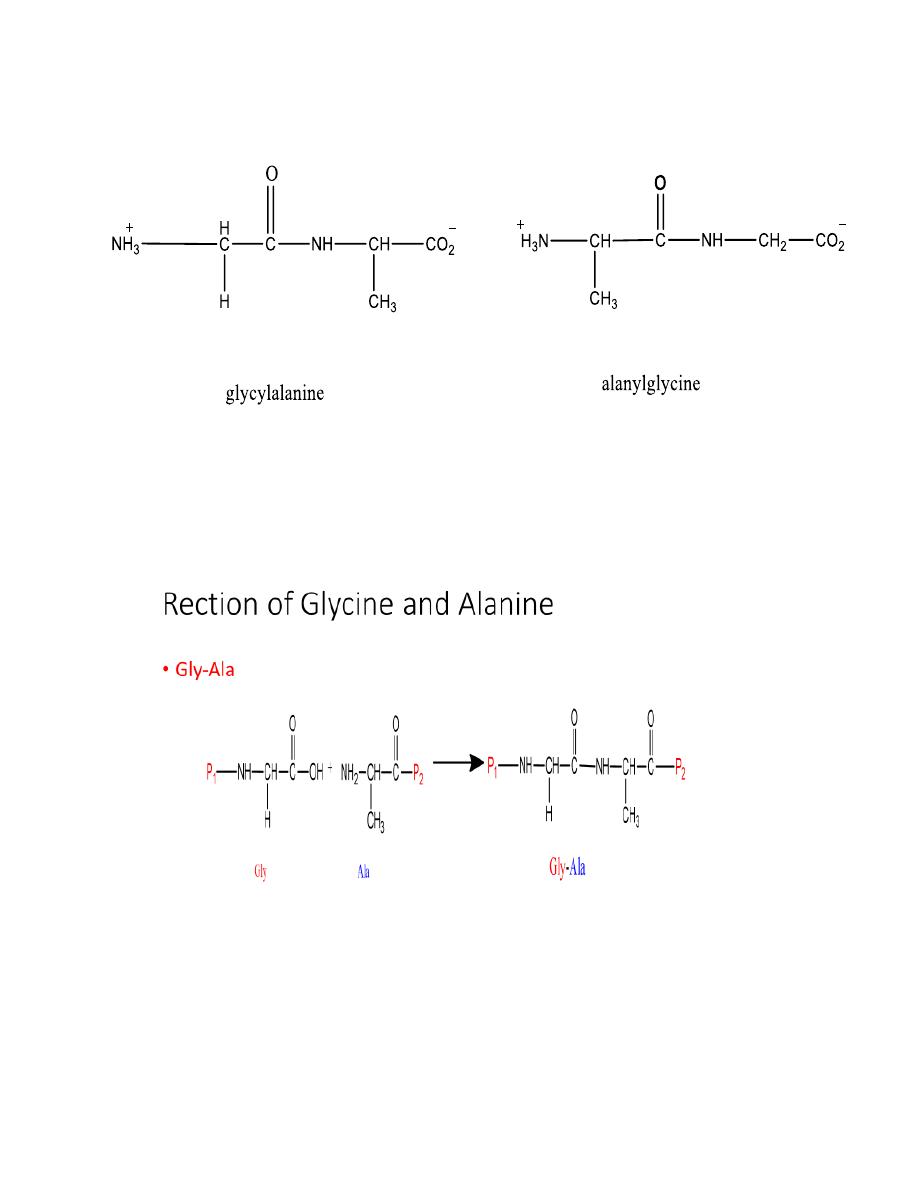
16
17
Gly-Gly Ala-Ala
Gly -
Ala
Ala-
Gly
Peptide synthesis linking amino acids in a controlled
manner
18
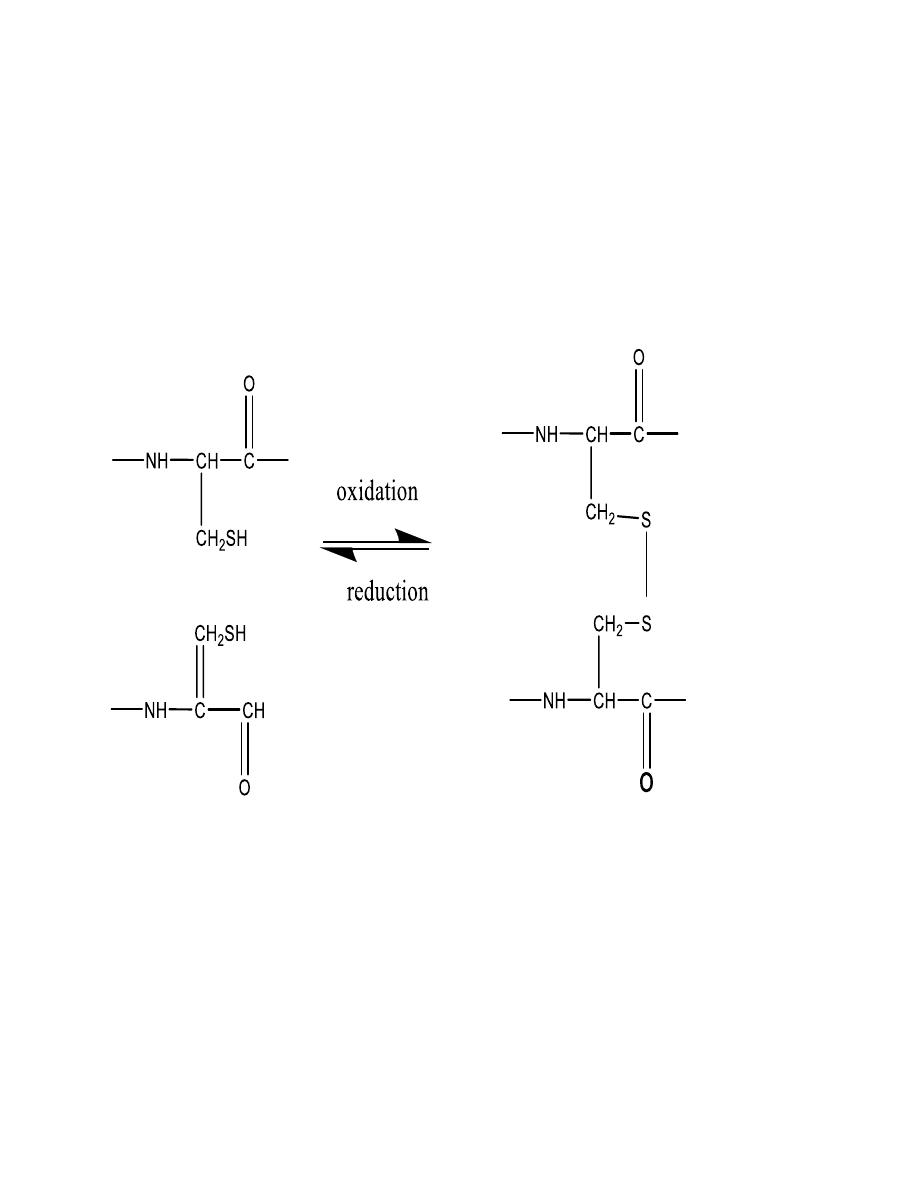
Oxidation and reduction
Aside from the peptide bond, the only other type of
covalent bond between amino acids in peptides and
proteins is the disulfide bond. It links two cysteine units.
