By
Dr Dhafer A. Farhan
Ph. D. Cancer research/UK
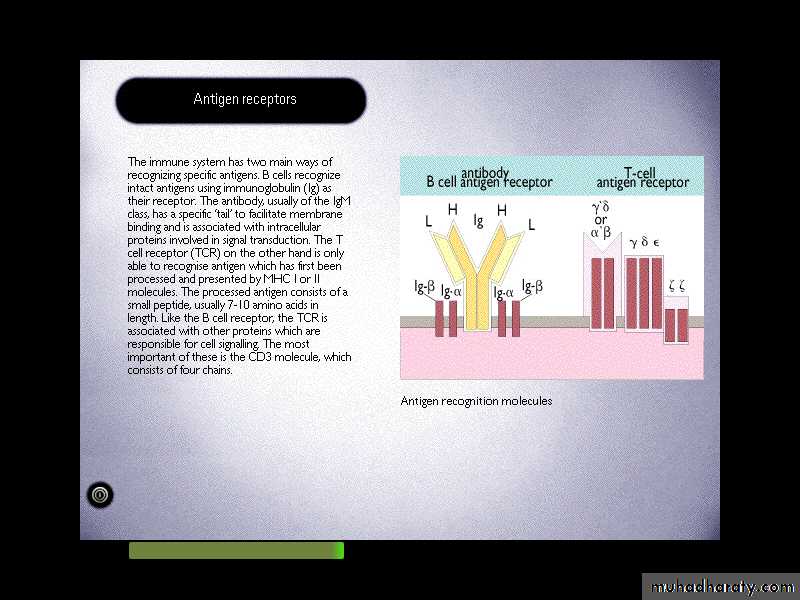
It's another antigen recognition molecule.
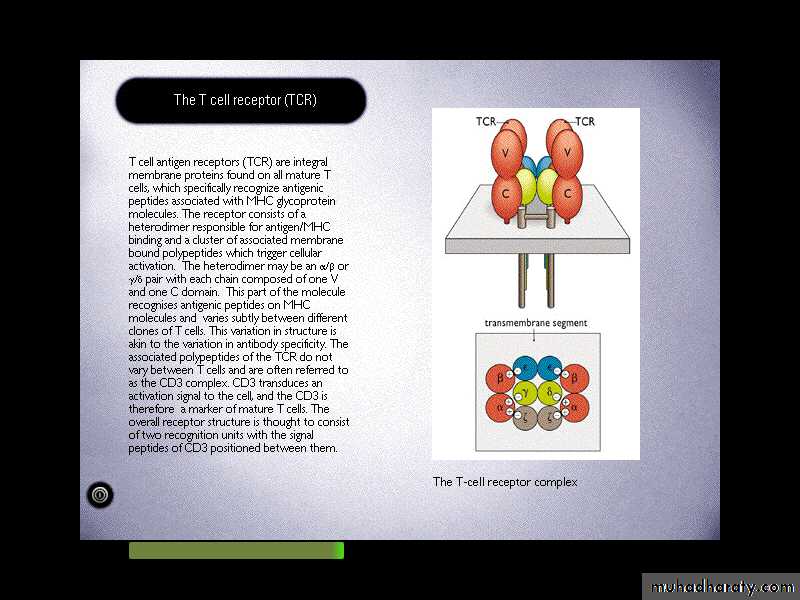
TCR is a molecule found on the surface of T cells, or T lymphocytes that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules.T-cell receptor
T-cell receptorIt is a heterodimeric molecules comprising of alpha & beta chains or gamma and delta chains linked by a disulphide bond.
(the great majority with alpha& beta).
Each poly peptide chain comprises two extracellular Ig-like domains anchored into the plasma membrane.
The N-terminal domains known as complementarity determine region (CDR) or hypervariable region(HVR)
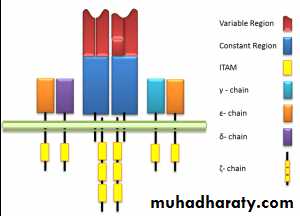
TCR - Complex
TCR associated physically with a series of polypeptides called CD3, which comprises of four invariant polypeptides, called gamma, delta, epsilon, and sigma.The total structure of TCR-complex is (, )2,2,,2,
CD3 chains are negatively charged.TCR chains are positively charged.
* , TCR present in 95% of T-cells.
While, , TCR are abundant in various epithelia like epidermis, intestinal epithelia, uterus and tongue.The T cell antigen receptor
Va
Vb
Ca
Cb
Carbohydrates
HingeMonovalent
Resembles an Ig Fab fragment
Fab
VH
VL
Fc
CL
CH
VL
VH
CH
CL
CH
CH
CH
CH
No alternative constant regions
Transmembrane region
Never secretedDomain structure: Ig gene superfamily
Heterodimeric, chains are disuphide-bonded
Cytoplasmic tailVery short intracytoplasmic tail
++
+
Positively charged amino acids in the TM region
Antigen
combining siteAntigen combining site made of juxtaposed Va and Vb regions
30,000 identical specificity TcR per cell
Intracytoplasmic section of sigmma chain called immunoreceptor
Tyrosine activation motifs (ITAM) of T-cells, while ITAM of B-cell
Is the Ig , (CD79), whereas, CD16 is the ITAM of NK-cell & macrophage.
