Graves Disease
Graves disease is the most common cause of endogenous hyperthyroidism. It is
characterized by a triad of clinical findings:
1.Hyperthyroidism owing to hyperfunctional, diffuse enlargement of the thyroid.
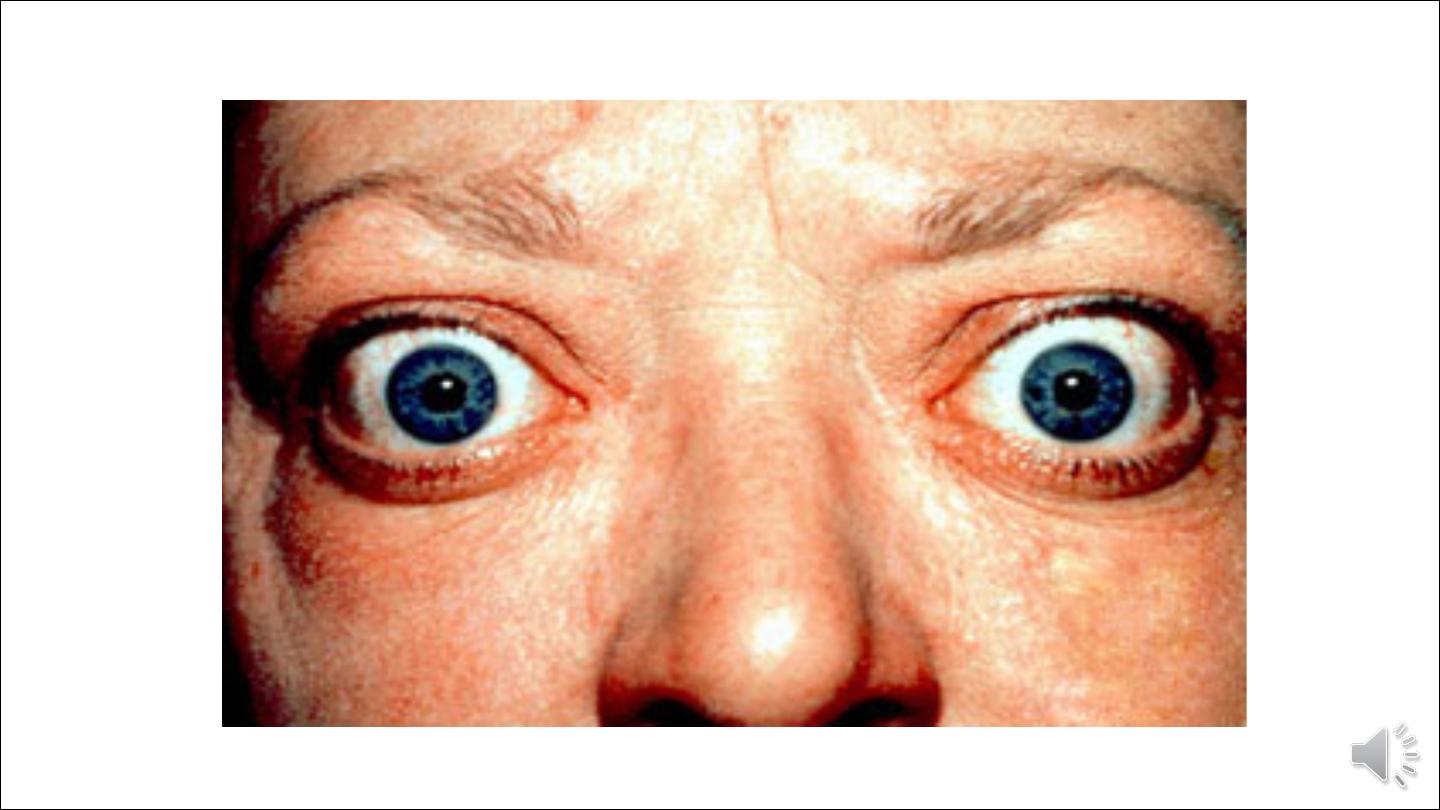
2.Infiltrative ophthalmopathy with resultant exophthalmos.
3.Localized, infiltrative dermopathy, sometimes called pretibial myxedema, which
is present in a minority of patients.
Lecture 2
Dr Afraa Mamoori

Aetiology:
Graves disease has a peak incidence between the ages of 20 and 40, women being affected up to seven
times more frequently than men. Genetic factors are important in the aetiology of Graves disease.
An increased incidence of Graves disease occurs among family members of affected patients, and the
concordance rate in monozygotic twins is as high as 60%. A genetic susceptibility to Graves disease
associated with the presence of certain major histocompatibility haplotypes, specifically HLA-B8 and
-DR3. Polymorphisms in the cytotoxic T-lymphocyte-associated-4 (CTLA-4) gene are also linked to
Graves disease. Recall that the HLA proteins are a critical component of antigen presentation to T
cells, while CTLA-4 is an inhibitory receptor that prevents T-cell responses to self-antigens
.

Pathogenesis:
Graves disease is an autoimmune disorder in which a variety of antibodies may be present in the
serum, including antibodies to the TSH receptor, thyroid peroxisomes, and thyroglobulin. Of
these, autoantibodies to the TSH receptor are central to disease pathogenesis, although the
specific effects of the antibodies vary depending on which TSH receptor epitope they are directed
against:
• Thyroid-stimulating immunoglobulin (TSI): TSI directed against the TSH receptor. Almost all
patients with Graves disease have detectable levels of this autoantibody to the TSH receptor. TSI is
relatively specific for Graves disease, in contrast to thyroglobulin and thyroid peroxidase antibodies.
• Thyroid growth-stimulating immunoglobulins (TGI): Also directed against the TSH receptor, thyroid
growth-stimulating immunoglobulins have been implicated in the proliferation of thyroid follicular
epithelium.

• TSH-binding inhibitor immunoglobulins (TBII): These anti-TSH receptor antibodies
prevent TSH from binding normally to its receptor on thyroid epithelial cells. In so doing,
some forms of TSH-binding inhibitor immunoglobulins mimic the action of TSH, resulting in
the stimulation of thyroid epithelial cell activity, whereas other forms may actually inhibit
thyroid cell function.
.
It is not unusual to find the coexistence of stimulating and inhibiting immunoglobulins in the
serum of the same patient, a finding that could explain why some patients with Graves disease
spontaneously develop episodes of hypothyroidism.

