ENZYMES
Assistant Prof. Dr. Ban Mahmood Al-joda

• Biological catalysts which speed up the rate of reaction
without becoming part of the reaction but themselves cannot
initiate any chemical reaction.
• Enzymes :
• First name is of substrate
• second, ending in “ASE” indicating type of reaction catalyzed.
• Clarify the reaction , e.g. Malic Enzyme
• L- Malate + NAD → Pyruvate + NADH-H + CO
2
• Malate NAD oxidoreductase (Decarboxylating)
ENZYMES

Trival name
• Gives no idea of source, function or reaction
catalyzed by the enzyme.
• Example: trypsin, thrombin, pepsin.
Systematic Name
• According to the International union Of
Biochemistry an enzyme name has two parts:
-First part is the name of the substrates for
the enzyme.
-Second part is the type of reaction catalyzed
by the enzyme.This part ends with the suffix
“ase”.
Example: Lactate dehydrogenase
CLASSIFICATION OF ENZYMES
Formulated by the enzyme commission of I.U.B six major
classes based on the type of reactions catalyzed
1 Oxidoreductases
Catalyzing oxidation reduction reactions
2 Transferases
Catalyzing group transfer
3 Hydrolases
Catalyzing hydrolytic breakdown
Classification of Enzymes
4. Lyases
Catalysing removal of groups by mechanism other than
hydrolysis and leaving behind double bonds
5. Isomerases
Catalysing interconversion of isomers
6. Ligases
Catalysing formation of bonds and new compounds
1. Oxidoreductases
– Catalysing oxidation reduction reaction where one
substrate is oxidized and other is reduced
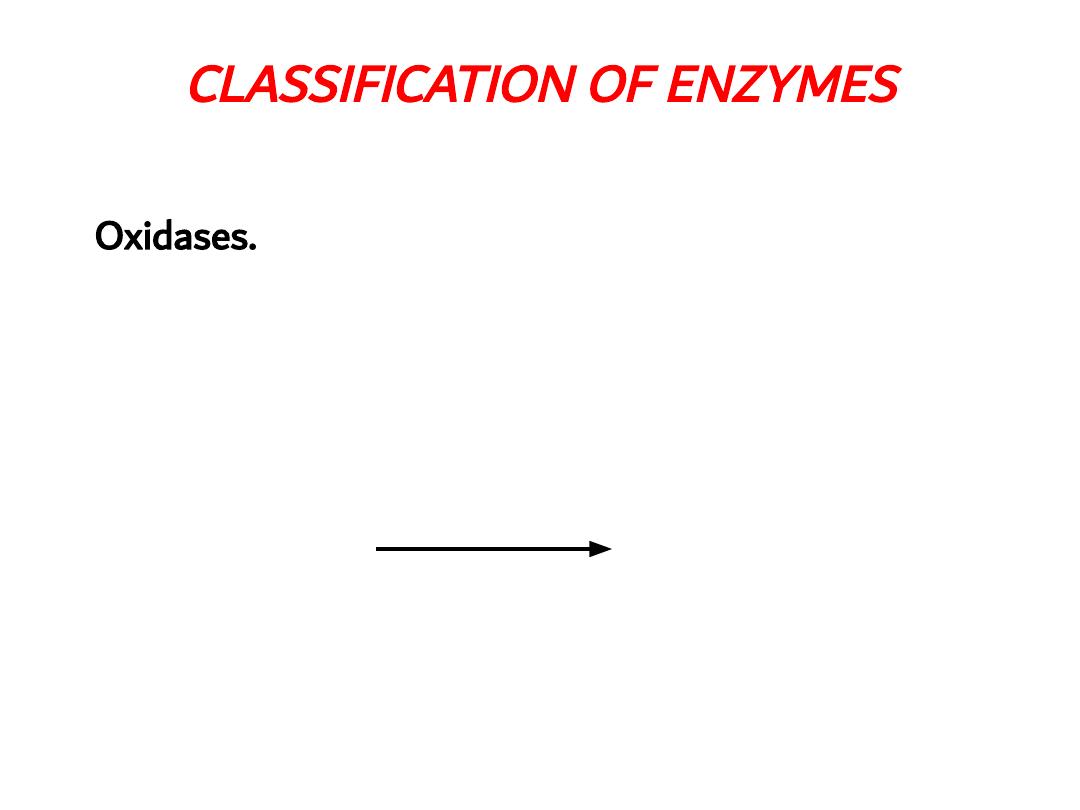
CLASSIFICATION OF ENZYMES
Oxidases. Catalyzing oxidation of the substrate and
atomic oxygen acts as recipient of hydrogen e.g.
