
1
Antiviral drugs
اﻟﻤﺮﺣﻠﮫ اﻟﺜﺎﻟﺜﮫ /ﻓﺎﯾﺮوﺳﺎت
د. اﻧﺘﻈﺎر ﻋﻼوي ﺟﻌﻔﺮ / ﻓﺮع اﻻﺣﯿﺎء اﻟﻤﺠﮭﺮﯾﮫ / ﻛﻠﯿﮫ اﻟﻄﺐ / ﺟﺎﻣﻌﮫ ذي ﻗﺎر
PhD. M.Sc. Microbiology
Introduction
The viruses, unlike most bacteria, are obligate intracellular pathogens that use
biosynthesis mechanisms and enzymes of the host cells for replication. Hence, it
was feared that it may not be possible to inhibit viral replication without also
being toxic to host cells. This close association between viruses and their host
cells is a source of some essential difficulties encountered when developing
virus-specific chemotherapeutics:
•
Since any interference with viral synthesis is likely to affect physiological
cellular synthetic functions as well.
•
Another problematic aspect is the necessity of administering
chemotherapeutics early, preferably before clinical symptoms manifest,
since the peak of viral replication is then usually already past but
currently newer antiviral drugs are used successfully for treatment of few
viral diseases without causing much of toxicity or serious side effects.
•
Recently used antiviral drugs, however, act specifically against virus-
coded enzymes or structures of the virus that are important for
replication of the viruses.
•
The first antiviral drugs to be used were like selective poisons that
targeted cells with intensive DNA and RNA synthesis.
•
Marboran was the first antiviral drug used clinically for effective
treatment of poxvirus infection in 1960. The compound was used
successfully against eczema vaccinatum and smallpox.
•
Subsequently in 1962, an antineoplastic agent idoxuridine was found to
be effective for treatment of herpetic eye infection.
•
In 1970s, Acyclovir (ACV) was used most successfully for treatment of
herpes virus infection by administering the drug parenterally.
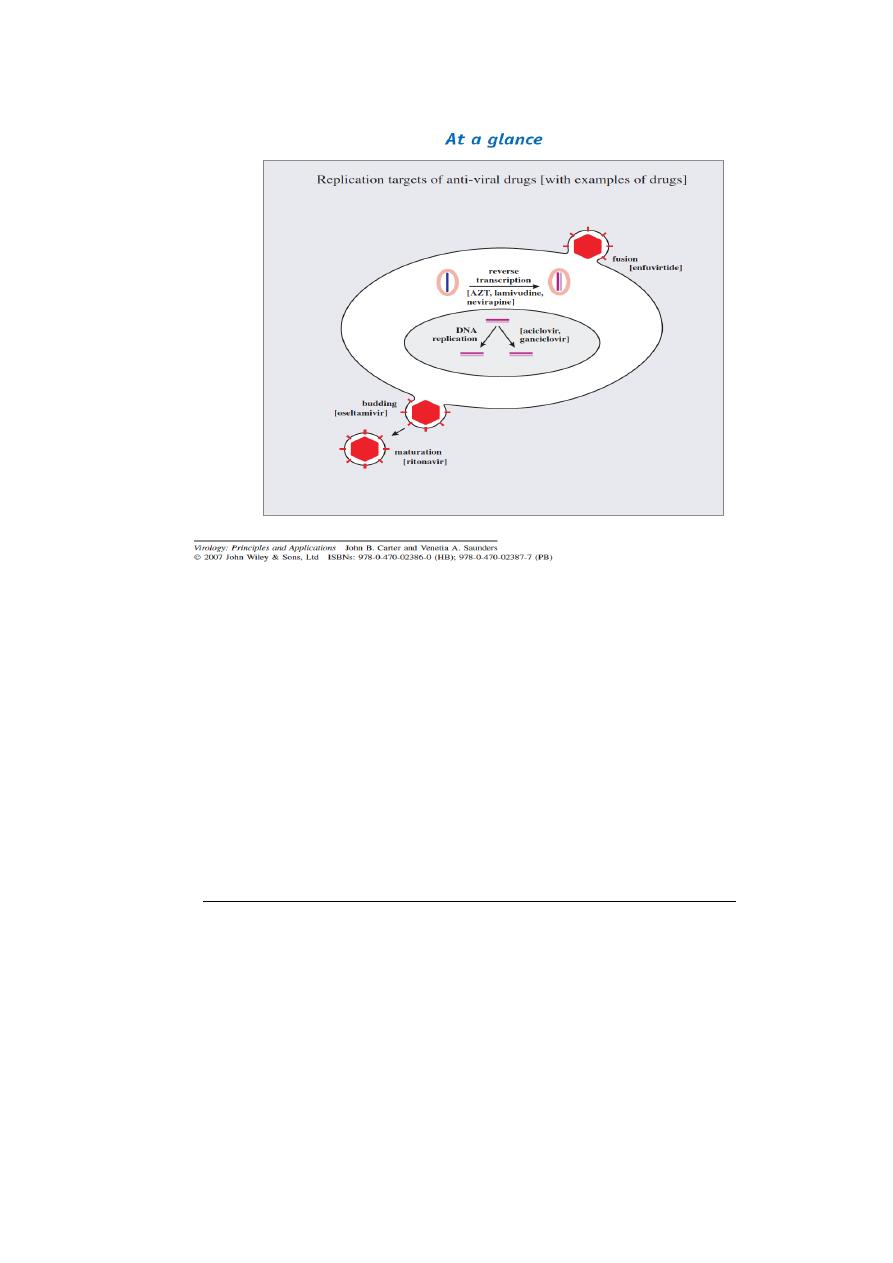
Mechanism of Action of Antiviral Drugs
Recently, many antiviral drugs have been developed against
viruses that are associated with high morbidity and mortality in humans. These
viruses provide potential targets during the cycle of replication for action by
antiviral drugs.
The viral infection may be inhibited at the level of
