
Diarrhea
Epidemiology of Childhood Diarrhea
Diarrheal disorders in childhood account for a large proportion (18%) of childhood
deaths, with an estimated 1.5 million deaths per year globally, making it the second most
common cause of child deaths worldwide. The World Health Organization (WHO) and
UNICEF estimate that almost 2.5 billion episodes of diarrhea occur annually in children
<5 yr of age in developing countries, with more than 80% of the episodes occurring in
Africa and South Asia (46% and 38%, respectively)
The decline in diarrheal mortality, despite the lack of significant changes in incidence, is
the result of preventive rotavirus vaccination and improved case management of diarrhea,
as well as improved nutrition of infants and children. These interventions have included
widespread home- and hospital-based oral rehydration therapy and improved nutritional
management of children with diarrhea.
The term diarrheal disorders is more commonly used to denote infectious diarrhea
in public health settings, although several noninfectious causes of GI illness with
vomiting and/or diarrhea are well recognized
Definition:
Diarrhea is defined as increased total daily stool output, its usually associated with
increased stool water content, for infant and children this would result in stool output
greater than 10 gm/kg/24 hours.
Pathophysiology
The basis for all diarrhea is disturbed intestinal solute transport; water movement across
intestinal is passive and is determined by both active and passive fluxes of solute,
particularly sodium, chloride and glucose. The pathogenesis of most episodes of diarrhea
can be explained by secretory, osmotic or motility abnormalities or a combination of
these. Secretory diarrhea is often caused by a secretagogue, such as cholera toxin,
binding to a receptor on the surface epithelium of the bowel and thereby stimulating
intracellular accumulation of cyclic adenosine monophosphate. Diarrhea not associated
with exogenous secretagogue may also have a secretory component ( e.g. congenital
microvillus inclusion disease). Secretory diarrhea tend to be watery and of large volume
and generally persist even when no feeding are given by mouth, it has normal osmolarity
with ion gap less than 100 mosm/kg and no stool leukocytes
Ion gap = stool osmolarity –[{stool Na + stool K}x 2]
E.g. ; cholera, toxigenic E. coli, carcinoid, vip, neuroblastoma, congenital chloride
diarrhea, clostridium difficile, cryptosporidiosis (AIDS)
Osmotic diarrhea occur after ingestion of a poorly absorbed solute. The solute may be one
that is normally not well absorbed (e.g. magnesium, phosphate, lactolose or sorbitol), or
one that is not well absorbed because of a disorder of the small bowel (e.g. lactose with
lactase deficiency or glucose with rotavirus diarrhea), also with laxative abuse this form
of diarrhea is usually of lesser volume than secretory diarrhea and stops with fasting, The
stool is watery, acidic with reducing substances and ion gap is > 100 mosm/kg, stool
osmolality is > 50 mosm, and there is increased breath hydrogen and no stool leukocyte.
Acute diarrhea
causes:
1. common:
Gastroenteritis, systemic infection (parenteral diarrhea), antibiotic associated and food
poisoning in older children.

2. rare:
Primary disaccharidase deficiency, hirschsprung toxic colitis, adrenogenital syndrome,
toxic ingestion and hyper thyroidism.
Gastroenteritis:
Mean infection of the gastrointestinal tract, it is caused by a wide variety of
enteropathogens, including bacteria, viruses and parasites. Gastroenteritis is due to
infection acquired through the feco-oral route or by ingestion of contaminated food or
water. Enteropathogens that are infectious in a small inoculum (Shigella, Escherichia
coli, noroviruses, rotavirus, Giardia lamblia, Cryptosporidium parvum, Entamoeba
histolytica) can be transmitted by person-to-person contact, whereas others such as
cholera are generally a consequence of contamination of food or water supply
. Salmonella, Shigella, and, most notably, the various diarrhea-producing E. coli
organisms are the most common pathogens in developing countries . Clostridium
difficile (by toxin production) is linked to antibiotic-associated diarrhea and
pseudomembranous colitis
PATHOGENESIS OF INFECTIOUS DIARRHEA
Enteropathogens elicit noninflammatory diarrhea through enterotoxin production
by some bacteria, destruction of villus (surface) cells by viruses, adherence by
parasites, and adherence and/or translocation by bacteria. Inflammatory diarrhea is
usually caused by bacteria that directly invade the intestine or produce cytotoxins with
consequent fluid, protein, and cells (erythrocytes, leukocytes) that enter the intestinal
lumen. Generally inflammatory diarrhea is associated with aeromonas, campylobacter
jejuni, clostridium difficile, enteroenvasive E.coli, shigatoxin-producing E.coli,
salmonella, shigella and yersinia. Non inflammatory diarrhea may be caused by
enteropathogenic E.coli, enterotoxigenic E.coli, vibrio cholera. Some enteropathogens
possess more than one virulence property.
