
Lecture -1-
Diabetic disease in pregnancy& it’s complication
.د
سها وتىت
Definition: is defined as carbohydrate disturbance characterized by
hyperglycemia & either peripheral insulin resistance or insulin
deficiency.
There are
2
types of D.M : 1.IDDM(type 1)
2.NIDDM( type 2).
In general 90% of DM in adult is NIDDM & 10% is IDDM while in
pregnancy only 27% is NIDDM& 73% is IDDM.
Where there may be a serological evidence of autoimmune destruction of
pancreatic islets & genetic marker in type 1 DM like HLA-DR4& HLA-
DR3, type 2 may be hyperglycemia for long period without clinical
symptoms( familial) with more genetic predisposition than type 1.
The symptoms of D.M is closely related to the degree of hyperglycemia&
these include polyuria, polydipsia, weight loss, recurrent vulvar boils&
bleured vision.
Incidence of both pre- existing DM and GDM is increasing.
Insulin secretion is increase during 1
st
& 3
rd
trimester due to hyperplasia
of pancreatic islands. However there is increase in insulin secretion in the
1
st
trimester but there is hypoglycemia due to release of insulin resistance
factors like HPL, cortisol .. so FBS decrease by 10-20% & more in post
prandial in addition to nausea & vomiting during 1
st
trimester.
Incidence:
It complicate 1-2% of pregnancy with high PNM& mortality reach about
65% but after introduction of insulin ,this percentage fall as in non-
diabetic mother in a well-controlled D.M.so if the mother is only had
IDDM only 2% of baby affected& if the father had DM , 8% affected &
if both parents , 30% will affected.
Pathophysiology:
During pregnancy ,there is significant change in carbohydrate
metabolism& glucose homeostasis secondary to complex hormonal

changes like estrogen ,progesterone ,human placental lactogen & cortisol.
HPL lead to increase lipolysis in the adipose tissue, this result in the
production of glycerol & fatty acid which are used as a source of energy
by the mother & this lead that glucose up take in the cell is inhibited ,so a
lot of glucose is available to the fetus.
The placenta not only produce diabetogenic hormones but also break
down insulin.
Reduced threshold for glucose & increase GFR result in glycosuria in
about 5-50% of women.
Classification of D.M:
White classification include
A. asymptomatic but abnormal GTT, equal to impaired GTT.
B. Onset age equal or more than 20 years, duration less than 10 years ,no
vascular complication.
C. Onset age 10-19 years ,duration 10-19 years, no vascular complication.
D. Onset age less than 10 years, duration equal or more than 20 years,
vascular disease ,benign retinopathy, leg artery calcification.
E. Nephropathy
F. Proliferate retinopathy.
Diagnosis of D.M in pregnancy:
The onset of IDDM is commonly between the age of 11-14 years&
therefore in most patient the diagnosis is made before pregnancy.
So serial blood glucose estimation & glycosylated Hemoglobin will be
useful in making the diagnosis.
So the WHO diagnostic criteria for overt D.M are:
1. symptoms of D.M plus RBS equal or more than 11.1 mmol\L
2. FBS equal or more 7.0 mmol\L
3. 1-hour plasma glucose equal or more11.1 mmol\L during OGGT.
Complication of pre-existing D.M :
1. Maternal effect:
a. exacerbation of pre-exiting disease like retinopathy ,so ophthalmic
assessment is recommended during pregnancy. As exacerbation of
retinopathy is more likely with 1. Increase the severity of pre- existing

DM 2. Long duration of DM 3. Poor glycemic control 4.rapid
improvement in control so try to do retinal assessment (pupil dilatation
)at booking & at 28 weeks and then 6 weeks postpartum
b. Diabetic nephropathy affect 5-10% of people as nephropathy
diagnosed when there is 1. Micro albuminemia 2. Protein urea 3.renal
failure 4.increase serum creatinine & blood urea 5. May lead to IUGR,
PET , PTL so need to maintain blood pressure between 130-120/ 70-
80mmgh & uterine artery Doppler study for fetal monitoring.
c. Cardiac disease :circulatory& CVS changes during pregnancy are
responsible for the deterioration of cardiac function & mortality rate may
reach 50% in patient with previous MI.
Late maternal complication like PIH&PET.
