
Leukaemias:
Leukaemias are a group of malignant disorders of the
haematopoietic tissues characteristically associated with increased
numbers of white cells in the bone marrow and/or peripheral blood.
The incidence of leukaemias approximately 10/100.000 per
annum.
Males to females ratio being about 3:2 in acute leukaemias

2:1 in chronic lymphocytic leukaemia (CLL) and 1.3:1 in chronic
myeloid leukaemia (CML).
Geographic variation in incidence does occur. Acute
lymphoblastic leukaemia (ALL) shows a peak of incidence in the 1-
5 age group while acute myeloid leukaemia have a striking rise over
the age of 50. Chronic leukaemias occur mainly in middle and old
age.
-

Aetiology:
The cause of leukaemia is unknown in the majority of patients.
Several factors are associated with the development of leukaemia:
1- Ionizing radiation.
2-Cytotoxic drugs particularly alkylating agent which induce myeloid
leukaemia.
3- Exposure to benzene in industry.
4- Retroviruses.
5- Genetics (increase incidence in down's syndrome).
6- Immunological ex. Hypogammaglobulinaemia.

Classification:
Leukaemias are classified according to the clinical behavior of the
disease into four main groups acute lymphoblastic, acute myeloid,
chronic lymphocytic, and chronic myeloid.
A more detailed classification developed for example the
subclassification of lymphoblastic type is of value in the treatment as
the common type responds well to treatment and carries the best
chance of long-term remission.

Other classification might indicate the variable degree of
maturation ex. In AML.
Subclassification of leukaemia:
Acute lymphoblastic:
* Common type (pre-B).
* T. cell.
* B. cell.
* Undifferentiated.

Acute myeloid:
FAB classification
* M0 undifferentiated
* M1 minimal differentiation.
* M2 differentiated.
* M3 promyelocytic.
* M4 Myelomonocytic.
* M5 Monocytic.
* M6 erythrocytic.
* M7 Megakaryocytic.

Chronic lymphocytic:
* B- cell – common.
* T- cell – rare.

Chronic myeloid:
* Ph
2
positive.
* Ph
2
negative, BCR- abI
3
positive.
* Ph
2
negative, BCR- abI3 – negative.
* Eosinophilic leukaemia.
1 - FAB= French – American – British.
2 - Ph= Philadelphia chromosome.
3 - BCR= break point cluster region, abl = Abelson oncogene.

Acute Leukaemias
•
Learning objectives
•
1
-
Definition and presentations
•
2
-
Investigations
•
3
-
managements
•
4
-
Prognosis
•
5
-
Indications for bone marrow transplantation
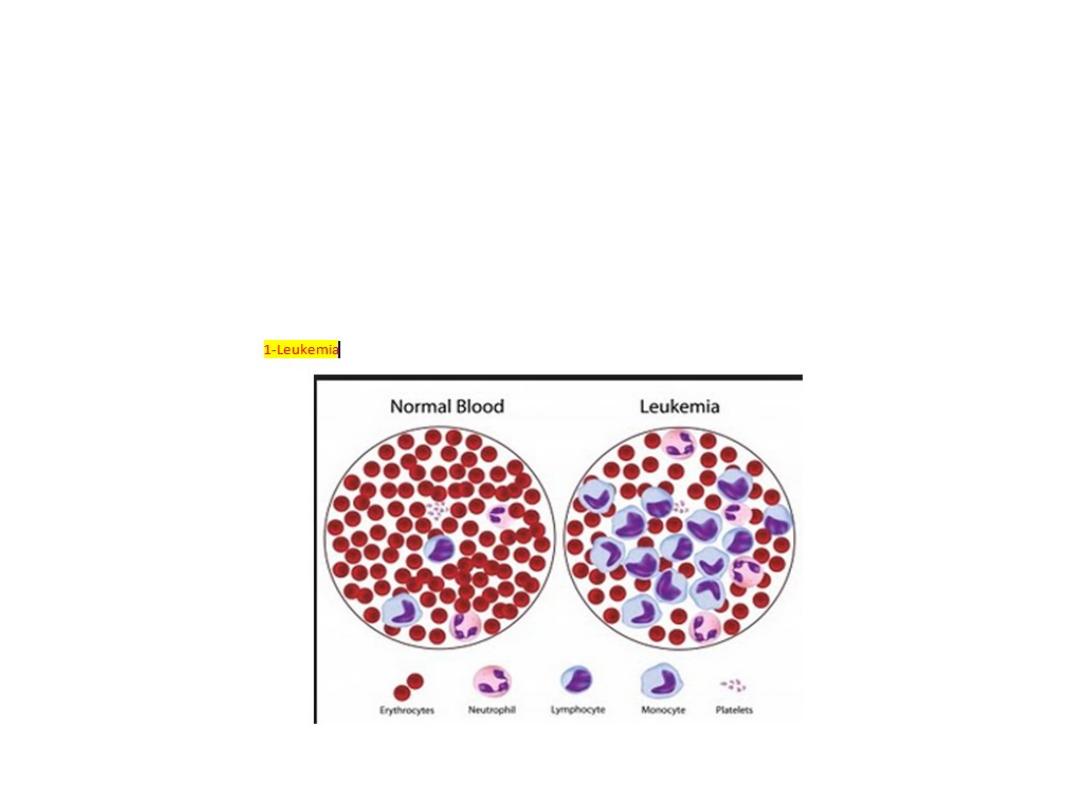
Acute leukaemias (AL):
There is a failure of cell maturation in AL. this immature cells
accumulate in the bone marrow at the expense of the normal
haematopoietic elements. Eventually this proliferation spills into the
blood
.

