
1
Disaccharides with
examples

2
DISACCHARIDES
When two monosaccharides are combined together
by glycosidic linkage, a disaccharide is formed. The
important disaccharides are
1. Sucrose
2. Maltose and isomaltose
3. Lactose.
3
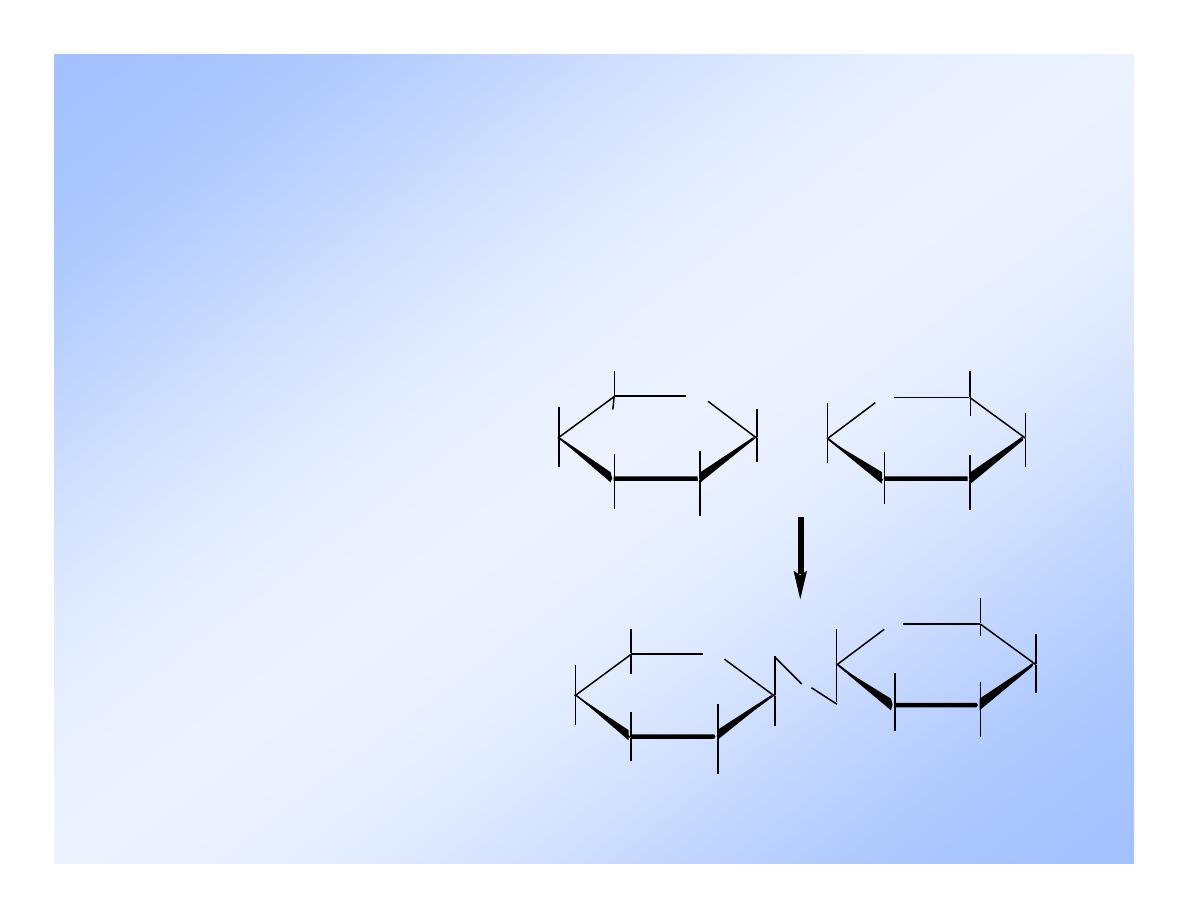
A disaccharide
• consists of two monosaccharides linked together.
• is formed when two monosaccharides combine in a
dehydration reaction.
Monosaccharides
Disaccharide
glucose + glucose
maltose + H
2
O
glucose + galactose
lactose + H
2
O
glucose + fructose
sucrose + H
2
O
The most common disaccharides are maltose, lactose,
and sucrose.