(1 ) Attachment,
(2 ) Penetration and uncoating,
(3 ) Transcription of viral nucleic acid,
(4 ) Translation of viral mRNA and protein synthesis,
(5 ) Replication of viral genome
(6 ) Nucleoside biosynthesis .
(7 ) Assembly and release of viral progeny.
2
Rational design of anti-viral drugs
The process of designing an anti-viral drug starts with deciding upon a target
activity for the drug . Once this has been selected, it is necessary to choose a
target protein, such as a viral enzyme, that is involved in that activity.
A detailed picture of the three-dimensional structure of the protein is derived
using techniques such as X-ray crystallography, and a target site in the protein
is selected. Computer programs are then used to design compounds that will
bind to the target site with the aim of inhibiting the activity of the virus protein.
Safety of anti-viral drugs
The potential value of a compound can be assessed by determining the ratio of
its cell toxicity to its antiviral activity. This ratio is known as the selectivity
index (SI) and is expressed by the formula
SI=
Minimum concentration inhibiting DNA synthesis
Minimum concentration inhibiting virus replication
A compound with a low IC50 and a high SI is most likely to have value as an
anti-viral drug.

3
Table 1: Mechanisms of action of antiviral drugs
Virus
Antiviral drug
Mechanism of action
Herpes simplex virus
Acyclovir
Vidarabine
Viral polymerase inhibitors
(nucleoside analog)
Varicella zoster virus
and herpes simplex
virus
Valacyclovir
Viral polymerase inhibitors
(nucleoside analog)
Cytomegalovirus
Ganciclovir
Foscarnet
Viral polymerase inhibitors
Human
immunodeficiency
virus
Zidovudine
Didanosine
Zalcitabine
Nucleoside reverse
transcriptase inhibitors
Influenza A virus
Amantadine
Rimantidine
Oseltamivir
Blocks viral uncoating
Neuraminidase inhibitor
Hepatitis C virus
Interferon
alpha
Inhibits protein synthesis
Respiratory syncytial
virus
Ribavirin
Blocks capping of viral mRNA
Classification of antiviral drugs
The antiviral agents available against viruses can be classified as:
(a ) Nucleoside analogs.
(b ) Non-nucleoside polymerase inhibitors.
(c ) Protease inhibitors.
