Definitions
Pharmacology: The word pharmacology is derived from the Greek: pharmakon meaning drug or poison and logos: word or discourse. It is the study of interaction of drugs with living organisms. It covers the use of drugs in the prevention (prophylaxis) and treatment of diseases.It also includes history, source, physicochemical properties, dosage forms, methods of administration, absorption, distribution mechanism of action, biotransformation, excretion, clinical uses and adverse effects of drugs.
Clinical Pharmacology: It evaluate the pharmacological action of drug preferred route of administration and safe dosage range in human by clinical trails.
Pharmacotherapeutics: It deals with the proper selection and use of drugs for the prevention and treatment of disease.
Drugs: Drugs are chemicals that alter functions of living organisms. Drugs are generally given for the diagnosis, prevention, control or cure of disease.
Pharmacy: It is the science of identification, selection, preservation, standardisation,
compounding and dispensing of medical substances.
Toxicology: It is the science of poisons. It is the area of pharmacology concerned with the undesirable effects of chemicals and biologicals on cellular functions.
Poisons are substances that cause harmful, dangerous or fatal symptoms in living substances. Many drugs in larger doses may act as poisons.
Origin and sources of drugs:1. Minerals: Liquid paraffin, magnesium sulfate, magnesium trisilicate, kaolin, etc.2. Animals: Insulin, thyroid extract, and antitoxin sera, etc.3. Plants: Morphine, digoxin, atropine, castor oil, etc.4. Synthetic source: Aspirin, sulphonamides, paracetamol, zidovudine, etc.5. Micro organisms: Penicillin, streptomycin and many other antibiotics.6. Genetic engineering: Human insulin, human growth hormone etc.
Drugs formulations:1- Injections ( Ampoules, vials)2. Liquid ( SYRUP, SUSPENSION)3. Tablets, capsule4. Suppositories, pessaries5. Sprays and inhalants6. Ointments and creams7. Trans dermal patches8. Drug implants9. Micro and nanoparticles10. Targeted drug delivery
Drug Nomenclature: Every drug has at least three names - a chemical name (e.g. 6-dimethylamino-4,4-diphenyl-3-heptanone hydrochloride),- a generic name (e.g., methadone hydrochloride) and -a proprietary (or trade) name (e.g., Dolophine). Chemical and generic names are written in lower case whereas trade names are capitalized. If drug is marketed by more than one pharmaceutical company, then the same drug may have several trade names but only one official generic name.
Pharmacopoeia: An official code containing a selected list of the established drugs and medical preparations with descriptions of their source, chemical nature, physical properties and tests for their identity, purity and potency, route of administration, dynamic effects and kinetic parameters. It also included: Indications (under what circumstances is the drug used). Drug action (what clinical effect does the drug have. Adverse effects (are there clinically relevant side effects of the drug). Contraindications (are there circumstances in which the drug should not be administered to certain patient populations e.g: the elderly, those with renal insufficiency, pregnant women etc).
Such as [British Pharmacopoeia (B.P),
United States Pharmacopoeia (USP)…etc
Drugs administration:
A. Oral:Oral route is the most common route of drug administration. It is mostly used for the neutral drugs. It may be in the form of tablets, capsules, syrup, emulsions or powders.
Advantages:
It is convenient, It is the cheapest available route, It is easy to use, It is safe.
Disadvantages:
Not suitable for all drugs, Some drugs are destroyed by gastric juices. eg: insulin., Absorption is slow, so is not preferred during emergency., May cause gastric irritation, May objectionable in taste., May cause discoloration of teeth e.g. tetracyclines below 14 cause brown discoloration so are not advisable during pregnancy., First pass effect : hepatic metabolism of drug when absorbed and delivered through portal blood. Greater the first pass effect, less amounts of the drug reach the systemic circulation
B. Parenteral Routes 1-Intravenous injections:Intravenous injections might be applied to the cubital, basilic and cephalic veins.Advantages: Immediate action takes place , This route is preferred in emergency situations, This route is preferred for unconscious patients., Titration of dose is possible., Large volume of fluids might be injected by this route, Diluted irritant might be injected. , Absorption is not required.,No first pass effect takes place., Blood plasma or fluids might be injected.Disadvantages:There is no retreat. , This method is more risky. , suitable for insoluble prSepsis-Infection might occur. , Phlebitis(Inflammation of the blood vessel) might occur., Infiltration of surrounding tissues might result., This method is not suitable for oily preparations. ,This method is not eparations2-Intramuscular injection: Drugs may be injected into the arm (deltoid), thigh (vastus lateralis) or buttocks (gluteus maximus). The volume used is 3 ml.Advantages:Absorption is rapid than subcutaneous route., Oily preparations can be used. ,Slow releasing drugs can be given by this route.DisadvantagesSome time painful, Not suitable for irritant drugs., Using this route might cause nerve or vein damage.3. Subcutaneous injection: Some drugs, notably insulin, are routinely administered SC. Drug absorption is generally slower SC than IM, due to poorer vascularity. Absorption can be facilitated by heat, massage or vasodilators. It can be slowed by co-administration of vasoconstrictors, a practice commonly used to prolong the local action of local anesthetics. As above, arm > thigh.4- Intraperitoneal injection: is the injection of a substance into the peritoneum (body cavity). In general , it is rarely used in human (fearing from peritonitis), it is preferred when large amounts of replacement fluids are needed, or when low blood pressure or other problems prevent the use of a suitable blood vessel for intravenous injection5- Intradermal injection: is injection of drug in the dermis, it is one of the routes used for administration vaccins and for the test of allergy.
