
CHRONIC INFLAMMATION
Chronic inflammation is inflammation of prolonged
duration (weeks to months to years) in which active
inflammation, tissue injury, and healing proceed
simultaneously
.
In contrast to acute inflammation, which is distinguished
by vascular changes, edema, and a predominantly
neutrophilic infiltrate, chronic inflammation is
characterized by
:
1.
Infiltration with mononuclear cells, including
macrophages, lymphocytes, and plasma cells
2.
Tissue destruction, largely induced by the products of
the inflammatory cells
3.
Repair, involving new vessel proliferation (angiogenesis)
and
fibrosis

acute inflammation may progress to chronic
inflammation. This transition occurs when the
acute response cannot be resolved, either
because of the persistence of the injurious
agent or because of interference with the
normal process of healing. For example, a
peptic ulcer of the duodenum initially shows
acute inflammation followed by the beginning
stages of resolution
.
Alternatively, some forms of injury (e.g., viral
infections) engender a response that involves
chronic inflammation from the onset
.

Causes of chronic inflammation
:
1. Persistent infections
:
by microbes that are difficult to eradicate. These include
mycobacteria ,
Treponema pallidum, (
causative
organism of syphilis), and certain viruses and fungi, all
of which tend to establish persistent infections and elicit
a
delayed-type hypersensitivity
. In fact, most viral
infections elicit chronic inflammatory reactions
dominated by lymphocytes and macrophages
2. hypersensitivity disease
diseases that are caused by excessive and inappropriate
activation of the immune system are increasingly
recognized as being important health problems)

under certain conditions, immune reactions develop
against the individual's own tissues, leading to
autoimmune diseases
. In these diseases, autoantigens
evoke a self-immune reaction that results in chronic
tissue damage and inflammation. Inflammation
secondary to autoimmunity plays an important role in
several common and debilitating chronic diseases,
such as rheumatoid arthritis and inflammatory bowel
disease. Immune responses against common
environmental substances are the cause of
allergic
diseases ,
such as bronchial asthma.
3. Prolonged exposure to potentially toxic agents .
:
either exogenous materials such as inhaled
particulate silica, which can induce
silicosis
or
endogenous agents such as chronically elevated
plasma lipid components, which may contribute to
atherosclerosis
)
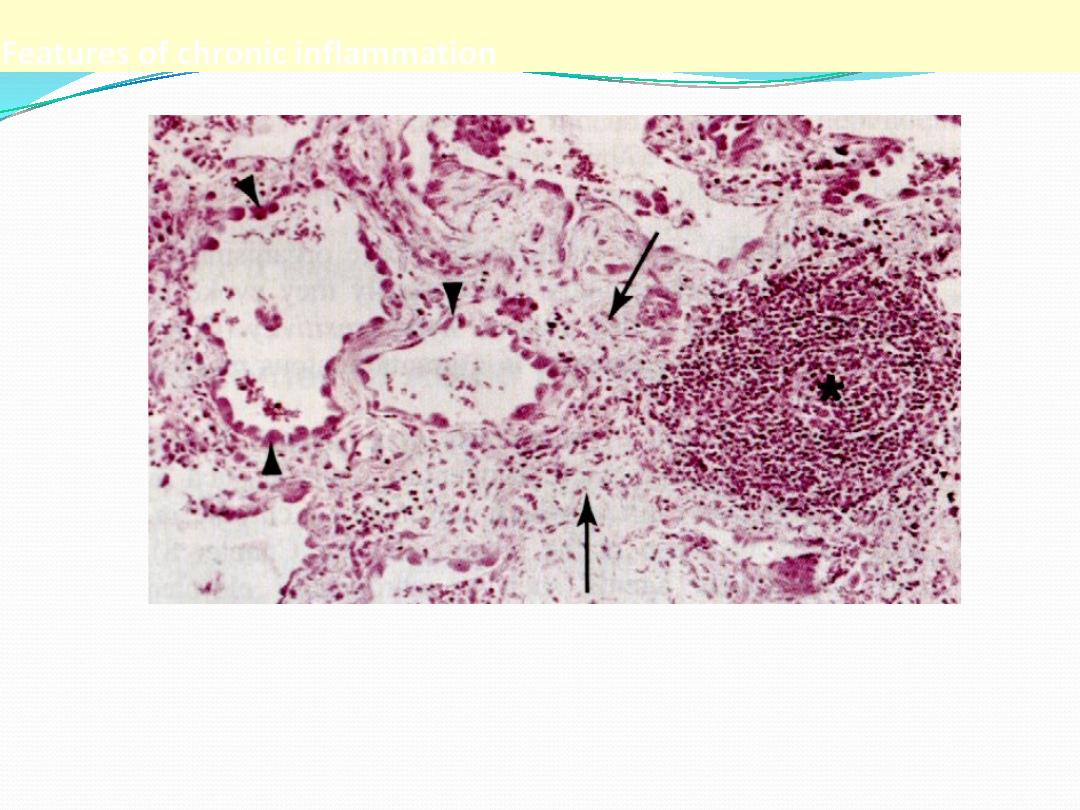
Features of chronic inflammation
The three characteristic features of chronic inflammation (in the lung)
1. Chronic inflammatory cells infiltration* 2. destruction of the normal tissue
(normal alveoli are replaced by spaces lined by cuboidal cells (arrow heads)
3. Replacement by fibrosis (arrows)

