Peptides and Proteins
BY
Assistant. Prof. Dr. Ban Mahmood Shaker Al-joda

Objectives:
1- Define peptide bond , dipeptide , tripeptide and
polypeptide and explain how they are formed .
2- Explain the formation of disulfide linkage
between two cysteine residues .
3- Describe the basic structure of protein
including both simple and conjugated proteins.
4- Classify of proteins .
Peptides and Proteins
20 amino acids are commonly found in protein.
These 20 amino acids are linked together through “peptide bond
forming peptides and proteins (what’s the difference?).
- The chains containing less than 50 amino acids are called
“peptides”,
while those containing greater than 50 amino acids
are called
“proteins”.
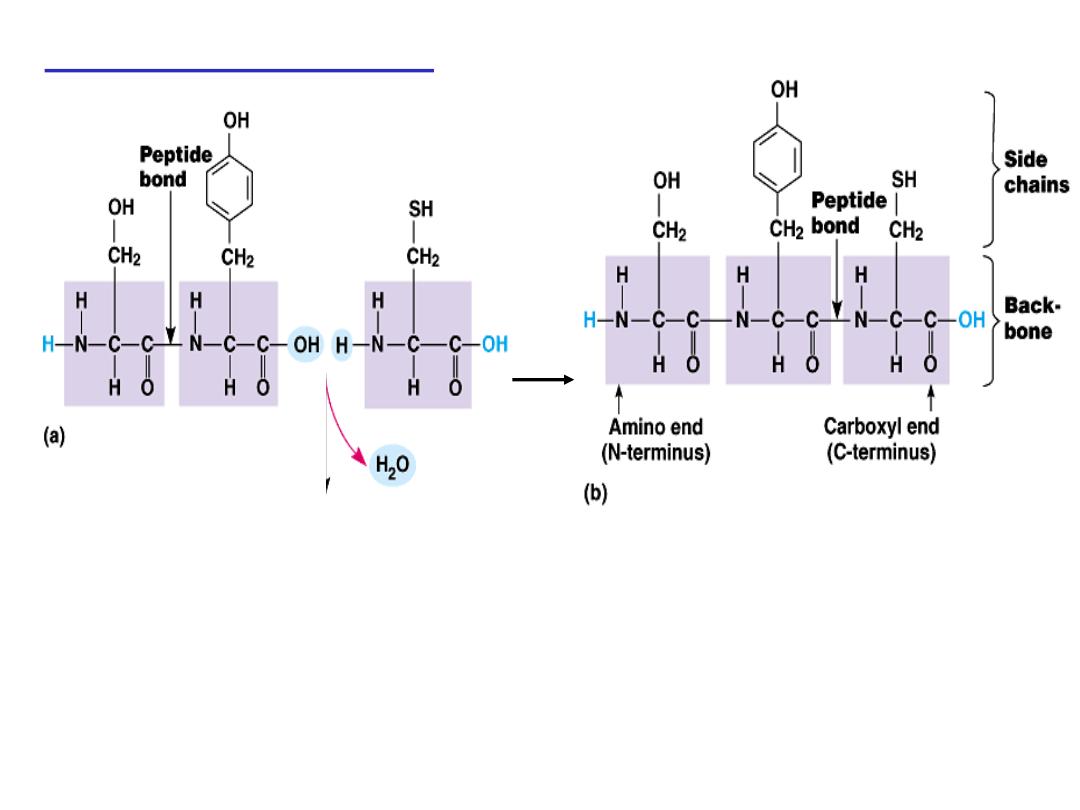
Peptide bond formation:
α-carboxyl group of one amino acid (with side chain R1)
forms a covalent peptide bond with α-amino group of another
amino acid ( with the side chain R2) by removal of a molecule of
water. The result is : Dipeptide ( i.e. Two amino acids linked by
one peptide bond).
By the same way,
the dipeptide can then
forms a second peptide bond with a third amino acid (with side
chain R3) to give Tripeptide. Repetition of this process generates
a polypeptide or protein of specific amino acid sequence.
Peptide bond formation:
- Each polypeptide chain starts on the left side by free amino group
of the first amino
acid enter in chain formation . It is termed
(N- terminus).
- Each polypeptide chain ends on the right side by free COOH group
of the last amino acid and termed
(C-terminus)
.
Examples on Peptides:
1- Dipeptide
( tow amino acids joined by one peptide bond):
Example:
Aspartame
which acts as sweetening agent being used in
replacement of cane sugar. It is composed of aspartic acid and
phenyl alanine.