RISK FACTORS FOR GASTROENTERITIS
Major risks include environmental contamination and increased exposure to
enteropathogens. Additional risks include young age, immune deficiency, measles,
malnutrition, and lack of exclusive or predominant breast-feeding. Malnutrition
increases severalfold the risk of diarrhea and associated mortality.The risks are
particularly higher with micronutrient malnutrition; in children with vitamin A
deficiency, the risk of dying from diarrhea, measles, and malaria is increased by 20–
24%. Zinc deficiency increases the risk of mortality from diarrhea, pneumonia, and
malaria by 13–21%.\
CLINICAL MANIFESTATION OF DIARRHEA
include: asymptomatic infection, abdominal cramps, vomiting, watery diarrhea,
bloody diarrhea and extra intestinal manifestation of the infection (e.g. hypotonia
from clostridium botulinum, hemolytic anemia from infection with E. coli or
shigella).
Several organisms, including Salmonella, Shigella, Campylobacter jejuni, Yersinia
enterocolitica, enteroinvasive E. coli, and Vibrio parahaemolyticus, produce diarrhea
that can contain blood as well as fecal leukocytes in association with abdominal
cramps, tenesmus, and fever; these features suggest bacterial dysentery.
COMPLICATIONS
dehydration with associated complications.
-
-malnutrition and complications such as secondary infections and micronutrient
deficiencies (iron, zinc).
-

Bacteremias
-
DIAGNOSIS
The diagnosis of gastroenteritis is based on clinical recognition, and confirmation by
appropriate laboratory investigations, if indicated.
CLINICAL EVALUATION OF DIARRHEA.
includes:
The evaluation of a child with acute diarrhea
Assess the degree of dehydration and acidosis and provide rapid resuscitation and
rehydration with oral or intravenous fluids as required .
Obtain appropriate contact or exposure history. This includes information on
exposure to contacts with similar symptoms, intake of contaminated foods or water,
child-care center attendance, recent travel to a diarrhea-endemic area, and use of
antimicrobial agents
Clinically determine the etiology of diarrhea for institution of prompt antibiotic
therapy, if indicated. Although nausea and vomiting are nonspecific symptoms, they
are indicative of infection in the upper intestine. Fever is suggestive of an
inflammatory process but also occurs as a result of dehydration or co-infection (e.g.,
urinary tract infection, otitis media). Fever is common in patients with inflammatory
diarrhea. Severe abdominal pain and tenesmus are indicative of involvement of the
large intestine and rectum. Features such as nausea and vomiting and absent or low-
grade fever with mild to moderate periumbilical pain and watery diarrhea are
indicative of small intestine involvement and also reduce the likelihood of a serious
bacterial infection
STOOL EXAMINATION;
Stool specimens should be examined for mucus, blood, and leukocytes. Fecal
leukocytes are indicative of bacterial invasion of colonic mucosa. In endemic areas,
stool microscopy must include examination for parasites causing diarrhea, such as G.
lamblia and E. histolytica
XTAG GPP is an FDA-approved gastrointestinal pathogen panel
using multiplexed nucleic acid technology that detects Campylobacter,
C. difficile, toxin A/B, E. coli 0157, enterotoxigenic E. coli, Salmonella,
Shigella, Shiga-like toxin E. coli, norovirus, rotavirus A, Giardia, and Cryptosporidium.
Stool cultures should be obtained as early in the course of disease as possible from
children with bloody diarrhea in whom stool microscopy indicates fecal leukocytes;
in outbreaks with suspected hemolytic-uremic syndrome (HUS); and in
immunosuppressed children with diarrhea.The yield and diagnosis of bacterial
diarrhea can be significantly improved by using molecular diagnostic procedures
such as PCR. Blood can be send for assessment of Hb level, Bl urea and serum
electrolyte like Na and K.
Assessment of dehydration
In all children with diarrhea, decide if dehydration is present and give appropriate
treatment.