Intrapartum complication: related to operative morbidity consequent to
fetal macrosomia, obstructed labor& operative delivery.
2. Fetal effect:
A. early complication: 1. congenital malformation: these depend on the
degree & timing of glycemic control, where there is optimum control pre-
pregnancy ,at conception& in the first trimester, the rate approximate to
that of un complicated pregnancy, while it is 5-10 times higher in
uncontrolled D.M as well as it increased if HbA1c increase or increment
in post prandial blood sugar.
these anomalies include cardiac anomalies increase 3-5 folds(ASD
,VSD, TGA) NTD increase 2-10 folds , caudal regression syndrome(
sacral agenesis which is pathognomonic& increase 200 folds ) ,polycystic
kidney, double ureter, tracheo- esophageal fistula, bowel atresia
&imperforated anus.
2. increase incidence of miscarriage& missed abortion.
B. late complication :
1. polyhydramnia occur in 22-26% of uncontrolled D.M due to fetal
polyurea
2. macrosomia: (asymmetrical) body weight > 4 kg or varies from 4-4.5
kg. or >90 centile on growth chart.
It is ten times higher in D.M . as there is a greater adiposity & muscle
mass in macrosomic baby ,the head is spared
3.Iatrogenic preterm delivery : is three fold higher in D.M. but also

increase risk of preterm labor due to polyhydramnia so some time we
need to give dexamethasone to decrease risk of RDS but when given it
increase blood sugar 4 folds so need to increase the dose of SC
insulin(increase at 8-15 hours after insulin )
4. stillbirth: due to poorly controlled D.M, fetal macrosomia, co-exiting
vasculopathy, PET. Stillbirth is about 17% at 24 weeks & is commonly
occur after weeks 36(41% ) due to :1. chronic hypoxia
2. Reduction in uterine blood flow in vasculopathy 3.frequant fluctuation
in RBS level
4. ketoacidosis & infection.
C. intrapartum complication: this related to macrosomia like shoulder
dystocia, increase rate of C\S, long bone fracture, Erb’s pulsy &
Klumpk’s pulsy.
D. neonatal complication: jaundice ,polycythemia ,tetany,
hypoglycemia, hypocalcaemia& hypomagnesaemia.
Management of D.M during pregnancy:
Usually achieved by team consist of physician, obstetrician, GP &
diabetic midwife.
Pre-pregnancy management is necessary as when control is optimum, the
PNM & mortality is similar to non-diabetic one.
So 1.mother education about tight control.
2.screening & treating the complication like retinopathy, nephropathy &
cardiac dysfunction.
3. pre-pregnancy & first trimester folic acid supplement.
4.modified diet.
5. stop smoking& adequate exercise.
6.glycosylated Hb (HbA1c) should be <6.1%. as if it is between 8.5-
10.5%, congenital anomalies increase by 20% and if it is more than 10%,
it is better to avoid pregnancy.
7.women with DM should be offered screening for Down syndrome
including nuchal translucency, B-HCG, PAPP-A in the 1
st
trimester & if
late presentation , do triple test or quadruplet test.
So in general the aims are : 1. HbA1c is <7%( ie: 6.1%) in preconception
time and in the 1
st
trimester
2. pre-prandial BS between 3.5-5.9 mmol/l
3. 1 hour post-prandial BS <7.8mmol/l& monitoring through 1.FBS and
1 hour post-prandial 2. Bed time test 3. Ketone test 4. Medical visit at 1-
2 weeks.

Maternal management
Most patient are usually on insulin before pregnancy & this should be
maintained during pregnancy. Other modality is insulin analog like
Lispro but some study show that it is teratogen.
Types of insulin: 1. Insulin analog( rapid acting within 15 min.) like
lispro. It is not widely used because its safety during pregnancy not
proved.
2. Short acting insulin start within 30 m0n. its peal for 2-4 hours and last
for 4-8 hours.
3.intermediate type start within 2-6 hours its peak after 4-14 hours and
last for 14-20 hours. Combination of both preparation called mixtard.
4. long acting start action after 1-2 hours and last for 24 hours.
During first trim, Sometime need to decrease the dose of insulin because
of Nausea &Vomiting, however insulin requirement need to increase in
the second & third trim.