Pathogenesis of ophthamopathy associated with gravis disease:
A T cell-mediated autoimmune phenomenon plays a role in the development of the infiltrative
ophthalmopathy that is characteristic of Graves disease. In Graves ophthalmopathy, the volume of the
retro-orbital connective tissues and extraocular muscles is increased owing to several causes,
including
(1) marked infiltration of the retro-orbital space by mononuclear cells, predominantly T cells.
(2) inflammatory edema and swelling of extraocular muscles.
(3) accumulation of extracellular matrix components.
(4) increased numbers of adipocytes (fatty infiltration).

Morphology:
Gross features
The thyroid gland is usually symmetrically enlarged because of diffuse hypertrophy and hyperplasia of
thyroid follicular epithelial cells. Increases in weight to over 80 gm are not uncommon. The gland is usually
smooth and soft, and its capsule is intact.
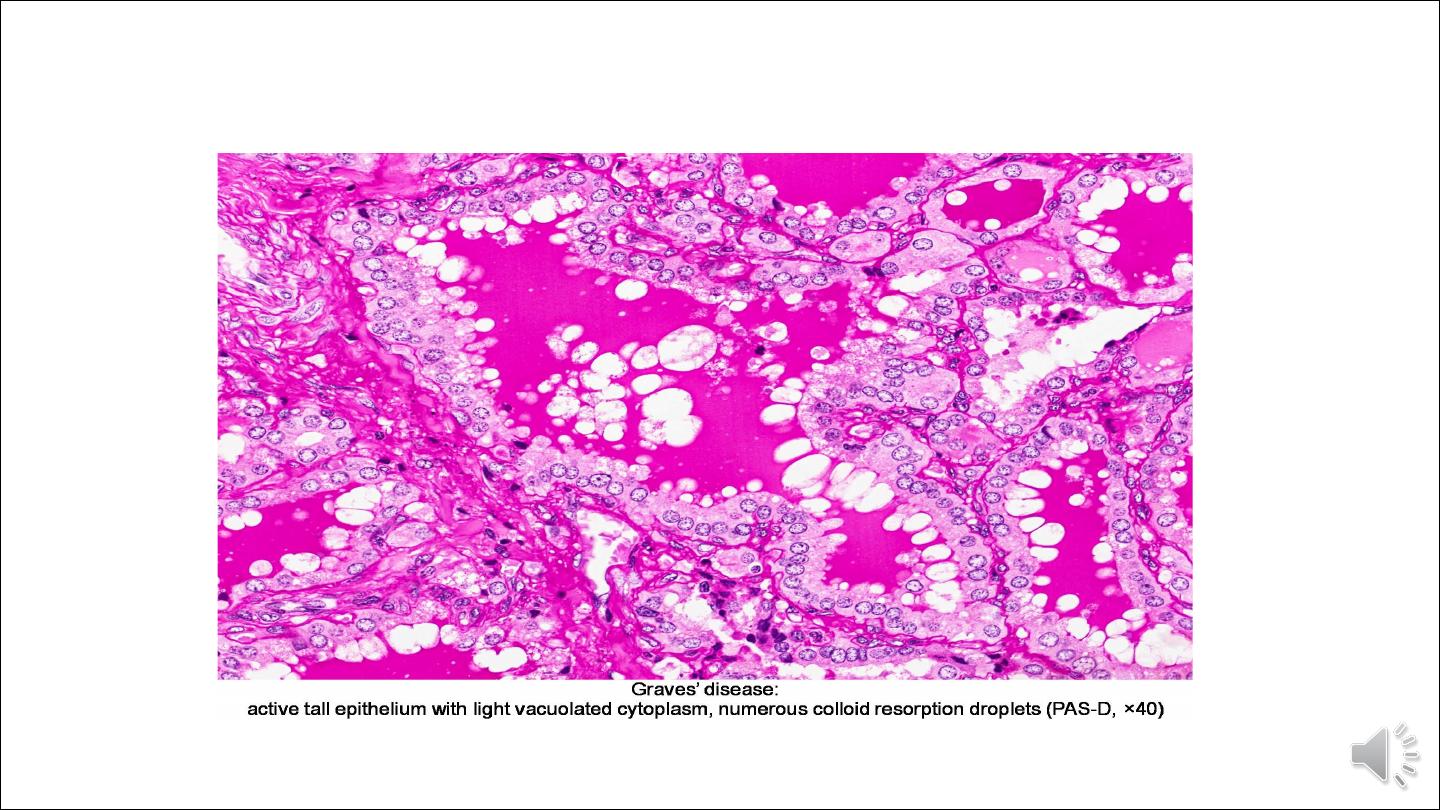
Histologically
The dominant feature is too many cells. The follicular epithelial cells in untreated cases are tall and more
crowded than usual. This crowding often results in the formation of small papillae, which project into the
follicular lumen, sometimes filling the follicles. Such papillae lack fibrovascular cores, in contrast to those
of papillary carcinoma. The colloid within the follicular lumen is pale, with scalloped margins. Lymphoid
infiltrates, consisting predominantly of T cells, with fewer B cells and mature plasma cells, are present
throughout the interstitium; germinal centers are common.

Laboratory findings in Graves disease
Laboratory findings in Graves disease include elevated free T
4
and T
3
levels and depressed TSH levels.
Because of ongoing stimulation of the thyroid follicles by thyroid-stimulating immunoglobulins,
radioactive iodine uptake is increased, and radioiodine scans show a diffuse uptake of iodine.

Neoplasms of the Thyroid
Benign neoplasms outnumber thyroid carcinomas by a ratio of nearly 10:1. Carcinomas of the thyroid are
thus uncommon, accounting for under 1% of solitary thyroid nodules. Several clinical criteria might
provide a clue to the nature of a given thyroid nodule:
• Solitary nodules, in general, are more likely to be neoplastic than are multiple nodules.
• Nodules in younger patients are more likely to be neoplastic than are those in older patients.
• Nodules in males are more likely to be neoplastic than are those in females.
• A history of radiation treatment to the head and neck region is associated with an increased incidence of thyroid
malignancy.
• Nodules that take up radioactive iodine in imaging studies (hot nodules) are more likely to be benign
than malignant