4
Glycoside formation
Glycoside formation
or -OH group of cyclic monosaccharide
can form link with another one (or more).
glycosidic bond
sugar -O- sugar
oxygen bridge
O
H
OH
OH
H
H
H
OH
CH
2
OH
H
O
H
OH
H
OH
H
OH
H
OH
CH
2
OH
H
OH
O
H
OH
H
H
H
OH
CH
2
OH
H
O
H
OH
H
H
OH
H
OH
CH
2
OH
H
OH
o
+ H
2
O
5
Glycosidic bonds
Glycosidic bonds
O
O
Type is based on the position of the C-1 OH
glycosidic bond
- linkage between a C-1
OH and a C-4 OH
glycosidic bond
- linkage between a C-1
OH and a C-4 OH
bonds
bonds
O
O
C-4 end can be either up or down depending
on the orientation of the monosaccharide.
6
Glycosidic bonds
Glycosidic bonds
General format used to describe bond.
OH type carbon# of carbon# of
(
or ) first sugar second sugar
As we work through the next few examples
this will become clear.
For disaccharides
- the sugar is either
or
based on form of the
remaining C-1 OH.
(
)
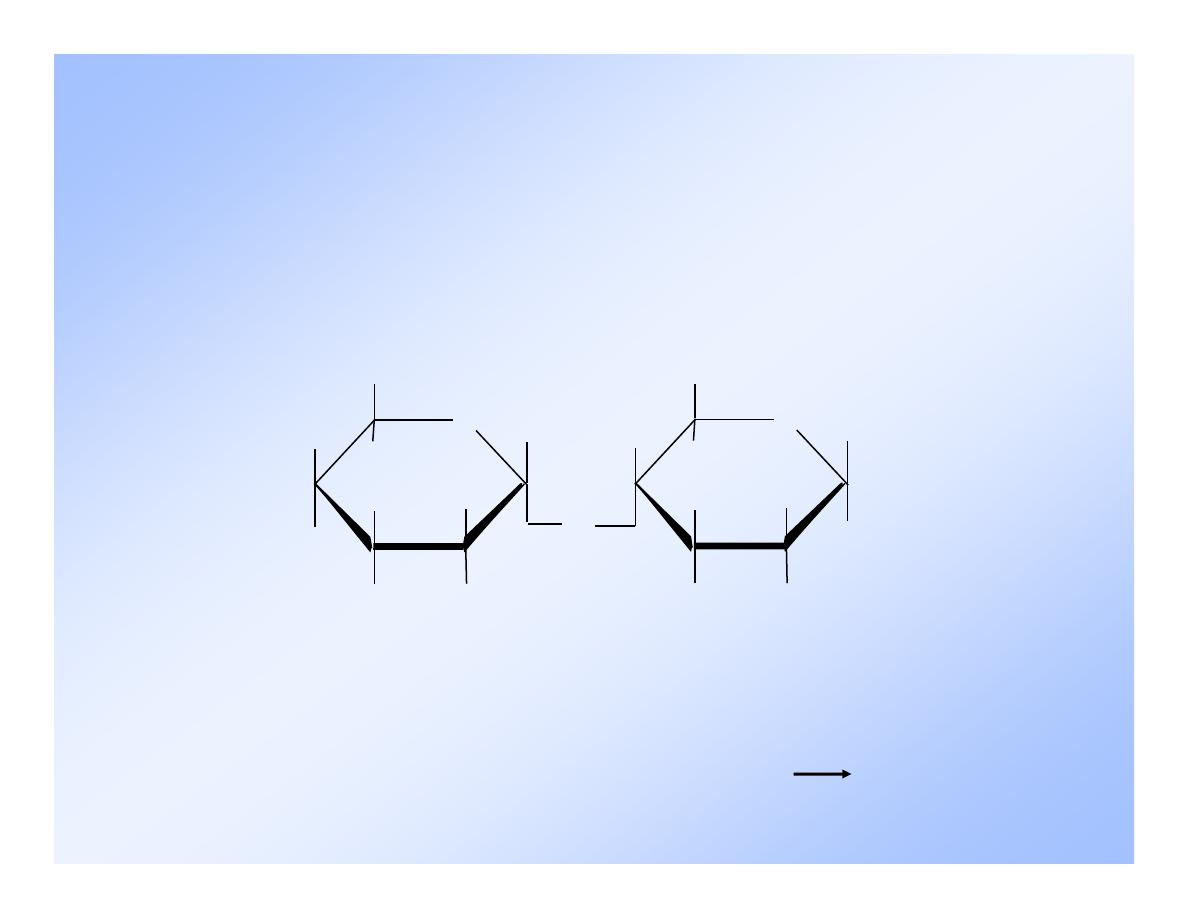
7
-Maltose
-Maltose
Malt sugar.