2- Tripeptides
( 3 amino acids linked by two peptide bonds).
Example:
Glutathione (
GSH) is an antioxidant in plants, animals,
fungi, and some bacteria and archaea.Which is formed from 3
amino acids:
glutamic acid
,
cysteine
and
glycine
. It helps in
absorption of amino acids, protects against hemolysis of Red Blood
Cell (RBC) by breaking H
2
O
2
which causes cell damage.
3- octapeptides: (8 amino acids)
Examples:
Two hormones; oxytocin and vasopressin Antidiuretic
Hormone (ADH).
4- polypeptides
: 10- 50 amino acids: e.g. Insulin hormone
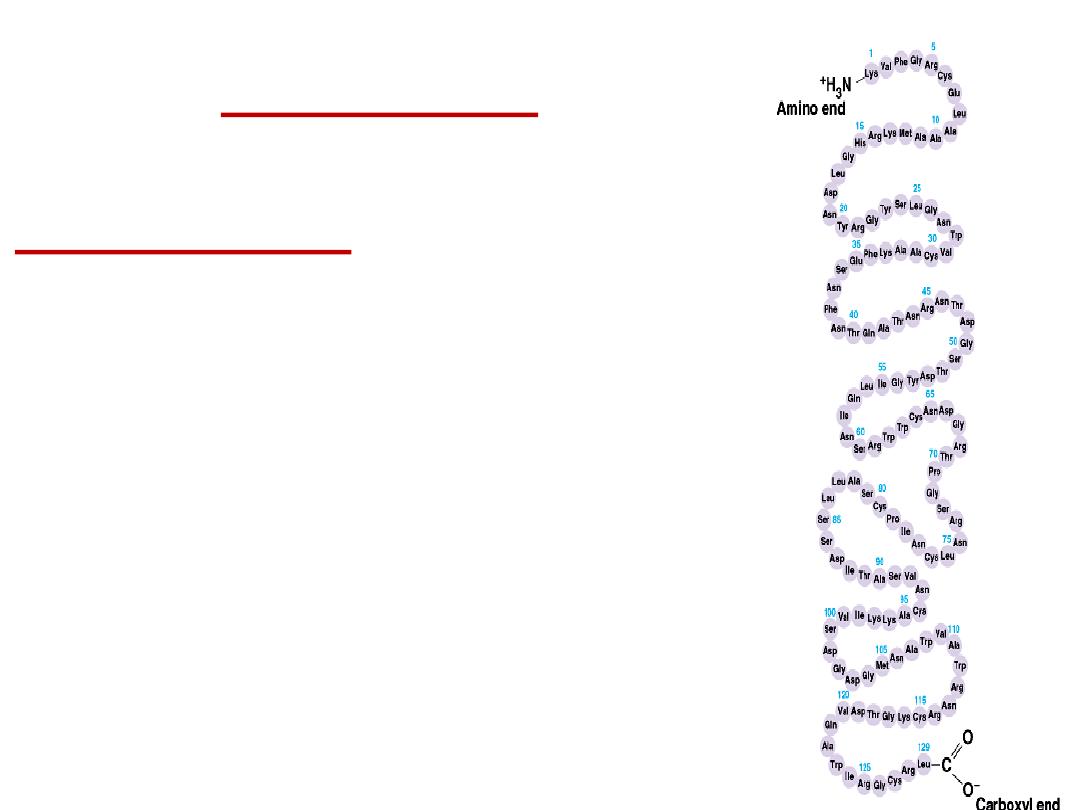
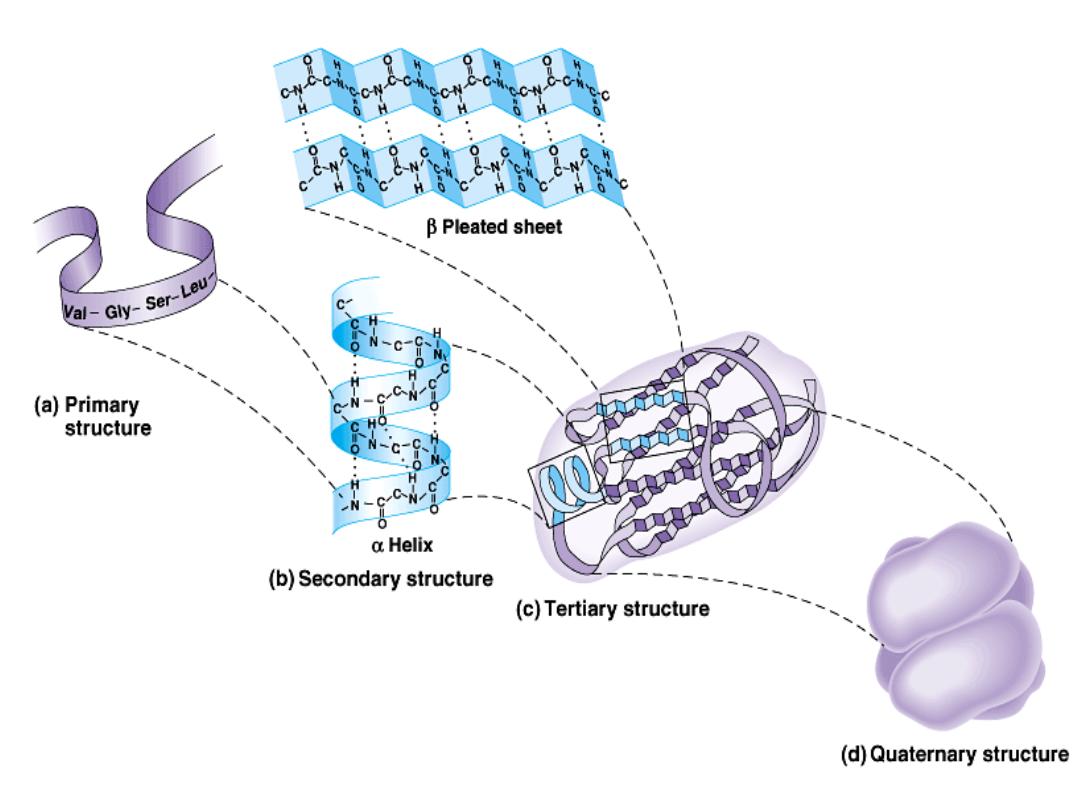
Protein structure:
There are four levels of protein structure (primary,
secondary, tertiary and quaternary)
Primary structure:
•
The primary structure of a protein is its
