
Biochemistry lectures
First stage
By
Assistant professor Dr.Suhayr Aessa Hussein
phD. Clinical Biochemistry
College of Medicine University of Babylon

Lecture 2
Fatty Acids

:
Objectives
Know the essential and nonessential fatty acids
Know the clinical significance of trans and cis fatty
acids
Glycerol structure and clinical importance

Structure of fatty acids
➢
Fatty acids are synthesized from linear acetogenins of
acetate pathway
➢
Fatty acids are carbon chains with a methyl group at
one end of the molecule (designated omega, ω) and a
carboxyl group at the other end.

The carbon atom next to the carboxyl group is called
the α
carbon, and the subsequentone the β carbon
The systematic nomenclature for fatty acids may also
indicate the location of double bonds with reference to
the carboxyl group (Δ).

CH3 –
(CH2 )n
CH2
–
CH2
– COOH
ω
β
α
Fatty acids may be named according to systematic
nomenclature.
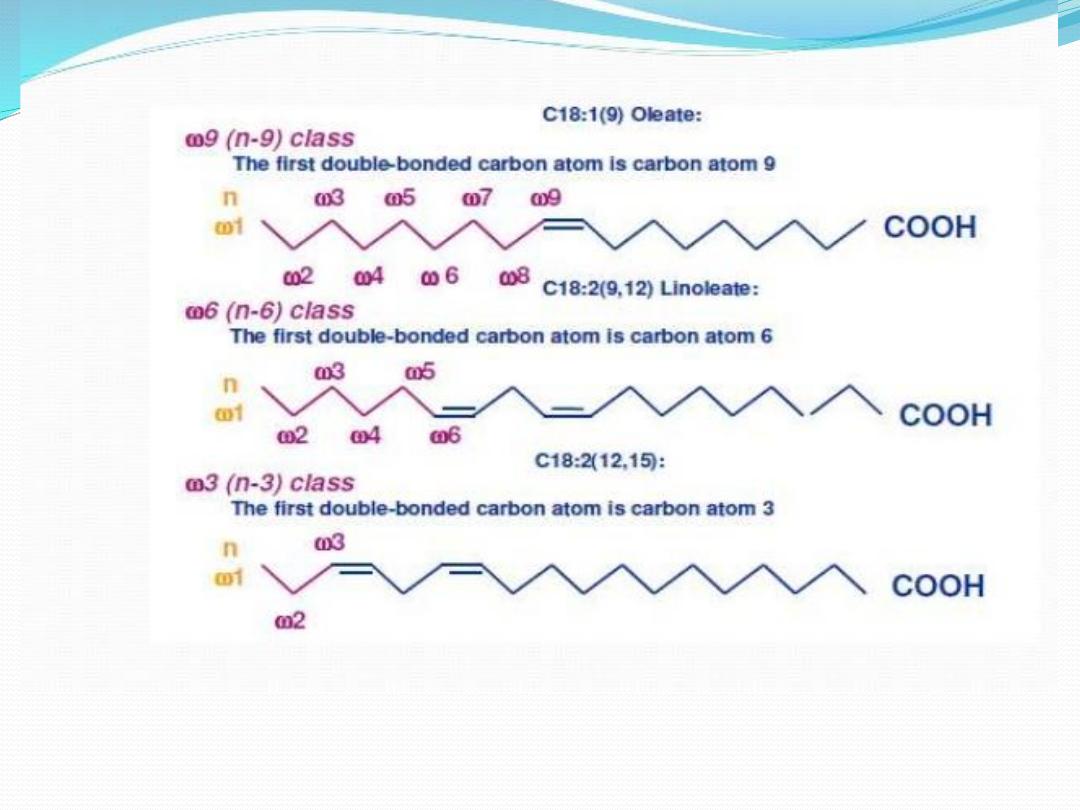
One systematic way to describe fatty acids is related to
the methyl (ω) end. This is used to describe the
position of double bonds from the end of the fatty
acid.

1- Essential and Nonessential fatty acids
1- Nonessential fatty acids: can be synthesized from
products of glucose oxidation and do not ;therefore;
have to be included in the diet.
2- Essential fatty acids such as linoleic and linolenic
families must be obtained from the diet

2- Saturated and unsaturated Fatty
Acids
A- Saturated fatty acids
❖
Saturated fatty acids may be based on acetic acid (CH3 —
COOH) as the first member of the series in which —CH2
— is progressively added between the terminal CH3 —
and —COOH groups.
❖
Fatty acids in biological systems usually contain an even
number of carbon atoms, typically between 14 and 24. The
16- and 18-carbon fatty acids are most common.
❖
The hydrocarbon chain is almost invariably
unbranched in animal fatty acids. A few branched-chain
fatty acids have also been isolated from both plant and
animal sources.