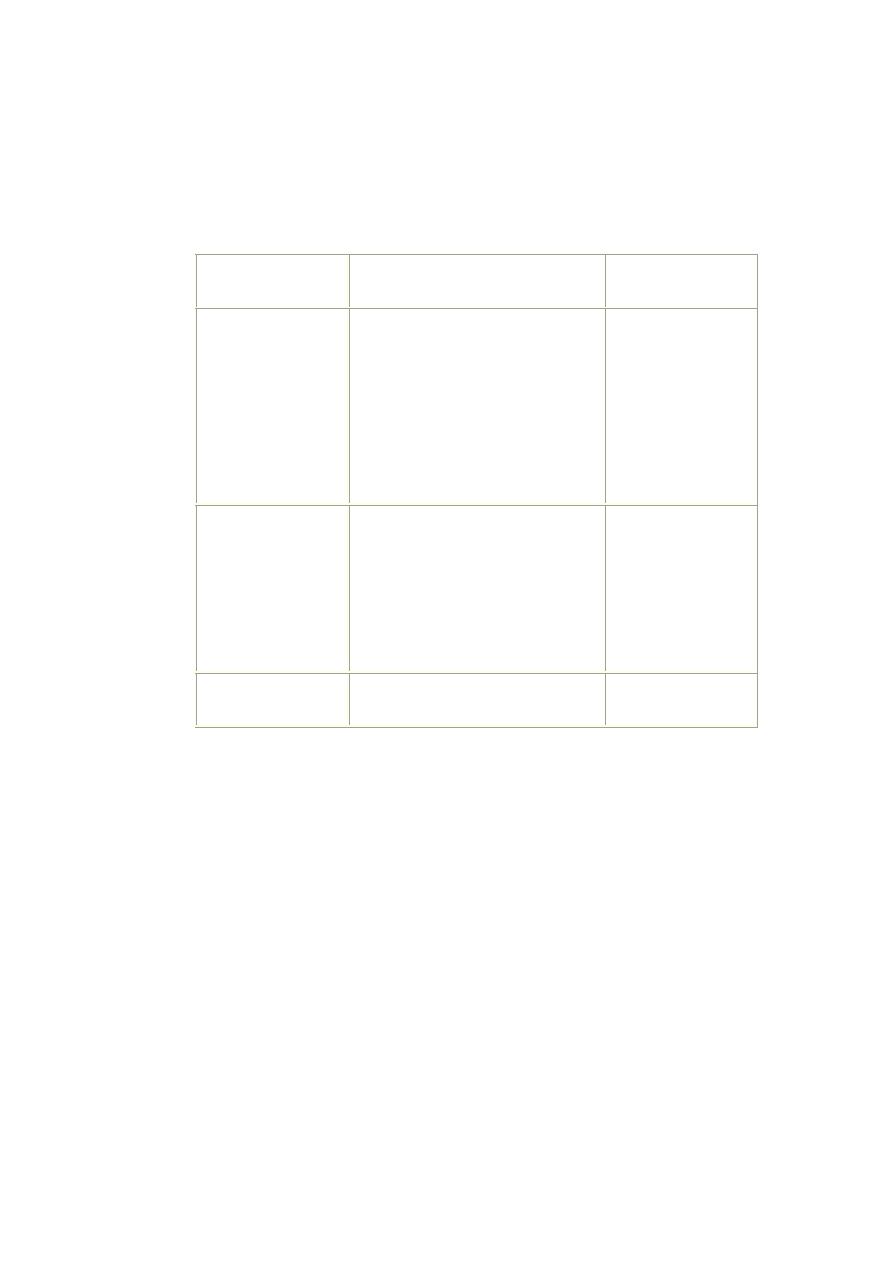
Classification of the severity of dehydration in children with diarrhea:
Treatment
Signs or symptoms
Classification
Treatment plan C
Two or more of the following
signs:
-lethargy/unconsciousness
- tear drops not present.
- sunken eyes.
- unable to drinks /poorly.
-skin pinch goes back very slowly
( ≥2 seconds)
-mouth is very dry
Sever dehydration
Treatment plan B
Two or more of the following
signs:
- restlessness, irritability.
-tear drops not present.
- sunken eyes.
- drinks eagerly.
- skin pinch goes back slowly
-Mouth is dry
Some dehydration
Treatment plan A
Not enough signs to classify as
some or severe dehydration.
No dehydration
TRETMENT:
Diarrhea treatment plan A:
Treat diarrhea at home
- Explain the 4 rules of home treatment:
1. give extra fluid (as much as the child will take)
Tell the mother:
- breastfeed frequently and for longer at each feed.
- if the infant is less than 6 m. old , give ORS or clean water in addition to breastmilk.
- if the child is older, give one or more of the followings: ORS solution, food-based fluids
(such as soup, rice water, and yoghurt drinks), or clean water.