Insulin given in a different regimen either short acting giving pre-meal &
long acting at bed time so blood sugar need to be monitored by doing
FBS, &1hour post prandial so at least 4 reading of blood sugar is taken in
addition to measurement of HbA1c which give idea about control in the
previous 3 months.
The patient should be educated about signs & symptoms of hypoglycemia
which is more common in IDDM than NIDDM and it is more common in
the 1
st
trimester before 10-15 weeks GA as blood sugar should be less
than 3.5 mmol/l& should be treated by either oral dextrose( either 4
teaspoon full of sugar or 1/2 can of juice or 3 glucose tablets if the patient
is conscious but if unconscious give injectable glucagon (I.M or
S.C)(50gm or 1ml) or give 150ml of 10% of GW& if not response you
can repeat the process with decrease insulin dose .
Also the woman should be alert about signs &symptoms of ketoacidosis
which has high PNM reach to 30%. It diagnosed when blood sugar
>12mmol/l, PH<7.3, with ketone urea. Can be triggered with insufficient
insulin dose , infection and stress, and associated with CTG
abnormalities. So should be treated by giving insulin on sliding scale and
CTG monitoring. Diabetic ketoacidosis DKA : occur when there is
insuffient insulin to metabolite glucose as in 1. Missed insulin dose
2.infection, steroid, stress 3. Defined when blood sugar>12mmol/l,

PH<7.3, +ve ketone urea, poor maternal &fetal outcome , CTG
abnormality.
Treatment : 1. Treatment of underlying cause 2. Give insulin on sliding
scale 3. Continuous CTG 4. Control of hyperglycemia 5. Decide whether
to deliver the patient or not after stabilization .
Fetal management:
1. dating U\S should be done
2. anomaly scan at 20-22 weeks & fetal echo.
3. regular U\S for fetal growth& AFI to identify macrosomia &
polyhydramnia respectively.
4. close fetal surveillance during third trimester because of high incidence
of still birth. Also fellow up by kick count ,NST, umbilical artery Doppler
are recommended.
Management of labor & delivery:
If diabetic control is optimum & there is no fetal or maternal
complication ,induction of labor at 38-39 weeks is done . the mode of
delivery is by NVD& C\S is done for obstetric indication however the
rate may reach to 50% because of fetal macrosomia & failure of induction
so when fetal weight is > 4.5 kg, elective C\S is done.
Insulin requirement fluctuate considerably during labor & fall quickly
after delivery so continuous I.V infusion of glucose & insulin is
recommended, prolonged labor is avoided.
So at day of induction advice for 1. normal diet
2.no overnight fasting & normal dose of insulin.
3. at day of induction give half morning dose of insulin& commence I.V
infusion of5% or 10% dextrose. Insulin infusion given through infusion
pump.
4. measure capillary blood glucose every 30-60 minutes to maintain
blood sugar between 4.0-7.0 mmol\L
5. continuous CTG.
6. using PG pessary for cervical ripening, then do ARM & oxytocin
augmentation when indicated.
During C\S: 1.woman fasting from 12 midnight
2.omit morning dose of insulin
3. give 5% dextrose & insulin infusion during C\S.
4. monitoring blood sugar just like NVD.

Post-partum management:
1. continue with insulin infusion until the patient start eating.
2. re commence the patient to pre-pregnancy insulin dose or 1/3 the initial
dose.
3. patient with NIDDM. Can recommence to their oral hypoglycemic
drugs when start oral intake.
4. monitor blood sugar hourly post-operative & then after each meal for
the next 48 hours.
5. advice the mother about contraceptive like low dose oral contraceptive
pills , progesterone only pills , condom & other barrier methods , if she
complete her family sometime do sterilization.
.سها
وتىت
LEC:2
Gestational Diabetes:
G.D.M.: is defined as a carbohydrate intolerance that begins or first
diagnosed during pregnancy& in most cases resolves after
pregnancy.(therefore woman with previously unrecognized NIDDM or
newly diagnosed IDDM is considered also as G.D.M .
In most cases diagnosis is made in the late second & early third trimester
after screening for various indication.it carry more genetic predisposition
than type one , if the mother affected , 15% of babies will affected and if
the father only affected ,15% of baby will affected and if both parents ,
30% of babies will affected.