B-Unsaturated fatty acids
: may further be divided as
follows-
(1) Monounsaturated (monoethenoid, monoenoic) acids,
containing one double bond.
(2) Polyunsaturated (polyethenoid, polyenoic) acids,
containing two or more double bonds. The configuration of
the double bonds in most unsaturated fatty acids is cis.
The double bonds in polyunsaturated fatty acids are
separated by at least one methylene group.

❖
Polyunsaturated fatty acids such as Linoleic and Linolenic
acids are essential for normal life functions. They are
therefore characterized as essential fatty acids.
❖
Arachidonic acid is considered as semi essential fatty acid
since it can be synthesized from Linoleic acid .
❖
Essential polyunsaturated fatty acids can be classified as
belonging to
A.
one of two "families", the omega-6 family or the
omega-3 family.
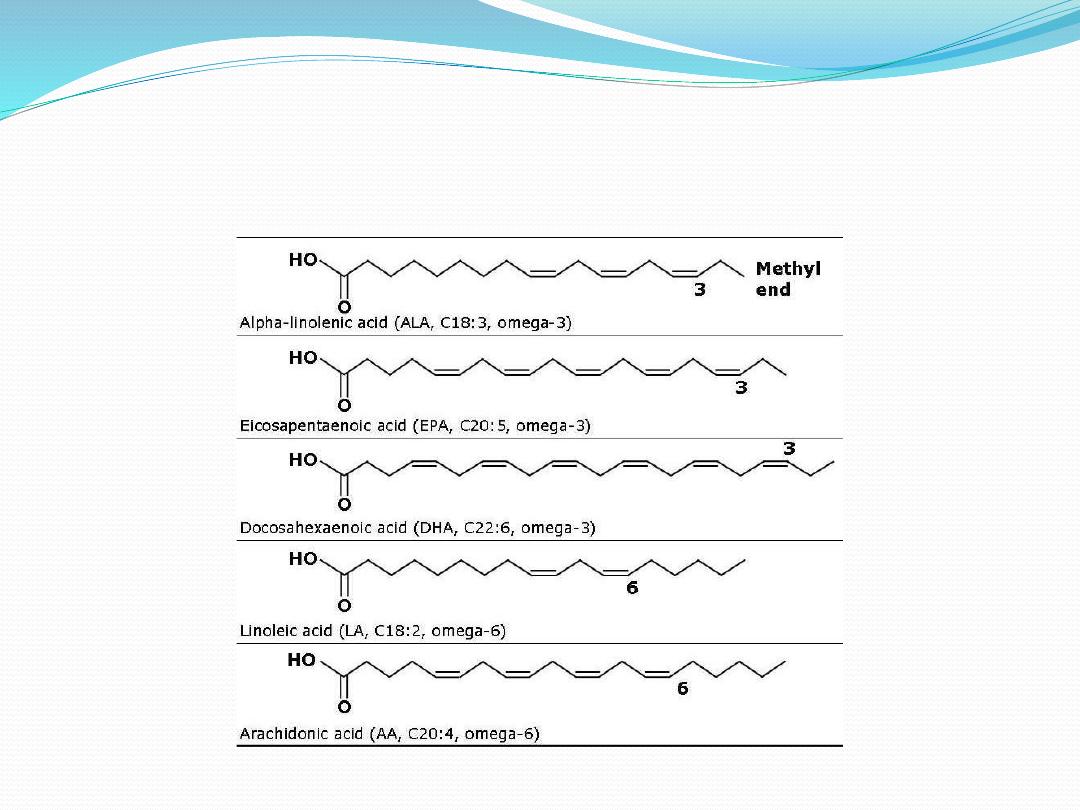
Omega 3 and Omega 6 Fatty Acids

Benefits Using Omega-3?
• freedom from pain and inflammation
• better brain function
• feel better with much less depression
• lower incidence of childhood disorders
•superior cardiovascular health
• protection from heart attack and stroke
• reduction of breast, prostate, and colon cancers
• slows biologic aging