(d ) Neuraminidase inhibitors.
(e ) M2 channel blockers.
(f ) Interferons.
Nucleoside analogs
Numerous analogs of naturally occurring nucleosides have been
synthesized in the laboratory for their possible use against viruses.
These nucleoside analogs that act by inhibiting the enzyme viral
polymerase are generally activated by phosphorylation by cellular or
viral kinases.

4
Nucleoside analogs cause selective inhibition of viral replication
by:
binding better to viral DNA polymerase, rather than to the cellular
DNA polymerase; and by being utilized more extensively in virus-
infected cells due to the more rapid synthesis of DNA in infected cells.
Examples of nucleoside Analogs are:
Acyclovir, valacyclovir, penciclovir, and famciclovir, ganciclovir,
azidothymidine (AZT),and ribavirin.
Azidothymidine (AZT)
Is the synthetic analog of thymidine. It acts by blocking the synthesis
of proviral DNA by inhibiting viral reverse transcriptase. It is widely
used for the management of HIV with reduced CD4 T-cell counts
(400–500 or less) to prevent progression of the disease.
•
It is also used for treatment of pregnant HIV-infected women. It
has been shown to reduce or prevent the transmission of HIV
from the mother to the baby.
Ribavirin
•
It is a synthetic analog of the nucleoside guanosine. It is
effective against many DNA and RNA viruses.
•
It acts mainly by preventing replication of the viruses by
inhibiting nucleoside biosynthesis, mRNA capping, and other
processes essential for viral replication.
•
When administered as an aerosol, ribavirin has been shown to
be effective for treatment of severe respiratory syncytial viral
infection in children and for treatment of severe influenza and
measles in adults.
•
Intravenous ribavirin is also effective for treatment of
infections caused by influenza B virus and Rift Valley virus,
Lassa, Crimean-Congo, and other hemorrhagic fevers.
* Ribavirin in combination with interferon-alpha (IFN-α) is shown
to be effective against the infection caused by hepatitis C virus.
Dideoxynucleosides
(e.g. lamivudine) have been synthesized for use against HIV. These
agents act by inhibiting HIV replication by blocking the enzyme
reverse transcriptase. by preventing DNA chain elongation.
Interferon
Interferons (IFNs) are produced by leukocytes and many other cells
in response to infection by virus, double-stranded RNA (dsRNA),

5
endotoxin, mutagenic and antigenic stimuli. The dsRNA is a potent
stimulator. The viruses that replicate slowly and viruses that do not
inhibit synthesis of host proteins are usually good inducers of the
interferons.
There are three classes of interferon:
(i ) interferon alpha (IFN-α ).
(ii ) interferon beta (IFN-β ).
(iii ) interferon gamma (IFN-γ ).
Interferons are now being increasingly used for treatment of chronic
hepatitis B and C virus carriers who are at risk to progress to
cirrhosis and hepatocellular carcinoma.
Currently, synthetic IFN- is actively used against hepatitis A, B, and C
viruses; papilloma virus; HSV; and rhinovirus.
Interferons exert antiviral effect by several pathways as
follows:
1.They cause increased expression of class I and class II MHC (major
histocompatibility complex) glycoproteins, thereby facilitating the
recognition of viral antigens by immune system.
2. They activate the cells, such as natural killer cells and
macrophages, the cells with the ability to destroy virus infected
targets.
3. They directly inhibit replication of viruses.
Development of resistance to chemotherapeutics
Antiviral resistance means that a virus has changed in such a
way that the antiviral drug is less effective in preventing illness.
Antiviral resistance is indicated if a patient is taking an antiviral drug
that has been proven in vitro to be effective against a virus, but the
patient shows no improvement and continues to deteriorate
clinically.
Reasons of antiviral drug resistance
•
Prolonged antiviral drug exposure
•
Ongoing viral replication due to immunosuppression are key
factors in the development of antiviral drug resistance e.g in
HIV infection
•
Viral mutation frequency e.g in HBV

6
Consequences of drug resistance
Is range from toxicity inherent in use of second-line antivirals, to
severe disease and even death from progressive viral infection when
no effective alternative treatments are available.
