
Biochemistry lectures
By
Assistant.Prof Dr. Ban Mahmood Al-joda
Clinical Biochemistry
College of Medicine University of
Babylon
Structure of amino acids and
classification

Objectives:
1-
Define amino acids, essential and non- essential
amino acids.
2. List the aliphatic, aromatic, acidic and basic amino
acids.
3. Name essential amino acids.
4. Classify amino acids based on structure, based on
side chain character, based on metabolic fate, based
on nutritional requirements.
5. Describe the basic structure and general
properties of amino acids.

REFERENCES
1. Lippincott’s Reviews of Biochemistry, 3
rd
ed. ,
2018.
2. Lehninger Principle of Biochemistry, 4
th
ed.
2005.
Amino Acids
Amino Acids are the building units of proteins. Proteins are
polymers of amino acids linked together by what is called “
Peptide bond” .
❑ There are about 300 amino acids occur in nature. Only 20 of
them occur in proteins.
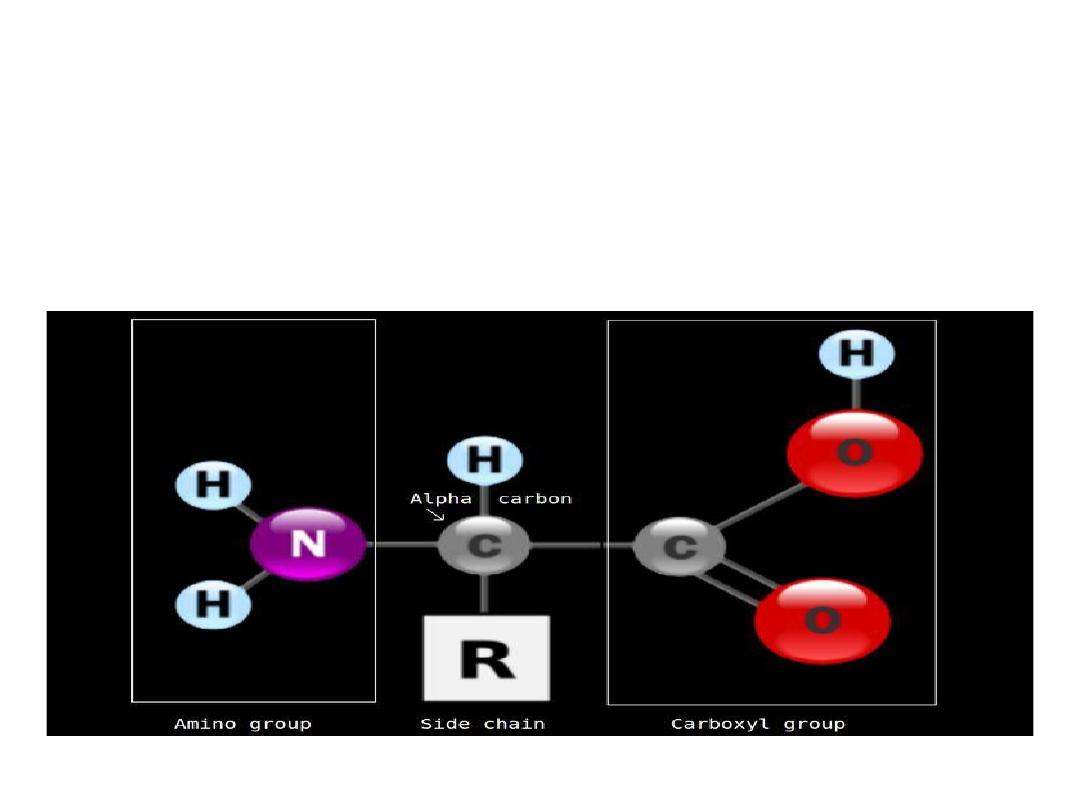
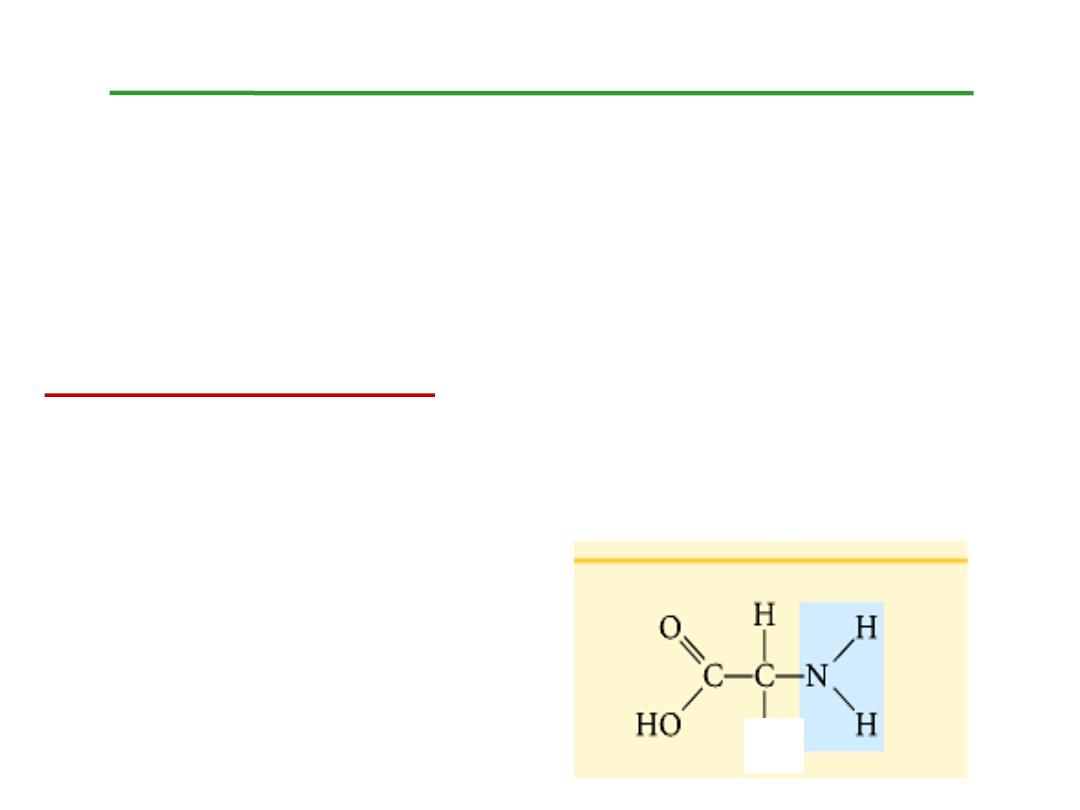
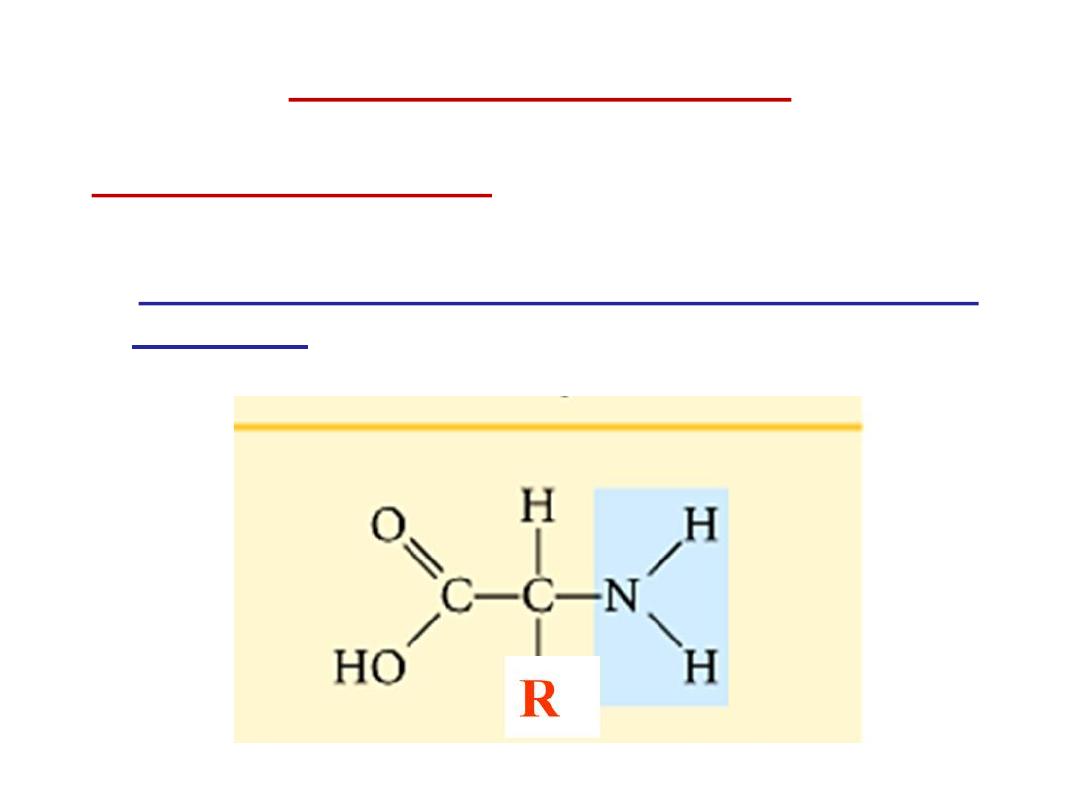
Structure of amino acids:
Each amino acid has 4 different groups attached to α- carbon (
which is C-atom next to COOH). These 4 groups are : amino
group, COOH
Hydrogen atom and side
Chain (R)
R
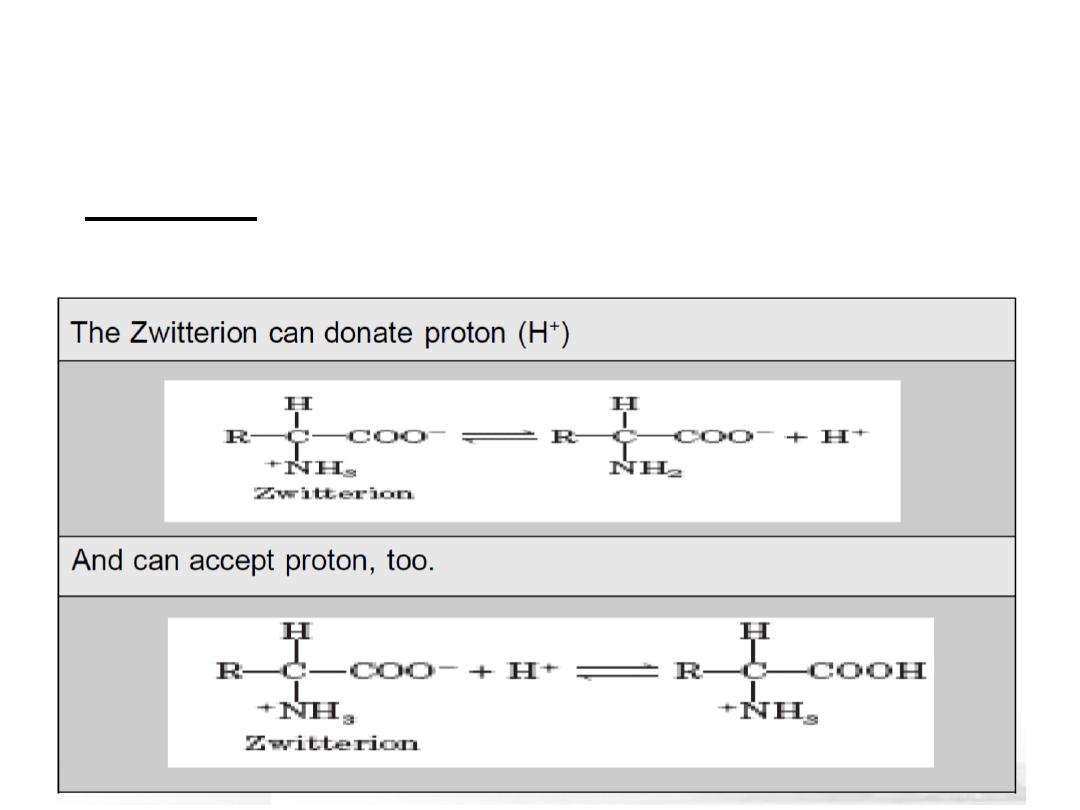
•
At physiological PH (7.4), -COOH is dissociated forming a
negatively charged carboxylate ion
(COO
-
)
and amino group
is protonated forming positively charged ion
(NH
3
+
)
forming
Zwitter ion
Classification of amino acids
I- Chemical classification:
According to number of COOH
and NH
2
groups i.e. according to net charge on amino acid.
A- Monobasic, monocarboxylic amino acids i.e. neutral or
uncharged:
Subclassification of neutral amino acids:
A)-ll structures are required
1- Glycine
R= H
2- Alanine
R= CH
3
3- Branched chain amino acids:
R is branched such as in:
a - Valine
R= isopropyl gp
b- Leucine
R= isobutyl gp
c- Isoleucine R = is isobutyl
R is isobutyl in both leucine and isoleucine but branching is
different:
in leucine →
branching occurs on γ carbon
in isoleucine→ branching occurs on β- carbon
4- Neutral Sulfur containing amino acids:
e.g. Cysteine and Methionine.
5- Neutral, hydroxy amino acids:
e.g. Serine and Threonine
6- Neutral aromatic amino acids:
a- Phenyl alanine :
It’s alanine in which one hydrogen of CH
3
is
substituted with phenyl group.
b- Tyrosine
: - it is P- hydroxy phenyl alanine
- it is classified as
phenolic amino acid
c- Tryptophan:
as it contains indole ring so it is classified as
heterocyclic amino acid
7- Neutral heterocyclic amino acids:
a- Tryptophan: contains indole ring
b- Proline:
In proline, amino group enters in the ring formation
being α-imino gp so proline is an α-imino acid
rather than α-amino acid
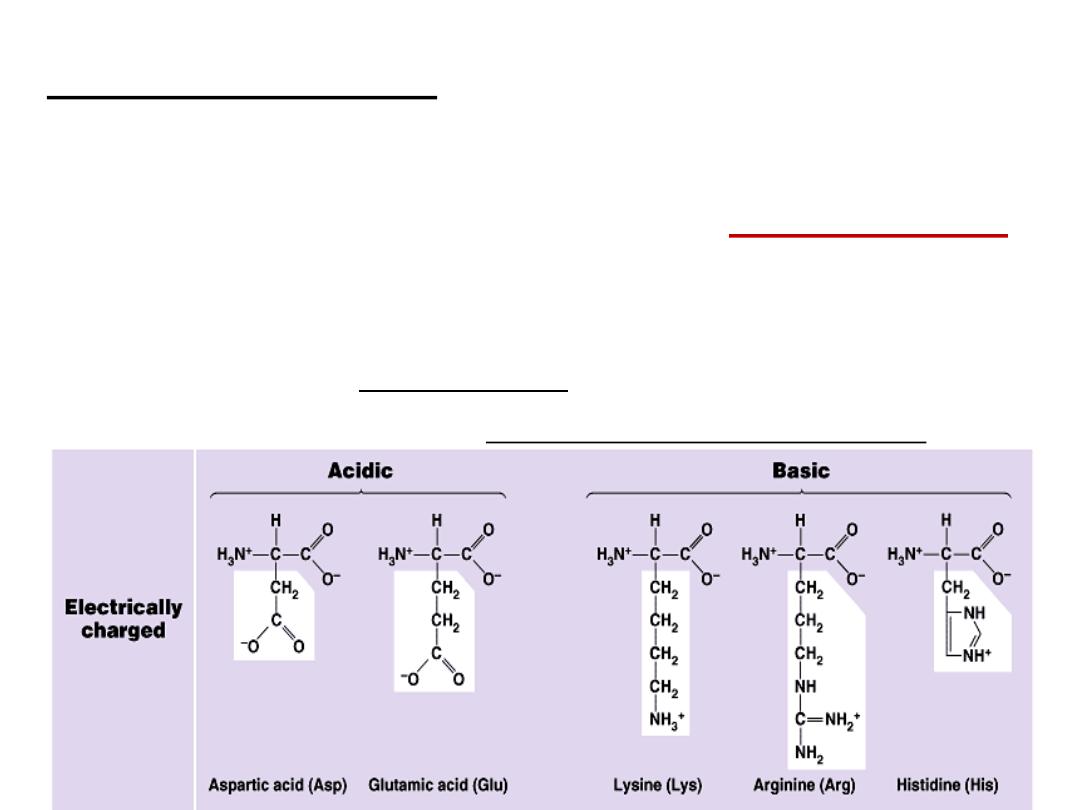
B- Basic amino acids: Contain two or more NH
2
groups or
nitrogen atoms that act as base i.e. can
bind proton.
At physiological pH, basic amino acids will be
positively charged
.
e.g.
a- Lysine
b- Arginine: contains guanido group
c- Histidine: is an example on basic heterocyclic amino acids

C
-
Acidic Amino acids:
at physiological pH will carry negative
charge.