Not common in nature except in
germinating grains.
-D-glucose
-D-glucose
O
H
OH
H
H
H
OH
CH2OH
H
OH
O
H
OH
H
H
H
OH
CH2OH
H
OH
O
-D-glucose and
-D-glucose, (1
4) linkage.
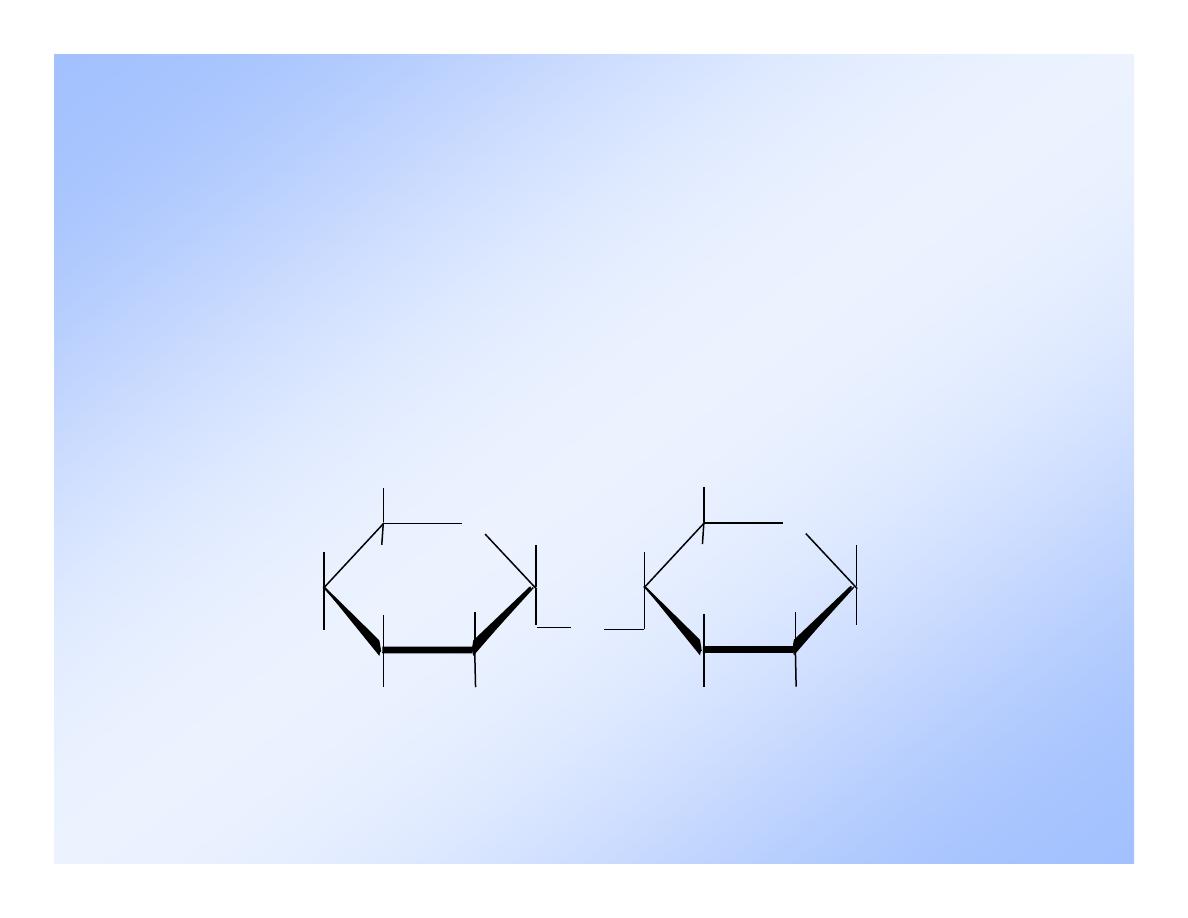
8
-Maltose
-Maltose
It is referred to as
-maltose because the
unreacted C-1 on
-D-glucose is in the
position.