Clinical features:
The clinical features are usually those of bone marrow failure
(anaemia, bleeding and infection). 50% presented with fatigue as
the first symptoms, anorexia and weight loss are also common.
Fever with or without infections is the initial symptoms in 10% of
patients. 5% of patients show signs of abnormal haemostasis.

Occasionally bone pain, lymphodenopathy, nonspecific cough,
headache, or diaphoresis is the presenting symptoms, rarely patients
may present with symptoms from a mass lesion which represent a
tumour of leukaemic cells and is called a (granulocytic Sarcoma or
Chloroma).
Physical signs such as fever, splenomegaly, hepatomegaly,
lymphadenopathy, sternal tenderness, and evidence of infection and
haemorrhage are often found at diagnosis.
M3 AML may present with significant haemorrhage.
M5 AML may present with bleeding associated with coagulopathies
.

Investigations:
1- Blood examination usually shows anemia with a normal or raised
MCV (normochromic normocytic), reduced reticulocyte count due to
decreased erythropoiesis.
2- The leukocyte count may vary from as low as 1x10
9
/L to as high as
500x10
9
/L or more. In the majority of patients the leukocyte count is
below 100x10
9
/L.
3- Thrombocytopenia is usually severe.

4- Blast ells and other primitive cells in the peripheral blood is
diagnostic. Sometimes the blast cells count could be very low in the
peripheral blood and bone marrow examination is necessary for
diagnosis.
5- The bone marrow is the most valuable diagnostic investigation
and provide material for cytology, cytogenetics and immunological
phenotying. A trephine biopsy should be taken if no marrow is
obtained (dry tap). The leukaemic blast cells represent more than
20% of the marrow cells.

* The presence of Auer rods in the cytoplasm of blast cells indicate
myeloblastic type of leukaemia.
* Other investigation required such as:
Haemostatic function, renal function, hepatic function, and cellular
proliferation (LDH and uric acid).

Management:
The first decision must be whether or not to give specific
treatment. It may not be appropriate for the elderly or patients with
other serious disorders.

Specific therapy:
Adequate survives should be available for the specific therapy to
be given:
1- Treatment of infection.
2- Correction of anaemia with red cell concentrate.
3- Control of bleeding by platelets transfusion.
4- If possible central venous catheter insertion to facilitate the
circulation.

The aim of treatment is to destroy the leukaemic clone of cells
without destroying the residual normal stem cell. Specific therapy
include three phases:
1- Remission induction: the bulk of the tumour is destroyed by
combination chemotherapy. The patients goes through a period of
severe bone marrow hypoplasia requiring intensive support.
2- Remission consolidation: residual disease is destroyed during this
phase resulting in a periods of marrow hypoplasia.

3- Remission maintenance: repeating cycle of drug administration
used in acute lymphoblastic leukaemia.
This specific therapy may extend for up to 2 years if relapse does
not occur thereafter specific therapy is discontinued and the patient
observed.
In patients with ALL it is necessary to give therapy to the CNS
(consist of a combination of cranial irradiation and intrathecal
methotrexate).

