Medical biology
Lecture 3
Electron Microscopy
And other cellular techniques
Transmission Electron
Microscopy
Types of Electron Microscopy
Scanning Electron Microscopy

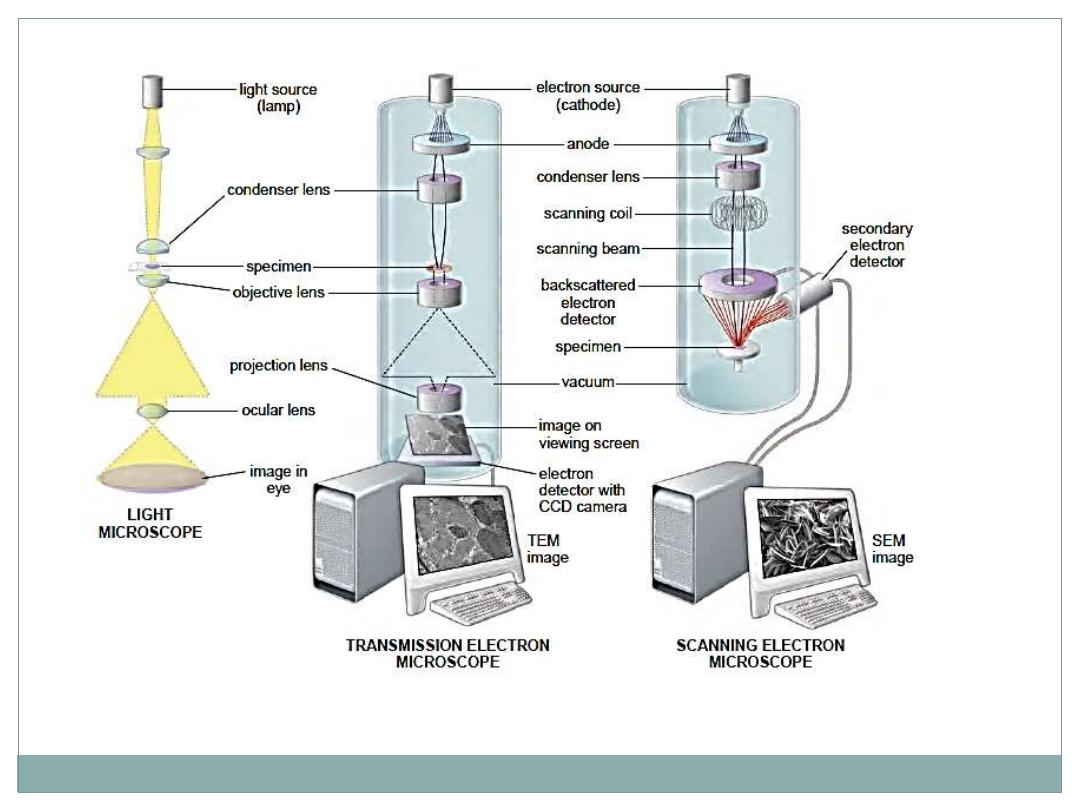
Transmission and scanning electron
microscopes
are
based
on
the
interaction of electrons and tissue
components
.
The wavelength in the electron beam is
much
shorter than of light
, allowing a
1000 -fold
increase in resolution
.