Recall that thiols are easily oxidized to disulfides in (eq.) .
two cysteine units can be linked by disulfide bond.
Proteins
•
Proteins are major components of
:
•
Structural tissues
(muscle, skin, nails, hair)
•
Transport molecules
(Hemoglobin)

19
•
Enzymes
(biological catalysts)
•
Structure of Peptides and Proteins
:
•
Primary structure
: Amino acids and sequence
•
Secondary, tertiary
and quaternary structures:
•
three dimensional
aspects of the structure
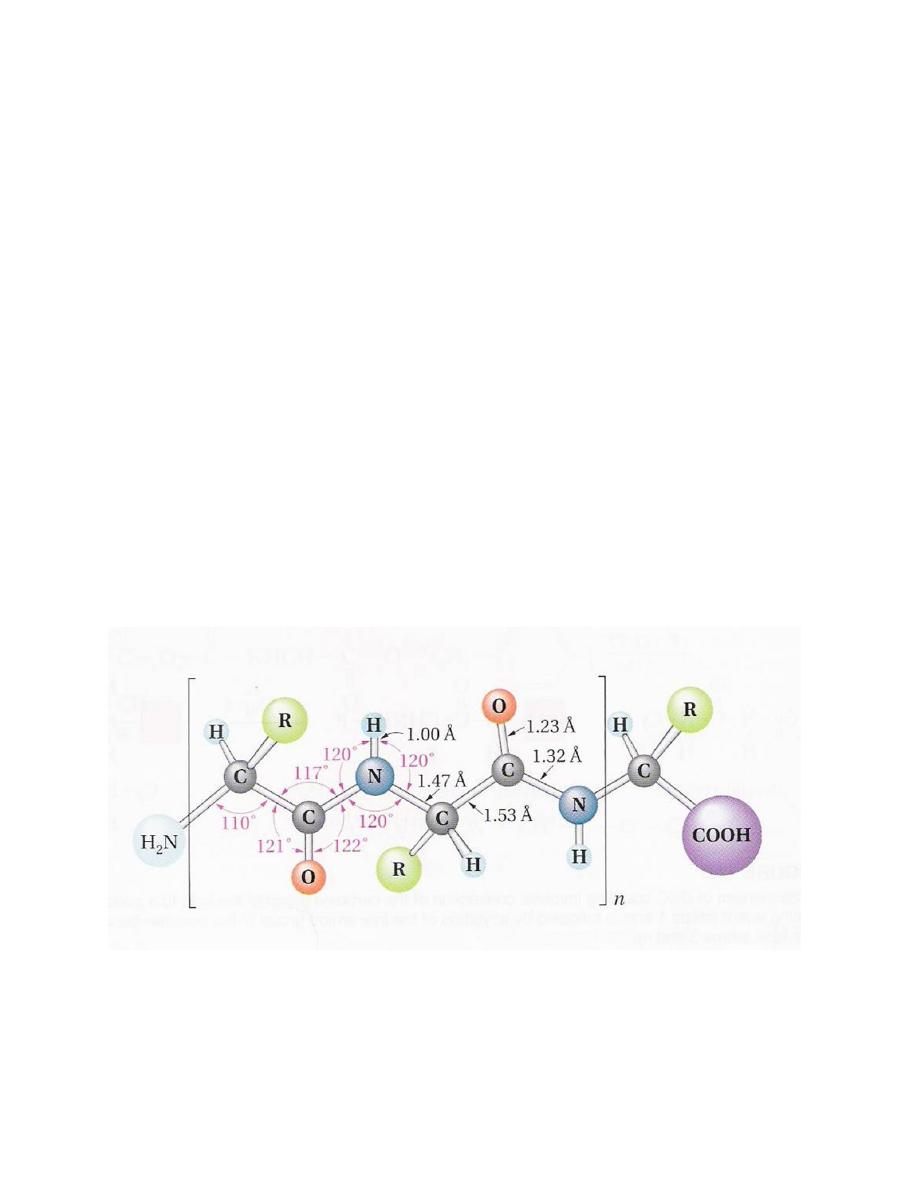
