Definition of healingThe word healing refers to the body replaced the destroyed tissue by living tissue.
III. Processes of healing
The healing involves two distinct processes:
Regeneration, the replacement of lost tissue by tissues similar in type
Repair (healing by scaring), the replacement of lost tissue by granulation tissue which matures to form scar tissue.
Healing by fibrosis is inevitable when the surrounding specialized cells do not possess the capacity to proliferate.
Whether healing takes place by regeneration or by repair (scarring) is determined partly by the type of cells in the damaged organ & partly by the destruction or the intactness of the stromal frame work of the organ. Hence, it is important to know the types of cells in the body.
Types of cellsBased on their proliferative capacity there are three types of cells.
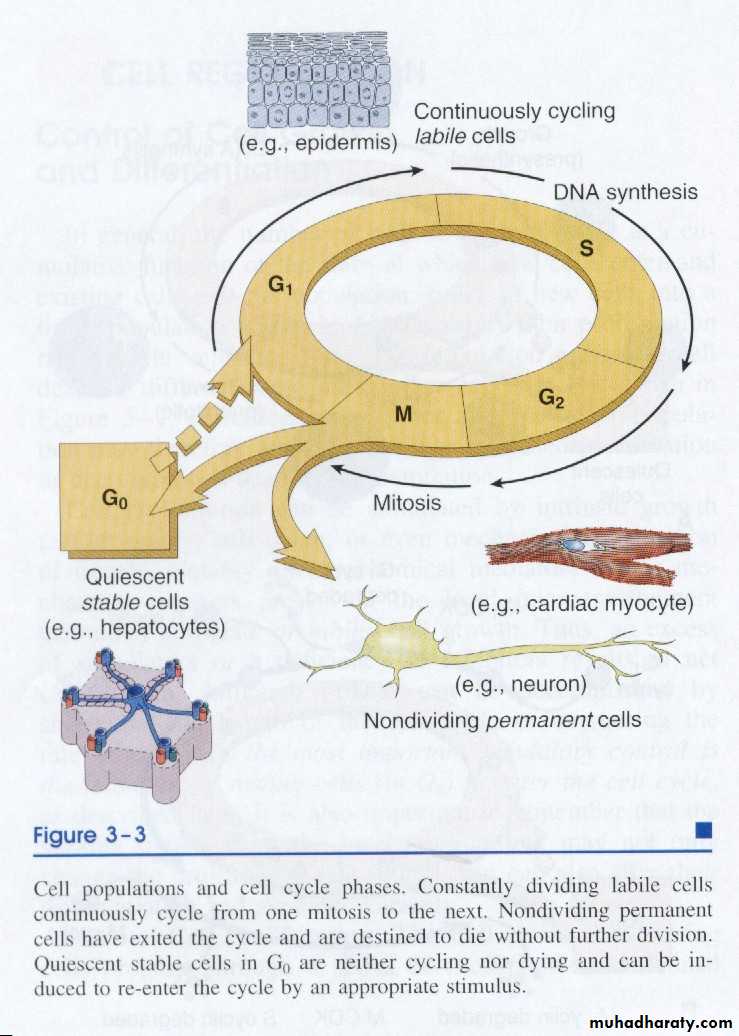
1. Labile cellsThese are cells which have a continuous turn over by programmed division of stem cells. They are found in the surface epithelium of the gastrointestinal treat, urinary tract or the skin. The cells of lymphoid and haemopoietic systems are further examples of labile cells. The chances of regeneration are excellent.
2. Stable cells (Quiescent cells)
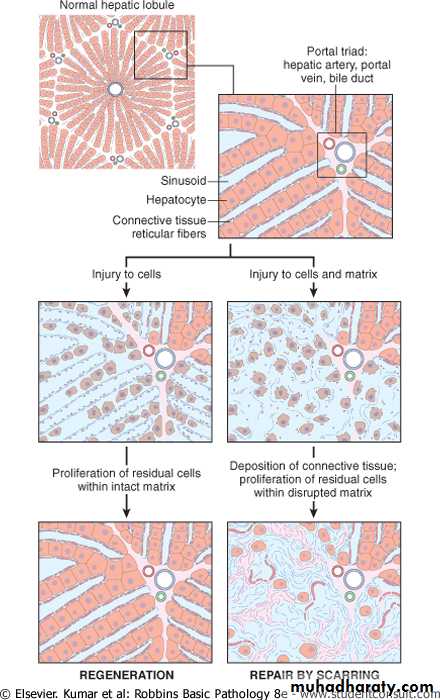
Tissues which have such type of cells have normally a much lower level of replication and there are few stem cells. However, the cells of such tissues can undergo rapid division in response to injury. For example, mesenchymal cells such as smooth muscle cells, fibroblasts, osteoblasts and endothelial cells are stable cells which can proliferate. Liver, endocrine glands and renal tubular epithelium has also such type of cells which can regenerate. Their chances of regeneration are good.
3. Permanent cells
These are non-dividing cells. If lost, permanent cells cannot be replaced, because they don not have the capacity to proliferate. For example: adult neurons, striated muscle cells, and cells of the lens. Having been introduced to the types of cells, we can go back to the two types of healing processes & elaborate them.
