
27
College of Medicine/Babylon University
Medical Physics Module
Session 3
Lecture 5:
Pressure
Objectives: after the end of this lecture, the student must know:
1- What is pressure and how can it affect our various body parts and organs
like skull, heart, lung….etc
2- Changes in pressure after diving
3- Principles of use of hyperbaric oxygen in the treatment of certain diseases
The pressure of column of liquid calculated
P = ρ g h
ρ = density
g = gravity acceleration
h = height of column
The peak systolic blood pressure 120 mmHg.
Pressure is defined force per unit area in gases or liquid while for solids the
quantity force per unit area is referred to as stress, the atmospheric pressure is
about 10 N/m² or 760 mm Hg.
Since we live in a sea of air with pressure 1 atm, it is easier to measure
pressure relative to atmospheric pressure.
There are places in the body where the pressure are lower than atmospheric
or negative for example:
When we breath (inspire) the pressure in the lung must be lower than
atmospheric pressure or the air would not flow to the lungs.

28
College of Medicine/Babylon University
Medical Physics Module
When person drink through straw the pressure in his mouth must be negative by
an amount equal to the height of his mouth above the level of the liquid he is
drinking.
Pressure inside skull
The brain contains approximately 150 cm³ of cerebrospinal fluid (CSF) in
series of interconnected cavities called ventricles, at birth if this opening is
blocked for any reason, CSF is trapped inside skull and increase internal pressure.
Pressure causes skull to enlarge, this called hydrocephalus and is common in
infant. The crude method for detecting hydrocephalus is to measure
circumference of the skull just above the ears, normal value of newborn infant
are from (32-37)cm, larger value indicate hydrocephalus.
Eye Pressure
The fluids in the eye ball (aqueous and vitreous humors) that transmit light to