O
H
OH
H
H
H
OH
CH2OH
H
OH
O
H
OH
H
H
H
OH
CH2OH
H
OH
O
9
-Maltose
-Maltose
Uses for
-maltose
Ingredient in infant formulas.
Production of beer.
Flavoring - fresh baked aroma.
It is hydrolyzed the in body by:
maltose + H
2
O 2 glucose
maltase
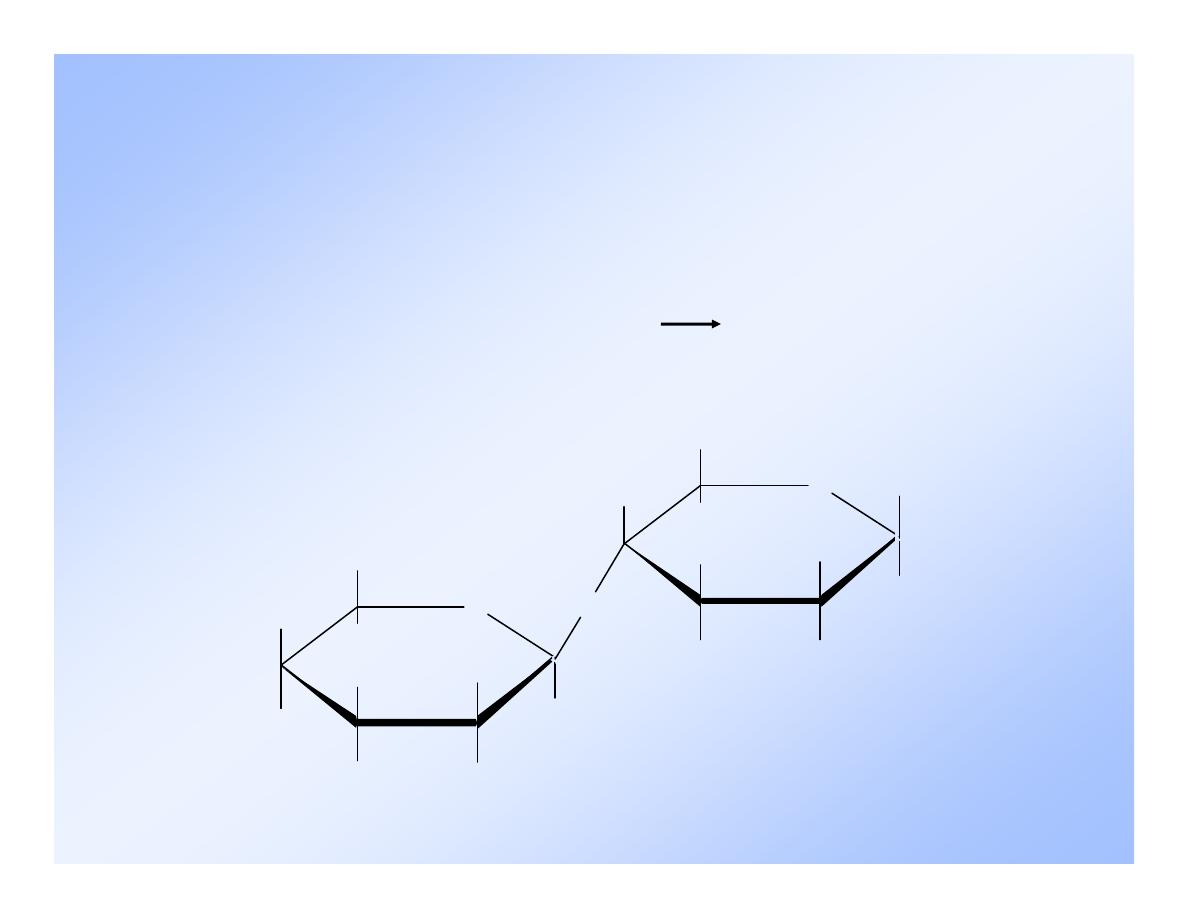
10
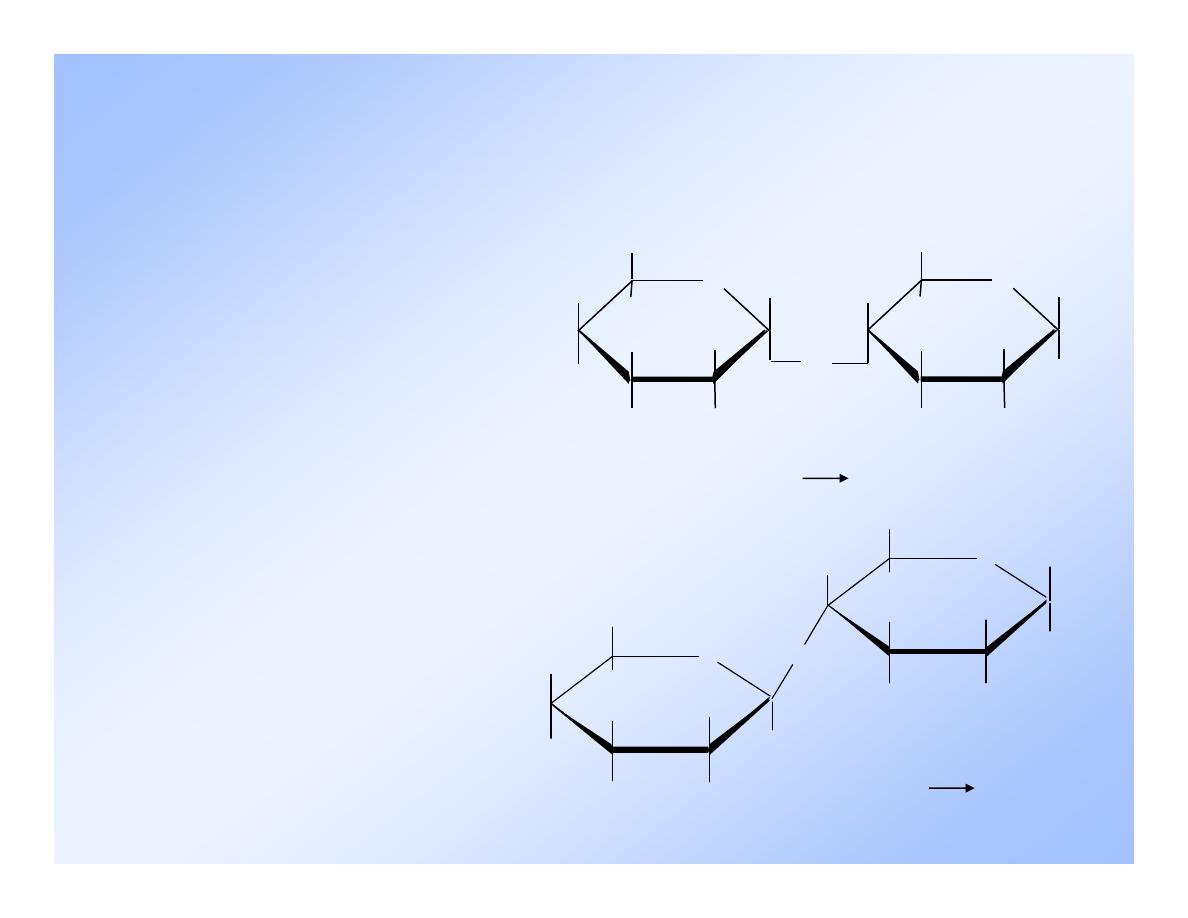
Cellobiose
Cellobiose
Like maltose, it is composed of two molecules of
D-glucose - but with a
(1 4) linkage.
