Regulation of the intracellular Ca2+
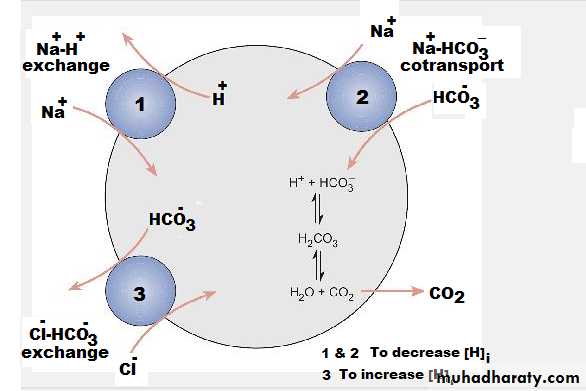
Regulation of intracellular [H]:Cell volume regulation
It depends on the total amount of intracellular solute (Na+ and K+ ionic gradients) generated by the Na+/K+ pumpFollowing cell shrinkage Intracellular solute concentration increasing mechanism is activated The synthesis of osmotically active molecules
(eg, sorbitol or taurine)
The transport of ions inside the cell through the
Na+-H+ exchanger or the Na+-H+-Cl−
cotransporter
Following cell swelling transport mechanisms that extrude solutes out of the cell (eg, K+ or Cl- channels or the K+-Cl- cotransporter) will be activated.
Cell Apoptosis = Cell suicide Genetically determined
Cell Necrosis = Cell murder External causeCell structure and Functions
Interesting facts about body fluids and water:
Water is key to life. Humans can survive more than a month without food, but only a few days without water.Transportation: Water transports glucose, oxygen, and fat to body tissues and waste products, such as carbon dioxide, and lactic acid away from body tissues.
Body temperature regulation: Your body produces enough heat in only thirty minutes to boil a half-gallon of water. The body wants to keep a constant temperature of approximately 37ºC. If the body temperature increases to >41ºC, cells will die.
To prevent overheating, the body regulates temperature by sweating. Water absorbs heat from the working muscles and dissipates this heat to circulating blood and ultimately through the skin and by sweat evaporation.
Digestion: Water is an important component of saliva, intestinal, and gastric juices, which help digest food.
Lubrication: Water is a good lubricator of joints, organs, and tissues.
Drinking too much water too quickly can lead to water intoxication. Water intoxication occurs when water dilutes the sodium level in the bloodstream and causes an imbalance of water in the brain. Water intoxication is most likely to occur during periods of intense athletic performance.
Pure water (solely hydrogen and oxygen atoms) has a neutral pH of 7, which is neither acidic nor basic. Water dissolves more substances than any other liquid
Wherever it travels, water carries chemicals, minerals, and nutrients with it.
By the time a person feels thirsty, his or her body has lost over 1 percent of its total water amount.
After the end of these lectures, you should be able to . . .
Know the composition of extracellular and intracellular body fluids. Explain osmosis, osmolarity and osmotic pressure, and tonicity of the body fluids.
Know the forces producing movement of substances between compartments.
Describe the process of endocytosis and exocytosis.
Describe the primary factors (Starling forces) that determine fluid movement through the capillary membrane and the formation of interstitial fluid and lymph.
Describe the intake versus output of water.
