Medical Chemi
s
tr
y
Lecture B
y
:
Asst.
Lect.
Tariq Al Mgheer
College of Medicine- Bab
y
lon Universit
y
Matter And Energy
M
atter define as anything that has mass and occupies space.
PROPERTIES OF MATTER -
A particular kind of matter, such as water, gold, silver, salt, or sugar, is called a
substance. Every substance has a characteristic set of properties that makes it different
from all other substances. These properties give it a unique identity. For example, sugar
is an odorless, white solid that has a sweet taste. When heated, sugar melts and turns
brown. The properties of a substance that are characteristic of that substance are called
its intrinsic properties.
The^e
properties do ,
".
ot depend on the size or shape of the
substance.
Some
i
ntrinsic properties are more
uSefU
i
ha
n
others
i
n de
s
cribing matter, such as
melting, bo
i
ling points and densities. They are easi
l
y measured and expressed in
numbers, it is give numerical values. For example t
h©
specific
pi-operties
o
f water are its
boil
i
ng po
i
nt (100°
C),
melting point (0°
C),
and d
e
n
s
ity (1.0
g/rnL
at 4°C
)
. No other
substance has exactly this set of propertie
s
Th
e
se intrin
s
ic pr
o
perties are called physical
properties.
Many substances react, either alone or with other matter, to form new materials.
These reactions are c
a
lled
tne
chemical prop
srties
of a
s
ubst
a
nc
e
.
CLSSIFYING
MATTER
Matter is
ciassified
a
s
either a m
i
xture or a pure substance. A mi
x
ture contains a
number of different sub
s
tances mixed together. A mixture has no unique set of propert
i
es.
Rath
e
r,
i
t has the properties of a
!!
the substances that are a part of it.
A m
i
xtur
e
i
s either heterogeneous or homogeneous. The parts of a heterogeneous
mixture ;
r
e visibly different, and the parts of a h
o
mogeneous mixture cannot be
detected even with a microscope.
A
mi/ture
can be separated into its parts by using the di
f
ferences in the physical
properties of the parts. For
exampic,
when the temperature of air is lowered, water vapor
sepa
r
ates as liquid (rain) or soli
d
water (snow).
W
hen the air is cooled stil
l
furt
h
er solid
carbon dioxid
e
(dry ice) form. Fina
l
ly,
^
oven lower temperatures, the rest of the a
i
r
becomes liquid. Thus, the differences in the physical propertie
s
of the substances in the
mixture
a
llow us to separate them
Pure substances are cl
ass
if
i
ed as e
i
ther elements or compounds. COMPOSITION OF
MATTER
Atoms are
^:»
fundamenta
l
un
i
t
s
of
l.
l
ler
rl
erlts.
An
e
l
e
ment is a substance that con
t
a
i
ns
only one kind of atom. Today, there are only 106 elements.
Medical Chemistry Lecture By
:
Asst.
Lect.Tariq Al mgheer-
College of
Medicine- Babylon University

Atoms combine to form molecules. A substance that contains only one kind of
molecule is called a pure compound. A molecule is the smallest particle that has the
properties of a pure compound. STATES OF MATTER
All the elements and most simple compounds can exist as a gas, liquid, or, sold.
These are the three states of matter. Water is a familiar example of a compound that
exists in the three states. Liquid water is the most common form. Yet when the
temperature is lowered, water freezes to form ice, the solid form of water. When
water evaporates or is heated to its boiling point, it exists as water vapor, the gaseous
form of water.
Not all substances can exist in three states. Many large and complex molecules
exist only in the solid or liquid state because they are unstab
l
e when heated to their
melt
i
ng or boiling point. For example, sugar decomposes instead of melting when
heated.
A plasma is typically an ionized gas, and is usually considered to be a distinct
phase of matter (the fourth state of matter) in contrast to so
l
ids, liquids, and gases
because of its unique properties
.
Chemist and physicist use the name plasma to
describe this region containing balanced ch
a
rges of ions and electrons. PHYSICAL
AND CHEMICAL CHANGES
Everything in the world undergoes change. These changes can be classified as
either physical or chemical. Chemical changes result in the disappearance of one or
more substances and the formation of new ones.