If a patient fails to go into remission with induction treatment,
alternative drug combination may be tried.
The early relapses the worse the prognosis.
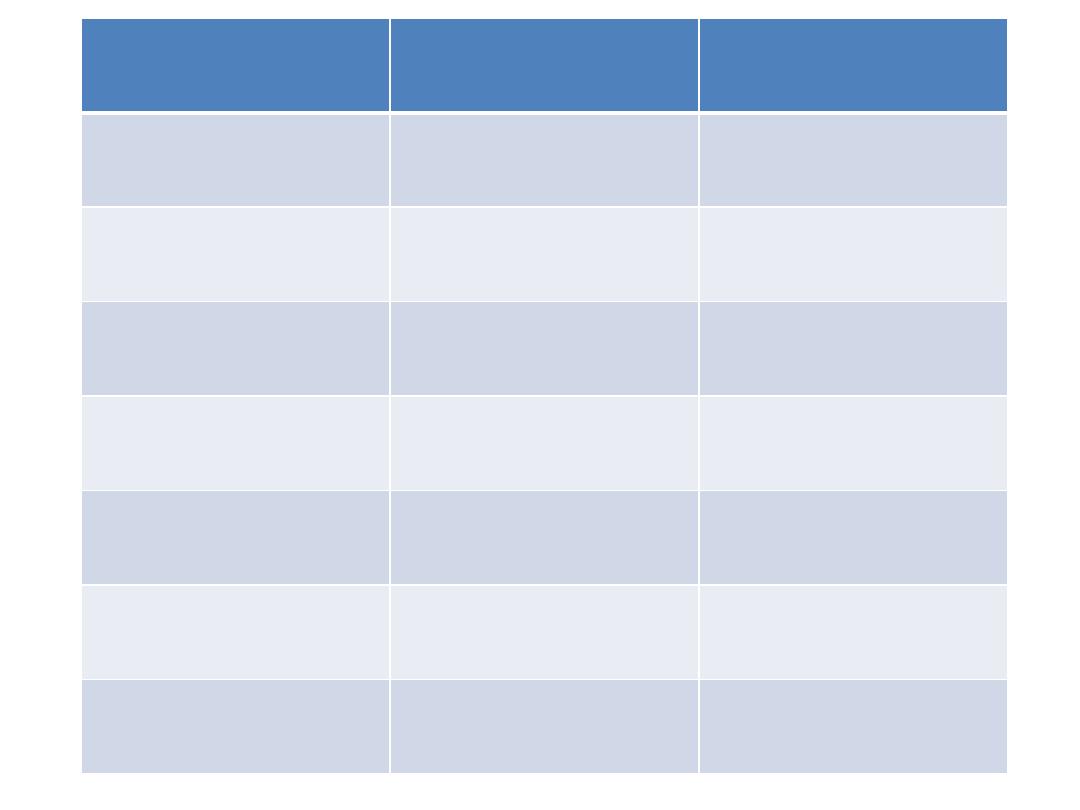
Myeloid
Lymphoblastic
Phase
Daunorubicin (I.V)
Vincristine (I.V)
Induction
cytarabin (I.V)
Prednisolon oral
Etoposide (I.V)
L. asparaginase
(I.V)
Tioguanine (oral)
Daunorubicin (I.V)
Methotrexate
(intrathecal)
cytarabine I.V
Daunorubicin (I.V)
Consolidation
Amsacrine IV
Cytarabine (I.V)
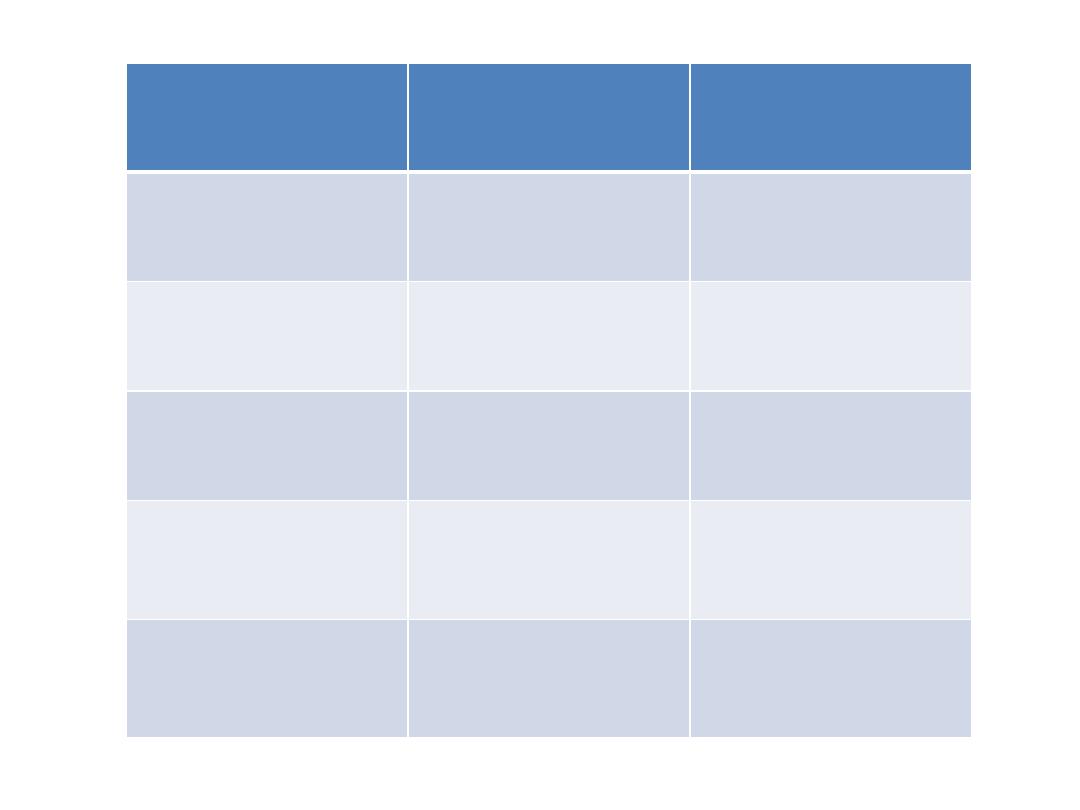
Mitoxantrone
Etoposide (I.V)
Mitozantrone
(I.V)
Methotrexate
(I.V)
prednisolon
(oral)
Maintenance
Vincristine (I.V)
Mercaptopurine
(oral)
Methotrexate
(oral)