retina. The dimensions of the eye are critical to good vision, a change of 0. 1 mm
in its diameter has effect on clarity of vision.
Pressure in normal eye 12—33 mmHg. The eye continuously produce aqueous
humor and the drainage system allows surplus to escape. If partial blockage of
this drain system occurs, the pressure increases and restrict the blood supply to
retina, and thus effect vision, this condition called glaucoma producing tunnel
vision
in moderate cases , and blindness in severe cases.
Pressure In The Digestive System
The pressure is greater than atmospheric in most gastrointestinal system. The
pylorus valve prevent the flow of food back into stomach from small intestine,
occasionally blockage forms in small or large intestine, and the pressure builds
College of Medicine/Babylon University
Medical Physics Module
up between blockage and pylorus valve, this pressure become greater enough
to restrict blood flow to critical organs, it can cause death. Intubations, the
passing of hollow tube through the nose, stomach, and pylorus is usually used to
relieve the pressure. If intubation does not work it is necessary to relive the
pressure surgically. The pressure gastrointestinal GI system is coupled to that in
the lungs through flexible diaphragm.
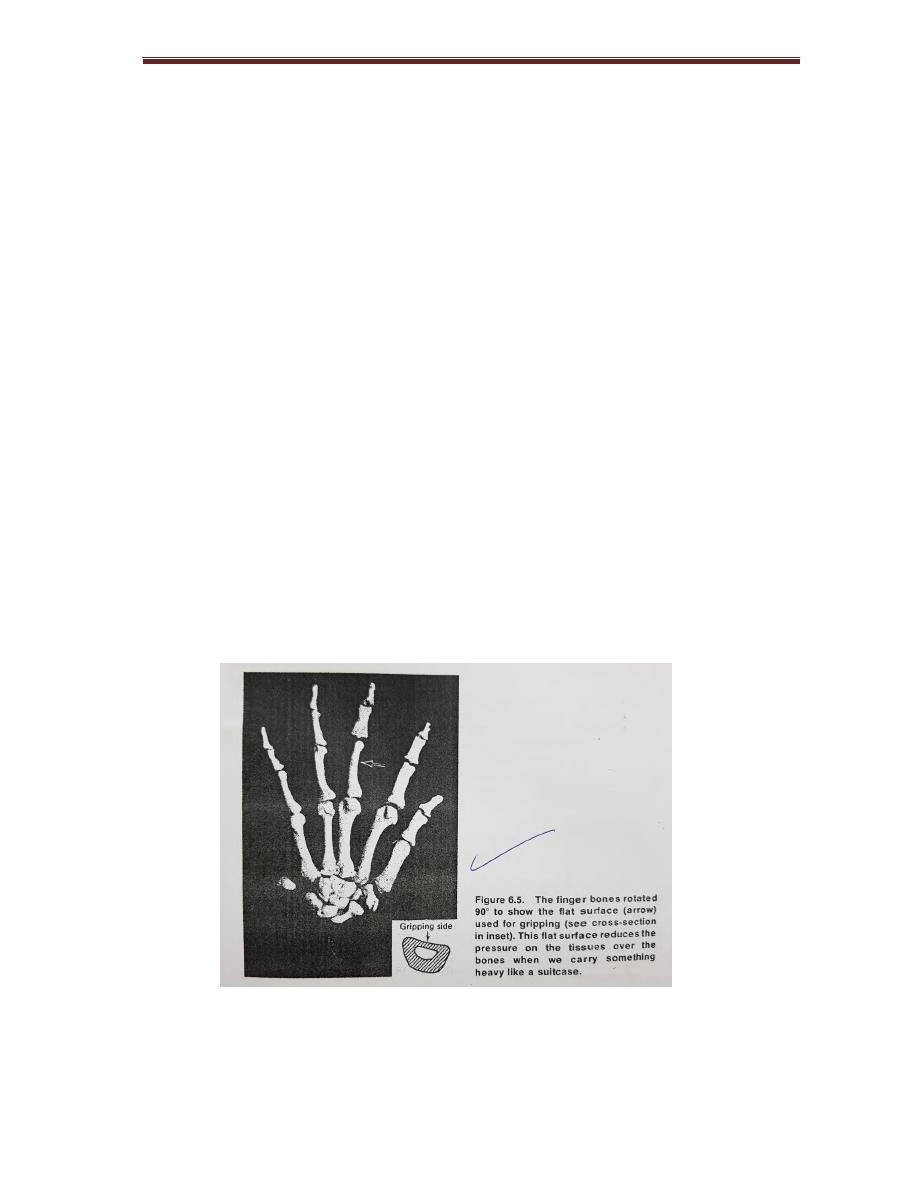
Pressure In The Skeleton
Since pressure is the force per unit area, the pressure is reduced as area
increased
The surface area of bone at joint˃ its area above or below the joint, thus
reducing pressure.
Finger bones are flat rather than cylindrical on the griping side , and the force
spread over large surface, this reduces pressure in the tissue over bones
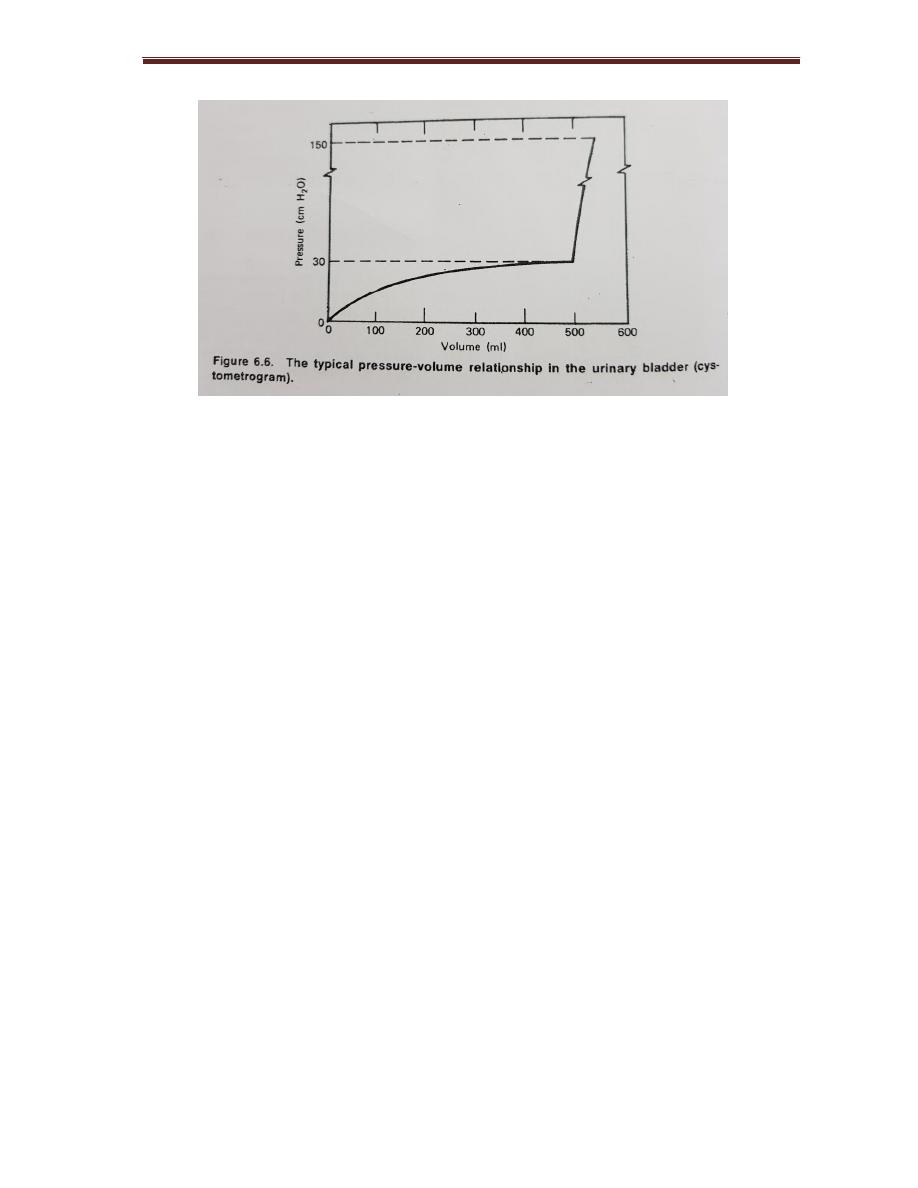
Pressure in the urinary bladder
There are internal pressure p in the bladder due to accumulation of urine, Fig
shows(P- volume) curve for bladder. For given increase in radius R, the volume

