1
أ.د. حممد حمسن عبد العزيز
Vesicoureteric reflux (VUR)
VUR is the abnormal retrograde flow of urine from the bladder into the upper urinary
tract with or without dilatation of the ureter, renal pelvis, and calyces. It can cause symptoms
and may lead to renal failure (reflux nephropathy).
Epidemiology
Overall incidence in children is >10%; younger > older; girls > boys (female: male
ratio 5:1); Caucasian > African. Siblings of an affected child have a 40% risk of reflux, and
routine screening of siblings is recommended.
Pathophysiology
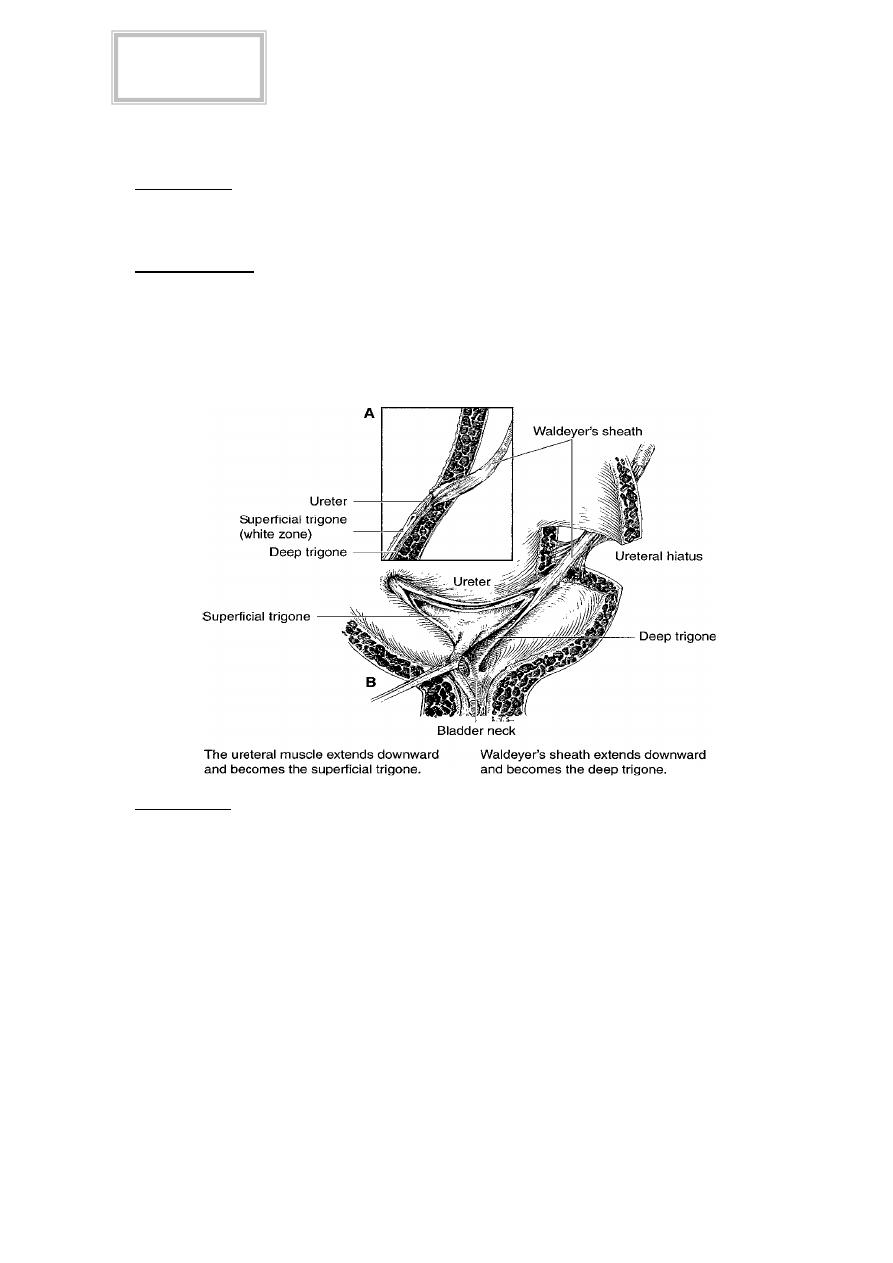
Reflux is normally prevented by low bladder pressures, efficient ureteric peristalsis,
and the ability of the vesicoureteric junction (VUJ) to occlude the distal ureter during bladder
contraction. This is assisted by the ureters passing obliquely through the bladder wall
(the intramural ureter), which is about 1.5cm long. Normal intramural ureteric length to ureteric
diameter ratio is 5:1. VUR of childhood tends to resolve spontaneously with increasing age
because as the bladder grows, the intramural ureter lengthens.