Immunoreceptor Tyrosine activation motifs
(ITAM)The B & T cell receptors also have several similarities
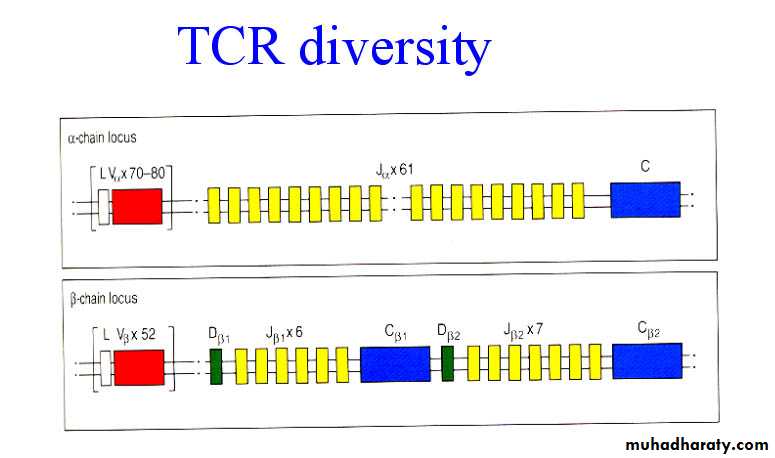
Even though the TCR is a heterodimer the TCR chains have constant and variable regions.TCR germ line DNA for each of the chains is organized into multigene families with multiple V region segments (with rearrangement of V,J – for a&g chains and V,D,J for b&d chains) with one or more C region gene segments.
The mechanisms for T cell V region gene segment rearrangement are similar to B cells rearranging Ig germline DNA.
The mechanisms generating TCR diversity are similar to those seen in generating Ab diversity.
Its structurally related since both are folded into domains.
The T cell receptor differs from the B cell receptor in SEVERAL ways:
The T cell does not secrete its receptor like the B cell does so any assessment of receptor structure and specificity has to rely on complex cellular assays.TCR has one antigen binding site, whereas, Igs have at least two.
T cell can recognize and bind antigens just when are presented by MHC, whereas, Ig can recognize free antigens.
TCR can be activated by protein antigens (not CHO antigens). In contrast, Ig can recognize intact molecules proteins, carbohydrates, and lipids.
Genes of the TCR