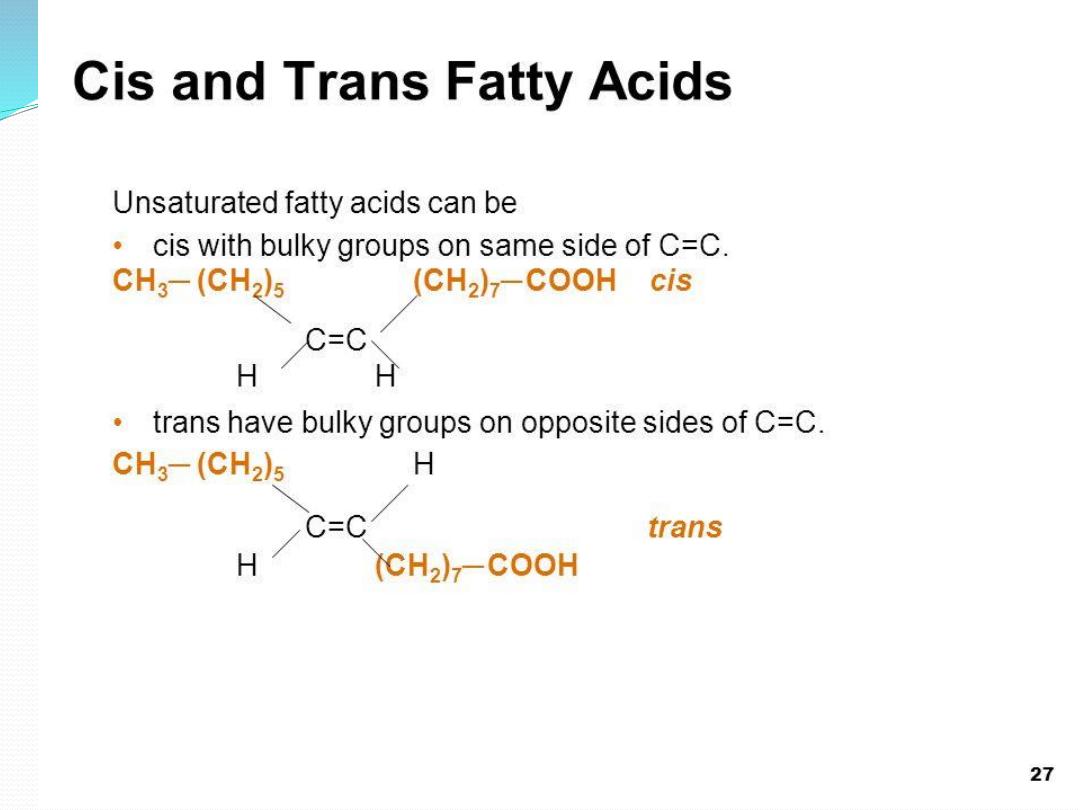
Natural unsaturated fatty acids have
➢
cis double bonds When unsaturated vegetable oils are
hydrogenated to form more saturated oils (as in
margarine), some of the cis fatty acids are isomerized
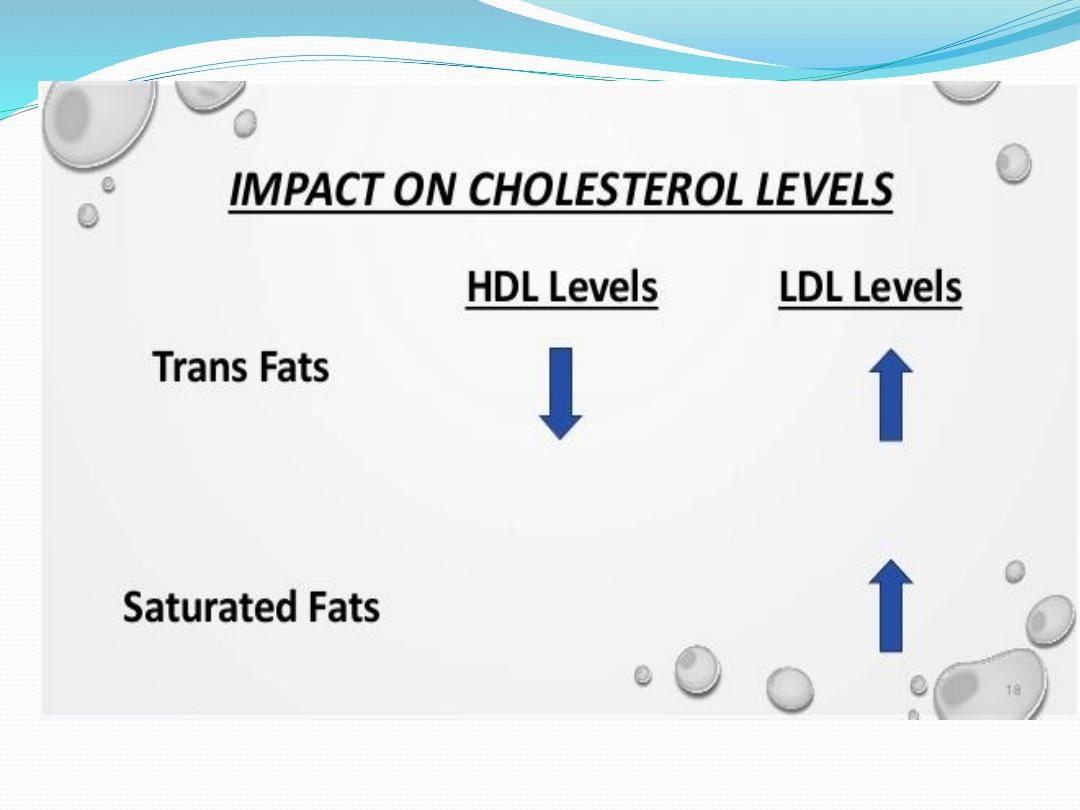
to trans fatty acids
➢
Trans fatty acids are much more linear than cis fatty
acids, so their melting points are higher and studies
have shown that trans fats may act similarly to
saturated fats and could contribute to heart disease
and some cancers
•
B- Cis and Trans Unsaturated Fatty
Acids

