By
Dr. Ali Jasim Alsultany
Internist and Nephrologist
M.B.Ch.B_F.I.C.M.S(Med.)_C.A.B.N(Nephro.)
College of medicine_Babylon university

The aim
of RRT
is to replace the excretory
functions of the kidney, and to maintain normal
electrolyte concentrations and fluid balance.
Various options
are available including:
1.Haemodialysis.
2.Haemofiltration and haemodiafiltration.
3.Peritoneal dialysis.
4.Renal transplantation.
Since the advent of long-term RRT in the 1960s,
the numbers of patients with ESRD who are kept
alive by dialysis and transplantation have
increased considerably.
Survival
on dialysis is strongly influenced
by
age
and presence of
complications
such
as diabetes. For this reason,
conservative
care rather than RRT
may be a more
appropriate option for older patients or
those with extensive comorbidities.

Preparing for renal replacement
therapy
This involves ensuring that they are referred to a
nephrologist in a timely manner, as those who
are referred late, when they are either at the
stage of or very close to requiring dialysis (about
20% of referrals in the UK), tend to have poorer
outcomes.
Several decisions need to be taken in discussion
with the patient and their family.
The first
is to decide whether
RRT
is an appropriate
choice or whether
conservative treatment
might
be preferable . This is especially relevant in older
people with significant comorbidity.
For those who decide to go ahead with RRT, there
are further choices between
haemodialysis
and
peritoneal dialysis
, between hospital and home
treatment, and on referral for renal transplantation.

Since there is no evidence that early
initiation of RRT improves outcome, the
overall aim
is to commence RRT by the
time symptoms of uraemia have started to
appear but before serious complications
have occurred.
While there is wide variation between
patients, this typically occurs when the
eGFR approaches 10 mL/min/1.73 m2.

Preparations for the initiation of RRT
should be
started
at least 12 months
before the predicted
start date.
Preparation for RRT involves providing the
patient with psychological and social support,
assessing home circumstances and discussing
the various choices of treatment.
Physical preparations
include establishment of
timely access for haemodialysis or peritoneal
dialysis and vaccination against hepatitis B.

In older patients with multiple comorbidities,
conservative treatment of stage 5 CKD, aimed at
limiting the adverse symptomatic effects of
ESRD without commencing RRT is increasingly
viewed as a positive choice.
survival of these patients without dialysis can be
similar or only slightly shorter than that of
patients who undergo RRT, but they avoid the
hospitalization and interventions associated
with dialysis

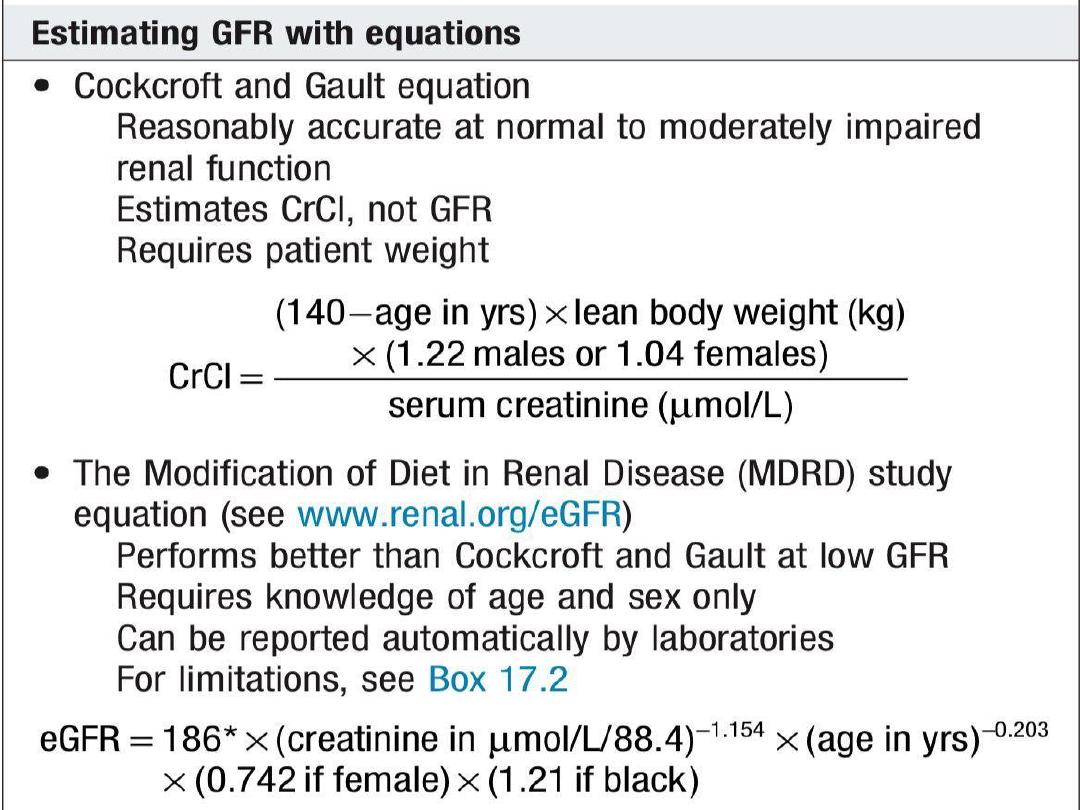
All patients with eGFR below 20 ml/min/1.73
m2 and/or who are likely to progress to ESRD
within 12 months should receive education and
counseling to aid their selection of the most
appropriate RRT modality.

If hemodialysis is the preferred option, an
arteriovenous fistula should be constructed,
remembering that it may take 8 to 12 weeks
for veins to become adequately arterialized
before needling can be attempted.
Peritoneal dialysis catheters can be inserted
and completely buried subcutaneously some
time before patients require dialysis, then
superficialized
for
use
once
clinical
circumstances dictate

Early kidney transplantation may be associated
with improved long-term outcome.
KDIGO
recommends
that
living
donor
preemptive kidney transplantation in adults be
considered when the GFR is below 20
ml/min/1.73 m2 and there is evidence of
progressive and irreversible CKD over the
preceding 6 to 12 months.
Planned early initiation of dialysis is not
associated with improvement in outcomes
compared with commencement when indicated
by signs and symptoms of uremia.