unique sequence of amino acids.
–
Lysozyme, an enzyme that attacks bacteria,
consists of a polypeptide chain of 129
amino acids.
–
The precise primary structure of a protein is
determined by inherited genetic
information.
–
At one end is an amino acid with a free
amino group the (the N-terminus) and at the
other is an amino acid with a free carboxyl
group the (the C-terminus).

•
A functional protein is not just a polypeptide chain, but one or
more polypeptides precisely twisted, folded and coiled into a
molecule of unique shape (conformation). This conformation is
essential for some protein function e.g. Enables a protein to
recognize and bind specifically to another molecule .
•
Example:
hormone/receptor ; enzyme/substrate and
antibody/antigen.
•
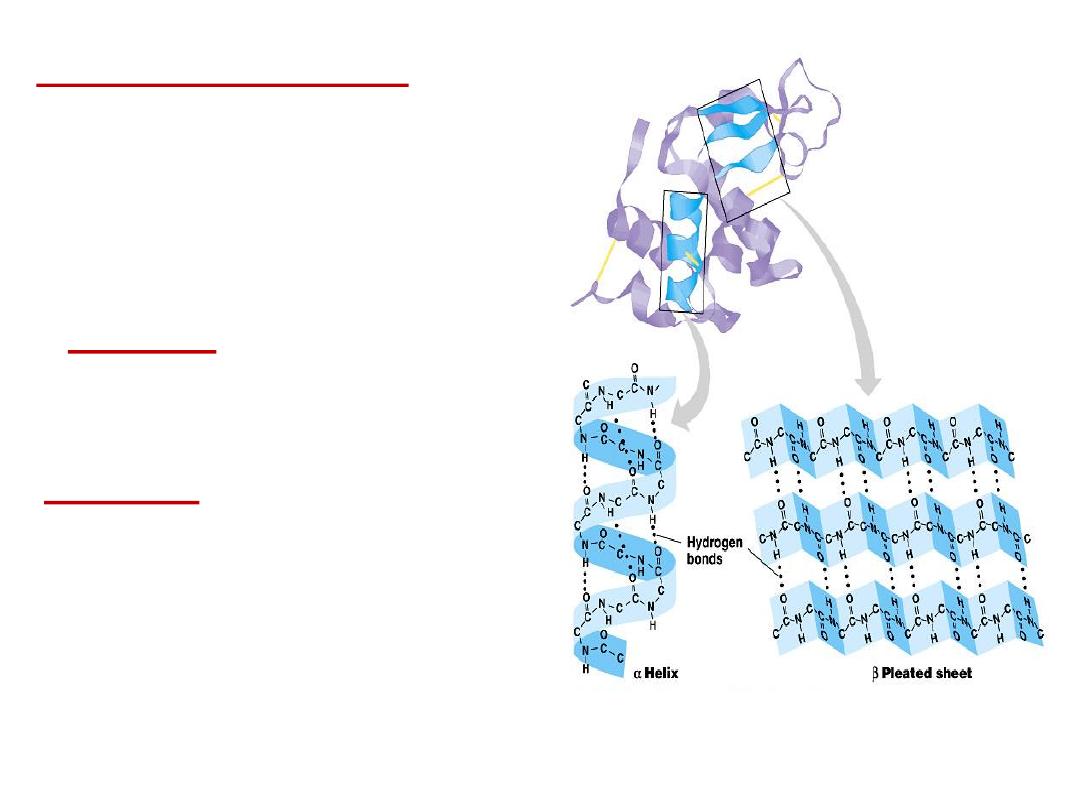
2- Secondary structure:
Results from hydrogen bond
formation between hydrogen of –NH
group of peptide bond and the carbonyl
oxygen
of
another
peptide
bond.
According to H-bonding there are two
main forms of secondary structure:
α-helix:
It is a spiral structure
resulting
from
hydrogen
bonding
between one peptide bond and the fourth
one
β-sheets:
is another form of secondary
structure
in
which
two
or
more
polypeptides (or segments of the same
peptide chain) are linked together by
hydrogen bond between H- of NH- of one
chain and carbonyl oxygen of adjacent
chain (or segment).
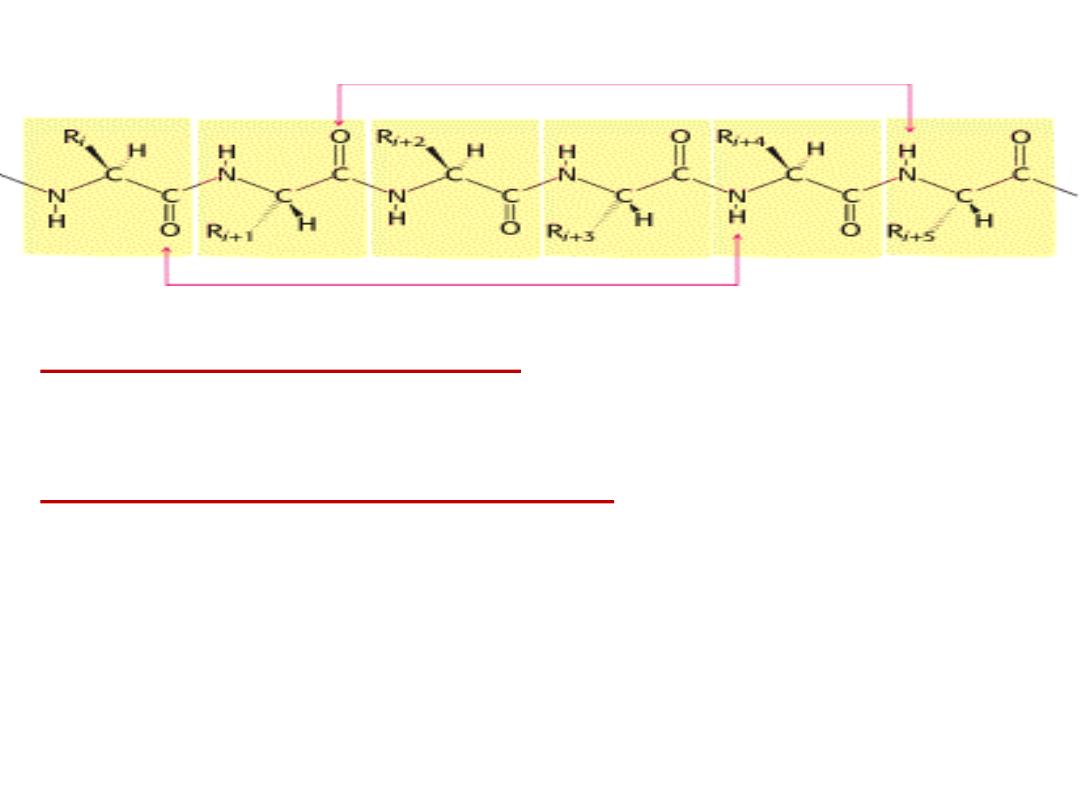
Hydrogen bonding in α-helix:
In the α-helix CO of the one amino
acid residue forms H-bond with NH of the forth one.