Significance of Unsaturated Fatty
Acids
❖
Because of the kinks in the hydrocarbon tails, unsaturated
fats can‘t pack as closely together, making them liquid at
room temperature.
❖
The membrane lipids, which must be fluid at all
environmental temperatures, are more unsaturated than
storage lipids.
❖
Lipids in tissues that are subject to cooling, eg, in hibernators
or in the extremities of animals, are more unsaturated.
❖
At higher temperatures, some bonds rotate, causing chain
shortening, which explains why biomembranes become
thinner with increases in temperature.
❖
The carbon chains of saturated fatty acids form a zigzag
pattern when extended, as at low temperatures.

3- Waxes
❖
They are esters of higher fatty acids with higher mono
hydroxy aliphatic alcohols(e.g. Acetyl alcohol).
❖
Have very long straight chain of 60-100 carbon atoms
❖
They can take up water without getting dissolved in it
❖
Used as bases for the preparation of cosmetics, ointments,
polishes, andlubricants.
❖
In nature, they are found on the surface of plants and
insects.

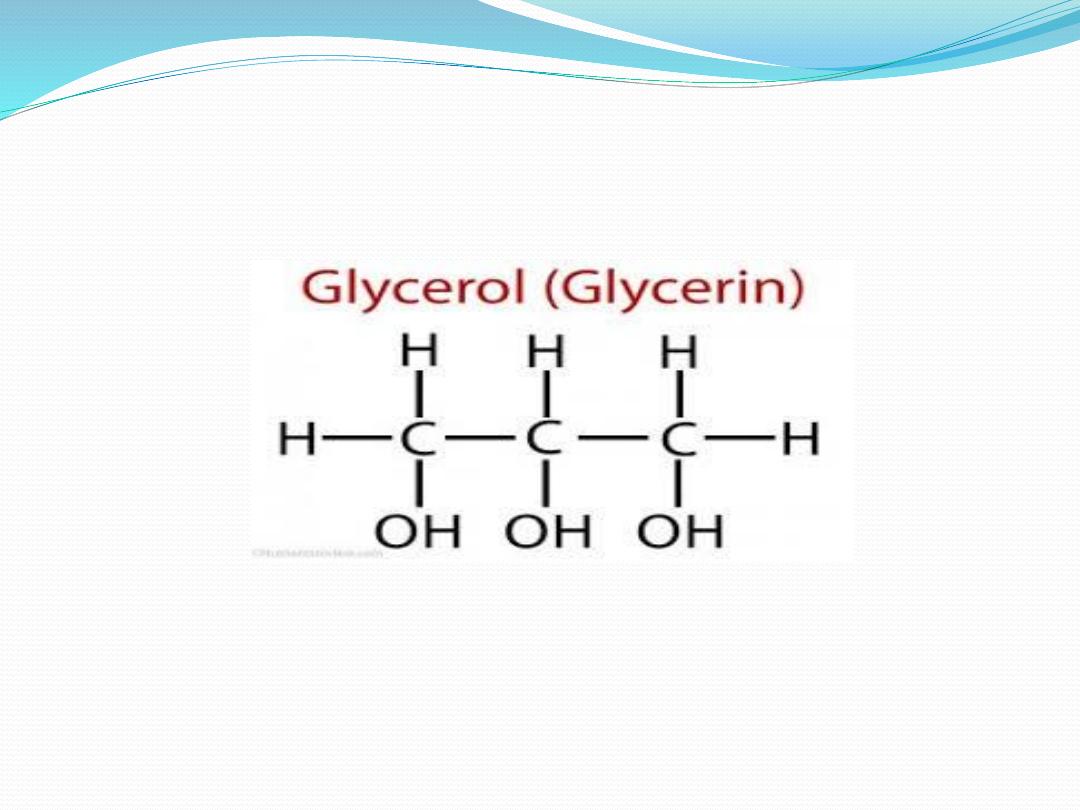
Glycerol-Structure and Significance
❖
Also called “Glycerin”.
❖
Trihydric alcohol as it contains three hydroxyl groups