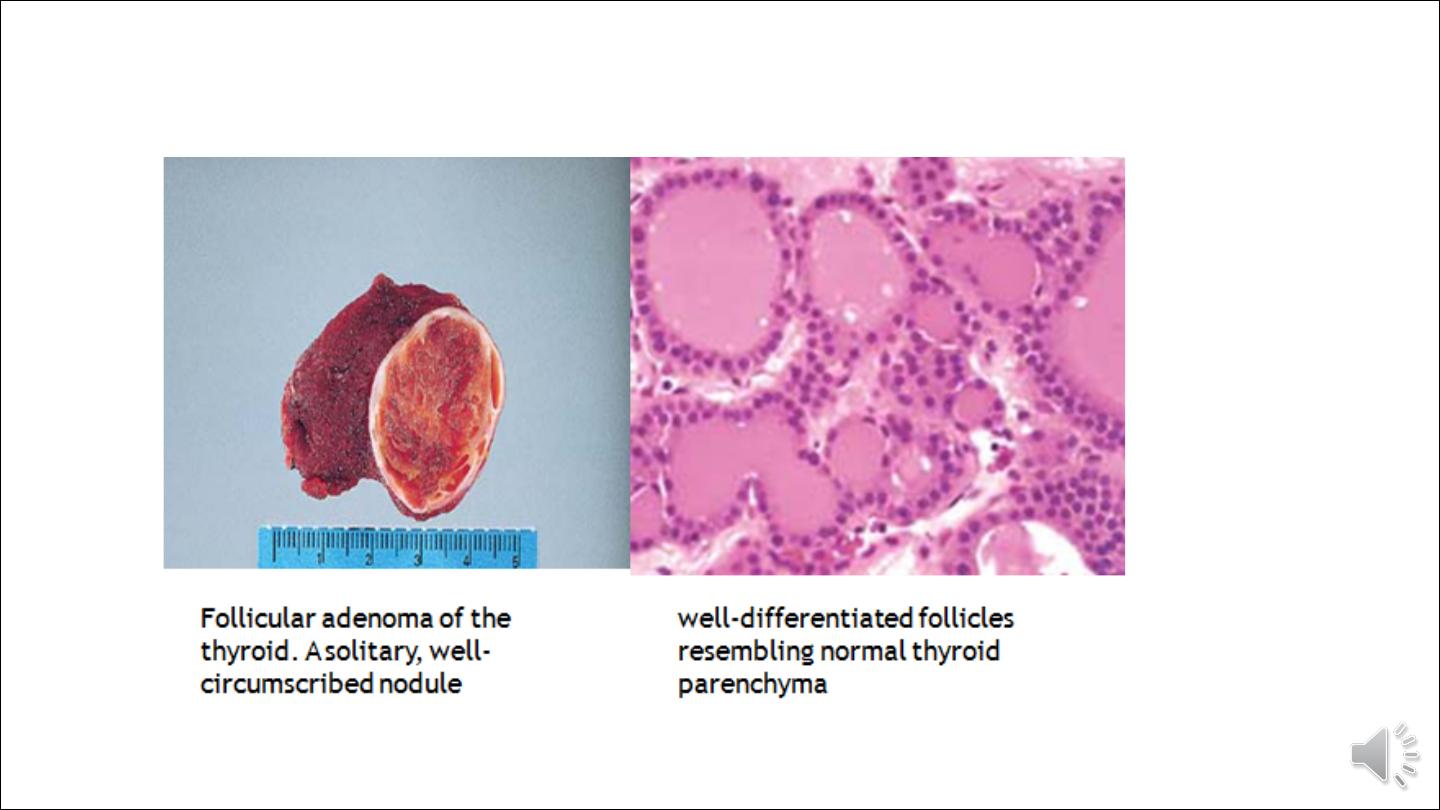
ADENOMAS
Adenomas of the thyroid are typically discrete, solitary masses. With rare exception, they are derived from follicular
epithelium and so might all be called follicular adenomas. A variety of terms have been proposed for classifying
adenomas on the basis of degree of follicle formation and the colloid content of the follicles. Simple colloid
adenomas (macrofollicular adenomas), a common form, resemble normal thyroid tissue; others recapitulate stages in
the embryogenesis of the normal thyroid (fetal or microfollicular, embryonal or trabecular). There is limited utility
in these classifications because mixed patterns are common, and most of these benign tumors are non functional.
Clinically, follicular adenomas can be difficult to distinguish from dominant nodules of follicular hyperplasia or
from the less common follicular carcinomas. Numerous studies have made it clear that adenomas are not
forerunners of cancer except in rare instances. Although the vast majority of adenomas are non functional, a
small proportion produces thyroid hormones and cause clinically apparent thyrotoxicosis.

Pathogenesis:
The TSH receptor signaling pathway plays an important role in the pathogenesis of toxic adenomas.
Activating ("gain of function") somatic mutations in TSH receptor itself cause chronic
overproduction of cAMP, generating cells that acquire a growth advantage. This results in clonal
expansion of follicular epithelial cells that can autonomously produce thyroid hormone and cause
symptoms of thyroid excess.

Morphology.
Gross features
The typical thyroid adenoma is a solitary, spherical, encapsulated lesion that is well demarcated from
the surrounding thyroid parenchyma. Follicular adenomas average about 3 cm in diameter, but some
are smaller and others are much larger (up to 10 cm in diameter). The color ranges from gray-white to
red-brown, depending on the cellularity of the adenoma and its colloid content. Areas of haemorrhage,
fibrosis, calcification, and cystic change, are common in follicular adenomas, particularly within larger
lesions.