Classification
Primary: a primary anatomical (and therefore functional) defect where the intramural length of
the ureter is too short (ratio <5:1).
Secondary to some other anatomical or functional problem:
Bladder outlet obstruction (BPH, posterior urethral valves, urethral stricture) which
leads to elevated bladder pressures.
Poor bladder compliance or the intermittently elevated pressures of detrusor
hyperreflexia (due to neuropathic disorders e.g. spinal cord injury, spina bifida).
Iatrogenic reflux following TURP or TURBT (a tumor overlying the ureteric orifice);
ureteric meatotomy (incision of the ureteric orifice) for removal of ureteric stones at the
VUJ; following incision of a ureterocele; ureteroneocystostomy; post pelvic
radiotherapy.
Inflammatory conditions affecting function of the VUJ: TB, schistosomiasis, UTI.
Tikrit Medical College
Urology
Fifth year
2
أ.د. حممد حمسن عبد العزيز
Associated disorders
VUR is commonly seen in duplex ureters (the Meyer Weigert law). Cystitis can cause
VUR through bladder inflammation, reduced bladder compliance, increased pressures, and
distortion of the VUJ. Coexistence of UTI with VUR is a potent cause of pyelonephritis & reflux
of infected urine under high pressure causes reflux nephropathy, resulting in renal scarring,
