
College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3rd 2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 24/11/2021 No.4
Pag
e
1
Sleeping sickness
Objectives
• Aetiology
• Biology :morphology, habitat ,host and infective stage
• Transmission and reservoirs
• Life cycle
• Pathogenesis
• Clinical picture
• Diagnosis
• Treatment
• Prevention
Aetiology
:
Two subspecies those are morphologically indistinguishable:
• T. brucei rhodesiense
• T. brucei gambiense.
Biology
Morphology
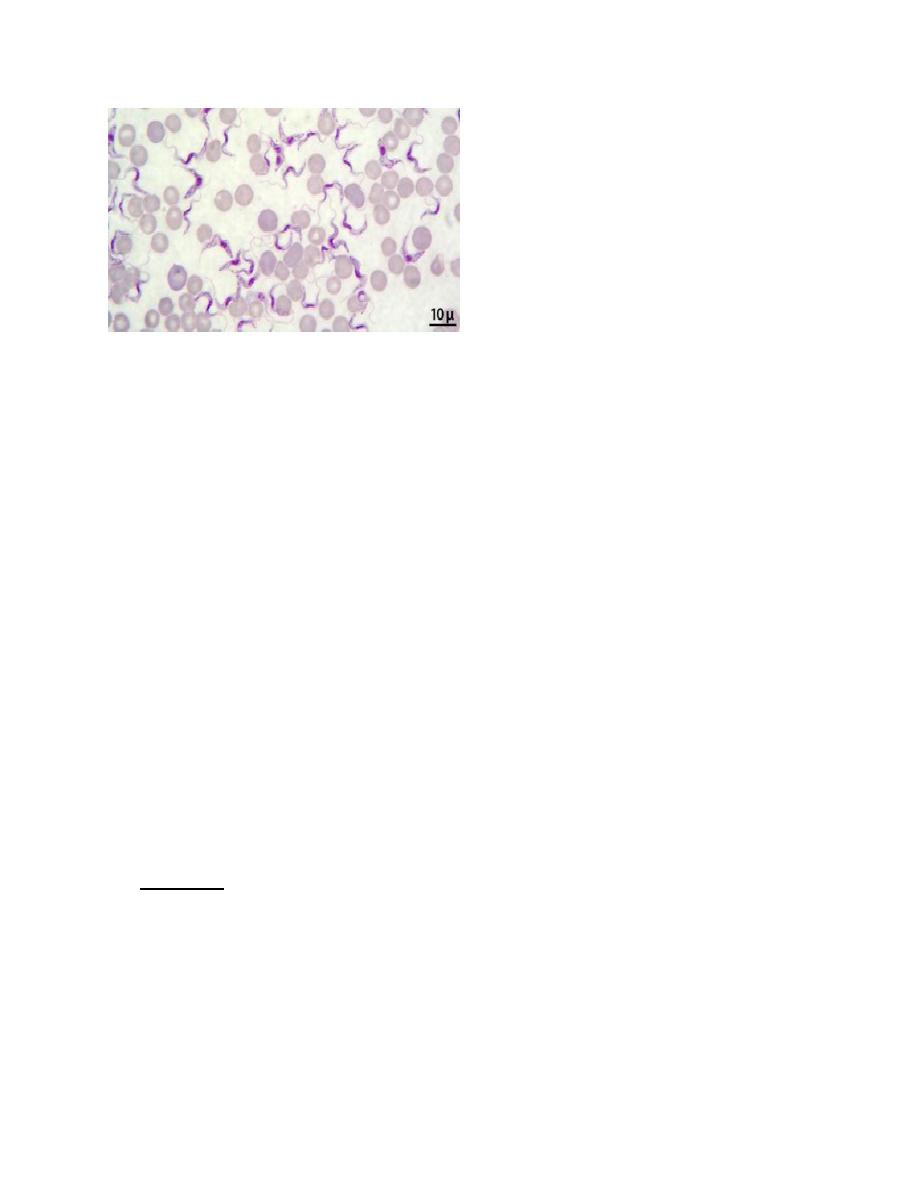
• Trypomastigotes: The size of Trypanosoma gambiense or rhodesiense trypomastigotes
is 14-33 μm.
• The trypomastigote appears as wavy or curved S shaped.
• Central nucleus and posterior kinetoblast but not bulging.
• Undulating membrane at hole length of the trypomastigote, which arises from the
kinetoblast
Habitat
:
• They are essentially parasites of connective tissue, where they multiply rapidly and then
invade regional lymph nodes, blood and finally may involve central nervous system
College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3rd 2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 24/11/2021 No.4
Pag
e
2
Trypanosome brucei
Host
:
T. brucei passes its life cycle in two hosts:
1. Vertebrate host: Man, game animals and other domestic animals.
2. Invertebrate host: Tsetse fly.
• Both male and female tsetse fly of Glossina species
• G. palpalis are capable of transmitting the disease to humans.