Teach the mother how to mix and give ORS. Give the mother 2 packets of ORS to use
at home. Tell her to mix one packet with one litter of water
Show the mother how much fluid to give in addition to the usual fluid intake:
Up to 2 years 50 to 100 ml after each loose stool
2 years or more 100 to 200 ml after each loose stool
Tell the mother to:
- give the frequent small sips from a cup. or by cup and spoon
- if the child vomits, wait 10 minutes. Then continue, but more slowly.
- continue giving extra fluid until the diarrhea stop.
2. give zinc supplements
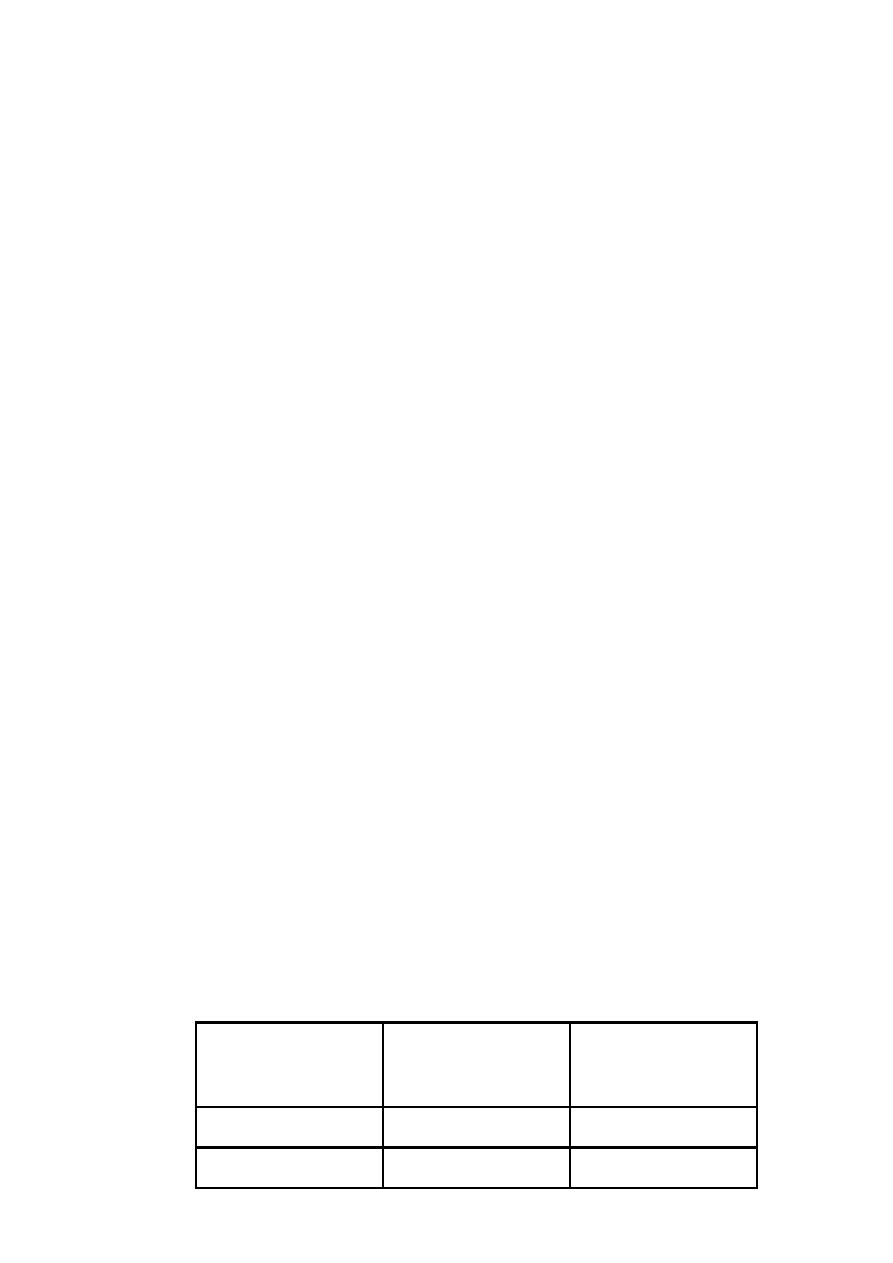
There is strong evidence that zinc supplementation in children with diarrhea in
developing countries leads to reduce duration and severity of diarrhea and could
potentially prevent 300,000 deaths. WHO and UNICEF recommend that all children
with acute diarrhea in at-risk areas should receive oral zinc in some form for 10–14
days during and after diarrhea (10 mg/day for infants <6 mo of age and 20 mg/day for
those >6 mo). In addition to improving diarrhea, administration of zinc in community
settings leads to increased use of ORS and reduction in the use of antimicrobials.