hypertension, and renal impairment.
Presentation
VUR may be symptomless, being identified during VCUG, IVU, or renal ultrasound
(which shows ureteric and renal pelvis dilatation) done for some other cause.
UTI symptoms.
Loin pain associated with a full bladder or immediately after micturition.
Symptoms of recurrent UTI or of loin pain may have been present for many years before the
patient seeks medical advice.
Investigation
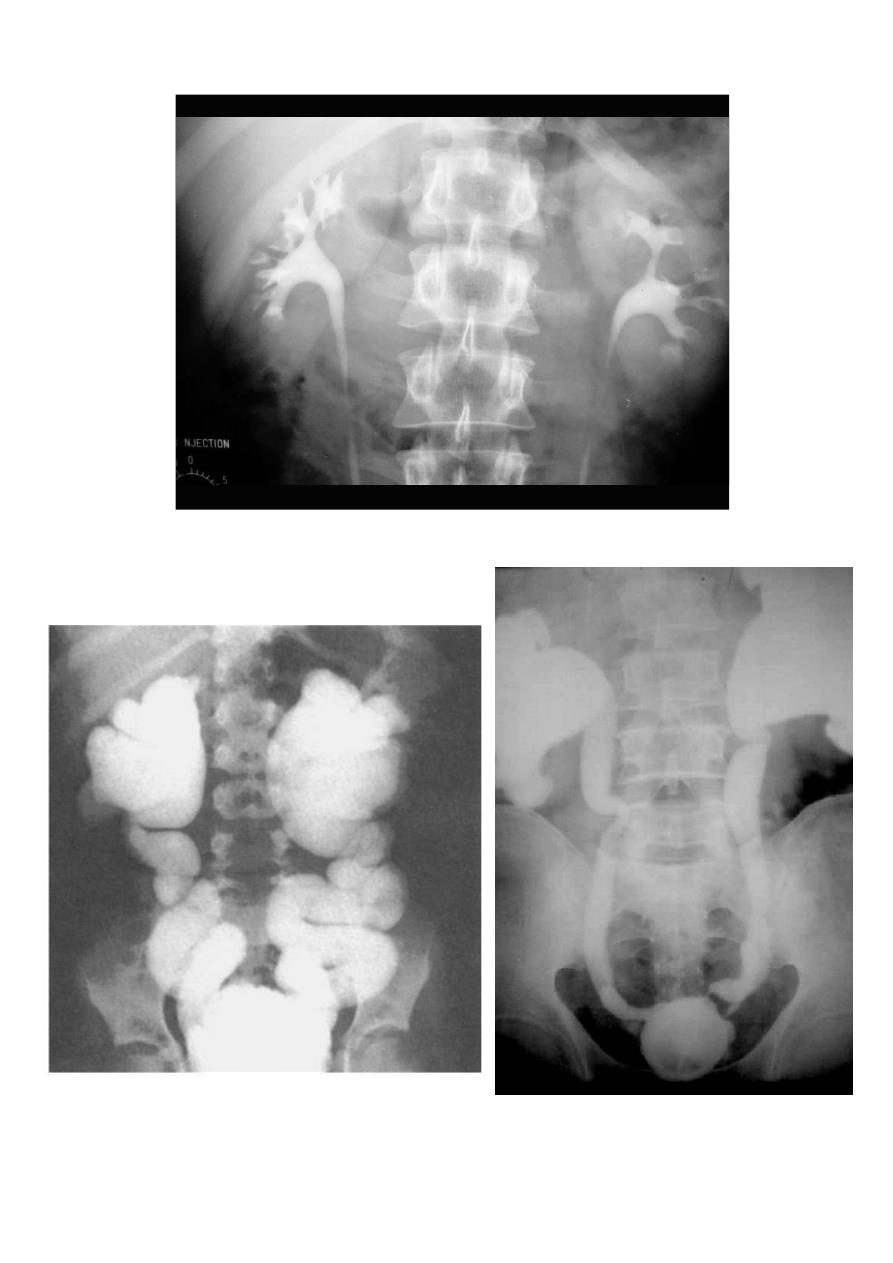
The definitive test for the diagnosis of VUR is cystography. VUR may be apparent during
bladder filling or during voiding (voiding cystourethrography VCUG also known as micturating
cystourethrography, MCUG). Urodynamics establishes the presence of voiding dysfunction if
this is suspected from the clinical picture. If there is radiographic evidence of reflux
nephropathy check blood pressure, check the urine for proteinuria, measure serum creatinine,
and arrange a
99m
Tc-DMSA isotope study to assess renal cortical scarring.
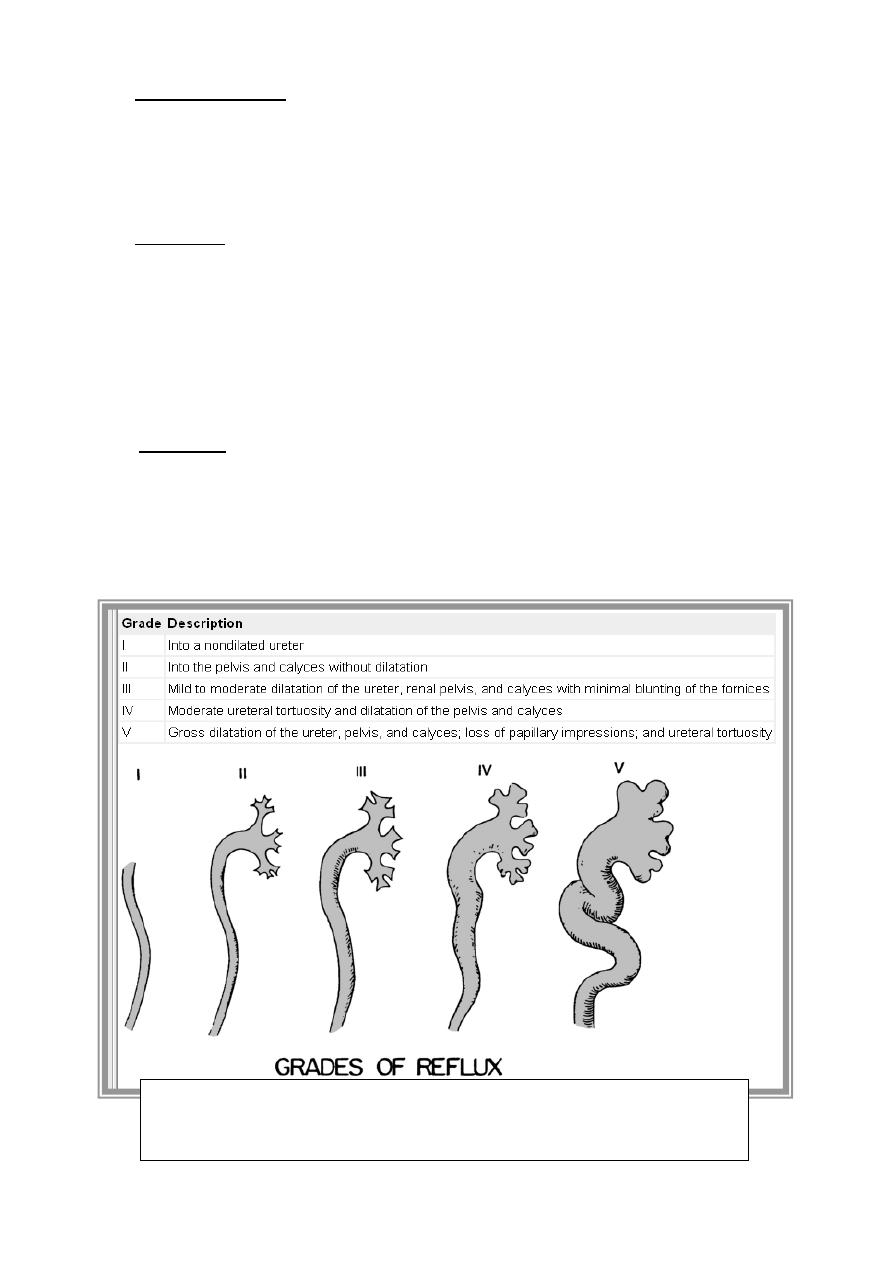
Grading System
The international grading system for reflux is based primarily on the radiographic
appearance of the calyces on voiding cystourethrography (VCUG).