The alpha and gamma loci have sets of V and J-genes (like Ig-light chain) and beta and delta loci have set of V, D, and J-genes (like Ig-heavy chain). Diversification of the TCR gene occurs by recombination between V, D, and J segments. Extensive diversity is generated in the joining process, as not only are VDJ-arrangement possible, but also V-J and VDDJ-joins.-the TCR a and b locus are composed of multiple gene segments
-the variable portion of the TCR a and b chains is encoded by different gene segmentsTCR diversity
-random association of V, D and J-flexible joining of gene segments
-N nucleotide addition
- a and b association
-no somatic hypermutation
1015 TCR
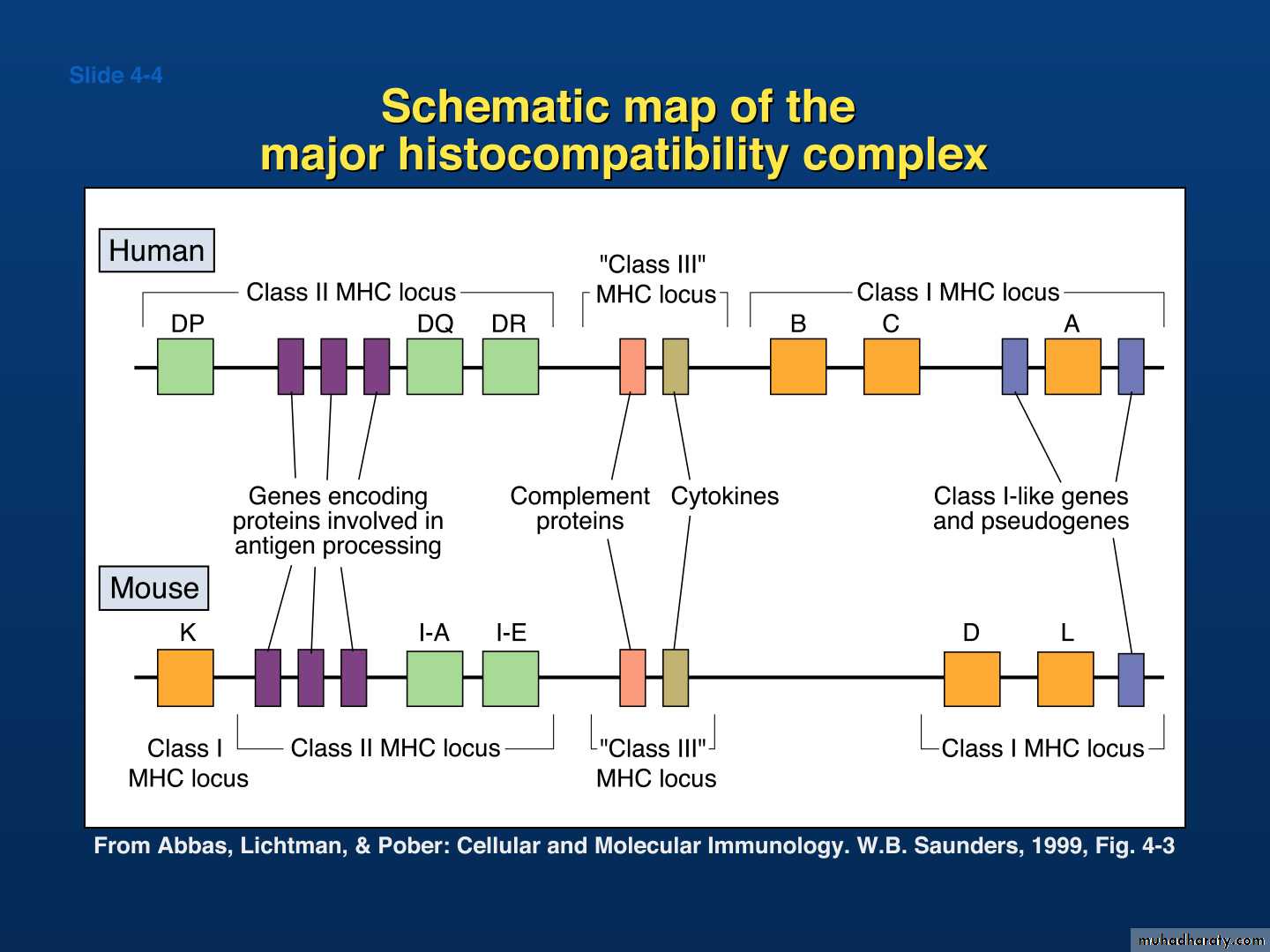
a cluster of genes that are located on a short arm of the chromosome 6.
The molecules which present Ag to T-cells are mostly encoded within the MHC.This gene complex containing more than 100 separate gene loci.
Human: Human leukocyte antigens (HLA)
Mouse: H-2Major histocompatibility complex(MHC)
Genes of the Major
Histocompatibility ComplexThe molecules which present Ag to T-cells are mostly encoded Within the MHC. This gene Complex containing more than 100 separate loci, the product of This complex :
1- Two highly polymorphic cell – surface molecules, called MHC-I &
MHC-II.