Supersecondary structure or Motifs :
occurs by combining secondary structure.
The combination may be: α-helix- turn- α-helix- turn…..etc
Or: β-sheet -turn- β-sheet-turn………etc
Or: α-helix- turn- β-sheet-turn- α-helix
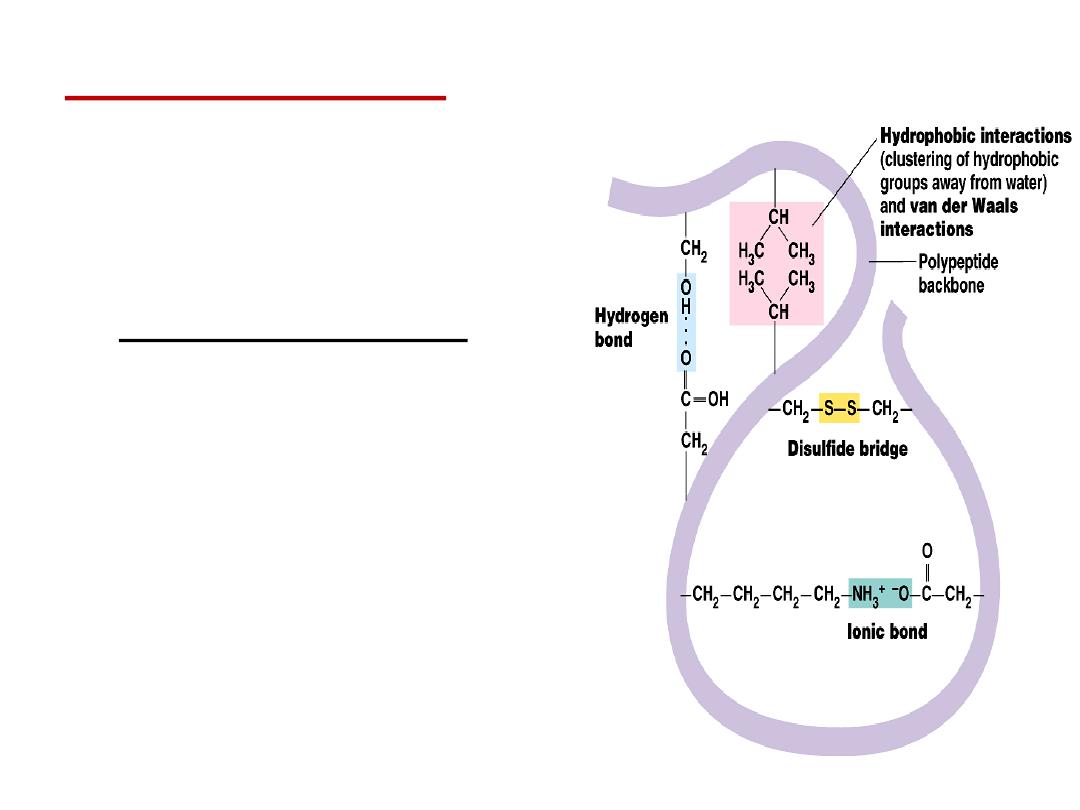
•
Tertiary structure
is
determined by a variety of
interactions (bond formation)
among R groups and between R
groups and the polypeptide
backbone.
a.
The weak interactions include:
▪
Hydrogen bonds among polar
side chains
▪
Ionic bonds between
charged R groups ( basic and
acidic amino acids)
▪
Hydrophobic
interactions among
hydrophobic ( non polar) R
groups.