Chemical changes are usually called chemical reaction.
No new substances are formed in a physical change However, a physical
change
often results in the change of some intrinsic properties. For example, the density of
ice is 0.917 g/mL
,
which is different from that of liquid water.
During chemical reactions and physical
c
hanges, energy is either released or
absorbed. We get the energy we need for our world from chemical reactions. Our
bodies get the energy they need from the food we eat. But what is energy?

Medical Chemistr
y
Lecture B
y
:
Asst.
Lect.
Tariq Al Mgheer-
College of Medicine- Bab
y
lon Universit
y
ENERGY AND ITS TRANSFER
The word energy is used to denote activity. We also speak of the energy that can
be obtained from petroleum. But we cannot see, taste, or smell energy. Unlike matter,
energy does not occupy space; yet we can feel its effects. Energy is not a th
i
ng but
is more like a characteristic of a substance.
All m&iter has energy. This energy has many forms. potential, kinetic, chemical,
atomic, and radiant energy are common types. An object has potential energy
because
of its position. For example, water at the top of a waterfall has potential energy as a result
of its position above the surface of the earth. An object has kinetic energy as a result of
its motion. A moving car and an airplane in flight all have kinetic energy. Chemical energy
is the energy stored in the molecule as a result of the kinds and pos
i
t
i
ons of its atoms.
Atomic or nuclear energy is associated with the structure of atoms. Radiant energy is
the energy of light.
The energy of any object changes whenever it undergoes a chemical reaction or
phys
i
cal change. This energy change occurs by transferring energy. For example, part of
the energy of wood is transferred to the surrounding air when
i
t is burned. The energy is
transferred by several methods. Four of the most common are work, heat, sound, and
light These are all visible signs of a transfer of energy.
It is
i
mportant to realize that, although a substance may contain energy, it never
contains heat or work. Heat and work are evident only when the energy of the material
changes and a transfer of energy occur. The terms heat content and work content of
substances are often used. These terms mean that the change in energy of a substance
will occur in the form of heat or work.
Chem
i
cal reactions involved in our body also demonstrate the transfer of energy. The
food we eat provides the energy that allows our bodies to carry out the normal work of
contraction and motion Food also provides us with heat to maintain a constant body
temperature. Food undergoes a series of chemical reactions in our bodies that result in
the transfer of part of the chemical energy to muscles to do work and to the surroundings
as heat.

UNITS OF ENERGY
Heat
i
s one of the most easily measured forms of energy. The most common unit of
measurement of heat is the calorie. Its symbol is
cal.
A calorie is defined as the amount
of heat needed to raise the temperature of 1 g of water from 14.5° to 15.5° C
.
Although is
the exact definition of a calorie, approximately 1 calorie is needed to ra
i
se the temperature
of 1 g of water by 1° C at any temperature between 0 and 70° C. Most chem
i
cal reactions
release several thousand calories; as a result, quantities of heat are usually reported as
kilocalones
(1
kcal
=
1000 cal). The
Sl
unit of energy is the joule. Its symbol is
J
There
are
exacdy
4
.
184
J
in 1 cal. Be careful in using the calor
i
e as a unit, because there are
actually two calories in use:
the one defined above, and the Calorie (with a capital C), used in nutrition. One
Calorie is equal to 1 kcal.
EXARCISE:
How much heat, in
cai
and kcal, is needed to ra
i
se the temperature of 1000
g
of water from 1°to25
0
C?
1 calorie is needed to raise the temperature of 1 g of water
1000 g
X
1
calone/1
g
=
1000
cal
or 1
kcal
1 kcal X24=
24kcal
=
24000 cal
THE BODY AND HEAT TRANSFER
The human body at rest gets its energy by means of a series of complex chemical
reactions called metabolism. The body gets its heat from a part of this energy. The
temperature of the body must stay fairly constant to function properly. Either too much or
too little heat can be lethal. The body gets rid of excess heat by transferring it to the
surroundings in a number of ways.
Evaporation of water from the skin is one way the body loses heat. This process takes
advantage of the fact that heat is needed to transform liquid water into its vapor. The heat
needed to carry out this physical change is called the heat of vaporization It
i
s defined
as the number of calories needed to change 1 g of substance from the liquid to the vapor
state at its normal boiling point. The heats of vaporization of a number of compounds used
in medic
i
ne are given in Table 1.