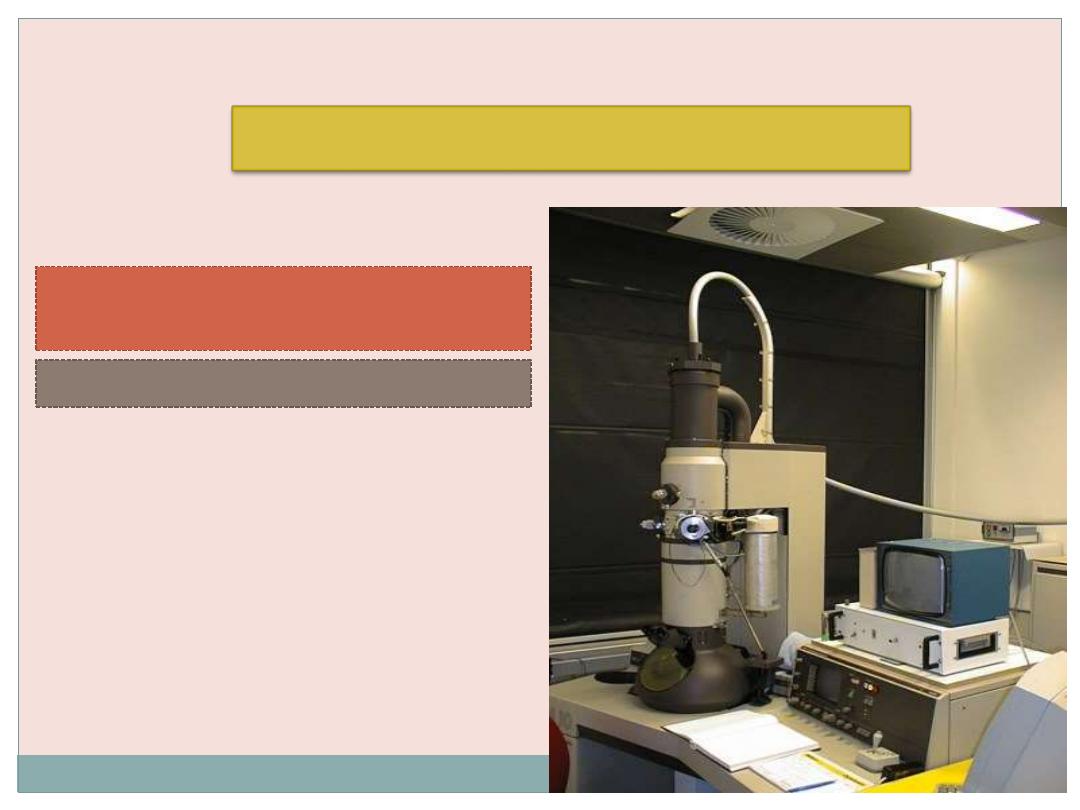
Transmission Electron Microscopy (TEM)
(TEM) is an imaging system that permits resolution
around
3 nm
.This high resolution allows magnifications of
up to
400,000 times
to be viewed with details.
Unfortunately, this level of magnification applies
only to
isolated molecules or particles
.
Very thin tissue sections can be observed with
details at magnifications of up to about
120,000
times.
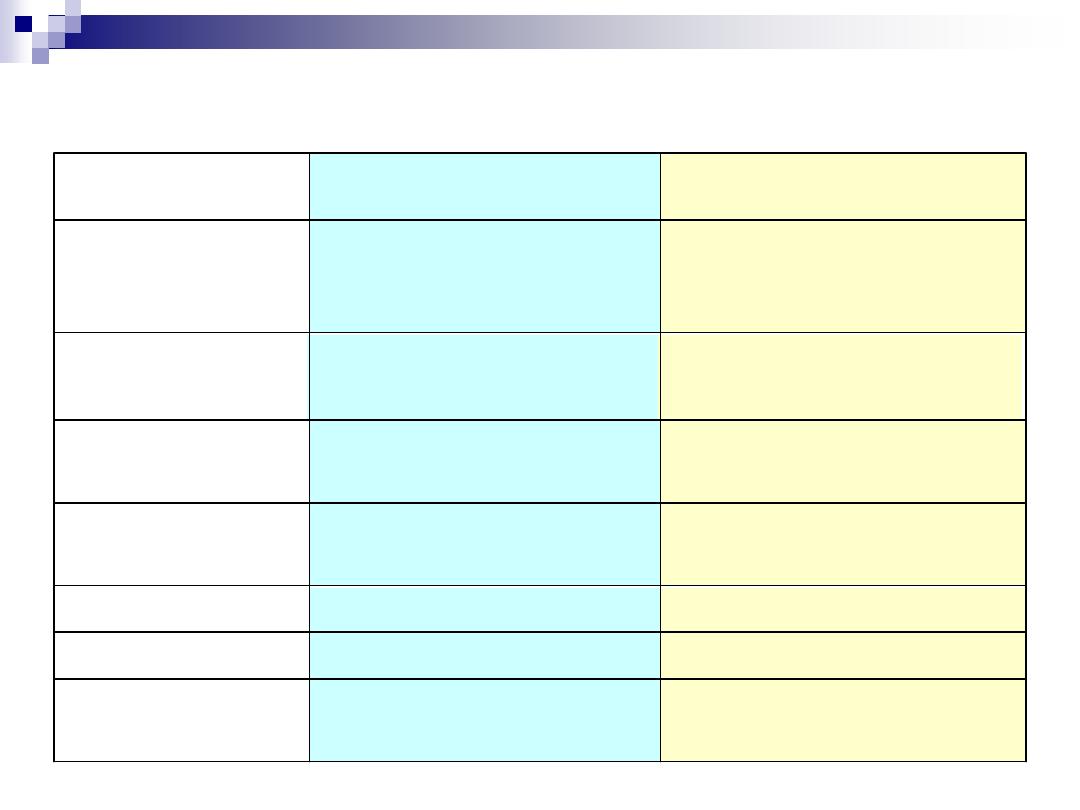
THE LIGHT MICROSCOPE v THE ELECTRON MICROSCOPE
fluorescent (TV) screen,
photographic film
Human eye (retina),
photographic film
Focussing
screen
Vacuum
Air-filled
Interior
Magnets
Glass
Lenses
High voltage (50kV)
tungsten lamp
Tungsten or quartz
halogen lamp
Radiation
source
x400 000
x1500
Maximum
magnification
3nm
Fine detail
app. 200nm
Maximum
resolving power
Electrons
app. 4nm