Ascorbic acid oxidase, Cytochrome oxidase, Tyrosinase
Ascorbic acid
Oxidase
Ascorbic acid + O
2
Dehydro ascorbic acid
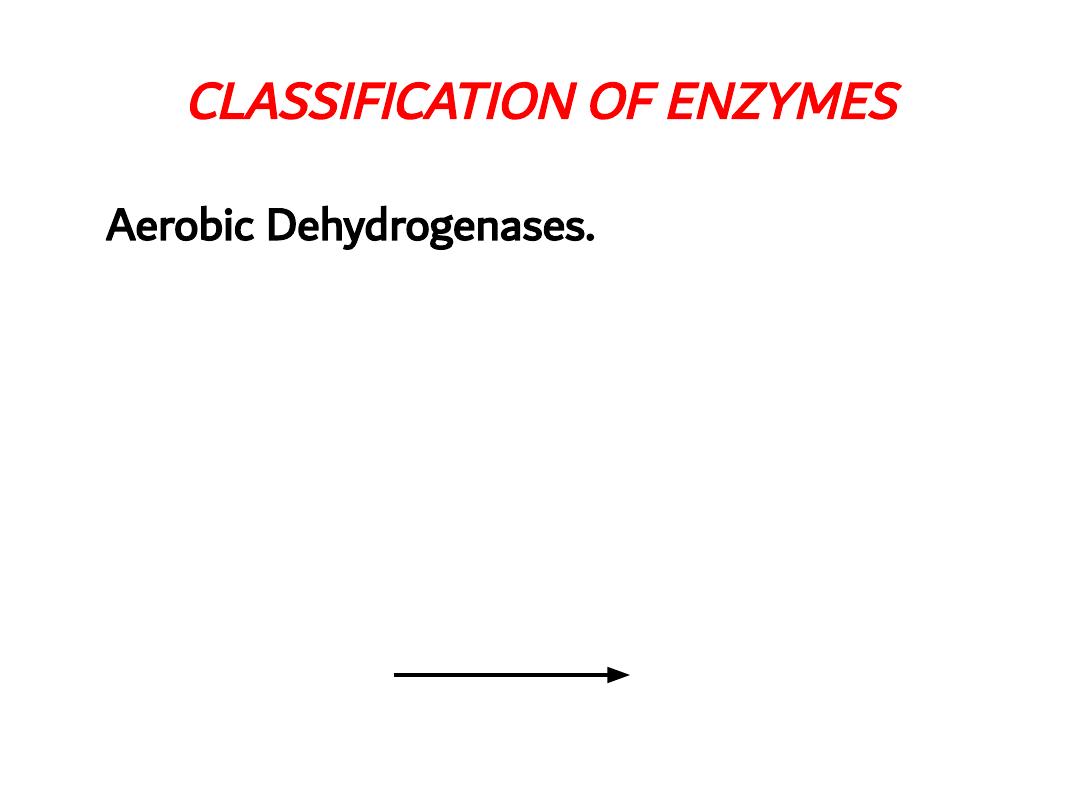
CLASSIFICATION OF ENZYMES
Aerobic Dehydrogenases.