Incidence:
It affect about 2-5% of pregnant woman & about 50% of them will
develop overt type 2 D.M in the future.
Screening for G.D.M:
Because G.D.M is associated with adverse effect on pregnancy &
significant no. of patient will develop overt D.M so it is important to
screen for G.D.M.
There are 2 approach for screening: universal screening & selective one.

Selective screening adopted for high risk pregnancy but it will miss 50%
of woman with G.D.M.
HIGH RISK GROUP ARE:
1. glucose urea on one occasion in the first trim. & 2 occasion in the
second trim.
2. polyhydramnia in current pregnancy
3. macrosomia (abdominal circumference >95th centile) in current
pregnancy.
4. history of big baby > 4.0 kg.
5. previous unexplained still birth
6. positive family history of D.M( first degree relative)
7. obesity BMI > 25
8. old age >35 years
9. Asian race 10.previous history of G.D.M 11. history of recurrent
abortion. 12.patient with PCOS.
Timing of oral glucose tolerance test(OGTT) is between 26-28 weeks of
gestation because insulin resistance at its maximum value& there is
variable absorption of glucose from GIT after this gestation.
Other method for screening is FBS, RBS, but those are not sensitive tests.
So if blood sugar level within 2 hour is >7mmol\
L or FBS is >4.8 mmol\L then do full GTT.
Other screening test is mini GTT & blood sugar >7.8 mmo\l so do full
GTT.
OGTT
mini GTT
We use 75 mg of oral glucose
50 gm. is used in mini type
Overnight fasting
no fasting
Associated with N & vomiting
less N& V
Measure blood sugar at 1\2 hour, 1
hour ,2 hour so more accurate
mini GTT measure blood sugar at
1 hour
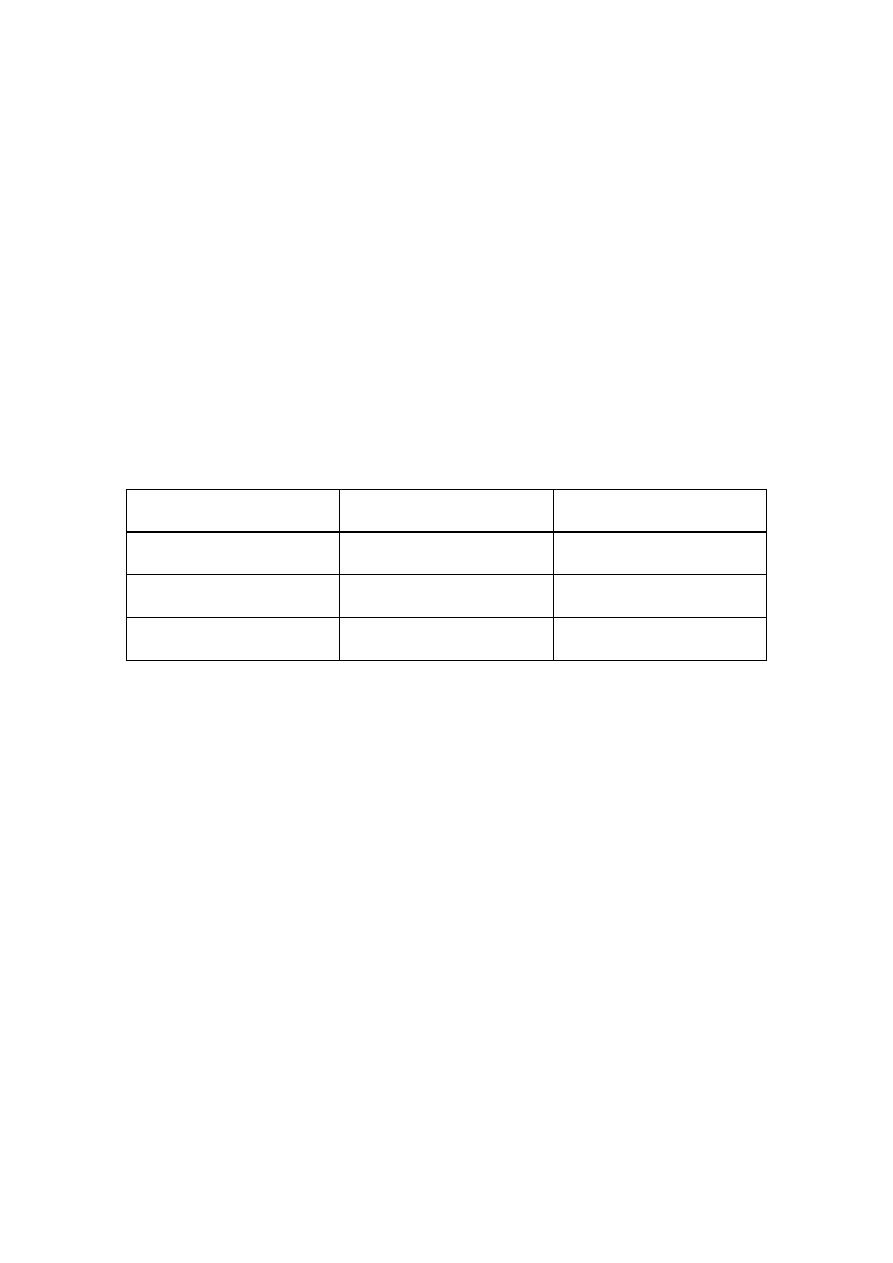
Venous blood sample is better than capillary blood for fallow up the
patient blood sugar because capillary blood sugar is higher after meal
than venous blood.
Diagnosis of G.D.M:
The diagnosis is usually depend on OGTT ( using 75 gm. glucose )
However many units in USA still used 100 gm. loading test.
The WHO recommended criteria for diagnosis are:
1.FBS equal or >5.5 mmol\L
2. 2 hour post prandial equal or >8.0 mmol\L.
Or WHO criteria for OGTT as following:
Fasting blood sugar
2 hour post prandial
Normal OGTT
<7.0 mmol\l