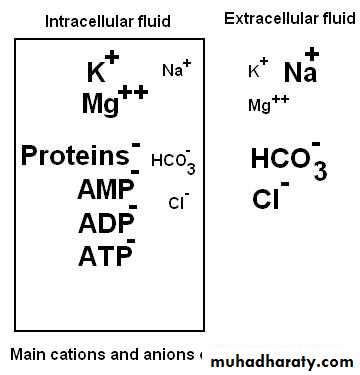
Body fluids and compartments
Water = 60% of TBW
ECF
20%
ICF
40%
Water = 60% of TBW
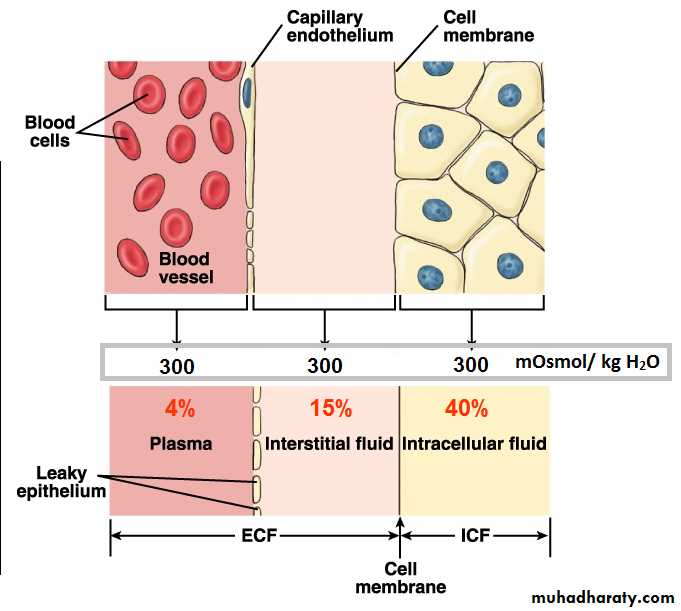
Body fluids and compartmentsExtracellular fluid (20% of the TBW):
1. The interstitial fluid 15% of the TBW.2. Plasma 4% of the TBW.
3. Transcellular fluids 1% of the TBW.
Intracellular fluid (40% of the TBW
15%4%
40%
Body fluids and compartments
Extracellular fluid (20% of the TBW):1. The interstitial fluid 15% of the TBW.
2. Plasma 4% of the TBW.
3. Transcellular fluids 1% of the TBW.
Intracellular fluid (40% of the TBW
15%4%
40%
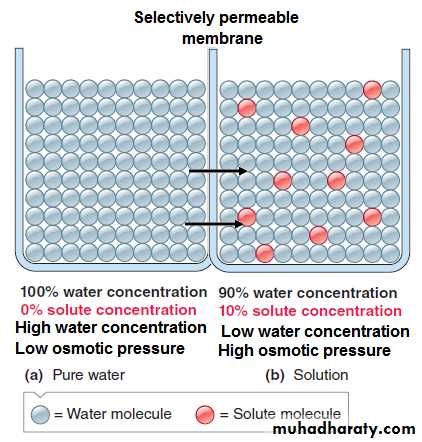
Osmosis, Osmolarity and Osmotic pressure of the body fluids
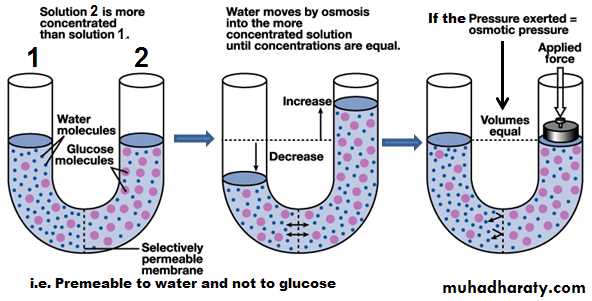
Osmosis is the diffusion or flow of water molecules across a membrane into a region in which there is a high concentration of a solute to which the membrane is impermeable
The number of the solute particles determines the magnitude of the osmotic pressure of the solution in which it is dissolved.
Each mole of a substance contains Avogadro's constant (6.022×1023) of molecules.
Molarity of a substance is the concentration of non-dissociated substance (in moles) per one L (kg) of water. The osmolarity (osmole) is the concentration of osmotically active particles (the molecules or the particles which attract water to it) in one liter of a solution (mol/L H2O).
The osmolality (osmole) is the concentration of osmotically active particles (the molecules or the particles which attract water to it) in one kg of a solution (mol/Kg H2O).
The number of osmotically active particles in any fluid is determined by: The number of moles per liter (or kg) of water X the numbers of osmotically active
particles released into solution when the solute is dissolved.
Therefore;
One Avogadro's constant molecules of a substance/L H2O = 1 Mole of substance/L H2O (MOLARITY) = 1 Mole X the numbers of osmotically active particles released into solution when the solute is dissolved (OSMOLARITY, Osmole of substance /L H2O).
1M glucose = 1 OsM/L
1M NaCl = 2 OsM NaCl/L, because NaCl dissociates to 2 ions in solution, Na+ and Cl-.Examples:
ʘ 5% of glucose in water has a molarity of 300 mMoles/L H2O, and because the glucose molecules in water do not dissociated, it has an osmolarity of 300 mOsmol/L H2O.ʘ 0.9% NaCl in water has a molarity of 150 mMoles/L H2O, and because each NaCl molecule in water is dissociated into two osmotically active particles (Na+ and Cl- ions); it has an osmolarity of 300 mOsmol/L H2O.