Catalyzing oxidation of the substrate and molecular
oxygen acts as recipients of hydrogen e.g. Glucose
oxidase, L amino acid dehydrogenase, Xanthene
dehydrogenase
glucose oxidase
Glucose
Gluconolactone
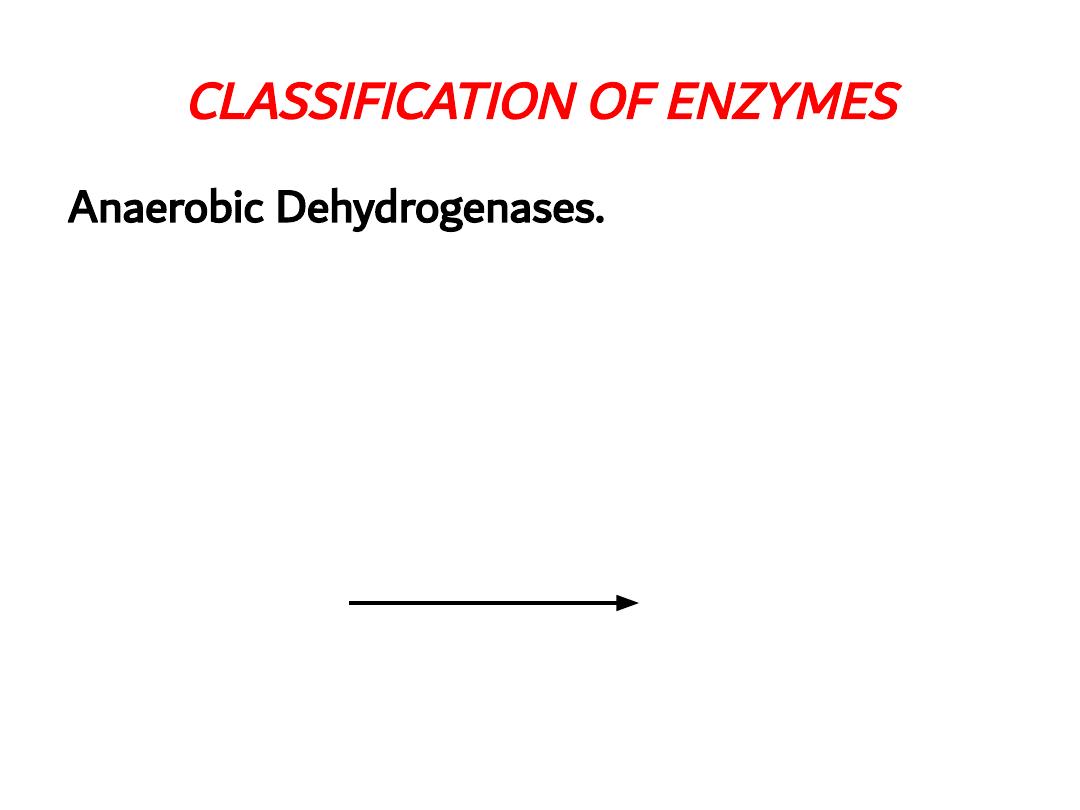
CLASSIFICATION OF ENZYMES
Anaerobic Dehydrogenases.
Catalyzing oxidation of
the substrate and coenzymes act as recipients of
hydrogen e.g. Lactate Dehydrogenase with NAD and
Glucose 6 phosphate dehydrogenase with NADP
Lactate
dehydrogenase
Lactic acid
Pyruvic acid
+ NAD
+ NADH – H
+

CLASSIFICATION OF ENZYMES
Oxygenases:
Is an enzyme that oxidizes a substrate by transferring
the oxygen from molecular oxygen O
2
(as in air) to it.
Types :
a. Monooxygenases:
Transfer one oxygen atom to the substrate, and reduce the
other oxygen atom to water.
b. Dioxygenases:
Incorporate both atoms of molecular oxygen (O
2
) into the
product(s) of the reaction.