3
أ.د. حممد حمسن عبد العزيز
NORMAL
VUR
VUR

4
أ.د. حممد حمسن عبد العزيز
Management
The management of patients is based on the observation that VUR has a natural
tendency for Spontaneous resolution. Surgical correction can often be avoided by maintaining
the patient on careful medical surveillance and continuous antibiotic prophylaxis to prevent
secondary renal damage. The decision for medical versus surgical management is made after
a careful diagnostic evaluation, with particular focus on the grade of reflux and age of the
patient. Most grade I to III refluxes resolve spontaneously, as do some in grade IV. Grade V
refluxes seldom resolve on their own. Reflux is more likely to resolve spontaneously in younger
children regardless of grade.
Medical
Medical management consists of continuous low-dose antibiotic prophylaxis, regular
urine cultures (every 3 months), and a yearly cystogram and renal ultrasound. Medical
surveillance is appropriate for young children with mild-to-moderate grades of reflux (I & III)
and very young children with grade IV reflux. Ampicillin or amoxicillin is appropriate for children
younger than 6 weeks, whereas trimethoprim & sulfamethoxazole can be used after 6 weeks
(mature biliary system). Grade V reflux also is managed medically in newborns until the child is
old enough for surgical management.
Secondary reflux:
treat the underlying cause & relieve BOO, improve bladder compliance.
Surgical
The success rate for antireflux procedures is greater than 95% in experienced hands.
Indications for early surgical intervention follow.
Breakthrough infection despite antibiotic prophylaxis
Poor compliance with medical regimen
Progressive renal scarring with antibiotic prophylaxis
A refluxing orifice within a diverticulum
Severe reflux (grade IV or V)
VUR that persists after puberty
VUR associated with congenital anomalies like double ureter
Techniques include laparoscopic repair and open ureteric re-implantation:
Intravesical methods involve mobilizing the ureter and advancing it across the trigone
(Cohen repair) or reinsertion into a higher, medial position in the bladder
(Politano & Leadbetter repair).
Extravesical techniques involve attaching the ureter into the bladder base and suturing
muscle around it (Lich & Gregoir procedure).
Alternatively, endoscopic subtrigonal injections into the ureteric orifice has 70%
success, rising to 95% with repeated treatments.
Reflux into a non-functioning kidney (<10% function on DMSA scan) with recurrent
UTIs and/or hypertension: nephroureterectomy.
5
أ.د. حممد حمسن عبد العزيز
Renal Transplantation
The 50th anniversary of the first successful kidney transplant from a live donor to his
identical twin was celebrated in 2004. During this interval, kidney transplantation has
progressed from an experimental procedure to the preferred method of renal replacement
therapy worldwide. The first long-term success with human renal allografting, in which the
patient survived for over a year, occurred in Boston in 1954, when a kidney from one twin was
transplanted into the other, who had ESRD. Thirty-six years after the first long-term success of
human-to-human kidney transplantation, Joseph E. Murray received the Nobel Prize in
Medicine in 1990 for his pioneering work in renal transplantation.
Renal transplantation is clearly the preferred treatment for patients with permanent
renal failure (GFR, <10 mL/minute, or serum creatinine, >8 mg/dL). It provides significantly
longer survival over dialysis and a better quality of life. Because of the increasing numbers of
patients requiring transplantation and the long waits for cadaveric kidneys, living related-donor
renal transplantation is increasing. Living related donors have a better probability of graft
survival and can allow patients to have preemptive renal transplantation before ever needing to
go on dialysis.