Therefore, 5% of glucose in water and 0.9% NaCl in water have different molarity but identical osmolarity and consequently identical osmotic pressure.
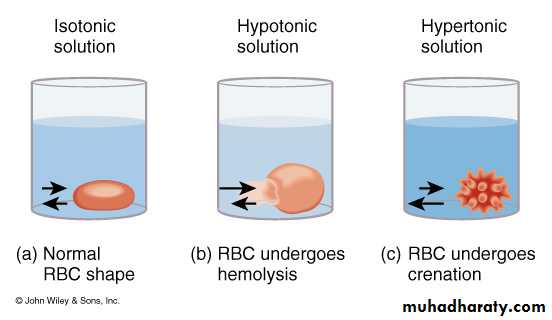
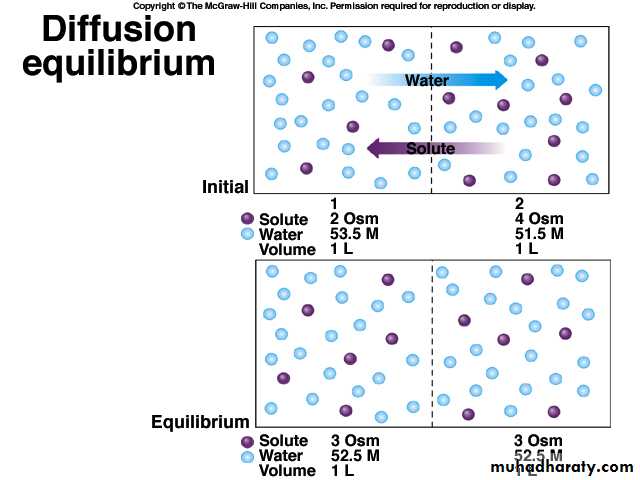
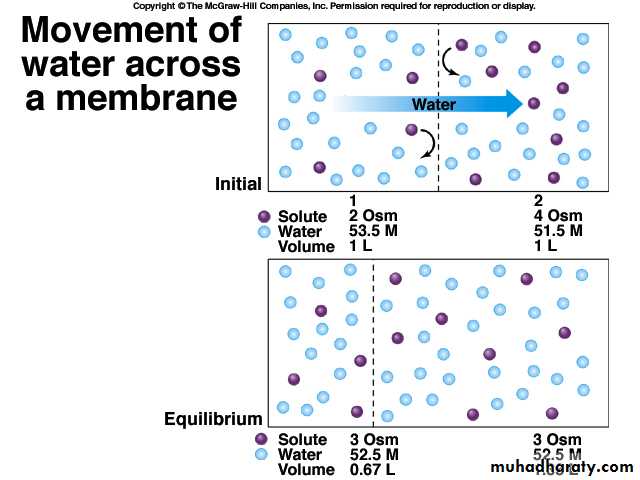
Cell keeps the same volume in an isotonic solution.
Cell gains volume in a hypotonic solutionCell looses volume in a hypertonic solution
Isotonic - both solutions have the same solute concentrations.
Hypotonic - solution with the lower solute concentration.
Hypertonic - solution with the higher solute concentration.