Cell types and cell cycle phases
Constantly dividing labile cells continuously cycling from one mitosis to the next. Nondividing permanent cells have exited the cycle and are distended to die without further division. Quiescent stable cells in G0 are neither cycling nor dying and can be induced to re-enter the cycle by an appropriative stimulus.
a. Healing by regeneration
Definition: Regeneration (generare=bring to life) is the renewal of a lost tissue in which the lost cells are replaced by identical ones.Regeneration involves two processes
1. Proliferation of surviving cells to replace lost tissue
2. Migration of surviving cells into the vacant (empty) space.
The capacity of a tissue for regeneration depends on its
1) proliferative ability,
2) degree of damage to stromal framework and
3) on the type and severity of the damage.
Tissues formed of labile and stable cells can regenerate provided that stromal frame work are intact.
b. Repair (Healing by connective tissue)
Definition:- Repair is the orderly process by which lost tissue is eventually replaced by a scar.
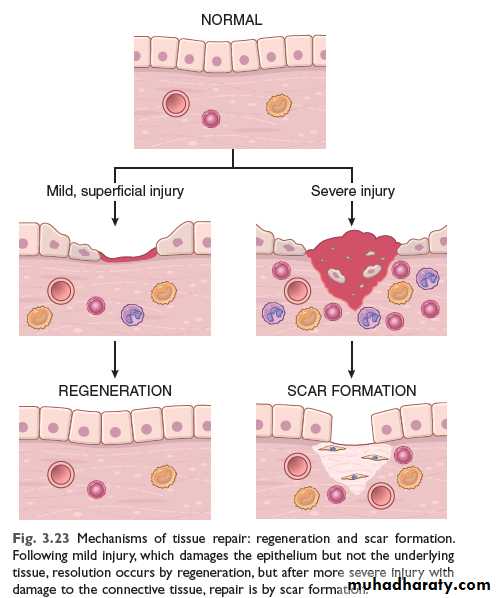
A wound in which only the lining epithelium is affected heals exclusively by regeneration. In contrast, wounds that extend through the basement membrane to the connective tissue, for example, the dermis in the skin or the sub-mucosa in the gastrointestinal tract, lead to the formation of granulation tissue and eventual scarring.
Tissues containing terminally differentiated (permanent) cells such as neurons and skeletal muscle cells can not heal by regeneration. Rather the lost permanent cells are replaced by formation of granulation tissue.
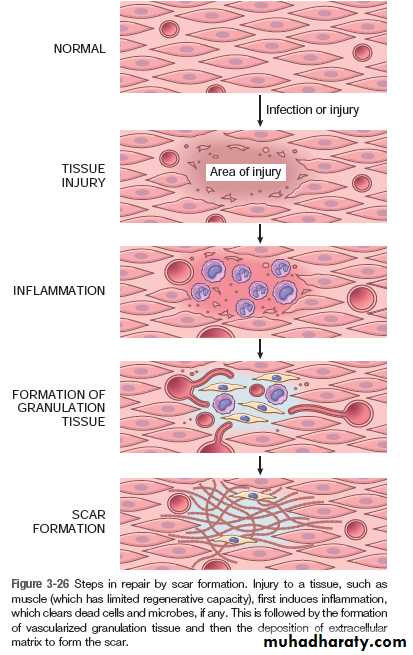
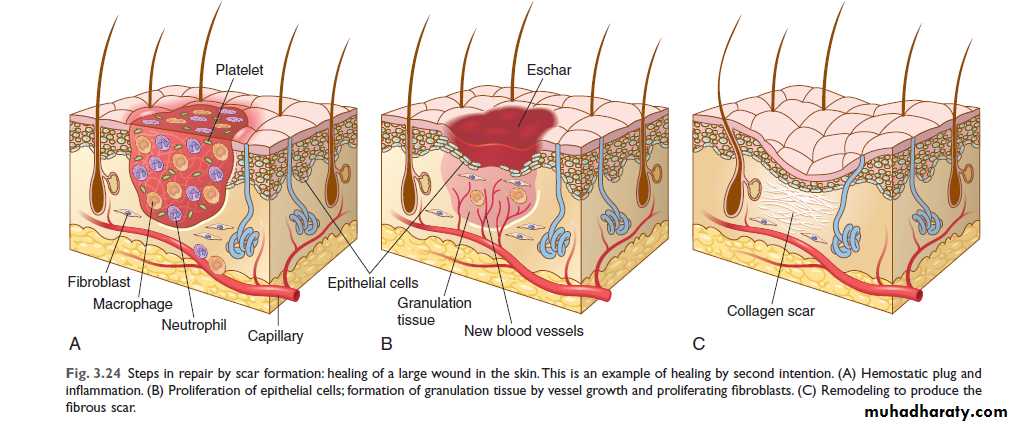
In granulation-tissue formation, three phases may be observed.
1. Phase of inflammation
At this phase, inflammatory exudate containing polymorphs is seen in the area of tissue injury. In addition, there is platelet aggregation and fibrin deposition.