<7.8 mmol\l
Impaired OGTT
<7.0mmol\l
>7.8 mmol\l
Overt G.D.M
>7.0 mmol\l
>11.1 mmol\l
Glycosylated HbA1c has no role in the diagnosis of G.D.M but useful in
the screening.
Complication of G.D.M:
In poorly controlled diabetes ,the PNM is 4 times higher than controlled
one.
1. maternal complication:
1. PET& PIH.
2. recurrent vulvovaginitis &candidacies
3. obstructed labor
4. increase incidence of operative deliveries( forcipes, ventose &C\S)
5. D.M in the future.
2. fetal complication:

a. macrosomia
b. polyhydramnia
c. RDS
d. Preterm labor
e. Unexplained IUD
f. Traumatic delivery
g. Neonatal complication like jaundice ,polycythemia , tetany,
hypoglycemia , hypocalcaemia ,hypomagnesaemia.
Management:
1. monitoring of glycemic control & this done by measuring pre & post
prandial blood sugar at least 4-7 times \day.
2. fetal monitoring :it is generally accepted that antenatal fetal monitoring
to minimize the risk of adverse outcome is essential in the following
groups:
a. poorly controlled D.M
b. D.M on insulin
c. Woman with history of previous still birth
d. Woman with history of chronic hypertension& PIH
Monitoring of fetal wellbeing is done by different approach: 1. regular
U\S
2.biophysical profile
3.NST
4. Doppler velocimetry
3. glucose control:
The aim is to maintain normoglycemia &this achieved by :
1. dietary control
2. insulin therapy
3. Oral hypoglycemic drugs
1. dietary control:
This is best achieved by counseling of dietitian & patient is generally
advice to consume 50% or more of their energy from carbohydrates, 30%
of protein & less than 20% from fat.
As the dietary restriction is advised to be 30-35%Kcal\kg\day or equal
to2000-2400 kcal\day.
During pregnancy ,there is accelerated starvation , therefore at least three
meals & 4 snacks are given with the last snack at bed time to avoid the
risk of overnight hypoglycemia.
2.insulin therapy:

Is indicated in a. FBS>5.8 mmol\l
b. 2 hour post prandial >7.8 mmol\l
c. Fetal macrosomia at third trim. Diagnosed by presence of abdominal
circumference of > 95centile at 29-33 weeks of gestation.
d. Failed dietary treatment : failure of control blood glucose after 2 weeks
of diet ,indicate switch to insulin.