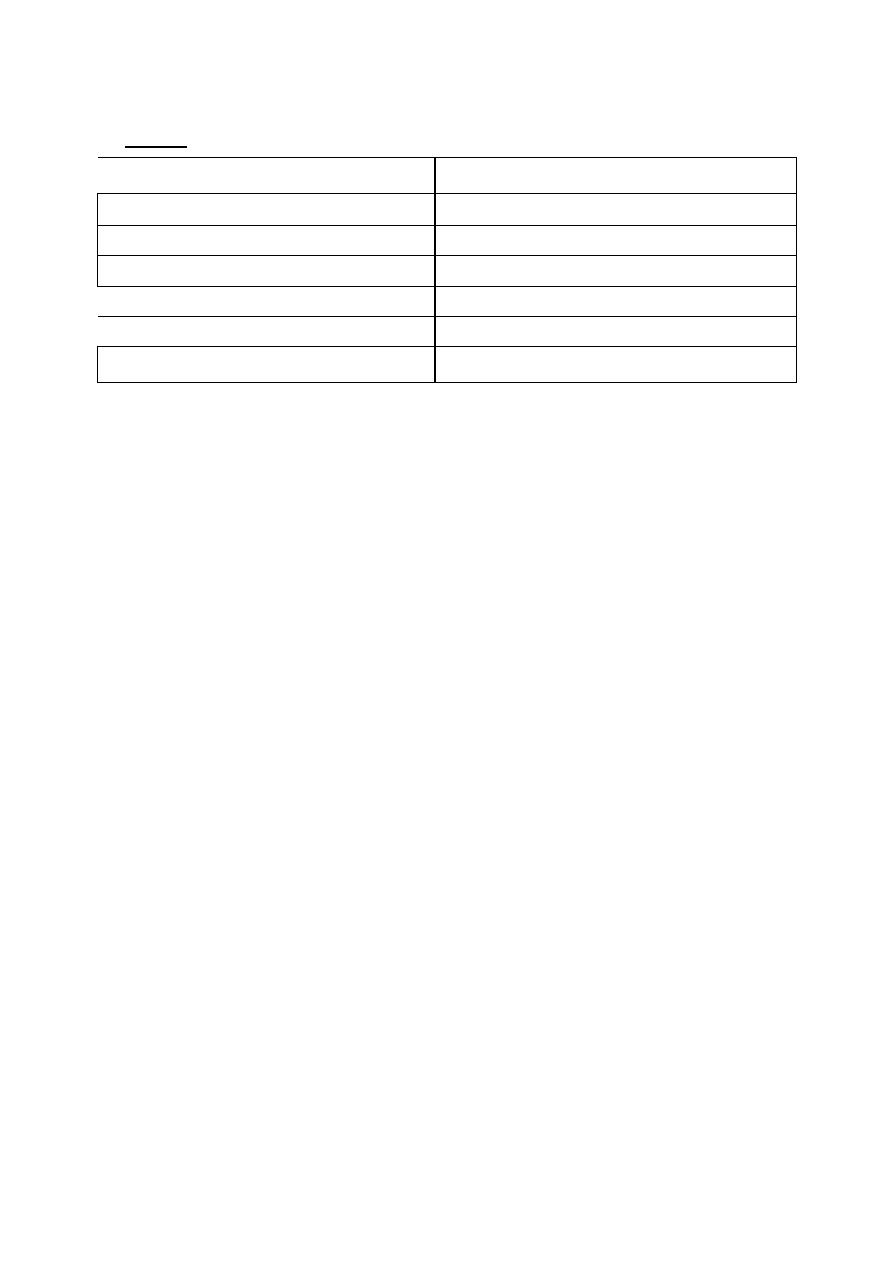
Table 1 Heats of Vaporization of Various Compounds
Compounds
Hear of Vaporization Compound (cal
/
g)
Water
540
IsopropyI
alcohol
159
Diethyl
ether
84
Ethyl chloride
93
Chloroform
59
Ethyl alcohol
204
Notice that the heat of vaporization of water is higher than that of the other
compounds. This means that it takes more heat to vaporize 1g of water than 1g
OT
most
other compounds.