2. Phase of demolition
The dead cells liberate their autolytic enzymes, and other enzymes (proteolytic) come from disintegrating polymorphs. There is an associated macrophage infiltration. These cells ingest particulate matter, either digesting or removing it.
3. In growth of granulation tissue
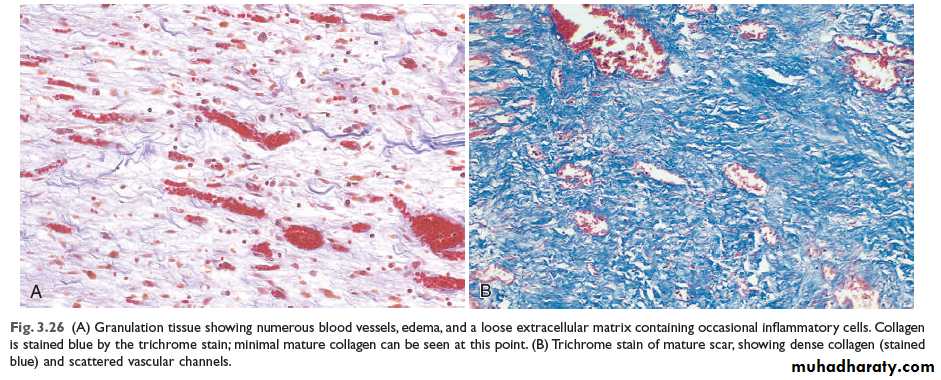
This is characterized by proliferation of fibroblasts and an in growth of new blood vessels into the area of injury, with a variable number of inflammatory cells.Fibroblasts actively synthesize and secrete extra-cellular matrix components, including fibronectin, proteoglycans, and collagen types I and III. The fibronectin and proteoglycans form the ‘scaffolding’ for rebuilding of the matrix. Fibronectin binds to fibrin and acts as a chemotactic factor for the recruitment of more fibroblasts and macrophages.
The synthesis of collagen by fibroblasts begins within 24 hours of the injury although its deposition in the tissue is not apparent until 4 days. By day 5, collagen type III is the predominant matrix protein being produced; but by day 7 to 8, type I is prominent, and it eventually becomes the major collagen of mature scar tissue. This type I collagen is responsible for providing the tensile strength of the matrix in a scar.
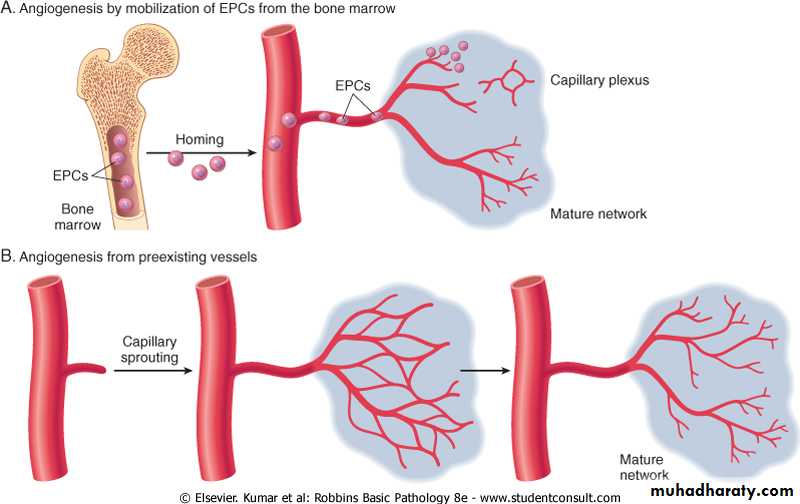
Coincident with fibroblast proliferation there is angiogenesis (neovascularization), a proliferation and formation of new small blood vessels. Vascular proliferation starts 48 to 72 hours after injury and lasts for several days.
With further healing, there is an increase in extracellular constituents, mostly collagen, with a decrease in the number of active fibroblasts and new vessels. Despite an increased collagenase activity in the wound (responsible for removal of built collagen), collagen accumulates at a steady rate, usually reaching a maximum 2 to 3 months after the injury.
The tensile strength of the wound continues to increase many months after the
collagen content has reached a maximum. As the collagen content of the wound
increases, many of the newly formed vessels disappear. This vascular involution which takes place in a few weeks, dramatically transforms a richly vascularized tissue in to a pale, avascular scar tissue.
Wound contraction
Wound contraction is a mechanical reduction in the size of the defect. The wound is reduced approximately by 70-80% of its original size. Contraction results in much faster healing, since only one-quarter to one-third of the amount of destroyed tissue has to be replaced. If contraction is prevented, healing is slow and a large ugly scar is formed.Causes of contraction
It is said to be due to contraction by myofibroblasts. Myofibroblasts have the features intermediate between those of fibroblasts and smooth muscle cells. Two to three days after the injury they migrate into the wound and their active contraction decrease the size of the defect.
Summary
Following tissue injury, whether healing occurs by regeneration or scarring is determined by the degree of tissue destruction, the capacity of the parenchymal cells to proliferate, and the degree of destructon of stromal framework as illustrated in the diagram below (See Fig. 4.2).