3.oral hypoglycemic drugs:
previously it was not recommended during pregnancy because they
1. are teratogenic
2. cause fetal hypoglycemia
3. it is difficult to achieve tight control with these agents but now it is
used. but nowadays it is widely used and the main 2 drugs used are 1.
Metformin (Biguinide group) 2. Clibenclimide (sulfonurea group)which
is less pass through the placenta because of high molecular weight . a lot
of studies compare between insulin. Metformin & Clibenclimide, they
found no differences in term of congenital anomalies and fetal
hypoglycemia. Current NICE guideline considered that metformin is an
alternative to insulin in type 2 DM.
4. delivery:
There is contraversal about the time of delivery in patient with G.D.M but
when there is good glycemic control& uncomplicated maternal & fetal
condition, delivery is done at 38-39 weeks& because of the risk of RDS
,the use of steroid is considered in all deliveries before 36-37weeks.
Usually NVD is the mode of delivery & but fetal macrosomia & risk of
shoulder dystocia lead to the increase rate of C\S to 50%. So C\S is
indicated in
1. fetal weight > 4.5kg
2. fetal weight between 4-4.5 kg but there is other risk factor like past
obstetric history, failure of progress of labor.
Note:
Management of labor , delivery & when doing C\S should be the same as
patient with pre-existing D.M .
Puerperium:
Insulin requirement after delivery usually return to pre-pregnancy level
after the placenta has been delivered so after delivery ,the woman :
1. on diet :stop diet restriction
2. on insulin: must reduce their insulin requirement & insulin stopped
once patient start oral intake.
3. contraceptive can be used :a. low dose o.cc.p for woman non lactating.

b. progesterone only pills
c. barrier
d. rarely IUCD) for lactating one.
Long term follow up:
Because about 50% of them will develop type 2 D.M later in life ,so
screening with full OGTT after 6 weeks ( ie: after Puerperium ) is
required.
Oral hypoglycemic drugs:
Since GDM and Type 2 diabetes are characterized by
insulin resistance and relatively decreased insulin secretion, a treatment with oral
hypoglycemic agents could be of potential interest. The use of oral agents is a
pragmatic alternative to insulin therapy in pregnancy because of easy administration
and patient's satisfaction due to a noninvasive treatment. sulfonylureas are currently
the only drugs to be studied in GDM women as that glyburide does not cross the
placenta in a significant amount (4% ex vivo). In mothers treated with therapeutic
doses of glyburide, the drug was undetectable in the cord blood of their neonates.
Since glyburide has been demonstrated to have minimal transfer across the human
placenta, it cannot determine neonatal hypoglycemia or fetal anomalies. In addition,
most GDM patients are identified between 24 and 28 weeks' gestation and the fetus
could be exposed to the drug during organogenesis. Glyburide is not present in the
milk of lactating mothers when measured in vivo and in vitro
1.Glyburide increases insulin secretion, and it decreases insulin resistance by reducing
glucose toxicity. Its onset of action is approximately 4 h, and its duration of action is
approximately 10 h. The starting dose is 2.5 mg orally in the morning. If the targeted
level of glycemic is not reached, 2.5 mg could be added to the morning dose. If
indicated (after 3-7 days), a further 5 mg could be added in the evening. Thereafter,
the dose could be increased by 5 mg to a total of 20 mg/day. If patients fail to achieve
glycemic objectives, long-acting insulin can be added to the regimen. its success is
comparable to insulin therapy in term of control of blood sugar, , incidence of
macrosomia & C/S& PN Mortality
2. Glucophage (metformin) : its widely used in the treatment of poly cystic ovarian
syndrome as it decrease insulin resistance & increase sensitivity to insulin. Previously
it was unsafe during pregnancy as its associated with congenital anomalies in about
1.7% which was similar to that of general population . it act by :1. Decrease glucose
level by decrease peripheral insulin resistance 2. Decrease hepatic production &
intestinal absorption of glucose . 3. Decrease peripheral uptake of glucose by the cells
. it should be taken within the meal to decrease GIT intolerance . peak of action 4
hour. SE : diarrhea , gastritis
Doses 500-850 mg up to2000mg/day. Not cause hypoglycemia as it not cause
increase insulin secretion.
GOOD LUCK