Microscopically
The constituent cells often form uniform-appearing follicles that contain colloid. The follicular growth
pattern within the adenoma is usually quite distinct from the adjacent non-neoplastic thyroid. The
epithelial cells composing the follicular adenoma reveal little variation in cell and nuclear morphology.
Mitotic figures are rare, and extensive mitotic activity warrants careful examination of the capsule
to exclude follicular carcinoma. Similarly, papillary change is not a typical feature of adenomas
and, if extensive, should raise the suspicion of an encapsulated papillary carcinoma. Occasionally,
the neoplastic cells acquire brightly eosinophilic granular cytoplasm (oxyphil or Hürthle cell change;
the clinical presentation and behavior of a follicular adenoma with oxyphilia (Hürthle cell adenoma) is
no different from that of a conventional adenoma.
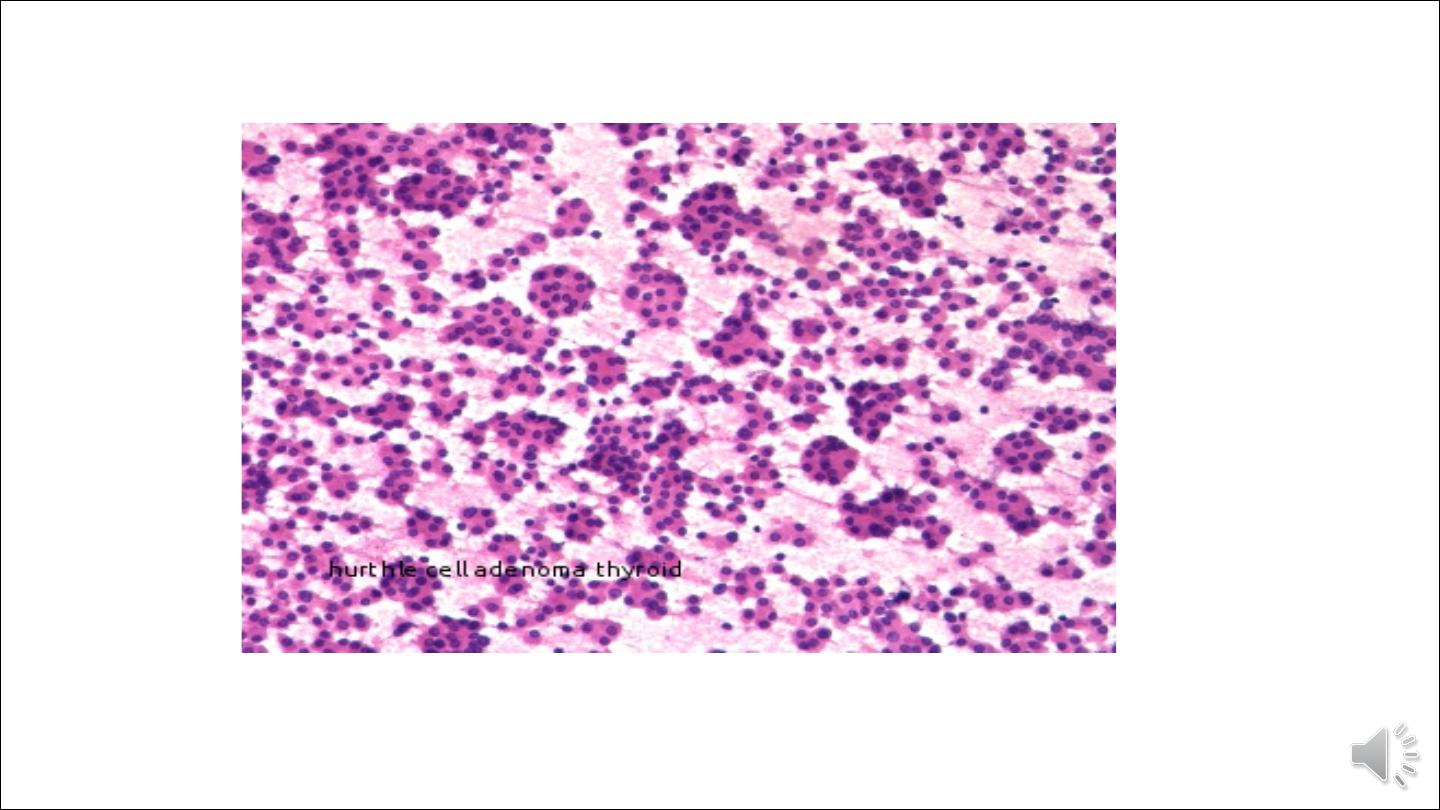
Hurthle cell adenoma of thyroid gland

OTHER BENIGN TUMORS
Solitary nodules of the thyroid gland may also prove to be cysts. Most of these lesions represent cystic
degeneration of a follicular adenoma. They are often filled with a brown, turbid fluid containing blood,
hemosiderin pigment, and cell debris. Additional benign rarities include dermoid cysts, lipomas,
hemangiomas, and teratomas (seen mainly in infants).

CARCINOMAS
Carcinomas of the thyroid are relatively uncommon. Most cases occur in adults, although some forms,
particularly papillary carcinomas, may present in childhood. A female predominance has been noted among
patients who develop thyroid carcinoma in the early and middle adult years, perhaps related to the expression of
estrogen receptors on neoplastic thyroid epithelium. In contrast, cases presenting in childhood and late adult life
are distributed equally among males and females. Most thyroid carcinomas are well-differentiated lesions. The
major subtypes of thyroid carcinoma and their relative frequencies include the following:
• Papillary carcinoma (75% to 85% of cases)
• Follicular carcinoma (10% to 20% of cases)
• Medullary carcinoma (5% of cases)
• Anaplastic carcinoma (<5% of cases)
Most thyroid carcinomas are derived from the follicular epithelium, except for medullary carcinomas; the latter
are derived from the parafollicular or C cells.

Pathogenesis
Genetic and environmental factors, implicated in the pathogenesis of thyroid cancers. The major risk
factor predisposing to thyroid cancer is exposure to ionizing radiation, particularly during the first two
decades of life. Genetic factors are important in both familial and non familial ("sporadic") forms of
thyroid cancer. Familial medullary cancers account for most inherited cases of thyroid cancer.
Familial non medullary thyroid cancers (papillary and follicular variants) are very rare. Distinct genes are
involved in the histologic variants of thyroid cancer as fallow:

• Approximately half of follicular thyroid carcinomas harbor mutations in the RAS family of oncogenes
(HRAS, NRAS, and KRAS), NRAS mutations being the most common.
• Like follicular thyroid carcinomas, papillary carcinomas also appear to arise by multiple distinct, non
overlapping molecular pathways. One pathway involves rearrangements of the tyrosine kinase receptors
RET or NTRK1 (neurotrophic tyrosine kinase receptor 1) and another involves activating mutations in the
BRAF oncogene. A third pathway involves RAS mutations (10% to 20% of papillary carcinomas).
• Familial medullary thyroid carcinomas occur in multiple endocrine neoplasia type 2 (MEN-2) and are
associated with germ-line RET protooncogene mutations. RET mutations are detectable in approximately
95% of families with MEN-2. RET mutations are also seen in non familial (sporadic) medullary thyroid
cancers
• Inactivating point mutations in the p53 tumor suppressor gene are common in anaplastic thyroid
carcinomas.

Papillary Carcinoma
Papillary carcinomas are the most common form of thyroid cancer. They occur at any age but most often in the
twenties to forties, and account for the majority of thyroid carcinomas associated with previous exposure to
ionizing radiation. Most papillary carcinomas present as asymptomatic thyroid nodules, but the first
manifestation may be a mass in a cervical lymph node. The carcinoma, which is usually a single nodule,
moves freely during swallowing and is not distinguishable from a benign nodule. Hoarseness, dysphagia,
cough, or dyspnea suggests advanced disease. In a minority of patients, hematogenous metastases are
present at the time of diagnosis, most commonly in the lung. Papillary thyroid cancers have an excellent
prognosis, with a 10-year survival rate in excess of 95%.