Example: Aspartic acid (aspartate) and Glutamic acid (glutamate).
Aspargine and Glutamine:
They are amide forms of aspartate and
glutamate in which side chain COOH groups are amidated.
They are classified as neutral amino acids.
II- Classification according to polarity of side chain (R):
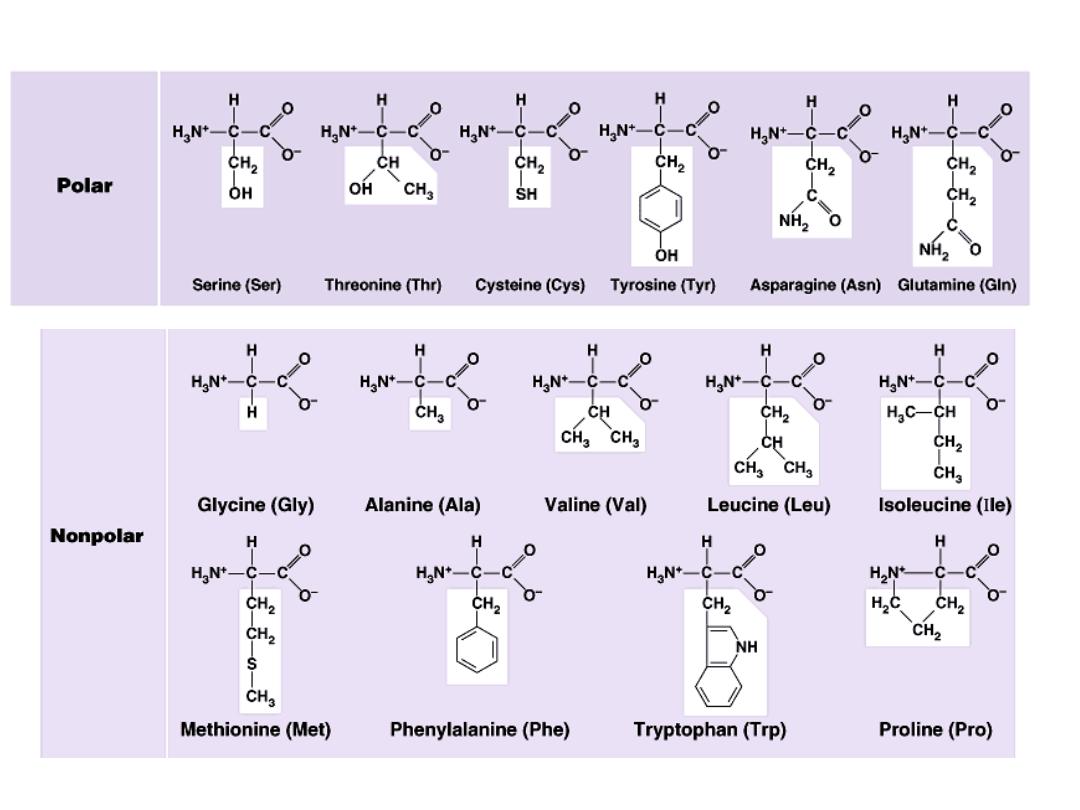
A- Polar amino acids:
in which R contains polar hydrophilic group so
can forms hydrogen bond with H
2
O. In those amino acids, R may
contain:
1- OH group : as in serine, threonine and tyrosine
2- SH group : as in cysteine
3- amide group: as in glutamine and aspargine
4- NH
2
group or nitrogen act as a base (basic amino acids ): as lysine,
arginine and histidine
5- COOH group ( acidic amino acids): as aspartic and glutamic .
B- Non polar amino acids:
R is alkyl hydrophobic group which can’t enter in hydrogen bond
formation. 9 amino acids are non polar ( glycine, alanine, valine, leucine,
isoleucine, phenyl alanine, tryptophan, proline and methionine)
III- Nutritional classification:
1- Essential amino acids:
These amino acids can’t be formed in the