KDIGO
suggests that dialysis be initiated when
one or more of the following are present (not
urgent indications):
• Symptoms or signs attributable to kidney failure
(serositis, acid-base or electrolyte abnormalities,
pruritus).
• Inability to control volume status or blood
pressure.
• Progressive deterioration in nutritional status
refractory to dietary intervention.
• Cognitive impairment.
These problems often but not invariably occur
when the GFR is below 15 ml/min/1.73 m2

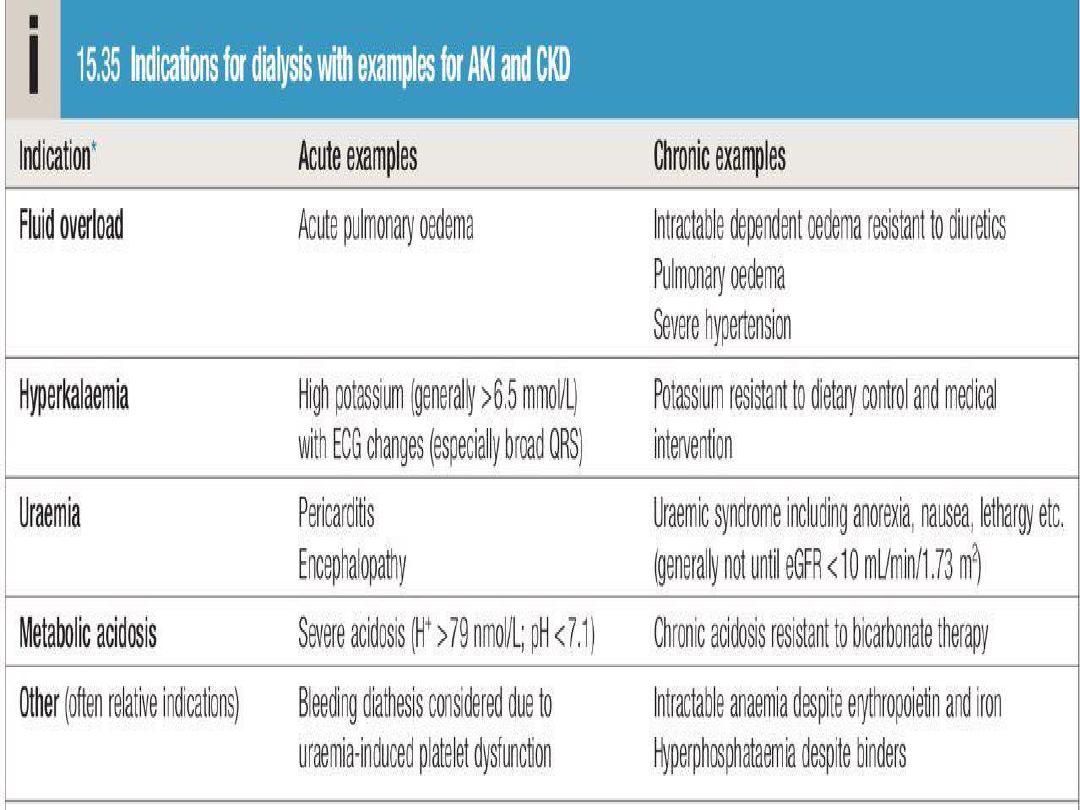
Urgent indications for RRT
• Circulatory overload
especially pulmonary edema
which is unresponsive to loop diuretics (e.g.frusemide).
• Metabolic acidosis not respond to conservative
measure.
• Uraemic encephalopathy.
• Pericarditis
• Bleeding diathesis.
• Hypertension unresponsive to treatment.
• Hyperkalaemia (serum K+ level > 7 mEq./litre).
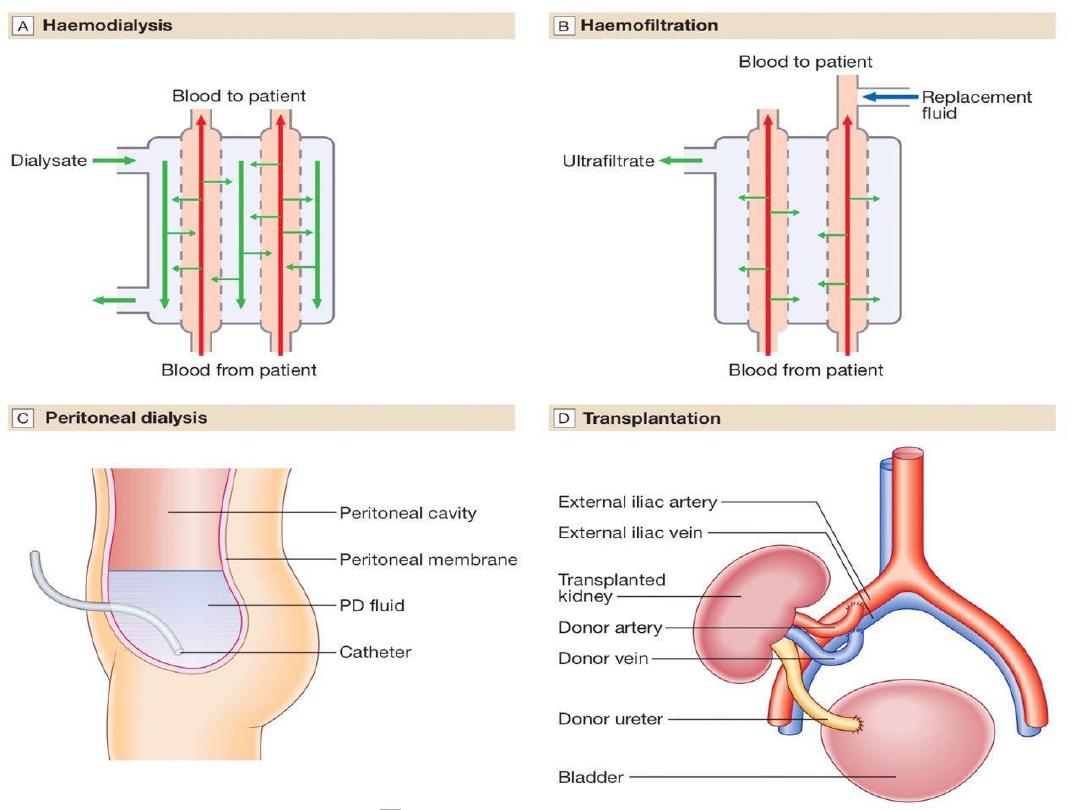

