
Monosaccharides
and their reactions
By
Assistant. Prof Dr. Ban Mahmood Shaker Al-joda

Monosaccharides (Simple Sugar)
A carbohydrate that cannot be split or hydrolyzed into
smaller carbohydrates
Monosaccharides
are carbohydrates with:
•
The simplest carbohydrates
•
3-9 carbon atoms
•
A carbonyl group (aldehyde or ketone)
•
Several hydroxyl groups
C
n
(H
2
O)
n
C
n
H
2n
O
n
║
C ─ H
│
H─ C ─ OH
│
H─ C ─ OH
│
CH
2
OH

Monosaccharides and their
reactions
Oxidation-Reduction.
Required for their
complete metabolic breakdown.
Esterification.
Production of phosphate
esters.
Amino derivatives.
Used to produce
structural components and glycoprotein.
Glycoside formation.
Linkage of
monosaccharides to form
polysaccharides.
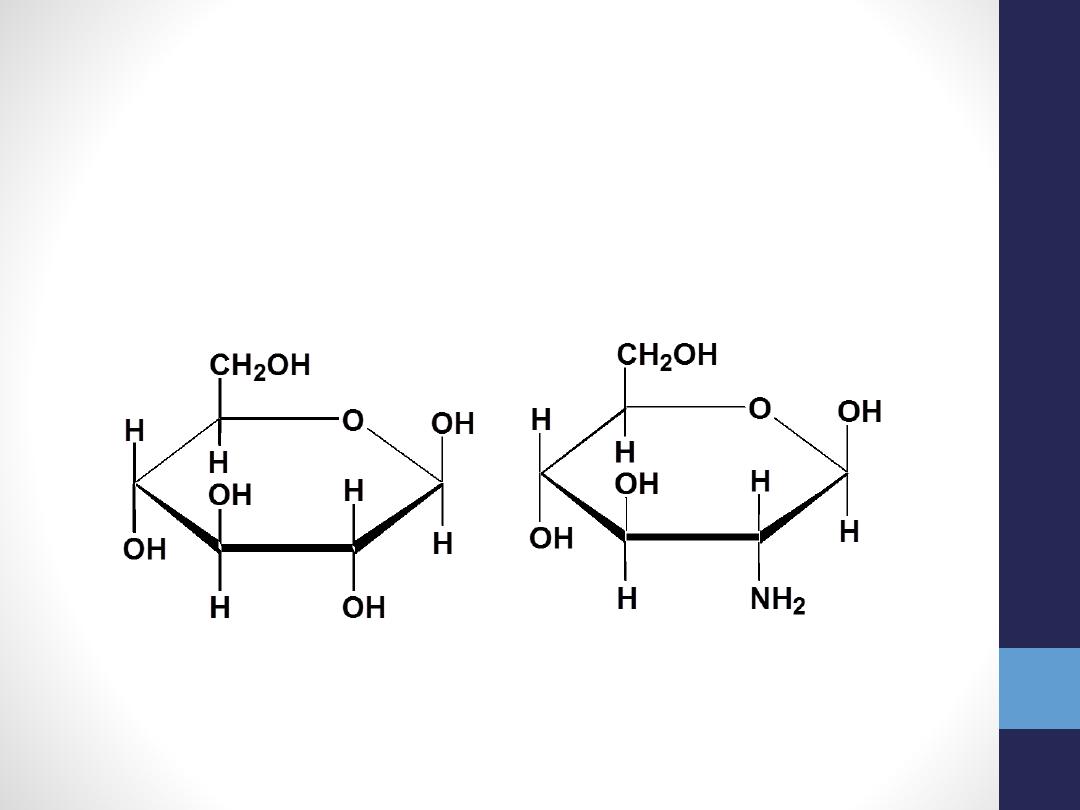
•
1-Amino sugar
(replacement of one
hydroxyl
group by
amino
group e.g,
glucose amine
(in
glycoprotein and glycolipid and
glycosaminoglycan
a heteropolysaccharide
erythromicine(antibiotic)
-D-glucose -D-2-aminoglucose
(glucosamine)