Recipient Selection
Contraindications to Transplantation
Active infection
Recent malignant disease
Active glomerulonephritis
Presensitization to donor class I human leukocyte antigen (HLA) antigens
Donor Selection
Living Related Donors
Living related and unrelated donors are an increasing source of kidney transplants in
the because of superior graft survival and a shortage of cadaveric kidneys. Donors are
expected to be in perfect physical and mental health with negative blood and tissue
cross-matches. Compatibility by mixed lymphocyte culture is demonstrated by negative HLA
antigenic stimulation.
Cadaver Donors
Cadaver donors are generally persons between ages 2 and 60 years without evidence
of significant hypertension, atherosclerosis, renal disease, malignancy, or infection. Blood and
urine cultures should be negative, with creatinine less than 2 mg/dL, and hepatitis B antigen
(HBsAg) negative. The heart must be pumping, and no longer than 10 minutes of warm
ischemia should exist. After removal, kidneys are flushed with ice-cold Collins' solution and
stored in ice slush (for 24-48 hours) or preserved by pulsatile perfusion (for 48-72 hours).
Tissue Matching
The HLA gene products (cell-surface antigens) from each haplotype are classified as
class I serologically defined HLA-A, -B, and -C and the class II HLA-DR (D-related) antigens.
Matching for major HLA antigens among closely related living donors has been shown to
predict results superior to those with cadaveric donors because of compatibility among other
less important gene products of the major histocompatibility complex (MHC) region. Living
related transplants between HLA identical siblings (two-haplotype match) yield the highest
1-year graft survival (up to 90%), followed next by HLA semi-identical (one-haplotype match).
The ABO (H) blood group antigens are expressed on vascular endothelium and
therefore must be matched for renal transplantation. However, Rh antigens are not expressed
on nucleated cells and need not be of concern.
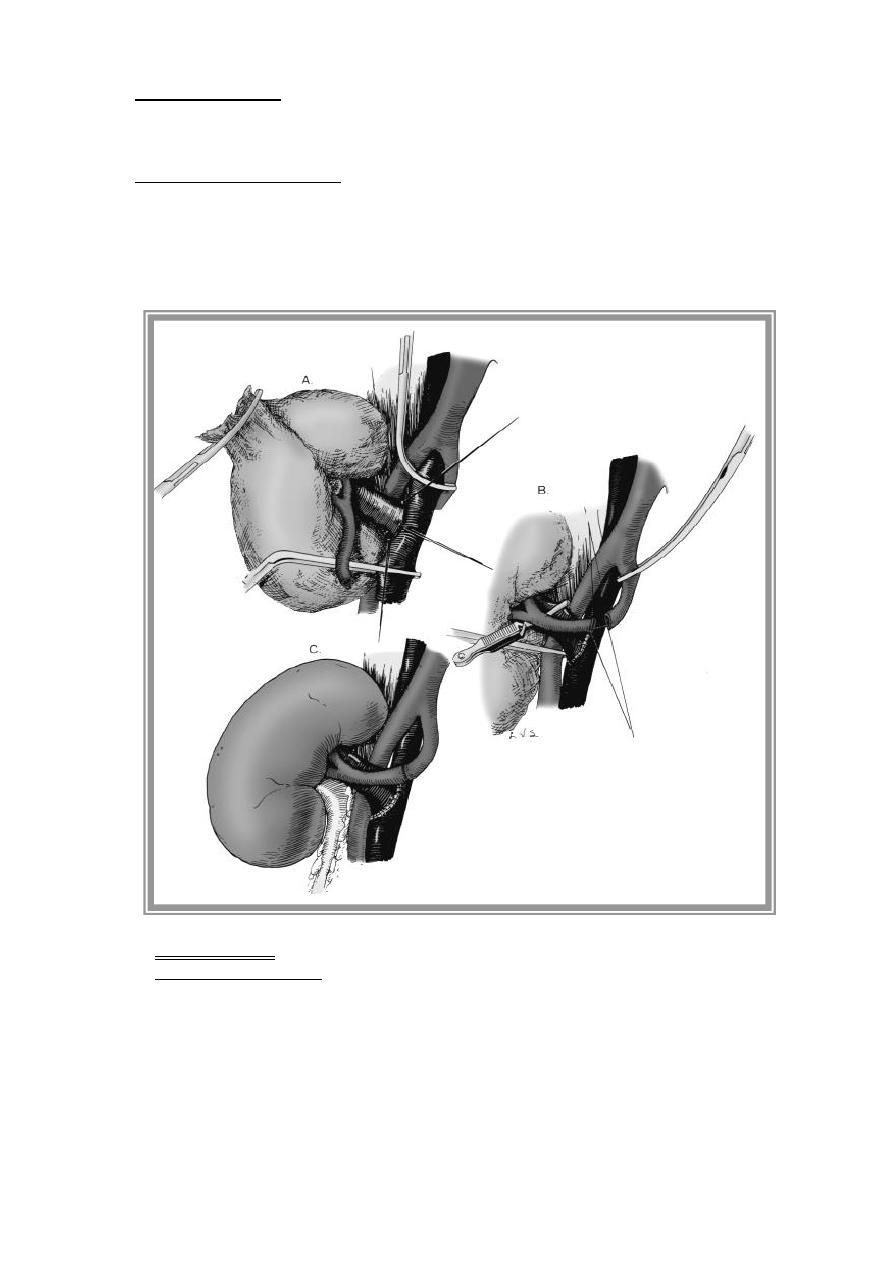
6
أ.د. حممد حمسن عبد العزيز
Immunosuppression
The allograft response is directed primarily against mismatched HI.A antigens and is
T cell dependent. Immunosuppression is important to prevent rejection.