C. Topical application to the skin and mucus membranes: a. Eye For desired local effects. b. Intravaginal for infections or contraceptives. c. Intranasal for alleviation of local symptoms. d. Respiratory mucus membrane: inhalors e. Skin Topical drug administration for skin disorders minimizes systemic exposure. However, systemic absorption does occur and varies with the area, site, drug, and state of the skin. f-Sublingual (buccal) Certain drugs are best given beneath the tongue or retained in the cheek pouch and are absorbed from these regions into the local circulation. These vascular areas are ideal for lipid-soluble drugs that would be metabolized in the gut or liver, since the blood vessels in the mouth bypass the liver (do not undergo first pass liver metabolism), and drain directly into the systemic circulation. This route is usually reserved for nitrates and certain hormones.g. Rectal: The administration of suppositories is usually reserved for situations in which oral administration is difficult. This route is more frequently used in small children. The rectum is devoid of villi, thus absorption is often slow.h. Inhalation Volatile anesthetics, as well as many drugs which affect pulmonary function, are administered as aerosols. Other obvious examples include nicotine and tetrahydrocannabinol (THC), which are absorbed following inhalation of tobacco or marijuana smoke. The large alveolar area and blood supply lead to rapid absorption into the blood. Drugs administered via this route are not subject to first-pass liver metabolism.
D- Administration of drugs by special routes: -Intrathecal and epidural injection: drugs are given via lumbar puncture and injection into the subarachnoid space such as using life-threatening, antibiotics, antifungals and anticancer and also in spinal anesthesia to by bass blood-brain barrier -Intra-arterial injection: Used in certain special situations, notably with anticancer drugs, in an effort to deliver a high concentration of drug to a particular tissue. Typically, the injected artery leads directly to the target organ.-Intra-articular: Injected directly into a joint e.g. hydrocortisone
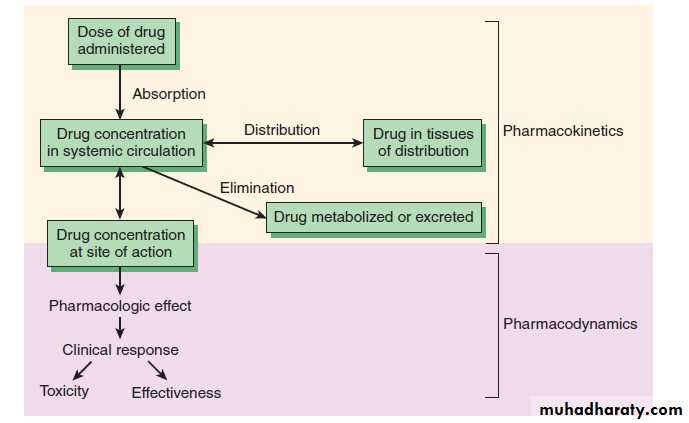
Pharmacodynamics: what the drug does to the body : The study of the biological and therapeutic effects of drugs. Pharmacokinetics: what the body does to the drug: Study of the absorption, distribution metabolism and excretion
Pharmacokinetics: Absorption
Absorption is movement of the drug from its site of administration into the circulation. Not only the fraction of the administered dose that gets absorbed, but also the rate of absorption is important. Except when given i.v., the drug has to cross biological membranes; absorption is governed by the above described principles. Other factors affecting absorption are:Biological Membrane:
Biological membranes consist of a lipid bilayer separating different compartments, with protein molecules acting as enzymes, channels or carrier proteins.
Drugs have to cross the biological membranes to get absorbed.