Monochrome
Visible light
760nm (red)
– 390nm
Colours
Illumination type
ELECTRON MICROSCOPE
LIGHT MICROSCOPE
FEATURE
The TEM functions on the principle that a beam of
electrons can be
deflected by electromagnetic fields
in a
manner similar to light deflection in glass lenses.
The beam is produced by a cathode at the top of the
instrument and passes down through the chamber in a
vacuum.
Because electrons change their path when submitted to
electromagnetic fields, the beam can be focused by
passing through electric coils which can be considered as
electromagnetic lenses.
The first lens is a
condenser
focusing the beam of
electrons on the specimen section.
Some electrons
interact
with atoms in the section and
their course is
modified
, while others simply cross the
specimen without interacting.
Electrons
passing
through the specimen reach the
objective lens, which forms a focused, magnified image
that is then magnified further through other lenses and
captured on a viewing screen.
The image of the specimen shows areas of
white
,
black
,
and
shades of gray
corresponding to areas through which
electrons readily passed (appearing brighter or electron
lucent) and areas where electrons were
absorbed
or
deflected
(appearing darker or more electron dense).
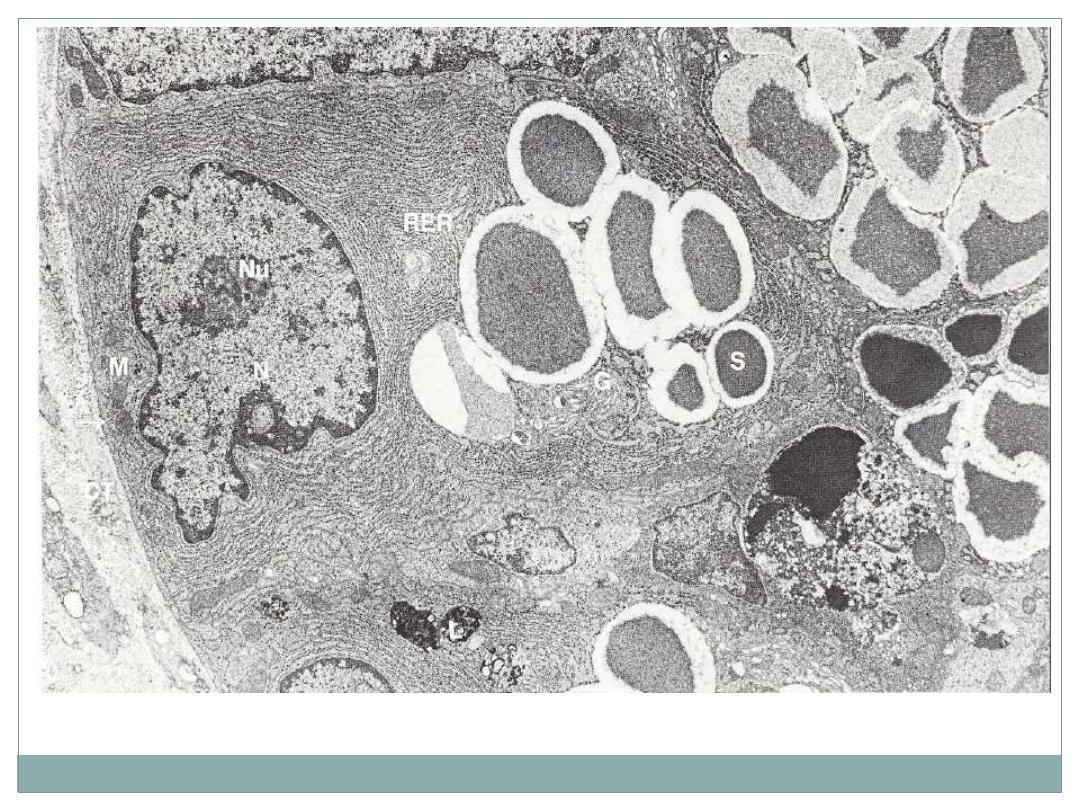
electron micrograph of a cell from the mouse. G, Golgi apparatus; L, lysosome; M, mitochondrion; N, nucleus; Nu,
nucleolus; RER, rough endoplasmic reticulum; S, secretory granule; arrow, base of Paneth cell
To improve contrast and resolution in TEM, compounds
with
heavy metal ions
(like
osmium tetroxide, lead citrate)
are often added to the fixative or dehydrating solutions used
to
prepare
the
tissue.
