1
.أ
.د
حممد حمسن عبد العزيز
Male Infertility
Male reproductive physiology
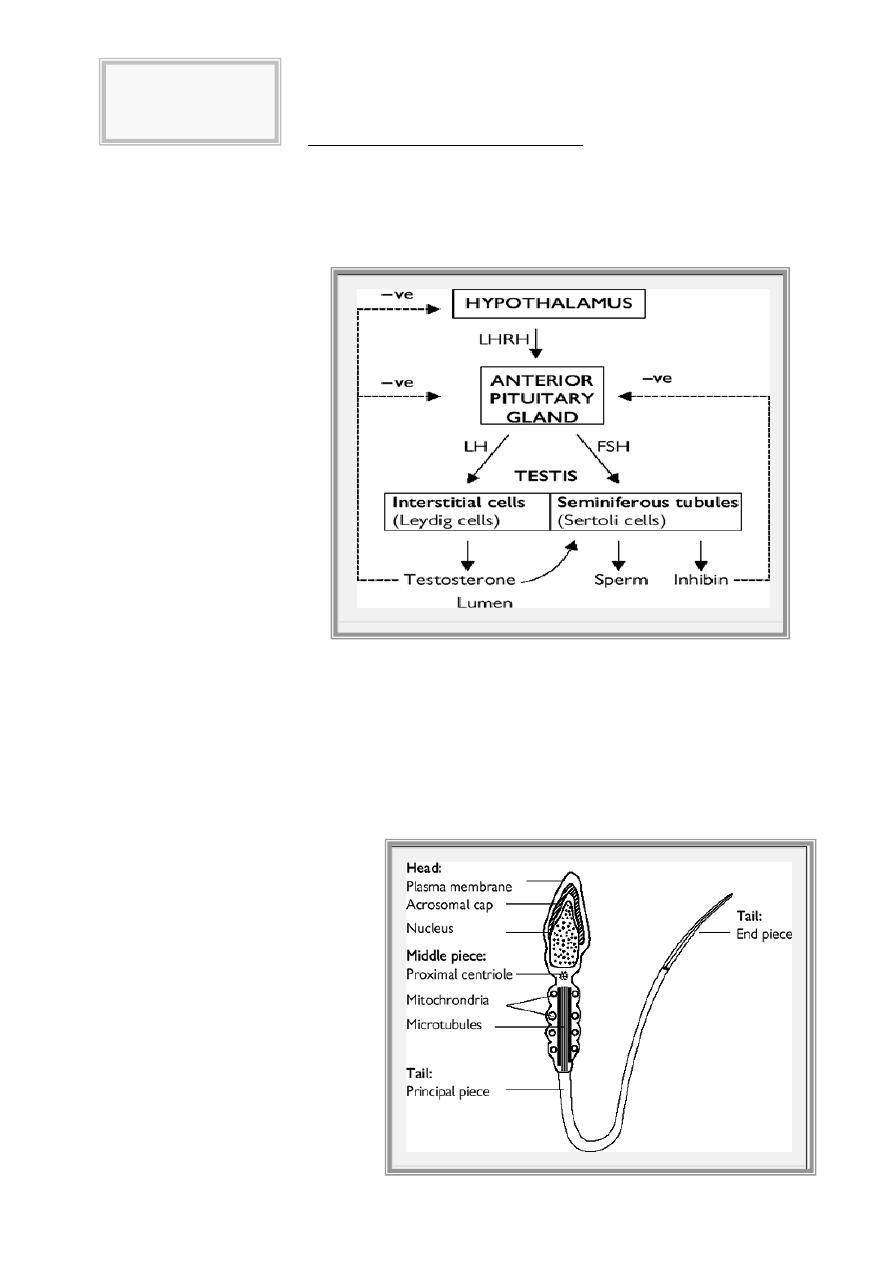
Hypothalamic & pituitary & testicular axis
The hypothalamus secretes luteinizing hormone-releasing hormone (LHRH), also known as
gonadotrophin-releasing hormone (GnRH). This causes pulsatile release of anterior pituitary
gonadotrophins, called follicle stimulating hormone (FSH) and luteinizing hormone (LH), which act on
the testis. FSH stimulates the seminiferous tubules to secrete inhibin and produce sperm; LH acts on
Leydig cells to produce
testosterone.
Testosterone
is secreted by the interstitial
Leydig cells, which lie adjacent
to the seminiferous tubules in
the
testis.
It
promotes
development of the male
reproductive
system
and
secondary
sexual
characteristics.