Tell the mother how much zinc to give:
Up to 6 months 1/2 tablet (10 mg) per day for 10-14 days.
6 months and more 1 tablet (20 mg) per day for 10-14 days.
Show the mother how to give the zinc supplements:
- infants, dissolve the tablet in a small amounts of clean water, expressed milk or ORS in
a small cup or spoon.
- older children, tablet can be chewed or dissolved in a small amount of clean water in a
cup or spoon.
Remind the mother to give the zinc supplements for the full 10-14 days.
3. continue feeding.
• FEEDING IN DIARRHEA:
o
Children should continue to be fed during diarrhea.
o
. Milk should not be diluted with water during any phase of acute diarrhea.
o
Milk can also be given as milk cereal mixture e.g. milk-rice mixture.
o
To make foods-energy dense some of preparation are:-
- Mashed banana with milk or curd
- Mashed potatoes with oil.
o
–fresh cooked vegetable
A child who is recovering from diarrhoea needs an extra meal every day for at least 2 W.
Breast feeding should be continued uninterrupted even during rehydration with ORS.
4. when to return
■ when her baby develop repeated vomiting.
■ develop fever.
■ become irritable and restless.
■ has blood in stool.
ORAL REHYDRATION THERAPY.
Although, in general, the standard WHO oral rehydration solution (ORS) is adequate,
lower osmolality oral rehydration fluids can be more effective in reducing stool
output. Compared with standard ORS, lower sodium and glucose ORS (containing 75
mEq of sodium and 75 mmol of glucose per liter, with total osmolarity of 245 mOsm
per liter) reduces stool output, vomiting, and the need for intravenous fluids without
substantially increasing the risk of hyponatremia.
Composition of oral rehydration solution:
Reduced osmolarity ORS
mmol/l
Standard ORS solution
mEq or mmol/l
Minerals
75
111
Glucose
75
90
Sodium
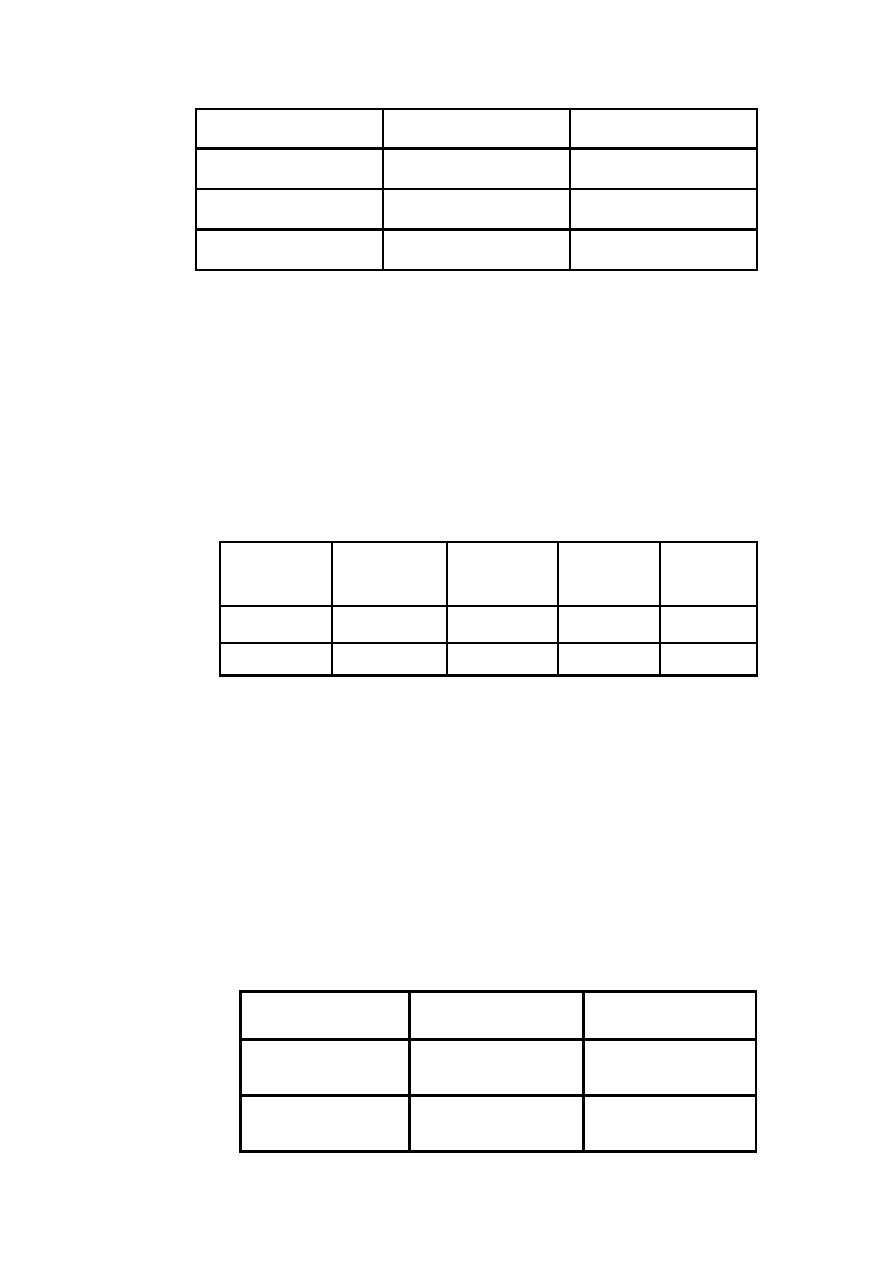
65
80
chloride
20
20
Potassium
10
10
Citrate
245
311
osmolartiy
Cereal-based oral rehydration fluids can also be advantageous in malnourished
