Catabolism of Amino Acids
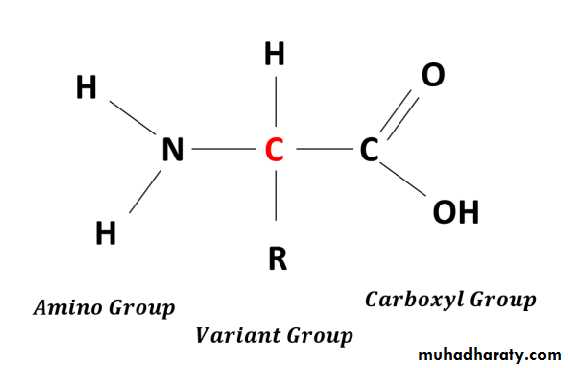
• By Dr.Ula Abbas ZekiAmino acids are the basic structural units of peptides and proteins play variable roles in the provision of energy and the formation of a number of important biomolecules, including hormones, neurotransmitters, and signaling molecules.
Unlike fats and carbohydrates, the body does not store amino acids, and there is no protein store exists to maintain a supply of amino acids for future use.
amino acids must be obtained either from diet, synthesized de novo, or produced from the degradation of body protein.
Any excess more than the biosynthetic needs will be rapidly degraded.
first phase : the removal of the α-amino groups forming ammonia and the corresponding α-keto acids, which the carbon skeletons of amino acids.
A portion of the free ammonia is excreted in the urine, but most is used in the synthesis of urea
second phase : the carbon skeletons of the α-keto acids are converted to intermediates of that can be metabolized to carbon dioxide (CO2) and water (H2O), glucose, fatty acids, or ketone bodies by the central pathways of metabolism .
•
OVERALL NITROGEN METABOLISM
• A.A catabolism is part of the larger process of the metabolism of nitrogen-containing molecules.• N enters the body in a variety of compounds present in food, most a. a are contained in dietary protein and leaves as urea, ammonia, and other products derived from amino acid metabolism.
• The role of body proteins in these transformations involves two important concepts: the amino acid pool and protein turnover
1) by the degradation of endogenous (body) proteins, most of which are reutilized 2) amino acids derived from exogenous (dietary) protein 3) nonessential amino acids synthesized from simple intermediates of metabolism
• 1) synthesis of body protein 2) consumption of amino acids as precursors of essential nitrogen-containing small molecules3) conversion of amino acids to glucose, glycogen, fatty acids, and ketone bodies or oxidation to CO2 + H2O
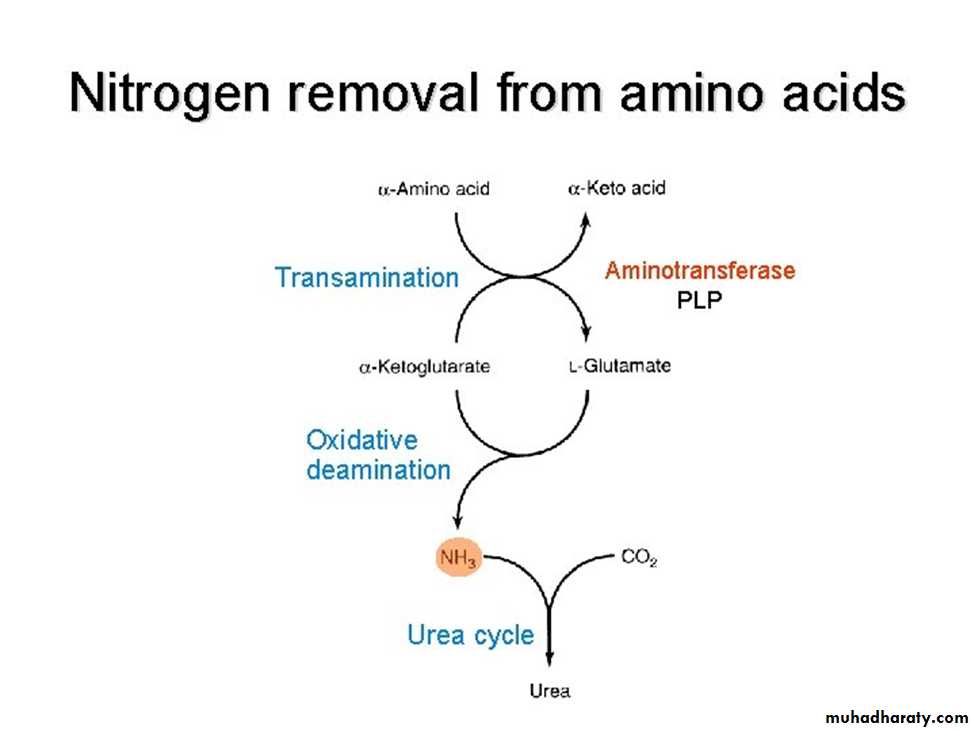
NITROGEN REMOVAL FROM AMINO ACIDS
• 01• The presence of the α-amino group keeps amino acids safely locked away from oxidative breakdown, also removing this group is essential for producing energy from any amino acid and it is an obligatory step in the catabolism of all a. a.