Among the most important monooxygenases are
the cytochrome P450 oxidases, responsible for breaking
down numerous chemicals in the body.
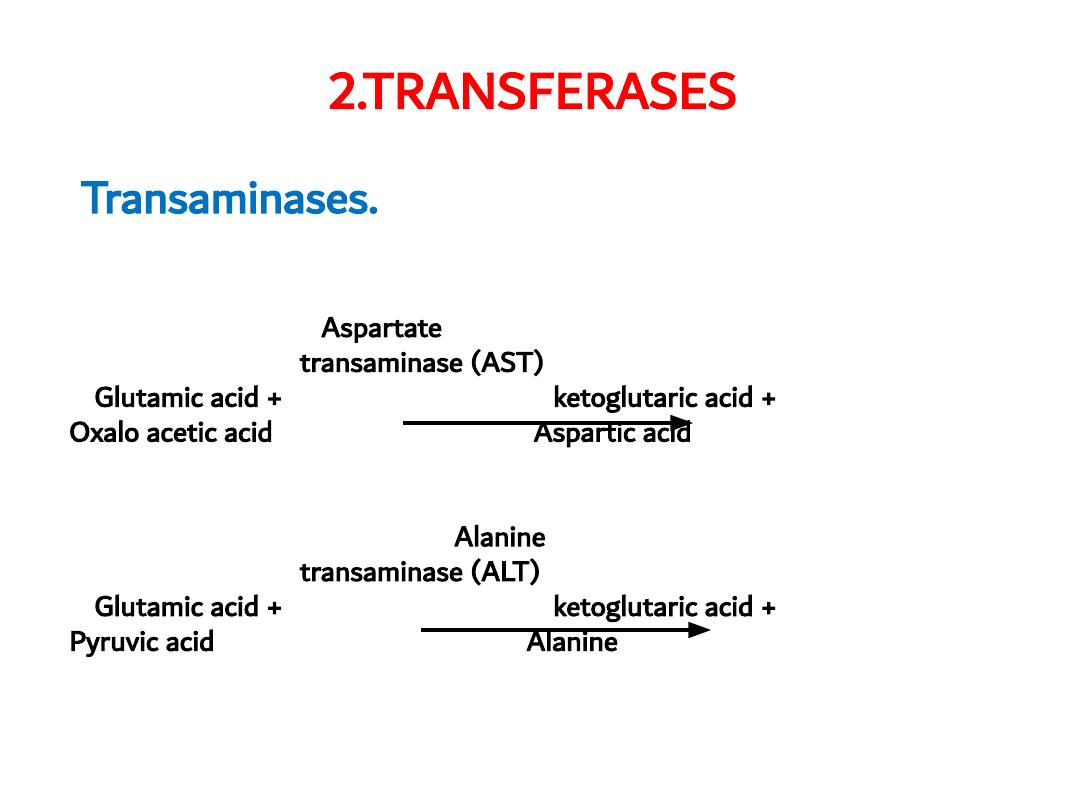
2.TRANSFERASES
Transaminases.