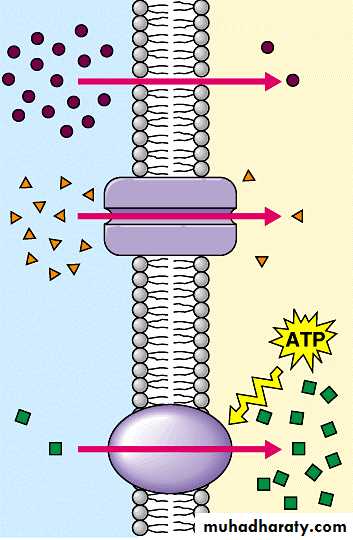
Drugs are transported across the membranes by:
(a) Passive: Simple diffusion and filtration:(b) Specialized transport ( carrier transport):
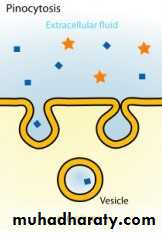
(c) Pinocytosis:
(a) Passive: Simple diffusion and filtration:
1-Simple diffusionThe drug diffuses across the membrane in the direction of its concentration gradient, the membrane playing no active role in the process. This is the most important mechanism for majority of drugs. Depend on: concentration gradient, lipid solubility and molecular weight.
Influence of pH Most drugs are weak electrolytes, i.e. their ionization is pH dependent (contrast strong electrolytes that are nearly completely ionized at acidic as well as alkaline pH). The ionization of a weak acid HA is given by the equation: I pH= pKa + log ------- …………………….. (1) I isonized, UI unionized UIpKa is the negative logarithm of acidic dissociation constant of the weak electrolyte. While for weak base UI pH= pKa + log ------- …………………….. (2) I (a) Acidic drugs, e.g. aspirin (pKa 3.5) are largely unionized at acid gastric pH and are absorbed from stomach, while bases, e.g. atropine (pKa 10) are largely ionized and are absorbed only when they reach the intestines.(b) The unionized form of acidic drugs which crosses the surface membrane of gastric mucosal cell, reverts to the ionized form within the cell (pH 7.0) and then only slowly passes to the extracellular fluid. This is called ion trapping. (c) Basic drugs attain higher concentration intracellularly (pH 7.0 vs 7.4 of plasma).(d) Acidic drugs are ionized more in alkaline urine-do not back diffuse in the kidney tubules and are excreted faster. Accordingly, basic drugs are excreted faster if urine is acidified.To enhance acidic drug excretion alkalinize urine, and to enhance alkaline drug excretion acidify urine.
2-FiltrationFiltration is passage of drugs through aqueous pores in the membrane or through paracellular spaces. This can be accelerated if hydrodynamic flow of the solvent is occurring under hydrostatic or osmotic pressure gradient, e.g. across most capillaries including glomeruli. Lipid-insoluble drugs cross biological membranes by filtration if their molecular size is smaller than the diameter of the pores. Majority of cells (intestinal mucosa, RBC, etc.) have very small pores (4 A) and drugs with MW > 100 or 200 are not able to penetrate. However, capillaries (except those in brain) have large paracellular spaces (40 A) and most drugs can filter through these. As such, diffusion of drugs across capillaries is dependent on rate of blood flow through them rather than on lipid solubility of the drug or pH of the medium.
(b) Specialized transport ( carrier transport): 1. Facilitated transport The transporter, belonging to the super-family of solute carrier (SLC) transporters, operates passively without needing energy and translocates the substrate in the direction of its electrochemical gradient, i.e. from higher to lower concentration . lt mearly facilitates permeation of a poorly diffusible substrate, e.g. the entry of glucose into muscle and fat cells by GLUT 4. 2. Active transport It requires energy, is inhibited by metabolic poisons, and transports the solute against its electrochemical gradient (low to high), resulting in selective accumulation of the substance on one side of the membrane. It is saturable and follows the Michaelis-Menten kinetics. The maximal rate of transport is dependent on the density of the transporter in a particular membrane, and its rate constant (Km), i.e. the substrate concentration at which rate of transport is half maximal, is governed by its affinity for the substrate. Genetic polymorphismcan alter both the density and affinity of the transporter protein for different substrates and thus affect the pharmacokinetics of drugs. Moreover, tissue specific drug distribution can occur due to the presence of specific transporters in certain cells. (c) Pinocytosis:Large macromolecules (e.g., proteins, viruses, lipoprotein particles) require more complex mechanisms to traverse membranes, and are transported into and out of cells selectively via endocytosis and exocytosis (secretion). Interestingly, endocytosis and exocytosis are not only important for the import/export of large molecules. Often, essential small molecules that are hydrophobic or toxic (e.g., iron) travel through the bloodstream bound to proteins, which enter and exit cells via these mechanisms. Receptor-mediated endocytosis will be discussed in more detail in the Organs block.