• Once removed, this nitrogen can be incorporated into other compounds or excreted as urea, with the carbon skeletons being metabolized.
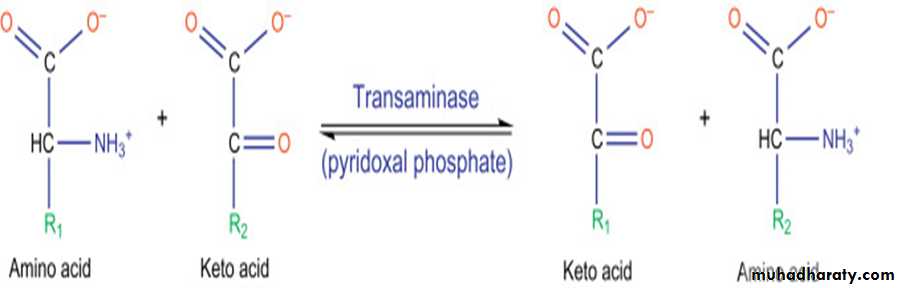
• The reaction is catalyzed by aminotransferase enzymes that found in high concentrations in liver and requires coenzyme pyridoxal phosphate (active form of B6).
• Glutamate produced by transamination can be oxidatively deaminated or used as an amino group donor in the synthesis of nonessential amino acids.
• All amino acids, with the exception of lysine and threonine, participate in transamination at some point in their catabolism.
Two important aminotransferase enzymes are alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
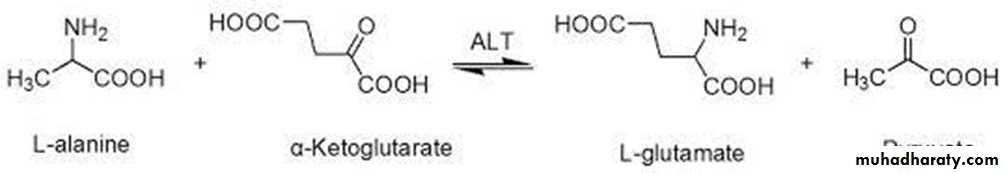
• ALT is present in many tissues mainly liver, also in kidney, skeletal muscle and heart.
• ALT transfers an amino group from alanine to alpha-ketoglutarate, forming pyruvate and glutamate.
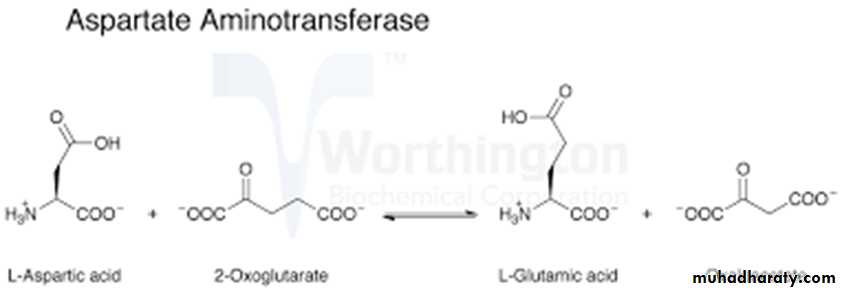
AST present in the heart, skeletal muscle, liver, kidney and RBC. AST transfers an amino group from aspartate to alpha-ketoglutarate, forming oxaloacetate and glutamate.
These enzymes are normally intracellular, and low levels that found in plasma represent the release of cellular contents during normal cell turnover.
Elevated plasma levels indicate damage to cells rich in these enzymes. For example, physical trauma or a disease process can cause cell lysis, resulting in release of intracellular enzymes into the blood.
Two aminotransferases, AST and ALT, are of particular diagnostic value when they are found in the plasma.
Diagnostic value of Aminotransferases
a.Hepatic disease
b. Nonhepatic disease:elevated in nearly all hepatic diseases but are particularly high in conditions that cause extensive cell necrosis, such as severe viral hepatitis, toxic injury.
Aminotransferases may be elevated in nonhepatic diseases such as those that cause damage to cardiac or skeletal muscle.
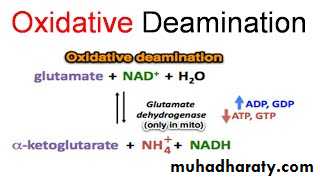
• Glutamate, that resulted from the transamination , is unique in that it is the only amino acid that undergoes rapid oxidative deamination, a reaction catalyzed by glutamate dehydrogenase [GDH].
Oxidative deamination by glutamate dehydrogenase.
The sequential action of transamination and the oxidative deamination of that glutamate (regenerating α-ketoglutarate) provide a pathway whereby the amino groups of most amino acids can be released as ammonia.