This
will
bind
cellular
macromolecules, increasing their electron density and
visibility.
To provide a useful interaction between the specimen and
the electrons, TEM
requires very thin sections (40–90 nm)
;
therefore,
embedding
is performed with a
hard epoxy
and
sectioning
is done with a
glass or diamond knife
.
The extremely thin sections are collected on
small metal
grids
and transferred to the interior of the microscope to be
analyzed.
Scanning Electron Microscopy (SEM)
(SEM) permits
pseudo–three-dimensional
views of the
surfaces of cells, tissues, and organs.
Like the TEM this microscope produces and focuses a very
narrow beam of electrons, but in this instrument the beam
does not pass through the specimen.
Instead the surface of the specimen is first dried and
coated
with a very thin layer of
metal
atoms through which
electrons
do not pass
readily.
When the beam is scanned from point to point across
the specimen it interacts with the metal atoms and
produces
reflected
electrons or
secondary
electrons
emitted from the metal.
These are captured by a detector and the resulting signal
is processed to produce a
black-and-white
image on a
monitor.

SEM images are usually easily understood, because they
present a
3D view
that appears to be illuminated from
above
, in the same way that large objects are seen with
highlights and shadows caused by light from above.

Autoradiography
Autoradiography
Is a method of
localizing
newly synthesized
macromolecules
(DNA, RNA, protein, glycoproteins, and polysaccharides) in
cells or tissue sections.
Radioactively
labeled
metabolites
(nucleotides, amino acids,
sugars)
incorporated
into the macromolecules
emit
weak
radiation
that is
restricted
to the cellular regions where the
molecules are
located
1-
Incorporation
of Radioactively labeled metabolites into
the macromolecules
2-
Coating
of radiolabeled cells or mounted tissue sections
with photographic emulsion in a darkroom
3-
Development
of the slides photographically after an
adequate exposure time
silver bromide crystals reduced by the radiation to small
black grains of metallic silver,
indicating locations of
radiolabeled macromolecules in the tissue
Steps of Autoradiography
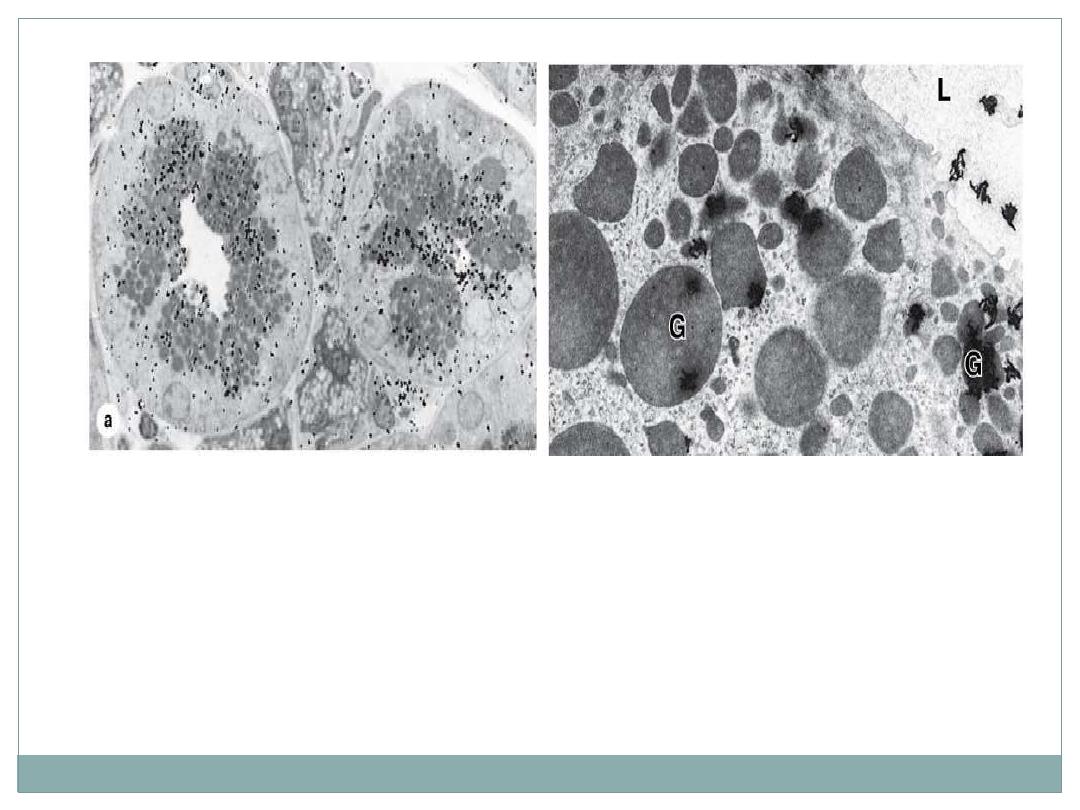
Autoradiography:
(a): Black "silver grains" are visible over regions with secretory granules and
the duct indicating glycoprotein locations. X1500.
(b): The same tissue prepared for TEM autoradiography shows silver grains
with a coiled or amorphous appearance again localized mainly over the
granules (G) and in the gland lumen (L). X7500.
•
If a
radioactive amino acid
is used, it is possible to know
which cells in a tissue produce more
protein
and which cells
produce less,
•
If a
radioactive precursor of DNA
(such as tritium-labeled
thymidine) is used, it is possible to know which cells in a
tissue (and how many) are preparing to divide
Histochemistry & Cytochemistry
The terms histochemistry and cytochemistry indicate
methods for
localizing cellular structures
in tissue sections using
unique
enzymatic activity
present in those structures.
To preserve these enzymes histochemical procedures are usually
applied to
unfixed or mildly fixed tissue
, often sectioned on a
cryostat
to avoid adverse effects of heat and paraffin on enzymatic
activity

Enzyme histochemistry steps:
(1) Tissue sections are
immersed in a solution that contains the substrate
of the enzyme to be localized;
(2) The enzyme is
allowed to act on its substrate
;
(3) At this stage or later, the section is put in contact with
a marker
compound
;
(4) This compound
reacts with a molecule produced by enzymatic action
on the substrate
(the product)
(5) The final reaction product, which must be
insoluble
and which is
visible
by light or electron microscopy only if it is
colored
or
electron-
dense
,
precipitates
over the site that contains the enzyme. When
examining such a section in the microscope, one can see the cell regions
(or organelles) covered with a colored or electron-dense material.