❖
To number the carbon atoms of glycerol
unambiguously, the -sn (stereochemical
numbering) system is used.
❖
Carbons 1 and 3 of glycerol are not identical when
viewed in three dimensions.
❖
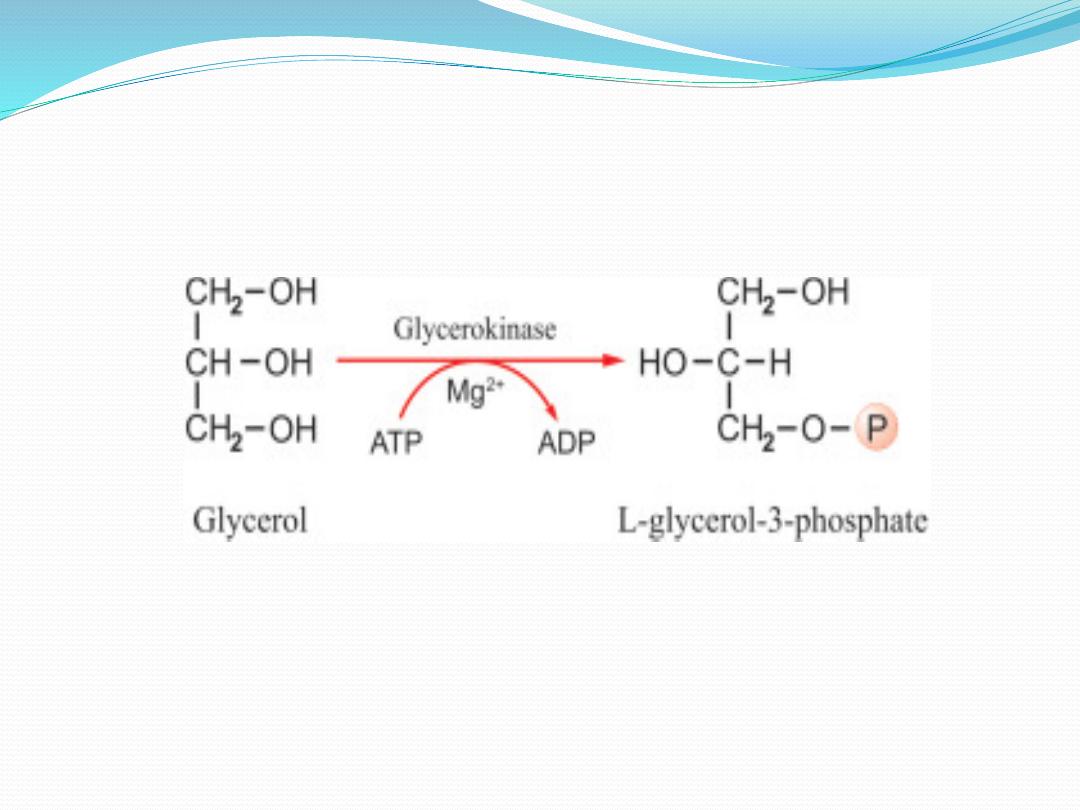
Enzymes readily distinguish between them and are
nearly always specific for one or the other carbon; eg,
glycerol is always phosphorylated on sn-3 by glycerol
kinase to give glycerol 3-phosphate and not glycerol 1-
phosphate.

❖
Can be obtained from diet, from lipolysis of fats in
adipose tissue and from glycolysis.
❖
Can be utilized for the synthesis of triacylglycerols,
phospholipids, glucose or can be oxidized to
provide energy
❖
Used as a solvent in the preparation of drugs and
cosmetics. Nitroglycerine is used as a vasodilator

Biochem /physiol Actions
Glycerol is hygroscopic in nature and is soluble in
water
owing
to
its
three
hydrophilic
alcoholic
hydroxyl groups. It can form both inter- and
intramolecular hydrogen bonds, making it a very
flexible molecule. The physiologic effect of glycerine
is due to cell-mediated immunity, increased IgG
production and increased histamine release

Please Stay home and read this
Lecture
بحفظه تعالى