Chronic Inflammatory Cells and Mediators
chronic inflammation results from complex interactions between the cells that are
recruited to the site of inflammation and are activated at this site.
1. Macrophages:
the dominant cells of chronic inflammation, are tissue cells derived from
circulating blood monocytes after their emigration from the bloodstream.
Macrophages are normally diffusely scattered in most connective tissues, and are
also found in organs such as the liver (where they are called Kupffer cells), spleen
and lymph nodes (called sinus histiocytes), central nervous system (microglial
cells), and lungs (alveolar macrophages). Together these cells comprise the so-called
mononuclear phagocyte system, also known by the older name of reticulo-
endothelial system
2. Lymphocytes :
are mobilized to the setting of any specific immune stimulus (i.e., infections) as well
as non-immune-mediated inflammation (e.g., due to infarction or tissue trauma).
Both T and B lymphocytes migrate into inflammatory sites using some of the same
adhesion molecule pairs and chemokines that recruit other leukocytes.
Lymphocytes and macrophages interact in a bidirectional way, and these
interactions play an important role in chronic inflammation. Macrophages display
antigens to T cells that stimulate them . Activated T lymphocytes, in turn, produce
cytokines, and one of these, IFN-γ, is a powerful activator of macrophages

The half-life of circulating monocytes is about 1 day; under the influence
of adhesion molecules and chemotactic factors, they begin to migrate to
a site of injury within 24 to 48 hours after the onset of acute
inflammation,. When monocytes reach the extravascular tissue, they
undergo transformation into larger macrophages, which have longer half-
lives and a greater capacity for phagocytosis than do blood monocytes.
Macrophages may also become activated
,
resulting in increased cell size,
increased content of lysosomal enzymes, more active metabolism, and
greater ability to kill ingested organisms. By light microscopy, activated
macrophages appear large, flat, and pink (in H&E stains); this
appearance may be similar to that of squamous epithelial cells, and cells
with such an appearance are therefore sometimes called epithelioid cells
.
Activation signals include bacterial endotoxin and other microbial
products, cytokines secreted by sensitized T lymphocytes (in particular
the cytokine IFN-γ), various mediators produced during acute
inflammation, and ECM proteins such as fibronectin. After activation,
macrophages secrete a wide variety of biologically active products that, if
unchecked, can result in the tissue injury and fibrosis that are
characteristic of chronic inflammation
) Fig. 2-21 .(These products
include

Acid and neutral proteases
.
Recall that the latter were also implicated as
mediators of tissue damage in acute inflammation. Other enzymes, such as
plasminogen activator
,
greatly amplify the generation of proinflammatory
substances
.
ROS and NO
AA metabolites.
Cytokines
such as IL-1 and TNF, as well as a variety of growth factors that
influence the proliferation of smooth muscle cells and fibroblasts and the
production of ECM
After the initiating stimulus is eliminated and the inflammatory reaction
abates, macrophages eventually die or wander off into lymphatics. In chronic
inflammatory sites, however, macrophage accumulation persists, and
macrophages can proliferate. Steady release of lymphocyte-derived
chemokines and other cytokines is an important mechanism by which
macrophages are recruited to or immobilized in inflammatory sites. IFN-γ can
also induce macrophages to fuse into large, multinucleated cells called giant
cells