Spermatogenesis
Seminiferous tubules are lined with Sertoli cells, which surround developing germ cells
(spermatogonium) and provide nutrients and stimulating factors, as well as secreting androgen-binding
factor and inhibin . Primordial germ cells divide to form primary spermatocytes. These undergo a first
meiotic division to create secondary spermatocytes (46 chromosomes), followed by a second meiotic
division to form spermatids (23 chromosomes). Finally, these differentiate into spermatozoa. This
process takes about 74 days. The non-motile spermatozoa leave the seminiferous tubules and pass to
the epididymis, for storage and maturation (until ejaculation). Spermatozoa that are not released are
reabsorbed by phagocytosis.
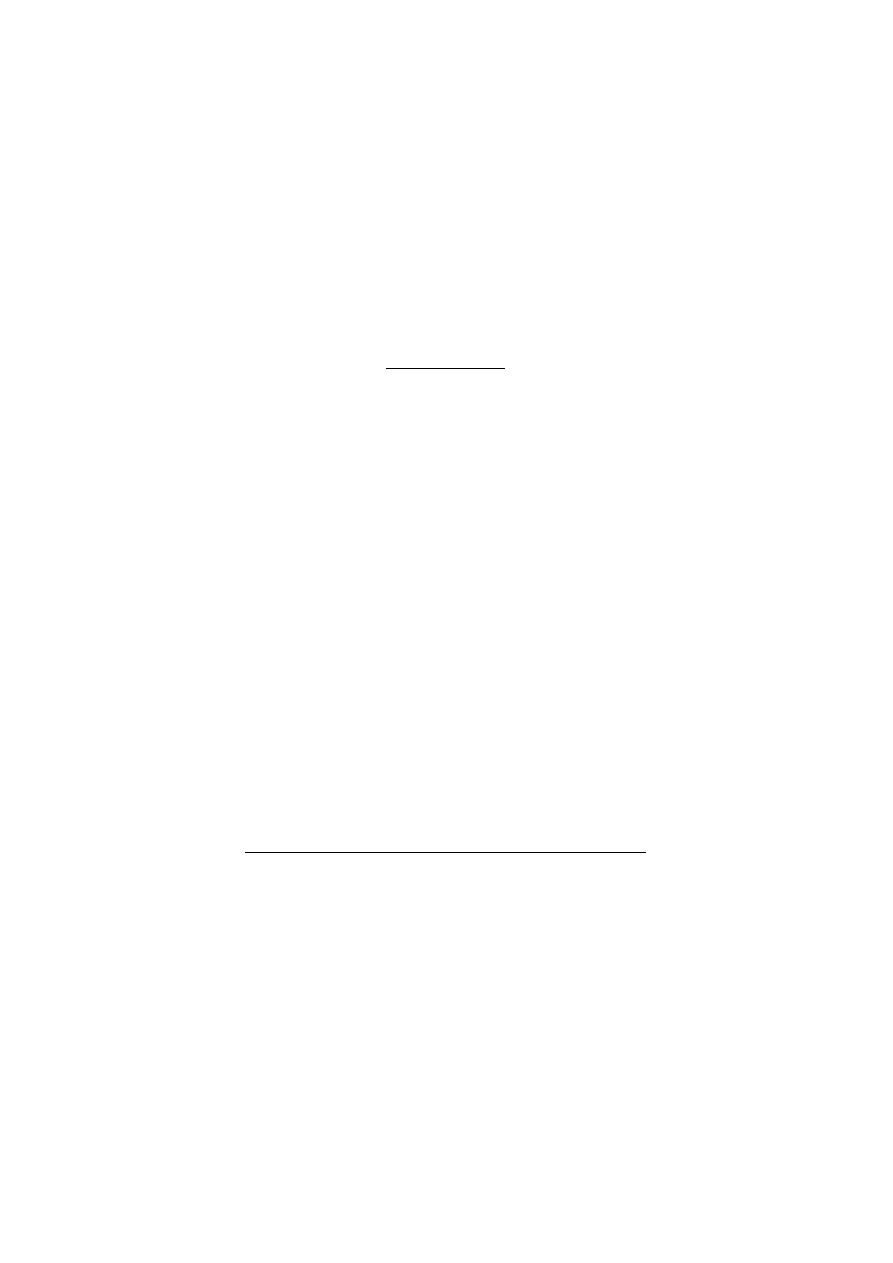
Mature sperm
have a head, middle piece, and
tail. The head is composed of a nucleus
covered by an acrosome cap,
containing vesicles filled with lytic
enzymes. The middle piece contains
mitochondria and contractile filaments,
which extend into the tail to aid motility.