2- Non-polymorphic products collectively called MHC-III, including
a- Complement component (C2,
C4, B-factor).
b- Cytokines (TNF-alpha & beta).
c- Heat shock proteins (Hsp70).
d- Some molecules involved in Ag processing.
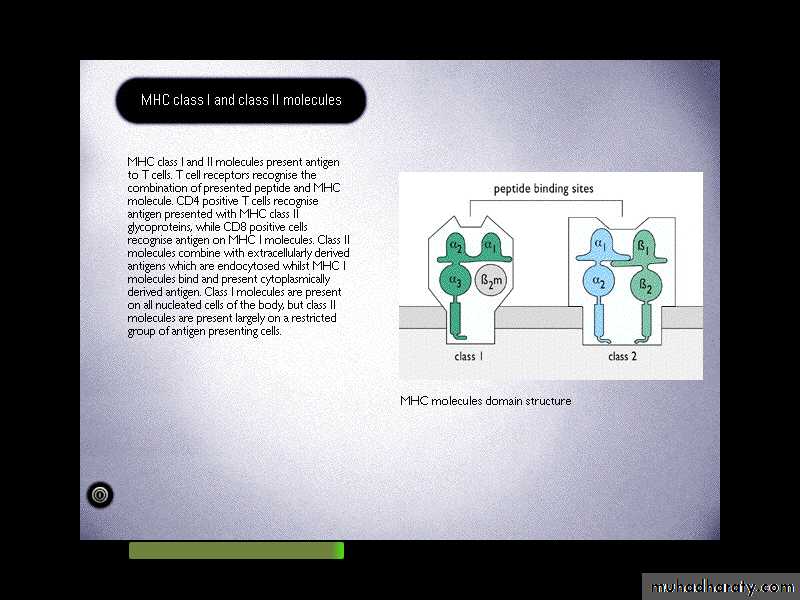
MHC-I molecules
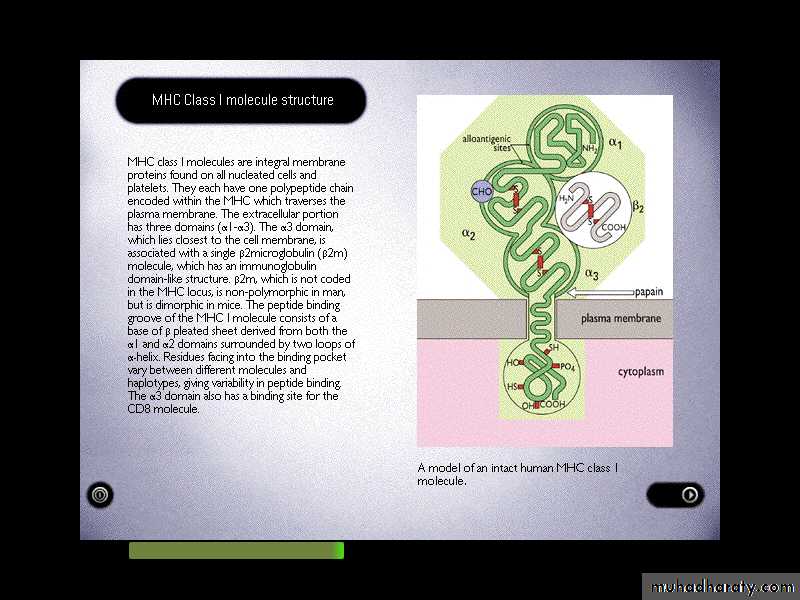
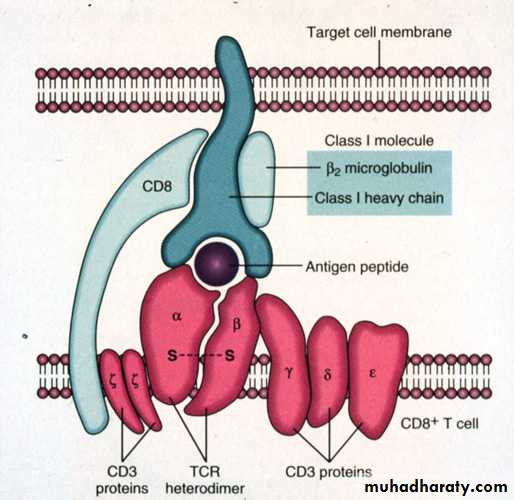
It comprises a glycosylated heavy chain which non-covalently associated with B2-microglobulin (B2MG).
MHC class - I heavy chain consists of three extracellular domains, designated alpha1,2&3, a transmembrane region and a cytoplasmic tail.
The alpha 2&3 domains have intrachain disulphide bonds.
The alpha 3 domain is structurally homologous to Ig-constant domains, and contains a site which interacts with CD8 on cytotoxic T-cells.B2MG is non-polymorphic in humans. This molecule also associates with a number of other class I like molecule, for example the products of the CD1-genes on chromosome 1 in man, and the Fc-R that mediates the uptake of IgG from milk in intestinal cells of neonate.
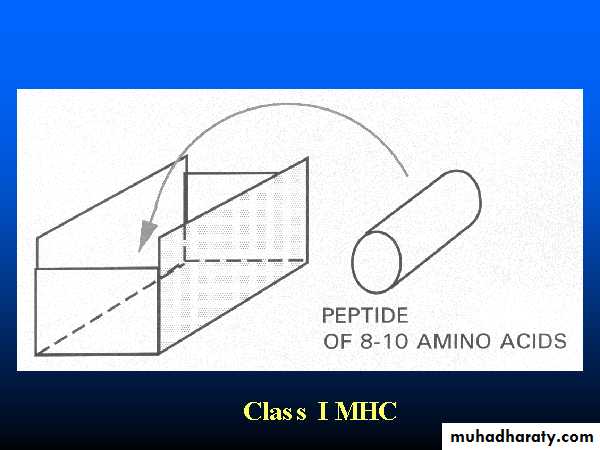
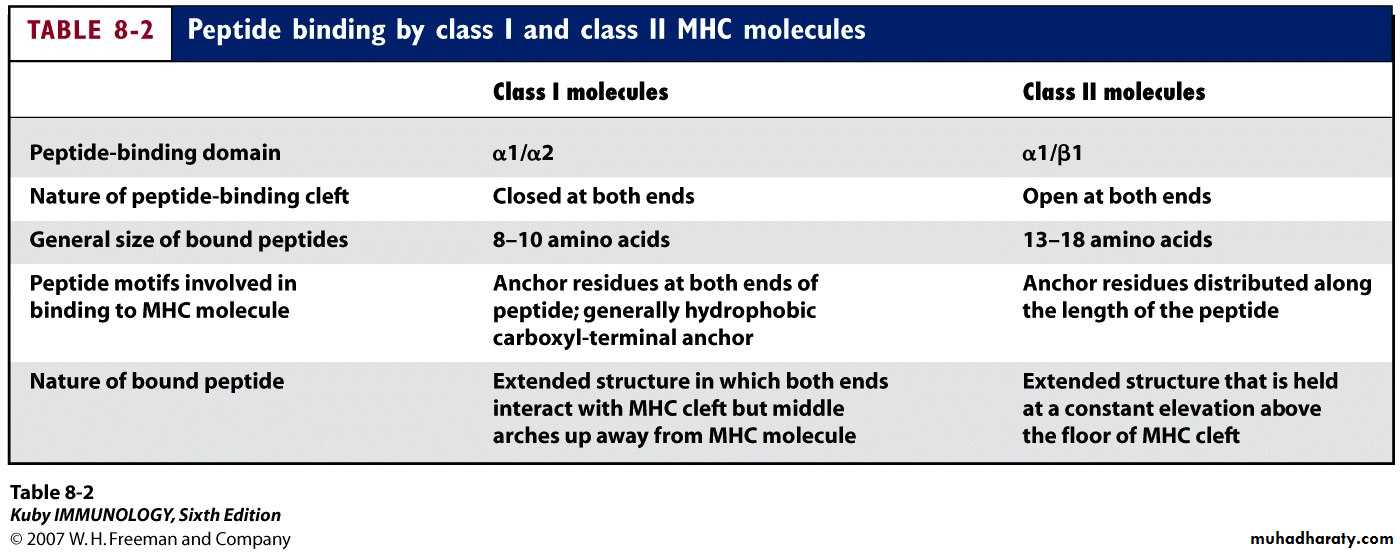
Heavy chain alpha1&2 domains form the Ag-binding groove. Typically the groove on an MHC I molecule will accommodate peptides of eight or nine residues.