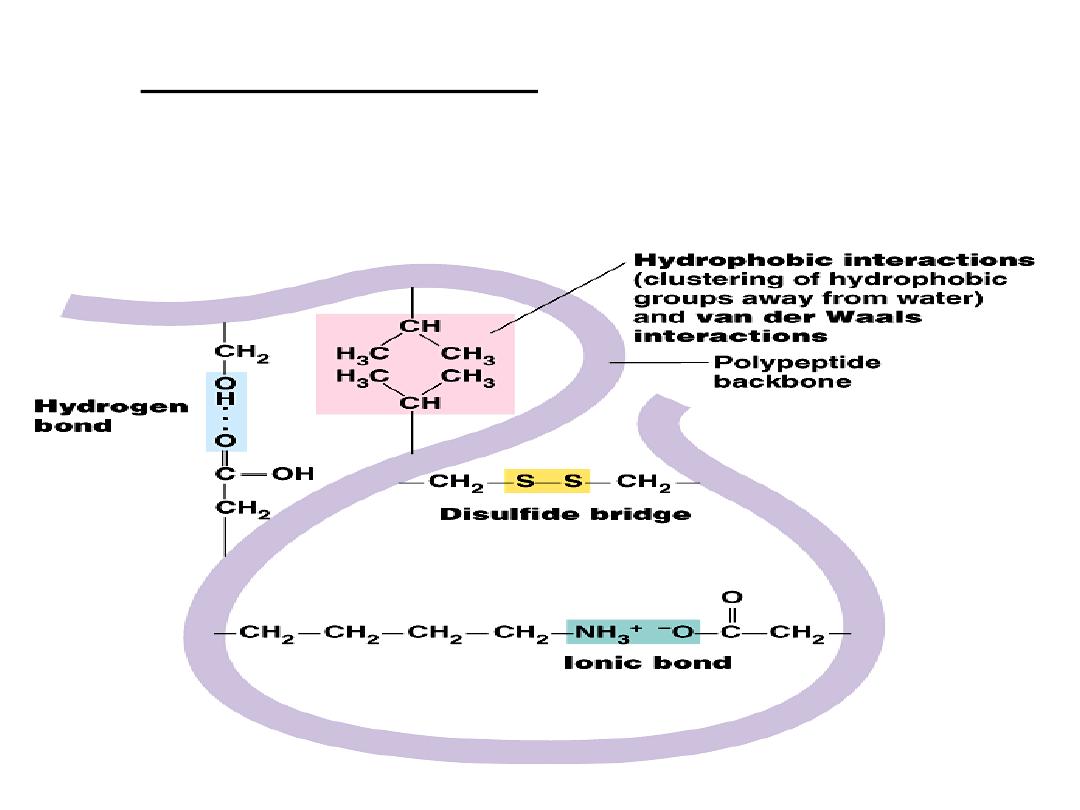
b.
Strong covalent bonds include disulfide
bridges, that form between the sulfhydryl
groups (SH) of cysteine monomers, stabilize
the structure.
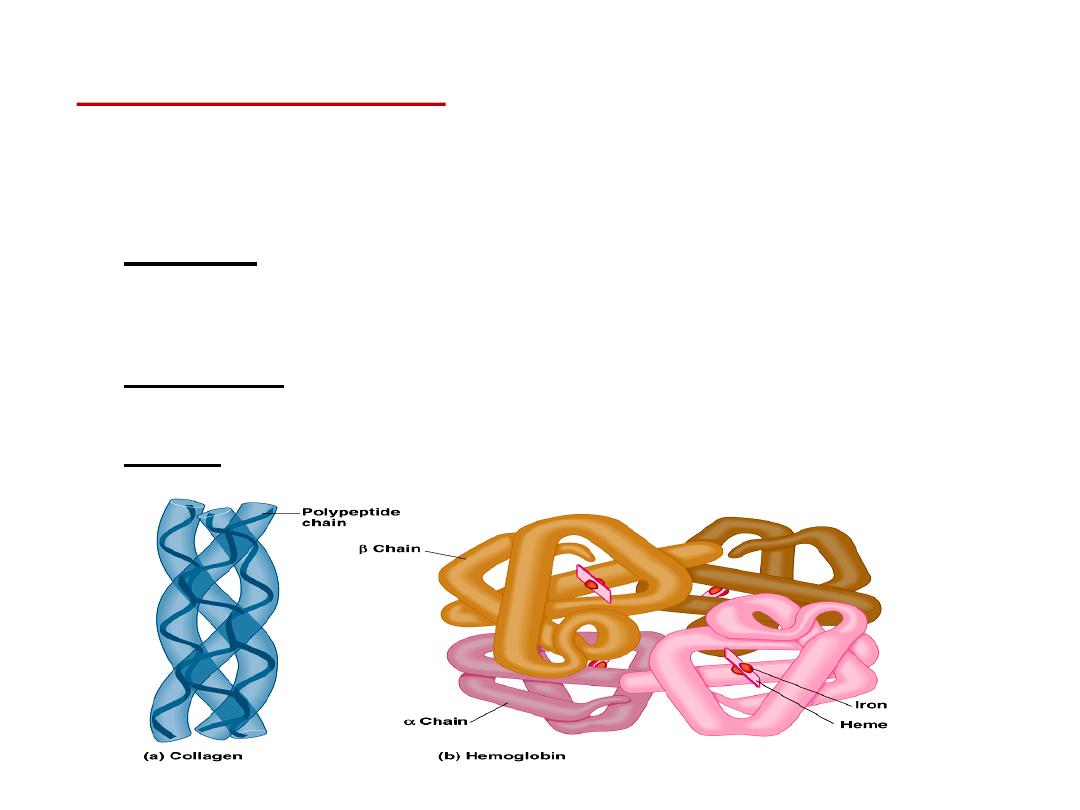
•
Quaternary structure:
results from the aggregation (combination) of two
or more polypeptide subunits held together by non-covalent interaction like H-
bonds, ionic or hydrophobic interactions.