body and so, it is essential to be taken in diet. Their deficiency
affects growth, health and protein synthesis.
2- Semiessential amino acids:
These are formed in the body but not
in sufficient amount for body requirements especially in children.
Summary of essential and semiessential amino acids:
V= valine
i= isoleucine
ly= lysine
l= leucine
A = arginine* H= histidine*
M= methionine
T= tryptophan Th= threonine P= phenyl alanine
*= arginine and histidine are semiessential
3- Non essential amino acids:
These are the rest of amino acids that
are formed in the body in amount enough for adults and children.
They are the remaining 10 amino acids.
IV- Metabolic classification:
according to metabolic or degradation
products of amino acids they may be:
1- Ketogenic amino acids:
which give ketone bodies . Lysine and
Leucine are the only pure ketogenic amino acids.
2- Mixed ketogenic and glucogenic amino acids:
which give both
ketonbodies and glucose.These are: isoleucine, phenyl alanine, tyrosine
and tryptophan.
3- Glucogenic amino acids:
Which give glucose. They include the rest
of amino acids. These amino acids by catabolism yields products that
enter in glycogen and glucose formation.
Chemical properties of amino acids:
1- Reactions due to COOH group:
-Salt formation with alkalis, ester formation with alcohols, amide
formation with amines and decarboxylation.
2- Reactions due toNH2 group:
deamination and reaction with
ninhydrin reagent.
-Ninhydrin reagent reacts with amino group of amino acid yielding
blue colored product. The intensity of blue color indicates quantity of
amino acids present.

Ninhydrine can react with imino acids as proline and hydroxy
proline but gives
yellow color.
3- Reactions due to side chain (R):
1- Millon reaction: for tyrosine gives red colored mass
2- Rosenheim reaction: for trptophan and gives violet ring.
3- Pauly reaction: for imidazole ring of histidine: gives yellow to
reddish product
4- Sakagushi test: for guanido group of arginine and gives red
color.
5- Lead sulfide test (sulfur test): for sulfur containing amino
acids as cysteine give brown
color.