Morphology
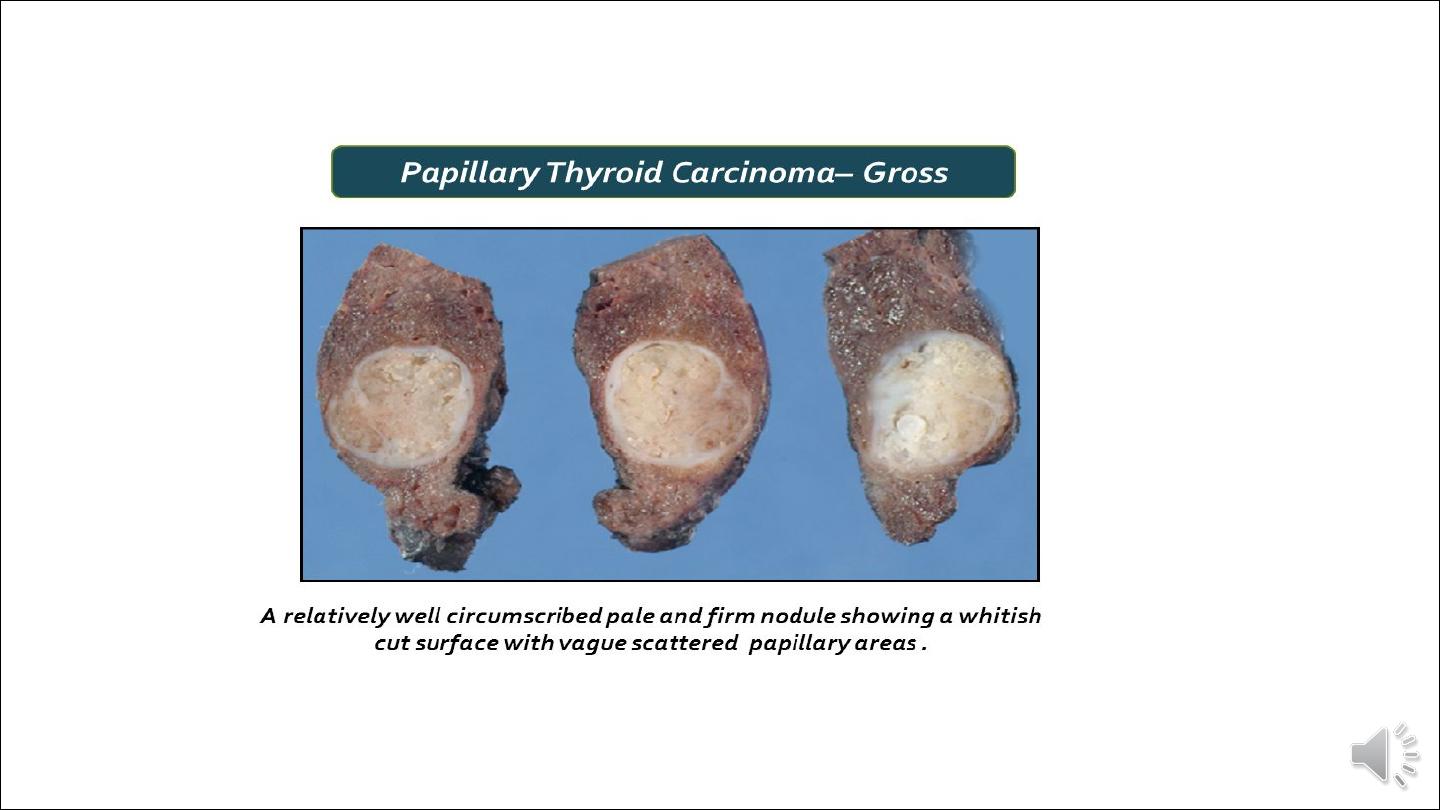
Gross appearance:
Papillary carcinomas are solitary or multifocal lesions. Some tumors may be well-circumscribed
and even encapsulated; others may infiltrate the adjacent parenchyma with ill-defined margins. The
lesions may contain areas of fibrosis and calcification and are often cystic. The definitive
diagnosis of papillary carcinoma can be made only after microscopic examination.