H
O
OH
H
OH
H
OH
CH
2
OH
H
H
O
H
O
H
OH
H
OH
CH
2
OH
H
H
OH
11
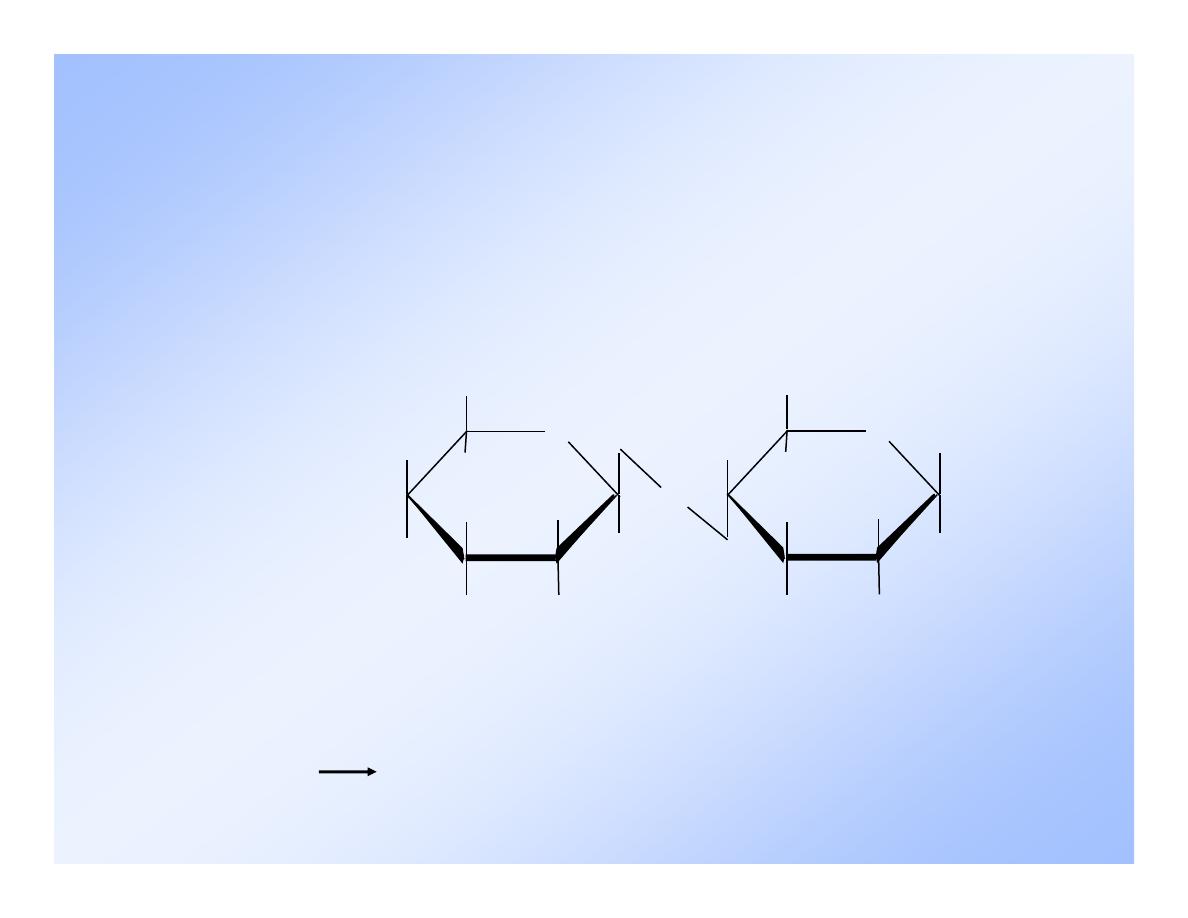
Cellobiose
Cellobiose
O
H
OH
H
H
H
OH
CH 2 OH
H
OH
O
H
OH
H
H
H
OH
CH 2 OH
H
OH
O
H
O
OH
H
OH
H
OH
CH
2
OH
H
H
O
H
O
H
OH
H
OH
CH
2
OH
H
H
OH
The difference in
the linkage results
in cellobiose
being unusable
We lack an enzyme
that can hydrolyze
cellobiose.