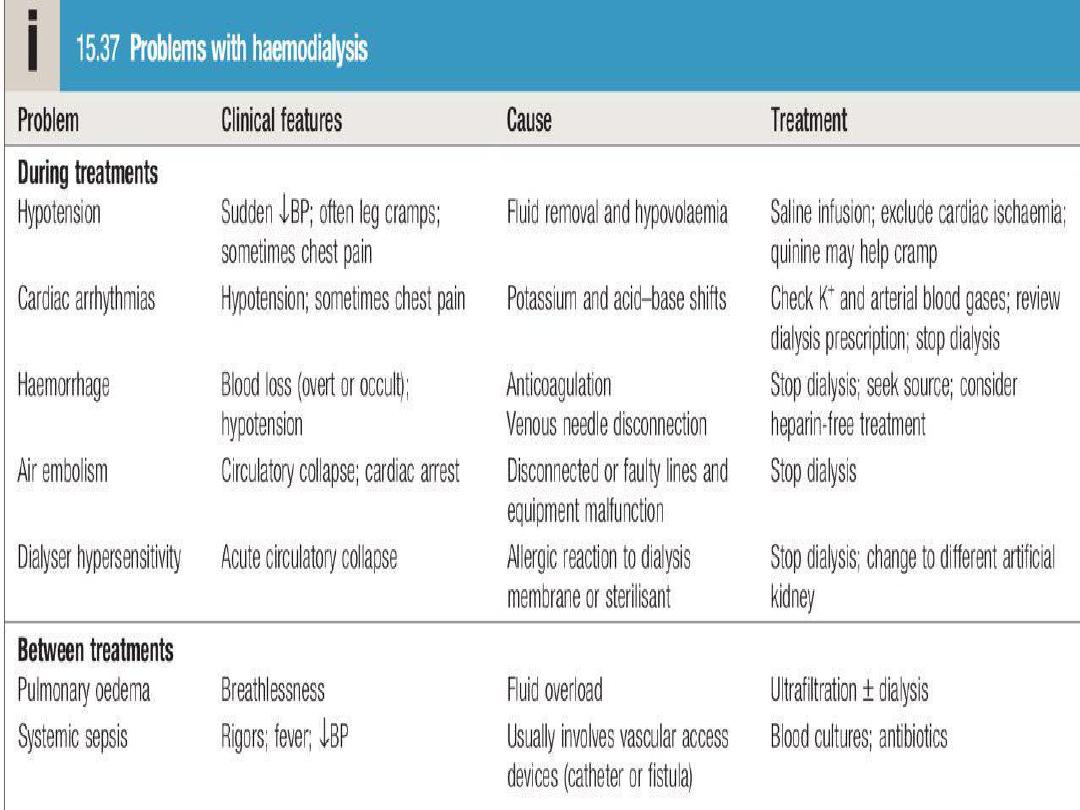
Haemodialysis
the most common form of RRT in
ESRD
and is also
used in
AKI
. Haemodialysis involves gaining access
to the circulation, either through an:
• arteriovenous fistula(AVF).
• central venous catheter(double lumen).
• arteriovenous shunt (graft).
Blood is pumped through a haemodialyser, which
allows bidirectional diffusion of solutes between
blood and the dialysate across a semipermeable
membrane down a concentration gradient.
Haemodialysis in AKI
Haemodialysis offers the best rate of small solute
clearance in AKI, compared with other techniques such
as haemofiltration, but should be started
gradually
because of the risk of
delirium and convulsions
due to
cerebral oedema (
dialysis disequilibrium
).
Typically,
1–2 hours
of dialysis is prescribed initially but,
subsequently,
patients
with
AKI
who
are
haemodynamically stable can be treated by
4–5 hours
of haemodialysis on alternate days
,
or 2–3 hours every day
.

During dialysis, it is standard practice to
anticoagulate patients with
heparin
but
the dose may be reduced if there is a
bleeding risk.
Epoprostenol
can be used as
an alternative but carries a risk of
hypotension
.
Note
In
patients
undergoing
short
treatments and in those with abnormal
clotting, it may be possible to avoid
anticoagulation altogether.
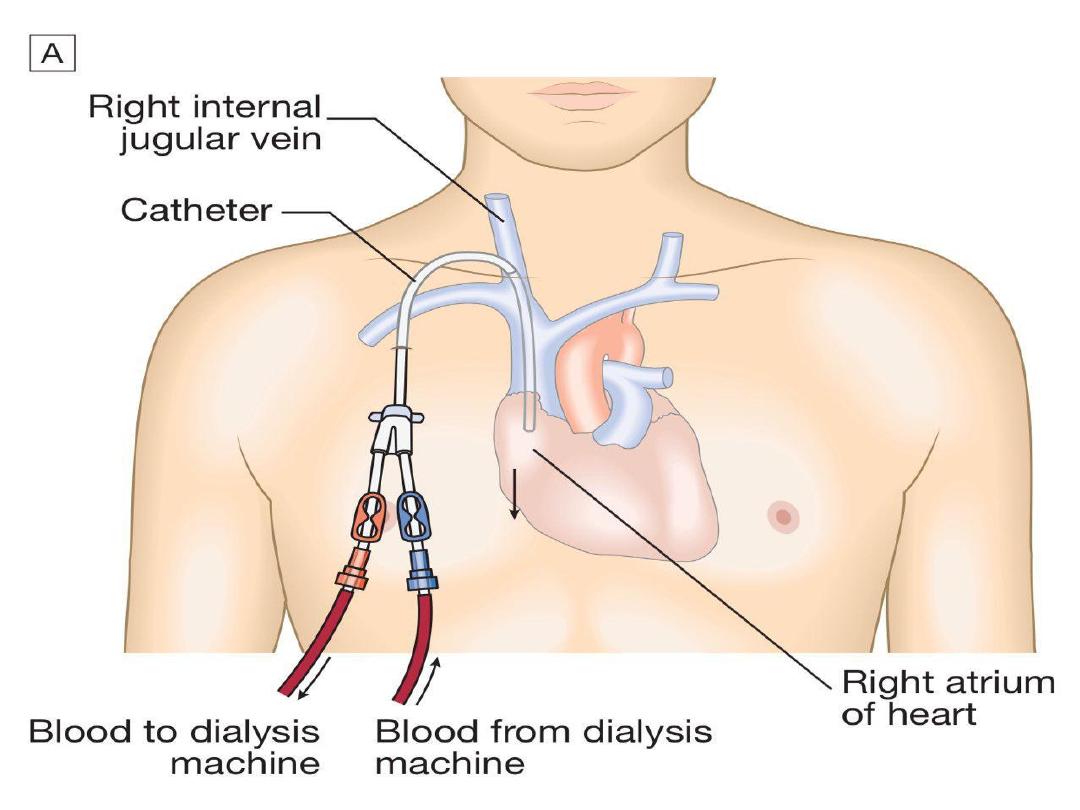
In AKI, dialysis is performed through a
large-bore, dual-lumen catheter inserted
into the
femoral
or
internal jugular vein
.
Subclavian
lines
are
avoided
where
possible, largely due to bleeding risk. Also,
thromboses
or
stenoses
here
will
compromise
the
ability
to
form
a
functioning fistula in the arm if the patient
fails to recover renal function and needs
chronic dialysis.