The characteristic hallmarks of papillary neoplasms include the following microscopic
features:
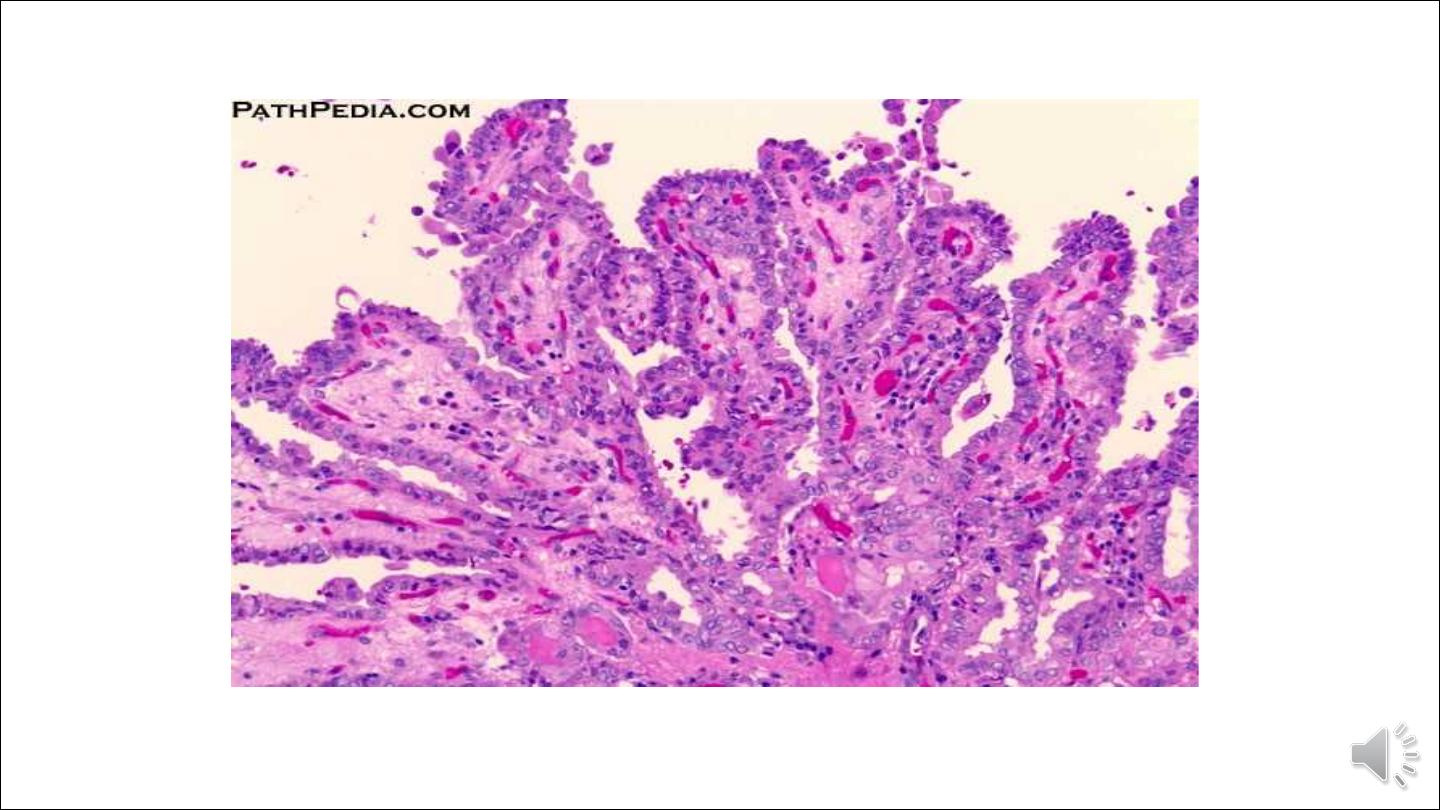
• Papillary carcinomas can contain branching papillae having a fibrovascular stalk covered by a single to multiple layers
of cuboidal epithelial cells. In most neoplasms, the epithelium covering the papillae consists of well-differentiated,
uniform, orderly, cuboidal cells.
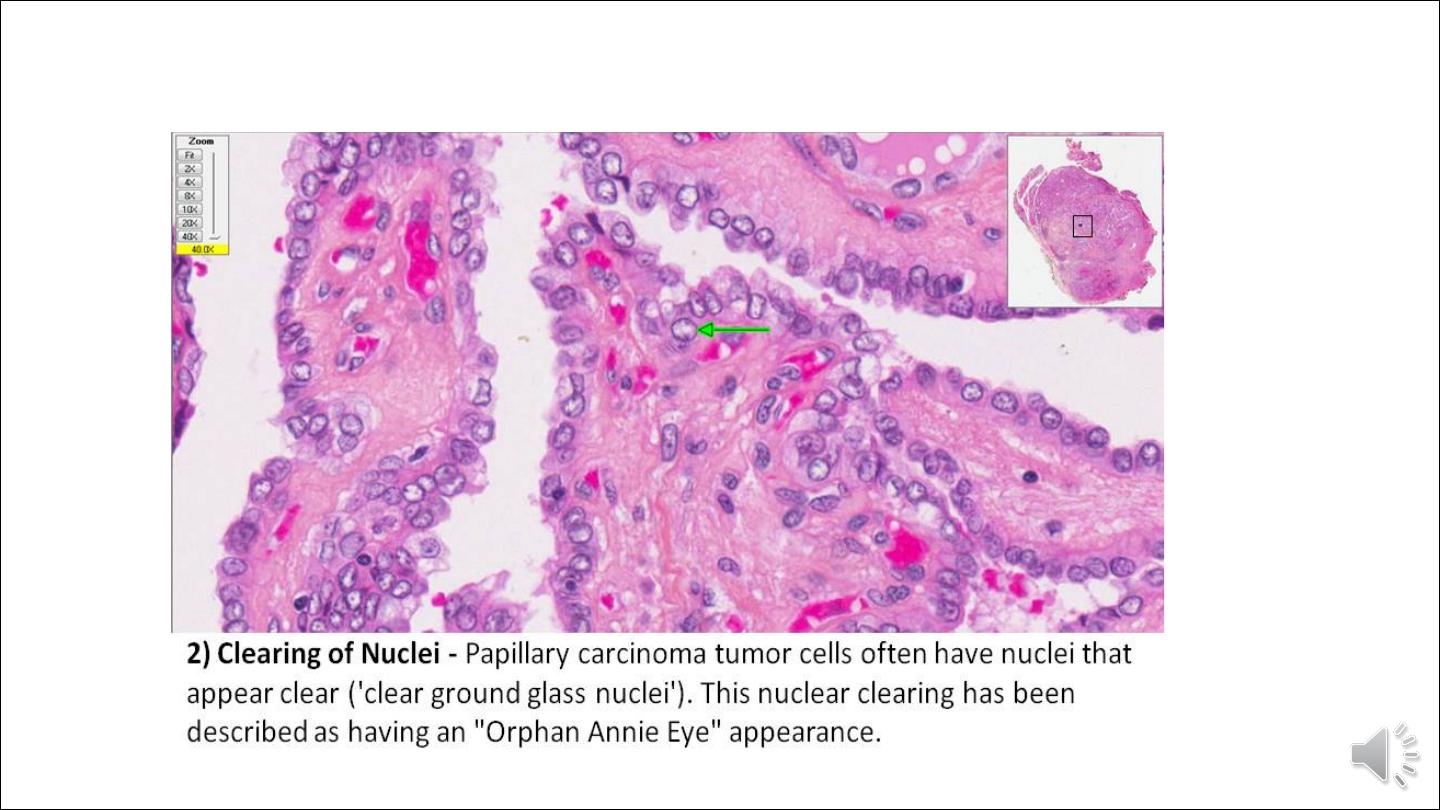
• The nuclei of papillary carcinoma cells contain finely dispersed chromatin, which imparts an optically clear or empty
appearance, giving rise to the designation ground glass or Orphan Annie eye nuclei. In addition, invaginations of the
cytoplasm may in cross-sections give the appearance of intranuclear inclusions ("pseudo-inclusions")