children and can be prepared at home. Home remedies including decarbonated soda
beverages, fruit juices, and tea are not suitable for rehydration or maintenance therapy
as they have inappropriately high osmolalities and low sodium concentrations. Oral
rehydration should be given to infants and children slowly, especially if they have
emesis. It can be given initially by a dropper, teaspoon, or syringe, beginning with as
little as 5 mL at a time. The volume is increased as tolerated.
Oral rehydration can also be given by a nasogastric tube if needed; this is not the usual
route. Limitations to oral rehydration therapy include shock, an ileus, intussusception,
carbohydrate intolerance (rare), severe emesis, and high stool output (>10 mL/kg/hr).
Diarrhea treatment plan B:
Treat some dehydration with ORS:
determine amount of ORS to give during first 4 hours.
2 years up
to
5 years
12 months
up to 2 years
4 months up
to 12 months
Up to 4
months
*
Age
12-19 kg
10-<12 kg
6-<10 kg
<6 kg
Weight
900-1400
700-900
400-700
200-400
In ml
* use the child's age only when do not know the weight. The approximate amount
of ORS required (in ml) can also be calculated by multiplying the child’s weight (in
kg) by 75
- if the child wants more ORS than shown, give more.
show the mother how to give ORS solution.
■ after 4 hours:
- reassess the child and classify the child for dehydration.
- select the appropriate plan to continue treatment.
-give zinc supplement
Diarrhea treatment plan C:
Treat sever dehydration quickly
start IV fluid immediately. If the child can drink, give ORS by mouth while the drip is
set up. Give 100 ml/kg Ringer's lactate solution (or, if not available, normal saline),
divided as follows:
Then give 70 ml/kg
in:
First give 30 ml/kg
in:
Age
5 hours
1 hour*
Infants (under 12
months)
2 1/2 hours
30 minutes*
Children (12 months
up to 5 years)
* repeat once if radial pulse is still very week or not detectable.