In the above discussion, regeneration, repair, and contraction have been dealt with separately. Yet they are not mutually exclusive processes. On the contrary, the three processes almost invariably participate together in wound healing.
…………………………………………………………………………………………………………………………………………….
Figure 4.2 Diagram showing healing process following acute inflammatory injury
IV. Molecular control of healing process
As seen above, healing involves an orderly sequence of events which includes regeneration and migration of specialized cells, angiogenesis, proliferation of fibroblasts and related cells, matrix protein synthesis and finally cessation of these processes. These processes, at least in part, are mediated by a series of low molecular weight polypeptides referred to as growth factors.These growth factors have the capacity to stimulate cell division and proliferation. Some of the factors, known to play a role in the healing process, are briefly discussed below.
Sources of Growth Factors:
Following injury, growth factors may be derived from a number of sources such as:1. Platelets, activated after endothelial damage,
2. Damaged epithelial cells,
3. Circulating serum growth factors,
4. Macrophages, or
5. Lymphocytes recruited to the area of injury
The healing process ceases when lost tissue has been replaced. The mechanisms
regulating this process are not fully understood. TGF-β acts as a growth inhibitor for both epithelial and endothelial cells and regulates their regeneration.
VI. Wound Healing
The two processes of healing, described above, can occur during healing of a diseased organ or during healing of a wound. A wound can be accidental or surgical. Now, we will discuss skin wound healing to demonstrate the two basic processes of healing mentioned above.
Healing of a wound demonstrates both epithelial regeneration (healing of the epidermis) and repair by scarring (healing of the dermis).
There are two patterns of wound healing depending on the amount of tissue damage:
1. Healing by first intention (Primary union)
2. Healing by second intention
These two patterns are essentially the same process varying only in amount.
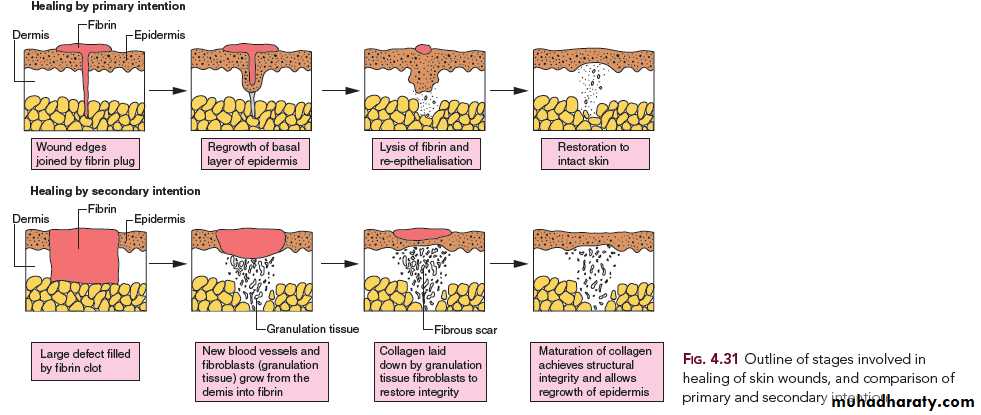
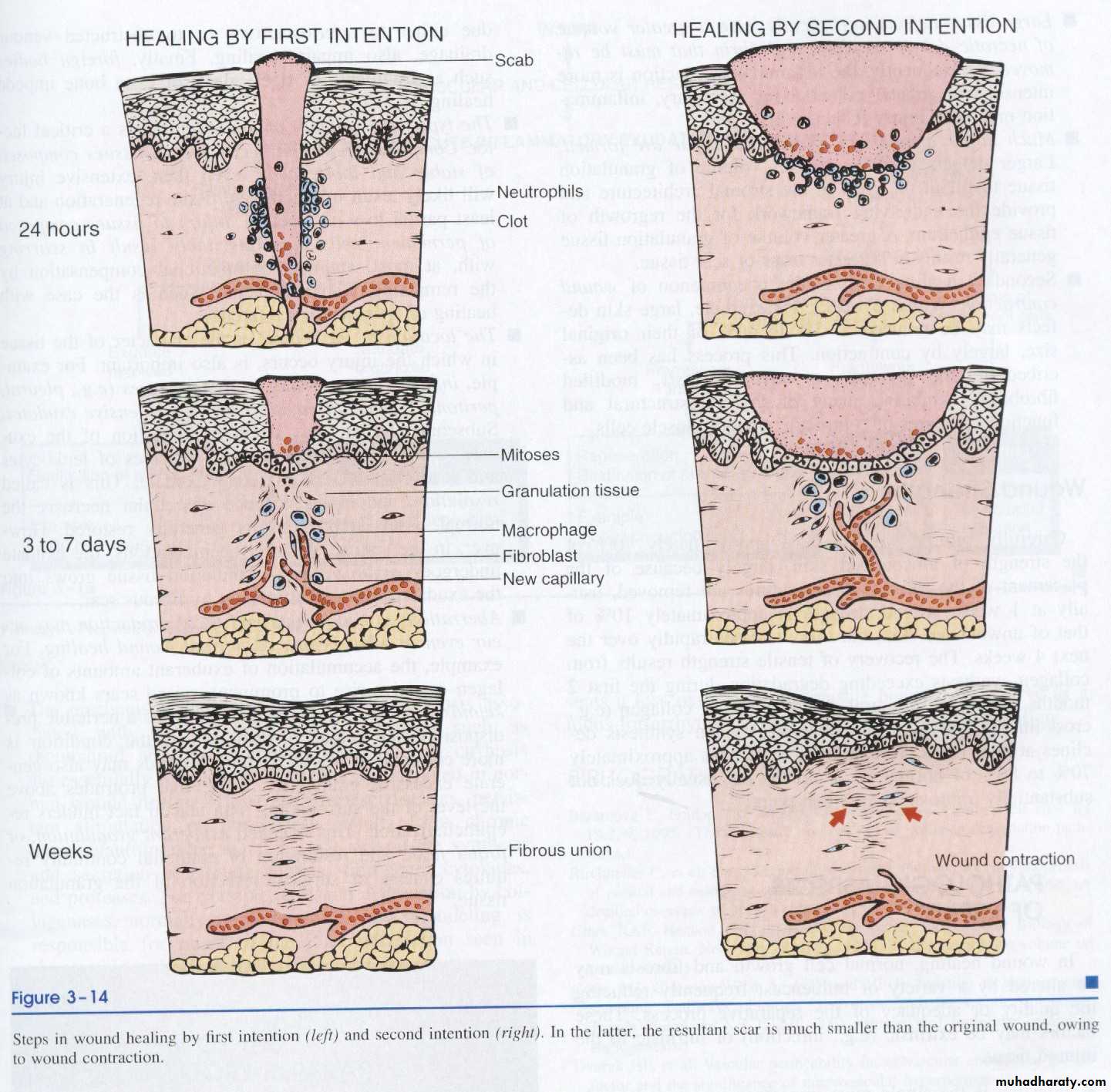
1. Healing by first intention (primary union)
The least complicated example of wound healing is the healing of a clean surgical incision (Fig. 4-4, left). The wound edges are approximated by surgical sutures, and healing occurs with a minimal loss of tissue. Such healing is referred to, surgically, as “primary union” or “healing by first intention”. The incision causes the death of a limited number of epithelial cells as well as of dermal adnexa and connective tissue cells; the incisional space is narrow and immediately fills with clotted blood, containing fibrin and blood cells; dehydration of the surface clot forms the well-known scab that covers the wound and seals it from the environment almost at once.