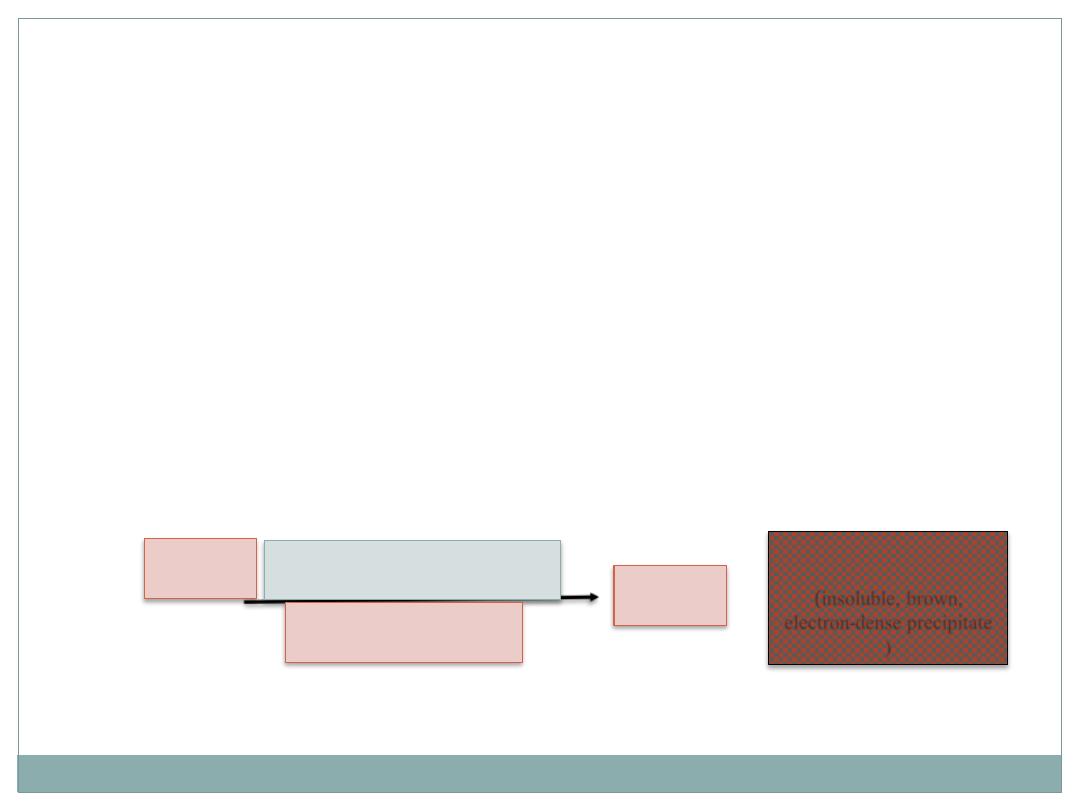
Example:
Detection of
Peroxidase enzyme
: sections of adequately fixed
tissue are incubated in a solution containing
hydrogen peroxide
and
3,3'-diamino-azobenzidine (DAB).
The latter compound is
oxidized in the presence of peroxidase, resulting in an insoluble,
brown, electron-dense precipitate that permits the localization of
peroxidase activity by light and electron microscopy.
H
2
O
2
3,3'-diamino-
azobenzidine (DAB)
H
2
O
+
Oxidized
(DAB)
(insoluble, brown,
electron-dense precipitate
)
Peroxidase
Immunocytochemistry
A highly specific interaction between molecules is that
between an antigen and its antibody. For this reason,
methods using labeled antibodies are extremely useful
in identifying and localizing specific proteins and
glycoproteins
.
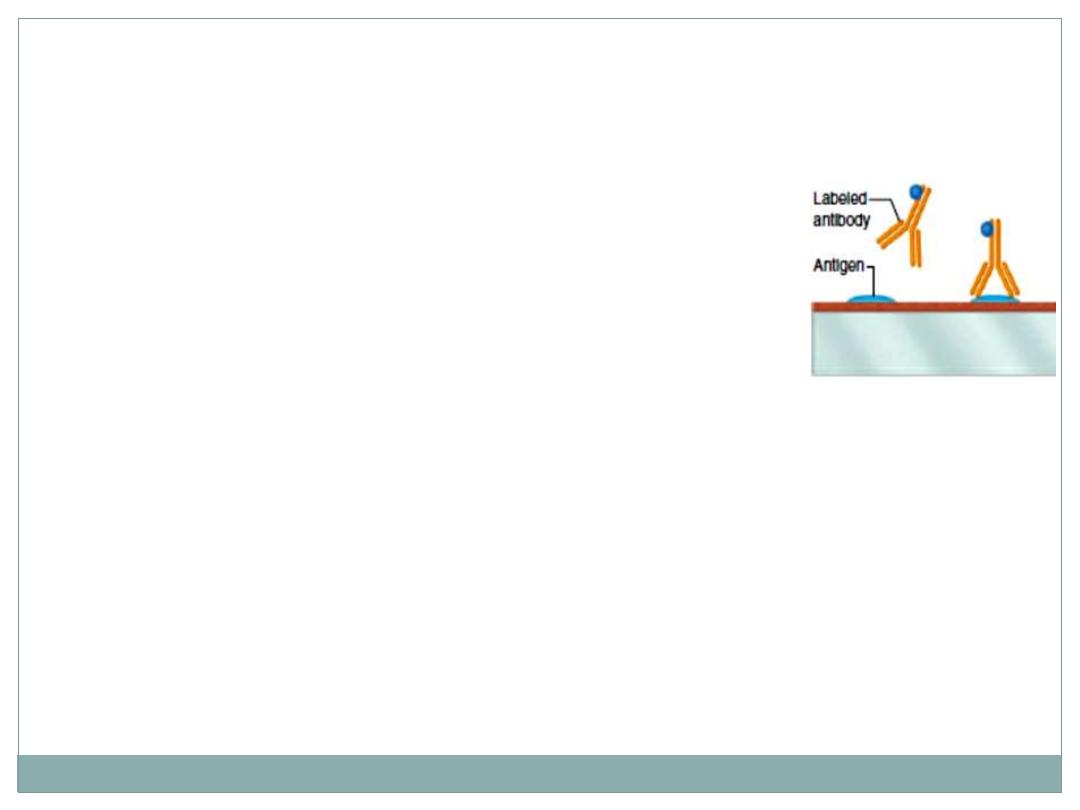
This procedure is done as following:
1-
isolation
and
purification
of the target protein.