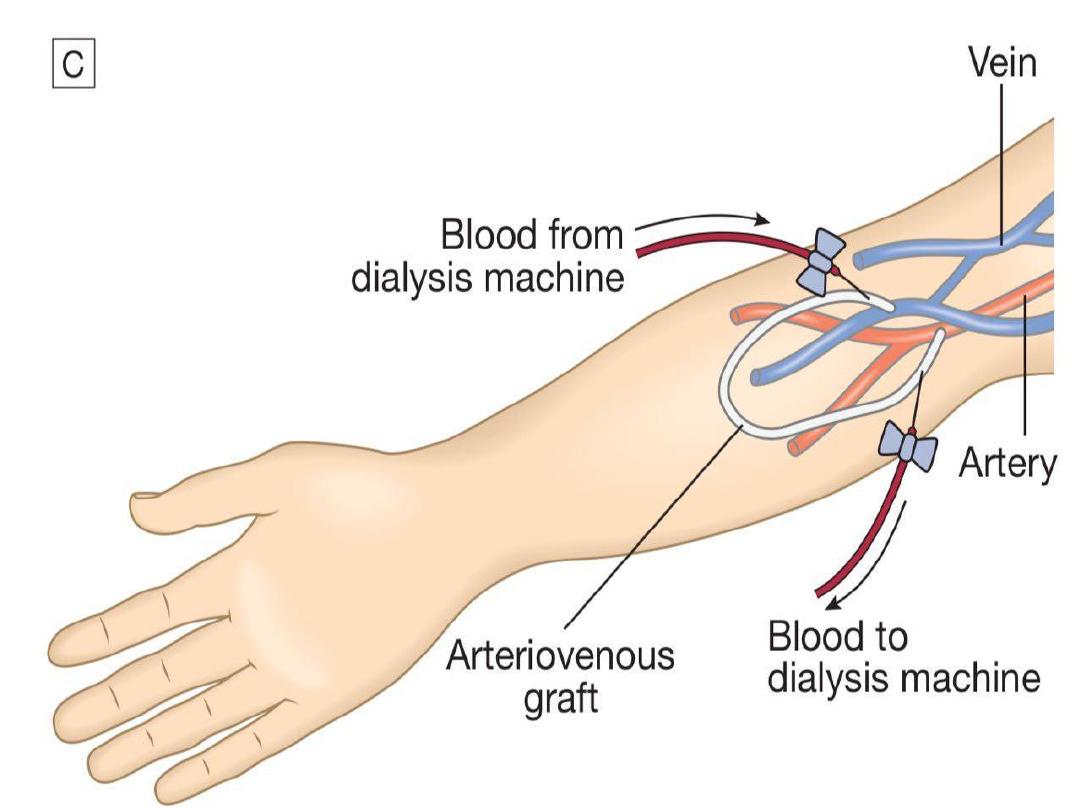
Haemodialysis in CKD
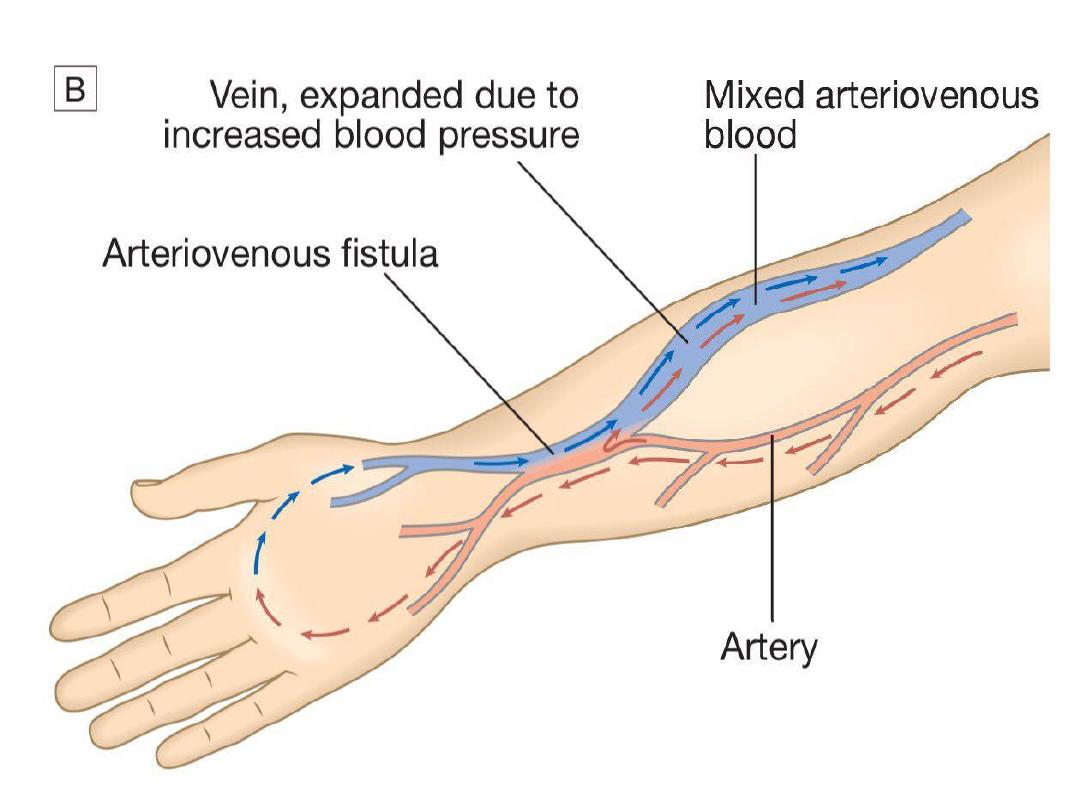
In CKD, vascular access for haemodialysis is
gained by formation of an arteriovenous fistula
(AVF), usually in the forearm, up to a year before
dialysis is contemplated (Fig. 15.27B). After 4–6
weeks, increased pressure transmitted from the
artery to the vein leading from the fistula causes
distension and thickening of the vessel wall
(arterialisation). Large-bore needles can then be
inserted into the vein to provide access for each
haemodialysis treatment.
Preservation of arm veins is thus very important
in patients with progressive renal disease who
may require haemodialysis in the future. If
creation of an AVF is not possible, synthetic
polytetrafluoroethylene (PTFE) grafts may be
fashioned between an artery and a vein, or
central venous catheters may be used for short-
term access. These are tunnelled under the skin
to reduce infection risk.
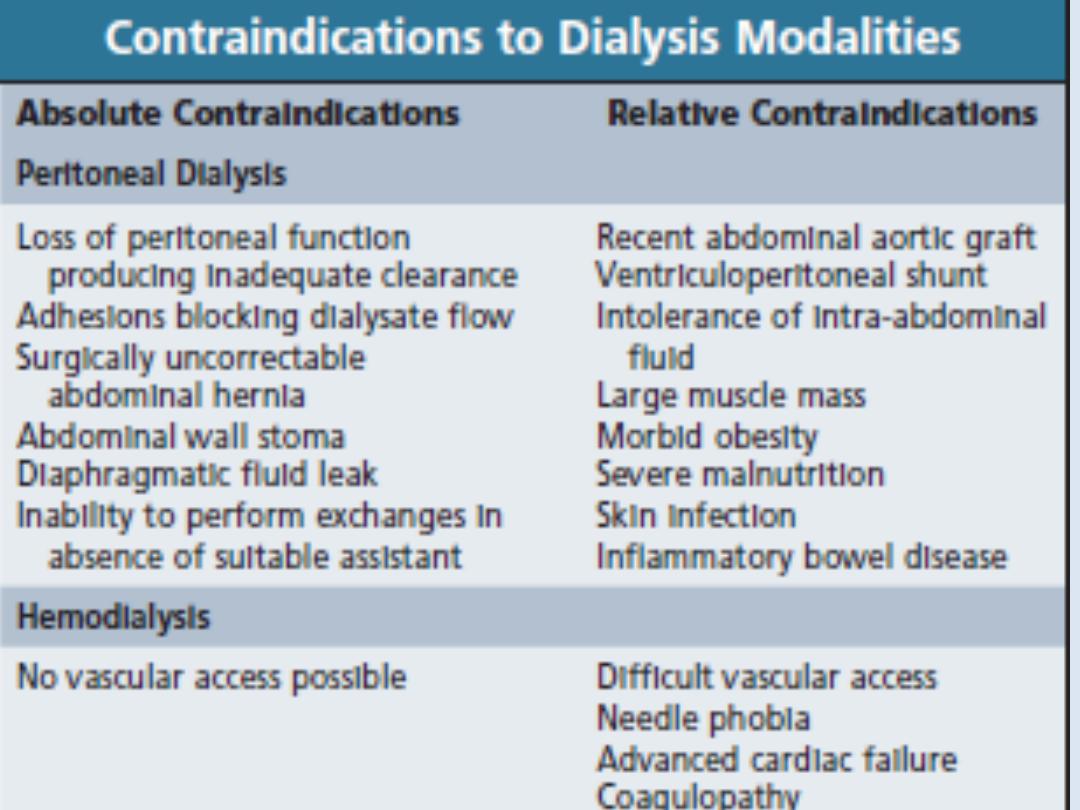
All patients
must be screened in advance for
hepatitis B, hepatitis C and HIV, and vaccinated
against hepatitis B if they are not immune.
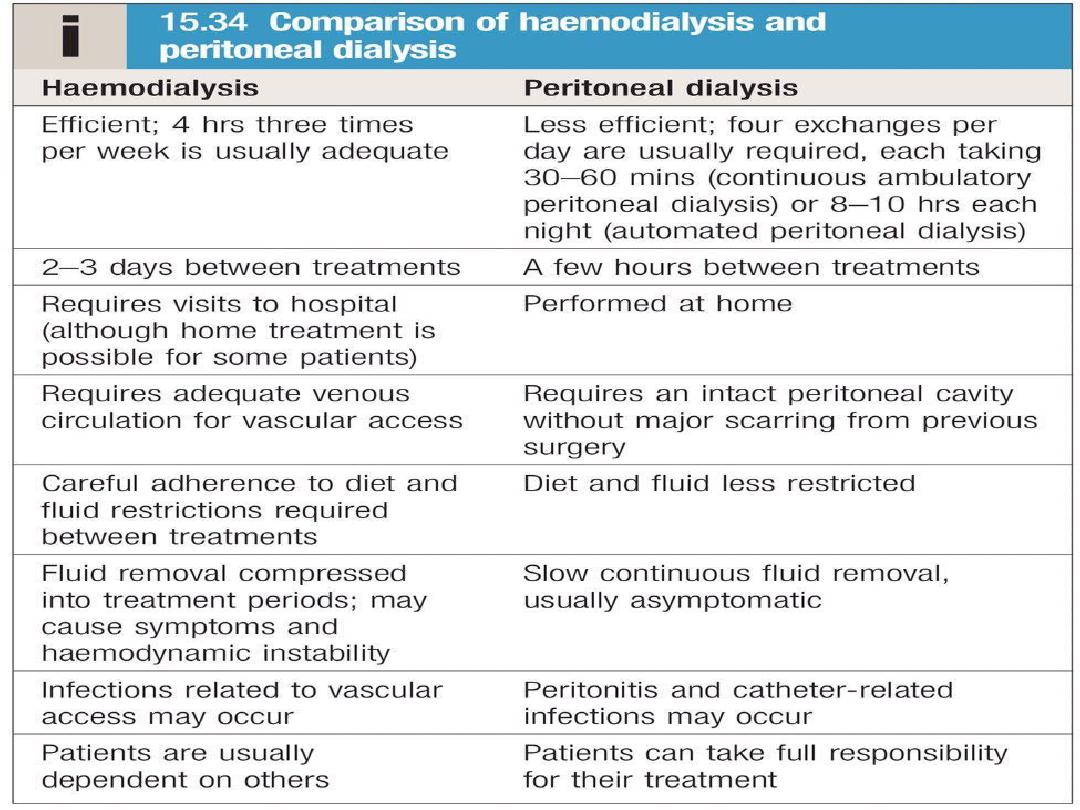
Haemodialysis is usually carried out
for 3–5
hours three times weekly
, either at home or in
an outpatient dialysis unit.
The intensity and frequency of dialysis should be
adjusted to achieve a reduction in urea during
dialysis (urea reduction ratio) of over 65%;
below this level there is an associated increase
in mortality.
Most patients notice an
improvement
in
symptoms during the
first 6 weeks
of treatment.