Within 24 hours, neutrophils appear at the margins of the incision, moving toward the fibrin clot. The epidermis at its cut edges thickens as a result of mitotic activity of basal cells and, within 24 to 48 hours, spurs of epithelial cells from the edges both migrate and grow along the cut margins of the dermis and beneath the surface scab to fuse in the midline, thus producing a continuous but thin epithelial layer.
By day 3, the neutrophils have been largely replaced by macrophages. Granulation tissue progressively invades the incisional space. Collagen fibers are now present in the margins of the incision, but at first these are vertically oriented and do not bridge the incision.
Epithelial cell proliferation continues, thickening the epidermal covering layer.
By day 5, the incisional space is filled with granulation tissue. Neovascularization ismaximal. Collagen fibrils become more abundant and begin to bridge the incision.
The epidermis recovers its normal thickness and differentiation of surface cells yields a mature epidermal architecture with surface keratinization.
During the second week, there is continued accumulation of collagen and proliferation of fibroblasts. Leukocytic infiltrate, edema, and increased vascularity have largely disappeared. At this time, the long process of blanching begins, accomplished by the increased accumulation of collagen within the incisional scar, accompanied by regression of vascular channels.
By the end of the first month, the scar comprises a cellular connective tissue devoid of inflammatory infiltrate, covered now by an intact epidermis. The dermal appendages that have been destroyed in the line of the incision are permanently lost.
Tensile strength of the wound increases thereafter, but it may take months for the wounded area to obtain its maximal strength.
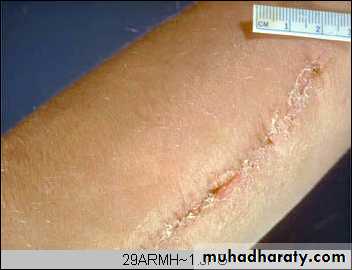
Arm, healing surgical incision. This image demonstrates healing by first intention which occur in clean, un infected wounds that have apposed edges.
2. Healing by second intention (secondary union)
When there is more extensive loss of cells and tissue, such as occurs in infarction,
inflammatory ulceration, abscess formation, and surface wounds that create large defects, the reparative process is more complicated. The common denominator in all these situations is a large tissue defect that must be filled. Regeneration of parenchymal cells cannot completely reconstitute the original architecture. Abundant granulation tissue grows in from the margin to complete the repair. This form of healing is referred to as “secondary union” or “healing by second intention.”
Secondary healing differs from primary healing in several respects:
Wound healing by primary and secondary union
1. Inevitably, large tissue defects initially have more fibrin and more necrotic debris and exudate that must be removed. Consequently, the inflammatory reaction is more intense.