The peptides which bind to MHC I molecules come from proteins synthesized within the cell.
1
32
MHC-encoded -chain of 43kDa
Overall structure of MHC class I molecules
3 domain & 2m have structural & amino acid sequence homology with Ig C domains Ig GENE SUPERFAMILY
2m
2-microglobulin, 12kDa, non-MHC encoded, non-transmembrane, non covalently bound to -chainPeptide antigen in a groove formed
from a pair of a-helicies on a floor of anti-parallel b strands-chain anchored to the cell membrane
1 and 2 domains: Interact to form a peptide-binding region which is a groove(cleft)Function of each domain
3 domain: Binding to CD8 on Tc cells2 microglobulin domain: To maintain proper conformation of class Ⅰ HLA molecules.
Trans-membrane region: Anchoring class Ⅰ HLA molecules
Intra-membrane region: Transmitting the signal
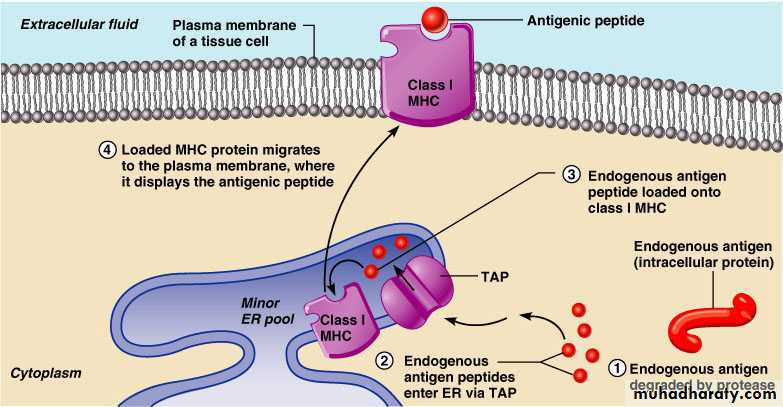
Endogenous antigens are:
Degraded by proteases and enter the endoplasmic reticulumTransported via TAP (transporter associated with antigen processing)
Loaded onto class I MHC molecules
Displayed on the cell surface in association with a class I MHC molecule
Figure by Eric A.J. Reits
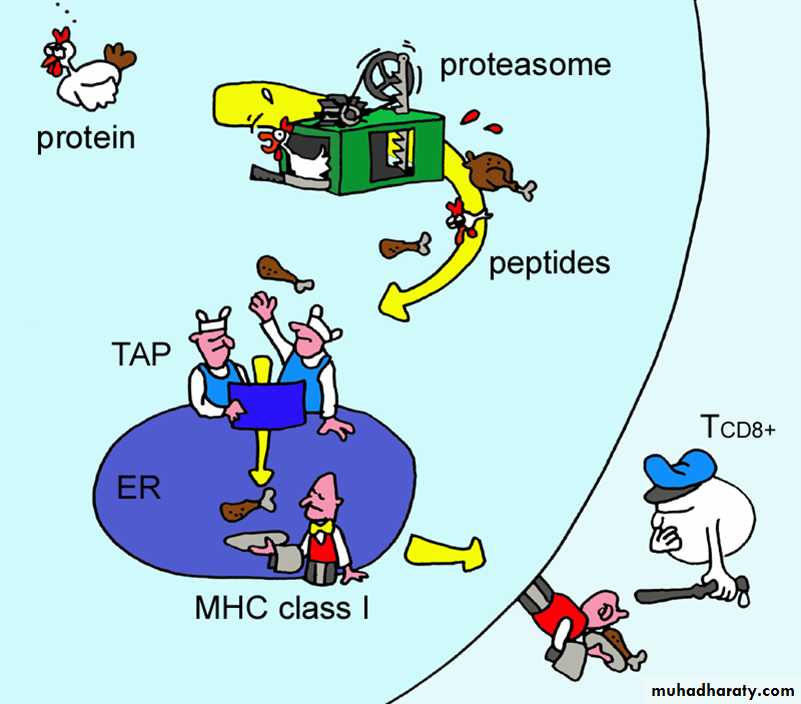
Generation of MHC Class I – Peptide ComplexesEndogenous Ags – generally synthesized within the cell
Generally viral or parasitic in origin.