GDH, a mitochondrial enzyme, is unusual in that it can use either nicotinamide adenine dinucleotide (NAD⁺) or its phosphorylated reduced form (NADPH) as a coenzyme.
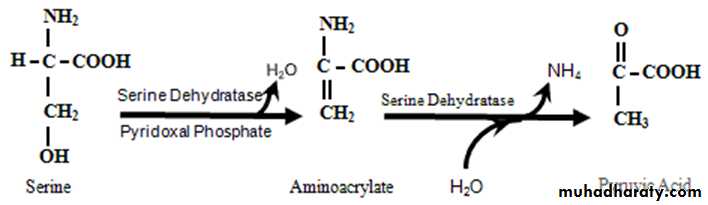
Nonoxidative deamination
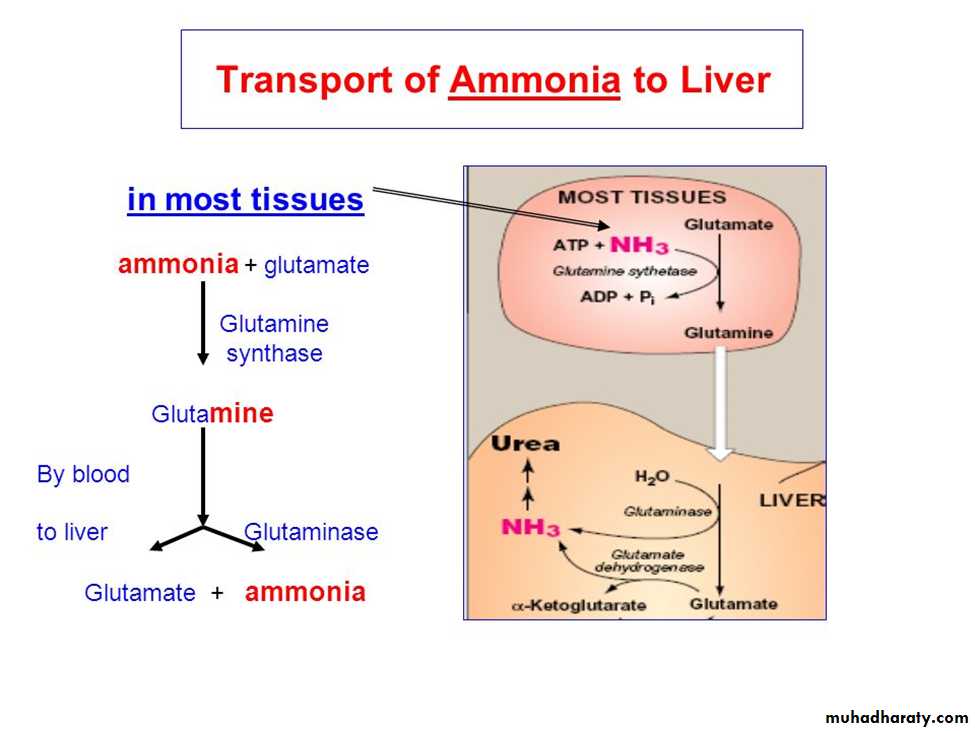
Certain a.a ex. serine , threonine, & cysteine are deaminated by specific lyases that require pyridoxal phosphate .Ammonia transport to the liver
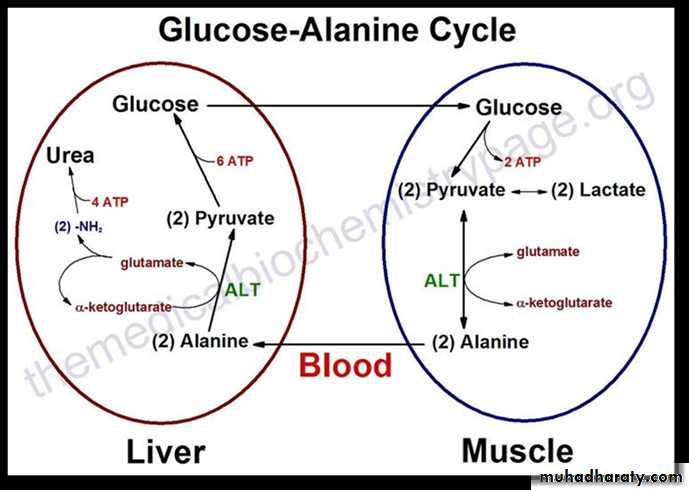
The second transport mechanism involves the formation of alanine by the transamination of pyruvate produced from both aerobic glycolysis and metabolism of the succinyl coenzyme A (CoA) .
Alanine is transported in the blood to the liver, where it is transaminated by ALT to pyruvate and glutamate.
The pyruvate is used in gluconeogenesis to synthesize glucose, which can enter the blood and be used by muscle, a pathway called the glucose–alanine cycle.
The glutamate product of ALT can be used to release ammonia via deamination by GDH.