Catalyzing transfer of amino group between
an amino acid and a ketoacid e.g. Aspartate transaminase (AST),
Alanine transaminase (ALT)
Aspartate
transaminase (AST)
Glutamic acid +
α
ketoglutaric acid +
Oxalo acetic acid
Aspartic acid
Alanine
transaminase (ALT)
Glutamic acid +
α
ketoglutaric acid +
Pyruvic acid
Alanine
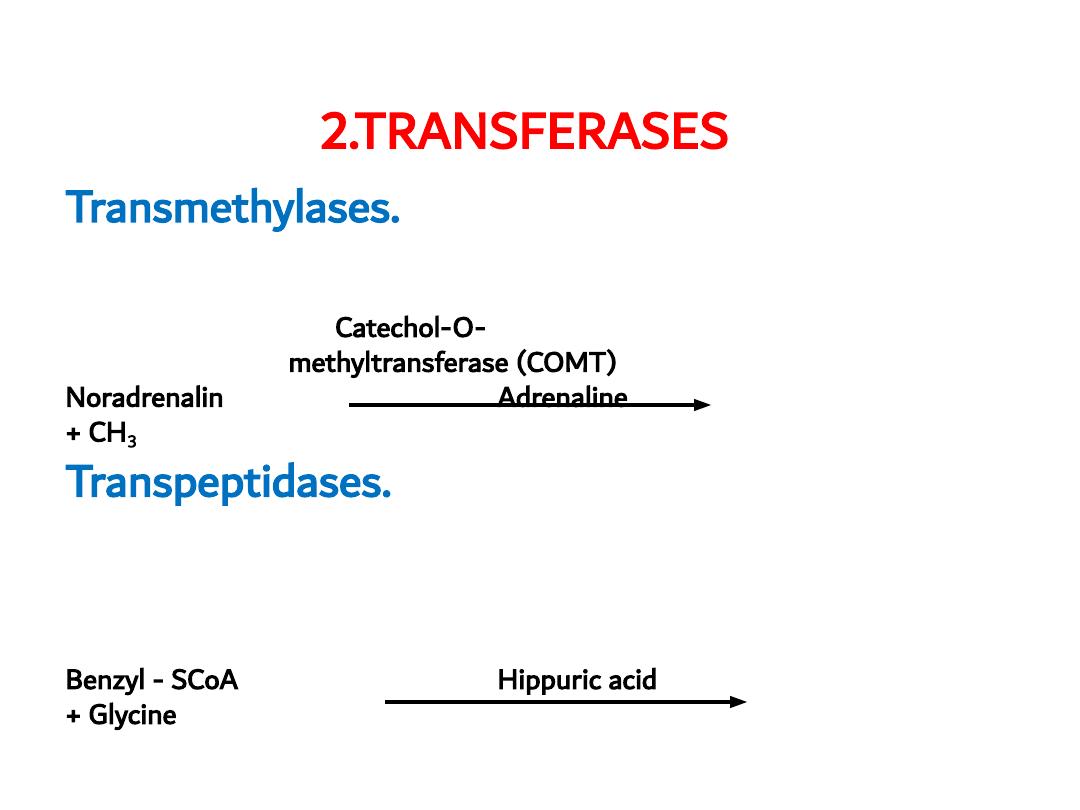
2.TRANSFERASES
Transmethylases.
Catalyzing transfer of methyl group
between to substrates e.g. COMT
Catechol-O-
methyltransferase (COMT)
Noradrenalin
Adrenaline
+ CH
3
Transpeptidases.
Catalyzing transfer of amino acids to
substrates e.g. Benzyl-SCoA transpeptidase
Benzyl-SCoA
transpeptidase
Benzyl - SCoA
Hippuric acid
+ Glycine

2. TRANSFERASES
Phosphotransferases
.
Catalyzing transfer of phosphate
group to substrates e.g. Hexokinase, glucokinase
* ATP D hexose 6 phosphotransferase [Hexokinase]
ATP + Glucose Hexokinase →ADP + D-Glucose –6-P
Acetyltransferase
.
