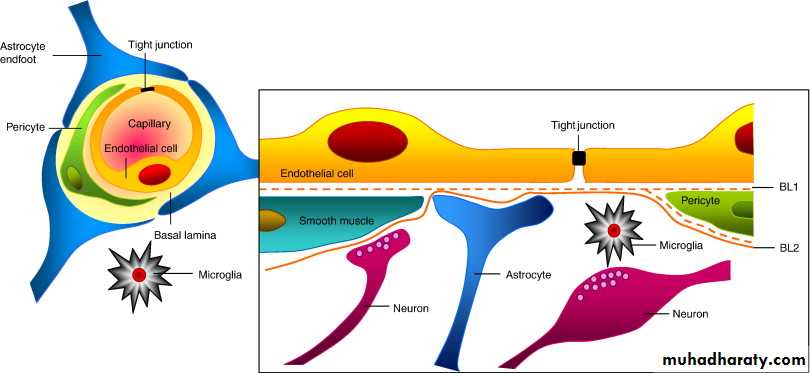
Blood-Brain Barrier The blood-brain barrier (BBB) is a functional barrier that allows much tighter control than that in most tissues over the passage of substances moving from blood into the CNS tissue. The main structural component of the BBB is 1- the capillary endothelium, in which the cells are tightly sealed together with well-developed occluding junctions and with little or no transcytosis activity. 2- The limiting layer of perivascular astrocytic feet that completely envelops the basal lamina of the capillaries in most CNS regions forms another BBB component and further regulates passage of molecules and ions from blood to brain. The BBB protects neurons and glia from bacterial toxins, infectious agents, and other exogenous substances, and helps maintain the stable composition and constant balance of ions in the interstitial fluid that is required for normal neuronal function. The components of the BBB are not found in the choroid plexus where CSF is produced, in the posterior pituitary which releases hormones, or in regions of the hypothalamus where plasma components are monitored.
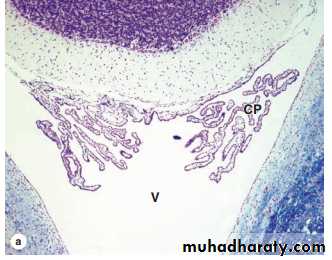
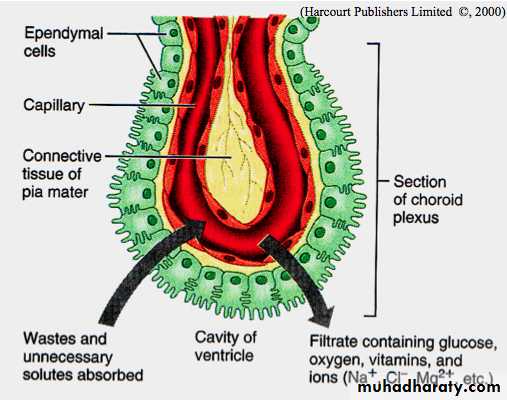
Choroid Plexus The choroid plexus consists of highly specialized tissue with elaborate folds and many villi projecting into the four large ventricles of the brain. It is found in the roofs of the third and fourth ventricles and in parts of the two lateral ventricular walls, all regions in which the ependymal lining directly contacts the pia mater. Each villus of the choroid plexus contains a thin layer of well-vascularized pia mater covered by cuboidal ependymal cells. The function of the choroid plexus is to remove water from blood and release it as the CSF. CSF is clear, contains Na+, K+, and Cl– ions but very little protein, and its only cells are normally very sparse lymphocytes. It is produced continuously and it completely fills the ventricles, the central canal of the spinal cord, the subarachnoid and perivascular spaces. It provides the ions required for CNS neuronal activity and in the arachnoid serves to help absorb mechanical shocks. Arachnoid villi provide the main pathway for absorption of CSF back into the venous circulation. There are no lymphatic vessels in CNS tissue.
MEDICAL APPLICATION: A decrease in the absorption of CSF or a blockage of outflow from the ventricles during fetal or postnatal development results in the condition known as hydrocephalus (Gr. hydro, water + kephale, head), which promotes a progressive enlargement of the head followed by mental impairment.
PERIPHERAL NERVOUS SYSTEM The main components of the peripheral nervous system (PNS) are the nerves, ganglia, and nerve endings. Nerves are bundles of nerve fibers (axons) surrounded by Schwann cells and layers of connective tissue.