• These flies dwell on the banks of shaded streams, wooded Savanna and agricultural
areas.
Infective form:
Metacyclic trypomastigote forms.
Transmission
:
• By bite of tsetse fly.
• Congenital transmission also recorded.
• Sexual contact.
• Blood transfusion
Reservoirs:
• Humans are the main reservoir for Trypanosoma brucei gambiense
• Wild animals are the main reservoir of T. b. rhodesiense.
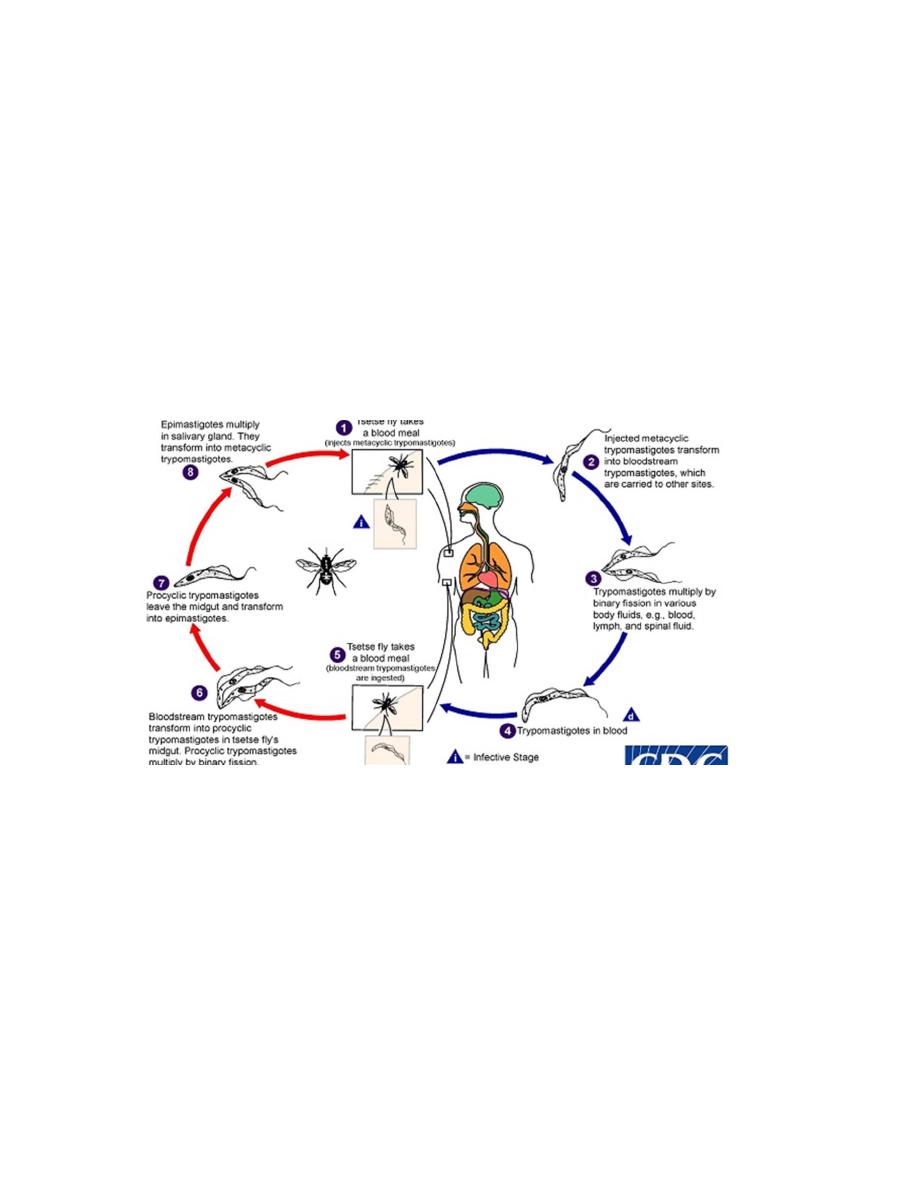
Life cycle:
• During a blood meal on the mammalian host, an infected tsetse fly injects the infective
stage (metacyclic trypomastigotes) into skin.
• The parasites enter the lymphatic system and pass into the bloodstream.
• Inside the host, they transform into blood stream trypomastigotes, which are carried to
other sites throughout the body, reach other body fluids (e.g., lymph, spinal fluid), and
continue the replication by binary fission.
• The tsetse fly becomes infected with bloodstream trypomastigotes when taking a blood
meal from an infected mammalian host.