2-
injection
of this protein into different animal or
different species.
3- the protein will induce immune response (
production
of
antibody against that protein) .
4- these antibody are
isolated
and
purified
5- the antibodies are
labeled
with fluorescent dyes and allow to react with the
protein wanted to be detected in the histological section.
6- finally, The site that contain the protein will
appear
fluorescent (brilliant) over
dark background.
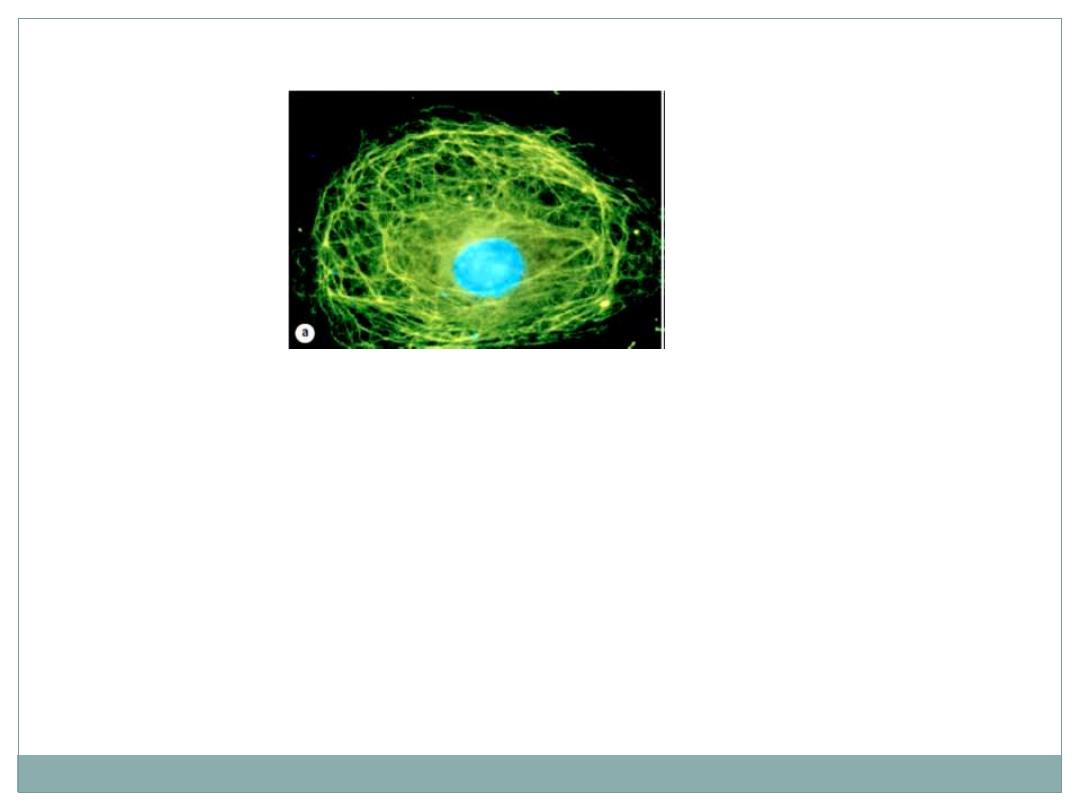
(a) A single cultured cell stained fluorescently to reveal a
meshwork of intermediate filaments (green) throughout
the cytoplasm.

THANK YOU