The primary structure
• The backbone of proteins is a repeating sequence
of one nitrogen and two carbon atoms
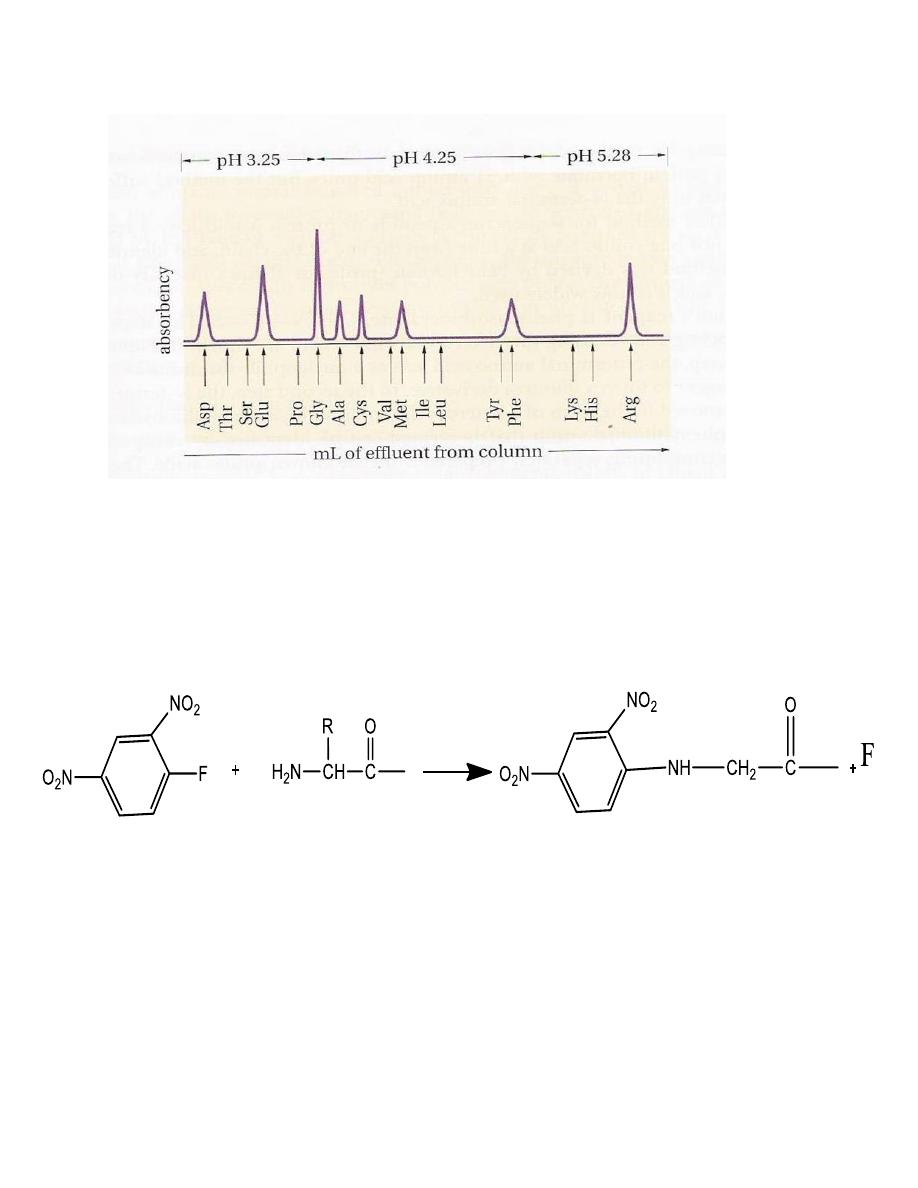
Hydrolysis of proteins and peptides (6 M HCl at 110 oC for 24
hours)
Amino acid analyzer
20
•
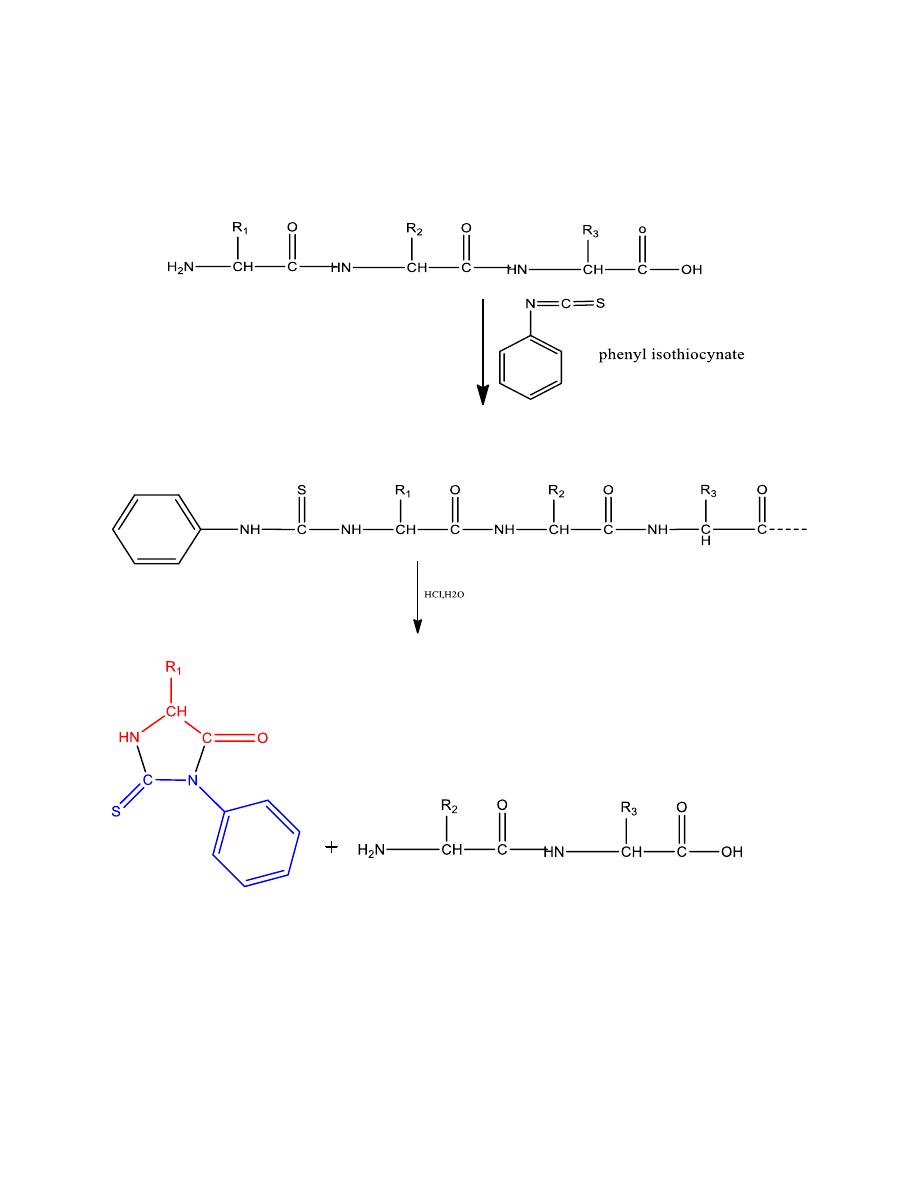
Sequence Determination
Sanger’s reagent is:
2,4dinitrofluorobenzene which
reacts with the NH2 group of amino acids and peptides
to give yellow 2,4-dinitrophenyl (DNP) derivatives
.