cellobiose
(1 4)
maltose,
(1 4)
12
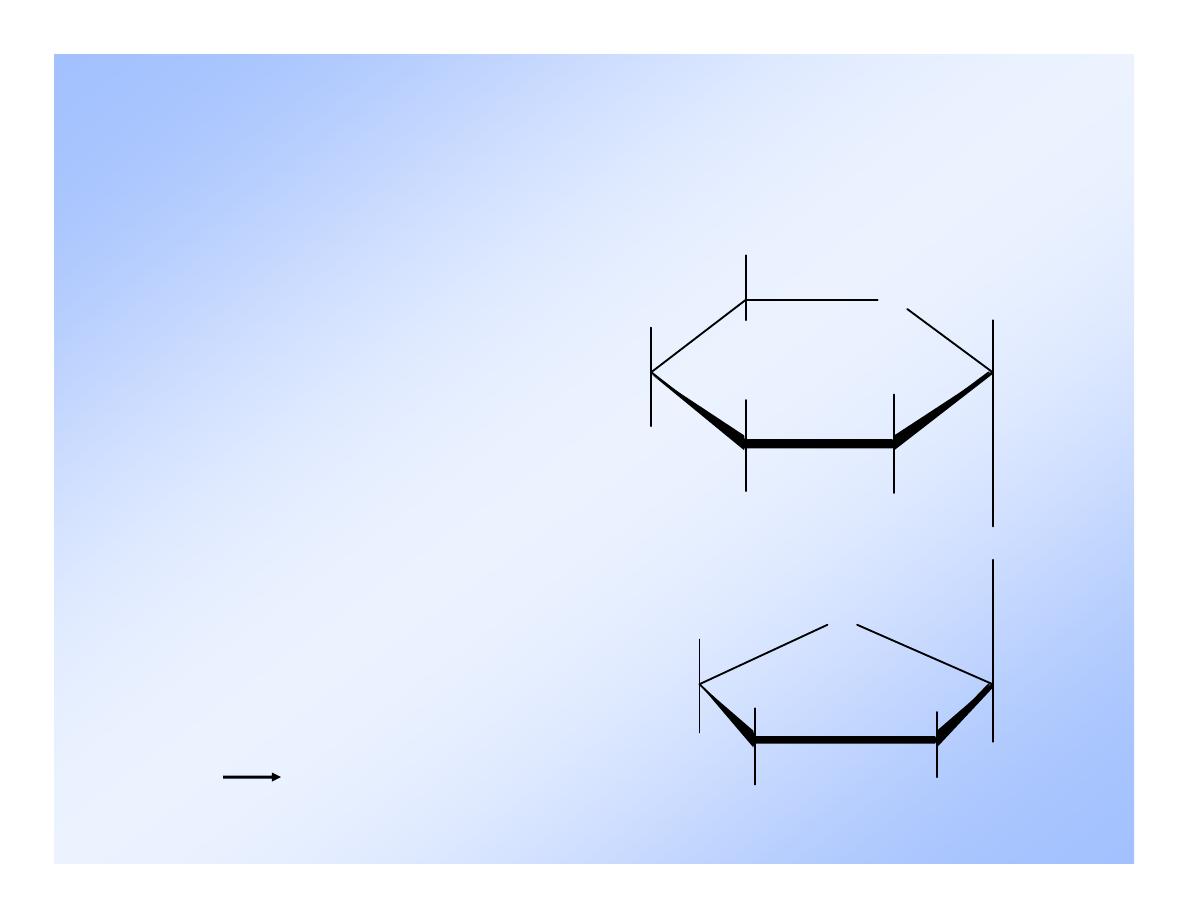
Lactose
Lactose
Milk sugar
- dimer of
-D-galactose and
either the
or - D-glucose.
-Lactose
O
OH
H
H
H
H
OH
CH2OH
H
OH
O
H
OH
H
H
H
OH
CH2OH
H
OH
O
-D-galactose
-D-glucose
(1 4) linkage,
disaccharide.

13
Lactose
Lactose
We can’t directly use galactose. It must be
converted to a form of glucose.
Galactosemia
- absence of needed enzymes
needed for conversion.

14
Lactose
Lactose
Lactase
Enzyme required to hydrolyze lactose.
Lactose intolerance
Lack or insufficient amount of the
enzyme.
If lactase enters lower (GI)Gastro intestinal,
it can cause gas and cramps.
15
Sucrose
Sucrose
Table sugar - most
common sugar in all
plants.
Sugar cane and beet,
are up to 20% by
mass sucrose.
Disaccharide of
-glucose and
-fructose.
(1 2) linkage
CH
2
OH O
CH
2
OH
H
OH
H
H
OH
H
O
OH
H
H
OH
H
OH
CH
2
OH
H
O