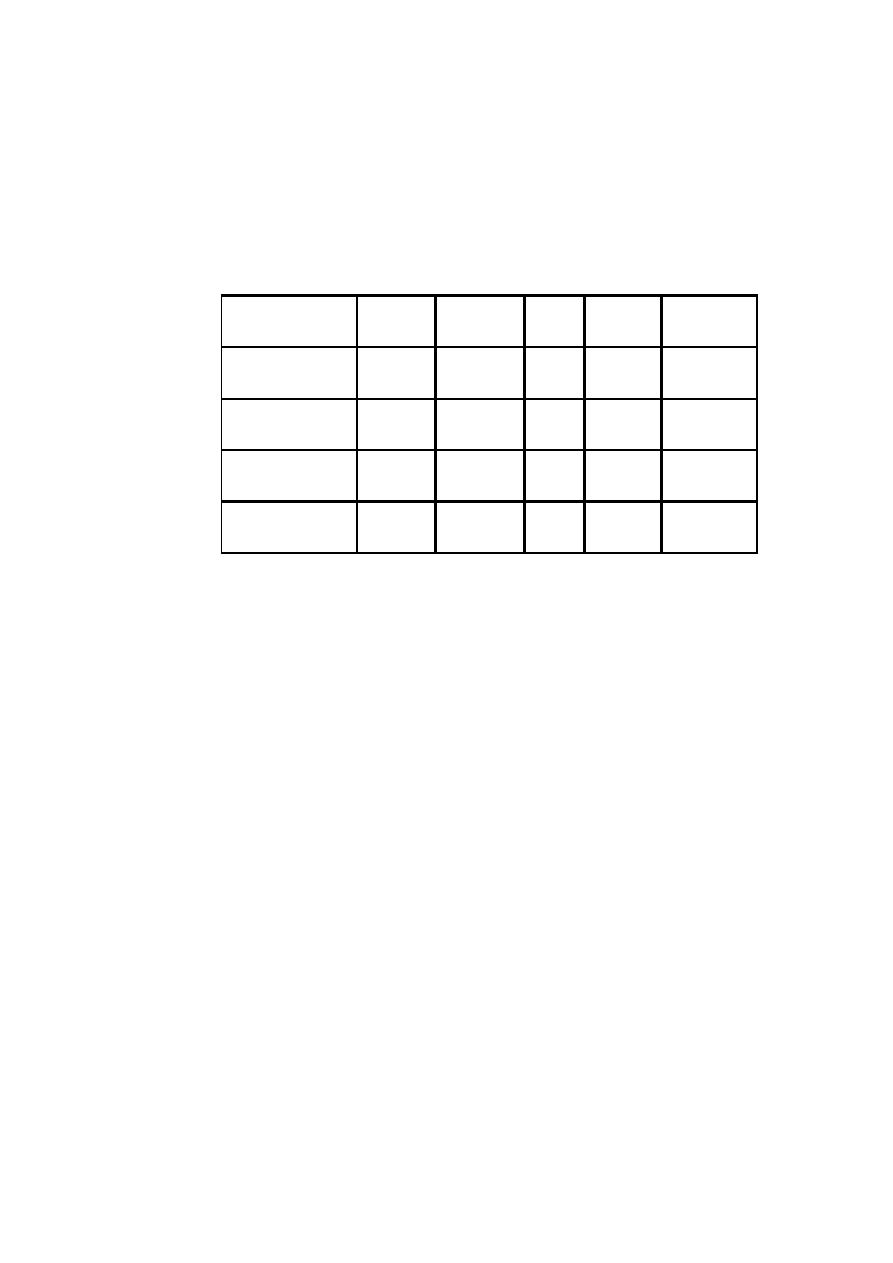
also give ORS (about 5 ml/kg/hour) as soon as the child can drink: usually after 3-4 hours
(infants) or 1-2 hours (children).
if IV fluid is not available, start rehydration by tube (or mouth) with ORS solution: give
20 ml/kg/hour for 6 hours (total of 120 ml/kg).
Types of fluids used intravenously:
In designing fluid management, it is important to know the component of the commonly
available solution.
lactate
Ca++
K+
Cl-
س
Na+
fluid
-
-
-
154
154
Normal saline
0.9 NaCl
-
-
-
77
77
Normal saline
0.45 NaCl
-
-
-
38.5
38.5
Normal saline
0.225 NaCl
28
3
4
109
130
Ringer lactate
The normal plasma osmolarity is 285-295 mosm/kg, infusing an intravenous solution
peripherally with a much lower osmolarity cause water to move into red blood cell
causing hemolysis, thus, intravenous fluids are generally designed to have an osmolarity
that is either close to 285 or moderately higher that is not cause problem, thus 1/4 N.S.
(osmolarity 77) should not be administered peripherally, but D5 1/4 N. S. (osmolarity
=355) or D5 1/2 N. S.+ 20 meq/l kcl (osmolarity 472) can be administered. The prefered
fluid for correction of dehydration is Ringer lactate and normal saline.
ADDITIONAL THERAPIES.
The use of probiotic nonpathogenic bacteria for prevention and therapy of diarrhea
has been successful in developing countries. There are a variety of organisms
(Lactobacillus, Bifidobacterium) that have a good safety record; therapy has not been
standardized and the most effective (and safe) organism has not been identified.