Processing occurs in the cytosolic compartment.
The major mechanism for generating peptide fragments in the cytoplasm is via a giant protein complex known as a proteasome
Cuts the protein into peptide fragments 8-10 aa long
Peptides selectively transported into the ER by transporter genes
TAP-1 and TAP-2
Generation of MHC Class I – Peptide Complexes
Binding of peptide to class I molecules takes place in the ERBinding of peptide is selective based on structure of the binding groove and the peptide
Preferentially binds peptides of 8-10 aa
Before peptide loading MHC class I and b2m chains synthesized in the ER associate with “chaperones”, which assist in the correct folding and direct the molecule through the ER
Peptide binds to MHC class I in the ER
Moves via the Golgi apparatus to the surface for interaction with CD8+ T cells expressing the appropriate receptors
Class I MHC Proteins
Figure 21.15aIs a heterodimers of heavy (alpha) and light (B) glycoprotein chains.
The alpha and beta chains have the same overall structures, An extracellular portion comprising two domains (alpha1&2 or beta1&2) is connected by a short sequence to a trans-membrane region & cytoplasmic domain.The products of the class II genes in human (DP, DQ & DR).
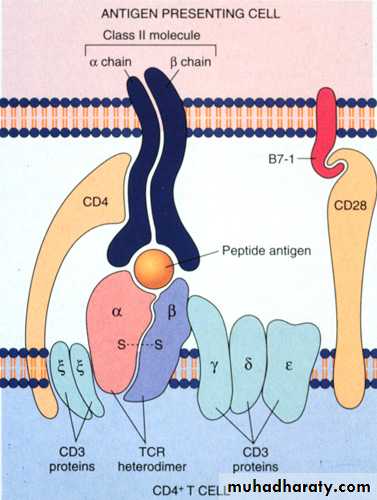
The B2-domain contain a binding site for CD4.
MHC-II molecule
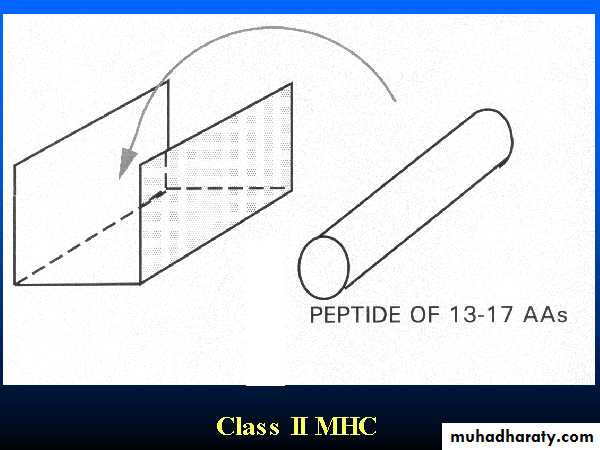
The class II groove is more open than that of class I, so that longer peptides can be accommodated. As the class II groove is not closed at the ends, peptides bound to MHC II molecule extend out of the ends of the groove.Peptides bind to MHC II come from proteins which have been internalized by the cell and then degraded. These peptides are less uniform in size and may be trimmed once they have bound to the MHCII-molecule.