2,4dinitrofluorobenzene N-terminal DNP-peptide, labeled
Edman’s reagent
21
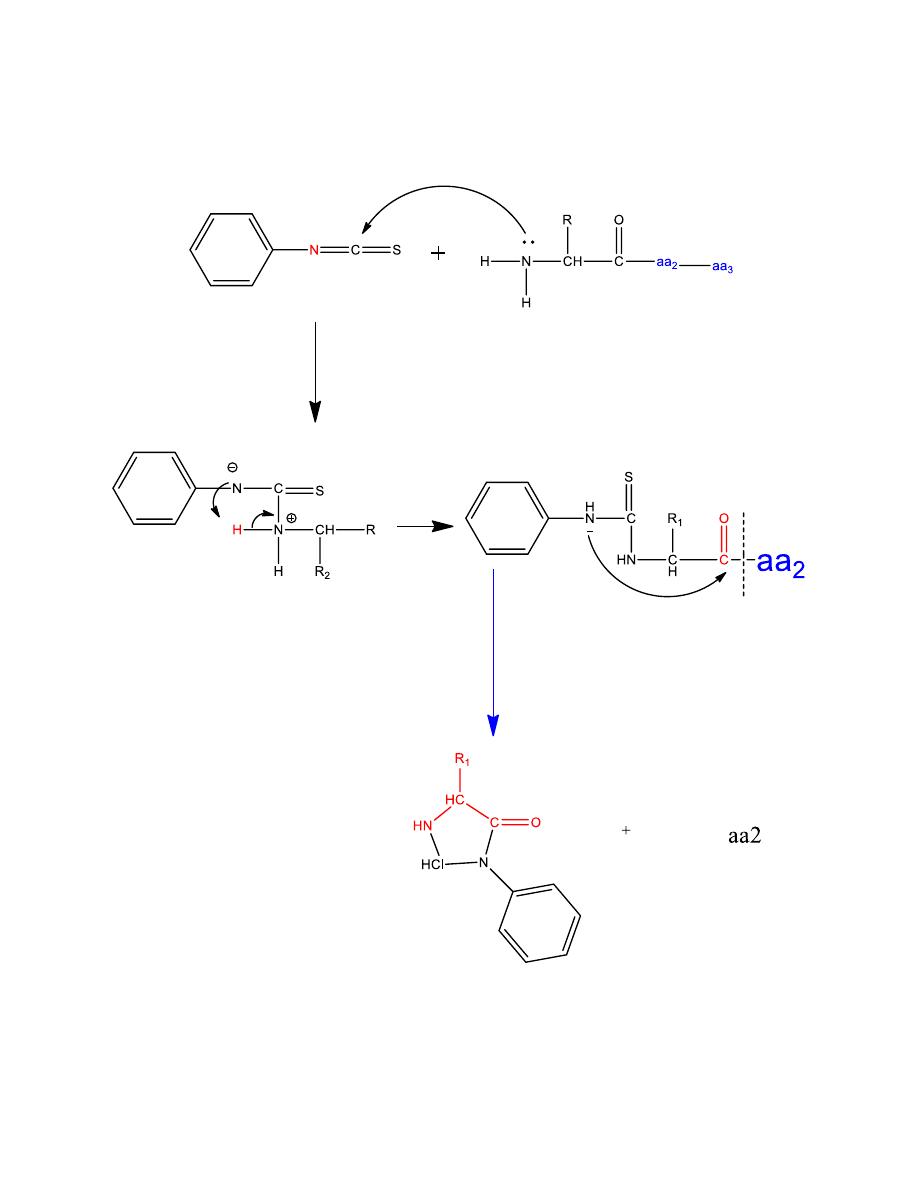
A reagent that clips off just one amino acid at a time
from the end of the chain
phenyllthiohydantion the next amino acid to be removed when the step sequence
N-terminal amino acid
Mechanism
22
C-terminal amino acid
23
Cleavage of selected peptide bonds

24
Proteins containing several hundred amino acid units are
better cleaved at particular peptide bonds using certain
chemicals or enzymes, then they
EXAMPLE:

25
Cinsider the following peptide
By referring top table determine what fragments will be
obtained when this peptide is hydrolyzed with
a. trypsin chymotrypsin cyanogen
SOLUTION
a. The enzyme trypsin will split the peptide on the
carboxyl side of lysine to give
b. the enzyme chymotrypsin will split the peptide on the
carboxyl sides of tyrosine and tryptophan, to give
cyanogen bromide will split the peptide on the carboxyl
side of methionine, thus splitting off the C-terminal
glycine and leaving the rest of the peptide untouched.
(carboxyl peptidase would do the same thing, confirming
that the C-terminal amino acid is glycine)
26
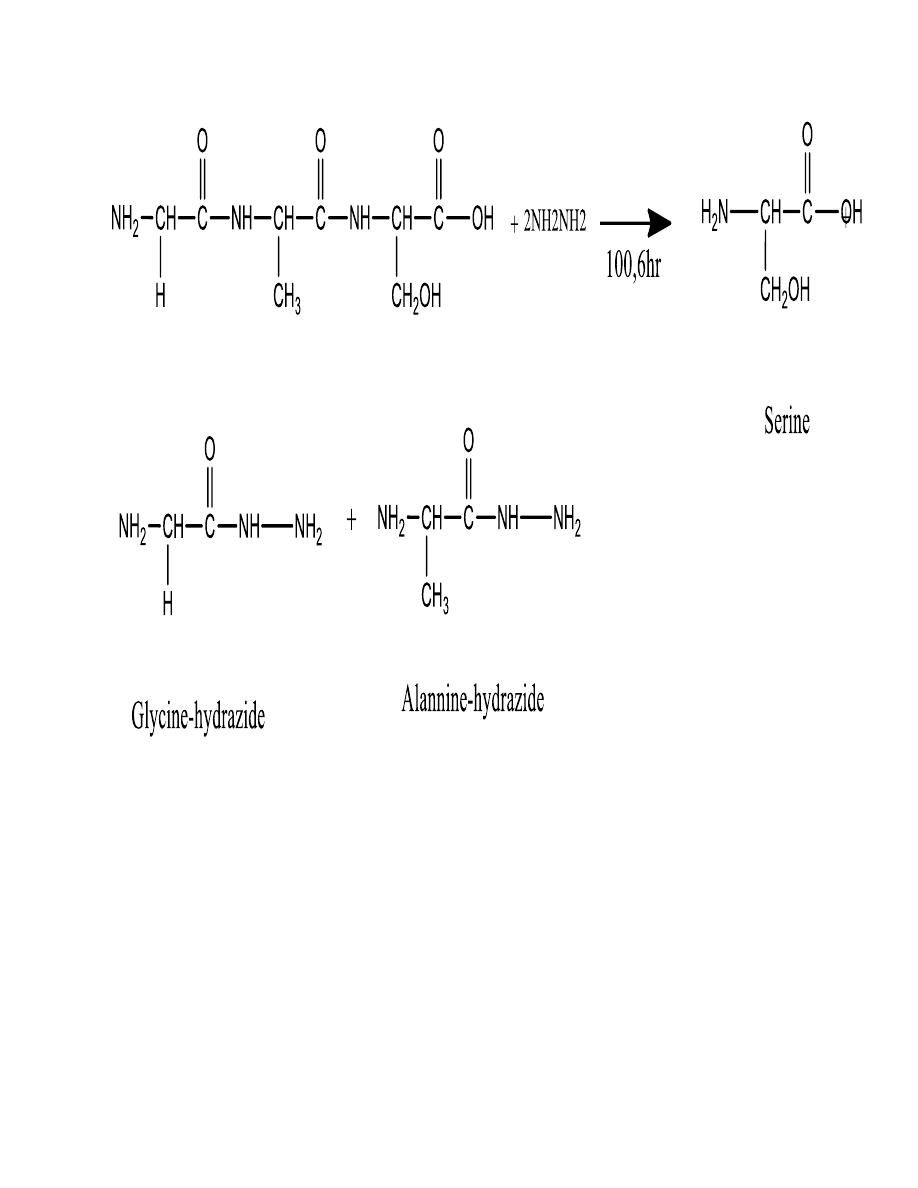
Insulin
Insulin (from Latin insula, island) is a peptide hormone produced by beta
cells of the pancreatic islets; it is considered to be the
main anabolic hormone of the body. It regulates
the metabolism of carbohydrates, fats and protein by promoting the
absorption of carbohydrates, especially glucose from the blood
into liver, fat and skeletal muscle cells. In these tissues the absorbed
glucose is converted into
either glycogen via glycogenesis or fats (triglycerides) via lipogenesis, or, in
the case of the liver, into both.