Nerve Fibers: Nerve fibers are analogous to tracts in the CNS, containing axons enclosed within sheaths of glial cells specialized to facilitate axonal function. In peripheral nerve fibers, axons are sheathed by Schwann cells, the sheath may or may not form myelin around the axons, depending on their diameter.
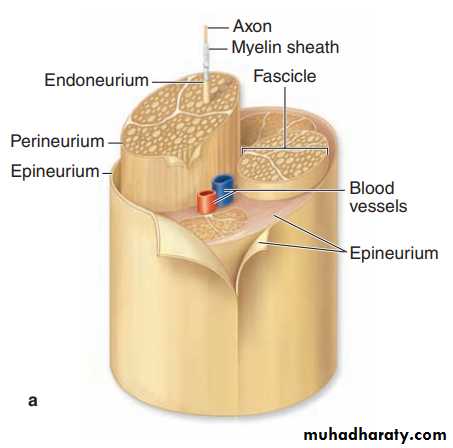
Nerve Organization In the PNS nerve fibers are grouped into bundles to form nerves. Except for very thin nerves containing only unmyelinated fibers, nerves have a whitish, glistening appearance because of their myelin and collagen content. Axons and Schwann cells are enclosed within layers of connective tissue. Immediately around the external laminae of the Schwann cells is a thin layer called the endoneurium, consisting of reticular fibers, scattered fibroblasts, and capillaries. Groups of axons with Schwann cells and endoneurium are bundled together as fascicles by a sleeve of perineurium, containing flat fibrocytes with their edges sealed together by tight junctions. From two to six layers of these unique connective tissue cells regulate diffusion into the fascicle and make up the blood-nerve barrier that helps maintain the fibers’ microenvironment. Externally, peripheral nerves have a dense, irregular fibrous coat called the epineurium, which extends deeply to fill the space between fascicles. Very small nerves consist of one fascicle. Small nerves can be found in sections of many organs and often show a winding disposition in connective tissue. Peripheral nerves establish communication between centers in the CNS and the sense organs and effectors (muscles, glands, etc). They generally contain both afferent and efferent fibers. Afferent fibers carry information from internal body regions and the environment to the CNS. Efferent fibers carry impulses from the CNS to effector organs commanded by these centers. Nerves possessing only sensory fibers are called sensory nerves; those composed only of fibers carrying impulses to the effectors are called motor nerves. Most nerves have both sensory and motor fibers and are called mixed nerves, usually also with both myelinated and unmyelinated axons.
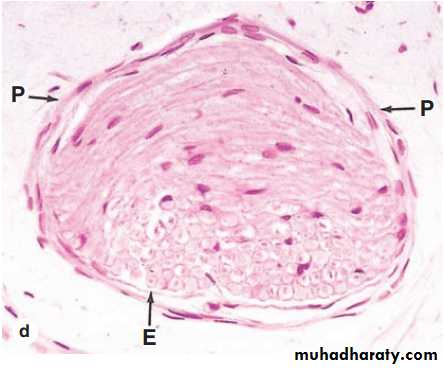
Ganglia Ganglia are typically ovoid structures containing neuronal cell bodies and their surrounding glial satellite cells supported by delicate connective tissue and surrounded by a denser capsule. Because they serve as relay stations to transmit nerve impulses, at least one nerve enters and another exits from each ganglion. The direction of the nerve impulse determines whether the ganglion will be a sensory or an autonomic ganglion.
Sensory Ganglia : Sensory ganglia receive afferent impulses that go to the CNS. Sensory ganglia are associated with both cranial nerves (cranial ganglia) and the dorsal roots of the spinal nerves (spinal ganglia). The large neuronal cell bodies of ganglia are associated with thin, sheetlike extensions of small glial satellite cells. Sensory ganglia are supported by a distinct connective tissue capsule and an internal framework continuous with the connective tissue layers of the nerves. The neurons of these ganglia are pseudounipolar and relay information from the ganglion’s nerve endings to the gray matter of the spinal cord via synapses with local neurons.