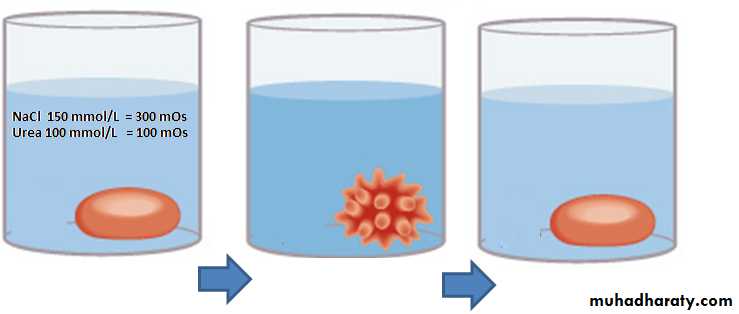
Solution containing:
NaCl 150 mmol/L = 300 mOs Non-Penetrating substancesUrea 100 mmol/L = 100 mOs Penetrating substance
Total Osmolarity = 400 mOs
Total Tonicity = 300 mOsDuring intavenous injection:
0.9% (normal) saline isotonic5% dextrose isotonic
0.45% saline hypotonic
Forces producing movement of substances between compartments
• Simple diffusion• Filtration
• Osmosis
• Carrier-mediated transport
• Endocytosis and exocytosis
Simple diffusion through cell membrane lipid bilayer or through cell membrane channel proteins
Affected by:
Concentration gradientElectrical gradient
Thickness of the boundary
Cross sectional area of the boundary
Temperature
Membrane permeability
Filtration Is the process by which water and water soluble substances is forced through an epithelial layer due to a difference in hydrostatic pressure on the two sides
Affected by the:
Pressure gradient
Surface area of the membrane
Diameter of the membrane pores
Size of the filtered molecules
Carrier-mediated transport
characterize by:[i] Stereospecificity
[ii] Saturation
[iii] Competition
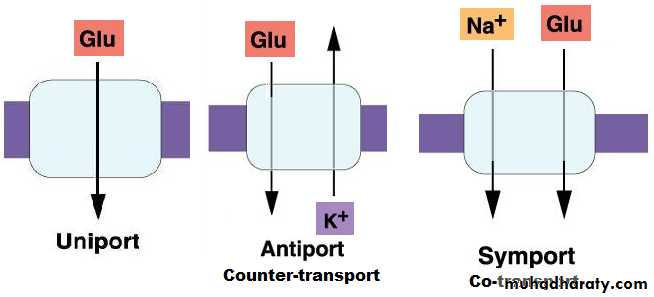
Classified according to the direction of transport:
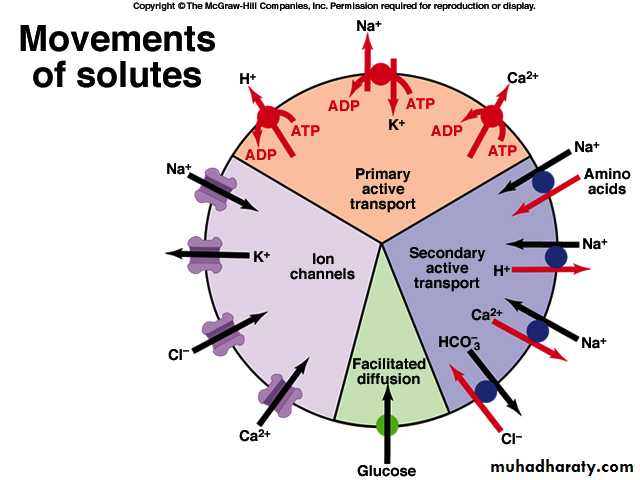
Carrier-mediated transportFacilitated diffusion
Active transportprimary active transport
Secondary active transport
Facilitated diffusion
Transport occurs according to electrochemical gradient, energy is not required (passive),
Animation
• Simple diffusion
• Facilitated diffusion• Diffusion according to electrochemical gradient
• Same
• Energy is not required
• Same
• Does not require a carrier protein
• Requires a carrier protein
• Simple diffusion is not saturable
• Have saturation limited (Tm)
Primary active transport
Transport occurs against electrochemical gradient, the process requires energy. High energy phosphate compound, ATP, provides directly the energy required for the transport processAnimation
Na-K-ATPase pump,
H-ATPase pump (proton pump)and Ca-ATPase pump
Animation
Animation
Secondary active transport: Co-transport or symport
The transport of one substances (e.g. Na+) provides the energy to transport another substance. Transport occurs against electrochemical gradient, the process requires energy.
Animation
Na co-transport of glucose and amino acids
Na-K-2Cl co-transport
Na-Ca counter-transport
Na-H counter transport
Secondary active transport: Counter-transport or antiport
The transport of one substances (e.g. Na+) provides the energy to transport another substance. Transport occurs against electrochemical gradient, the process requires energy.Animation
Secondary active transport: Co-transport or symport
• Facilitated diffusion• Active transport
• Transport according to electrochemical gradient
• Transport against electrochemical gradient
• Does not require energy
• Requires energy
• Uniport transport
• Uniport, Antiport, or symport transport