2. Much larger amounts of granulation tissue are formed. When a large defect occurs in deeper tissues, such as in a viscus, granulation tissue bears the responsibility for its closure, because drainage to the surface cannot occur.
3. Perhaps the feature that most clearly differentiates primary from secondary healing is the phenomenon of wound contraction, which occurs in large surface wounds.
4. Healing by second intention takes much longer than when it occurs by first
intention.
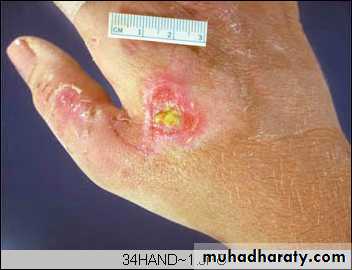
Wound with large tissue defect healed by secondary intention. Note the red granular appearance of the granulation tissue. A whitish- greenish- yellow neutrophilic exudates represent an inflammatory response to bacterial invasion of the wound.
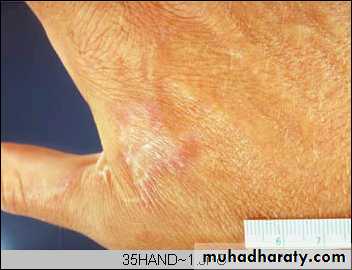
Hand healed by secondary intentionAs the granulation tissue matures, the myofibroblast contract to close the wound. They then become mature fibroblast, which produce collagen, forming a fibrous scar. The contraction that takes place can lead to decrease mobility.
VI. Factors that influence wound healing A number of factors can alter the rate and efficiency of healing. These can be classified in to those which act locally, and those which have systemic effects. Most of these factors have been established in studies of skin wound healing but many are likely to be of relevance to healing at other sites.
• Type, size, and location of the wound
A clean, aseptic wound produced by the surgeon’s scalpel heals faster than a wound
produced by blunt trauma, which exhibits aboundant necrosis and irregular edges. Small blunt wounds heal faster than larger ones. Injuries in richly vascularized areas (e.g., the face) heal faster than those in poorly vascularized ones (e.g., the foot). In areas where the skin adheres to bony surfaces, as in injuries over the tibia, wound contraction and adequate apposition of the edges are difficult. Hence, such wounds heal slowly.
• Vascular supply
Wounds with impaired blood supply heal slowly. For example, the healing of leg wounds in patients with varicose veins is prolonged. Ischemia due to pressure produces bed sores and then prevents their healing. Ischemia due to arterial obstruction, often in the lower extremities of diabetics, also prevents healing.
• Infection
Wounds provide a portal of entry for microorganisms. Infection delays or prevents healing, promotes the formation of excessive granulation tissue (proud flesh), and may result in large, deforming scars.
• Movement
Early motion, particularly before tensile strength has been established, subjects a wound to persistent trauma, thus preventing or retarding healing.
• Ionizing radiation
Prior irradiation leaves vascular lesions that interfere with blood supply and result in slow wound healing. Acutely, irradiation of a wound blocks cell proliferation, inhibits contraction, and retards the formation of granulation tissue.
Systemic Factors
• Circulatory statusCardiovascular status, by determining the blood supply to the injured area, is important for
wound healing. Poor healing attributed to old age is often due, largely, to impaired circulation.
• Infection
Systemic infections delay wound healing.
• Metabolic status
Poorly controlled diabetes mellitus is associated with delayed wound healing due to increase the risk of infection.
In diabetic patients, there can be impaired circulation secondary to arteriosclerosis and impaired sensation due to diabetic neuropathy. The impaired sensation renders the lower extremity blind to every day hazards. Hence, in diabetic patients, wounds heal the very slowly.
• Nutritional deficiencies
Protein deficiency
In protein depletion there is an impairment of granulation tissue and collagen formation, resulting in a great delay in wound healing.
Vitamine deficiency
Vitamin C is required for collagen synthesis and secretion. It is required in hydroxylation of proline and lysine in the process of collagen synthesis. Vitamin C deficiency (scurvy) results in grossly deficient wound healing, with a lack of vascular proliferation and collagen deposition.
Trace element deficiency
Zinc (a co-factor of several enzymes) deficiency will retard healing by preventing cell
proliferation. Zinc is necessary in several DNA and RNA polymerases and transferases; hence, a deficiency state will inhibit mitosis. Proliferation of fibroblasts (fibroplasia) is, therefore, retarded.
• Hormones
Corticosteroids impair wound healing, an effect attributed to an inhibition of collagen
synthesis. However, these hormones have many other effects, including anti-inflammatory actions and a general depression of protein synthesis. It also inhibits fibroplasia and neovascularization. Both epithelialization and contraction are impaired. It is, therefore, difficult to attribute their inhibition of wound healing to any one specific mechanism.
Thyroid hormones, androgens, estrogens and growth hormone also influence wound
healing. This effect, however, may be more due to their regulation of general metabolic status rather than to a specific modification of the healing process.