2
1and a -chain of 29kDa
MHC-encoded, -chain of 34kDa
2
1Overall structure of MHC class II molecules
a and b chains anchored to the cell membrane
2 & 2 domains have structural & amino acid sequence homology with Ig C domains Ig GENE SUPERFAMILY
No b-2 microglobulin
Peptide antigen in a groove formed from a pair of a-helicies on a floor of anti-parallel b strands
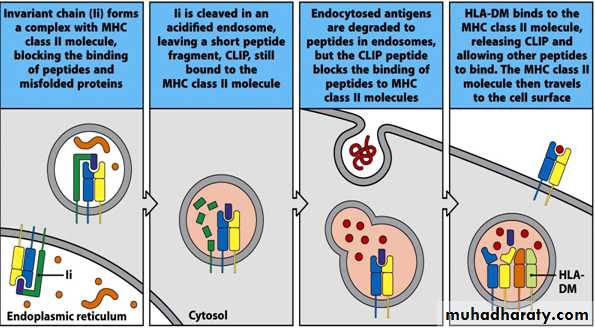
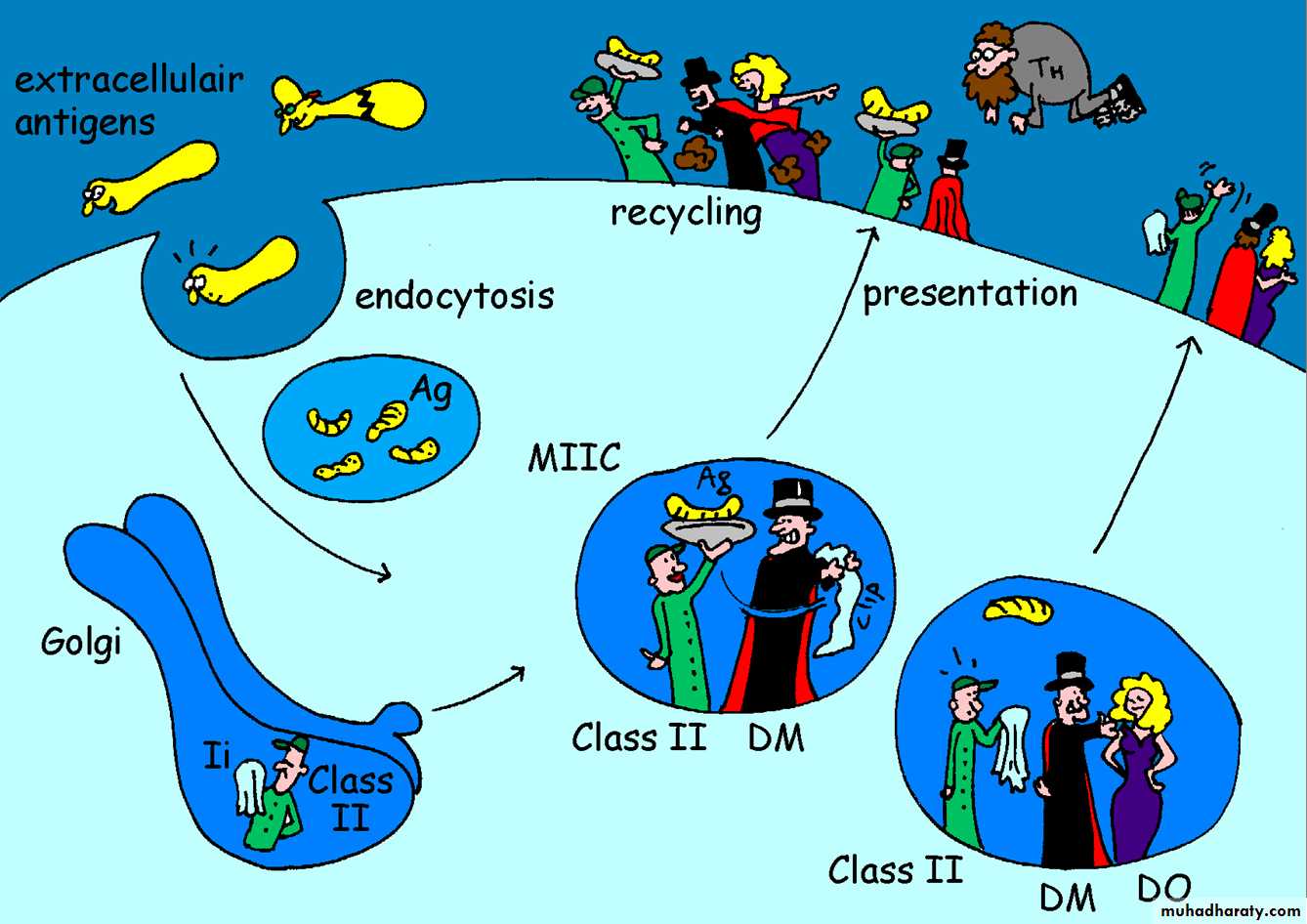
Invariant Chain (Ii, CD74)
In the ER – a region of Ii interacts with the binding groove to prevent binding of endogenous peptidesLi also acts as “chaperone” to allow the MHC class II molecule + Ii to leave the ER
Removal of Ii occurs in stages in acid vesicles
Li is degraded proteolytically to a fragment known as “CLIP”
Fusion of endosomes
HLA-DM facilitates peptide exchange – replacing CLIP with exogenous Ag
Peptide-MHC class II complex is generated and moves to cell surface to interact with CD4+ T cells expressing the appropriate receptor
Class II MHC Proteins
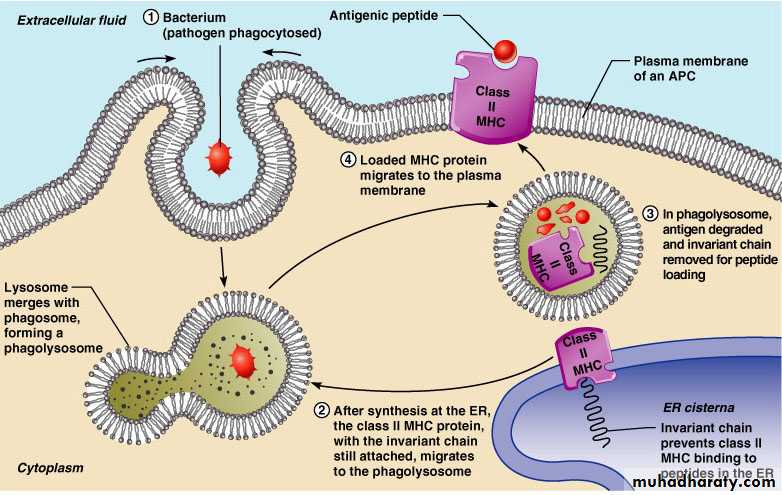
Class II MHC proteins are found only on mature B cells, some T cells, and antigen-presenting cellsA phagosome containing pathogens (with exogenous antigens) merges with a lysosome
Invariant protein prevents class II MHC proteins from binding to peptides in the endoplasmic reticulum
Class II MHC Proteins
Class II MHC proteins migrate into the phagosomes where the antigen is degraded and the invariant chain is removed for peptide loading
Loaded Class II MHC molecules then migrate to the cell membrane and display antigenic peptide for recognition by CD4 cells