After deposition at the cervix, sperm
penetrate cervical mucus and travel
through the uterus to the site of
fertilization in the fallopian tube.
Tikrit Medical College
Urology
Fifth year

2
.أ
.د
حممد حمسن عبد العزيز
Aetiology and evaluation of male infertility
Definition of infertility
Failure of conception after at least 12 months of unprotected intercourse. The chance of a
normal couple conceiving is estimated at 20-25% per month, 75% by 6 months, and 90% at 1 year.
Epidemiology
Up to 35% of infertility is due to male factors. Up to 25% of couples may be affected at some
point in their reproductive years.
Pathophysiology
Failure of fertilization of the normal ovum due to defective sperm development, function,
or inadequate numbers. There may be abnormalities of morphology (teratospermia), motility
(asthenospermia), low sperm numbers (oligospermia), or absent sperm (azoospermia). Abnormal
epididymal function may result in defective spermatozoa maturation or transport, or induce cell death.
Aetiology
Idiopathic (25%)
Varicocele (present in 40%)
Cryptorchidism (undescended testes)
Functional sperm disorders: immunological infertility (sperm antibodies); head or tail defects
Erectile or ejaculatory problems
Testicular injury: orchitis (post-pubertal, bilateral mumps orchitis); testicular torsion; trauma;
radiotherapy
Endocrine disorders: Kallmann's syndrome (isolated gonadotrophin deficiency causing
hypogonadism); pituitary gland adenoma, radiation, or infection
Hormone excess: excess prolactin (pituitary tumour); excess androgen (congenital adrenal
hyperplasia, anabolic steroids); excess oestrogens
Genetic disorders: Kleinfelter's syndrome (47XXY) involves azoospermia,
Male genital tract obstruction: congenital absence of vas deferens; epididymal obstruction or
infection; groin or scrotal surgery
Systemic disease: renal failure; liver cirrhosis; cystic fibrosis
Drugs: chemotherapy; alcohol; marijuana; sulphasalazine; smoking
Environmental factors: pesticides; heavy metals; hot baths
History
Sexual: duration of problem; frequency and timing of intercourse; previous successful
conceptions; previous birth control; erectile or ejaculatory dysfunction.
Developmental: age at puberty; history of cryptorchidism; gynaecomastia.
Medical and surgical: detailed assessment for risk factors, recent febrile illness; post-pubertal
mumps orchitis; varicocele; testicular torsion, trauma, or tumour; sexually transmitted diseases;
genitourinary surgery; radiotherapy; respiratory diseases associated with ciliary dysfunction;
diabetes.
Drugs and environmental: previous chemotherapy; exposure to substances which impair
spermatogenesis or erectile function; alcohol consumption; smoking habits; hot baths.
Family: hypogonadism; cryptorchidism.
Examination
Perform a full assessment of all systems, with attention to general appearance (evidence of
secondary sexual development; signs of hypogonadism; gynaecomastia). Urogenital examination
should include assessment of the penis (Peyronie's plaque, phimosis, hypospadias); measurement of
testicular consistency, tenderness, and volume with a Prader orchidometer (normal >18ml; varies with

3
.أ
.د
حممد حمسن عبد العزيز
race); palpate epididymis (tenderness, swelling) and spermatic cord (vas deferens present or
absent, varicocele); digital rectal examination of prostate.
Investigation of male infertility
Basic investigations
Semen analysis 2 or 3 specimens over several weeks, collected after 2-3 days of sexual
abstinence. Deliver specimens to the laboratory within 1h. Ejaculate volume, liquefaction time, and pH
are noted. Microscopy techniques measure sperm concentration, total numbers, morphology, and
motility .
The World Health Organization defines the following reference values
Volume: 2.0 mL or more
pH: 7.2 or more
Sperm concentration: 20 × 10
6
or more spermatozoa/mL
Total sperm number: 40 × 10
6
or more spermatozoa per ejaculate
Motility: 50% or more with grade “a + b” motility or 25% or more
with grade “a” motility
Morphology: 15% or more by strict criteria
Viability: 75% or more of sperm viable
WBCs: Less than 1 million/mL
Hormone measurement Serum FSH, LH, and testosterone . In cases of isolated low
testosterone level, it is recommended to test morning and free testosterone levels. Raised prolactin is
associated with sexual dysfunction, and may indicate pituitary disease.
Special investigations
Chromosome analysis: Indicated for clinical suspicion of an abnormality.
Testicular biopsy: Performed for azoospermic patients, to differentiate between idiopathic and
obstructive causes. May also be used for sperm retrieval.