Glucose production and secretion by the
liver is strongly inhibited by high concentrations of insulin in the
blood.
Circulating insulin also affects the synthesis of proteins in a wide
variety of tissues. It is therefore an anabolic hormone, promoting the
conversion of small molecules in the blood into large molecules inside the
cells. Low insulin levels in the blood have the opposite effect by promoting
widespread catabolism, especially of reserve body fat
27
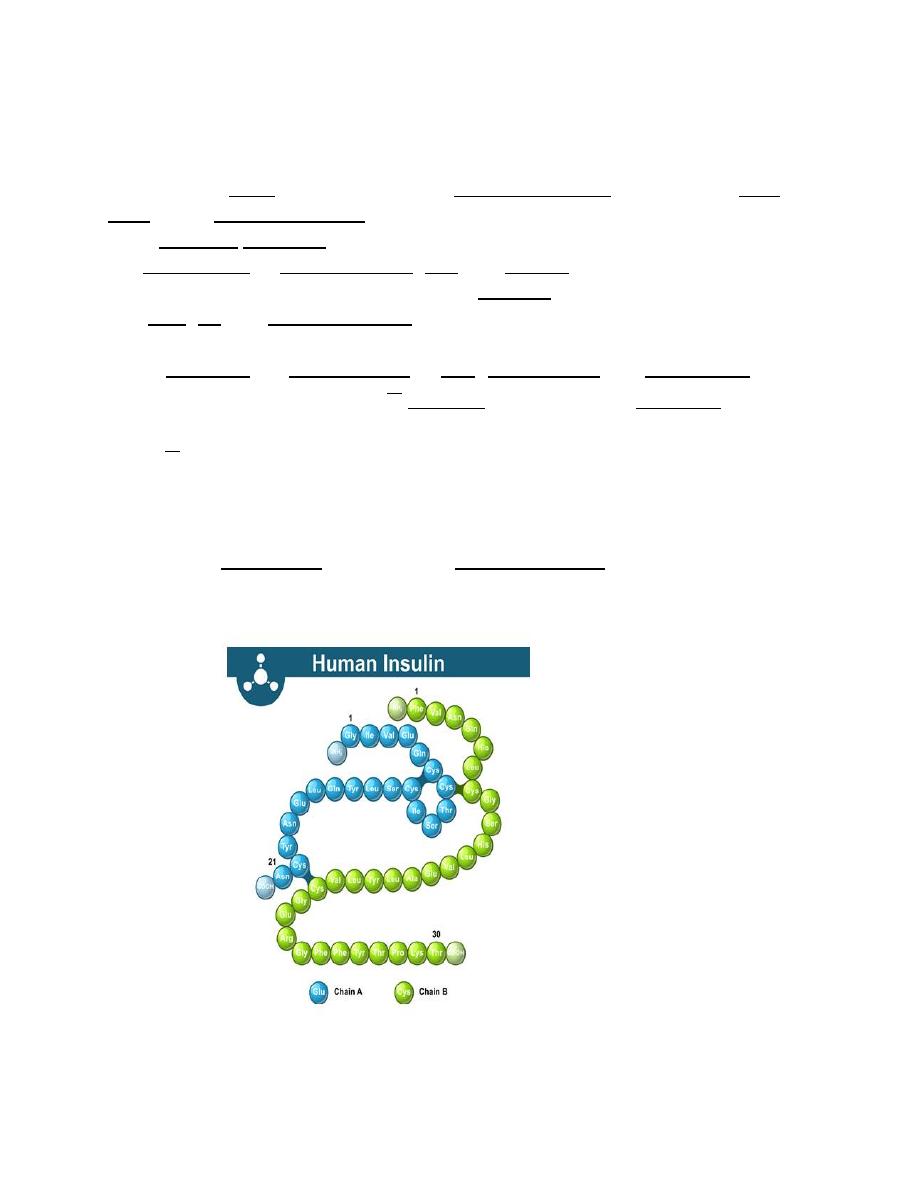
Vasopressin (Antidiuretic Hormone,
ADH)
Vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP)
or aggression is a hormone synthesized as a peptide prohormone in neurons in
the hypothalamus, and is converted to AVP. It then travels down the axon of that
cell, which terminates in the posterior pituitary, and is released from vesicles into
the circulation in response to extracellular fluid hypertonicity (hyperosmolality).
AVP has two primary functions. First, it increases the amount of solute-free water
reabsorbed back into the circulation from the filtrate in the kidney tubules of
the nephrons. Second, AVP constricts arterioles, which increases peripheral
vascular resistance and raises arterial blood pressure.