Uses for amino sugars.
Structural components of bacterial cell
walls.
A major structural unit of
chondroitin
sulfate
- a component of cartilage.
Component of
glycoprotein
and
glycolipids
.
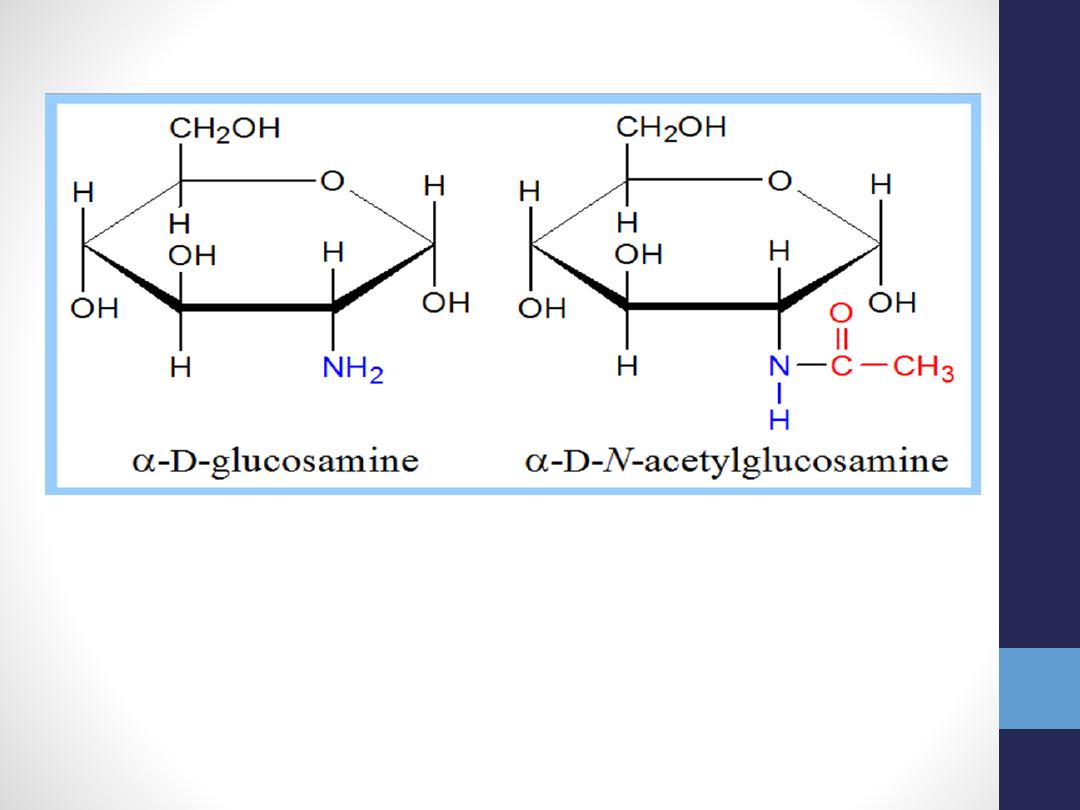
amino sugar - an amino group substitutes for a
hydroxyl. An example is glucosamine.
The amino group may be acetylated, as in
N-acetylglucosamine
.

2-Phosphate ester in metabolism.
3- Oxidation of glucose at carbon one , give
(
aldonic
) gluconic acid this is the bases of
Benedict
and
Fehling
,reaction in
detection
of
glucose
in
urine
the sugars are called
reducing sugar.
When the
anomeric
cabon
form bond
with
another molecule the sugar is
non reducing