Imaging
Scrotal ultrasound scan is used to confirm a varicocele and assess testicular abnormalities.
Transrectal ultrasound scan is indicated for low ejaculate volumes, to investigate seminal vesicle
obstruction or absence and ejaculatory duct obstruction.
Vasography Vas deferens is punctured at the level of the scrotum and injected with contrast. A normal
test shows the passage of contrast along the vas deferens, seminal vesicles, ejaculatory duct, and into
the bladder, which rules out obstruction.
Oligospermia
Defined as a sperm concentration of less than 20 million/ml of ejaculate.
Aetiology
Varicoceles; idiopathic; androgen deficiency. It is identified in ~60% of patients presenting with
testicular cancer or lymphoma.
4
.أ
.د
حممد حمسن عبد العزيز
Associated disorders
It is often associated with abnormalities of morphology and motility. The combined disorder is
called oligoasthenoteratospermia (OAT) syndrome. Common causes include varicoceles;
cryptorchidism; idiopathic; drug and toxin exposure; febrile illness.
Investigations
Semen analysis: sperm counts <5-10 million/ml (severe form) require hormone investigation,
including FSH and testosterone.
Treatment
Correct the underlying cause. Idiopathic cases may respond to empirical medical therapy or
require assisted reproductive techniques.
Azoospermia
Defined as an absence of sperm in the ejaculate fluid.
Aetiology
Obstructive Absent or obstructed vas deferens; epididymal or ejaculatory duct obstruction
(related to infection, cystic fibrosis).
Non-obstructive Hypogonadotrophism (Kallmann's syndrome, pituitary tumour); abnormalities
of spermatogenesis (chromosomal anomalies, toxins, idiopathic, varicocele, orchitis, testicular
torsion).
Investigations
Hormone assay (raised FSH indicates non-obstructive cause; normal FSH with normal testes
indicates increased likelihood of obstruction).
Testicular biopsy is performed to assess if normal sperm maturation is occurring, and for
sperm retrieval (for later therapeutic use).
Transrectal ultrasound scan assesses absence or blockage of vas deferens, and ejaculatory
duct obstruction. Exclude cystic fibrosis in patients with vas deferens defects.
Management
Treatment will depend on underlying aetiology.
Treatment options for male factor infertility
General
Modification of life style factors (reduce alcohol consumption; avoid hot baths).
Medical treatment
Correct any reversible causative factors.
Hormonal
Secondary hypogonadism (pituitary intact) may respond to human chorionic gonadotrophin
(hCG) which stimulates an increase in testosterone and testicular size. If the patient remains
azoospermic after 6 months of treatment, FSH is added (human recombinant FSH or human
menopausal gonadotrophin). Alternatively, pulsatile LHRH can be administered
subcutaneously via a minipump.
Testosterone deficiency requires testosterone replacement therapy.
Hyperprolactinaemia is treated with dopamine agonists.
Anti-oestrogens (clomiphene citrate & tamoxifen) are often used empirically to increase LHRH,
which stimulates endogenous gonadotrophin secretion.

5
.أ
.د
حممد حمسن عبد العزيز
Erectile and ejaculatory dysfunction
Erectile dysfunction may be treated conventionally (oral, intraurethral, intracavernosal drugs;
vacuum devices or prostheses). Ejaculatory failure may respond to sympathomimetic drugs or
electroejaculation (used in spinal cord injury)
Antisperm antibodies
Corticosteroids have been used, but assisted conception methods are usually required.
Surgical treatment
Genital tract obstruction
Epididymal obstruction can be overcome by microsurgical anastomosis between the
epididymal tubule and vas (epididymovasovasostomy).
Vas deferen obstruction is treated by microsurgical reanastomosis of ends of the vas, and is
used for vasectomy reversal. Ejaculatory duct obstruction requires transurethral resection of
the ducts.
Varicocele
Repaired by embolization or open/laparoscopic surgical ligation.
Assisted reproductive techniques (ART)
Assisted conception
Intrauterine insemination (IUI) Following ovarian stimulation, sperm are placed directly into
the uterus.
In vitro fertilization (IVF) Controlled ovarian stimulation produces oocytes which are then
retrieved under transvaginal USS-guidance. Oocytes and sperm are placed in a Petri dish for
fertilization to occur. Embryos are transferred to the uterine cavity. Pregnancy rates are
20-30% per cycle.
Intracytoplasmic Sperm injection (ICSI) A single spermatozoon is injected directly into the
oocyte cytoplasm. Pregnancy rates are 15-22% per cycle.