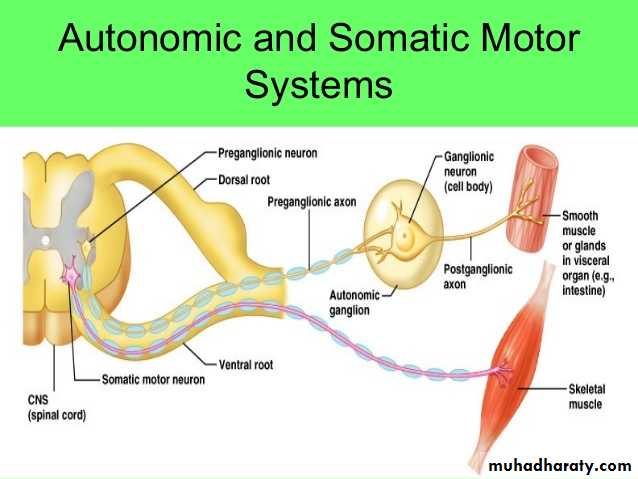
Autonomic Ganglia : Autonomic nerves effect the activity of smooth muscle, the secretion of some glands, heart rate, and many other involuntary activities by which the body maintains a constant internal environment (homeostasis). Autonomic ganglia are small bulbous dilations in autonomic nerves, usually with multipolar neurons. Some are located within certain organs, especially in the walls of the digestive tract, where they constitute the intramural ganglia. The capsules of these ganglia may be poorly defined among the local connective tissue. A layer of satellite cells also envelops the neurons of autonomic ganglia, although these may also be inconspicuous in intramural ganglia. Autonomic nerves use two-neuron circuits. The first neuron of the chain, with the preganglionic fiber, is located in the CNS. Its axon forms a synapse with postganglionic fibers of the second multipolar neuron in the chain located in a peripheral ganglion system. The chemical mediator present in the synaptic vesicles of all preganglionic axons is acetylcholine. As indicated earlier autonomic nerves make up the autonomic nervous system. This has two parts: the sympathetic and the parasympathetic divisions. Neuronal cell bodies of preganglionic sympathetic nerves are located in the thoracic and lumbar segments of the spinal cord and those of the parasympathetic division are in the medulla and midbrain and in the sacral portion of the spinal cord. Sympathetic second neurons are located in small ganglia along the vertebral column, while second neurons of the parasympathetic series are found in very small ganglia always located near or within the effector organs, for example in the walls of the stomach and intestines. Parasympathetic ganglia may lack distinct capsules altogether, perikarya and associated satellite cells simply forming a loosely organized plexus within the surrounding connective tissue.
NEURAL PLASTICITY & REGENERATION Despite its general stability, the nervous system exhibits neuronal differentiation and formation of new synapses even in adults. Embryonic development of the nervous system produces an excess of differentiating neurons, and the cells that do not establish correct synapses with other neurons are eliminated by apoptosis. In adult mammals after an injury, the neuronal circuits may be reorganized by the growth of neuronal processes, forming new synapses to replace ones lost by injury. Thus, new communications are established with some degree of functional recovery. This neural plasticity and reformation of processes are controlled by several growth factors produced by both neurons and glial cells in a family of proteins called neurotrophins. Neuronal stem cells are present in the adult CNS, located in part among the cells of the ependyma, which can supply new neurons, astrocytes, and oligodendrocytes. fully differentiated, interconnected CNS neurons cannot temporarily disengage these connections and divide to replace cells lost by injury or disease; the potential of neural stem cells to allow tissue regeneration and functional recovery within the CNS components is a subject of intense investigation. Astrocytes do proliferate at injured sites and these growing cells can interfere with successful axonal regeneration in structures such as spinal cord tracts. In the histologically much simpler peripheral nerves, injured axons have a much greater potential for regeneration and return of function. If the cell bodies are intact, damaged, or severed PNS axons can regenerate as shown in the sequence of diagrams . Distal portions of axons, isolated from their source of new proteins and organelles, degenerate; the surrounding Schwann cells dedifferentiate, shed the myelin sheaths, and proliferate within the surrounding layers of connective tissue. Cellular debris including shed myelin is removed by blood-derived macrophages, which also secrete neurotrophins to promote anabolic events of axon regeneration. The onset of regeneration is signaled by changes in the perikaryon that characterize the process of chromatolysis : the cell body swells slightly, Nissl substance is initially diminished, and the nucleus migrates to a peripheral position within the perikaryon. The proximal segment of the axon close to the wound degenerates for a short distance, but begins to grow again distally as new Nissl substance appears and debris is removed. The new Schwann cells align to serve as guides for the regrowing axons and produce polypeptide factors that promote axonal outgrowth. Motor axons reestablish synaptic connections with muscles and function is restored .