The body also transfers hear to its surroundings by radiation The body is like a hot water
radiator used in cars. Both give off heat. The heat radiated from the body accounts for
much of its heat loss, particularly during cold weather. Much heat is lost from an
uncovered head. Th
i
s loss can be greatly reduced simply by wearing a hat.
Heat is transferred from one substance to another substance that is colder. This is
called heat conduction This is another way that heat can be transferred to
'
or from the
body. For example, when an ice pack is placed on the skin, heat is transferred from the
skin to the
i
ce and the skin becomes cool.
Water is the most abundant compound in the body. Body water can act internally to
control body temperature because it can absorb a fairly large amount of heat with
relat
i
vely little change in temperature. The amount of heat needed to raise the
temperature of 1
g
of a substance by 1° Celsius is called its
specilic
heat. Water has a
higher specific heat than most compounds (1
cal/g
X
°C).
Forexample,
it takes 10 times
as much heat to raise the temperature of a quantity of water by 1° than it does to
i
ncrease
the temperature of the same amount of copper by 1
°
(0.0949
cal
/
g X °C). This means
that water in the body can absorb a fairly large amount of heat without changing
temperature. In this way, water acts as an internal temperature regulator.

Buffer solution:-
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its
conjugate base, or vice versa. Its pH changes very little when a small amount of strong
acid or base is added to it.
What is buffer solution example?
For example, a mixture of acetic acid and sodium acetate acts as a buffer solution
with a pH of about 4.75. ... For example, a mixture of ammonium chloride and
ammonium hydroxide acts as a buffer solution with a pH of about 9.25. Buffer
solutions help maintain the pH of many different things.
What is a buffer solution used for?
A buffer is a solution that can resist pH change upon the addition of an acidic or basic
components. It is able to neutralize small amounts of added acid or base, thus
maintaining the pH of the solution relatively stable. This is important for processes
and/or reactions which require specific and stable pH ranges.
Is blood a buffer?
Blood. Human blood contains a buffer of carbonic acid (H
2
CO
3
) and bicarbonate anion
(HCO
3
-
) in order to maintain blood pH between 7.35 and 7.45, as a value higher than 7.8 or
lower than 6.8 can lead to death. In this buffer, hydronium and bicarbonate anion are in
equilibrium with carbonic acid.
What is a buffer and how does it work in the blood?
Buffers in the Human Body help maintain the bloods pH at 7.4. If blood pH falls below
6.8 or rises above 7.8, one can become sick or die. The bicarbonate neutralizes excess
acids in the blood while the carbonic acid neutralizes excess bases
What is the buffer system in blood?
Buffer Systems in the Body. ... The buffer systems functioning in blood plasma
include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The
kidneys help control acid-base balance by excreting hydrogen ions and generating
bicarbonate that helps maintain blood plasma pH within a normal range.
How is pH maintained in the body?
The lungs control your body's pH balance by releasing carbon dioxide. Carbon dioxide
is a slightly acidic compound. ... Your brain constantly monitors this in order to
maintain the proper pH balance in your body. The kidneys help the lungs maintain
acid-base balance by excreting acids or bases into the blood.

Osmolality:-
refers to the concentration of dissolved particles of chemicals and minerals -- such as
sodium and other electrolytes -- in your serum. Higher osmolality means more
particles in your serum. Lower osmolality means they're more diluted.
What is the osmolality of blood?
Osmolality is a measure of the number of dissolved particles in a fluid. A test for
osmolality measures the amount of dissolved substances such as sodium, potassium,
chloride, glucose, and urea in a sample of blood and sometimes in urine.
What is the difference between osmolarity and osmolality?
Osmolarity refers to the number of solute particles per 1 L of solvent,
whereasosmolality is the number of solute particles in 1 kg of solvent.
What causes high blood osmolality?
In healthy people, when osmolality in the blood becomes high, the body releases
antidiuretic hormone (ADH). This hormone causes the kidneys to reabsorb water. This
results in more concentrated urine. ... Dilute urine is passed to get rid of the excess
water, which increases blood osmolality back toward normal.