The intensity of dialysis can be
increased by:
• escalating the number of standard sessions to
four or more per week.
• performing short, frequent dialysis sessions of
2–3 hours 5–7 times per week.
• performing nocturnal haemodialysis, when
low blood-pump speeds and single-needle
dialysis are used for approximately 8 hours
overnight 5–6 times per week.

More frequent dialysis and nocturnal
dialysis
can
achieve
better
fluid
balance
and
phosphate
control,
improve left ventricular mass and
possibly improve mortality, although
the latter has not yet been robustly
demonstrated
.

Haemofiltration
This technique is principally
used in the
treatment of AKI as CRRT
. Large volumes of
water are filtered from blood across a porous
semipermeable membrane under a pressure
gradient.
Solutes
are
removed
via
‘solvent
drag’.
Replacement fluid of a suitable electrolyte
composition is added to the blood after it exits
the haemofilter. If removal of fluid is required,
then less fluid is added back than is removed.

Haemofiltration may be either intermittent or
continuous, and typically 1–2 L of filtrate is
replaced per hour (equivalent to a GFR of 15–30
mL/min/1.73 m2); higher rates of filtration may
be of benefit in patients with sepsis and multi-
organ failure.
Issuesconcerning anticoagulation are similar to
those for haemodialysis, but may be more
problematic because longer or continuous
anticoagulation is necessary.
Haemodiafiltration
This
technique
combines
haemodialysis
with
approximately
20–30
L
of
ultrafiltration
(with
replacement of filtrate) over a 3–5-hour treatment.
It uses a large-pore membrane and combines the
improved
clearance
of
medium-sized
molecules
observed in haemofiltration with the higher small-
solute clearance of haemodialysis. It is sometimes used
in the treatment of AKI, often as continuous therapy It
is increasingly favoured in the treatment of CKD but is
more expensive than haemodialysis and the long-term
benefits are not yet established
.