28
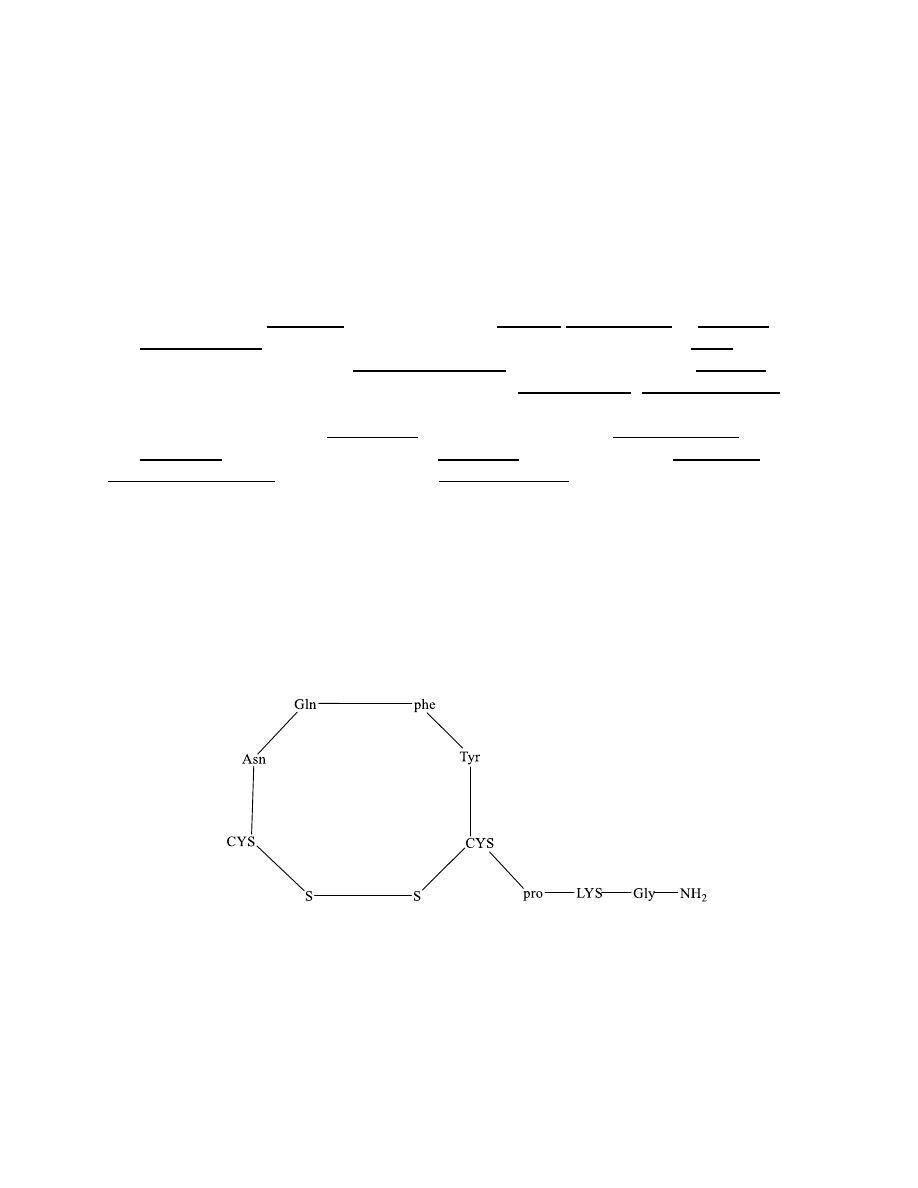
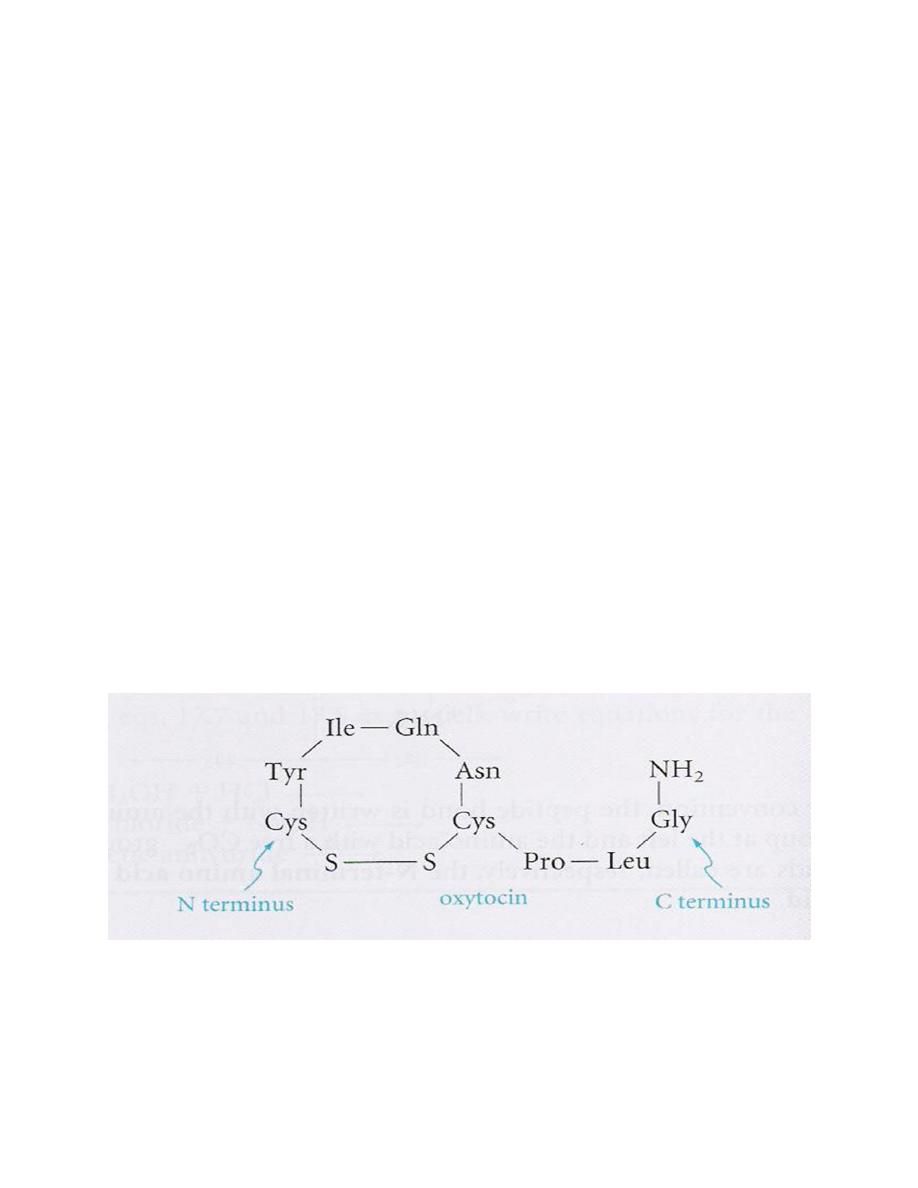
Oxytocin
To add more amino acids, we must selectively remove
one of the protecting groups and join the next amino acid
Oxytocin (prepared by Vincent du Vigneaud – Nobel
1955)
Oxytocin produced by posterior pituitary gland. It
regulates uterine contraction and lactation and may be
administered when it is necessary to induce labor at
childbirth
29
Secondary Structure of Proteins
Many polymers have been isolated in pure
crystalline form and are polymers with very well
defined shapes.