3. Eosinophils
are characteristically found in inflammatory sites around parasitic
infections or as part of immune reactions mediated by IgE, typically
associated with allergies
.
Their recruitment is driven by adhesion molecules similar to those used by
neutrophils, and by specific chemokines (e.g., eotaxin) derived from
leukocytes or epithelial cells. Eosinophil granules contain major basic
protein, a highly charged cationic protein that is toxic to parasites but also
causes epithelial cell necrosis
.
4. Mast cells
are sentinel cells widely distributed in connective tissues throughout the
body, and they can participate in both acute and chronic inflammatory
responses. In atopic individuals (individuals prone to allergic reactions),
mast cells are "armed" with IgE antibody specific for certain
environmental antigens.
When these antigens are subsequently encountered, the IgE-coated mast
cells are triggered to release histamines and AA metabolites that elicit the
early vascular changes of acute inflammation. IgE-armed mast cells are
central players in allergic reactions, including anaphylactic shock.
Mast cells can also elaborate cytokines such as TNF and chemokines and
may play a beneficial role in some infections.
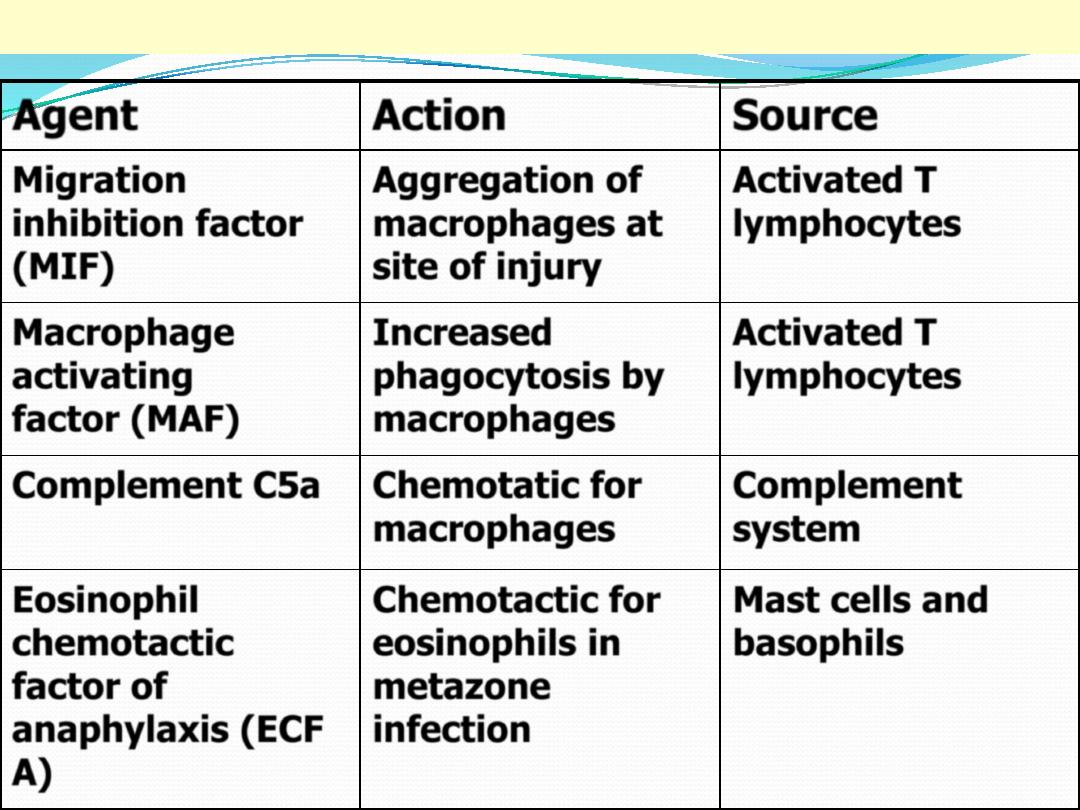
Mediators of chronic inflammation
Agent
Action
Source
Migration
inhibition factor
(MIF)
Aggregation of
macrophages at
site of injury
Activated T
lymphocytes
Macrophage
activating
factor (MAF)
Increased
phagocytosis by
macrophages
Activated T
lymphocytes
Complement C5a
Chemotatic for
macrophages
Complement
system
Eosinophil
chemotactic
factor of
anaphylaxis (ECF
A)
Chemotactic for
eosinophils in
metazone
infection
Mast cells and
basophils