• Anti-inflammatory drugs
Anti-inflammatory medications do not interfere with wound healing when administered at the usual daily dosages. Asprin and indomethalin both inhibit prostaglandin synthesis and thus delay healing.
VII. Complications of Wound HealingAbnormalities in any of the three basic healing processes – contraction, repair, andregeneration – result in the complications of wound healing.
1. Infection
A wound may provide the portal of entry for many organisms. Infectrion may delayhealing, and if severe stop it completely.
2. Deficient Scar Formation
Inadequate formation of granulation tissue or an inability to form a suitable extracellular matrix leads to deficient scar formation and its complications. The complications of deficient scar formation are:
a. Wound dehiscence & incitional hernias
b. Ulceration
a. Wound Dehiscence and Incisional Hernias:
Dehiscence (bursting of a wound) is of most concern after abdominal surgery. If insufficient extracellular matrix is deposited or there is inadequate cross-linking of the matrix, weak scars result. Dehiscence occurs in 0.5% to 5% of abdominal operations. Inappropriate suture material and poor surgical techiniques are important factors. Wound infection, increased mechanical stress on the wound from vomiting, coughing, or ileus is a factor in most cases of abdominal dehiscence. Systemic factors that predispose to dehiscence include poor metabolic status, such as vitamin C deficiency, hypoproteinemia, and the general inanition
that often accompanies metastatic cancer. Dehiscence of an abdominal wound can be a life threatening complication, in some studies carrying a mortality as high as 30%. An incisional hernia, usually of the abdominal wall, refers to a defect caused by poor wound healing following surgery into which the intestines protrude.
b. Ulceration:
Wounds ulcerate because of an inadequate intrinsic blood supply or insufficient
vascularization during healing. For example, leg wounds in persons with varicose veins or severe atherosclerosis typically ulcerate. Non healing wounds also develop in areas devoid of sensation because of persistent trauma. Such trophic or neuropathic ulcers are occasionally seen in patients with leprosy, diabetic peripheral neuropathy and in tertiary syphilis from spinal involvement (in tabes dorsalis).
3. Excessive Scar Formation
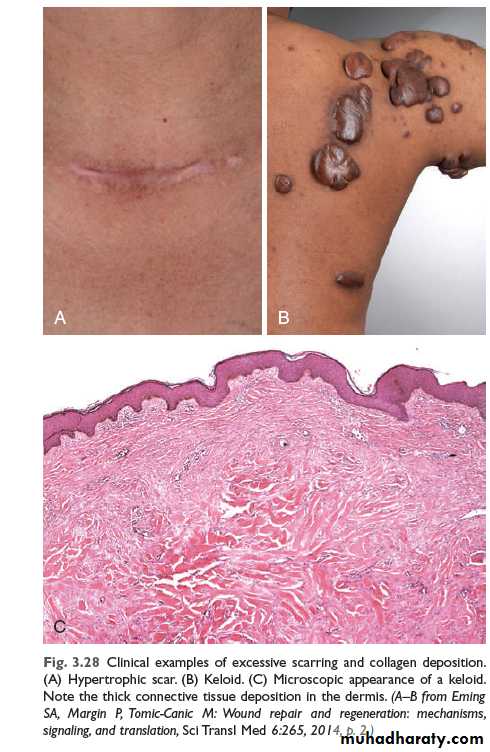
An excessive deposition of extracellular matrix at the wound site results in a hypertrophic scar or a keloid (See Figure 4-5 and 4-6). The rate of collagen synthesis, the ratio of type III to type I collagen, and the number of reducible cross-links remain high, a situation that indicates a “maturation arrest”, or block, in the healing process.
Keloid Formation
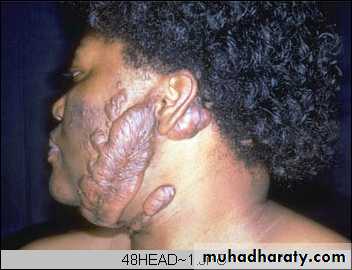
An excessive formation of collagenous tissue results in the appearance of a raised area of scar tissue called keloid. It is an exuberant scar that tends to progress and recur after excision. The cause of this is unknown. Genetic predisposition, repeated trauma, and irritation caused by foreign body, hair, keratin, etc., may play a part. It is especially frequent after burns. It is common in areas of the neck & in the ear lobes.
Hypertrophic Scar
Hypertrophic scar is structurally similar to keloid. However, hypertrophic scar never gets worse after 6 months unlike keloid, which gets worse even after a year and some may even progress for 5 to 10 years. Following excision keloid recurres, whereas a hypertrophic scar does not.
KeloidKeloid nodular masses of of hyperplastic scar tissue, occur when the wound healing process runs unchecked. They are more commonly in people of African descent. surgical excision leads to repeated keloid formation.