30
College of Medicine/Babylon University
Medical Physics Module
increase as R³ while pressure increases as R².
For adult the maximum volume in bladder before voiding is 500 ml
College of Medicine/Babylon University
Medical Physics Module
Figure 5-1:
typical pressure volume relationship of the bladder.
In men who suffer prostatic obstruction of urinary passage, it may be over (100
cm H
2
O).
The bladder pressure increasing during coughing and sitting up, during pregnancy,
the weight of the fetus over bladder increases the bladder pressure, and causes
frequent urination. A stressful situation may also produce pressure increase.
Pressure Effect While Diving
The body composed solid and liquid, and liquid incompressible so pressure
changes not effect, there are gas cavities where sudden pressure changes can
produce effects because Boyles law for fixed quantity of gas at fixed
temperature the product ( PV = constant )
The middle ear is one air cavity exist within the body, when divining many people
have difficulty in pressure equalization, and feel pressure in the ears.
A pressure of 120 mmHg across the eardrum can accrue in about (1.7 m) of
water can cause the eardrum to rupture. Rupture can be serious, since cold
water in the middle ear can affect the vestibular or balance mechanism and

32
College of Medicine/Babylon University
Medical Physics Module
cause nausea and dizziness.

33
College of Medicine/Babylon University
Medical Physics Module
If a diver has cold, the sinus cavities in the skull become closed off and not
equalize of pressure causing pain, another pain for small volumes of air trapped
beneath filling in the teeth.
Breathing air in the depth of (30 m) is dangerous because there is excess
nitrogen in the blood tissues.
Henry’s law
: the amount of gas that will dissolved in a liquid is proportional to
the partial pressure of gas in contact with the liquid, thus more nitrogen is
dissolved as deeper, when the diver ascends, the extra nitrogen inside tissue
must be removed via the blood and lungs, other problems occur during ascent.
One of the membrane that separate air and blood in the lung can burst allowing
air to go directly into the blood stream (air embolism) air becomes trapped
under the skin around the base of neck or in the middle of the chest, these
problems best treated by a physician.
Viscosity of blood is decreasing when shear stress increasing, the pure liquids
have Newton’s behavior and the suspension liquids are non-Newton’s behavior,
the blood belongs to the later type.
If a scuba diver at depth 10 m holds his breath and comes to the surface, the air
volume will expand by factor two thus causes serious pressure rise in the lungs
if the lungs filled to the capacity an ascent of only 1.2 m can cause lung damage.
All scuba divers learn during training to avoid breath holding during ascent
and to exhale continuously if rapid ascent is necessary.
The pressure in the lungs at any depth is greater than the pressure in the
lungs at sea level. The high pressure of oxygen causes more oxygen molecules to
be transferred into the blood, and oxygen poisoning results if the partial pressure

34
College of Medicine/Babylon University
Medical Physics Module
of oxygen gets too high. Usually oxygen poisoning occurs when the partial
pressure of oxygen is about 0.8 atm. When the absolute air pressure is about
8 atm. Or at depth 30 m.
Breathing air at depth 30 m is also dangerous because it may results in
excess nitrogen in the blood and tissues this causes two problems nitrogen
nacrosis.
Hyper baric oxygen Therapy
( HOT )
About one fifth oxygen and four fifth nitrogen in atmospheric, to greatly increase
the amount of oxygen medical engineering have constructed special high
pressure (hyper baric) oxygen chambers.
Gas gangrene is a disease that killed more than half of its victims before
HOT was developed, since the bacteria that causes gas gangrene cannot survive
in the presences of oxygen, almost all gas gangrene patients treated with HOT
are cured without the need for amputation, the previous best method of
treatment.
In carbon monoxide poisoning RBC cannot carry oxygen to the tissue
because carbon monoxide fastens to the hemoglobin at the places normally used
by oxygen.
The presence of few CO on RBC greatly reduces the ability of the
cell to transport O
2
. Normally the amount of O
2
dissolved in the blood is about
2% of that carried on the RBC with HOT the partial pressure of O
2
increased
by a factor 15.
Many victims of Co poisoning are saved with this technique.
HOT has been used in conjunction with radiation in the treatment of cancer .
The theory was the more O2 would make the poorly oxygenated radiation

35
College of Medicine/Babylon University
Medical Physics Module
resistant cells in the center of the tumor more susceptible to radiation damage