• Calcified structures termed psammoma bodies are often present within the lesion, usually within the cores of papillae.
These structures are almost never found in follicular and medullary carcinomas, and so, when present, they are a
strong indication that the lesion is a papillary carcinoma.
• Foci of lymphatic invasion by tumor are often present, but involvement of blood vessels is relatively uncommon.
Metastases to adjacent cervical lymph nodes are estimated to occur in up to half the cases.
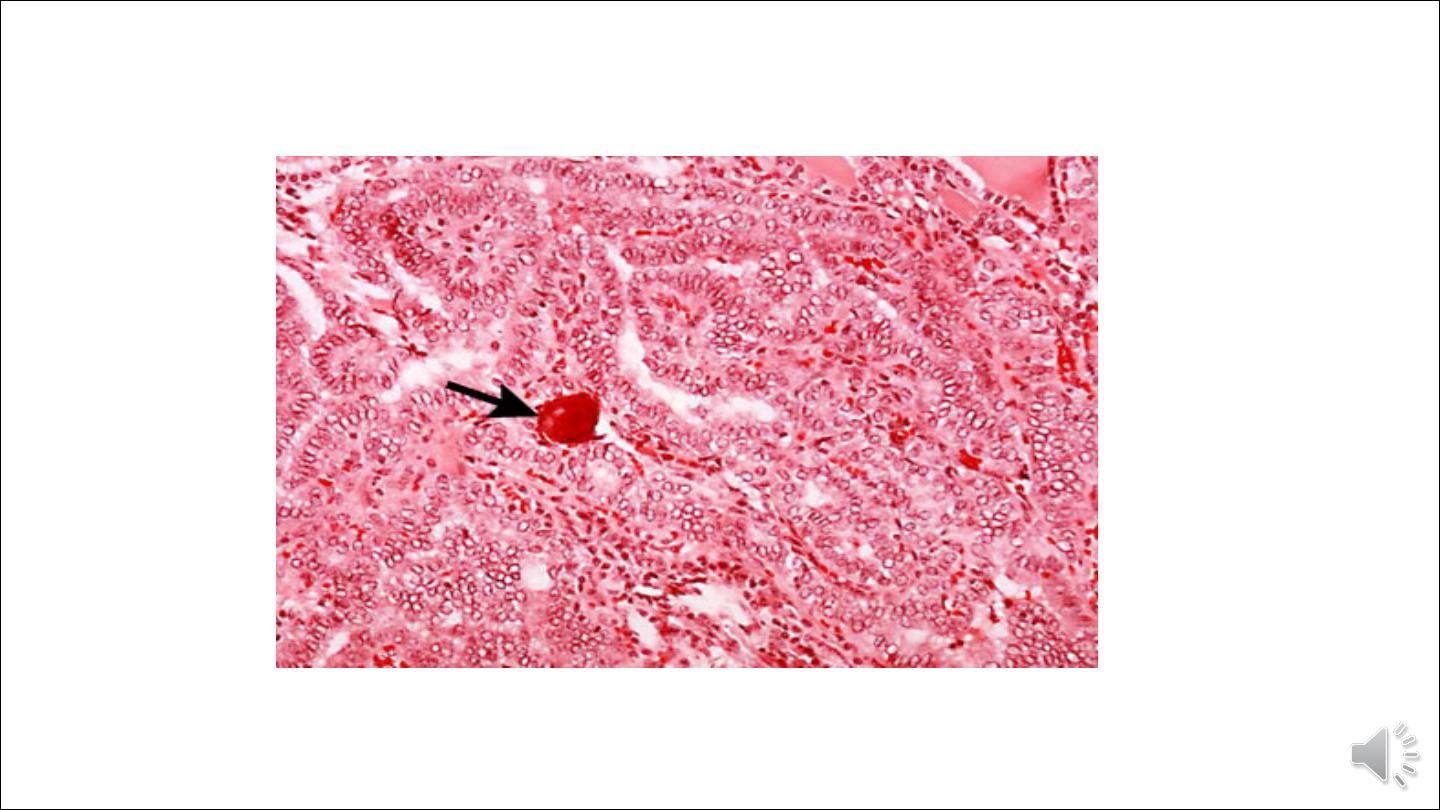
Histopathology of papillary carcinoma, thyroid: a psammoma body is visible (arrow)
Image Library/Dr Edwin P. Ewing,
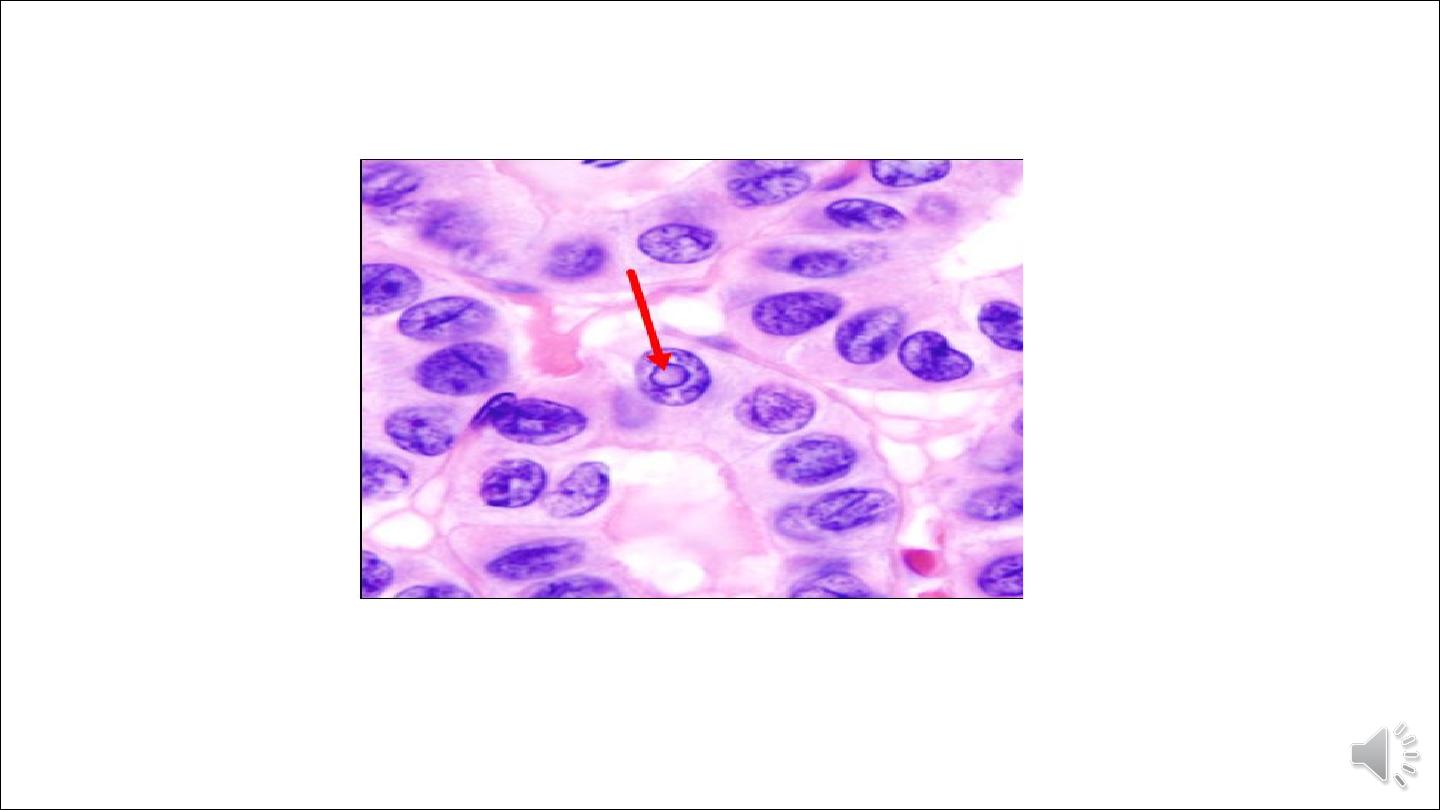
Intra nuclear inclusion