Pro myelocytic leukaemia (M3) can be treated by tretenoin
(ATRA) together with combination chemotherapy for remission
induction and then receive consolidation chemotherapy
(Daunorubincin and cytarabine) followed by maintenance tretenoin
or chemotherapy
.

Complete remission (CR) is defined after examination of both
blood and bone marrow and should last ≥ 4 weeks.
The blood neutrophil count must be ≥ 1500/microliter and the
platlets count ≥ 100.000/ML. circulating blast should be absent.
Bone marrow cellularity should be > 20% with trilineage maturation.
The bone marrow should contain <5% blast, and Aure rods
should be absent. Extramedullary leukaemia should not be present
.

Supportive therapy:
Potentially curative therapy which involves periods of severe
bone marrow failure would not be possible without adequate
supportive care.
Anaemia treated with red cell concentrate (Hb > 10mg/dL).
Bleeding: thrombocytopenic bleeding requires platlets transfusion
(prophylactic transfusion maintain the platlet count > 10 x 10
9
/L.
coagulation abnormalities may need treatment with FFP).

*
Infection: fever (> 38C°) lasting over 1 hour in aneutropenic
patient (neutrophil count < 1 x 10
9
/L) indicate possible septicaemia.
Parentral braod spectrum antibiotics therapy is essential example
aminoglycoside with braod spectrum pencilline. The most common
organisms associated with severe neutropenia are gram positive
bacteria such as staph. aureas and staph. Epidermidis . Gram
negative infection with E-coli, pseudomonas and klebsiella are more
likely to cause rapid clinical deterioration. Pneumocytic karinii may
affect patients with lymphoblastic leukaemia and may need
prophylasis with co-trimoxazol. Oral monilial infection is common
and prophylaxis with fluconazol is often consider

Systemic fungal infection with candida or pulmonary aspergillosis
may need intravenous amphotericin 0.5-1 mg/kg/day for at least 3
weeks.
Herpes simplex infection occurs frequently and may need
prophylactic treatment with acyclovir (200 mg 5 times/ day). The
value of isolation facilities is debatable.
Metabolic problems: continuous monitoring of renal, hepatic and
haemostatic function is necessary together with fluid balance
measurements.

Poor prognostic features in acute leukaemia:
1- Increasing age.
2- Male.
3- High leukocyte count of diagnosis.
4- Cytogenetic abnormality.
5- CNS involvement at diagnosis.
6- Antecedent haematological disorder.

Allogeneic bone marrow transplantation:
Indications:
1- Neoplastic disorders affecting totipotent or pluripotent stem cell
(ex. Leukaemia).
2- Failure of haematopoiesis ex. (aplastic anaemia).
3- Inherited defect in blood cell production ex. (thalassaemia,
immune deficiency diseases).

4- In born errors of metabolism.
Healthy marrow or stem cells collected from the peripheral blood of
a normal donar may be injected intravenously into a recipient who
has been suitably conditioned.
The conditioning therapy used most frequently is high dose
cyclophosphamide and total body irradiation.

The injected doner cells home to the marrow, engraft and
produce enough erythrocytes, granulocyte and platlets for the
patients need after about 3-4 weeks. It may take several years to
regain normal immunological function. The use of peripheral blood
stem cells is associated with more rapid engraftment and
immunological reconstitution making the procedure safer. The
donars immunological system can recognize malignant recipient cells
and destroy them. This immunological (graft versus disease) effect is
a powerful tool against many haematological tumours and can be
boosted in post transplantation relapse by the infusion of T cell
taken from the donar so called donar leukocyte infusion
.

Haematological indication for allogeneic B.M. transplant
:
AML in first remission – CML in chronic plase – lymphoblastic
leukaemia (T & B) in first remission – ALL (common type) in second
remission – a plastic anaemia – acute myelofibrosis – lymphoma –
myeloma and immune deficiency syndrome.