Geometry of the peptide bond
•
Planar geometry
•
Amide C-N bond (1.32 A) is shorter than
normal C-N bond (1.47 A)
•
Rotation around the amide bond is restricted
(double bond character)
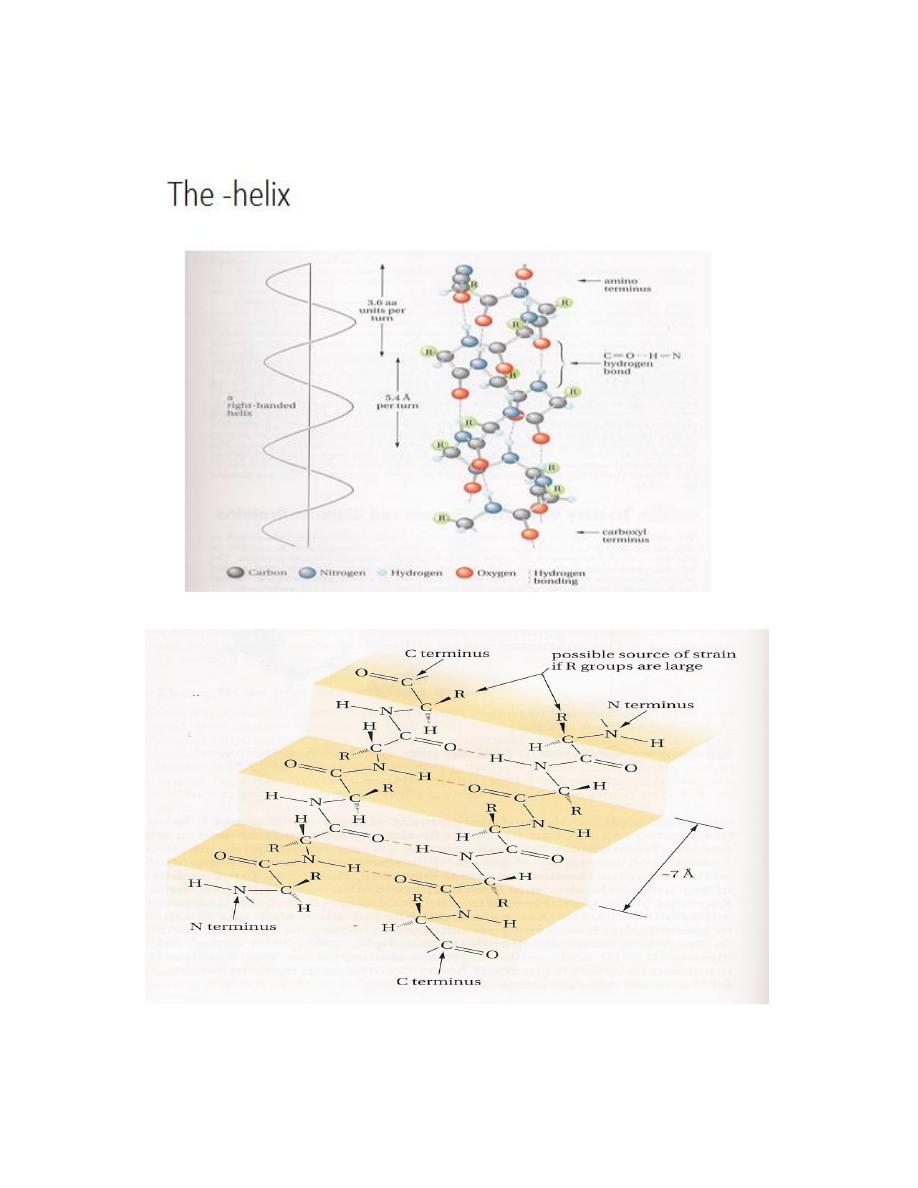
30
The – α helix
The pleated sheet
The Pleated sheet
31
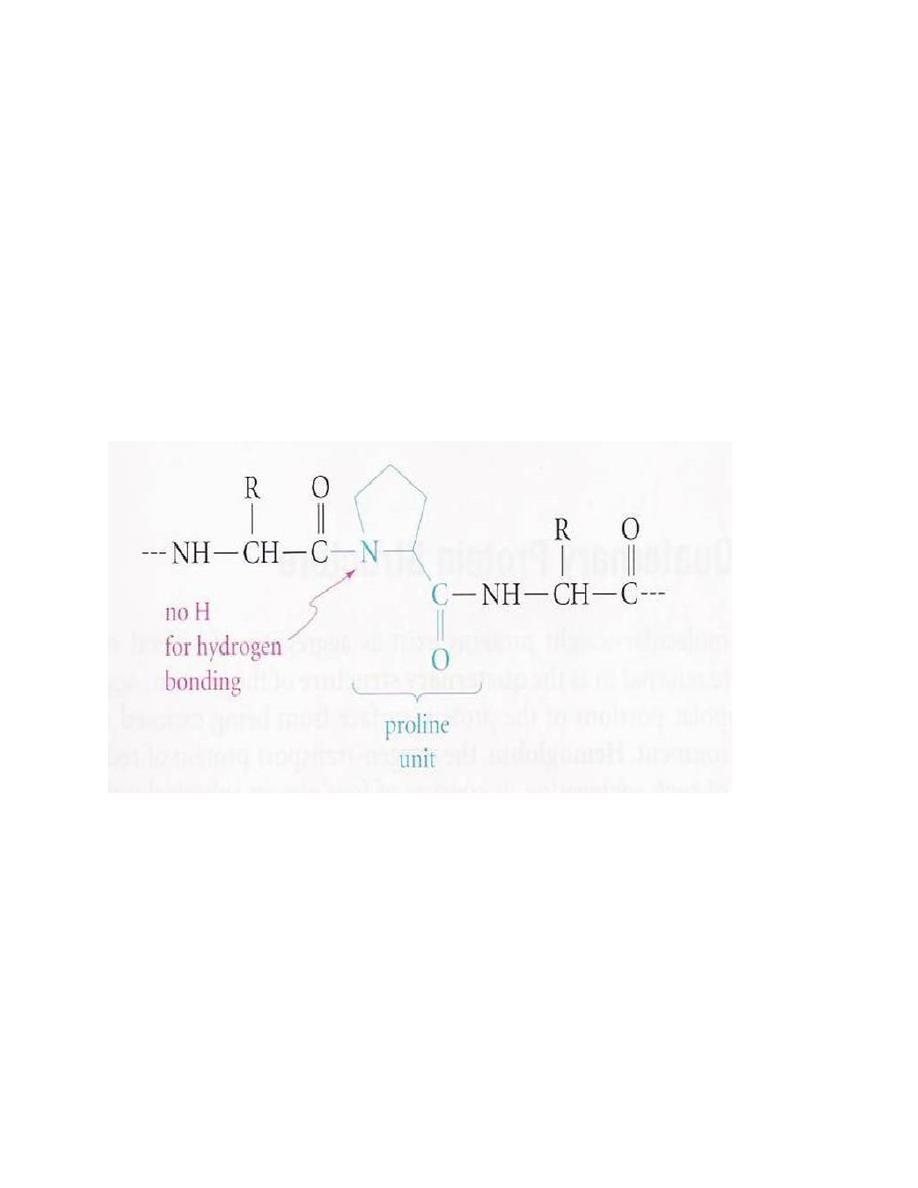
Tertiary structure: Fibrous and globular proteins
Three-dimensional structure of the protein which results
from the:
a)R groups b) the disulfide bonds
For example, turns are found at or near proline
(No H bonding)
Proteins
Fibrous (water insoluble) Globular (water soluble)
Keratins (protective tissues, hair, nails) Enzymes, hormones,
Collagens (connective tissues, blood, vessels) Transport proteins storage protein