•
Examples on protein having quaternary structure:
–
Collagen is a fibrous protein of three polypeptides (trimeric) that are
supercoiled like a rope.
•
This provides the structural strength for their role in connective tissue.
–
Hemoglobin is a globular protein with four polypeptide chains (tetrameric)
–
Insulin : two polypeptide chains (dimeric)
Classification of proteins
I- Simple proteins:
i.e. on hydrolysis gives only amino acids
Examples:
1- Albumin and globulins:
present in
egg, milk and blood
They are proteins of high biological value i.e. contain all essential
amino acids and easily digested.
Types of globulins:
α1 globulin:
e.g. antitrypsin
α2 globulin:
e.g. hepatoglobin: protein that binds hemoglobin to
prevent its excretion by the kidney
β-globulin
: e.g. transferrin: protein that transport iron
γ-globulins
=
Immunoglobulins
(antibodies) : responsible for
immunity.
2- Globins (Histones):
They are basic proteins rich in histidine amino
acid.
They are present in :
a - combined with DNA
b - combined with hem to form hemoglobin of
RBCs.
3- Gliadines : are the proteins present in cereals.
4- Scleroproteins:
They are structural proteins, not digested.
include: keratin, collagen and elastin.
a- α-keratin:
protein found in hair, nails, enamel of teeth and outer layer
of skin.
• It is α-helical polypeptide chain, rich in cysteine and hydrophobic
(non polar) amino acids so it is water insoluble.
b- collagens:
protein of connective tissues found in bone, teeth,
cartilage, tendons, skin and blood vessels.
• Collagen may be present as gel e.g. in extracellular matrix
or in vitreous humor of the eye.
• Collagens are the most important protein in mammals.
They form about 30% of total body proteins.
• There are more than 20 types of collagens, the most
common type is collagen I which constitutes about 90% of
cell collagens.
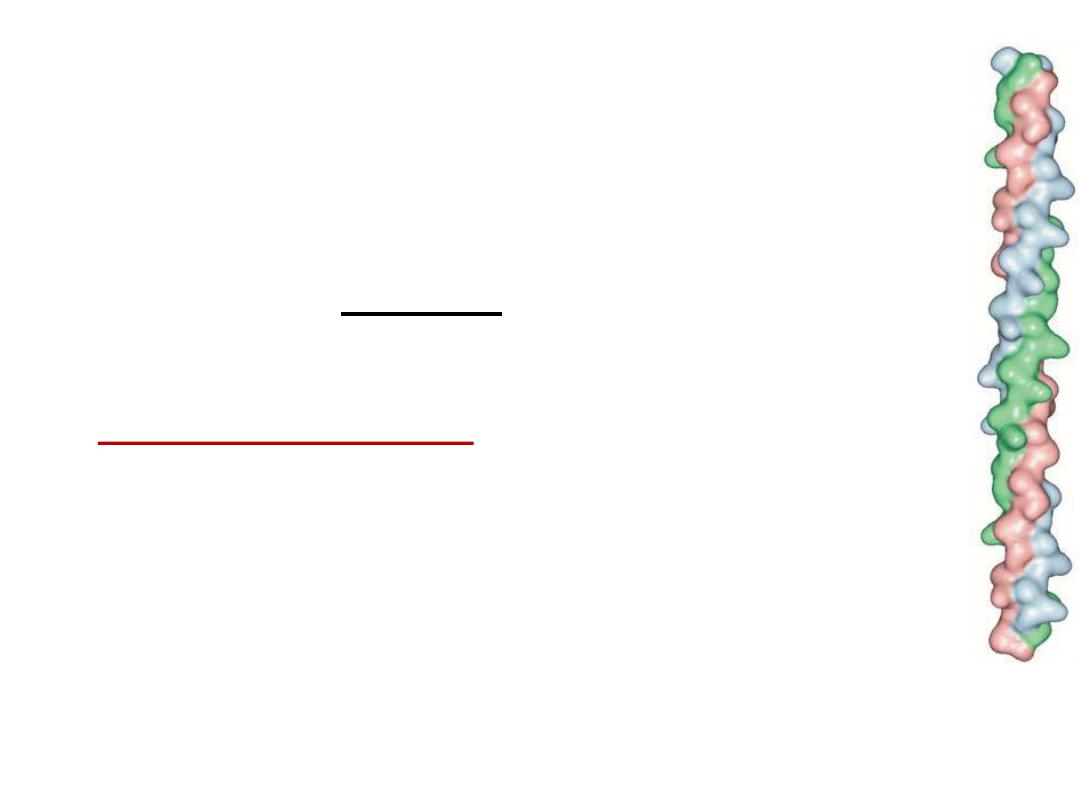
• Structure of collagen:
three helical polypeptide chains
(trimeric) twisted around each other forming triplet-helix
molecule.