Antimotility agents (loperamide) are contraindicated in children with dysentery and
probably have no role in the management of acute watery diarrhea in otherwise
healthy children. Similarly, antiemetic agents such as the phenothiazines are of little
value and are associated with potentially serious side effects (lethargy, dystonia,
malignant hyperpyrexia). Nonetheless, ondansetron is an effective and less toxic
antiemetic agent. Because persistent vomiting may limit oral rehydration therapy, a
single sublingual dose of an oral dissolvable tablet of ondansetron (2 mg children 8–
15 kg; 4 mg children <15–30 kg; 8 mg children >30 kg) may be given. However,
most children do not require specific antiemetic therapy; careful oral rehydration
therapy is usually sufficient
Racecadotril, an enkephalinse inhibitor, has been shown to reduce stool output in
patients with diarrhea. Experience with this drug in children is limited, and for the
average child with acute diarrhea it may be unnecessary.
ANTIBIOTIC THERAPY.
Timely antibiotic therapy in select cases of diarrhea may reduce the duration and
severity of diarrhea and prevent complications. While these agents are important to
use in specific cases, their widespread and indiscriminate use leads to the
development of antimicrobial resistance. Nitazoxanide, an anti-infective agent, has

been effective in the treatment of a wide variety of pathogens including
Cryptosporidum parvum, Giardia lamblia, Entamoeba histolytica, Blastocystis
hominis, C. difficile, and rotavirus
•
Dysentery
o
Requires antibiotic therapy
o
o
. Shigellae responds to cotrimoxazole
OR
o
Nalidixic acid 55 mg/kg/day in 4 doses x 5 days.
o
. Acute Amoebiasis:
o
Metronidazole -30 mg./kg/ day 3 doses x 5-10 days.
•
Cholera:-
Antibiotics used are:
o
Doxycycline- 6 mg/kg/day a single dose x 3 days or
o
. Tetracycline - 50 mg/kg/day 4 doses x 3 days or
o
. Erythromycin -30 mg/kg/day 3 doses x 3 days.
o
Note : doxycycline and tetracycline note used for children under 8 y. old
PREVENTION
In many developed countries, diarrhea due to pathogens such as Clostridum
botulinum, E. coli 0157 : H7, Salmonella, Shigella, V. cholerae, Cryptosporidium, and
Cyclospora is a notifiable disease and, thus, contact tracing and source identification
is important in preventing outbreaks.
PROMOTION OF EXCLUSIVE BREAST-FEEDING.
IMPROVED COMPLEMENTARY FEEDING PRACTICES.
There is a strong inverse association between appropriate, safe complementary
feeding and mortality in children age 6–11 mo; malnutrition is an independent risk for
the frequency and severity of diarrheal illness. Complementary foods should be
introduced at 6 mo of age while breast-feeding should continue for up to 1 yr (longer
period for developing countries). Complementary foods in developing countries are
generally poor in quality and frequently heavily contaminated, thus predisposing to
diarrhea. Contamination of complementary foods can be potentially reduced through
caregivers' education and improving home food storage. Vitamin A supplementation
reduces childhood mortality by 34%; improved vitamin A status reduces the
frequency of severe diarrhea.
ROTAVIRUS IMMUNIZATION.
Most infants acquire rotavirus diarrhea early in life; an effective rotavirus vaccine
would have a major effect on reducing diarrhea mortality in developing countries.
Rota virus vaccination:
Rota shield vaccine -1999 was licensed in America
. Withdrawn because of its association with intussusception
. Two new oral, live attenuated rotavirus vaccines were licensed in 2006 with very good
safety and efficacy
. The first dose administered between ages 6-10 weeks .
. subsequent doses at intervals 4-10 weeks.
. Vaccination should not be initiated before 6weeks and after 12 weeks of age.
. All doses should be administered before 32 weeks
Other vaccines that could potentially reduce the burden of severe diarrhea and
mortality in young children are vaccines against Shigella and ETEC
IMPROVED WATER AND SANITARY FACILITIES AND PROMOTION OF
PERSONAL AND DOMESTIC HYGIENE.

Much of the reduction in diarrhea prevalence in the developed world is the result of
improvement in standards of hygiene, sanitation, and water supply. In addition,
routine handwashing with plain soap in the home can reduce the incidence of diarrhea
in all environments.
IMPROVED CASE MANAGEMENT OF DIARRHEA.
Improved management of diarrhea through prompt identification and appropriate
therapy significantly reduces diarrhea duration, its nutritional penalty, and risk of
death in childhood. Improved management of acute diarrhea is a key factor in
reducing the burden of prolonged episodes and persistent diarrhea. The
WHO/UNICEF recommendations to use low osmolality ORS and zinc
supplementation for the management of diarrhea, coupled with selective and
appropriate use of antibiotics, have the potential to reduce the number of diarrheal
deaths among children.