α1 and 1: Interact to form the peptide-binding region which is a groove (cleft)
2 and 2 domain : Form the immunoglobulin-like region.
2 domain can bind to CD4 on Th cellsTrans-membrane region: Anchoring class Ⅱ HLA molecules
Intra-membrane region: Transmitting the signal
β
α
Class II MHC Proteins
Figure 21.15b
MHC class II pathway
Figure by Eric A.J. Reits
Generation of MHC Class II-Peptide ComplexesExogenous Ags – taken into the cell by endocytosis (soluble Ag) or phagocytosis (particulate Ag)
Bacteria, viruses, foreign proteins, foreign RBC.
Once internalized in an intracellular vesicle fuses with endosomal or lysosomal vesicles – highly acidic (pH~4) + degradative enzymes.
Acid vesicle containing the immunogenic peptide fuses with vesicles containing newly synthesized MHC class II a and b chains synthesized in the ER
Class I MHC and Class II MHC
• MHC Class I• MHC Class II
• Nomenclature
• HLA-A, HLA-B, HLA-C
• HLA-DP, HLA-DQ,
• HLA-DR
• Found on
• All nucleated somatic cells
• Macrophages, B-cells, Dentritic cells, langerhans cells of skin and activated T cells
• Recognized by
• CD8 TC cells
• CD4 TH cells
• Functions
• Presentation of Ag to TC cells leading to elimination of tumor or infected host cell
• Presentation of Ag to TH cells which secrete cytokines
• The biological importance of MHC
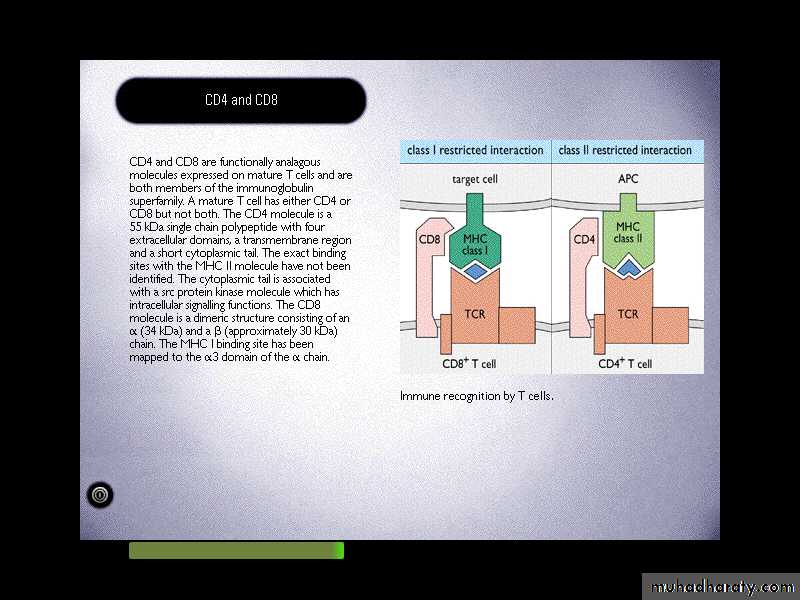
Antigen recognition by T cells:CD8 T cells …………… MHC I molecules
CD4 T cells……………...MHC II molecules
It’s also important in autoimmune diseases which occur in people who carry MHC genes such as HLA- B27 in Ankylosing spondylitis.
Success of organ transplantation is determined by compatibility of MHC genes of donor and recipient.