Perioperative Considerations
The usual preoperative laboratory tests, including a urine culture, chest radiograph,
and electrocardiogram (ECG), should be obtained. Most patients will require preoperative
dialysis. Immunosuppressive medications should be started preoperatively as dictated by the
prescribed protocol. Prophylactic antibiotics should be given on call to the operating room. A
living related donor should be kept well hydrated with optimal urine output.
Complications
Acute Tubular Necrosis
ATN is the most common cause of early oliguria or anuria after transplantation and is
the result of ischemic insult to the kidney. Recovery will generally occur within 3 to 6 weeks;
however, a renal biopsy may be helpful to rule out rejection. The diagnosis is made primarily by
exclusion.

7
أ.د. حممد حمسن عبد العزيز
Rejection
Hyperacute or Accelerated Rejection.
Hyperacute rejection is generally an irreversible process that begins within minutes to hours
after transplantation and is the result of presensitization of the recipient with cytotoxic
antibodies to donor ABO.
Acute Rejection
Acute rejection occurs within 3 months after transplantation and is clinically
characterized by fever, swelling, and tenderness over the graft, with decreased function and
urine output. Hypertension also may be present.
Chronic Rejection
Chronic rejection results in a gradual deterioration of function, months to years after
transplantation, without clear evidence of a rejection episode.
Surgical Complications
Vascular Complications
Vascular complications occur infrequently after transplantation; however, when they
occur, they can be disastrous and must be recognized immediately.
Thrombophlebitis
Thrombophlebitis is a relatively uncommon occurrence; however, if it is suspected,
ultrasound or phlebography should be performed.
Graft Rupture
Graft rupture usually occurs as spontaneous severe pain and shock from massive life-
threatening hemorrhage. It is believed to be caused by acute rejection and ischemia.
Immediate nephrectomy is indicated.
Lymphatic
Lymphatic drainage from the area of dissection may occasionally be excessive,
resulting in leakage from the incision or surgical drains. Occasionally, more prolonged drainage
can result in a lymphocele, which is best diagnosed with ultrasound.
Infectious Complications
Infections are the most common and life-threatening complications for the transplant
recipient. Immunosuppressive therapy coupled with leukopenia, hyperglycemia, and azotemia
markedly increases the risk of serious complicated infections. Bacterial sepsis is most
common; however, opportunistic infections such as those with cytomegalovirus (CMV),
Pneumocystis carinii, or Candida albicans are major problems.
Urinary Tract Infections
Urinary tract infections are a common source of bacterial sepsis in the post transplant
patient. Use of prophylactic antibiotics and early removal of the Foley catheter can help lessen
their incidence.
Wound Infection
Wound infection must always be suspected in the posttransplant patient with high
fever. The incision will often look benign, even in the face of a grossly purulent abscess. Do not
underestimate the masking effect of immunosuppressive agents.
Pulmonary Infection
Pulmonary infection with gram-negative bacteria, fungi, P. carinii, and CMV usually
appears with fever and pulmonary infiltrates. However, CMV and Pneumocystis can often
cause severe respiratory failure before radiographic evidence of pneumonia.
Meningitis
Meningitis will often be masked by immunosuppressive therapy. Patients with
unexplained fever, dull headache, photophobia, or mental-status changes should undergo
lumbar puncture. Look for Listeria, Candida, Cryptococcus, and Aspergillus.