Granulomatous inflammation
is a distinctive pattern of chronic inflammation
characterized by aggregates of activated macrophages
that assume an epithelioid appearance.
Granulomas are encountered in certain specific
pathologic states; consequently, recognition of the
granulomatous pattern is important because of the
limited number of conditions (some life-threatening)
that cause it
.
Granulomas can form in the setting of persistent T-cell
responses to certain microbes (such as Mycobacterium
tuberculosis, T. pallidum
,
or fungi), where T-cell-
derived cytokines are responsible for chronic
macrophage activation
.
Tuberculosis is the prototype of a granulomatous
disease caused by infection and should always be
excluded as the cause when granulomas are identified
.

Granulomas may also develop in response to
relatively inert foreign bodies (e.g., suture or
splinter), forming so-called foreign body
granulomas
.
The formation of a granuloma effectively "walls
off" the offending agent and is therefore a useful
defense mechanism.
However, granuloma formation does not always
lead to eradication of the causal agent, which is
frequently resistant to killing or degradation,
and granulomatous inflammation with
subsequent fibrosis may even be the major
cause of organ dysfunction in some diseases,
such as tuberculosis
.

Causes of granulomatous inflammation:
granulomatous inflammation is encountered in a number of
immunologically mediated infectious and non-infectious
conditions, these include:
1.
Tuberculosis
2.
Sarcoidosis
3.
Cat-scratch disease
4.
Lymphogranuloma inguinale
5.
Leprosy
6.
Brucelosis
7.
Syphilis
8.
Some fungal infection
9.
Reactions of irretant lipid