Catalyzing transfer of acetyl group to
substrates e.g. choline acetyltransferase
Acetyl-CoA+ Choline → CoA + Acetyl- Choline
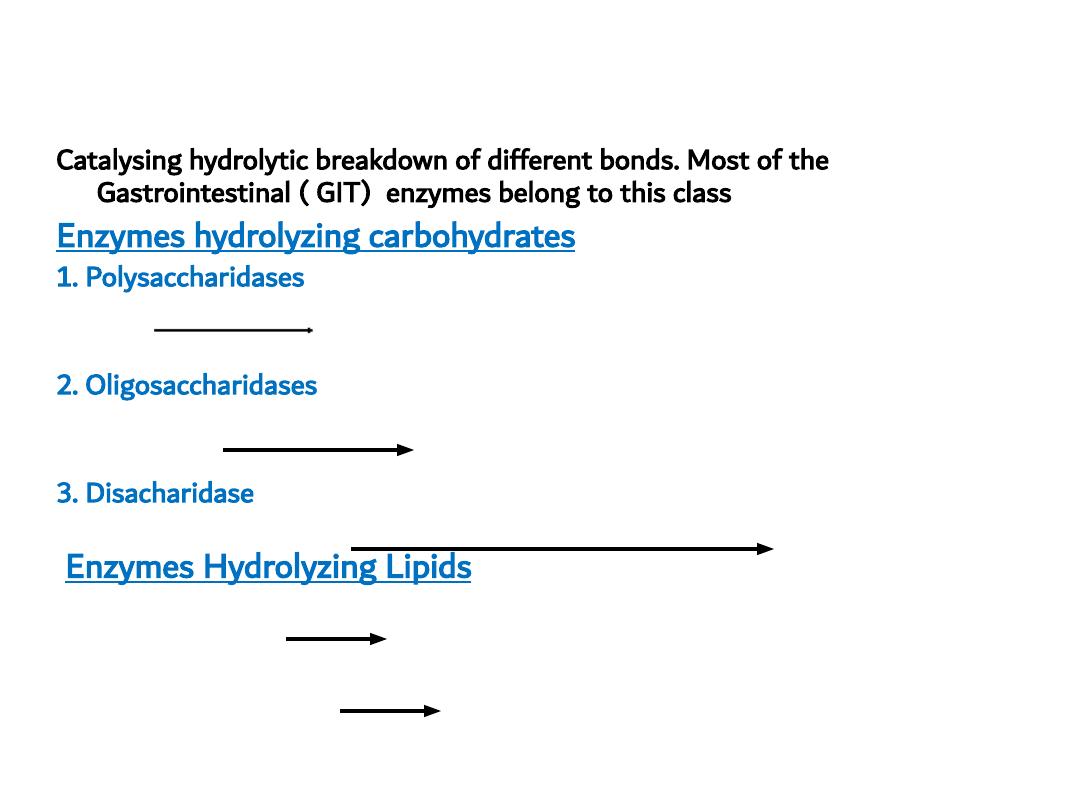
3. HYDROLASES
Catalysing hydrolytic breakdown of different bonds. Most of the
Gastrointestinal ( GIT) enzymes belong to this class
Enzymes hydrolyzing carbohydrates
1. Polysaccharidases
Starch Amylase
Maltose, maltrios , dextrin
2. Oligosaccharidases
Dextrin Dextrinase glucose
3. Disacharidase
Maltose, Lactose, Sucrose (Disacharidases) Maltase, Lactase, Sucrase monosaccharides
Enzymes Hydrolyzing Lipids
Triacyl glycerol lipase monoacyl glycerol + 2 F.F.A
Cholesterol ester cholesterol
free cholesterol + FFA
esterase
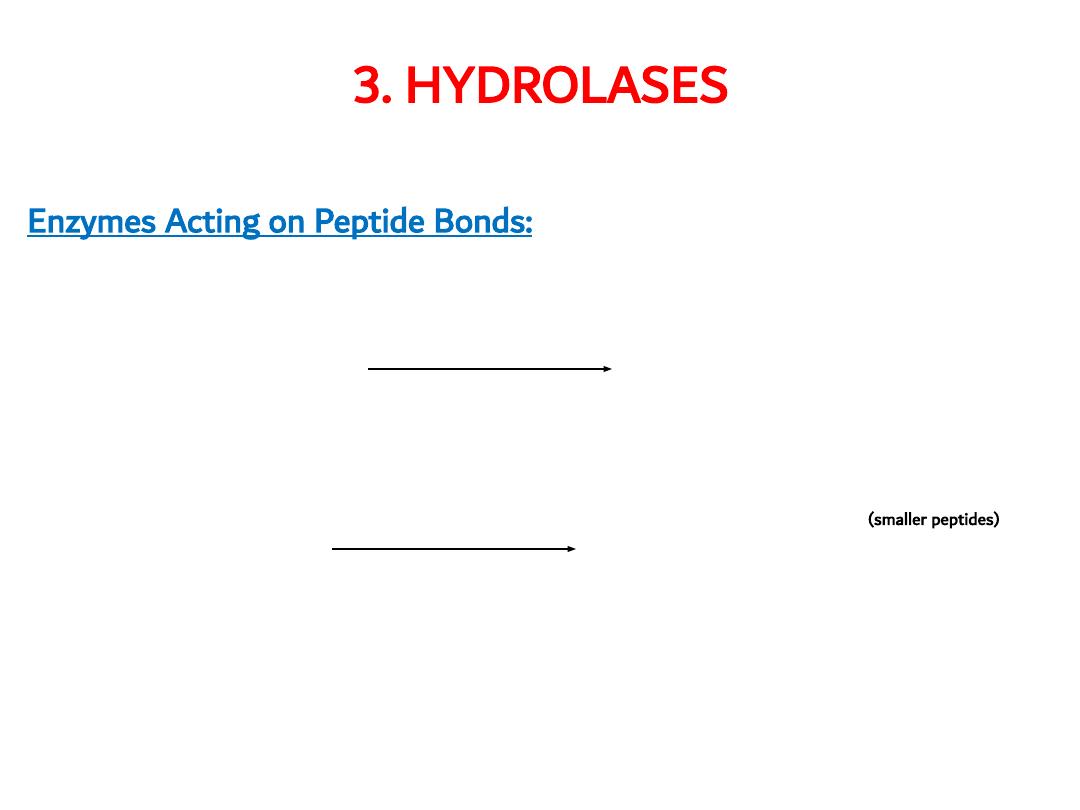
3. HYDROLASES
Enzymes Acting on Peptide Bonds:
exopeptidases
carboxypeptidase amino acids
endopeptidase aminopeptidases
e. g pepsin
(smaller peptides)
3. HYDROLASES
Tripeptidase : Tripeptide → A.A
Dipeptidase : Dipeptide → A.A
Phosphatases
1.Phosphomonoesterases:
Glucose – 6.P. + H
2
O Phosphatase
G 6. Phosphate Glucose
+Pi
2. Phosphodiesterases:
Removal of phosphate Group of diesters breakdown of 3’-5’ p