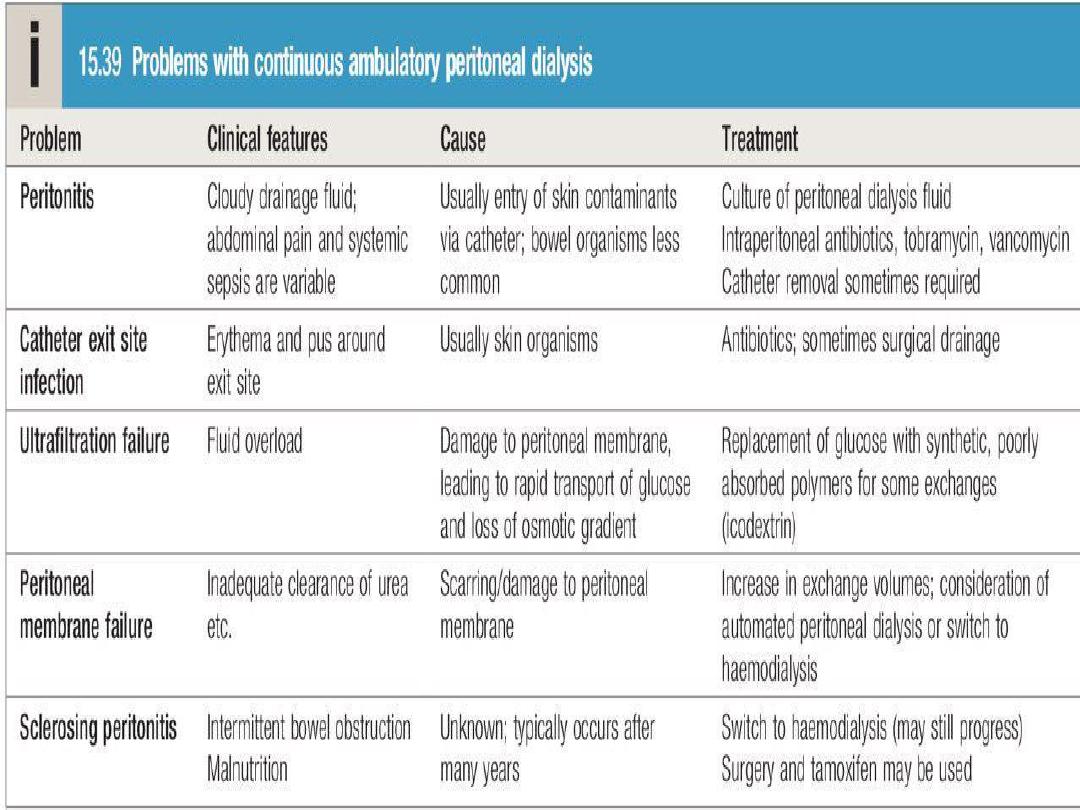
Peritoneal dialysis
Peritoneal dialysis is principally used in the
treatment of CKD, though it may occasionally be
employed in AKI. It requires the insertion of a
permanent Silastic catheter into the peritoneal
cavity.
Two types are in common use:
In continuous ambulatory peritoneal dialysis
(CAPD)
, about 2 L of sterile, isotonic dialysis
fluid are introduced and left in place for
approximately 4–6 hours.

Metabolic
waste
products
diffuse
from
peritoneal capillaries into the dialysis fluid down
a concentration gradient. The fluid is then
drained and fresh dialysis fluid introduced, in a
continuous
four-times-daily cycle.
Automated peritoneal dialysis (APD)
is similar
to CAPD but uses a mechanical device to
perform the fluid exchanges during the night,
leaving the patient free, or with
only a single
exchange to perform, during the day.
CAPD is particularly useful in
children
, as a
first
treatment in adults with residual renal function
,
and as a treatment for
elderly patients with
cardiovascular instability
.
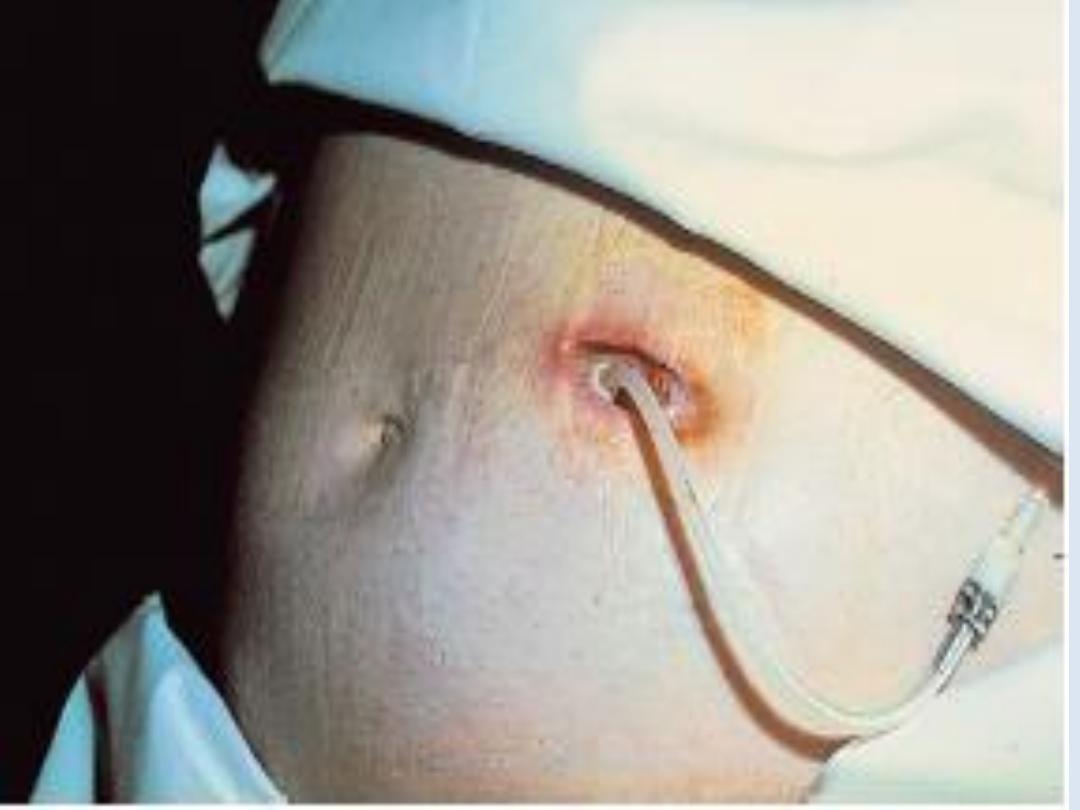
The long-term use of peritoneal dialysis
may be
limited by episodes of bacterial peritonitis and
damage to the peritoneal membrane, including
encapsulating peritoneal sclerosis, but some
patients have been treated successfully for more
than 10 years.