Recognition of granuloma in biopsy specimen is
important because it shorten the list of differetial
diagnosis .agranuloma is a focus of chronic
inflammation consist of aggregation of
macrophages that are transformed into epithelioid
cells surrounded by a collar of mononuclear
leukocytes principally lymphocytes and
occasionally plasma cells.older granulomas
develop an enclosing rim of fibroblasts and
connective tissue .frequently , epithelioid cells fuse
to form multinucleated giant cells in periphery or
some time in the center of the granuloma.they
have 20 or more small nuclei arranged either
peripherally (Langhans-type giant cell) or
haphazardlly ( foreign body
–type giant cell )
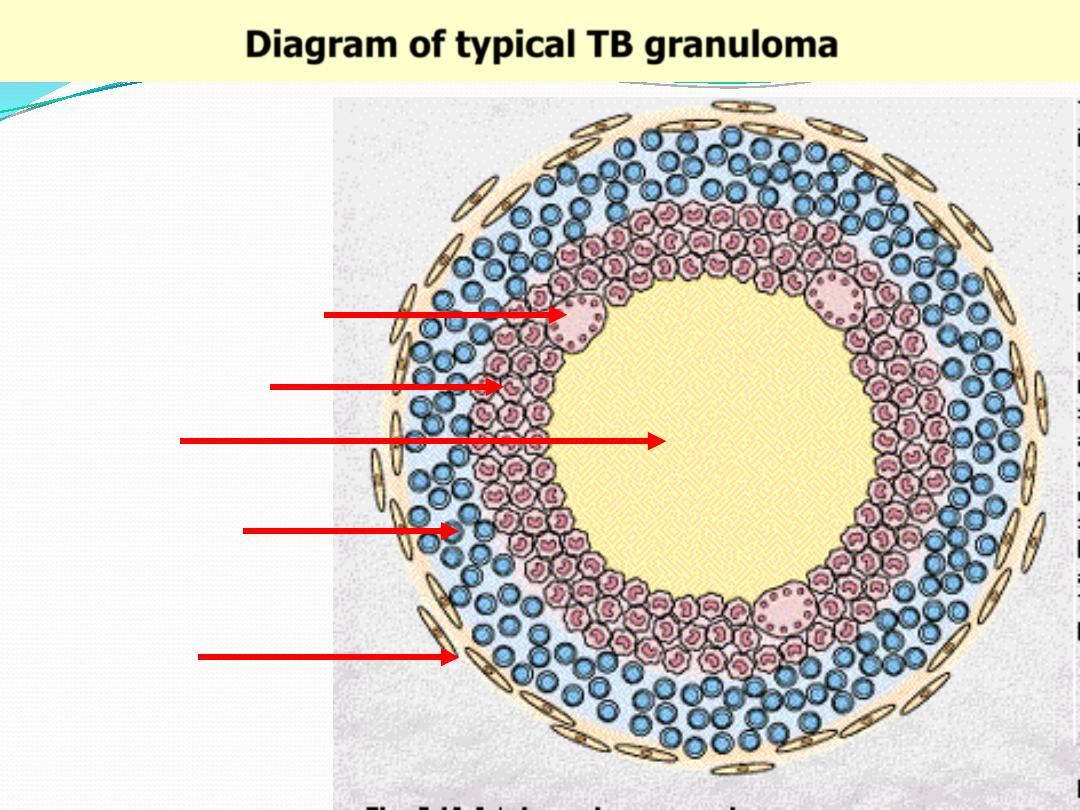