linkages in cyclic AMP
4. LYASES
• Catalysing reactions in which groups are removed without
hydrolysis leaving a double bond or add groups to already
existing double bonds.
CH3. CO. COOH CH3. CHO+ CO2
(pyruvate)
pyruvate decarboxylase
(acetaldehyde)
COOH.CH = CH. COOH Fumerase COOH-CHOH. CH2-COOH
(Fumaric acid) (malic acid)
5. ISOMERASES
• Involved in inter conversion of pair of isomeric compounds
• Glucose 6. P Phosphogluco mutase
glucose I.P
• Glucose 6.P
Phosphohexose isomerase
Fructose 6.P
• All trans retinene
Retinene 11- CIS retinene
Isomerase
6. LIGASES
• Catalyze reactions in which linking together of two
molecules occur coupled with the breakdown of a high
energy phosphate bonds like ATP, GTP
Acetate + CoA +ATP Acetyl CoA Acetyl CoA+AMP
Synthetase
Succinate + CoA + ATP Succinyl CoA Succinyl CoA + ADP+ Pi
Synthetase
Pyruvate + CO
2
+ ATP Pyruvate
Oxaloacetate + ADP + Pi
Carboxylase
Fatty acid + CoA + ATP Acyl CoA Acyl CoA (Activated fatty acid) + AMP + PiPi
Synthetase

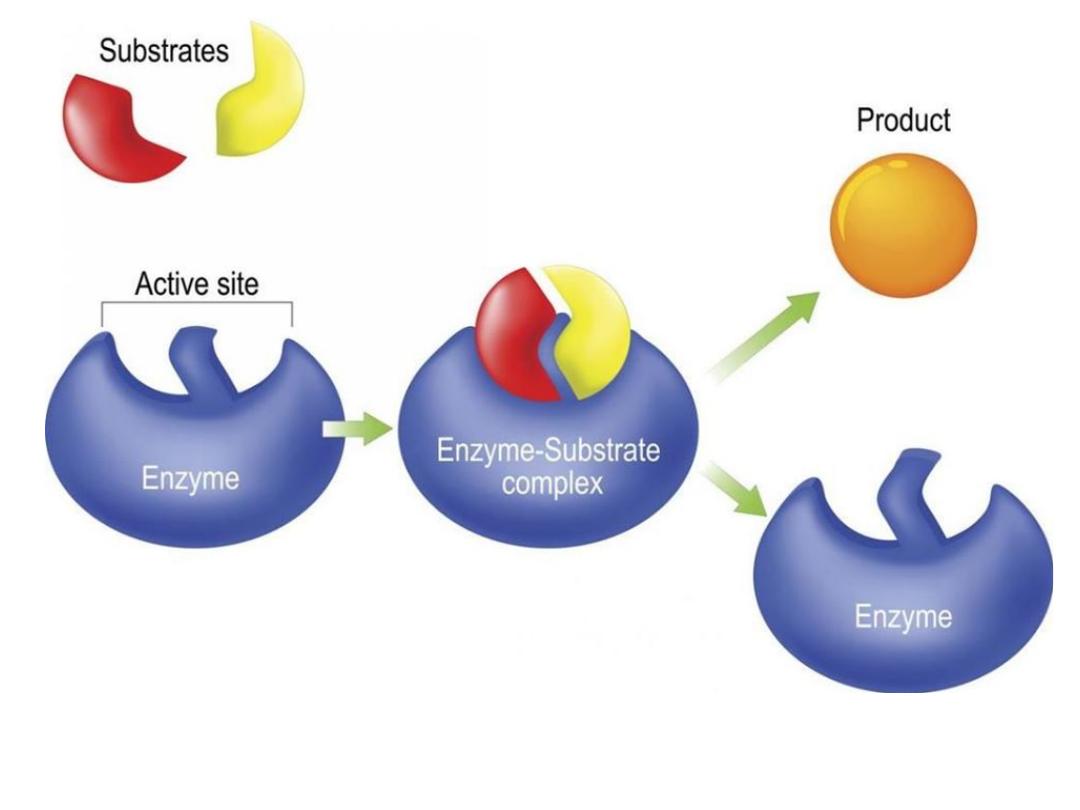
The active site
The active site is the region of an enzyme where
substrate molecules bind and undergo a
chemical reaction. The active site consists of
amino acid residues that form temporary bonds
with the substrate (binding site) and residues
that catalyze a reaction of that substrate
(catalytic site). Although the active site
occupies only ~10–20% of the volume of an
enzyme

The substrate
• The substrates of enzymes are the reactants
that are activated by the enzymes
• Enzymes are specific to their substrates
• The specificity is determined by the active site.

Cofactors
• An additional non-protein molecule needed by
some enzymes to help the reaction
• Tightly bound cofactors are called prosthetic
groups
• Cofactors that are bound and released easily
are called coenzymes
• Many vitamins are coenzymes.

Cofactors
• Cofactors can be divided into two types,
either inorganic ions, or complex organic
molecules called coenzymes. Coenzymes are
mostly derived from vitamins and other
organic essential nutrients in small amounts.

Factors affecting Enzymes
1-substrate concentration
2- pH
3- temperature
4- inhibitors

REFERENCES
• Lippincott’s Reviews of Biochemistry, 3rd ed. , 2018.