while oxidation at carbon 6 give (
uronic
)
glucuronic important in detoxification
reaction and in glycosaminoglycan a
heterpolysaccharide
4- Reduction of glucose at carbon one ,give
sugar alcohol sorbitol (which in high level
as in diabetic leads to cataract formation)
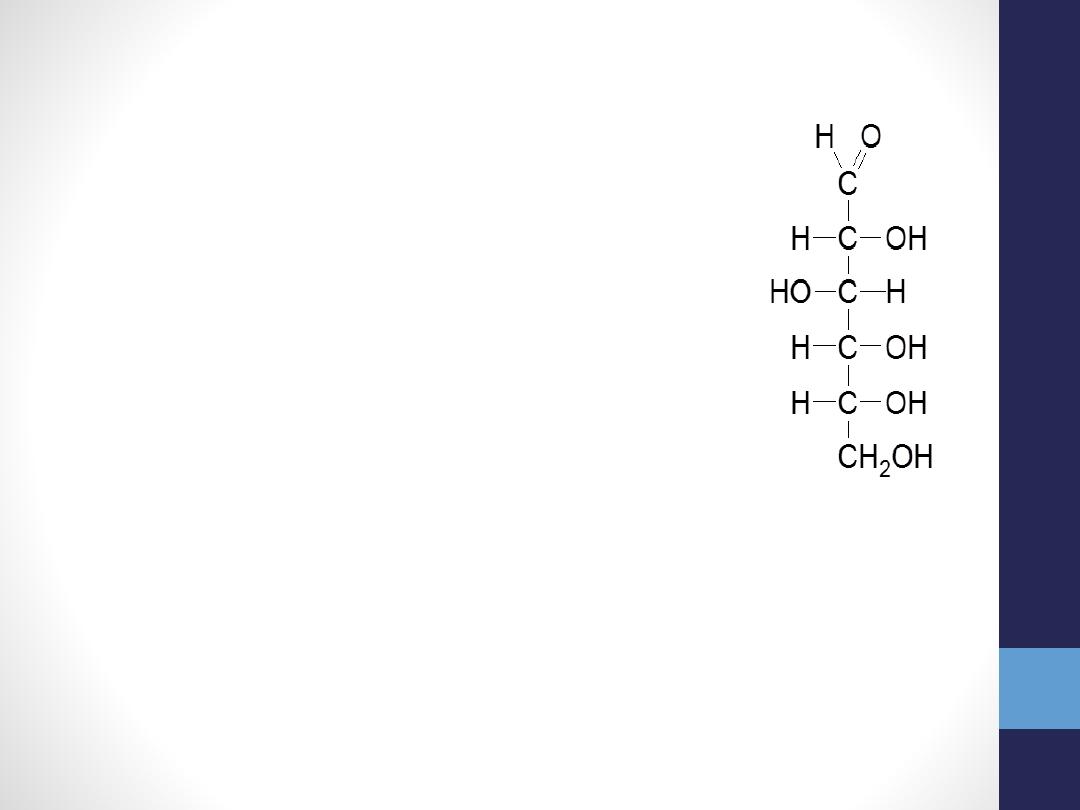
Some important Monosaccharides
Glucose
(Dextrose)
(C
6
H
12
O
6
, aldohexose)
– Blood sugar
•
The most abundant monosaccharide
•
Is found in fruits, vegetables,
corn syrup, and honey.
•
Is found in disaccharides such as sucrose, lactose, and maltose.
•
Makes up polysaccharides such as starch, cellulose, and glycogen

Glucose
(Dextrose)
Normal blood glucose levels are
70-110 mg/dL
.
Excess glucose is
stored
as the polysaccharide
glycogen
or as
fat
.
-
Insulin
(a protein produced in the pancreas)
regulates blood glucose levels by stimulating the
uptake of glucose into tissues or the formation of
glycogen.
- Patients with
diabetes
produce insufficient insulin to
adequately regulate blood sugar levels, so they must
monitor their diet
and/or
inject insulin daily
.
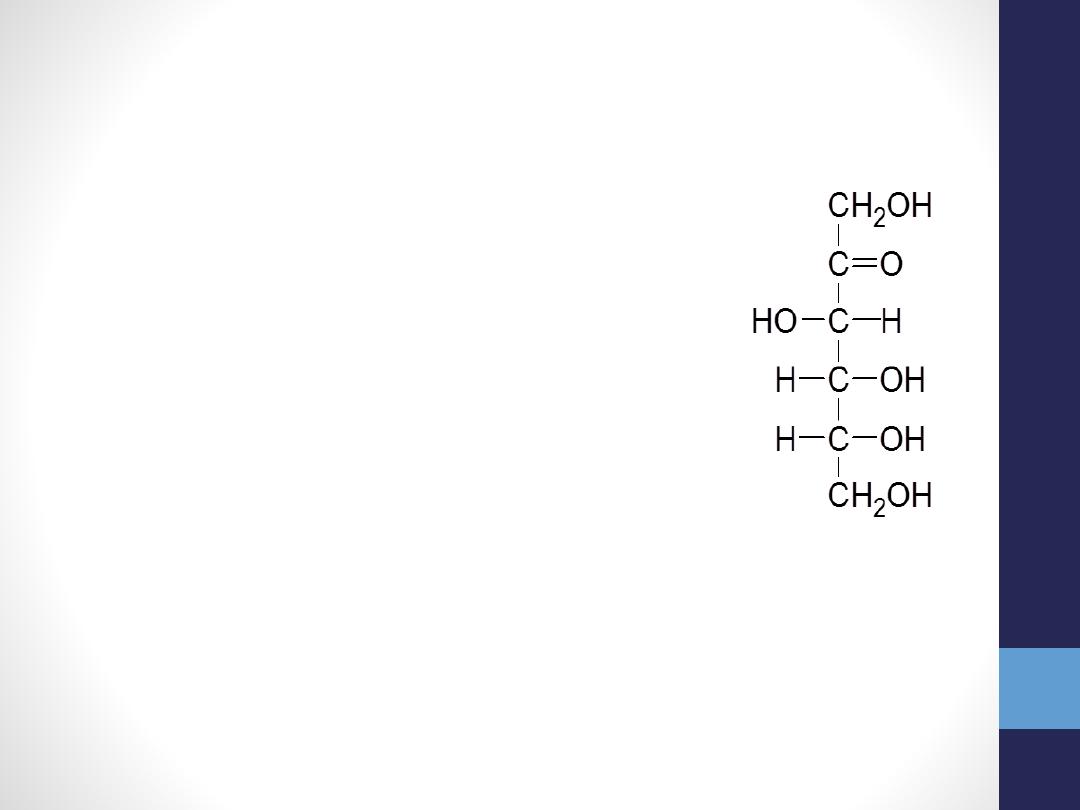
Some important Monosaccharides
Fructose
(C
6
H
12
O
6
, ketohexose),
•
Is the sweetest of the carbohydrates.
•
Is found in fruit juices and honey (fruit sugar).
•
In bloodstream, it is converted to its isomer, glucose.
•
Is bonded to glucose in sucrose (a disaccharide known as table
sugar).