Diagram of typical TB granuloma
Caseation
Epithelioid cells
Multinucleated GC
Lymphocytes
Fibroblasts
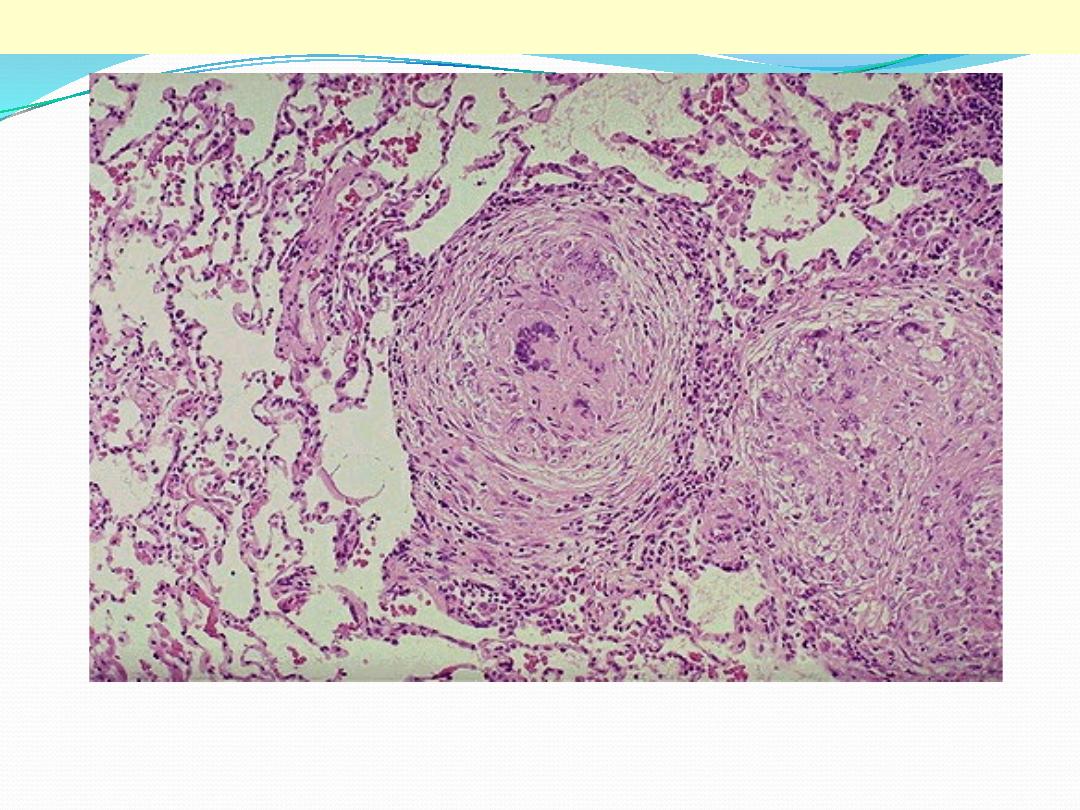
This is a low power view showing two, adjacent, well-defined, rounded granulomas . From this power
the presence of multinucleated giant cells is obvious (arrow).
TB granulomas lung
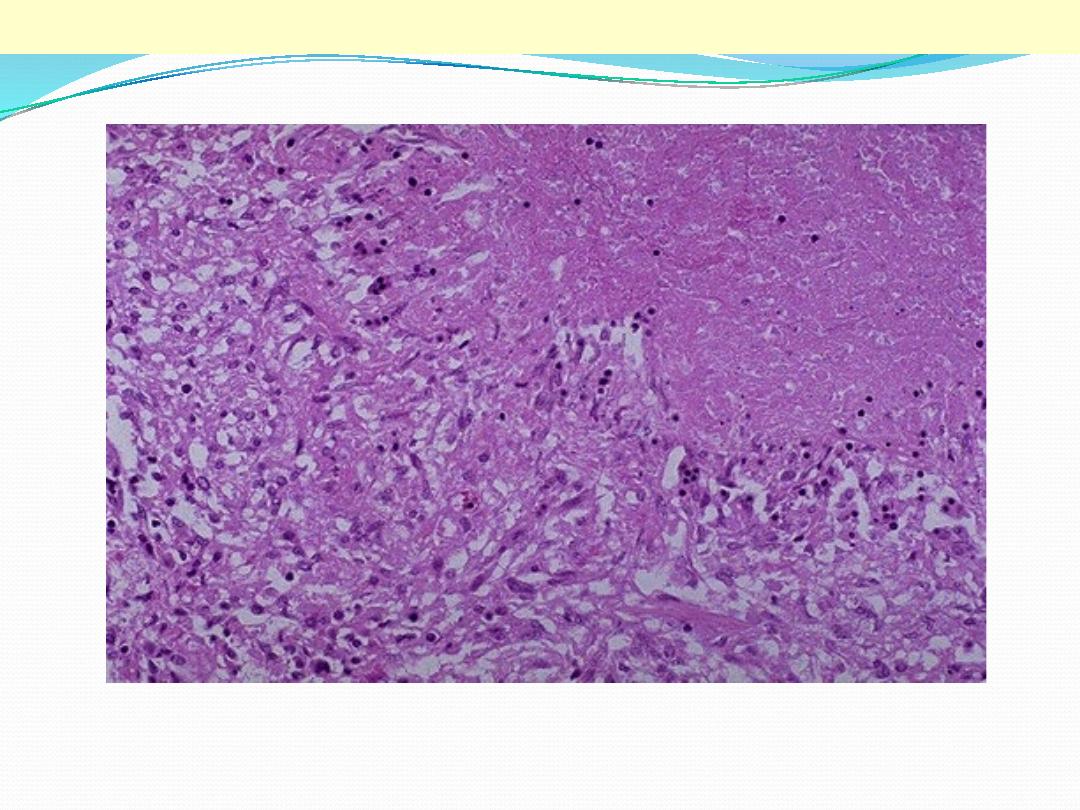
This is a high power view showing a portion of typical TB granuloma. Note the amorphous, pinkish
central caseation, which is surrounded by a rim of epithelioid cells.
TB granulomas
Epithelioid cells
Caseation
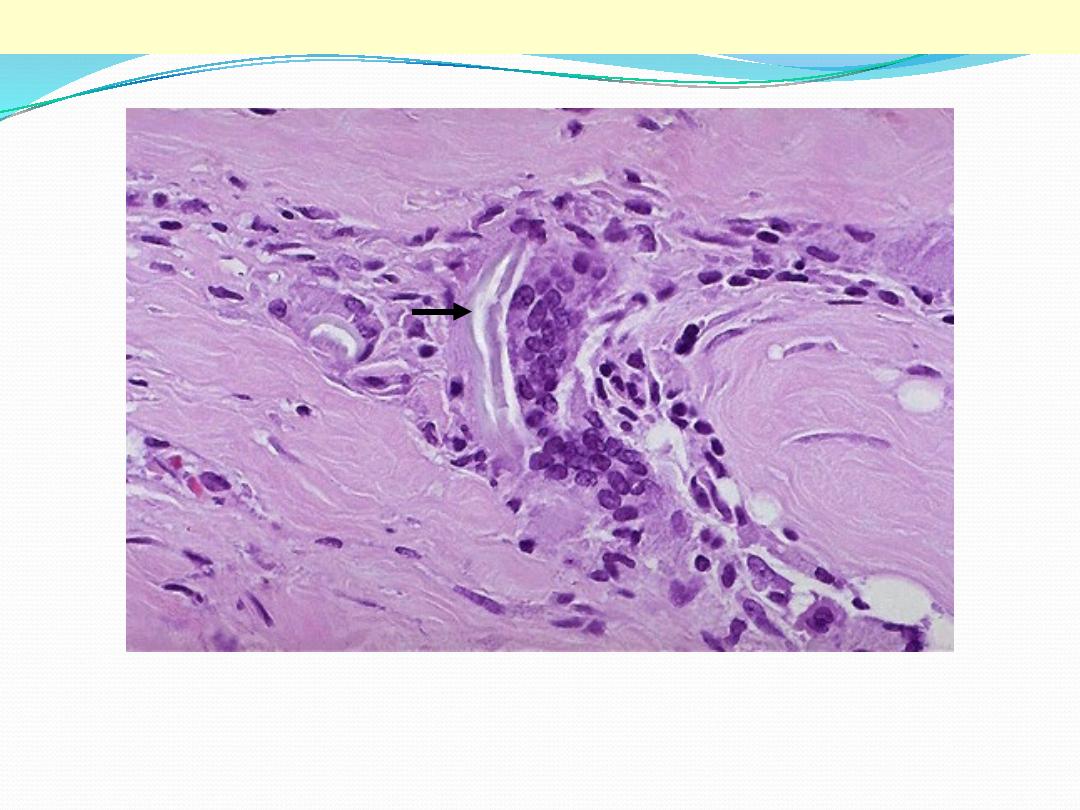
Two foreign body giant cells are seen, where there is a bluish strand of suture material (arrow) from a
previous operation
Foreign body giant cells in suture granuloma
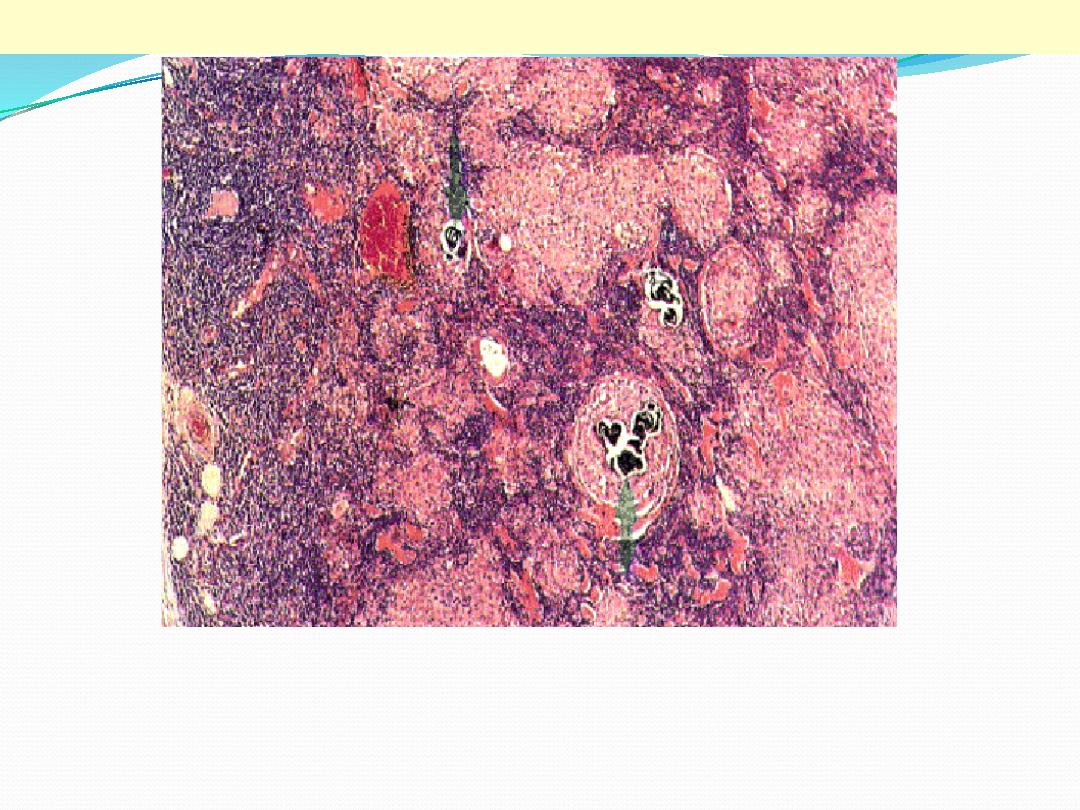
Sarcoidosis is a granulomatous inflammatory disease which affects many tissues, including lymphoid
tissue. The capsule of the node is on the left. The normal architecture of the node has been largely
destroyed, with some blue-staining lymphoid tissue surviving beneath the capsule and between the
round sarcoid granulomas. The latter vary widely in size, from a few cells to very large collections
(right) several mm in diameter. They consist of epithelioid histiocytes. There is no caseation, but some
contain calcified laminated Schaumann bodies (arrows).
Sarcoidosis lymph node