4. Excessive contraction
A decrease in the size of a wound depends on the presence of myofibroblasts, development of cell-cell contacts and sustained cell contraction. An exaggeration of these processes is termed contracture (cicatrisation) and results in severe deformity of the wound and surrounding tissues. Contracture (cicatrisation) is also said to arise as a result of late reduction in the size of the wound. Interestingly, the regions that normally show minimal wound contraction (such as the palms, the soles, and the anterior aspect of the thorax) are the ones prone to contractures. Contractures are particularly conspicuous in the healing of serious burns.Contractures of the skin and underlying connective tissue can be severe
enough to compromise the movement of joints. Cicatrisation is also important in hollow viscera such as urethra, esophagus, and intestine. It leads to progressive stenosis with stricture formation. In the alimentary tract, a contracture (stricture) can result in an obstruction to the passage of food in the esophagus or a block in the flow of intestinal contents.5. Miscellaneous
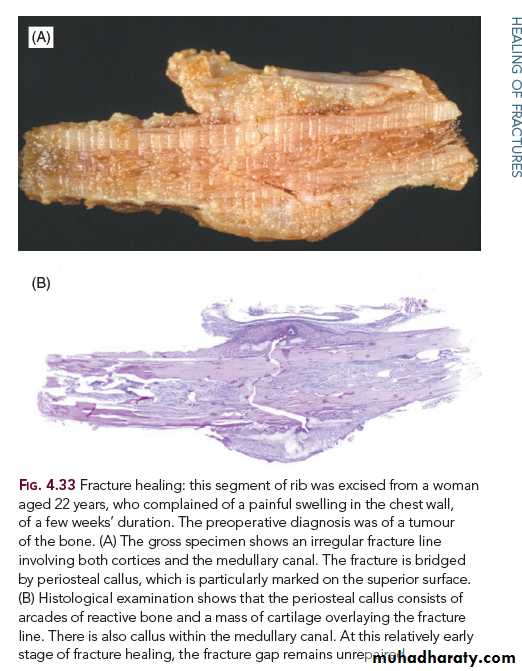
Implantation (or epidermoid cyst: Epithelial cells which flow into the healing wound may later sometimes persist, and proliferate to form an epidermoid cyst.VIII. Fracture HealingThe basic processes involved in the healing of bone fractures bear many resemblances to those seen in skin wound healing. Unlike healing of a skin wound, however, the defect caused by a fracture is repaired not by a fibrous “scar” tissue, but by specialized bone forming tissue so that, under favorable circumstances, the bone is restored nearly to normal.
Structure of bone
Bone is composed of calcified osteoid tissue, which consists of collagen fibers embedded in a mucoprotein matrix (osteomucin). Depending on the arrangement of the collagen fibers, there are two histological types of bone:1. Woven, immature or non-lamellar bone This shows irregularity in the arrangement
of the collagen bundles and in the distribution of the osteocytes. The osseomucin
is less abundant and it also contains less calcium.
2. Lamellar or adult bone In this type of bone, the collagen bundles are arranged in
parallel sheets.
Stages in Fracture Healing (Bone Regeneration)
Stage 1: Haematoma formation. Immediately following the injury, there is a variable
amount of bleeding from torn vessels; if the periosteum is torn, this blood may
extend into the surrounding muscles. If it is subsequently organized and ossified,
myositis ossificans results.
Stage 2: Inflammation. The tissue damage excites an inflammatory response, the exudates adding more fibrin to the clot already present. The inflammatory changes differ in no way from those seen in other inflamed tissues. There is an increased blood flow and a polymorphonuclear leucocytic infiltration. The haematoma attains a fusiform shape.
Stage 3: Demolition. Macrophages invade the clot and remove the fibrin, red cells, the inflammatory exudate, and debris. Any fragments of bone, which have become
detached from their blood supply, undergo necrosis, and are attacked by
macrophages and osteoclasts.
Stage 4: Formation of granulation tissue. Following this phase of demolition, there is an ingrowth of capillary loops and mesenchymal cells derived from the periosteum
and the endosteum of the cancellous bone. These cells have osteogenic potential
and together with the newly formed blood vessels contribute to the granulation –
tissue formation.
Stage 5: Woven bone and cartilage formation. The mesenchymal “osteoblasts” next differentiate to form either woven bone or cartilage. The term “callus”, derived
from the Latin and meaning hard, is often used to describe the material uniting the
fracture ends regardless of its consistency. When this is granulation tissue, the
“callus” is soft, but as bone or cartilage formation occurs, it becomes hard.
Stage 6: Formation of lamellar bone. The dead calcified cartilage or woven bone is next invaded by capillaries headed by osteoclasts. As the initial scaffolding
(“provisional callus”) is removed, osteoblasts lay down osteoid, which calcifies to
form bone. Its collagen bundles are now arranged in orderly lamellar fashion, for
the most part concentrically around the blood vessels, and in this way the
Haversian systems are formed. Adjacent to the periosteum and endosteum the
lamellae are parallel to the surface as in the normal bone. This phase of formation
of definitive lamellar bone merges with the last stage.
Stage 7: Remodelling. The final remodeling process involving the continued osteoclastic removal and osteoblastic laying down of bone results in the formation of a bone, which differs remarkably little from the original tissue. The external callus is slowly removed, the intermediate callus becomes converted into compact bone
containing Haversian systems, while the internal callus is hollowed out into a
marrow cavity in which only a few spicules of cancellous bone remain.