Renal transplantation
offers the best chance of long-term survival in
ESRD, and is the most cost-effective treatment.
Transplantation can restore normal kidney
function
and
correct
all
the
metabolic
abnormalities of CKD but requires long-term
immunosuppression with its attendant risks.
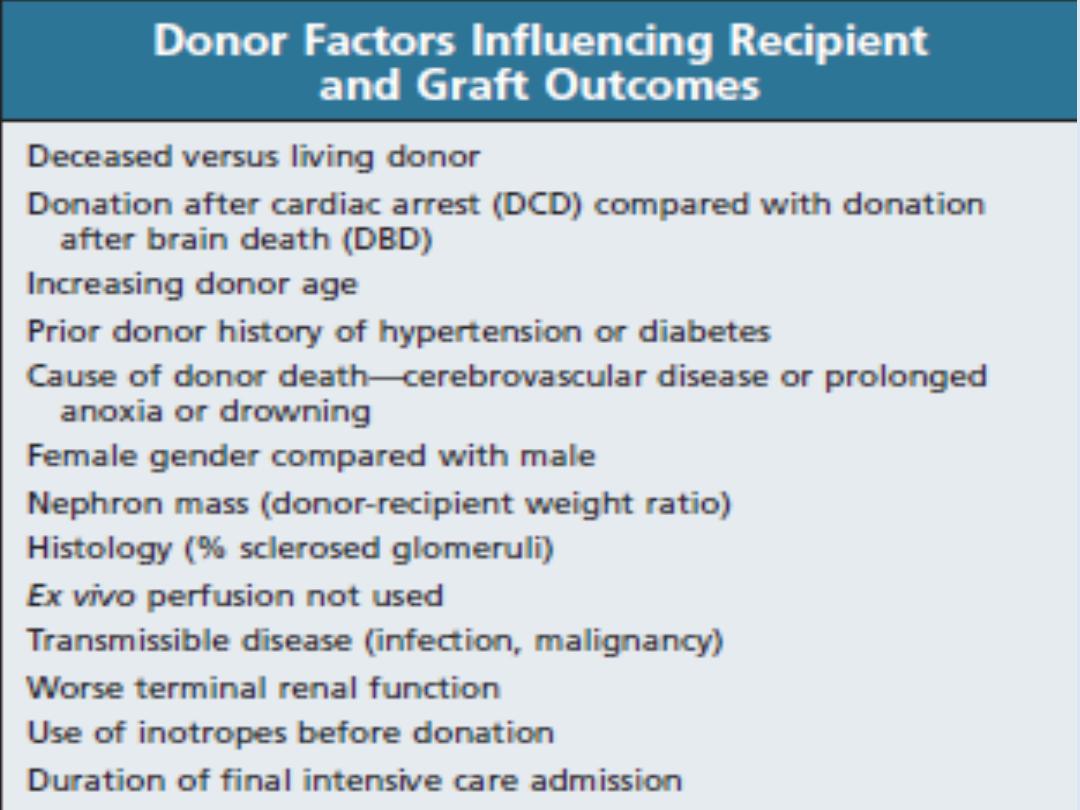
Kidney grafts may be taken from a cadaver in
the UK after brain death (51%) or circulatory
death (11%), or from a living donor (38%).
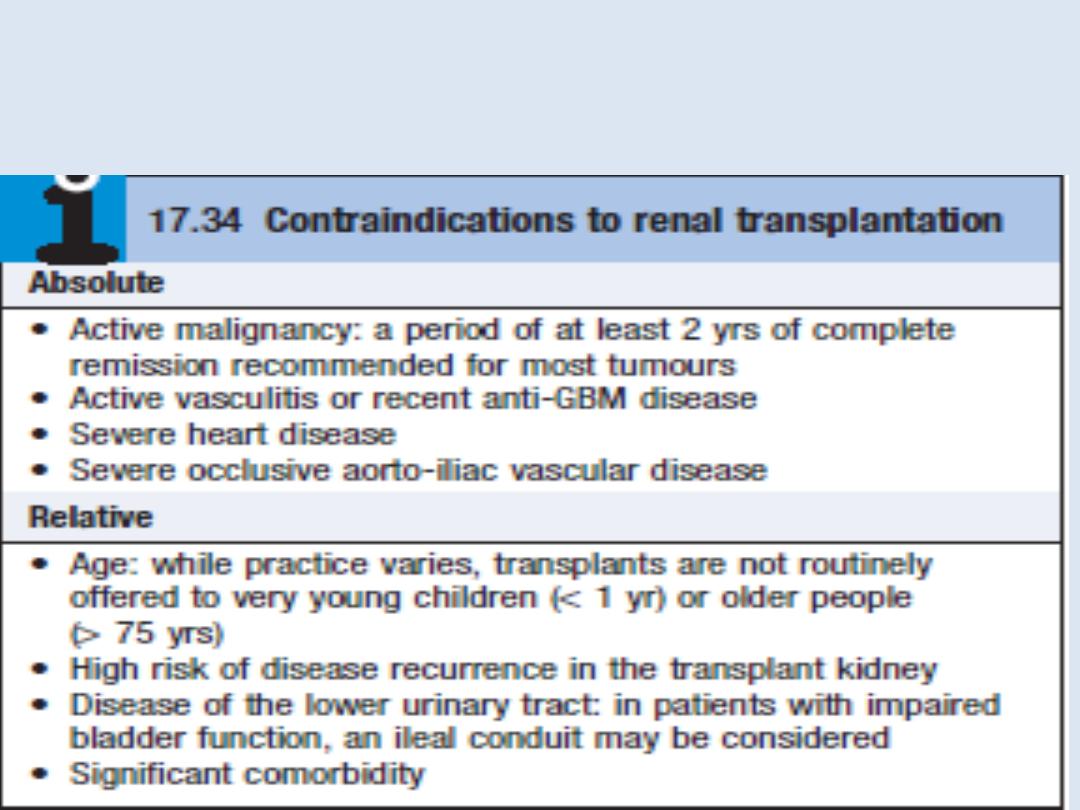
All patients with ESRD
should be considered for transplantation, unless there
are contraindications
Compatibility of ABO blood group between donor
and recipient is usually required and the degree of
matching for major histocompatibility (MHC)
antigens,
particularly
HLA-DR, influences the
incidence of rejection.
During the transplant operation
, the kidney is
placed in the pelvis; the donor vessels are usually
anastomosed to the recipient’s internal iliac artery
and vein, and the donor ureter anastomosed to the
bladder. The native kidneys are usually left in place
but may be
removed pre-transplant
if they are a
source of repeated sepsis or to make room for a
transplant in patients with very large kidneys due to
adult polycystic kidney disease.