Silks. Polar R groups outside
32
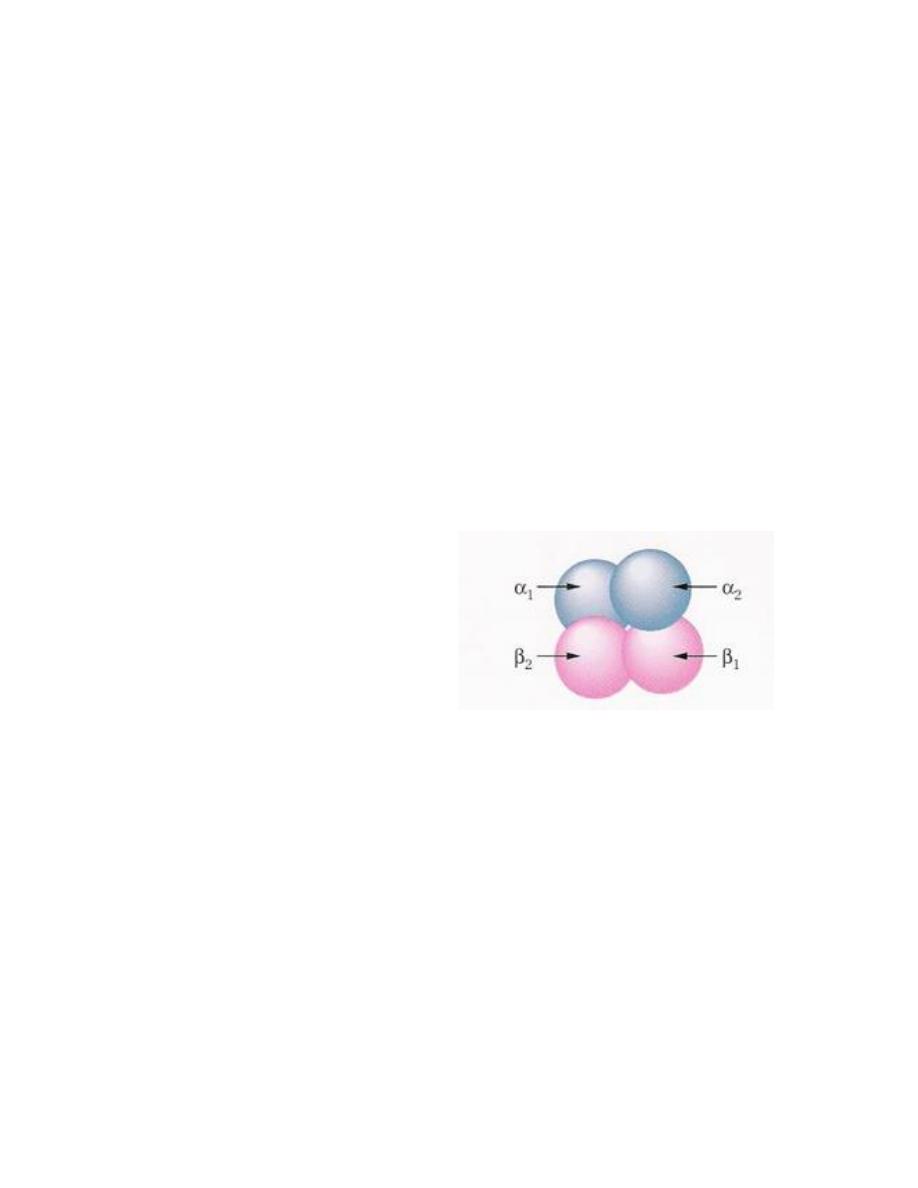
Quaternary Protein structure
Some high-molecular-weight proteins exist aggregates of several
subunits these aggregates are referred to as the quaternary
structure of the protein. Aggregation helps to keep nonpolar
portions of the protein surface from being exposed to the aqueous
cellular environment. Hemoglobin, the oxygen -transport protein of
red cells, provides an example of such aggregation. It consists of four
almost spherical units, two α units with 141 amino acids and two β
unit with 146 amino acids. The four units come together in a
tetrahedral array, as shown in this figure:
