
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
1
L1
A system of glands and hormone secreting cells that regulate body functions
through hormones.
Hormones: Chemical messengers that exert a regulatory effect on the cells of the
body bearing receptors for it.
Endocrine glands: Ductless structures that release hormones directly into the
blood, and then transported by the circulation to the target tissues.
Target tissue: Tissue possess specific receptors to which the hormone binds and
this receptor binding then elicits a series of events that influences cellular
activities.
Eicosanoids and Vitamins
Eicosanoids is a group of compounds that have a hormone like action. The most
important of these eicosanoids are the prostaglandins, leukotrienes, and
thromboxanes. These substances derives from arachidonic acid (a cell membrane
lipid), and act mainly by paracrine and autocrine mechanisms.
Vitamins also act in ways that resemble hormones. For example, vitamin D3.
Biochemical classification of hormones
Hormones are classified into three biochemical categories:
• Steroids
• Proteins/peptides
• Amines
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
2
Steroid hormones are produced by the adrenal cortex, testes, ovaries, and
placenta. Synthesized from cholesterol, these hormones are lipid soluble; therefore,
they cross cell membranes readily and bind to receptors found intracellularly.
However, because their lipid solubility renders them insoluble in blood, these
hormones are transported in the blood bound to proteins.
Furthermore, steroid hormones are not typically preformed and stored for future
use within the endocrine gland, because they are lipid soluble, they could diffuse
out of the cells and physiological regulation of their release would not be possible.
Finally, steroid hormones are absorbed easily by the gastrointestinal tract and
therefore may be administered orally.
Protein/peptide hormones are derived from amino acids. These hormones are
preformed and stored for future use in membrane-bound secretory granules. When
needed, they are released by exocytosis. Protein/peptide hormones are water
soluble, circulate in the blood predominantly in an unbound form, and thus tend to
have short half-lives. Because these hormones are unable to cross the cell
membranes of their target tissues, they bind to receptors on the membrane surface.
Protein/peptide hormones cannot be administered orally because they would be
digested in the gastrointestinal tract. Instead, they are usually administered by
injection (e.g., insulin).
Amine hormones include the thyroid hormones and the catecholamines.
The thyroid hormones tend to be biologically similar to the steroid hormones. They
are mainly insoluble in the blood and are transported predominantly (>99%) bound
to proteins. As such, these hormones have longer half-lives (triiodothyronine (T3)
=24h; thyroxine(T4) =7 days). Furthermore, thyroid hormones cross cell
membranes to bind with intracellular receptors and may be administered orally.
The catecholamines are biologically similar to protein/peptide hormones. These
hormones are soluble in the blood and are transported in an unbound form.
Therefore, the catecholamines have a relatively short half-life.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
3
Lipid- versus Water-Soluble Hormones
Hormones also can be classified depending on their solubility in the water or in the
lipid into Lipid-Soluble Hormones like (steroids, thyroid hormones) and Water-
Soluble Hormones like (peptides, proteins)
Feedback Control of Hormone Secretion
The endocrine system, like many other physiological systems, is regulated by
feedback mechanisms:
1. Negative Feedback,
2. Positive Feedback,
Lipid-Soluble
Hormones
(steroids,thyroid
hormones)
Water-Soluble Hormones (peptides,
proteins)
Receptors
Inside the cell
Outer surface of the cell membrane
Intracellular action
Stimulates synthesis of
new proteins
Production of second messengers,
e.g., cAMP•
Storage
Synthesized as
needed,
except thyroid hormone
Stored in vesicles
Plasma transport
Attached to proteins that
serve as carriers.
Dissolved in plasma, (free, unbound)
Half life
Long (hours, days)
Short (minutes)
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
4
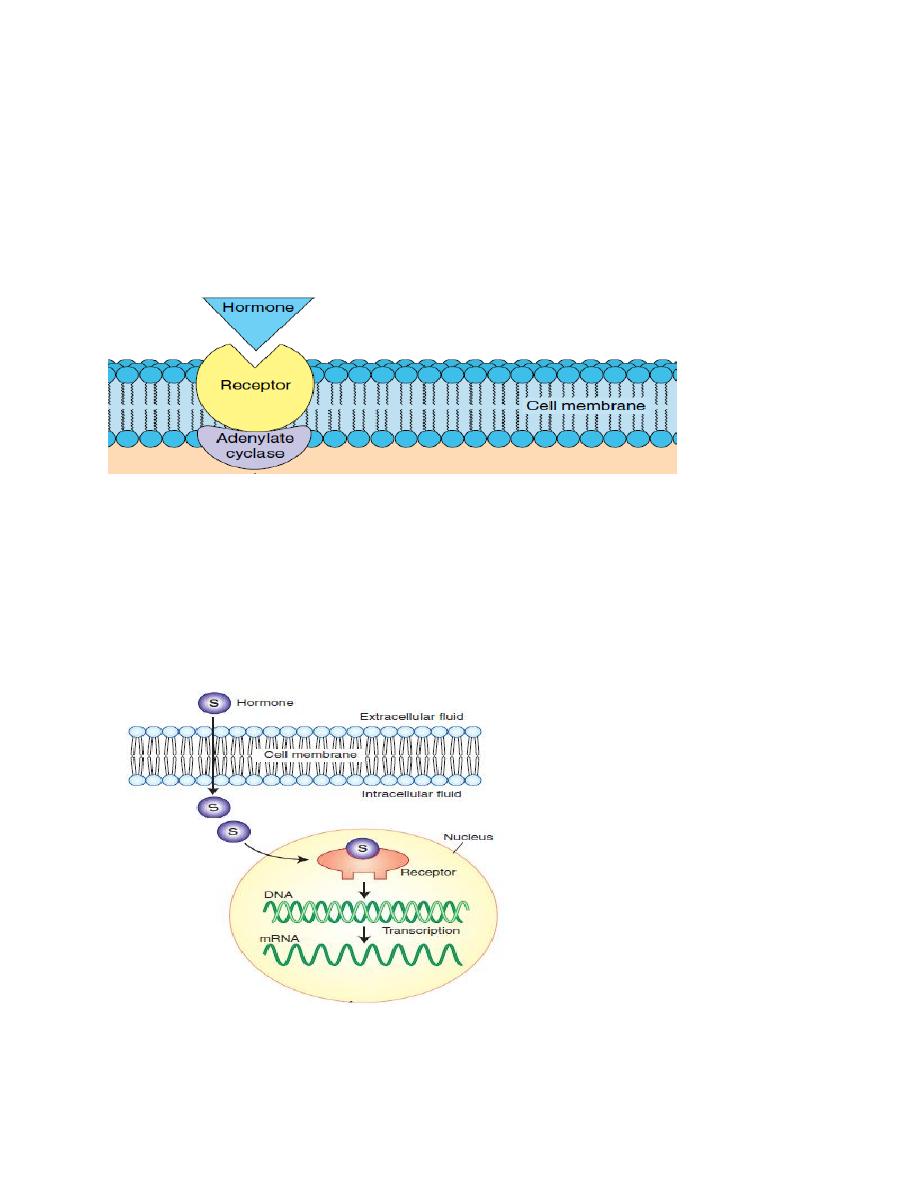
Hormone receptors locations:
The locations for the different types of hormone receptors are:
1- On the cell membrane like protein hormones receptors.
Figure. The cell membrane receptor.
2. In the cytoplasm like steroid hormones receptors (steroid hormone are lipid
soluble, thus they go directly into the target cell).
3. In the nucleus, like thyroid hormones receptors.
Figure . The nuclear receptor.

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
5
Receptors are dynamic structures. For example, decreased hormone levels often
produce an increase in receptor numbers by means of a process called up-
regulation; this increases the sensitivity of the body to existing hormone levels. In
other cases, prolonged exposure to high hormone concentrations decrease the
number of receptors to the specific hormones in target cells, so that they respond
less vigorously to hormonal stimulation, a phenomenon called down-regulation.
Transport of hormones
In most cases, 90% or more of steroid and thyroid hormones in the blood are
bound to plasma proteins. The liver produces proteins that bind lipid-soluble
hormones, e.g.:
• Cortisol-binding globulin
• Thyroid-binding globulin
• Sex hormone-binding globulin (SHBG).
Typically, for hormones that bind to carrier proteins, only 1 to 10% of the total
hormone present in the plasma exists free in solution. However, only this free
hormone is biologically active. The free hormone and carrier-bound hormone are
in a dynamic equilibrium with each other. When the concentration of the free form
of a hormone decreases, then more of this hormone will be released from the
binding proteins.
The binding of hormones to plasma proteins has several beneficial effects,
including:
• Facilitation of transport
Steroid and thyroid hormones are minimally soluble in the blood. Binding to
plasma proteins renders them water soluble and facilitates their transport.
• Prolonged half-life
Protein binding also prolongs the circulating half-life of these hormones. Because
they are lipid soluble, they cross cell membranes easily. As the blood flows

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
6
through the kidney, these hormones would enter cells or be filtered and lost to the
urine if they were not held in the blood by the impermeable plasma proteins.
• Hormone reservoir
Finally, the protein-bound form of the hormone serves as a “reservoir” of hormone
that minimizes the changes in free hormone concentration when hormone secretion
from its endocrine gland changes abruptly.
Modulation
Liver dysfunction and androgens can decrease the level of binding proteins while
estrogens can increase the circulating level of binding proteins. For example, a rise
in circulating estrogen during pregnancy increase the circulating level of binding
proteins, which binds more free hormone. The transient decrease in free hormone
stimulate gland to secrete more hormone quickly and returns the plasma free
hormone to normal. Thus, assays of total hormone content during pregnancy might
be misleading, since free hormone concentrations may be in the normal range. In
such cases, it is helpful to determine the extent of protein binding, so free hormone
concentrations can be estimated.
Functional classification of hormones
Hormones are classified into two functional categories:
• Trophic hormones
• Nontrophic hormones
A trophic hormone acts on another endocrine gland to stimulate secretion of its
hormone. For example, thyrotropin, or thyroid-stimulating hormone (TSH),
stimulates the secretion of thyroid hormones. Adrenocorticotropin, or
adrenocorticotropic hormone (ACTH), stimulates the adrenal cortex to secrete the
hormone cortisol. Both trophic hormones are produced by the pituitary gland; in
fact, many trophic hormones are secreted by the pituitary.
A nontrophic hormone acts on nonendocrine target tissues. For example,
parathormone released from the parathyroid glands acts on bone tissue to stimulate

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
7
the release of calcium into the blood. Aldosterone released from the cortical region
of the adrenal glands acts on the kidney to stimulate the reabsorption of sodium
into the blood.
Mechanisms of Hormone Action
Hormone action includes one or more of the following changes:
1. By opening or closing ion channels in plasma membrane
2. Stimulates synthesis of proteins or regulatory molecules
3. Activates or deactivates enzymes.
4. Induces secretory activity.
5. Stimulates mitosis.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
8
L2
Pituitary gland, or hypophysis, is a small gland-about 1cm. in diameter and 0.5 to 1
gram in weight, located at the base of the brain just below the hypothalamus. The
pituitary gland has usually been thought of as the ‘‘master gland’’ because its
hormone secretions control the growth and activity of other endocrine glands.
Physiologically, the pituitary gland is divisible into two distinct portions:
1. The anterior pituitary, also known as the adenohypophysis originate from
Rathke's pouch, which is an embryonic invagination of the pharyngeal epithelium.
2. The posterior pituitary, also known as the neurohypophysis originate from a
neural tissue outgrowth from the hypothalamus.
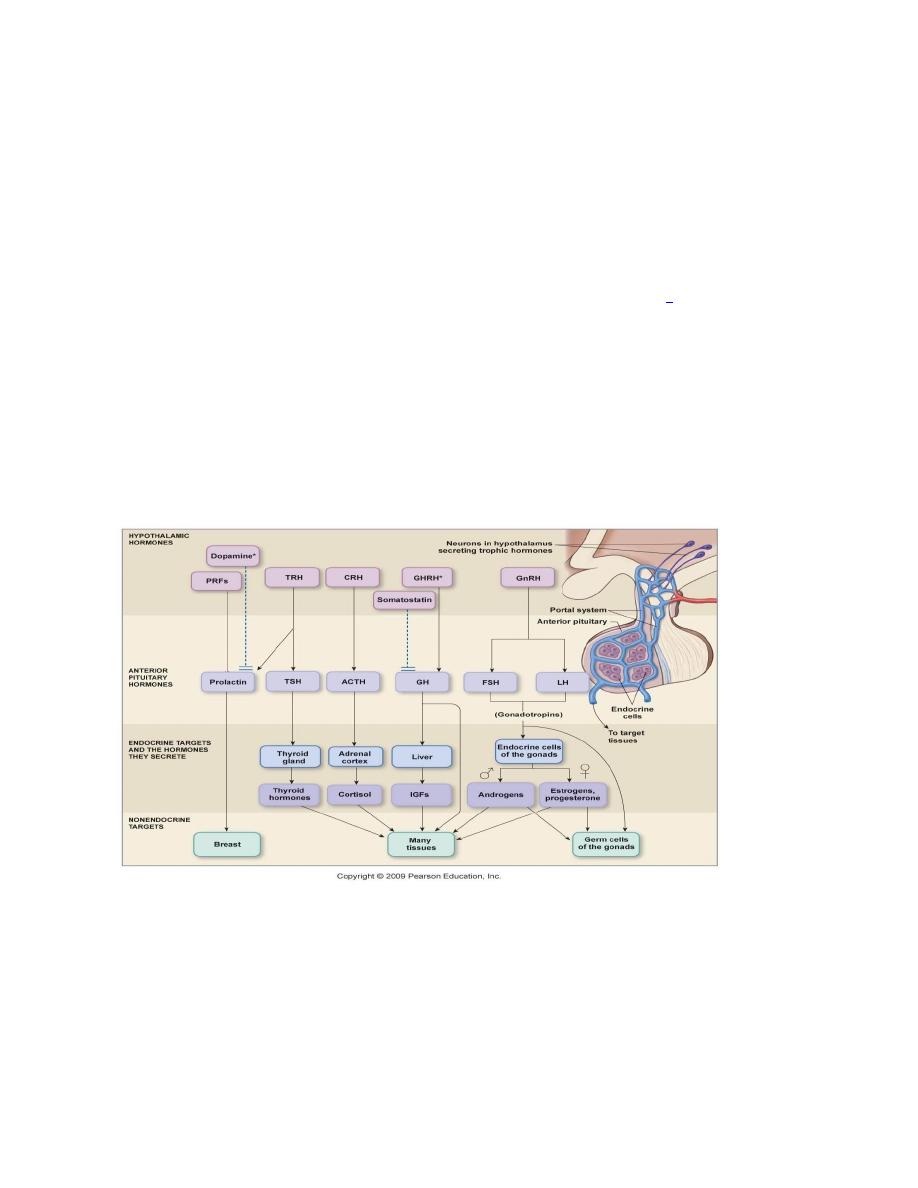
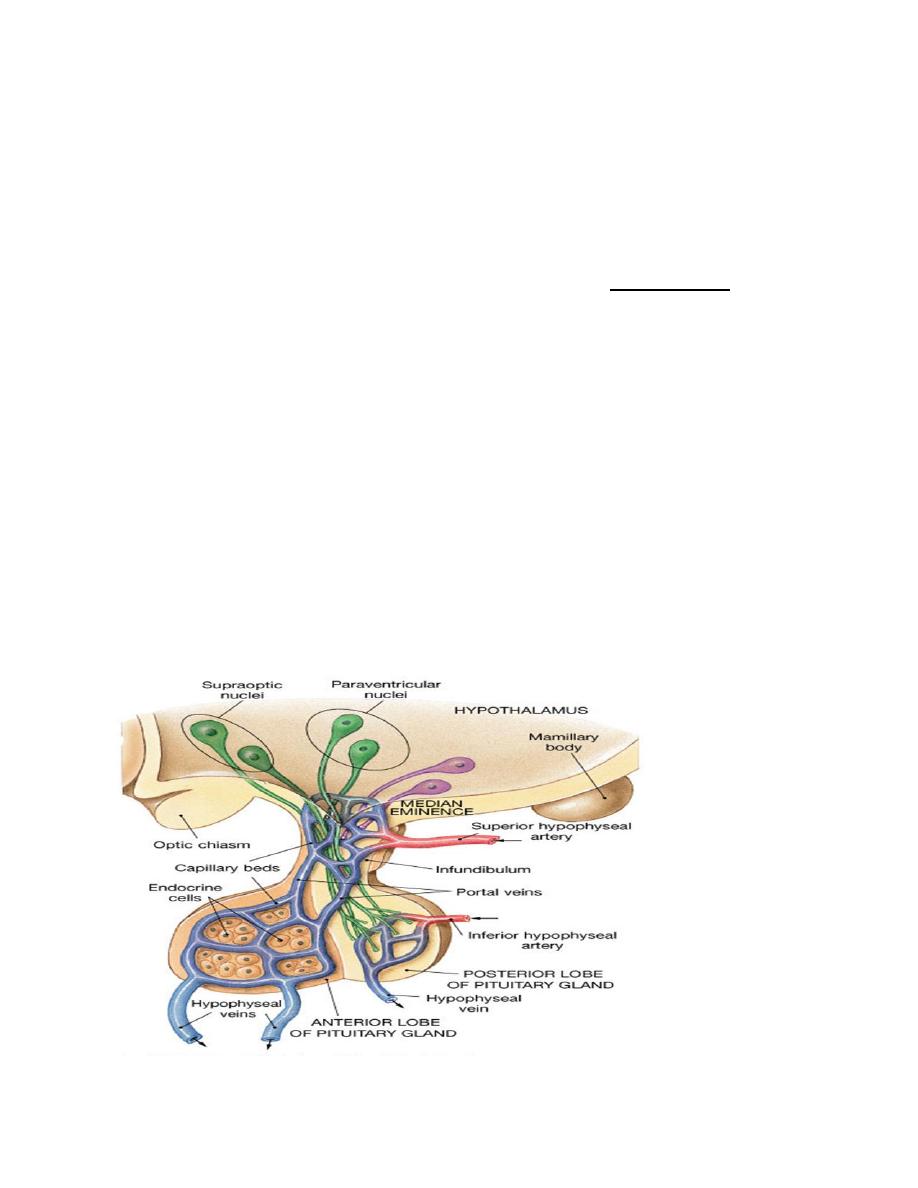
Hypothalamic-anterior pituitary axis
The hypothalamus oversees most endocrine activity. Special cells in the
hypothalamus secrete hormones influence the activity of the anterior pituitary
gland. The nerve endings all come together in the median eminence region of the
hypothalamus. The hormones are then secreted into the hypophyseal- portal system

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
9
and transported to the anterior pituitary. The hormones are either releasing
hormones (RH) or inhibiting hormones (IH). All hormones in this system are
water-soluble. The pattern of secretion of hormones in the hypothalamic-anterior
pituitary system, is mainly pulsatile, a possible exception is the thyroid system.
The pulsatile release of GnRH prevents down regulation of its receptors on the
gonadotrophs of the anterior pituitary.
Hypothalamic releasing and hypothalamic inhibitory hormones
The major hypothalamic releasing and inhibitory hormones are:
1. Thyrotropin-releasing hormone (TRH) stimulate secretion of TSH.
2. Corticotropin-releasing hormone (CRH) stimulate secretion of ACTH
3. Prolactin-inhibiting factor (PIF) inhibits secretion of prolactin.
4. Growth hormone inhibiting hormone (somatostatin) inhibits secretion of growth
hormone by somatotropes.
5. Growth hormone-releasing hormone (GHRH) stimulate secretion of growth
hormone.
6. Gonadotropin-releasing hormone (GnRH) stimulate secretion of luteinizing
hormone (LH) and follicle-stimulating hormone (FSH).
Hormones of anterior pituitary
There are five types of cells in the anterior pituitary:
1. Somatotrophs cells (50%) secrete growth (GH) by the effect of GHRH secreted
by hypothalamus. the secretion of GH inhibited by Somatostatin (GHIH).
2. Corticotrophs cells (10-25 %) secrete ACTH by the effect of CRH secreted by
hypothalamus. Adrenocorticotropin ACTH (corticotropin ) controls the secretion
of some of the adrenocortical hormones, which affect the metabolism of glucose,
proteins, and fats.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
10
3. Gonadotrophs cell (10- 15 %) secrete LH, FSH by the effect of GnRH secreted
by hypothalamus. Follicle-stimulating hormone (FSH) and luteinizing hormone
(LH), control growth of the ovaries and testes.
4. Thyrotrophs cell (10%) secrete thyroid-stimulating hormone (TSH) or
thyrotropin by the effect of TRH secreted by hypothalamus. TSH
controls the rate
of secretion of thyroxine and triiodothyronine by the thyroid gland, and these
hormones control the rates of most intracellular chemical reactions in the body.
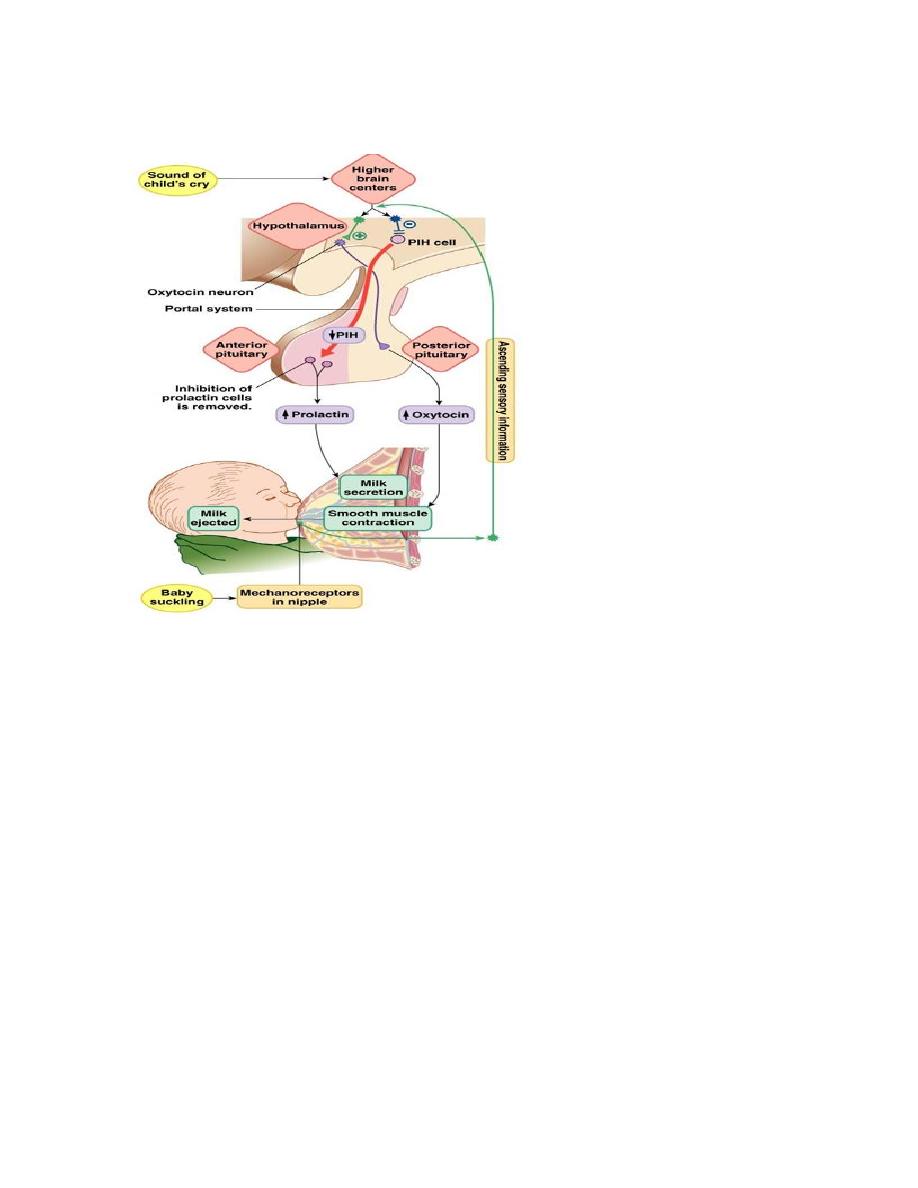
5. Lactotrophs cell (10-15%) secrete Prolactin. The secretion of prolactin inhibited
by Prolactin-inhibiting factor (PIF). Prolactin promote mammary gland
development and milk production.
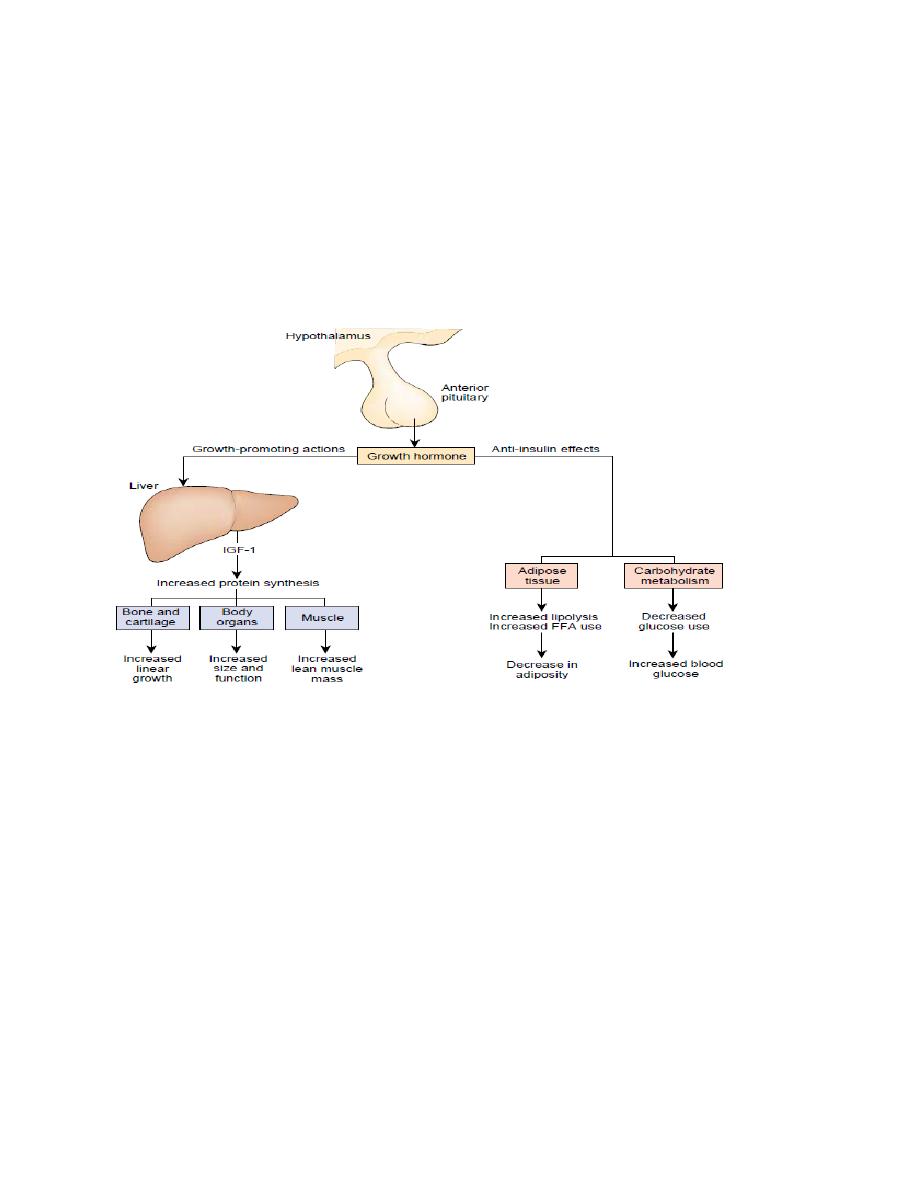
Growth Hormone (somatotropic hormone or somatotropin).
Growth hormone (GH, somatotropin) is a single chain polypeptide comprising 191
amino acids synthesized and secreted by cells in the anterior pituitary. Growth
hormone promotes growth of the entire body by affecting protein formation, cell
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
11
multiplication, and cell differentiation. This hormone is essential for normal
growth and development of the skeleton as well as visceral, or soft, tissues from
birth until young adulthood. Growth of the skeleton involves an increase in bone
thickness and an increase in bone length. The mechanism of this growth involves
stimulation of osteoblast (bone-forming cell) activity and proliferation of the
epiphyseal cartilage in the ends of the long bones. The growth of visceral tissues
occurs by hyperplasia (increasing the number of cells) and hypertrophy (increasing
the size of cells).
The effects of GH on linear growth occurs indirectly through stimulation of
insulin-like growth factor (IGF)-1 also known as somatomedin C which are
produced mainly by the liver. In children, with Laron-type dwarfism, GH levels are
normal or elevated, but there is a hereditary defect in
IGF production.
Metabolic effects of GH
Protein metabolism
• Increase in tissue amino acid uptake
• Stimulation of protein synthesis "protein sparer."
Lipid metabolism
• Increase in blood fatty acids
• Stimulation of lipolysis
• Inhibition of lipogenesis
Carbohydrate metabolism
• Increase in blood glucose
• Decrease in glucose uptake by muscle
• Increase in the hepatic output of glucose (glycogenolysis)
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
12
The net effects of these actions include enhanced growth due to protein synthesis;
enhanced availability of fatty acids for use by skeletal muscle as an energy source;
and glucose sparing for the brain, which can use only this nutrient molecule as a
source of energy.
Figure: Action of GH
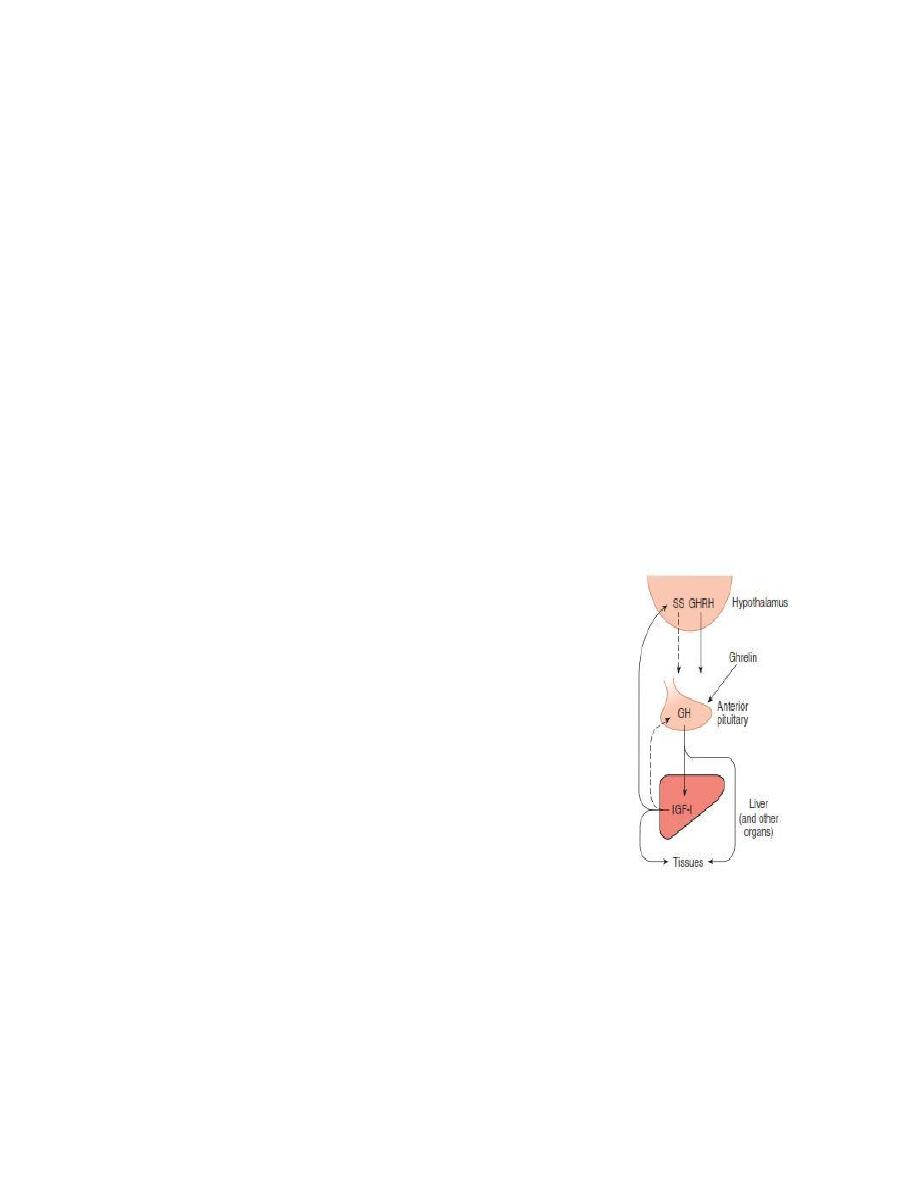
Control of Growth Hormone Secretion
GH secretion is stimulated by hypoglycemia, fasting, starvation, increased blood
levels of amino acids (particularly arginine), and stress conditions such as trauma,
excitement, emotional stress, and heavy exercise. GH is inhibited by increased
glucose levels, free fatty acid release, cortisol, and obesity. However, its primary
controllers are:
Growth hormone-releasing hormone (GHRH) stimulates both the synthesis
and secretion of growth hormone.

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
13
Somatostatin (SS) or Growth hormone-inhibitory hormone (GHIH)
it inhibits growth hormone release.
Ghrelin is a peptide hormone secreted from the stomach. Ghrelin binds to
receptors on somatotrophs and potently stimulates secretion of growth
hormone.
High blood levels of IGF-I lead to decreased secretion of GH.
Growth hormone also feeds back to inhibit GHRH secretion.
The secretion of GH follows a diurnal rhythm with GH levels low and constant
throughout the day and with a marked burst of GH secretion approximately one
hour following the onset of sleep.
In most individuals, production of GH decreases
after 30 years of age, this decrease in GH production is likely a critical factor in the
loss of lean muscle mass at a rate of 5% per decade and gain of body fat at the
same rate after 40 years of age.
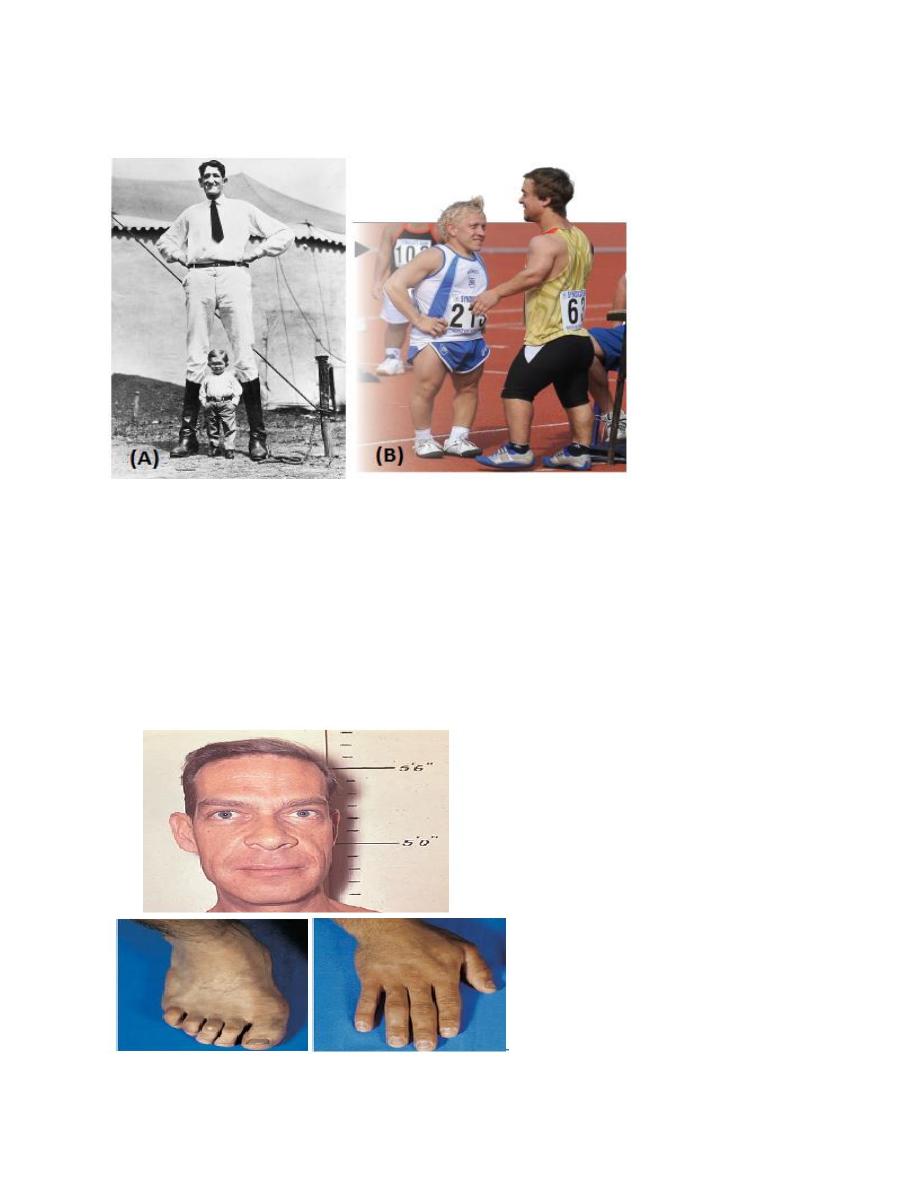
Gigantism
A tumor of the anterior pituitary existing prior to puberty causes secretion of too
much human growth hormone, resulting in gigantism. The acceleration of bone
growth in this condition results in a person with normal proportions but taller-than-
normal height.
Dwarfism
When the levels of secretion of human growth hormone (hGH) by the anterior
pituitary are insufficient or the production of IGF-1 is low prior to puberty, bone
growth is impaired and the individual does not grow to normal height. A person
with pituitary dwarfism has normal body proportions but overall shorter-than-
normal height.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
14
Acromegaly
Is a disorder in which too much growth hormone is produced in adults. This
disorder is caused by an increased production of growth hormone or by a tumor of
the pituitary gland. The primary signs and symptoms include enlargement of the
bones in the entire skull as well as in the hands and feet, and thickening of the skin
other symptoms include headache, fatigue, profuse sweating, weight gain,
excessive hair production, cardiovascular diseases, arthritis, and vision problems.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
15
Posterior Pituitary Gland
L3
The bodies of the cells that secrete the posterior pituitary hormones are not located
in the pituitary gland itself but are large neurons, called magnocellular neurons,
located in the supraoptic and paraventricular nuclei of the hypothalamus. The
hormones are then transported in the axoplasm of the neurons’ nerve fibers passing
from the hypothalamus to the posterior pituitary gland. The two hormones secreted
by the posterior pituitary are.
1. Antidiuretic hormone (also called vasopressin) controls the rate of water
excretion by the kidney, thus helping to control the concentration of water in the
body.
2. Oxytocin, this hormone has two functions:
(a) it helps express milk from the glands of the breast to the nipples during
suckling
(b) it possibly helps in the delivery of the baby at the end of gestation.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
16
Figure: hypothalamic-pituitary vasculature
Regulation of ADH (AVP) secretion
ADH (Arginine vasopressin) is a nine amino acid peptide, it increase water
reabsorption in the collecting ducts of the kidney. This is achieved by binding to
the G-protein-coupled V2 receptor in the basolateral membrane of the collecting
duct cells.
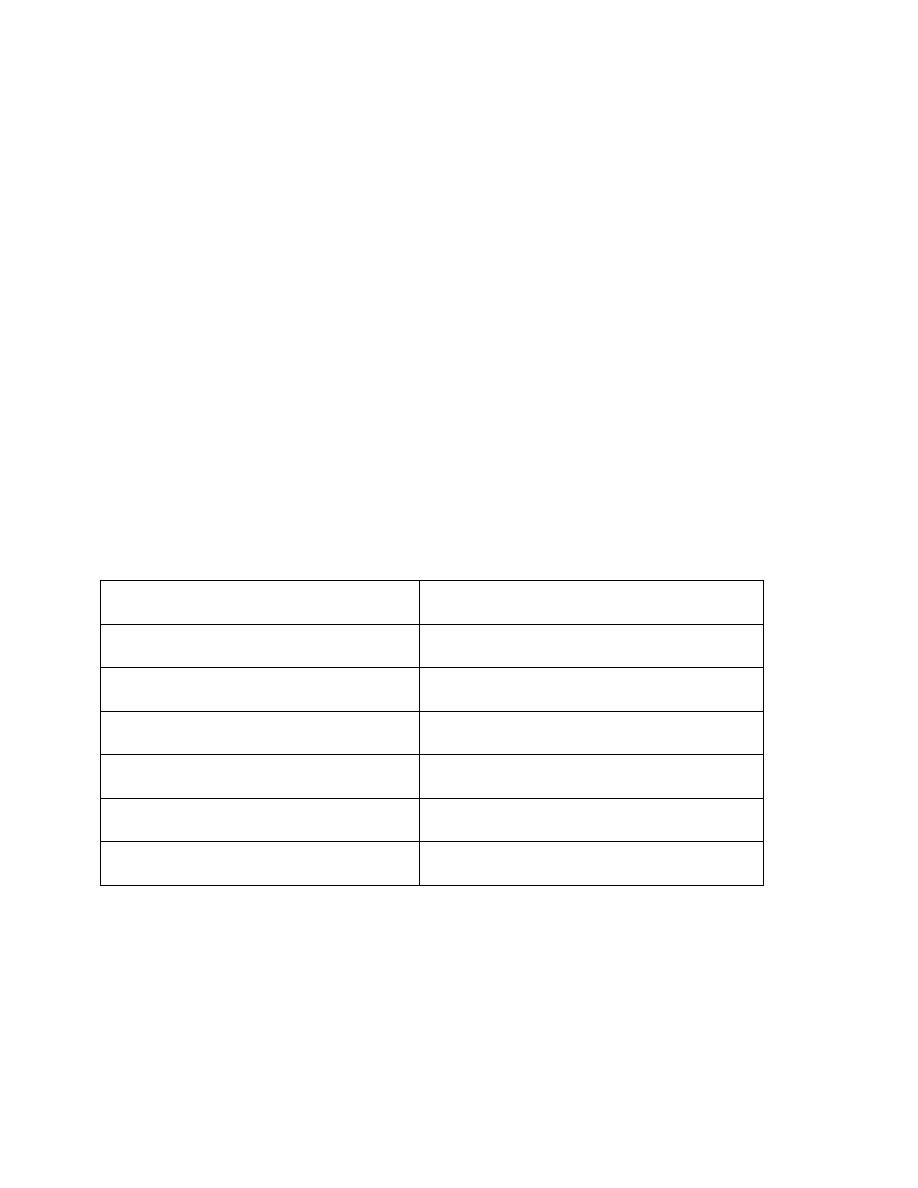
Antidiuretic hormone secretion is regulated by several factors:
• Plasma osmolarity
• Blood volume
• Blood pressure
• Alcohol
factors that increase ADH secretion factors that decrease ADH secretion
↑ Plasma osmolarity
↓ Plasma osmolarity
↓ Blood volume
↑ Blood volume
↓ Blood pressure
↑ Blood pressure
Hypoxia, Nausea
Alcohol
Morphine, Nicotine
Clonidine (antihypertensive drug)
Cyclophosphamide
Haloperidol (dopamine blocker)
Diabetes lnsipidus (DI) is a condition characterized by large amounts of dilute
urine and increased thirst. The amount of urine produced can be nearly 20 liters per
day.

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
17
-Central DI (CDI) is due to a lack of the hormone vasopressin (antidiuretic
hormone). This can be due to damage to the hypothalamus or pituitary
gland or genetics.
-Nephrogenic diabetes insipidus (NDI) occurs when the kidneys do not respond
properly to vasopressin
Oxytocin
Oxytocin stimulates contraction of the smooth muscle in the wall of the uterus.
During labor, this facilitates the delivery of the fetus and, during intercourse, may
facilitate the transport of the sperm through the female reproductive tract. Oxytocin
also causes contraction of the myoepithelial cells surrounding the alveoli of the
mammary glands. This results in “milk letdown” or the expulsion of milk from
deep within the gland into the larger ducts from which the milk can be obtained
more readily by the suckling infant.
The secretion of oxytocin is regulated by reflexes elicited by cervical stretch and
by suckling. The release of oxytocin may be inhibited by pain, fear, or stress.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
18
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
19
L4
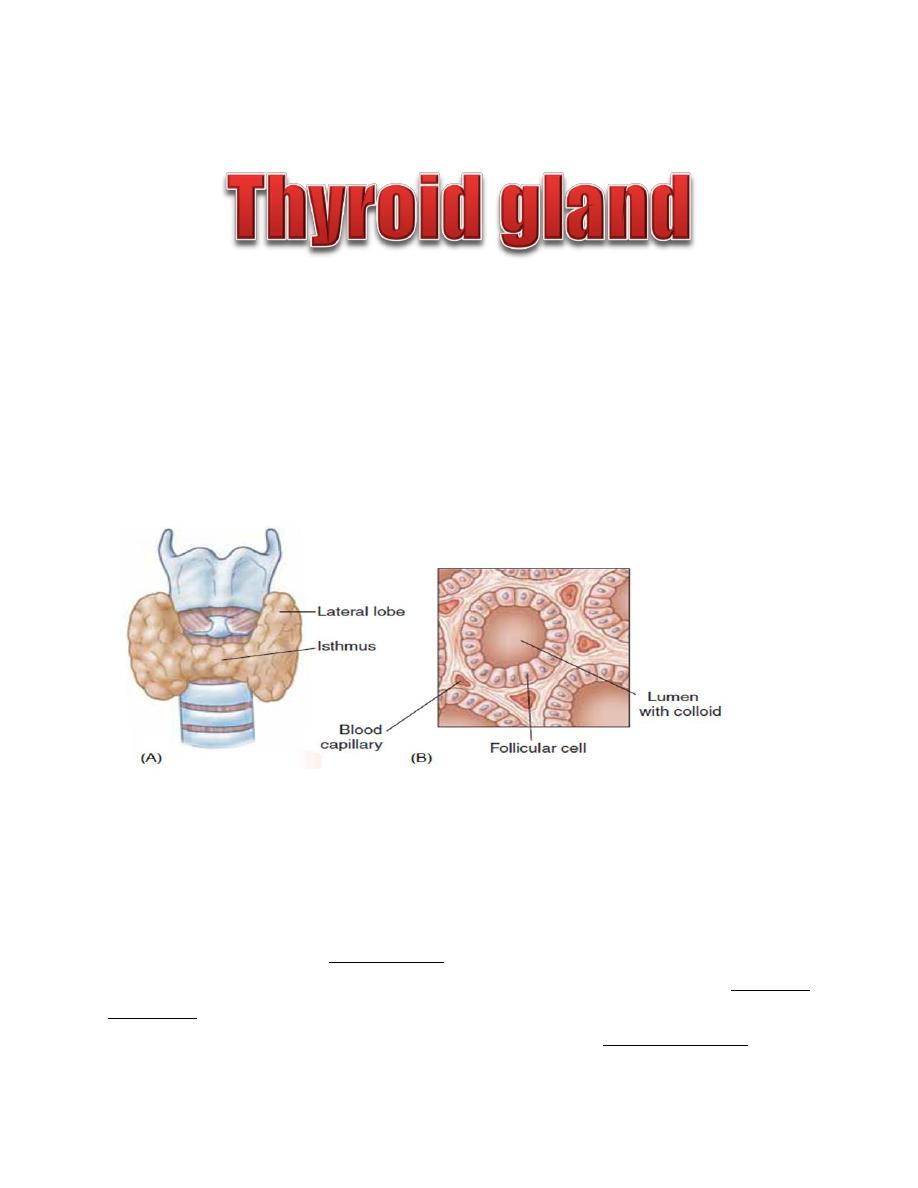
The thyroid gland is a butterfly-shaped structure lying over the ventral surface of
the trachea just below the larynx. This gland produces two classes of hormones
synthesized by two distinct cell types:
• Thyroid hormones (T3 and T4) synthesized by follicular cells
• Calcitonin synthesized by parafollicular cells
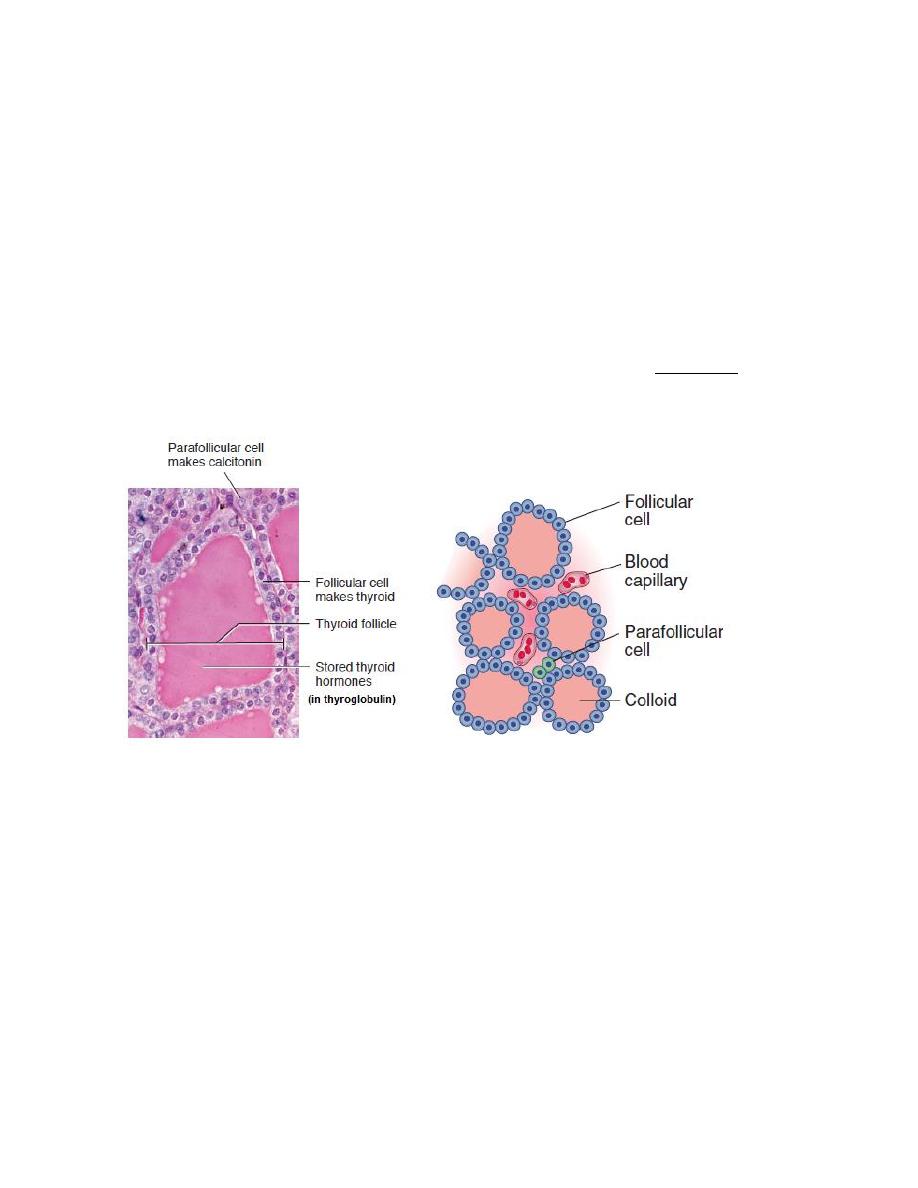
Thyroid Follicle
The thyroid gland is composed of a large number (3million) of tiny, saclike
structures called follicles. These are the functional units of the thyroid. Each
follicle is formed by a single layer of epithelial (follicular) cells and is filled with a
secretory substance called colloid, which consists largely of a glycoprotein-
tyrosine complex called thyroglobulin. Each follicle is surrounded by a dense
capillary network separated from epithelial cells by a well defined basement
membrane. The apical membranes of the follicular cells, which face the lumen, are
covered with microvilli and pseudopods formed from the apical membrane extend
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
20
into the lumen. The amount of thyroid hormones stored within the colloid is
enough to supply the body for 2 to 3 months.
Parafollicular Cells
In addition to the epithelial cells that secrete T4 and T3, the wall of the thyroid
follicle contains small numbers of parafollicular cells. The parafollicular cell is
usually embedded in the wall of the follicle, inside the basal lamina surrounding
the follicle. Parafollicular cells produce and secrete the hormone calcitonin.
Figure: thyroid follicle.
Synthesis of thyroid hormone
The steps involved in the synthesis of thyroid hormone are:
1. Synthesis and Secretion of the Thyroglobulin.
Thyroglobulin is synthesized in the follicular cells, then it enters the lumen via
exocytosis. This large protein contains tyrosyl groups.
2. Iodide Uptake
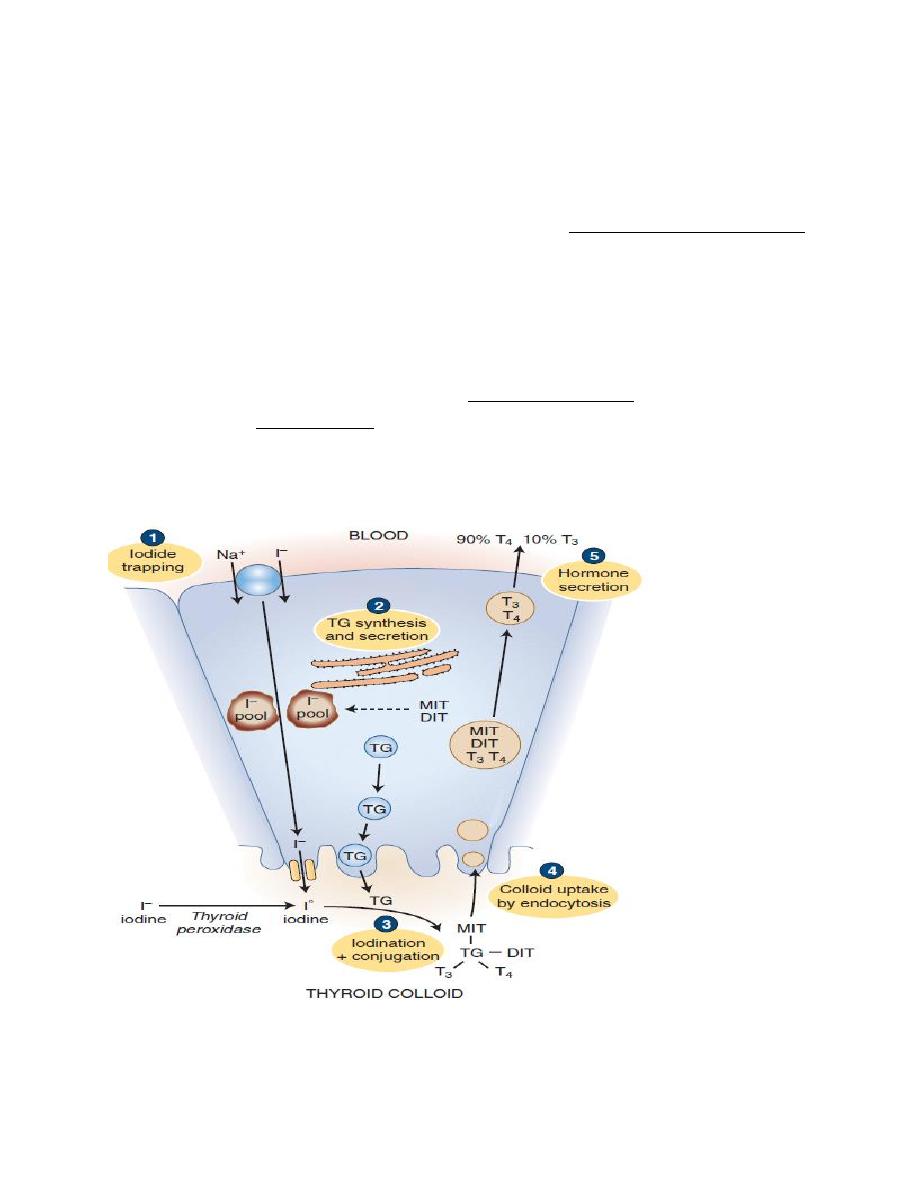
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
21
Iodide uptake is via a sodium/iodide symporter on the basal membrane (NIS). This
pump can raise the concentration of I within the cell to as much as 250 times that
of plasma. The pump can be blocked by anions like perchlorate and thiocyanate,
which compete with Iodine. The NIS derives its energy from Na
+
/ K
+
- ATPase,
which drives the process.
3. Oxidation of Iodide
Once Iodide is pumped into the cell, it traverses the cell to the apical membrane,
where it is oxidized into iodine
atoms, by Thyroid peroxidase. Thyroid peroxidase
is inhibited by polythiouracil (PTU), which blocks the synthesis of thyroid
hormones by blocking all of the steps catalyzed by thyroid peroxidase.
Figure: thyroid hormone synthesis and secretion

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
22
4. Iodination
At the apical membrane, just inside the lumen of the follicle, iodine
atoms
combines with the tyrosine moieties of thyroglobulin, to form monoiodotyrosine
(MIT). The iodination of thyroglobulin is catalyzed by the enzyme thyroid
peroxidase. A second iodine atom may be added to a MIT residue by this same
enzymatic process, forming a diiodotyrosine (DIT).
5. Coupling
Peroxidase enzyme promotes the coupling of iodinated tyrosine in the
thyroglobulin molecule. When two DITs couple, tetraiodothyronine (T4) is
formed. When one DIT and one MIT combine, triiodothyronine (T3) is formed.
The hormones stored in the follicular lumen as colloid until the thyroid gland is
stimulated to secrete its hormones. The thyroid is unique among endocrine glands
in that it stores its product extracellularly in follicular lumens as large precursor
molecules.
Secretion of T3 and T4 from the Thyroid Gland
1.Pinocytosis: Pieces of the follicular colloid are taken back into the follicle by
endocytosis.
2.Fusion: The endocytosed material fuses with lysosomes, which transport it
toward the basal surface of the cell.
3.Proteolysis of thyroglobulin: Within the lysosomes, the thyroglobulin is broken
into T4, T3, DIT, and MIT.
4.Secretion: T4 and T3 are secreted into the blood, with the T4:T3 ratio being as
high as 20:1 .
5.Deiodination: A microsomal deiodinase removes the iodine from iodinated
tyrosines (DIT and MIT) but not from the iodinated thyronines (T3 and T4). The
iodine is then available for resynthesis of hormone. (Individuals with a deficiency
of this enzyme are more likely to develop symptoms of iodine deficiency).
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
23
Conversion of T4 to T3
T4 is the major secretory product of the thyroid gland and is the predominant
thyroid hormone in the blood. However, about 40% of the T4 secreted by the
thyroid gland is converted to T3 by enzymatic removal of the iodine atom at
position 5
-
of the thyronine ring structure. This reaction is catalyzed by a 5
-
-
deiodinase located in the liver, kidneys, and thyroid gland. The T3 formed by this
deiodination and that secreted by the thyroid react with thyroid hormone receptors
in target cells; therefore, T3 is the physiologically active form of the thyroid
hormones.
Transport of Thyroxine and Triiodothyronine to Tissues
About 70% of the circulating thyroid is bound to thyroid-binding globulin (TBG).
The remainder is attached to thyroxine-binding pre albumin (transthyretin) and
albumin. T4 has the higher affinity for binding proteins; therefore, it binds more
tightly to protein than does T3, and consequently has a greater half-life thanT3.
• T4 half-life = 7 days • T3 half-life = 1 day
Metabolism of thyroid hormones
Both T4, T3 undergo enzymatic deiodinations, particularly in the liver and
kidneys, which inactivate them. T4 and, to a lesser extent, T3 are also metabolized
by conjugation with glucuronic acid in the liver. The conjugated hormones are
secreted into the bile and eliminated in the feces.
Physiologic actions of thyroid hormones
1. Metabolic Rate, thyroid hormones increase metabolic rate, as evidenced by
increased O
2
consumption and heat production.it also increase the activity of the
membrane-bound Na/K- ATPase in many tissues.
2. Thyroid hormones are essential for normal menstrual cycles.
3. Growth and Maturation (T 4 and T 3 are anabolic hormones), thyroid hormones
are absolutely necessary for normal brain maturation, without adequate thyroid

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
24
hormones during the prenatal period, abnormalities rapidly develop in nervous
system maturation. These abnormalities lead to mental retardation and lead to
cretinism unless replacement therapy is started soon after birth.
4. Lipid Metabolism, thyroid hormone accelerates cholesterol clearance from the
plasma.
5. CHO Metabolism, thyroid hormone increases the rate of glucose absorption
from the small intestine.
6. Cardiovascular Effects
Thyroid hormones increase cardiac output by increasing heart rate and stroke
volume. It increase contractility by increasing the number and affinity of β-
adrenergic receptors in the heart to catecholamines (positive inotropic), it act on
the SA node, and directly increase heart rate (positive chronotropic effects).
Disorders of thyroid function
Hypothyroidism
Hypothyroidism is a common endocrine
disorder that affects about 1% of the adult
population at some times. Inadequate
thyroid hormone production can result
from failure at the level of thyroid gland
itself (primary hypothyroidism), or it can
be due to a lack of stimulation from TSH.
Low TSH levels can result from pituitary
dysfunction (secondary hypothyroidism)
or from lack of pituitary stimulation by
hypothalamic
TRH
(tertiary
hypothyroidism).

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
25
Primary Hypothyroidism
L5
Most common cause is an autoimmune destruction of the thyroid with lymphocytic
infiltration like Hashimoto's thyroiditis; TSH increased while, T3 and T4
decreased. The condition characterized by:
• Decreased basal metabolic rate and oxygen consumption
• Plasma cholesterol and other blood lipids tend to be elevated.
• Anemia, constipation, horseness in speech, the skin is dry and cool
• Accumulation of subcutaneous mucopolysaccharides that give rise to a
nonpitting edema (myxedema).
Cretinism
Untreated postnatal hypothyroidism results in
cretinism, a form of dwarfism with mental
retardation. T4 supplementation begun in the
first 6 weeks of life results in normal
intelligence.
Acquired hypothyroidism during childhood
results in dwarfism but there is no mental
retardation.
A major way thyroid hormones
promote normal body growth is by stimulating
the expression of the gene for growth hormone
(GH) in the somatotrophs of the anterior
pituitary gland.
In a thyroid hormone-deficient
individual, GH synthesis by the somatotrophs is
greatly reduced and consequently GH secretion
is impaired; therefore, a thyroid hormone-deficient individual will also be GH-
deficient.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
26
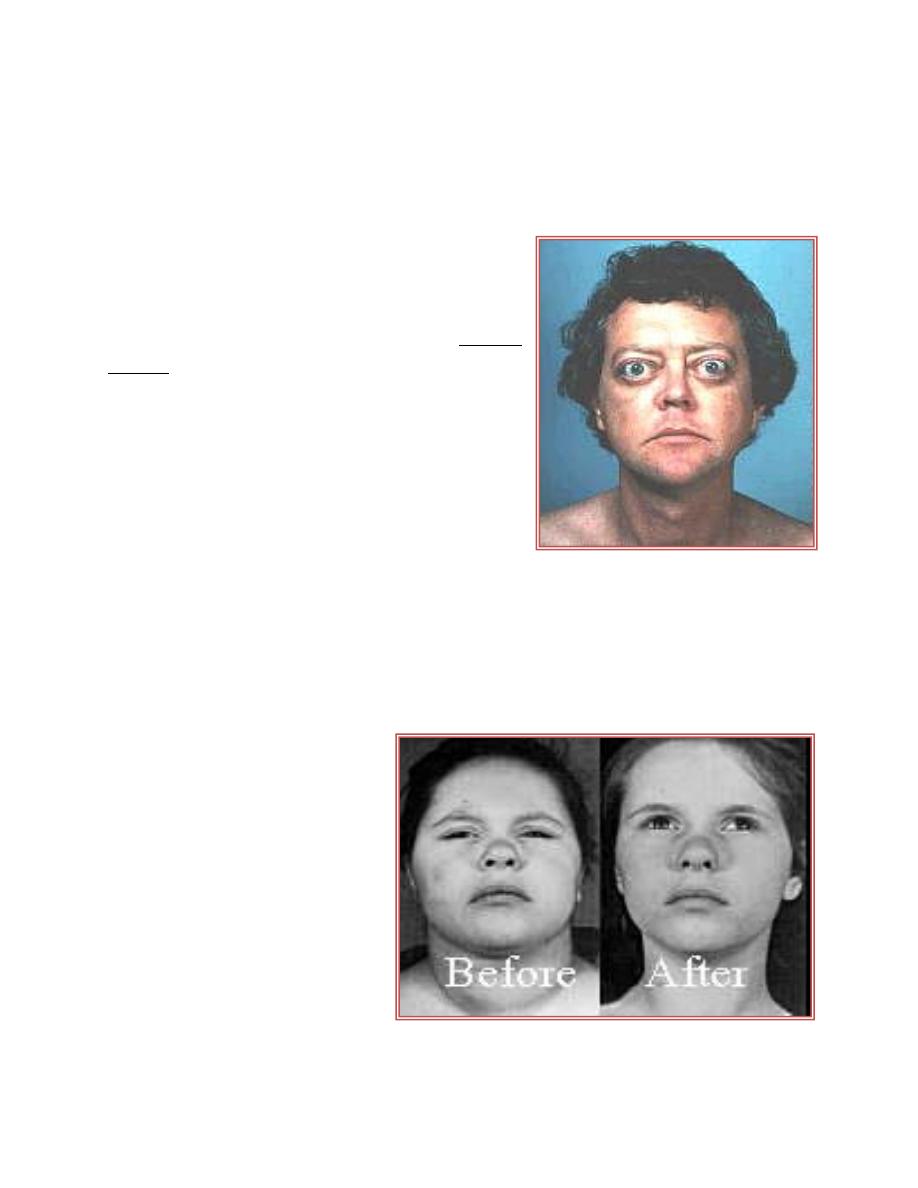
Primary Hyperthyroidism (Graves' Disease)
Thyrotoxicosis by definition is the clinical
syndrome whereby tissues are exposed to high
levels of thyroid hormone (hyperthyroidism).The
most common cause of thyrotoxicosis is Graves'
disease, an autoimmune problem due to antibody
formation against the TSH receptor in the
plasma membranes of thyroid follicular cells.
These antibodies bind to the TSH receptor, and
produces effects similar to those caused by the
action of TSH. In Graves' disease the thyroid is
symmetrically
enlarged.
Cardiac
output,
contractility, and heart rate are increased with
possibly palpitations and arrhythmias. Weight loss with increased food intake,
protein wasting and muscle weakness. Tremor, nervousness, and excessive
sweating. The wide-eyed stare (exophthalmos) in patients with Graves' is caused
by an infiltration of orbital soft tissues and extraocular muscles and the resulting
edema.
Goiter
A goiter is simply an enlarged
thyroid and does not designate
functional status. A goiter can
be present in hypo-, hyper-, and
euthyroid states.
There is no correlation between
thyroid size and function.
A generalized enlargement of
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
27
the thyroid is considered a "diffuse goiter. Diffuse enlargement often results from
prolonged stimulation by TSH or TSH-like factor; e.g., Hashimoto's thyroiditis,
Graves' disease, diet deficient in iodine.
About 99% of calcium is stored in the bones and only about 1% is in the cells, and
0.1 % of body calcium is in the extracellular fluid. The bones can serve as large
reservoirs, releasing calcium when extracellular fluid concentration decreases and
storing excess calcium. 40% of the total blood Ca
2+
is bound to plasma proteins,
mainly albumin, 10% is complexed to anions (e.g., phosphate, sulfate, and citrate)
and 50% are free ionized Ca
2+
and it is the only form of Ca
2+
that is biologically
active. The calcium concentration in the interstitial fluid is 100 times higher than
the intracellular calcium concentration.
Factors affect ECF calcium
Extracellular fluid calcium concentration normally is 2.4 mmol/L, calcium plays a
key role in contraction of muscles; blood clotting; and transmission of nerve
impulses. A number of factors can effect ECF calcium concentration:
1. Increases in plasma protein concentration increases total Ca
2+
concentration.
2. Increases plasma phosphate, decrease the ionized (free) Ca
2+
concentration.
3. Acidosis increases concentration of ionized (free) Ca
2+
.
4. Alkalosis decreases concentration of ionized (free) Ca
2+
.
The usual daily intake of calcium is about 1000 mg. Vitamin D promotes calcium
absorption by the intestines. About 90% (900 mg/day) of the daily intake of
calcium is excreted in the feces.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
28
Parathyroid hormone (PTH)
Physiologic Anatomy of the Parathyroid Glands.
There are four parathyroid glands in humans; they are located immediately behind
the thyroid gland. The parathyroid gland contains mainly chief cells and oxyphil
cells. The chief cells are believed to secrete most of the PTH.
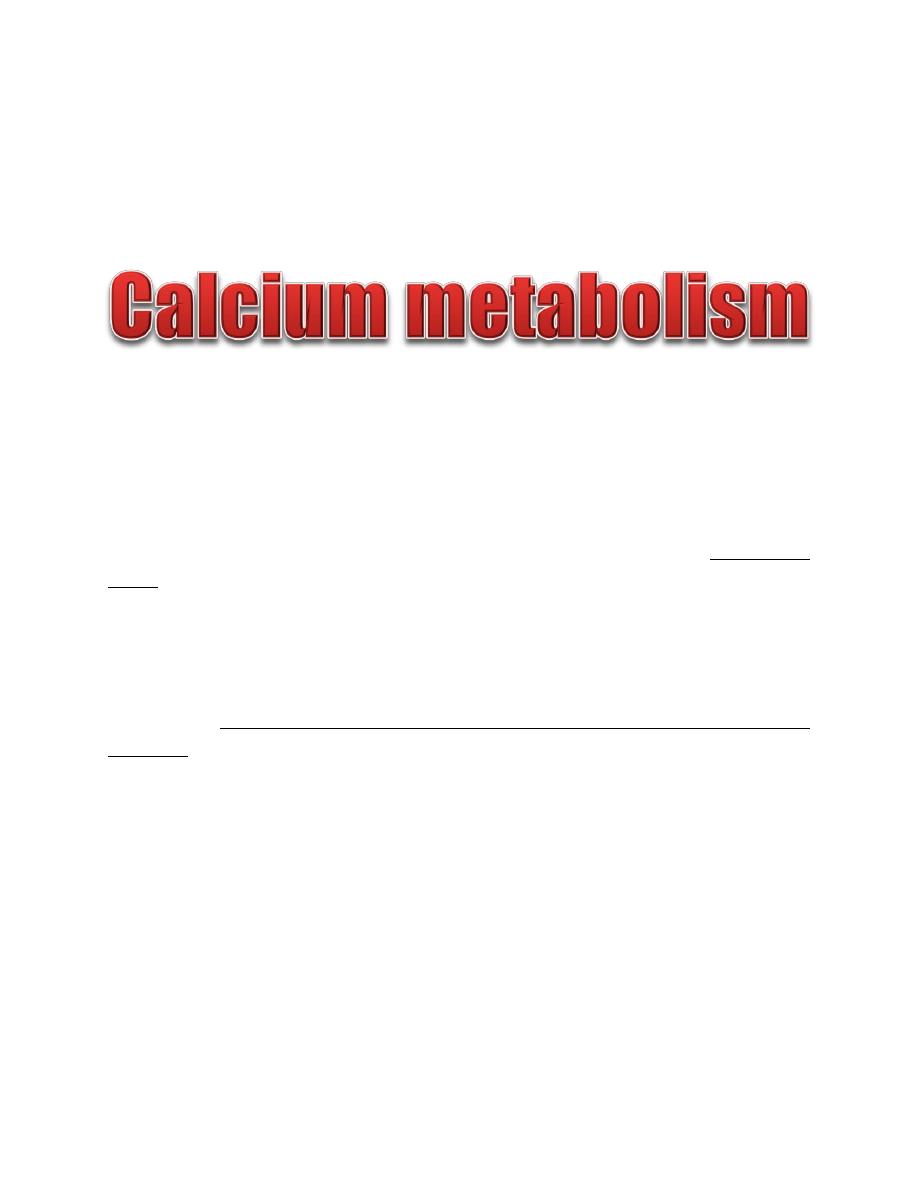
Control of PTH secretion
The rate of PTH secretion is regulated by the following three factors:
1. Plasma free Ca
2+
.
A decrease in the plasma Ca
2+
is the most potent stimulus for PTH secretion. Chief
cells sense plasma Ca
2+
concentration through expression of the extracellular Ca
2+
-
sensing receptor (CaSR).
2. Plasma phosphate.
A prolonged increase in phosphate concentration stimulates PTH secretion.
3. Vitamin D.
PTH stimulates vitamin D synthesis, which exerts negative feedback inhibition on
PTH secretion.
Figure: Control of PTH secretion
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
29
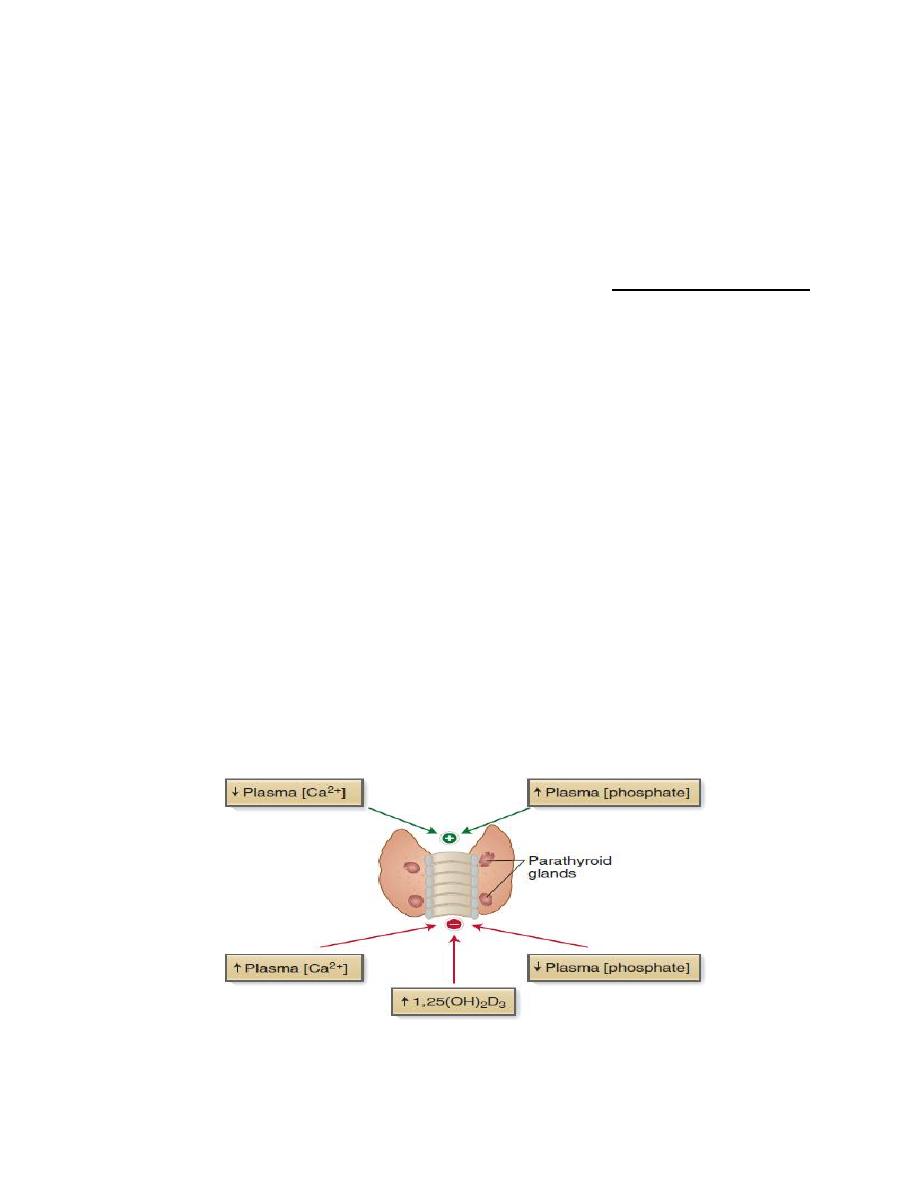
Calcium-sensing receptor (CaSR)
The cells of the parathyroid gland and the basolateral membrane of cells in Thick
segment of loop of Henle contain CaSR, this receptors influence by the plasma
concentration of calcium. when plasma calcium level is high CaSR is activated
which in turn lead to inhibition of the Na
+
-K
+
-2Cl
-
transporter• This will decrease
K
+
movement from cell into lumen, reducing the positive luminal potential which
in turn, decreases calcium reabsorption and return plasma calcium concentration
to normal level.
Figure. Calcium-sensing receptor (CaSR).
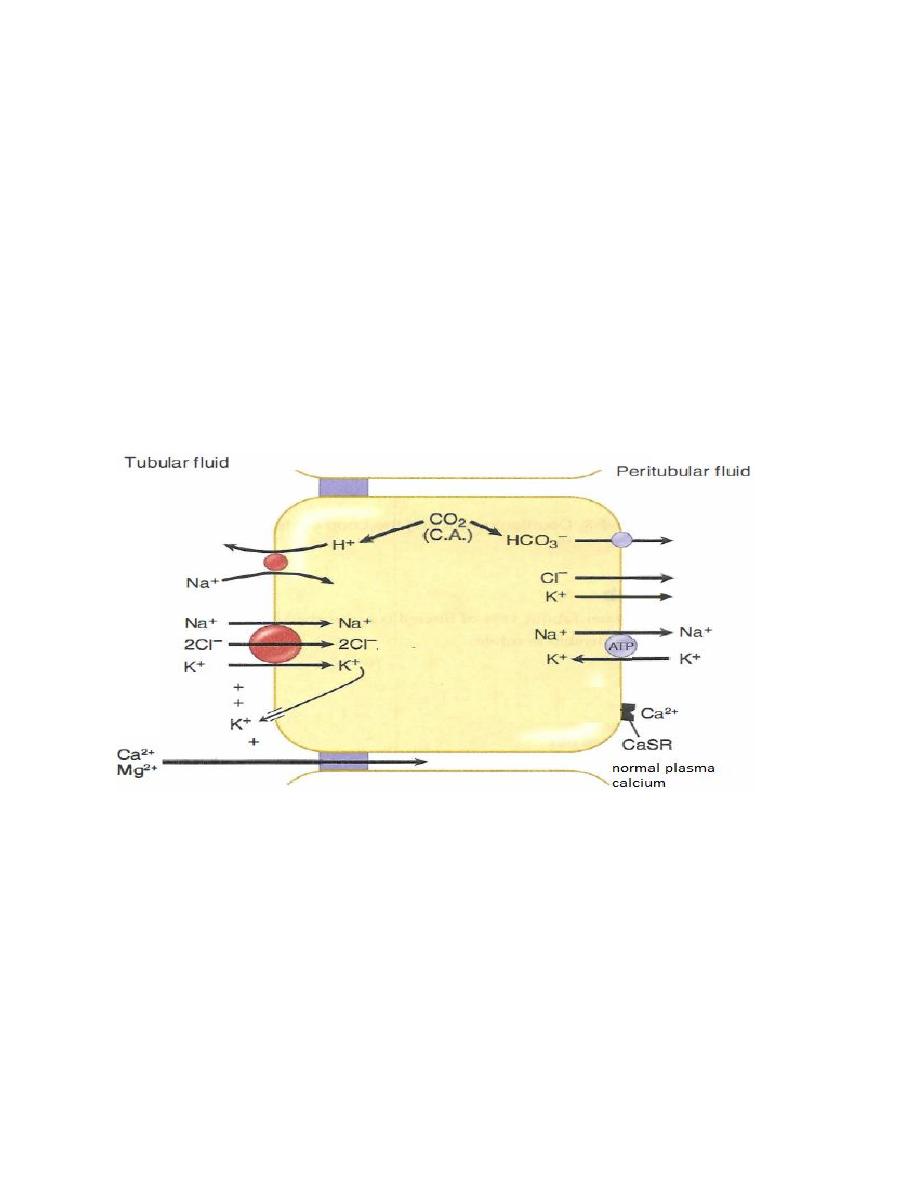
Actions of PTH
A decrease in the free calcium is the signal to increase PTH secretion and the
function of PTH is to raise free calcium, which it does by several mechanisms.
• Increases Ca
2+
reabsorption in distal tubule of the kidney.
• Inhibits phosphate reabsorption in proximal tubule of the kidney.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
30
• Stimulates the 1-alpha-hydroxylase enzyme in kidney, converting inactive
vitamin D to its active form which in turn increase intestinal reabsorption of Ca
2+
and phosphate.
• PTH bind to osteoblasts (cells responsible for osteoid synthesis) inducing the
synthesis of specific proteins, which activate the already present osteoclasts (cells
responsible for bone resorption) and stimulate the formation of new osteoclasts.
Osteoclasts in turn causes bone resorption, releasing Ca
2+
and phosphate into the
blood.
Calcitonin
Calcitonin (CT) is a peptide hormone secreted by the parafollicular cells of the
thyroid gland. It is released in response to elevated free calcium. Calcitonin lowers
plasma calcium by decreasing the activity of osteoclasts, thus decreasing bone
resorption, this results in less demineralization of the bone and therefore a decrease
in the release of calcium and phosphate from the bone into the blood. Calcitonin
has no direct effect on bone formation by osteoblasts.
Calcitonin is useful in the treatment of severe hypercalcemia, osteoporosis and
Paget's disease (disease characterized by a significant increase in osteoclast activity
and, thus, a high rate of bone turnover and hypercalcemia),
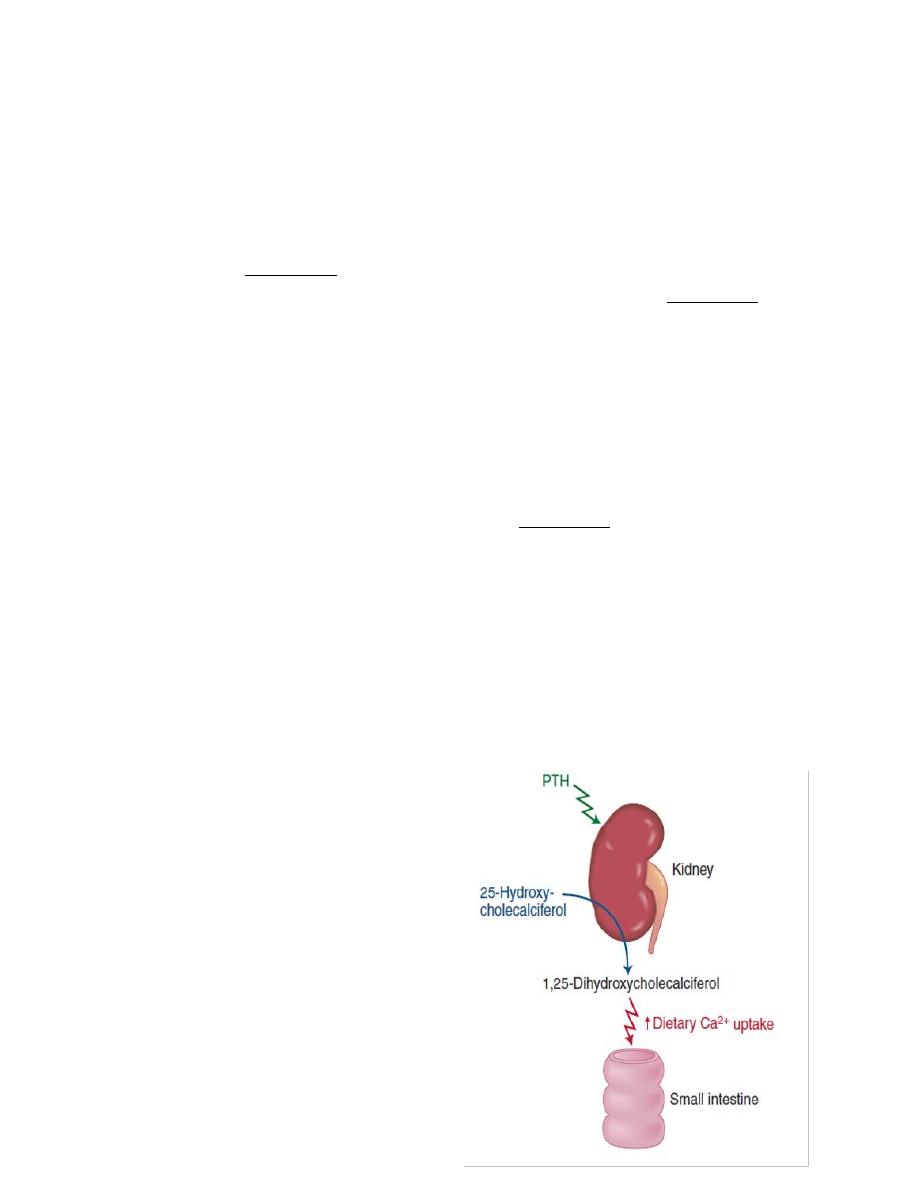
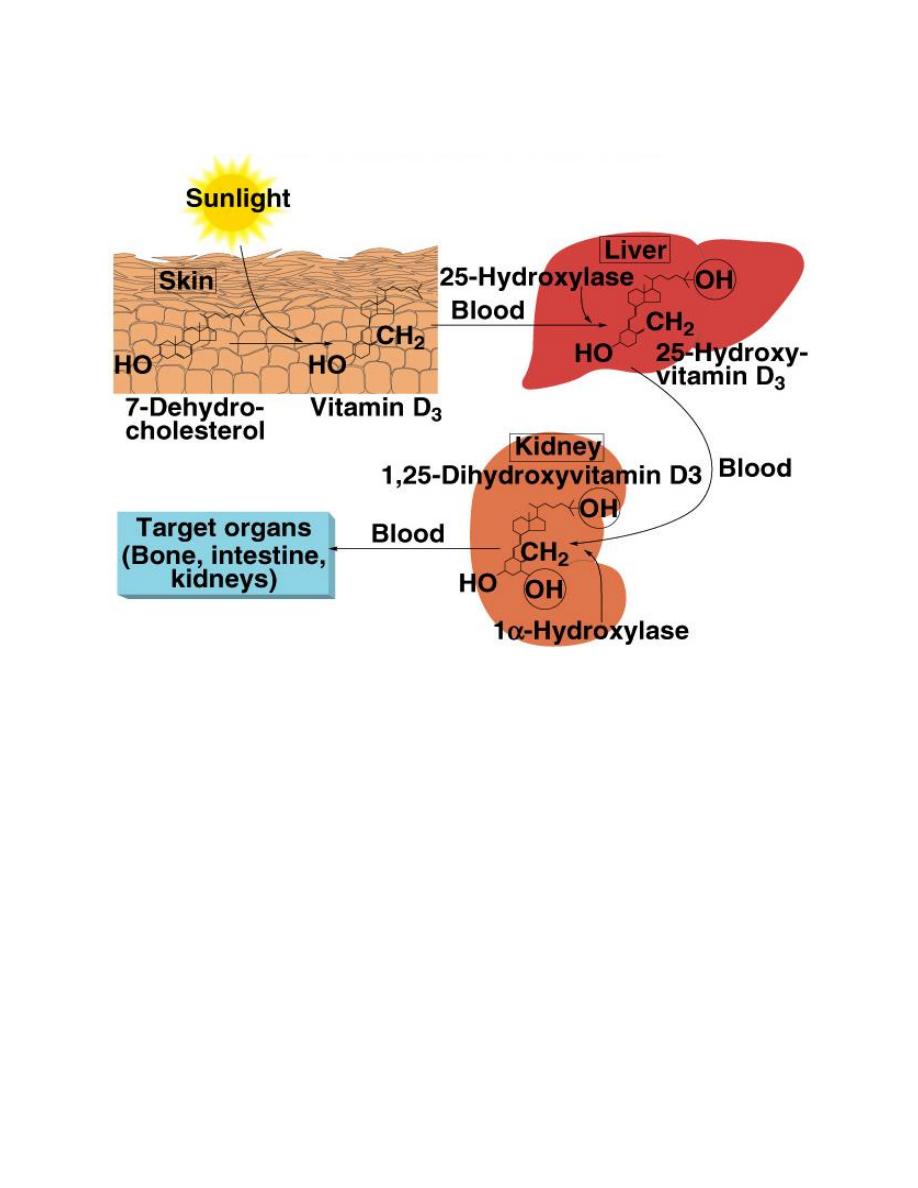
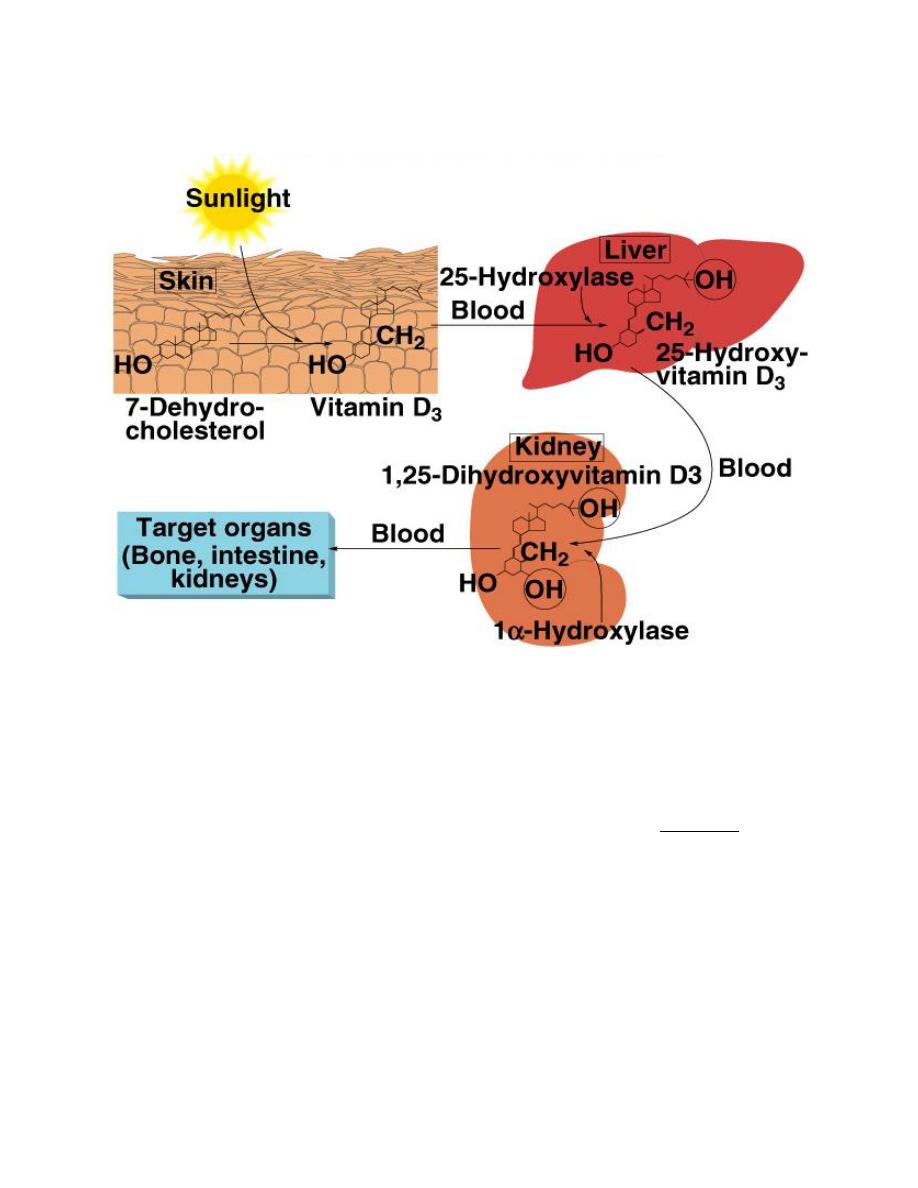
Vitami D (calcitriol)
Vitamin D3 (also called cholecalciferol)
is formed in the skin as a result of
irradiation of 7-dehydrocholesterol, by
ultraviolet rays from the sun. Vitamin D
also provided in the diet. The first step
in the activation of cholecalciferol is to
hydroxylate
it
into
25-
hydroxycholecalciferol, in the liver
which also is inactive. In the kidney, 25-
hydroxycholecalciferol hydroxylated at
the C1 position to produce 1,25-

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
31
dihydroxycholecalciferol, which is the physiologically active form. C1
hydroxylation is catalyzed by the enzyme 1α-hydroxylase, which is regulated by
several factors, including the plasma Ca
2+
concentration and PTH.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
32
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
33
Actions of Calcitriol
Vitamin D acts to raise plasma Ca
2+
and phosphate. Thus, vitamin D promotes
bone deposition. This is accomplished by
1. Calcitriol increases the absorption of Ca
2+
and phosphate by the intestinal
mucosa by increasing the production of the Ca
2+
-binding protein calbindin.
2. Calcitriol enhances PTH's action at the renal distal tubule.
Sex steroids and bone

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
34
The sex steroids estradiol (in females) and testosterone (in males) are required for
maintenance of normal bone mass. In postmenopausal women, there is a marked
decline in estradiol levels, which is associated with a loss of bone mass, called
osteoporosis, and a corresponding increase in bone fractures. Osteoporosis is less
common in males due to a smaller and more gradual decline in testosterone levels
with age.
Rickets
A deficiency of vitamin D during childhood causes a bone deformity called rickets,
which is due to the poor mineralization of bone. Classic clinical finding is bowing
of the lower legs.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
35
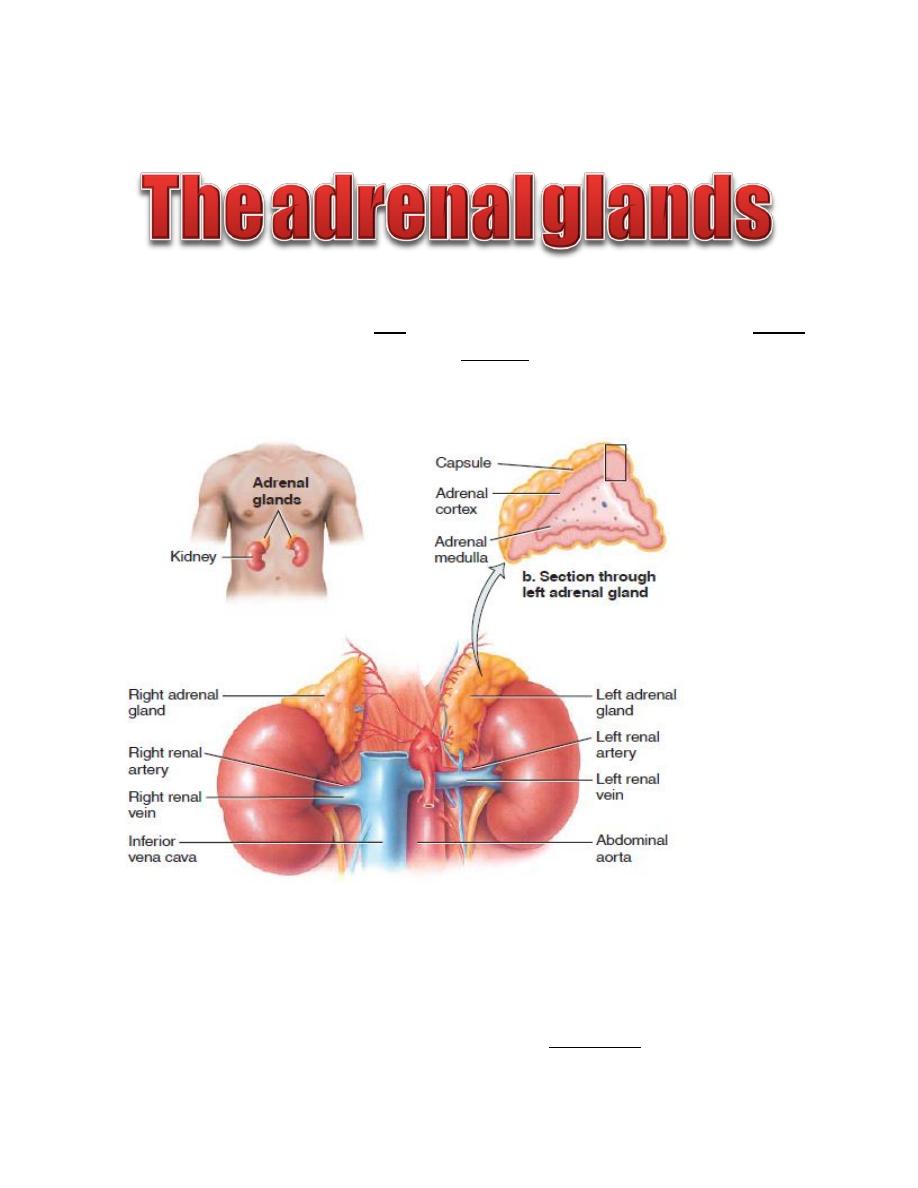
L6
The adrenal glands consist of two functionally distinct parts: the adrenal cortex,
which secretes steroids, and the adrenal medulla, which secretes catecholamines.
Each adrenal glands weighs about 4 grams, lie at the superior poles of the two
kidneys.
Figure: Anatomy of adrenal glands.
Synthesis of adrenocortical hormones
All Adrenocortical hormones are synthesized from cholesterol. Although the cells
of the adrenal cortex can synthesize small amounts of cholesterol from acetate,
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
36
approximately 80% of the cholesterol used for steroid synthesis is provided by
low-density lipoproteins (LDL) in the circulating plasma.
The adrenal medulla is distinct from the adrenal cortex and consists of
chromaffin cells, which are embryologically derived from the neuronal precursor
(neural crest) cells. The adrenal medulla is richly innervated by preganglionic
sympathetic neurons, which release acetylcholine as their neurotransmitter.
Chromaffin cells are the functional equivalent of the postganglionic neurons of the
sympathetic nervous system. Chromaffin cells mainly secrete epinephrine plus a
small amount of norepinephrine in response to preganglionic stimulation.
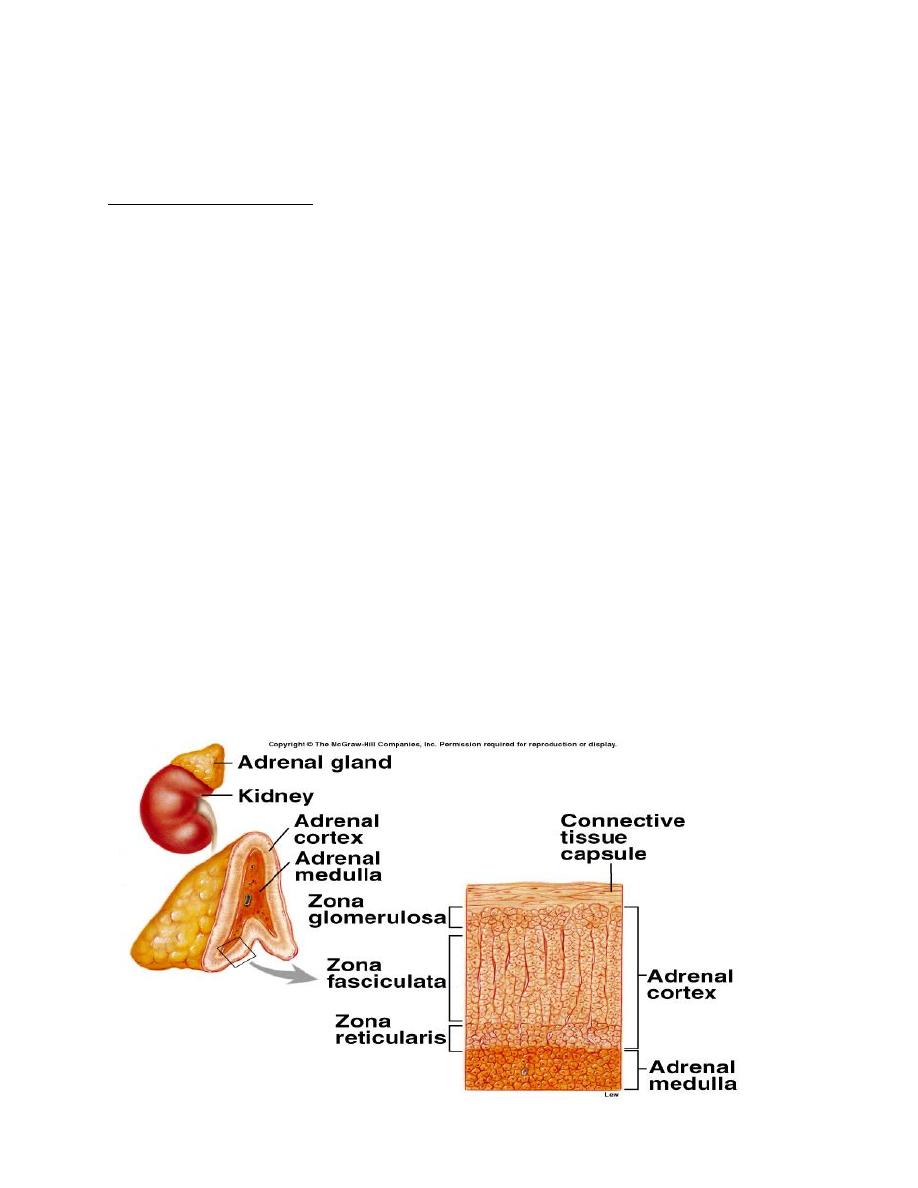
The adrenal cortex forms the outer portion of the adrenal gland and accounts for
80 to 90% of the weight of the gland. It composed of three layers:
1.The zona glomerulosa, which secrete aldosterone.
2.The zona fasciculate, and secretes the glucocorticoids, as well as small amounts
of adrenal androgens and estrogens.
3.The zona reticularis, secretes the adrenal androgens androstenedione and
dehydroepiandrosterone (DHEA).
M
inera

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
37
locorticoids
The primary mineralocorticoid is aldosterone. The actions of this hormone include:
• Stimulation of renal retention of sodium
• Promotion of renal excretion of potassium
Aldosterone synthesis takes place only in the outer (glomerulosa) cell layer where
Aldosterone synthase is normally expressed.
Aldosterone receptors
The effects of aldosterone are mediated via the mineralocorticoid receptor in
principal cells of renal tubule. Aldosterone increases the number of luminal ENaC
channels, increases their open time, and increases the synthesis of the basolateral
Na
+
-K
+
ATPase. The net effect is increased sodium reabsorption and potassium
secretion.
The release of aldosterone from the adrenal cortex is regulated by two important
factors:
• Serum potassium levels
• The Renin-Angiotensin-Aldosterone System (RAAS).
Increased potassium ion concentration in the extracellular fluid greatly increases
aldosterone secretion.
The Renin-Angiotensin-Aldosterone System
The main sensory stimulus for activation of RAAS start in the kidney at
juxtaglomerular cells which secrete the enzyme renin in response to these factors:
• Decrease in blood volume
• Decrease in blood pressure
• Sympathetic stimulation
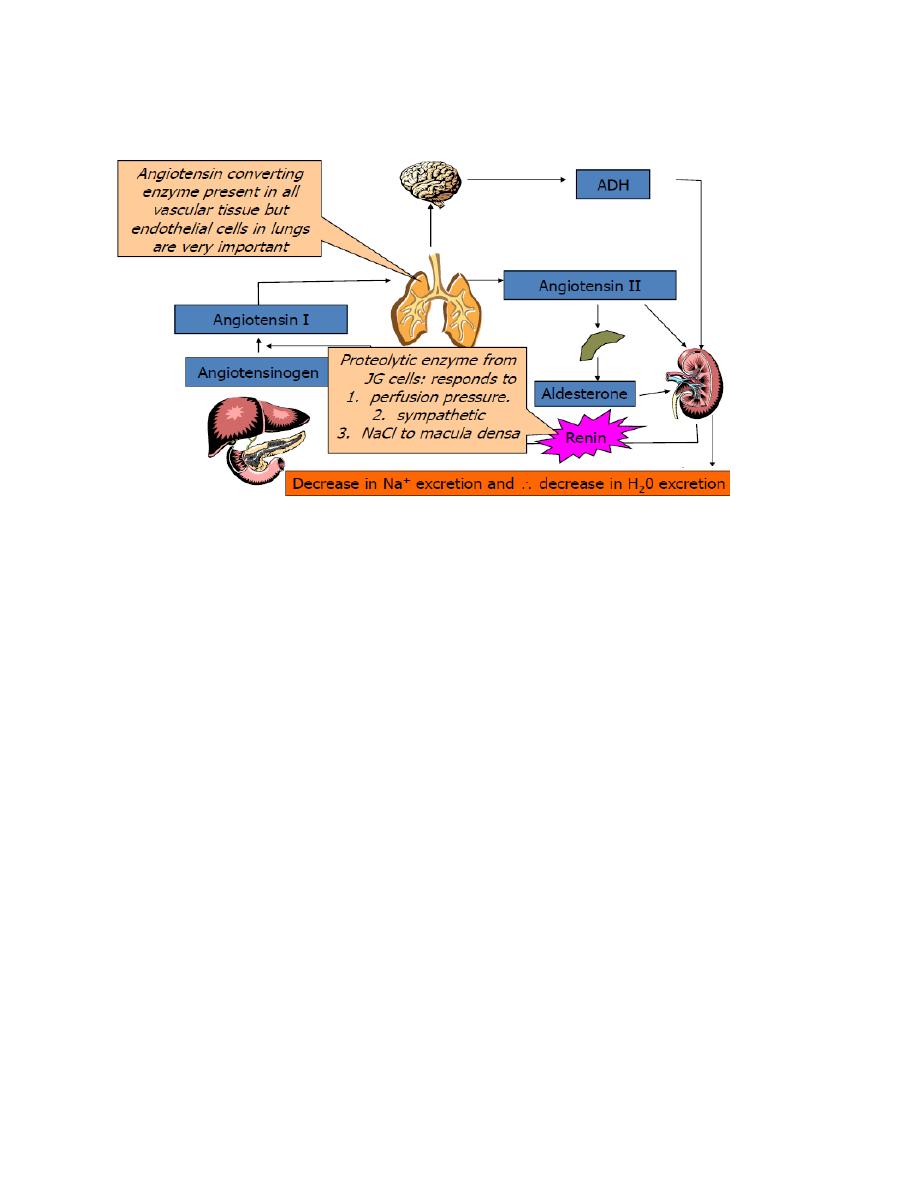
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
38
Figure: the renin angiotensin aldosterone system (RAAS).
The RAAS play important role in regulation of blood pressure and cardiac output.
Blood pressure is monitored by the juxtaglomerular apparatus. When renal
perfusion pressure decreases, secretion of renin increases. Any condition that
decreases pressure in the renal artery (e.g., hemorrhage, prolonged sweating)
stimulate renin secretion. Renin is an enzyme that converts a circulating protein
produced in the liver, angiotensinogen, also called renin substrate, into
angiotensinI. Angiotensin converting enzyme (ACE), found mainly in endothelial
cells of pulmonary vessels, converts angiotensin I into angiotensin II. Angiotensin
II has potent effects:
Cause arteriolar vasoconstriction.
Stimulate secretion of aldosterone, which in turn increase Na
+
reabsorption
by the renal tubule.
Increases ADH release from posterior pituitary
Increases thirst
It also directly stimulates reabsorption of sodium in the proximal tubule.

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
39
Volume-depleted states tend to produce metabolic alkalosis. Why?
Disorders of adrenal functions
-Primary hyperaldosteronism
Primary Aldosteronism is due to excessive and inappropriate aldosterone
production, the disease is usually the result of an aldosterone-producing adrenal
adenoma (Conn’s syndrome).
Symptoms include:
Hypertension due to excessive retention of Na
+
and fluids by the kidney.
Hypokalemia due to increased urinary K
+
excretion.
Potassium depletion is responsible for the muscle weakness and fatigue due to the
effect of potassium depletion on the muscle cell membrane.
Metabolic alkalosis due to increased urinary H
+
excretion.
-Secondary hyperaldosteronism
Secondary hyperaldosteronism occurs in response to activation of the renin-
angiotensin-aldosterone axis. Examples of conditions that result in secondary
hyperaldosteronism include renal artery stenosis, cirrhosis, and congestive heart
failure.

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
40
Glucocorticoids
The primary glucocorticoid is cortisol. Cortisol affects many cell types due to the
wide expression of glucocorticoid receptors. Free cortisol molecules diffuse into
the target cells and bind to the cytoplasmic glucocorticoid receptors.
The main physiologic actions of glucocorticoids include
Increase in blood glucose
Increase in blood free fatty acids
Suppress the immune system and block the inflammatory response to
allergic reactions.
Cortisol increases blood glucose by several mechanisms of action including:
1- Decrease in glucose utilization by many peripheral tissues (especially muscle
and adipose tissue)
2- Increase in availability of gluconeogenic substrates
• Increase in protein catabolism (especially muscle)
• Increase in lipolysis
3- Increase in hepatic gluconeogenesis.
Circadian variation of cortisol secretion
Cortisol secretion has a circadian variation, with hormone levels highest in the
early morning hours and lower during late afternoon and evening. The circadian
rhythm of cortisol helps the body in becoming active and alert in the morning and
in reducing activity prior to sleep. Variations in cortisol secretion reflect the
pulsatile release of CRH and ACTH.
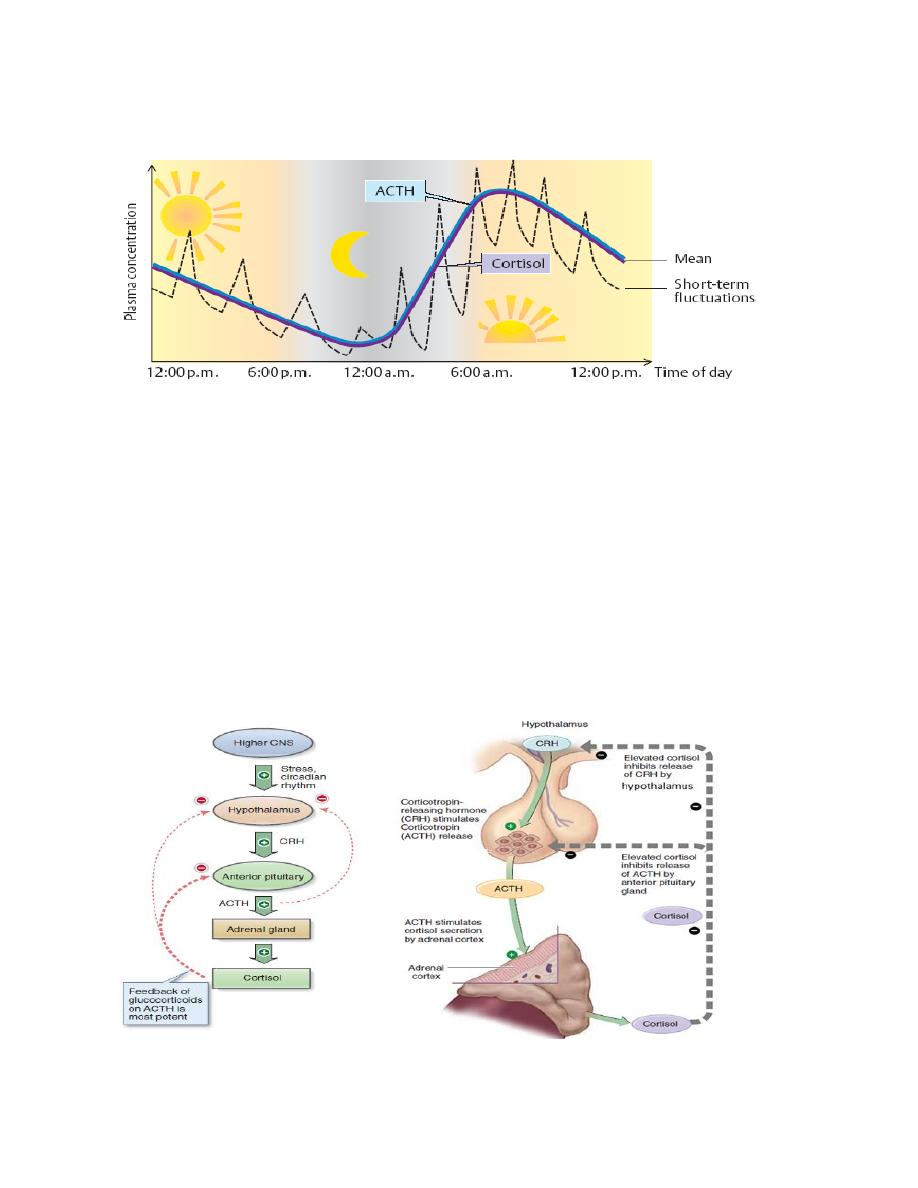
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
41
Figure: circadian rhythm of glucocorticoid secretion.
Cortisol and stress
Cortisol is an important component of the body’s response to physical and
psychological stress. Nervous signals regarding stress are transmitted to the
hypothalamus and the release of CRH is stimulated. The resulting increase in
cortisol increases levels of glucose, free fatty acids, and amino acids in the blood,
providing the metabolic fuels that enable the individual to cope with the stress. A
potent inhibitor of this system is cortisol itself. This hormone exerts a negative-
feedback effect on the hypothalamus and the adenohypophysis and inhibits the
secretion of CRH and ACTH, respectively.
Figure: control of adrenocorticotropin and cortisol secretion.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
42
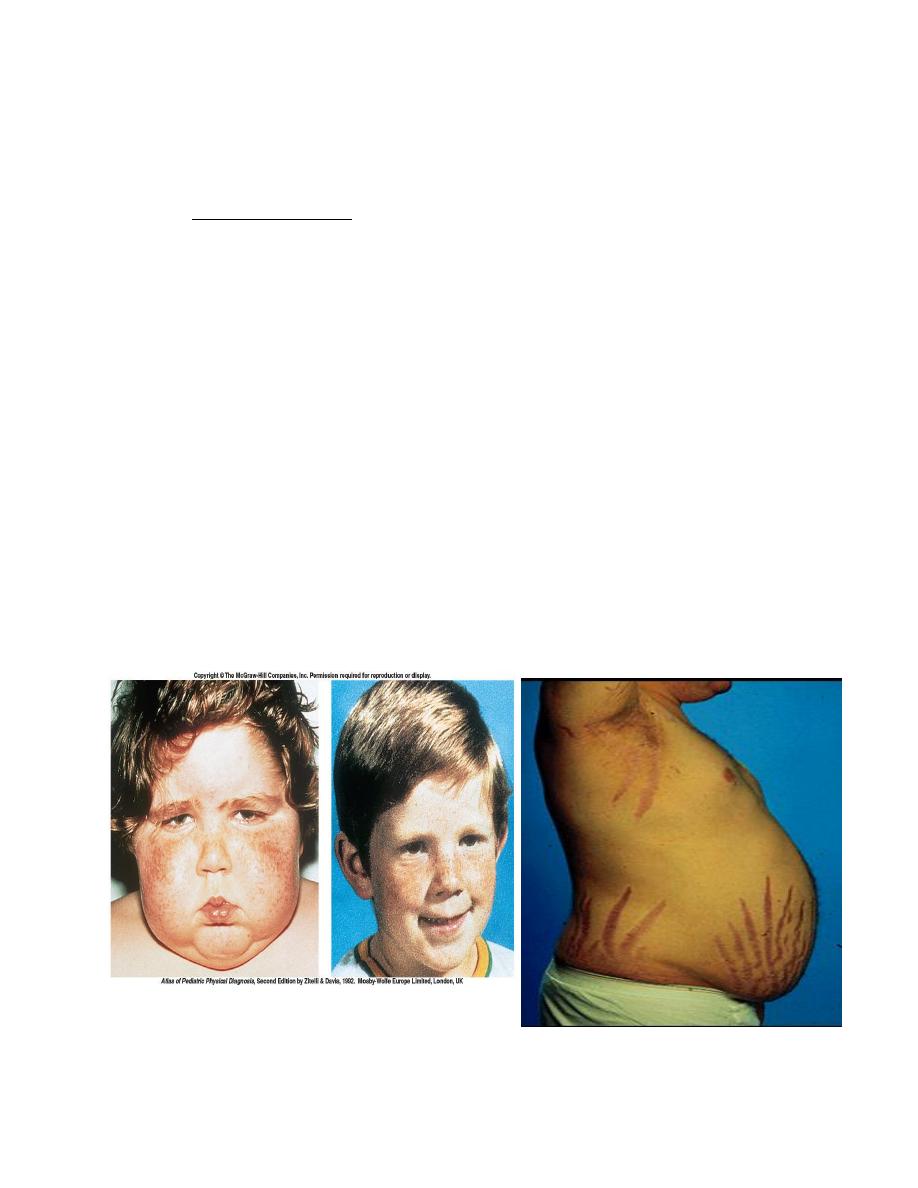
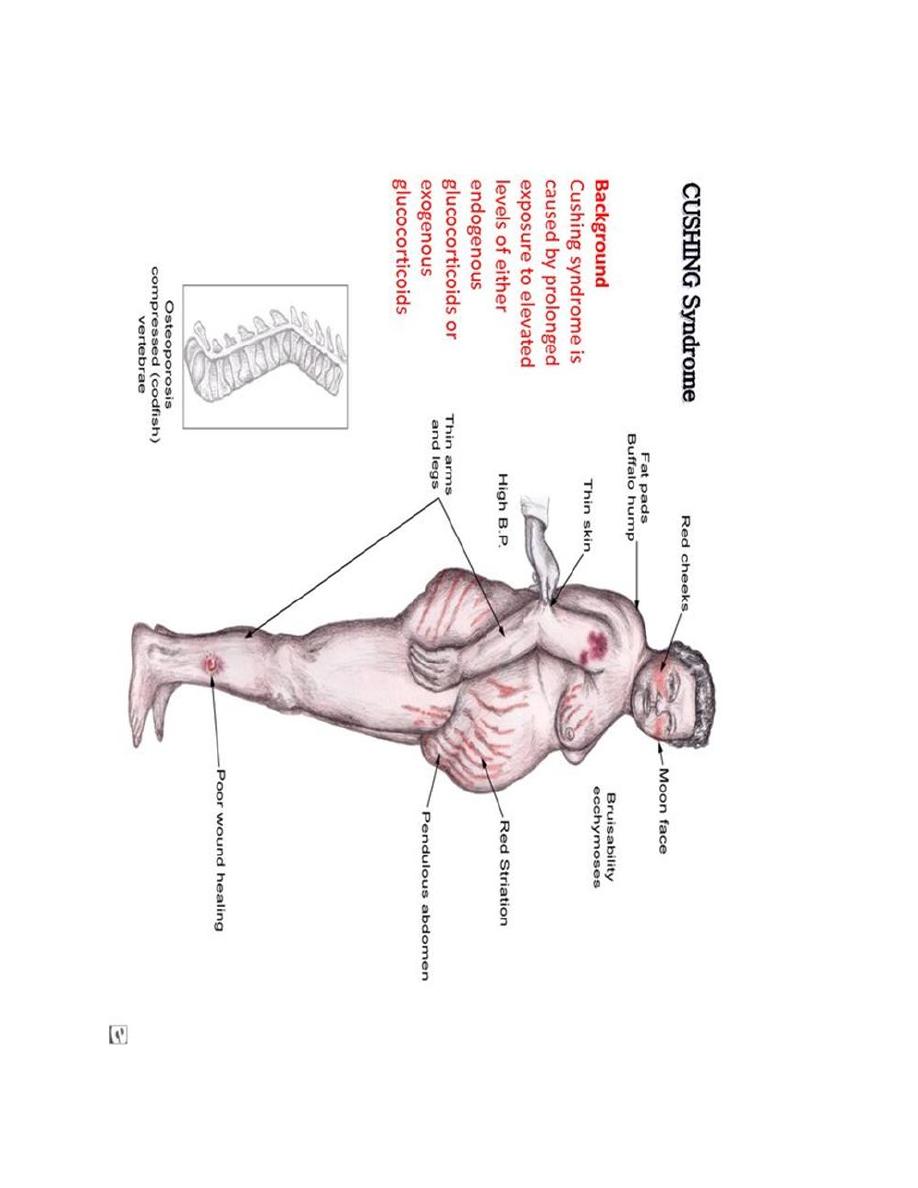
Glucocorticoid Hormone Excess
The term Cushing syndrome refers to the manifestations of hypercortisolism from
any cause.
Characteristics of Cushing Syndrome
Obesity because of hyperphagia, classically central affecting mainly the
face, neck, trunk, and abdomen: "moon face" and "buffalo hump"
Protein depletion as a result of excessive protein catabolism
Inhibition of inflammatory response and poor wound healing
Hyperglycemia leads to hyperinsulinemia and insulin resistance.
Hyperlipidemia
Bone breakdown and osteoporosis
Thinning of the skin with wide purple striae located around abdomen and
hips.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
43

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
44
Hypocortisolism (Addison's disease)
About 80% of cases of cortisol deficiency are of autoimmune origin, cortisol
deficiency leads to weakness, fatigue, anorexia, weight loss, hypotension,
hyponatremia, hypoglycemia.
ACTH has melanocyte-stimulating activity when present in the blood at high
concentrations. Humans who have high blood levels of ACTH, as a result of
Addison’s disease or an ACTH-secreting tumor are often hyperpigmented.
Adrenal androgens. The predominant androgens produced by the adrenal cortex
are dehydroepiandrosterone (DHEA) and androstenedione. These steroid
hormones are weak androgens; however, in peripheral tissues they can be
converted to more powerful androgens, such as testosterone, or even to estrogens.
The quantities of these hormones released from the adrenal cortex are very small.
Therefore, the contribution of this source of these hormones to androgenic effects
in the male is negligible compared to that of the testicular androgens. However, the
adrenal gland is the major source of androgens in females. These hormones
stimulate pubic and axillary (under arm) hair development in pubertal females. In
pathological conditions in which adrenal androgens are overproduced,
masculinization of females may occur.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
45
L7
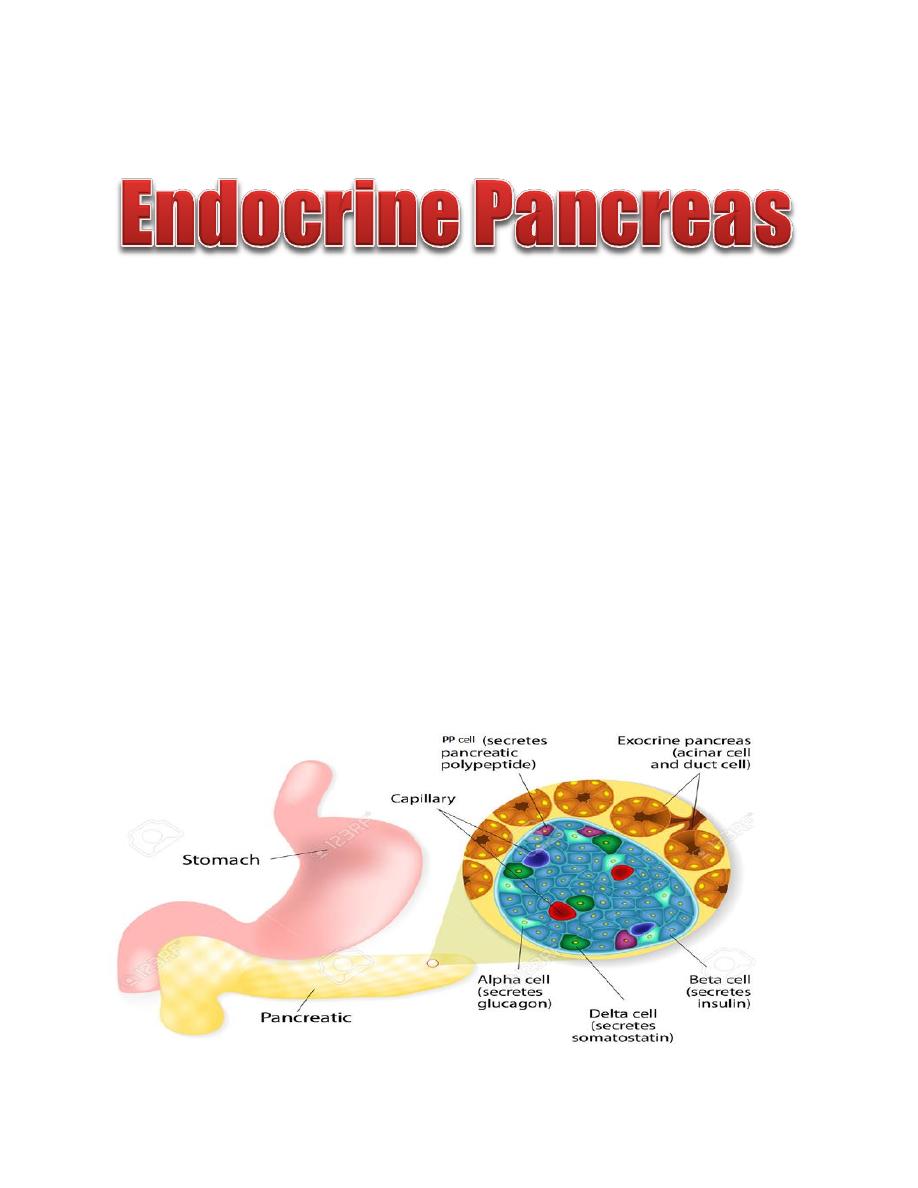
The pancreas is composed of two major types of tissues:
(1) The acini, which secrete digestive juices into the duodenum.
(2) The islets of Langerhans (Endocrine Pancreas).
The endocrine cells of the pancreas are arranged in clusters called the isletʹs of
Langerhans, which compose 1% to 2% of the pancreatic mass. There are
approximately 1-2 million islets of Langerhans, each about 0.3 mm in diameter.
The islets of Langerhans contain four cell types, and each cell secretes a different
hormone or peptide The β cells compose 60% of the islet and secrete insulin. The
α cells compose 25% of the islet and secrete glucagon. The delta (δ) cells compose
10% of the islet and secrete somatostatin, the PP cell, is present in small numbers
in the islets and secretes a hormone of uncertain function called pancreatic
polypeptide.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
46
Figure: physiologic anatomy of an islet of langerhans in the pancreas.
Structure and Synthesis of Insulin
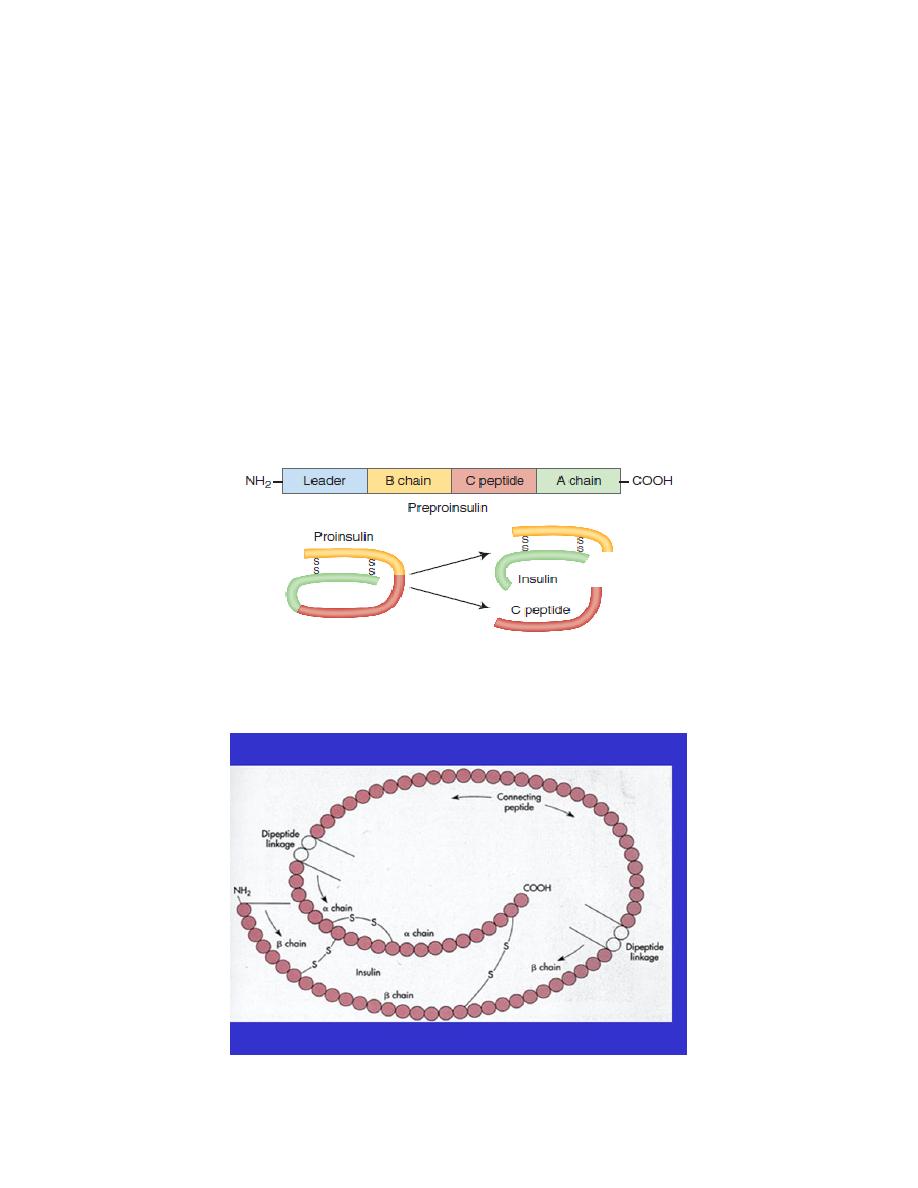
Insulin synthezised as preprohormone then cleaved in the endoplasmic reticulum to
form a proinsulin most of this is further cleaved in the Golgi apparatus to form
insulin and C- peptide fragments before being packaged in the secretory granules.
Both insulin and C- peptide are secreted together. C-peptide may serve a protective
role, helping to prevent the renal, neural, and microvascular pathologies. Urinary
excretion of C- peptide is a useful marker of insulin production.
Figure: Structure of insulin. The connecting peptide (C- peptide) is cleaved to form insulin.

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
47
A chain (21 amino acids) and a B chain (30 amino acids)
Specifically, insulin exerts its important actions on the following tissues:
Liver
• Increase in glucose uptake
• Increase in glycogenesis (formation of glycogen, the storage form of glucose)
• Increase in lipogenesis (formation of triglycerides, the storage form of lipids)
Adipose tissue
• Increase in glucose uptake
• Increase in free fatty acid uptake
• Increase in lipogenesis
Muscle
• Increase in glucose uptake
• Increase in glycogenesis
• Increase in amino acid uptake
• Increase in protein synthesis
Insulin is the only hormone that lowers blood glucose. (epinephrine, growth
hormone, cortisol, and glucagon increase blood glucose). It does so by stimulating
the uptake of glucose from the blood into the liver, adipose tissue, and muscle.
This glucose is first used as an energy source and then stored in the form of
glycogen in the liver and in muscle. Excess glucose is stored as fat in adipose
tissue.
Insulin is a major anabolic hormone, which is secreted in response to a
carbohydrate- and/or protein-containing meal. Anabolic hormones tend to promote
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
48
protein synthesis (increase lean body mass). Other anabolic hormones include,
Thyroid hormones, Growth hormone/IGF I and Sex steroids (androgens)
Metabolic Actions of Insulin
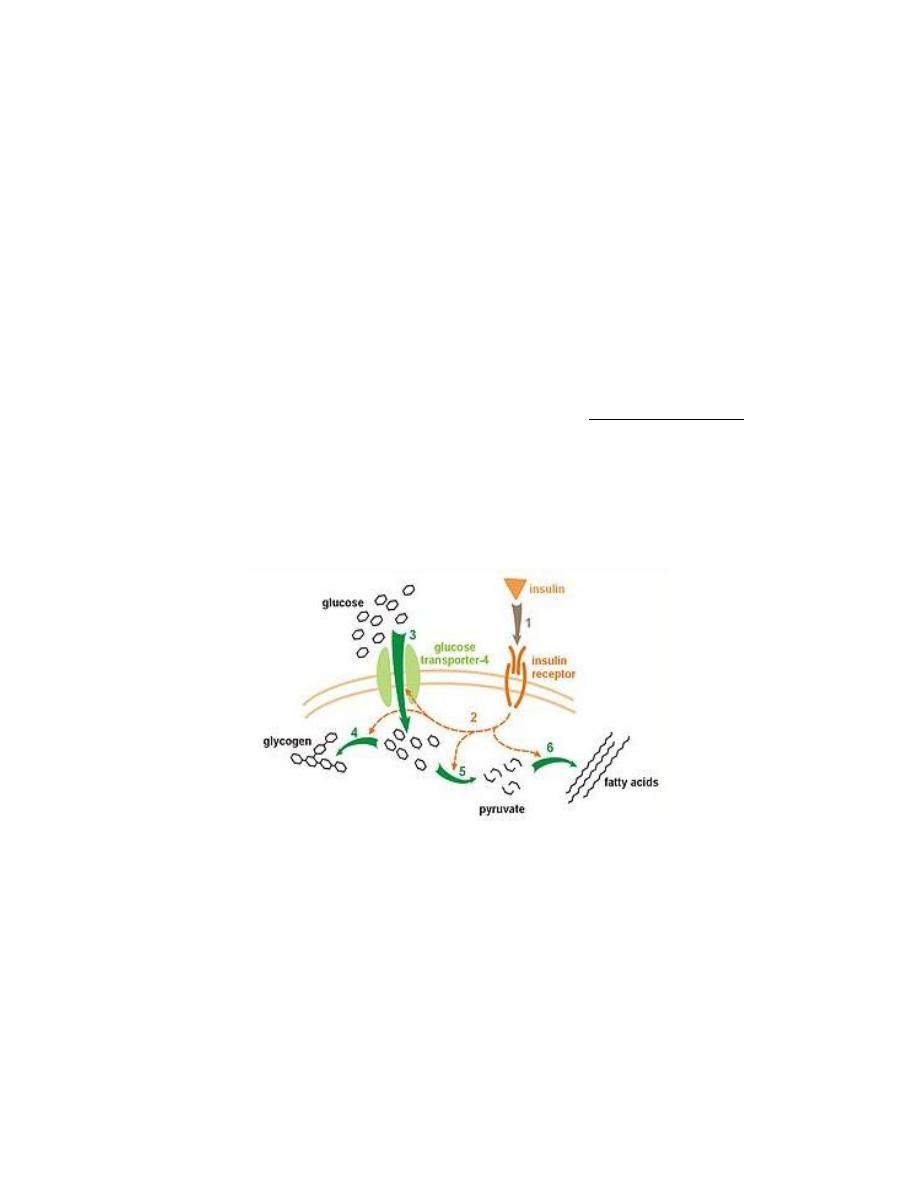
1. Effect of Insulin on Carbohydrate Metabolism
Insulin increases the uptake of glucose in muscle and fat tissue by directing
the insertion of glucose transporters (GLUT 4) into the cell membranes.
Insulin promotes the formation of glycogen from glucose in the liver and in
muscle by increasing the activities of the enzymes glycogen synthase,
Insulin inhibits glycogenolysis (glycogen breakdown).
Insulin also promotes conversion of excess glucose into fatty acids and
inhibits gluconeogenesis in the liver.
Figure: effect of insulin on carbohydrate metabolism
2.Effect of Insulin on Fat Metabolism
Insulin increase fat storage in adipose tissue by
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
49
Insulin increases the utilization of glucose by most of the body's tissues,
which automatically decreases the utilization of fat, thus functioning as a fat
sparer.
Insulin promotes the conversion of glucose to triglycerides.
Insulin decrease triglyceride breakdown (lipolysis) in adipose tissue by
decreasing the activity of hormone-sensitive lipase. This enzyme is activated
by stress hormones (i.e., cortisol, growth hormone, epinephrine, glucagon) .
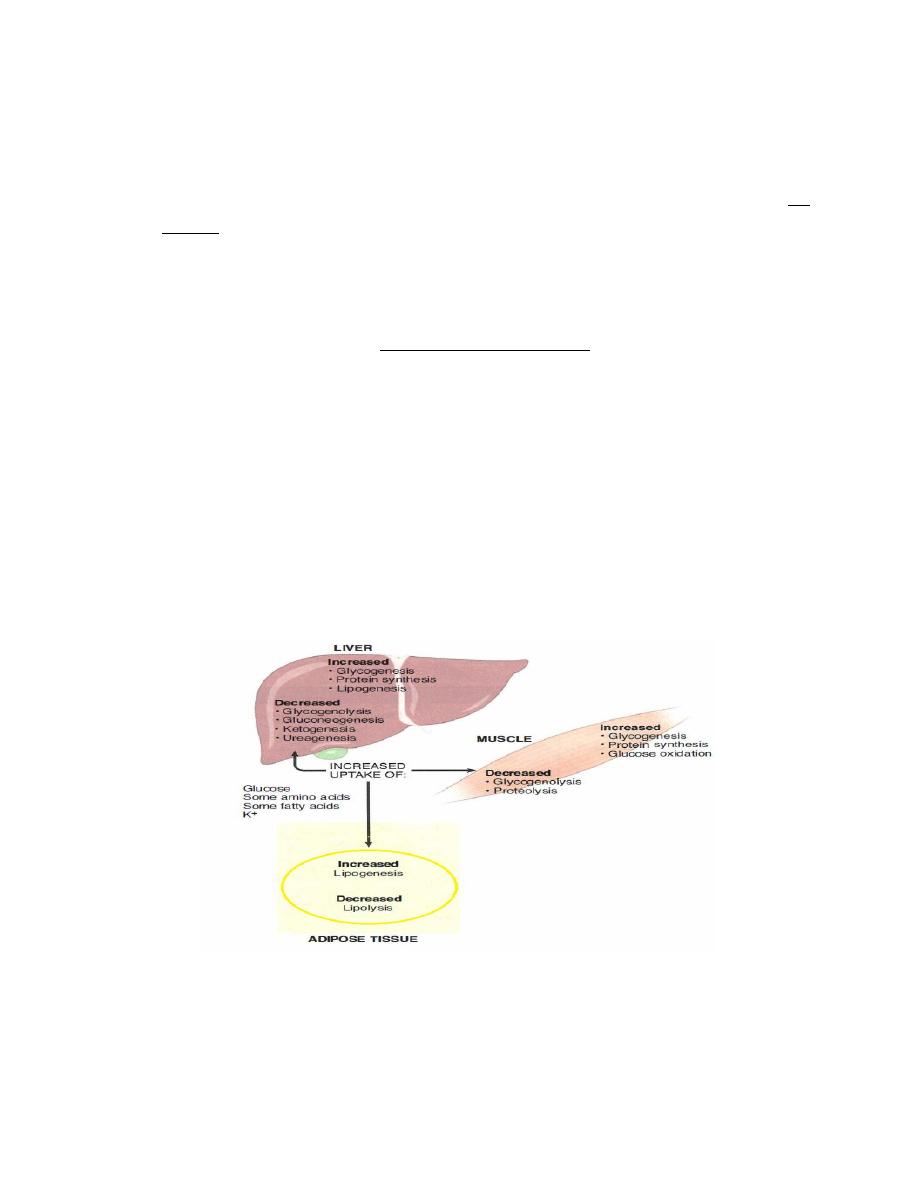
3. Effect of Insulin on Protein Metabolism
Insulin increase protein storage of the body as follow
Insulin stimulates transport of the amino acids into the cells.
Insulin increases the translation of messenger RNA.
Insulin also increases the rate of transcription of selected DNA.
Insulin inhibits the catabolism of proteins,
Figure: metabolic effect of the liver.
4. Insulin Effects on Potassium

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
50
Insulin promotes K
+
movement into cells, probably by increases the activity of
Na/K-ATPase in most body tissues. This K
+
-lowering action of insulin is used to
treat acute, life-threatening hyperkalemia by the simultaneous administration of
insulin and glucose.
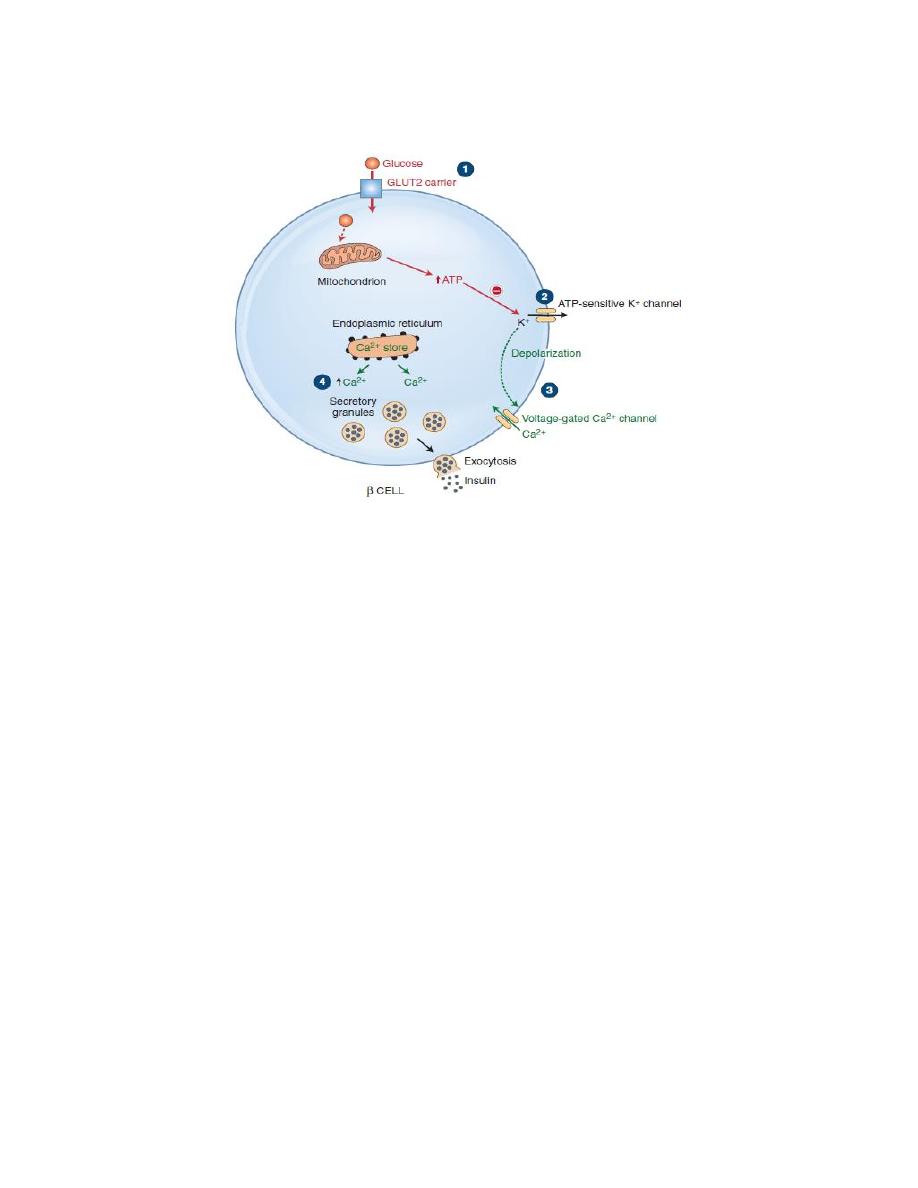
Mechanisms of Insulin Secretion:
1. Transport of glucose into the β cell by GLUT 2
2. Once inside the cell, glucose is phosphorylated to glucose-6-phosphate by
glucokinase, and glucose-6-phosphate is subsequently oxidized. ATP, one of the
products of this oxidation step.
3. ATP closes ATP-sensitive K
+
channels and depolarizes β-cell membrane.
4. Depolarization of the β-cell membrane opens voltage-sensitive Ca
2+
channels.
Ca
2+
flows into the β cell down its electrochemical gradient and the intracellular
Ca
2+
concentration increases which causes insulin secretion by exocytosis.
Link to pharmacology
Sulfonylureas (e.g., glipizide and glyburide) are pharmacologic agents that bind to
and inhibit the ATP-sensitive K
+
channels. Sulfonylureas, therefore, stimulate the
release of preformed insulin stored in vesicles, which results in reducing the blood
glucose concentration. (Note: sulfonylureas do not cause an increase in insulin
synthesis.)
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
51
Figure: Mechanisms of Insulin Secretion.
Control of Insulin Secretion
Blood glucose concentration is the primary regulator of insulin secretion. An
increase in blood glucose concentration stimulates insulin secretion. The actions of
insulin reduce the blood glucose concentration back to normal, thereby inhibiting
further insulin secretion. Other factors and conditions that increase insulin
secretion are: increase amino acid, fatty acid concentration, glucagon, cortisol,
Sulfonurea drugs and obesity, while low glucose concentration, fasting,
Somatostatin and α adrenergic agonist decrease insulin secretion.
Parasympathetic nervous system stimulates the secretion of insulin, sympathetic
nervous stimulation inhibits insulin secretion.
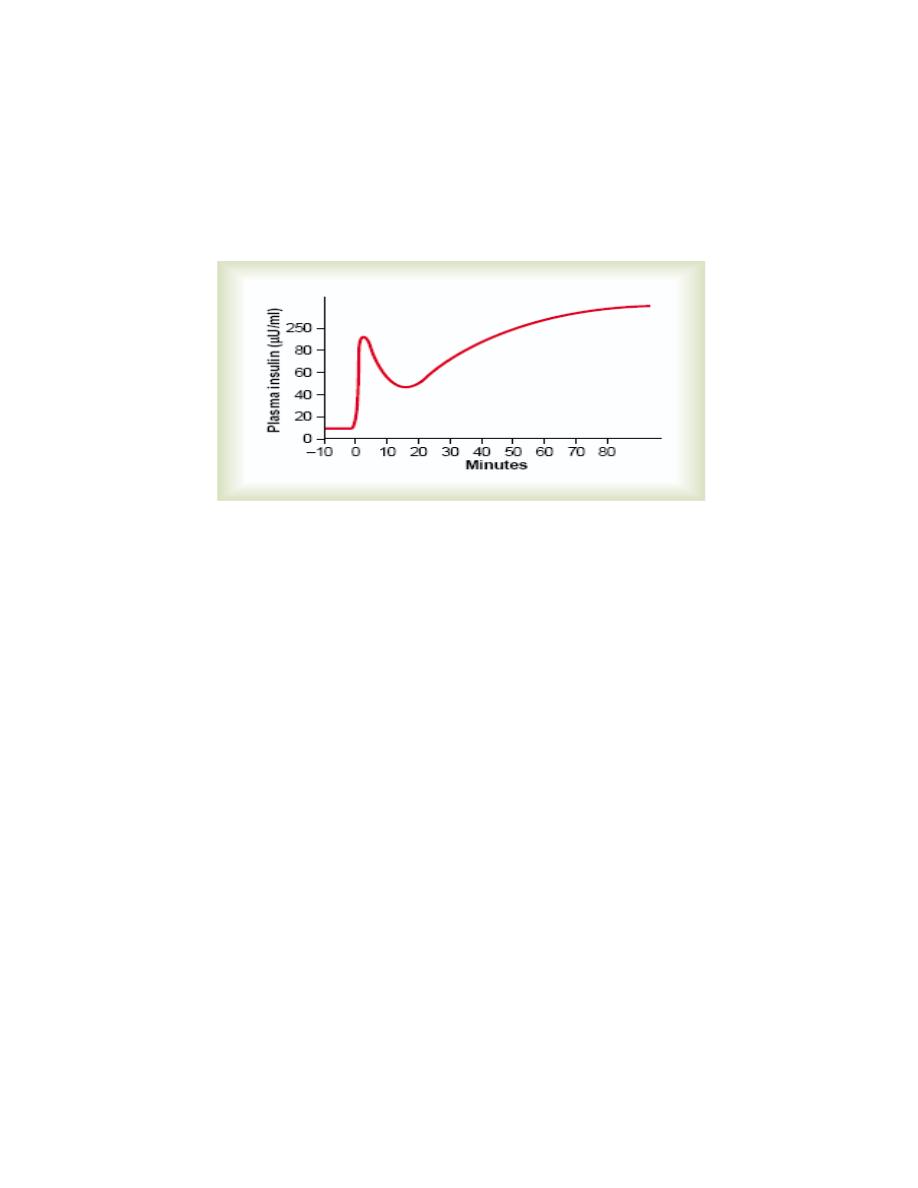
Insulin secretion increases markedly in two stages:
1. Plasma insulin concentration increases almost 10-fold within 3 to 5 minutes after
the acute elevation of the blood glucose; this results from immediate dumping of
preformed insulin from the beta cells of the islets of Langerhans.
2. At about 15 minutes, insulin secretion rises a second time and reaches a new
plateau in 2 to 3 hours, this time usually at a rate of secretion even greater than that
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
52
in the initial phase. This secretion results both from additional release of preformed
insulin and from activation of the enzyme system that synthesizes and releases new
insulin from the cells.
Figure: Biphasic insulin response to a constant glucose stimulus.
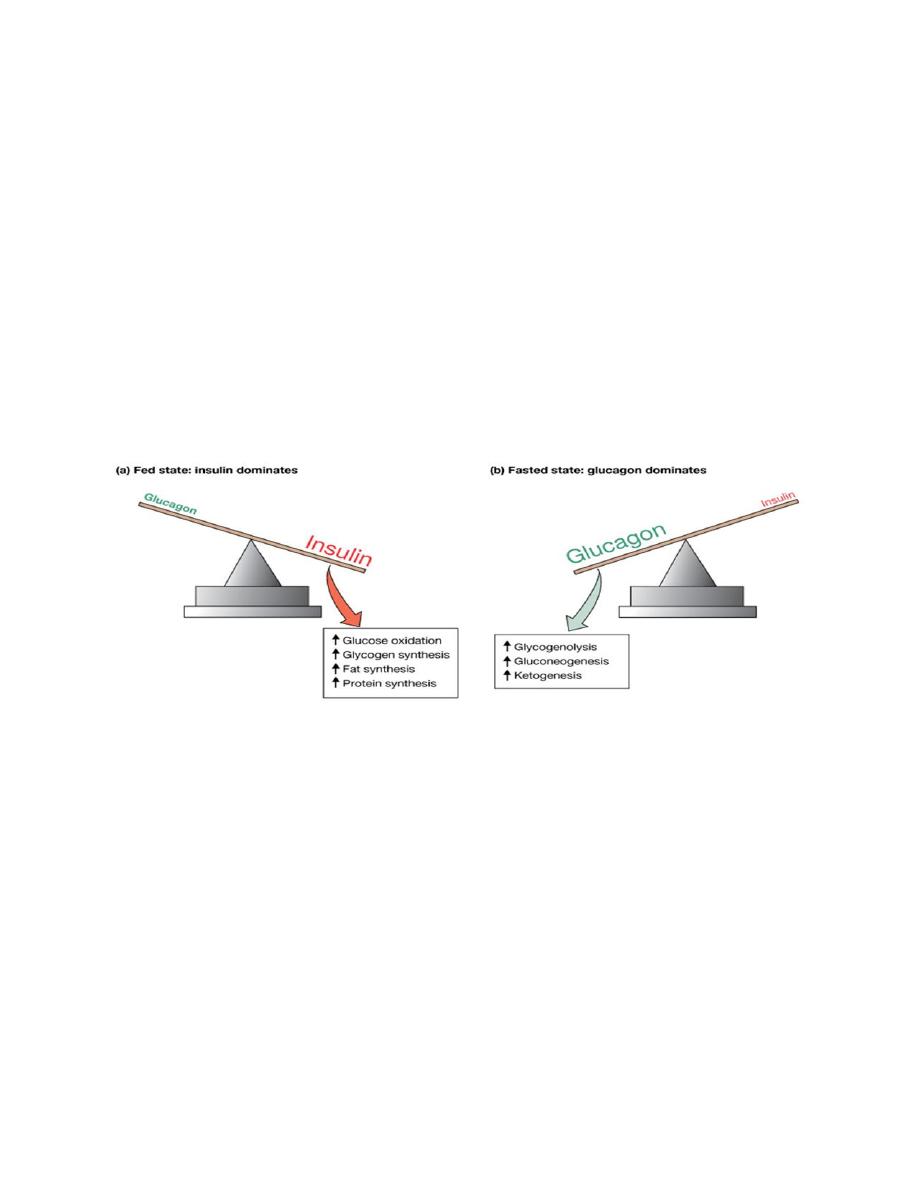
Glucagon
A peptide hormone, produced by α-cells of the islets of Langerhans. The overall
effects of glucagon includes:
• Increase in hepatic glucose production
• Glycogenolysis
• Gluconeogenesis
• Stimulation of lipolysis in the liver and in adipose tissue
The effects of glucagon on glucose metabolism are generally opposite to those of
insulin. Acting primarily on the liver, glucagon stimulates glycogenolysis
(breakdown of glycogen, the storage form of glucose) and gluconeogenesis, which
increase blood glucose levels. This hormone also stimulates lipolysis, which
increases the circulating concentration of free fatty acids. These molecules may
then be used as an alternative energy source by muscle or serve as gluconeogenic
substrates in the liver. Finally, glucagon stimulates the hepatic uptake of amino
acids, which also serve as substrates for gluconeogenesis.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
53
Factors that stimulate glucagon secretion include: a decrease in blood glucose; an
increase in blood amino acids; sympathetic nervous stimulation; stress; and
exercise. Factors that inhibit glucagon secretion include insulin and an increase in
blood glucose. Other factors which effect glucagon secretion are Fasting, CCK and
β adrenergic agonist, while insulin and somatostatin decrease glucagon secretion.
Factors that stimulate glucagon secretion include: a decrease in blood glucose; an
increase in blood amino acids; sympathetic nervous stimulation; stress; and
exercise. Factors that inhibit glucagon secretion include insulin and an increase in
blood glucose. Other factors which effect glucagon secretion are Fasting, CCK and
β adrenergic agonist, while insulin and somatostatin decrease glucagon secretion.
Diabetes mellitus
Diabetes mellitus is a group of disorders involved in the regulation of insulin
production or secretion or in the cellular actions of insulin; the result is
hyperglycemia. Diabetes mellitus, characterized by hyperglycemia, polyuria,
increased thirst and fluid intake.
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus,
divides diabetes into four clinical classes:
1. Type 1 diabetes mellitus, which is characterized by destruction of the
pancreatic beta cells and an absolute insulin deficiency and accounts for 5% to
10% of those with diabetes.

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
54
2. Type 2 diabetes mellitus, previously described as non– insulin-dependent
diabetes. It currently accounts for about 90% to 95% of the cases of diabetes. Most
people with type 2 diabetes are older and overweight. Recently, however, type 2
diabetes has become a more common occurrence in obese children and
adolescents. The metabolic abnormalities involved in type 2 diabetes include (1)
insulin resistance, (2) increased glucose production by the liver, and (3) deranged
secretion of insulin by the pancreatic beta cells.
3. Gestational diabetes mellitus (diabetes that develops during pregnancy).
4. Other specific types of diabetes, many of which occurs secondary to other
conditions (e.g., Cushing syndrome, acromegaly, and pancreatitis).

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
55
L8
We reproduce by sexual reproduction, a process in which sex cells (gametes) unite
to form offspring. The gametes are formed in the gonads. The gametes unite inside
the female in a process called fertilization. The female then nurtures the growing
embryo until birth.
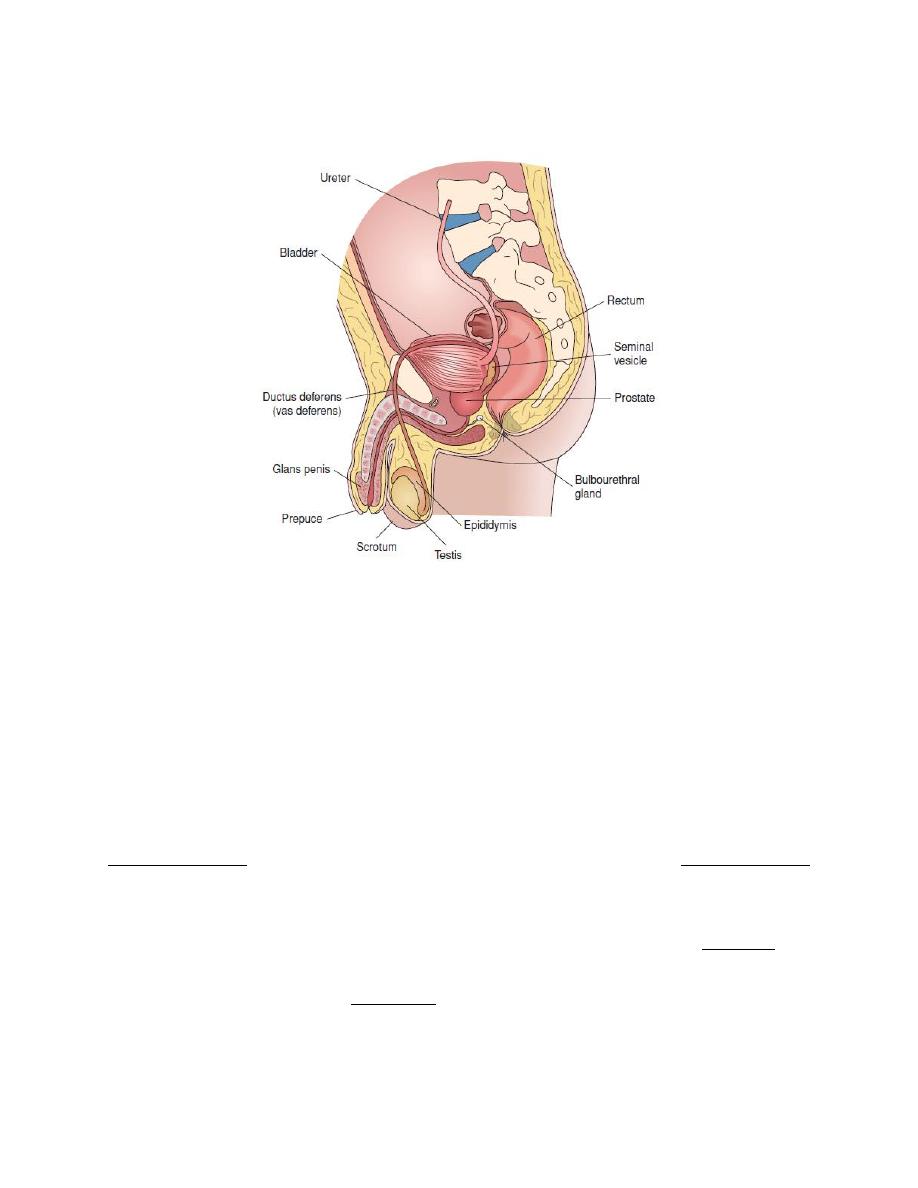
Male Reproductive system
The organs of the male reproductive system are the following.
The testes.
A system of ducts: epididymis, vas deferens, ejaculatory ducts, urethra.
Accessory glands: seminal vesicles, prostate, and bulbourethral glands.
Supporting structures: the scrotum and the penis.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
56
Figure. Structures of the male reproductive system,
The testes
The testes are paired ovoid organs that lie within the scrotum, which hangs in
scrotum outside the abdominal cavity. The scrotum keeps the sperm at an optimal
temperature for their development, slightly less than the core body temperature.
Higher temperature may harm the sperm and cause infertility.
The testes divided into 200 to 300 lobules. Each lobule contains one to four tightly
coiled seminiferous tubules. The testes have two primary functions,
spermatogenesis (process of producing mature sperm), and steroidogenesis
(synthesis of testosterone). Both processes are regulated by the pituitary
gonadotropins LH and FSH. Testosterone is the primary sex hormone in the male
and is responsible for primary and secondary sex characteristics. The primary sex
characteristics include those structures responsible for promoting the development
and delivery of sperm. The secondary sex characteristics are those structures and
behavioral features that make men externally different from women and include
the typical male hair pattern, deep voice, and large muscle and bone masses.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
57
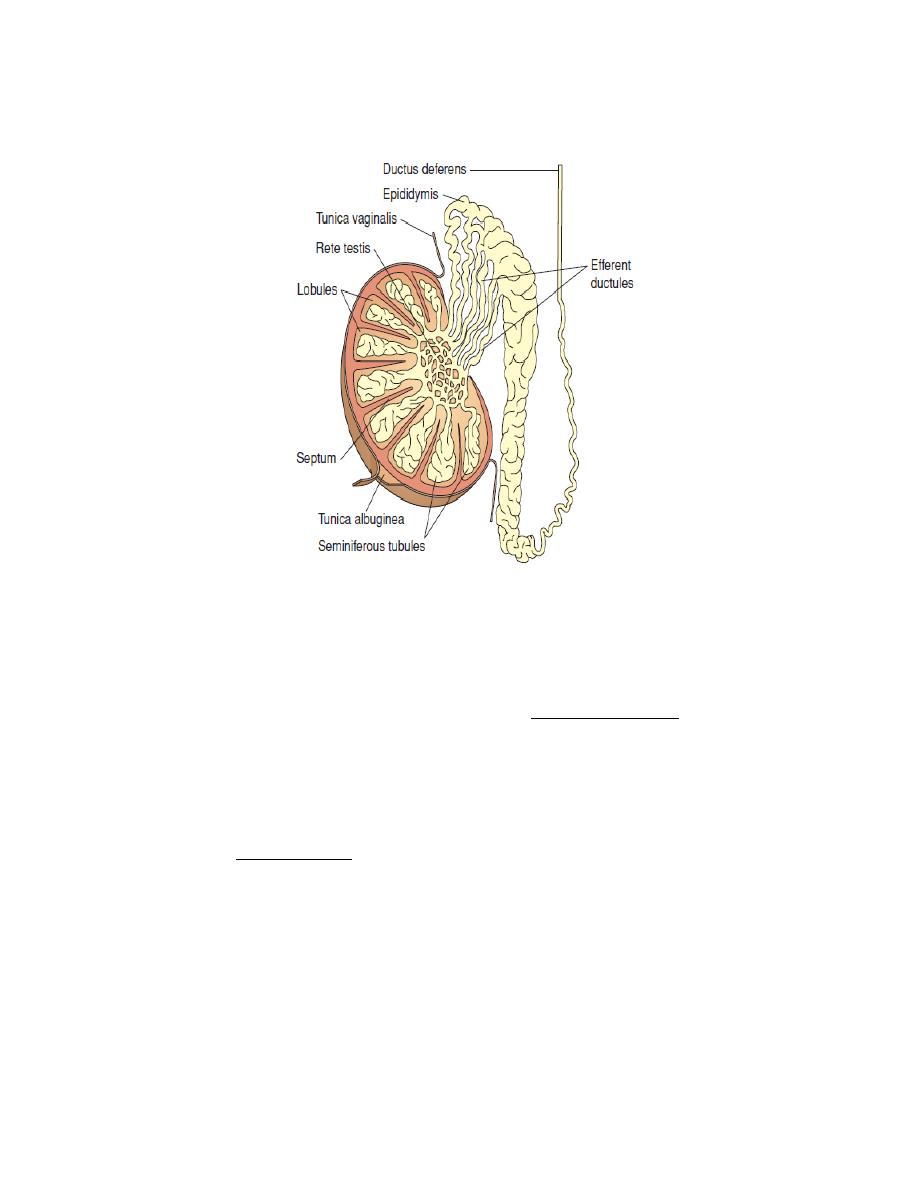
Figure 3. The parts of the testes and epididymis.
Epididymus
Each epididymus is a tightly coiled tube lying adjacent to the testis and leading
from the testis to the vas deferens. It is the site of sperm maturation.
The vas deference
The vas deferens is a muscular tube 45 centimeters in length leading from the
epididymis up into the ejaculatory duct where it combine with seminal vesicle it is
the area where sperms stored.
Seminal Vesicle
The seminal vesicle is a saclike structure attached to the vas deferens near the base
of the urinary bladder, it produce about 60% of semen (a mixture of sex gland
fluids and about 300 million sperm) which contains:
Fructose, to nourish sperm.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
58
Prostaglandins to cause muscular contractions in the female tract to help
propel sperm
High pH to help neutralize the acid environment of the urethra and vagina.
Prostate gland
The prostate gland surrounds the first portion of the urethra and drain directly into
the urethra through small ducts. It produces about 30% of semen which is a thin,
milky secretion, with slightly acidic pH, rich in acid phosphatase.
Bulb urethral gland: secretes a clear lubricating fluid that aids in sexual
intercourse.
Penis: A copulatory organ that is responsible for delivering the sperm to the
female reproductive tract. Contains 2 erectile tissues called corpus cavernosa and
corpus spongiosum.
During sexual excitement, parasympathetic nerves cause vasodilatation in the
penis, allowing erectile tissues to swell and erect the penis.
During ejaculation, sympathetic nerves cause vas deferens, urethra and erectile
tissues to contract, forcefully expelling semen outward.
Seminiferous Tubules
About 1,000 seminiferous tubules in each testis conduct spermatogenesis.
Between the tubules are specialized glandular cells called interstitial cells (or
Leydig's cells) which produce testosterone.
Inside the tubules are specialized cells called Sertoli's cells which support and
nourish the sperm.
Spermatogenesis
Spermatogenesis is the process of transformation of male germ cells into
spermatozoa, it occurs in the seminiferous tubules of the testes. The process begins
shortly before puberty and continues throughout a life. The seminiferous tubules
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
59
are composed of two types of cells: Sertoli cells and Spermatogenic cells.
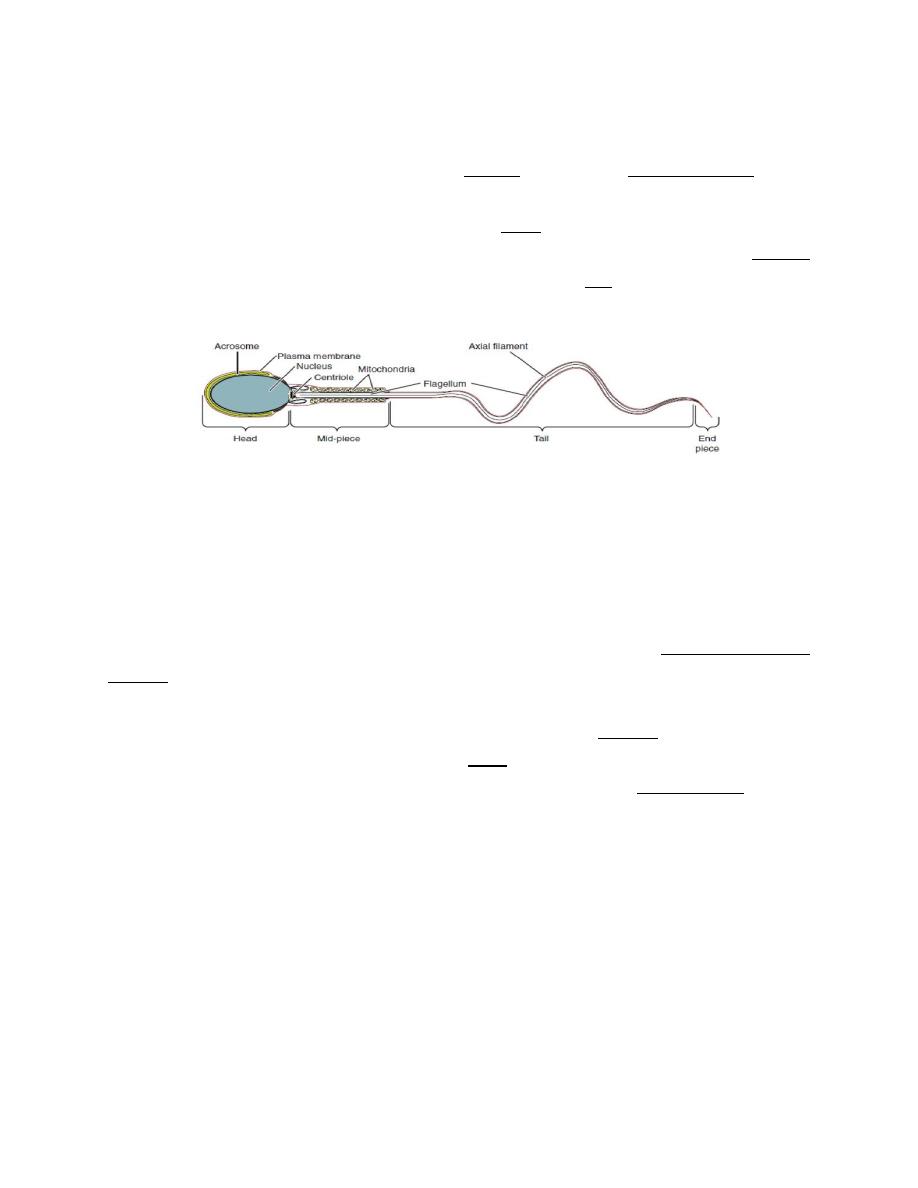
Approximately 64-74 days are needed to produce mature spermatozoa from
spermatogonia. Mature spermatozoa have a head region containing the nucleus,
the head is surrounded by a large secretory vesicle (acrosome); there is a middle
connecting section, which is rich in mitochondria, and a tail region, which provides
a swimming action.
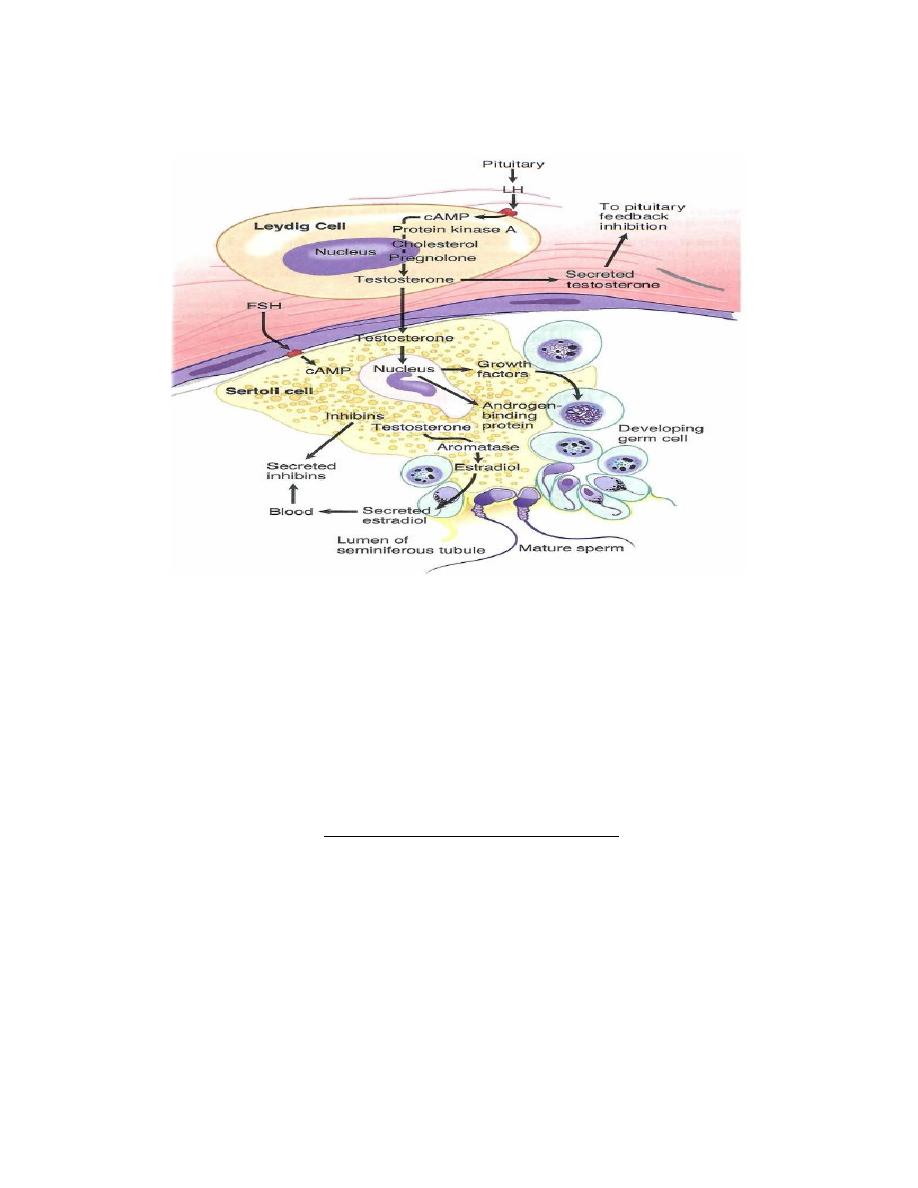
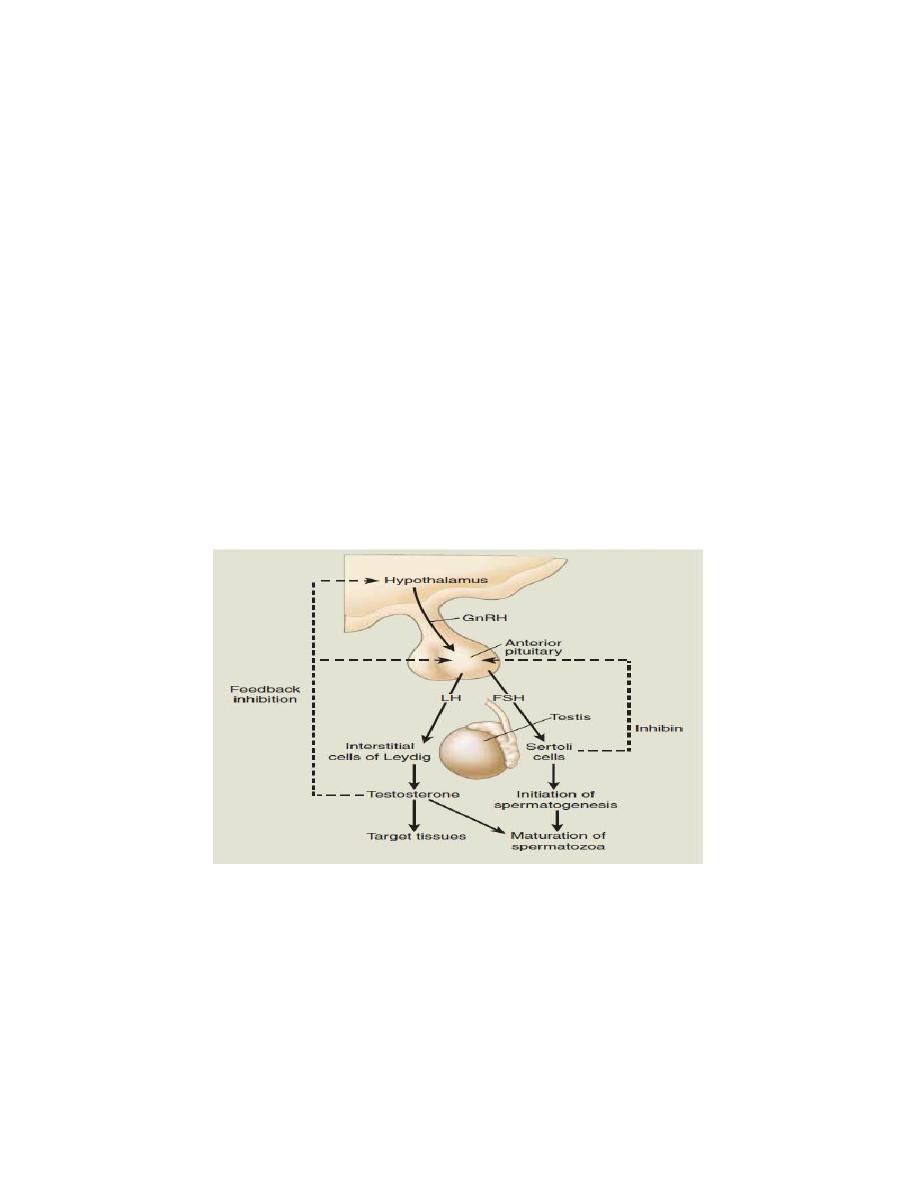
Endocrine control of spermatogenesis
1. LH and FSH are secreted from the anterior pituitary under the control of GnRH
from the hypothalamus.
2. LH stimulates Leydig cells to produce testosterone.
3. FSH stimulates the Sertoli cells, resulting in the secretion of androgen-binding
protein into the lumen of the seminiferous tubule. The function of androgen-
binding protein is to increase the local concentration of testosterone at the site of
spermatogenesis. FSH also stimulates the secretion of inhibin from the Sertoli
cells, which exerts negative feedback on FSH secretion by the anterior pituitary.
Testosterone exerts negative feedback on the secretion of both FSH and LH.
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
60
Figure. stages of development of spermatozoa.
Testosterone
Testosterone is secreted by Leydig cells, some testosterone enters the systemic
circulation and others diffuse locally into the seminiferous tubules. In the
bloodstream, most testosterone are bound to plasma proteins, and only
approximately 3% of circulating testosterone is unbound and therefore able to enter
the cell and exert its metabolic effects. In target tissue much of the testosterone is
converted to more potent dihydrotestosterone by α-reductase.
The main functions of testosterone are:
1. Induces differentiation of the male genital tract during fetal development
2. Induces development of primary and secondary sex characteristics
Gonadal function
External genitalia and accessory organs
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
61
Male voice timbre
Male skin characteristics
Male hair distribution
3. Anabolic effects
Promotes protein metabolism
Promotes musculoskeletal growth
Influences subcutaneous fat distribution
4. Promotes spermatogenesis and maturation of sperm
5. Stimulates erythropoiesis
Figure. Hypothalamic-pituitary feedback control of spermatogenesis and testosterone levels in the male.
Gonadal dysfunction in the male
The consequences of deficient testosterone production depend upon the age of
onset:

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
62
1. Testosterone deficiency in the second to third month of gestation results in that
patients have a short, blind-ended vagina without a cervix, uterus, or ovary. Failure
of masculinization during puberty is due to the lack of androgen receptors.
2. Testosterone deficiency in the third trimester leads to problems in testicular
descent (cryptorchidism) along with micro penis.
3. Pubertal testosterone deficiency leads to poor secondary sexual development.
4. Postpubertal testosterone deficiency leads to decreased libido, erectile
dysfunction, decrease in facial and body hair growth, low energy, and infertility.
Exogenous testosterone given to men would normally inhibit endogenous LH
release through a negative-feedback effect on the hypothalamic-pituitary axis, and
lead to a suppression of testosterone production by the Leydig cells and a further
decrease in testicular testosterone concentrations. Ultimately, because LH levels
decrease when exogenous testosterone is administered, testicular size decreases.
High levels of androgens have an anabolic effect on muscle tissue, leading to
increased muscle mass, strength, and performance, a desired result for body
builders and athletes. Androgen abuse has been associated with abnormally
aggressive behavior and the potential for increased incidence of liver and brain
tumors.
Leydig cells also contain receptors for prolactin that synergize with LH to
stimulate testosterone production by increasing the number of LH receptors, but
Hyperprolactinemia in men with pituitary tumors, is associated with decreased
testosterone levels. This condition is due to a direct effect of elevated circulating
levels of PRL on Leydig cells, reducing the number of LH receptors. In addition,
hyperprolactinemia may decrease LH secretion by reducing the pulsatile nature of
its release.
The female reproductive system
The female reproductive system functions to produce gametes and reproductive
hormones, just like the male reproductive system; however, it also has the

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
63
additional task of supporting the developing fetus and delivering it to the outside
world. The female reproductive system consists of the external and internal genital
structures:
The external structures, or genitalia, are situated in the anterior part of the
perineum which is collectively referred to as the vulva.
The internal structures (vagina, uterus, fallopian tubes, and ovaries) are
located in the pelvic cavity.
Vagina
Vagina is an elastic channel inferior to the cervix that serves as the "birth canal"
during parturition.
The uterus
The uterus is a thick-walled muscular organ, the inferior constricted part, called the
cervix. After fertilization, embryo adheres to the endometrial layer for further
development an event called implantation.
Fallopian (Uterine) Tubes
Fallopian tubes are slender cylindrical structures attached bilaterally to the uterus.
The most distal part of a fallopian tube is the fimbria, which receive the ovum
when it is released from the ovary at ovulation. The usual site of fertilization of the
oocyte is a dilated area called the ampulla. The fallopian tubes transport the zygote
to the site of implantation in the uterus.
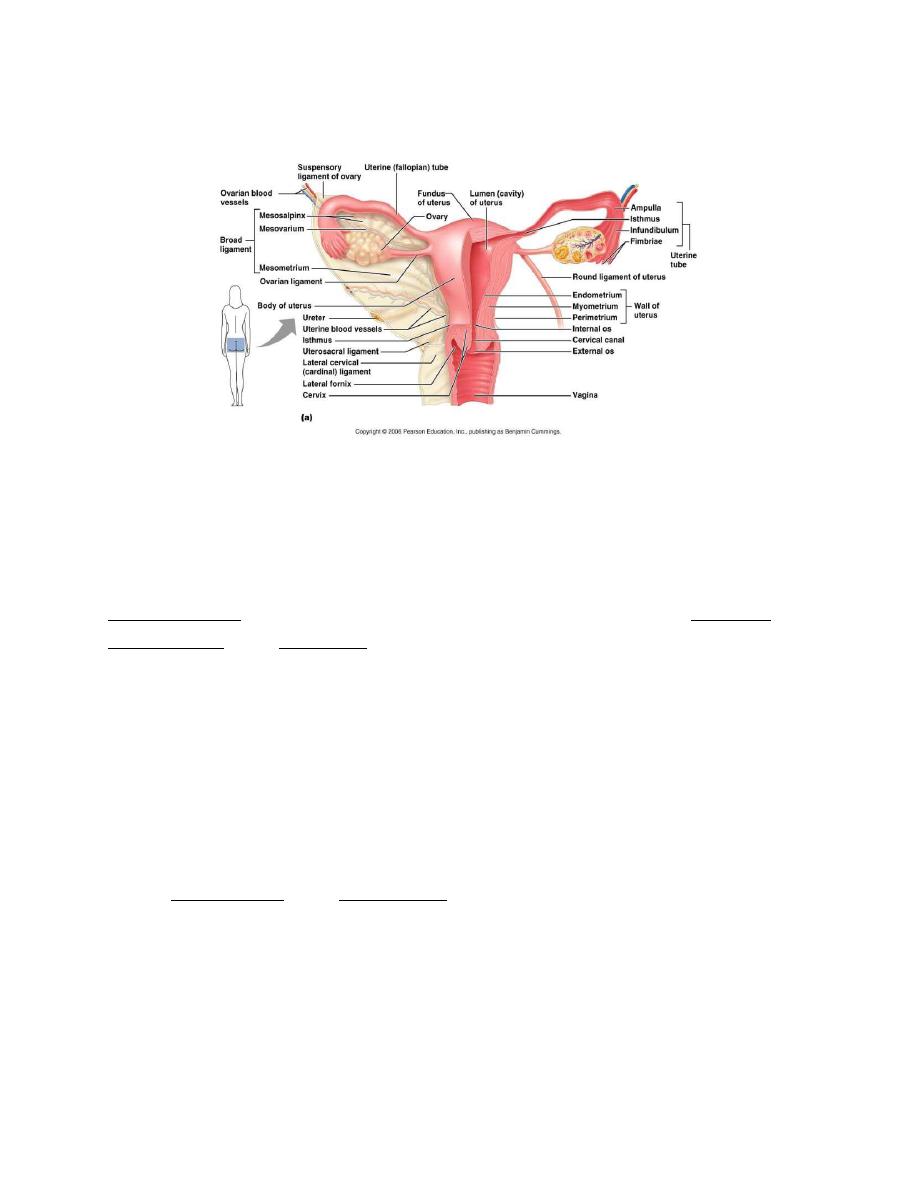
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
64
Figure: The female reproductive system.
Ovary
The paired ovaries are located on either side of the uterus below the ends of the
two fallopian tubes. The ovaries perform two interrelated functions:
Steroidogenesis (production of the female sex hormones such as estrogens and
progesterone), and Oogenesis (production of ova). Both of these functions are
regulated by follicle-stimulating hormone (FSH) and luteinizing hormone (LH)
from the anterior pituitary gland.
The female reproductive cycle
Towards the end of puberty, girls begin to release eggs as part of a monthly period
called the female reproductive cycle. The normal menstrual cycle is 28 days long.
In fact, only 15% of women have a perfect 28-day cycle. Any cycle of between 21
and 35 days long can be regarded as normal. The reproductive cycle can be divided
into an ovarian cycle and a uterine cycle.
Uterine cycle (Menstrual cycle)
The menstrual cycle (approximately 28 days) can be divided into the following
phases or events:
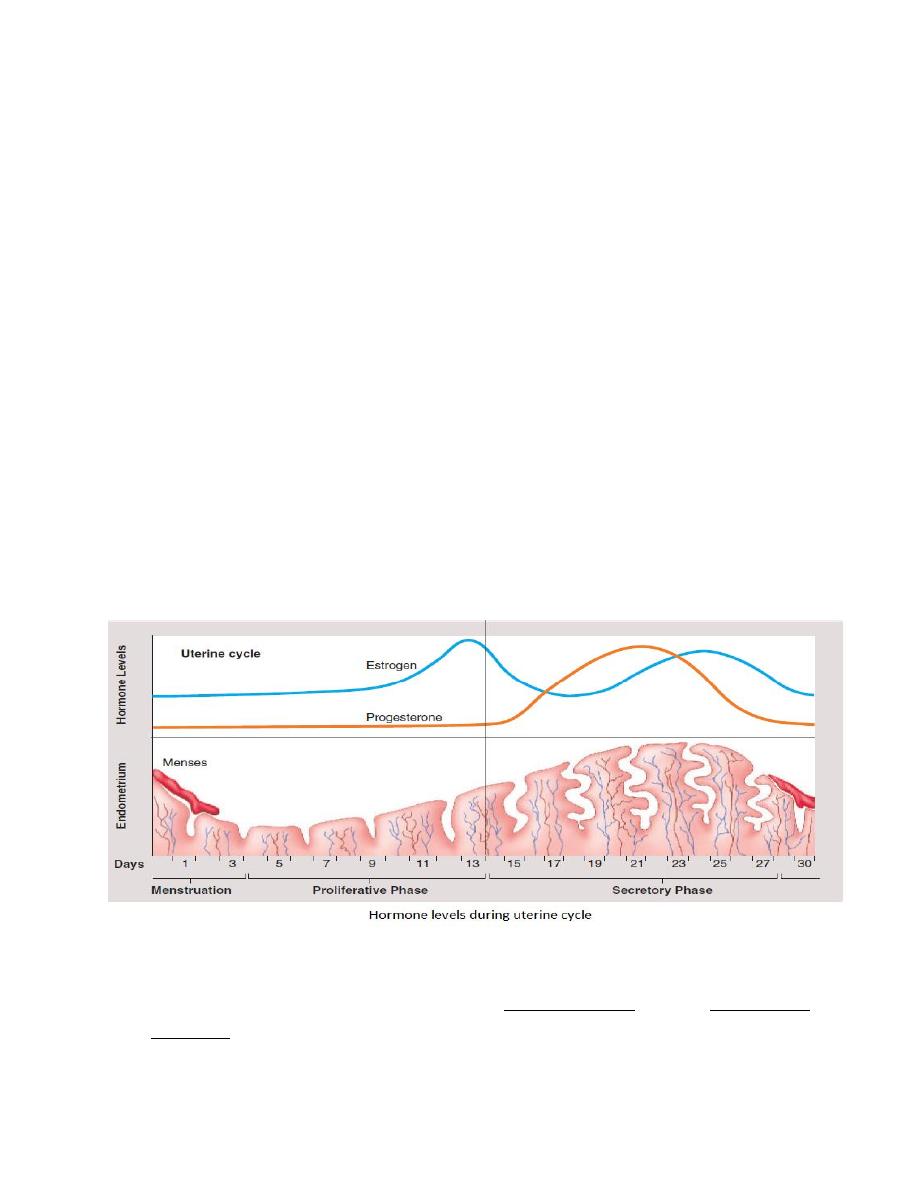
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
65
1. Menses Phase
The menses phase of the menstrual cycle is the phase during which the lining of
the uterus is shed. Although it averages approximately five days, the menses phase
can last from 2 to 7 days, or longer. The menses phase occurs during the early days
of the follicular phase of the ovarian cycle, when progesterone, FSH, and LH
levels are low.
2. Proliferative Phase
Once menstrual flow ceases, granulosa and theca cells of the follicles begin to
produce increased amounts of estrogen. These rising estrogen concentrations
stimulate the endometrial lining to rebuild.
3. Secretory Phase
Following ovulation, corpus luteum start to produce progesterone.
Ovarian cycle
There are two phases of the ovarian cycle the follicular phase and the luteal phase
with ovulation in between:
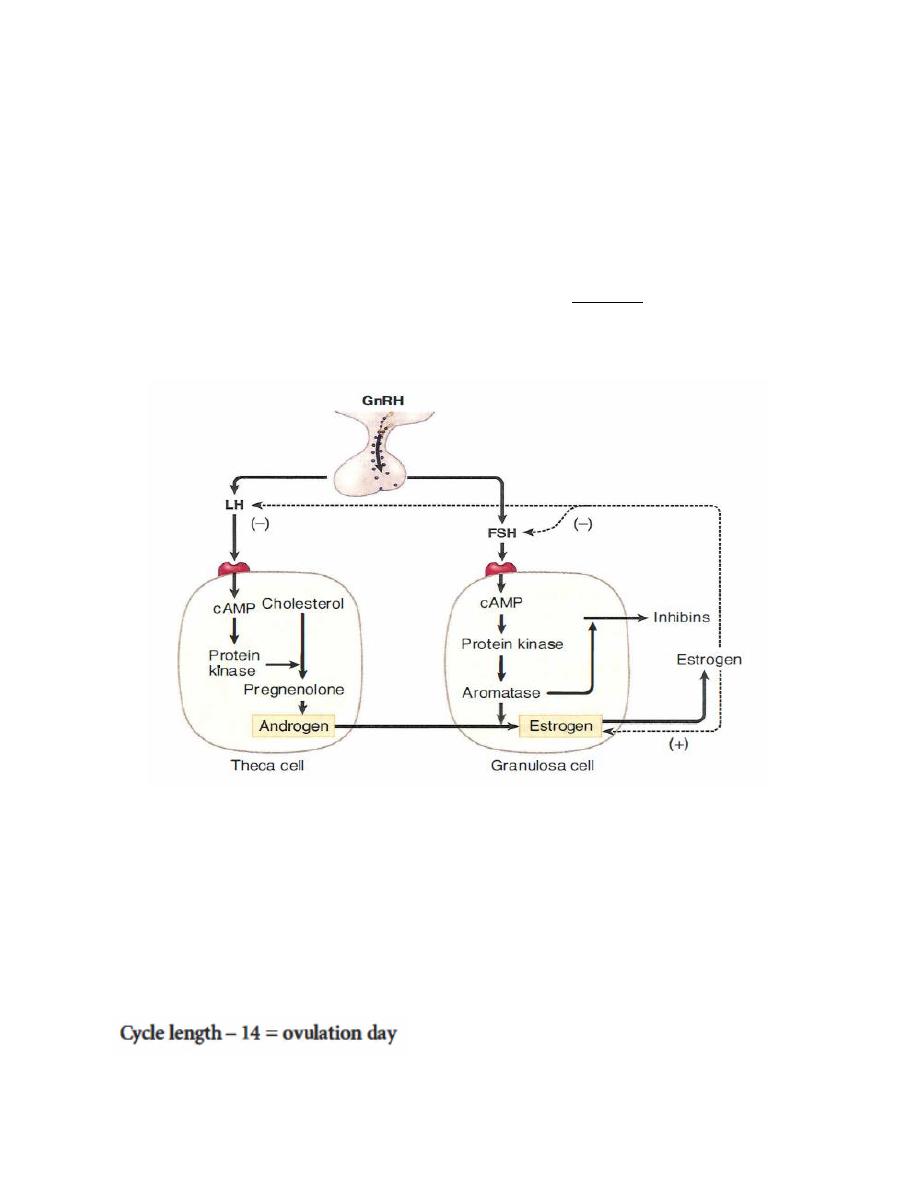
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
66
1. Follicular phase (first 2 weeks) is also called the proliferative or preovulatory
phase. In the follicular phase about 10-20 follicles are taken but only one follicle
destined to grow and rupture while the others undergo atresia and die, leaving a
single oocyte for release at ovulation.
This phase is dominated by the peripheral effects of estrogen, which include the
replacement of the endometrial cells lost during menses it also cause the cervical
mucus to be thin and watery, making the cervix easy for sperm to traverse.
Figure. follicular phase relationships approximately days 1 to 1 4
2. Ovulation
Ovulation takes place approximately on day 14. Near the end of the follicular
phase, there is a dramatic rise in circulating estrogen, this will stimulate the release
of LH and FSH. The LH surge induce ovulation and formation of the corpus
luteum. Follicular rupture occurs 24-36 hours after the onset of the LH surge.
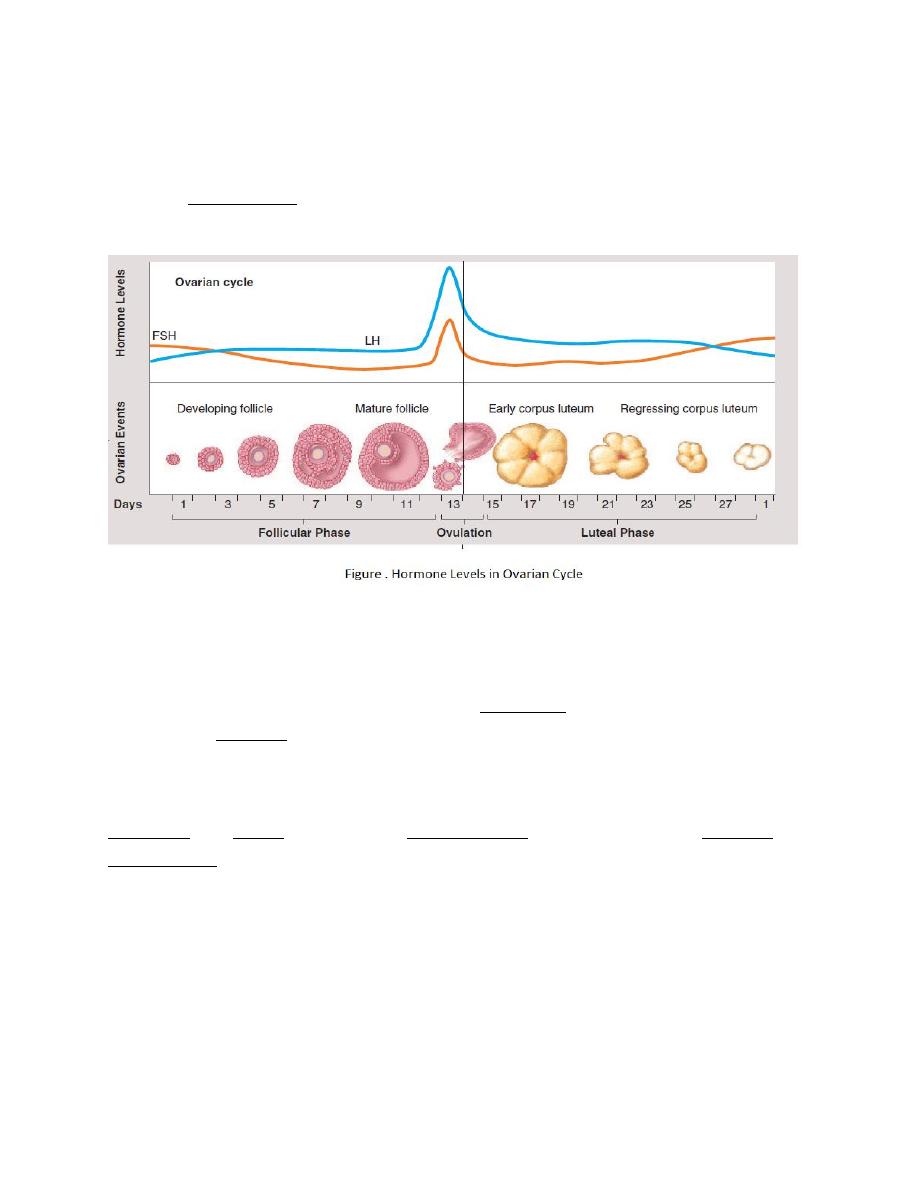
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
67
3. Luteal phase (approximately 2 weeks) is dominated by the elevated plasma
levels of progesterone, and along with lower levels of secreted estrogen, creates a
secretory quiescent endometrium which prepares the uterus for implantation.
Major events in menstrual cycle
1. The Ant. pituitary gland secretes FSH and LH.
2. FSH stimulates maturation of a follicle. Granulose cells of the follicle produce
and secrete estrogen. Estrogen maintains 2ndry sex traits & causes the uterine
lining to thicken.
3. The Ant. pituitary gland releases a surge of LH, which stimulates ovulation.
Follicular and thecal cells become corpus luteum cells which secrete estrogen and
progesterone.
a.Estrogen continues to stimulate uterine wall development.
b.Progesterone stimulates the uterine lining to become more glandular and
vascular.
c.Estrogen and progesterone inhibit secretion of FSH and LH from the Ant.
pituitary gland.

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
68
4. If the egg is not fertilized, the corpus luteum degenerates and no longer secretes
estrogen and progesterone (24th day of the cycle).
5. As the conc. of luteal hormones decline, blood vessels in the uterine lining
constrict.
6. The uterine lining disintegrates and sloughs off, producing a menstrual flow
(28th day of the cycle).
7. The Ant. pituitary gland, no longer inhibited, again secretes FSH and LH.
8. The menstrual cycle repeats.
Functions of Estrogens
The main functions of estrogens are:
1- Stimulate development of reproductive organs like vagina, uterus, and
fallopian tubes in utero and of secondary sex characteristics during puberty.
2- Promotes growth of ovarian follicles and Ovulation.
3- Alter the cervical secretions to favor survival and transport of sperm and
promote motility of sperm within the fallopian tubes by decreasing mucus
viscosity.
4- Helps in process of implantation by promoting development of endometrial
lining in the event of pregnancy.
5- Stimulate stromal development and ductal growth of breast.
6- Accelerate growth of long bones and closure of epiphyses at puberty it also
decrease rate of bone resorption and increase production of thyroid and other
binding globulins.
7- Increase high-density and slightly decrease low-density lipoproteins.
Inhibin
Inhibin, was originally described as a testicular product that inhibited pituitary
FSH production hence its name. However, inhibin is also produced by a variety of
other cell types, including granulosa cells within the ovary; production is

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
69
stimulated by FSH. Within the ovary, inhibin enhances LH-induced androgen
synthesis and attenuates FSH production.
The hypothalamo-pituitary-ovarian axis:
The output of LH and FSH from the pituitary gland is stimulated by pulses of
gonadotrophin-releasing hormone (GnRH) produced by the hypothalamus and
transported to the pituitary in the portal circulation. The response of the pituitary
modulated by ovarian hormones, particularly estrogen and progesterone. Low
levels of estrogen have an inhibitor effect on LH (negative feedback), whereas high
levels of estrogen actually stimulate pituitary LH production (positive feedback).In
the late follicular phase, serum levels of estrogen are sufficiently high that a
positive-feedback effect is triggered, thus generating the peri-ovulatory LH surge.
The mechanism of action of the positive-feedback effect of estrogen involves an
increase in GnRH receptor concentrations and an increase in GnRH production.
Low levels of progesterone prior to ovulation have a positive-feedback effect on
pitutary LH and FSH secretion and contribute to the LH surge. High levels of
progesterone (luteal phase) inhibit pituitary gonadotrophin production. Negative-
feedback effects of progesterone are generated both via decreased GnRH
production and via decreased sensitivity to GnRH at the pituitary level. Positive-
feedback effects of progeteroneoperate at the pituitary level only and involve
increased sensitivity to GnRH.
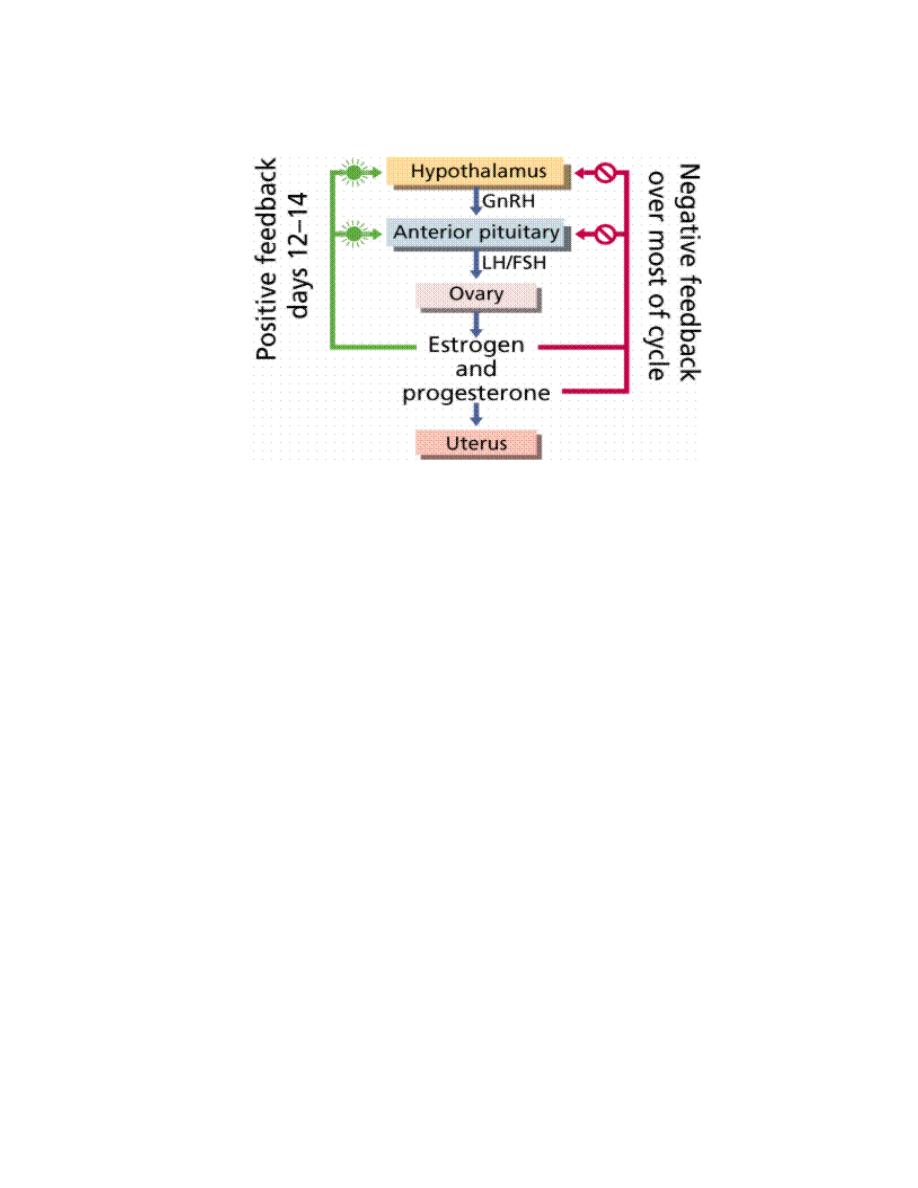
Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
70
Figure: hypothalamus pituitary ovarian axis.
Menopause
Menopause is a normal stage of life occurs as the ovaries stop producing estrogen,
cessation of menstruation is a universal feature of menopause. Important symptoms
of menopause, include insomnia, hot flushes, variable degrees of vaginal atrophy,
and decreased breast size. Estrogen deficiency causes bone loss and increases the
risk of osteoporosis. The loss of ovarian function removes negative feedback on
GnRH, producing high serum concentrations of FSH and LH.
Endocrine control of lactation
Prolactin and oxytocin are the two key hormones involved in the control of
lactation; prolactin is required for milk production, and oxytocin stimulates milk
ejection from the breast. During pregnancy, the high levels of plasma estrogen
greatly increase prolactin secretion, but milk synthesis does not occur because the
high level of estrogen (and progesterone) blocks milk synthesis. At parturition,
plasma estrogen drops, withdrawing the block on milk synthesis. As a result, the
number of prolactin receptors in mammary tissue increases several-fold, and milk
synthesis begins.

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
71
-Note: Detecting HCG in a woman’s urine or blood is used to confirm pregnancy.
The endocrinology of pregnancy
There are several hormones (e.g., peptides, amines, and steroids) that are essential
to the maintenance of pregnancy.
1. Human chorionic gonadotropin (hCG)
At the time of implantation, trophoblastic cells of the fetus begin to secrete hCG
which stimulate progesterone and estrogen secretion by the corpus luteum, thus
rescuing the corpus luteum, which was otherwise destined to regress. Loss of the
corpus luteum during this period would terminate the pregnancy.
2. Human chorionic somatomammotropin (hCS) also called human
placental lactogen
hCS is structurally similar to GH and prolactin. Its metabolic effects are similar to
those of GH, with suppression of maternal glucose use and reduced maternal
insulin responsiveness, which may preserve glucose for fetal use.
3. progesterone and estrogens
At about 8–9 weeks of gestation, the placenta will assume the production of
progesterone. Thereafter, the plasma hCG concentrations decrease to lower levels
but continue to be important for maintaining progesterone secretion by the
syncytiotrophoblast. High steroid levels are necessary to support pregnancy.
Maternally derived LDL cholesterol is necessary for placental steroidogenesis
because the placenta lacks enzymes needed to produce cholesterol from acetate.
The placenta can produce progesterone when provided with a supply of
cholesterol. The placenta lacks enzymes needed to produce estrogens but has very
high aromatase activity to convert androgens to estrogens. The fetal adrenal glands
supply the placenta with weak androgens for conversion to estrogens. These glands
produce dehydroepiandrosterone sulphate (DHEA-S).
(note:The fetus does not produce progesterone or estrogens, thereby avoiding
exposure to high concentrations of these steroids. Although the fetus produces

Medical physiology Endocrinology
Dr: ABDULHASAN ALNIYAZI
Ph.D. Medical Physiology
_____________________________________________________________________________________
72
large amounts of weak adrenal androgens, masculinization of the female fetus does
not occur because the placenta acts as a large sink for fetal androgens).
4. Relaxin secreted by the corpus luteum and the placenta softens the pubic
symphysis, relaxes sacroiliac ligaments, and dilates the cervix in preparation
for labor.
Infertility
Infertility is the failure of a couple to achieve pregnancy after one year of regular,
unprotected intercourse. The American Medical Association estimates that 15% of
all couples are infertile. The cause of infertility can be attributed to the male
(40%), the female (40%), or both (20%).The most common causes of infertility in
females are blocked oviducts and endometriosis. The most frequent cause of
infertility in males is low sperm count and/or a large proportion of abnormal
sperm.
Assisted reproductive technologies (ART)
consist of techniques used to increase the chances of pregnancy:
1. In Vitro Fertilization (IVF). During IVF, fertilization occurs in laboratory
glassware. After about two to four days, the embryos are ready to be transferred to
the uterus of the woman.
2. Intrauterine insemination (IUI) represents one of the assisted reproductive
technologies that involves insertion of sperms inside a lady’s uterus to
enhance fertilization. The aim of IUI procedures is to help deliver male sperms
closer to the female egg to increase the amount of sperms that reach the
fallopian tubes and then increase the fertilization chance
3. Intracytoplasmic Sperm Injection (ICSI). In this highly sophisticated procedure,
one single sperm is injected into an egg. It is used effectively when a man has
severe infertility problems