• Fluid balance
: Careful matching of input to
output is required. Patients can be very polyuric
in the initial period after transplantation.
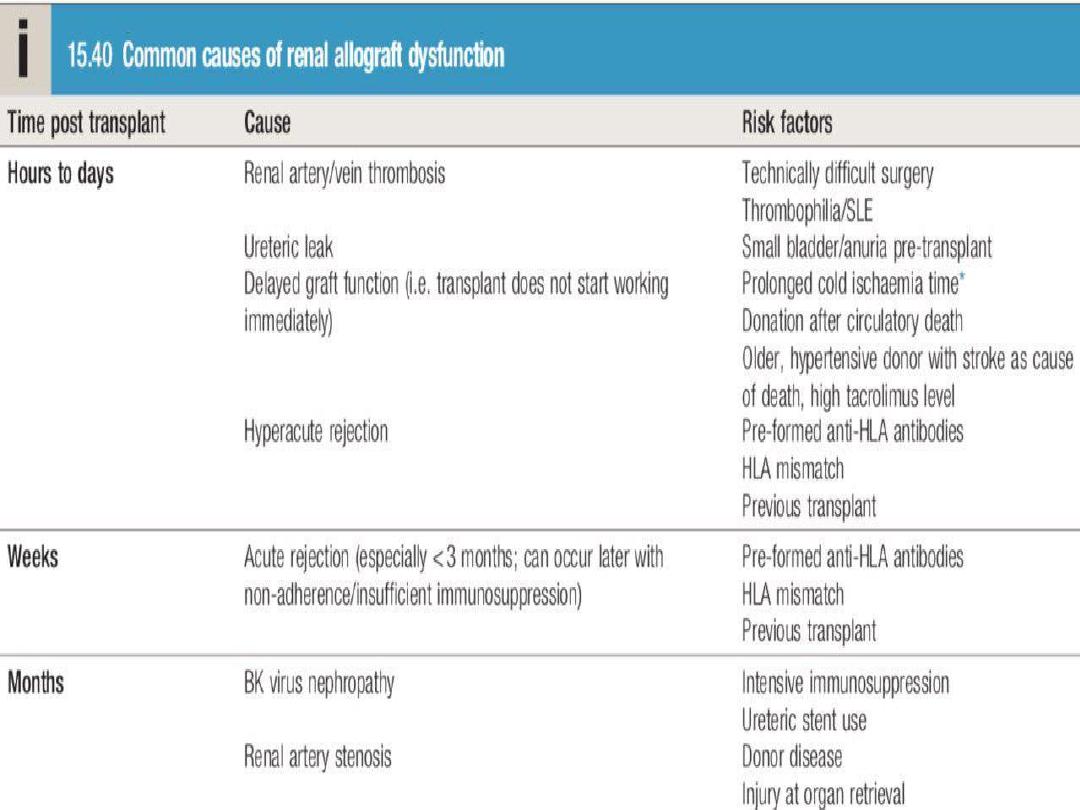
• Primary graft non-function
: Causes include
hypovolaemia, preservation injury/acute tubular
necrosis during storage and transfer, other pre-
existing renal damage, hyperacute rejection,
vascular occlusion and urinary tract obstruction.

• Sepsis
: In addition to risks of sepsis associated
with any operation, there is an increased risk
due to the uraemia and immunosuppression.
Once the graft begins to function, near-normal
biochemistry is usually achieved within days to
weeks.
All transplant patients require regular life-long
follow-up to monitor renal function and life-long
immunosuppressive therapy.
Allograft dysfunction is often asymptomatic and
picked up during routine surveillance blood
tests.
A common regimen is
triple therapy
with
prednisolone; ciclosporin or tacrolimus; and
azathioprine or mycophenolate mofetil.

Acute rejection
is usually treated, in the first
instance,
by
short
courses
of
high-dose
corticosteroids, such as methylprednisolone 500
mg IV on 3 consecutive days.
Other
therapies,
such
as
antilymphocyte
antibodies, intravenous immunoglobulin and
plasma exchange, can be used for episodes of
acute rejection that do not respond to high dose
corticosteroids.

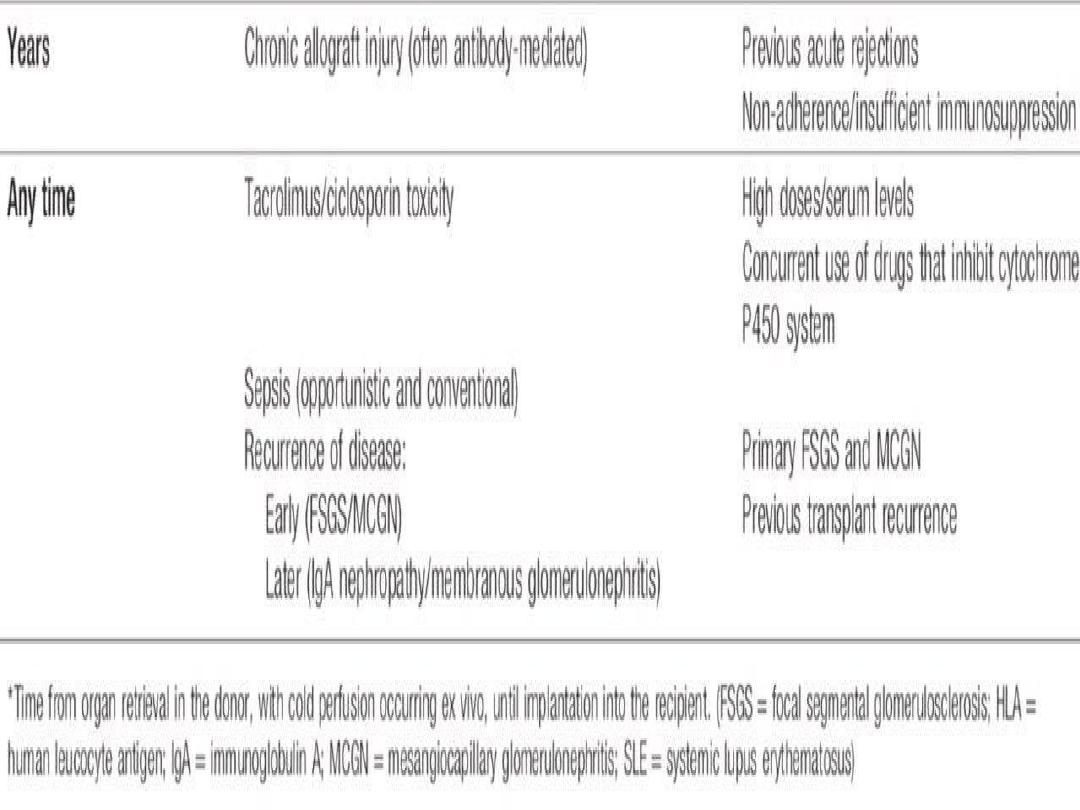
Complications of immunosuppression
include
infections and malignancy. Approximately 50% of
white patients develop skin malignancy by 15 years
after transplantation.
The prognosis
after kidney transplantation is good.
Recent UK statistics for transplants from cadaver
donors indicate 96% patient survival and 93% graft
survival at 1 year, and 88% patient survival and 84%
graft survival at 5 years. Even better figures are
obtained with living donor transplantation (91%
graft survival at 5 years).