Variant forms of papillary carcinoma:
1. encapsulated variant
2. follicular variant
3. tall cell variant
4. diffuse sclerosing variant
5. Hyalinizing trabecular tumors

Follicular Carcinoma
Follicular carcinomas are the second most common form of thyroid cancer, accounting for 10% to 20% of all thyroid
cancers. They tend to present in women, and at an older age than do papillary carcinomas, with a peak incidence in the
forties and fifties. The incidence of follicular carcinoma is increased in areas of dietary iodine deficiency. Follicular
carcinomas present as slowly enlarging painless nodules. Follicular carcinomas have little propensity for invading
lymphatics; therefore, regional lymph nodes are rarely involved, but vascular invasion is common, with spread to
bone, lungs, liver, and elsewhere. The prognosis is largely dependent on the extent of invasion and stage at presentation.

Morphology.
Gross appearance:
Follicular carcinomas are single nodules that may be well circumscribed or widely infiltrative.
Sharply demarcated lesions may be exceedingly difficult to distinguish from follicular adenomas by
gross examination. Larger lesions may penetrate the capsule and infiltrate well beyond the thyroid
capsule into the adjacent neck. They are gray to tan to pink on cut section and, on occasion, are
somewhat translucent when large. Degenerative changes, such as central fibrosis and foci of
calcification, are sometimes present.

Microscopically:
Most follicular carcinomas are composed of fairly uniform cells forming small follicles containing colloid, quite
reminiscent of normal thyroid. In other cases, follicular differentiation may be less apparent, and there may be nests or
sheets of cells without colloid. Occasional tumors are dominated by cells with abundant granular, eosinophilic cytoplasm
(Hürthle cells). Whatever the pattern, the nuclei lack the features typical of papillary carcinoma, and psammoma
bodies are not present. Unlike in papillary cancers, lymphatic spread is distinctly uncommon in follicular cancers. In
contrast to minimally invasive follicular cancers, extensive invasion of adjacent thyroid parenchyma or extra thyroidal
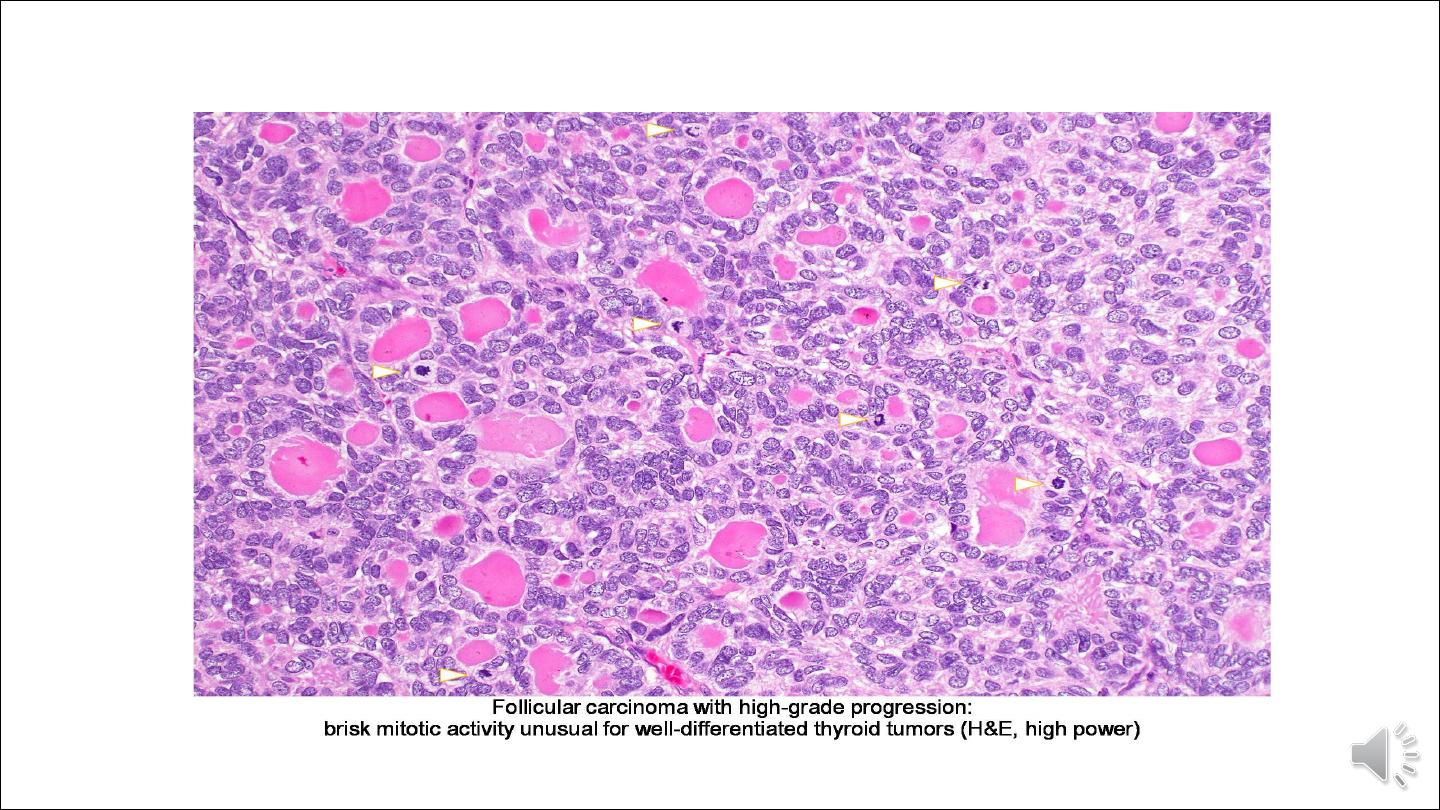
tissues makes the diagnosis of carcinoma obvious in widely invasive follicular carcinomas. Histologically, these cancers
tend to have a greater proportion of solid or trabecular growth pattern, less evidence of follicular differentiation, and
increased mitotic activity.