•
Oxidation of Sugars
Under mild oxidation conditions
(hypobromous acid, Br2/H2O), the aldehyde
group is oxidized to carboxyl group to produce
aldonic acid
. Thus, glucose is oxidized to
gluconic acid, mannose to mannonic acid and
galactose to galactonic acid.

•
When aldehyde group is protected, and the
molecule is oxidized, the last carbon becomes
COOH group to produce
uronic acid
. Thus glucose
is oxidized to glucuronic acid, mannose to
mannuronic acid and galactose to galacturonic acid.
The glucuronic acid is used by the body for
conjugation with insoluble molecules to make them
soluble in water for detoxification purpose and also
for synthesis of heteropolysaccharides.

•
Under strong oxidation conditions (nitric acid+ heat),
the first and last carbon atoms are coexisting
oxidized to form dicarboxylic acids, known as
saccharic acids
.Glucose is thus oxidized to
glucosaccharic acid, mannose to mannaric acid and
galactose to mucic acid. The mucic acid forms
insoluble crystals, and is the basis for a test for
identification of galactose.
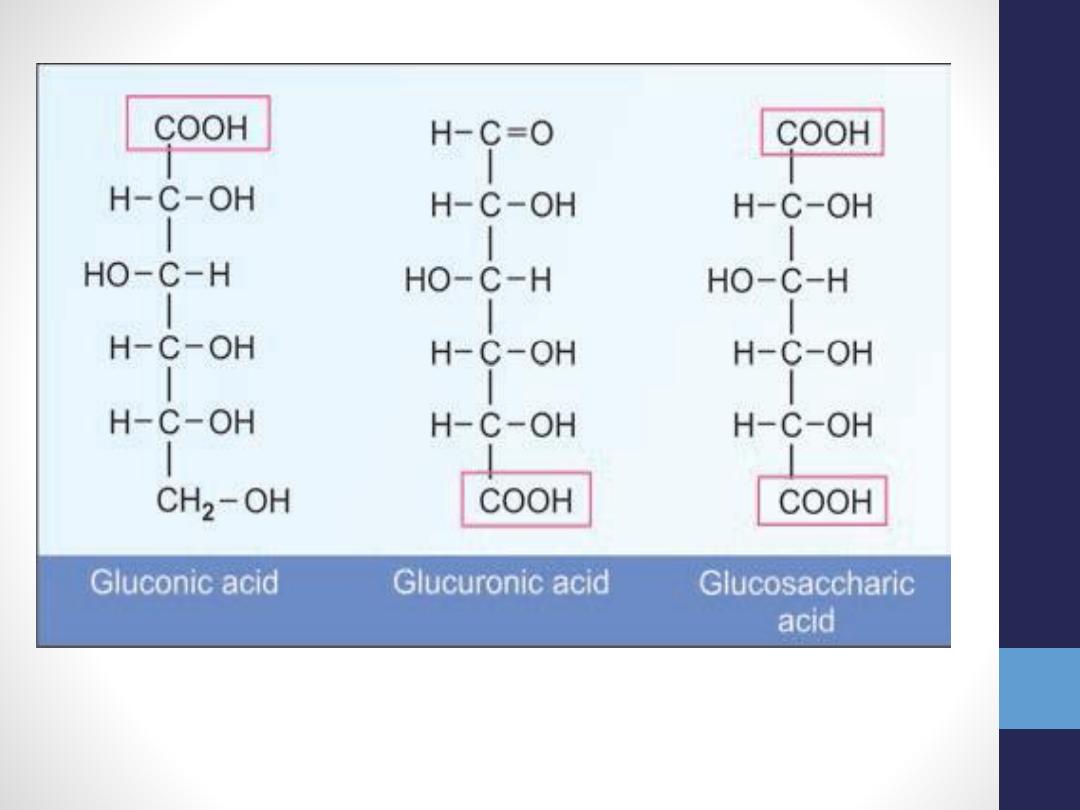
Oxidative products of glucose
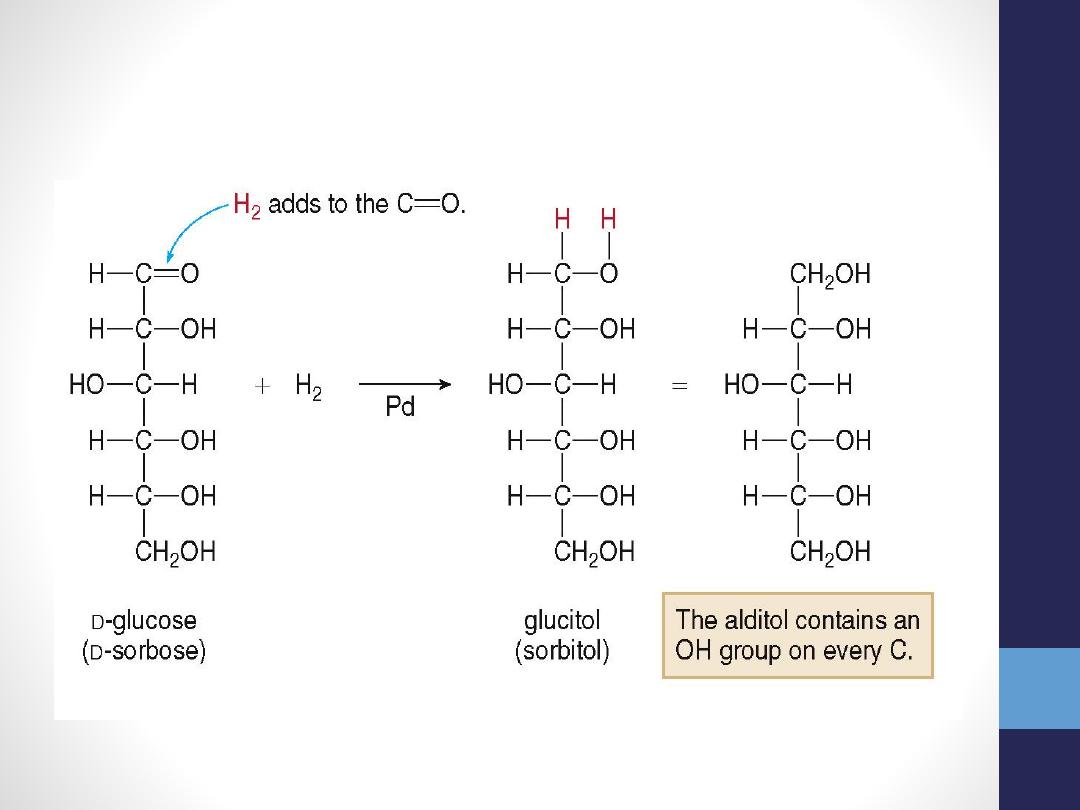
Reduction of Monosaccharides

Reference
TEXTBOOK OF BIOCHEMISTRY For Medical Students (Sixth
Edition) .
DM VASUDEVAN MBBS MD FAMS FRCPath Distinguished Professor of
Biochemistry College of Medicine, Amrita Institute of Medical Sciences,
Cochin, Kerala (Formerly Principal, College of Medicine, Amrita, Kerala)
(Formerly, Dean, Sikkim Manipal Institute of Medical Sciences, Gangtok,
Sikkim) E-mail: dmvasudevan@yahoo.co.in