SYSTEMIC EFFECTS OF INFLAMMATION
the systemic effects of inflammation, collectively called the acute-
phase reaction, or the systemic inflammatory response syndrome. The
cytokines TNF, IL-1, and IL-6 are the most important mediators of the
acute-phase reaction.
The acute-phase response consists of several clinical and pathologic
changes:
1. Fever :
is produced in response to substances called pyrogens that act by
stimulating prostaglandin (PG) synthesis in the vascular and
perivascular cells of the hypothalamus.
2. Acute-phase proteins:
which are plasma proteins, mostly synthesized in the liver, whose
concentrations may increase several 100-fold as part of the response to
inflammatory stimuli Three of the best-known of these proteins are C-
reactive protein (CRP), fibrinogen, and serum amyloid A (SAA) protein .

CRP and SAA, bind to microbial cell walls, and they may act as opsonins and fix complement,
thus promoting the elimination of the microbes. Fibrinogen binds to erythrocytes and
causes them to form stacks (rouleaux) that sediment more rapidly at unit gravity than do
individual erythrocytes
.
3.
Leukocytosis
is a common feature of inflammatory reactions, especially those induced by bacterial
infection. The leukocyte count usually climbs to 15,000 or 20,000 cells/μL, but sometimes it
may reach extraordinarily high levels, as high as 40,000 to 100,000 cells/μL. These extreme
elevations are referred to as leukemoid reactions because they are similar to the white cell
counts obtained in leukemia. The leukocytosis occurs initially because of accelerated
release of cells from the bone marrow postmitotic reserve pool (caused by cytokines,
including TNF and IL-1) and is therefore associated with a rise in the number of more
immature neutrophils in the blood (shift to the left). Prolonged infection also stimulates
production of colony-stimulating factors (CSFs), leading to increased bone marrow output
of leukocytes, which compensates for the loss of these cells in the inflammatory reaction.
Most bacterial infections induce an increase in the blood neutrophil count, called
neutrophilia. Viral infections, such as infectious mononucleosis, mumps, and German
measles, are associated with increased numbers of lymphocytes (lymphocytosis). Bronchial
asthma, hay fever, and parasite infestations all involve an increase in the absolute number
of eosinophils, creating an eosinophilia. Certain infections (typhoid fever and infections
caused by some viruses, rickettsiae, and certain protozoa) are paradoxically associated with
a decreased number of circulating white cells (leukopenia), likely because of cytokine-
induced sequestration of lymphocytes in lymph nodes.

4. Other manifestations of the acute-phase response include
increased heart rate and blood pressure; decreased sweating,
mainly because of redirection of blood flow from cutaneous to
deep vascular beds, to minimize heat loss through the skin; and
rigors (shivering), chills (perception of being cold as the
hypothalamus resets the body temperature), anorexia, and
malaise, probably because of the actions of cytokines on brain
cells. Chronic inflammation is associated with a wasting syndrome
called cachexia
,
which is mainly the result of TNF-mediated
appetite suppression and mobilization of fat stores
5. In severe bacterial infections (sepsis), the large amounts of organisms and
LPS in the blood or extravascular tissue stimulate the production of
enormous quantities of several cytokines, notably TNF, as well as IL-12 and
IL-1. As a result, circulating levels of these cytokines increase, and the
nature of the host response changes. High levels of TNF cause
disseminated intravascular coagulation (DIC), hypoglycemia, and
hypotensive shock. This clinical triad is described as septic shock.

Thank you