Factors Related to Drugs:1. Lipid water solubilityLipid water solubility coefficient is the ratio of dissolution of drug in lipid as compared to water. Greater the lipid water solubility coefficient, more is the lipid solubility of the drug and greater is the absorption. Less the coefficient, less is the lipid solubility and less is the absorption.Water film exists on the membranes so part of the drugs must be water soluble to cross this water film. Drugs with benzene ring, hydrocarbon chain, steroid nucleus and halogen groups in their structures are lipid soluble. 2. Molecular sizeSmaller the molecular size of the drug, rapid is the absorption. Those with a large molecular size undergo active transport or endocytosis, while those with smaller molecular sizes utilize aqueous diffusion or lipid channels. 3. Particle sizeLarger particle size, slower will be the diffusion and absorption and vice versa. 4. Degree of IonizationDrugs are either acidic or basic and are present in ionized or unionized form. In the body, the ratio of the ionized and unionized forms depend on the pH of the medium. Acidic drugs are unionized in the acidic medium and basic drugs are unionized in the basic medium. Acidic drugs are better absorbed from the acidic compartment and basic drugs from alkaline compartment. 5. Physical FormsDrugs may exist as solids, liquids or gases. Gases are rapidly absorbed than the liquids, while the liquids are rapidly absorbed than the solids. Volatile gases used in general anesthesia are quickly absorbed through the pulmonary route. Syrup or suspension form are rapidly absorbed than the tablets or capsules.
Factors affected absorption:
6. Chemical NatureChemical nature is responsible for the selection of the route of administration of drug. Drugs that cannot be absorbed from GIT are given by the parenteral route, eg: heparin, insulin and benzyl penicillin is degraded in the GIT, so is given parenterally. 7. Dosage FormsDosage forms affect the rate and extent of absorption. A drug can be given in the form of tablets, capsules or transdermal forms. Examples nitroglycerin which when given by sublingual route, disintegrates rapidly but stays for a shorter duration. When it is given orally, it disintegrates slowly and stays for longer duration. When given by transdermal route, the drug can cover an even longer duration. IM injections may be aqueous or oily. Oily was slowly absorbed.a. Disintegration:Disintegration is the breaking up of the dosage form into smaller particles. When rapid is the disintegration, rapid will be the absorption.b. Dissolution:After disintegration, the drug dissolves in the gastric juices, which is called dissolution. It is only then that the drug can be absorbed. When these two processes occur rapidly, the rate of absorption increases. 8. FormulationWhen the drugs are formed, apart from the active form some inert substances are included. These are the diluents, excipients and the binders. Normally they are inert, but if they interact, they can change the bioavailability. 9. ConcentrationThe higher the concentration more flux occurs across the membrane. The rate is less affected than the extent of absorption.Factors Related to Body
1. Area of Absorptive SurfaceArea of absorptive surface affects oral as well as other routes. Most of the drugs are given orally because of the large area of absorptive surface, so that greater absorption occurs. Similarly, when the topically acting drugs are applied on a large surface area, they are better absorbed.
2. Vascularity
More the vascularity, more is the rate and extent of absorption and vice versa. In shock, the blood flow to the peripheries is decreased, so absorption in those areas is diminished, intravenous route is preferred.
Vasoconstrictors decrease the blood supply of an area, thus are useful to restrict the local anesthesias so that they remain for a longer duration. Their wash away as well as their toxic effects are decreased in this way.
Massage in intramuscular injections improves vascular supply to enhance absorption.
3. pH
Acidic pH favors acidic drug absorption while basic pH is better for basic drugs.
4. Presence of other Substances
Foods or drugs may interact with the drugs to alter their rate of absorption. Especially for the drugs given orally, food can increase or decrease the absorption.
Antihyperlipidemic drugs like the statins are better absorbed when taken with the food.
Iron when given with milk has decreased absorption.
Vitamin C enhances the absorption of iron.
Milk decreases the absorption of tetracyclines. Calcium salts when given with iron salts or tetracyclines interfere with their absorption
Aspirin is given with food while antibiotics are given in empty stomach. Liquid paraffin may affect drug absorption. Some acidic drugs bind with cholestyramine to from a complex which is not absorbed in GIT.
5. GI MobilityGI mobility must be optimal for absorption of oral drugs. It should be neither increased nor decreased which may affect the rate or extent of absorption.Different diseases or drugs may alter the mobility. Diarrhea causes rapid peristalsis, decreasing contact time and thus the extent of absorption is affected more. Constipation affects disintegration and dissolution so decreases motility. 6. Functional Integrity of Absorptive SurfaceFlattening and edema of mucosa decreaes absorption. Burns and wounds in the skin affects the absorption of topical drugs.Parasympathomimetic drugs can decrease drug absorption and parasympatholytic drugs can increase absorption. Prokinetic drugs prevents vomiting and accelerates gastric emptying. It increases gastric emptying increasing drug absorption. 7. Diseasesa. Diarrhea and vomiting: Decreases absorption.b. Malabsorptive syndrome : Decreases absorptionc. Achlorhydria: Decrease stomach acid secretion decrease absorption of acidic drugs. Methods for Delaying Absorption1. Vasoconstrictors: Vasoconstrictors decrease absorption.2. Formulation: Slow releasing (SR) preparations and Slowly absorbed oily preparations.