• ⅓
of structure is glycine, 10% proline, 10% hydroxyproline
and 1% hydroxylysine. Glycine is found in every third
position of the chain. The repeating sequence –Gly-X-Y-,
where X is frequently proline and Y is often hydroxyproline
and can be hydroxylysine.
Solubility:
collagen is insoluble in all solvents and not digested.
• When collagen is heated with water or dil. HCl it will be converted
into
gelatin
which is soluble , digestible and used as diet ( as jelly).
Gelatin is classified as derived protein.

C- Elastin:
present in walls of large blood vessels (such as aorta).
It is very important in lungs, elastic ligaments, skin,
cartilage, ..
It is elastic fiber that can be stretched to several times as its normal
length.
Structure: composed of 4 polypeptide chains (tetramer), similar to
collagen being having 33% glycine and rich in prolin
but in that it has low hydroxyprolin and absence of
hydroxylysine .
II-Conjugated proteins
i.e. On hydrolysis, give protein part and non protein part and
subclassified into:
1- Phosphoproteins:
These are proteins conjugated with phosphate
group. Phosphorus is attached to OH group of serine or threonine.
e.g. Casein of milk .
2- Lipoproteins:
These are proteins conjugated with lipids.
Functions: a- help lipids to transport in blood
b- Enter in cell membrane structure helping lipid
soluble substances to pass through cell membranes.
3- Glycoproteins:
proteins conjugated with sugar (carbohydrate)
Example : Mucin
Functions of Glycoproteins :
- Some hormones such as erythropoeitin
- present in cell membrane structure
- blood groups.
4- Nucleoproteins:
These are basic proteins ( e.g. histones)
conjugated with nucleic acid (DNA or RNA).
Examples :
a- chromosomes: are proteins conjugated with DNA
b- Ribosomes: are proteins conjugated with RNA
5- Metalloproteins:
These are proteins conjugated with metal like
iron, copper, zinc, ……
a- Iron-containing proteins: Iron may present in heme such as in
- hemoglobin (Hb)
- myoglobin ( protein of skeletal muscles and cardiac muscle),
- cytochromes ,
- catalase, peroxidases (destroy H2O2)
- tryptophan pyrrolase (destroy indole ring of tryptophan).
Iron may be present in free state ( not in heme) as in:
-
Ferritin: Main store of iron in the body. ferritin is present in liver,
spleen and bone marrow.
-
Hemosidrin: another iron store.
-
Transferrin: is the iron carrier protein in plasma.
b- Copper containing proteins:
e.g. Oxidase enzymes such as cytochrome oxidase.
c- Zn containing proteins: e.g. Insulin and carbonic anhydrase
d- Mg containing proteins: e.g. Kinases and phosphatases.
6-Chromoproteins:
These are proteins conjugated with pigment. e.g.
- All proteins containing heme (Hb, myoglobin, ………..)
- Melanoprotein : e.g. proteins of hair or iris which contain melanin.
II-Derived proteins
Produced from hydrolysis of simple proteins.
e.g. - Gelatin: from hydrolysis of collagen
- Peptone: from hydrolysis of albumin

REFERENCES
Lippincott’s Reviews of Biochemistry, 3
rd
ed , 2018.