College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3rd 2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 24/11/2021 No.4
Pag
e
3
• In the fly’s midgut, the parasites transform into procyclic trypomastigotes, multiply by
binary fission, leave the midgut, and transform into epimastigotes.
• The epimastigotes reach the fly’s salivary glands and continue multiplication by binary
fission.
• The cycle in the fly takes approximately 3 weeks.
Pathogenesis
:
• The parasites evade the humoral immune response by extensive antigenic variation of
parasite surface glycoproteins known as Major variant surface glycoprotein (VSG).
• This evasion of the humoral immune responses contributes to parasite virulence.
• The trypomastigotes spread from the skin through the blood to the lymph nodes and
the brain.
• The pathological changes may be the result of immune-mediated reactions against
antigens on red blood cells, cardiac tissue, and brain tissue, resulting in hemolysis,
anemia, pericarditis, and meningoencephalitis.
• The typical somnolence (sleeping sickness) progresses to coma is a result of a
demyelinating encephalitis.
Clinical picture:
• The clinical course of human African trypanosomiasis has Two stages:
• 1-The first stage: The parasite is found in the peripheral circulation.
• 2-The second stage: Once the parasite crosses the blood-brain barrier and infects the
central nervous system.
• Although, infection with either form at the end will lead to coma and death if not
treated, the clinical course depends on form of the parasite (T. b. rhodesiense or T. b.
gambiense) causing the infection.
T. b. rhodesiense infection (East African sleeping sickness):
• Progresses rapidly.
• In some patients, a large sore (a chancre) will develop at the site of the tsetse bite.
• Most patients develop: Headache, muscle, joint aches, fever, and enlarged lymph nodes
within 1-2 weeks of the infective bite.
• Some people develop a rash.
• Myocarditis
• After a few weeks of infection, the parasite invades the central nervous system and
eventually causes mental deterioration and other neurologic problems. Daytime and
nighttime sleep disturbance, and progressive confusion and coma.
• Death occurs usually within months.

College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3rd 2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 24/11/2021 No.4
Pag
e
4
T. b. gambiense infection (West African sleeping sickness)
• Progresses more slowly.
• At first, there may be only mild symptoms.
• Infected persons may have intermittent fevers, headaches, muscle and joint aches,
malaise, itching of the skin, swollen lymph nodes mainly cervical (winterbottoms sign),
and weight loss.
• Usually, after 1-2 years, there is evidence of central nervous system involvement, with
personality changes, daytime sleepiness with nighttime sleep disturbance, and
progressive confusion. Other neurologic signs, such as partial paralysis or problems with
balance or walking may occur, as well as hormonal imbalances.
• The course of untreated infection rarely lasts longer than 6-7 years and more often kills
in about 3 years.
Last stages of sleeping sickness (2)
Finally the late stage leads to death
The host enters a terminal coma or sleep, giving the disease its name “sleeping sickness”
Diagnosis
:
Nonspecific findings:
• Anemia and monocytosis.
• Raised ESR due to rise in gamma globulin levels.
• Reversed albumin: globulin ratio.
• Increased CSF pressure and raised cell count and proteins in CSF.
Specific findings:
Definitive diagnosis of sleeping sickness is established by the demonstration of trypanosomes
in peripheral blood, bone marrow, lymph node, CSF and chancre fluid.
Microscopy:
• Wet mount preparation of lymph node aspirates and chancre fluid are the rapid method
for demonstration of trypanosomes.
• Examination of Giemsa stained thick peripheral blood smears reveals the presence of
the trypomastigotes.
• If parasitemia is low, then examination of concentrated blood smear is a highly sensitive
method.
• Examination of wet mount and stained smear of the CSF may also show trypanosomes
College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3rd 2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 24/11/2021 No.4
Pag
e
5
Culture
:
•
The organisms are difficult to grow; so culture is not routinely used for primary isolation
of the parasite
.
Animal inoculation:
Is a highly sensitive procedure for detection of T. brucei rhodesiense infection.
Serodiagnosis
:
Antibody detection:
o Patients with African trypanosomiasis have very high levels of total serum IgM and later, CSF
IgM antibodies.
o Various serological methods have been developed to detect these antibodies and are as follows:
Indirect hemagglutination (IHA)
Indirect immunotluorescence (llF)
Enzyme-linked immunosorbent assay (ELISA)
Card agglutination trypanosomiasis test (CATT)
Complement fixation test (CFT)
o Specific antibodies are detected by these tests in serum within 2-3 weeks of infection.
o These serological tests are useful for field use and mass screening.
Antigen detection:
Antigens from serum and CSF can be detected by ELISA.
Molecular diagnosis as PCR.
Imaging:
CT scan of the brain shows cerebral edema and MRI shows white matter enhancement in late
stages of disease.
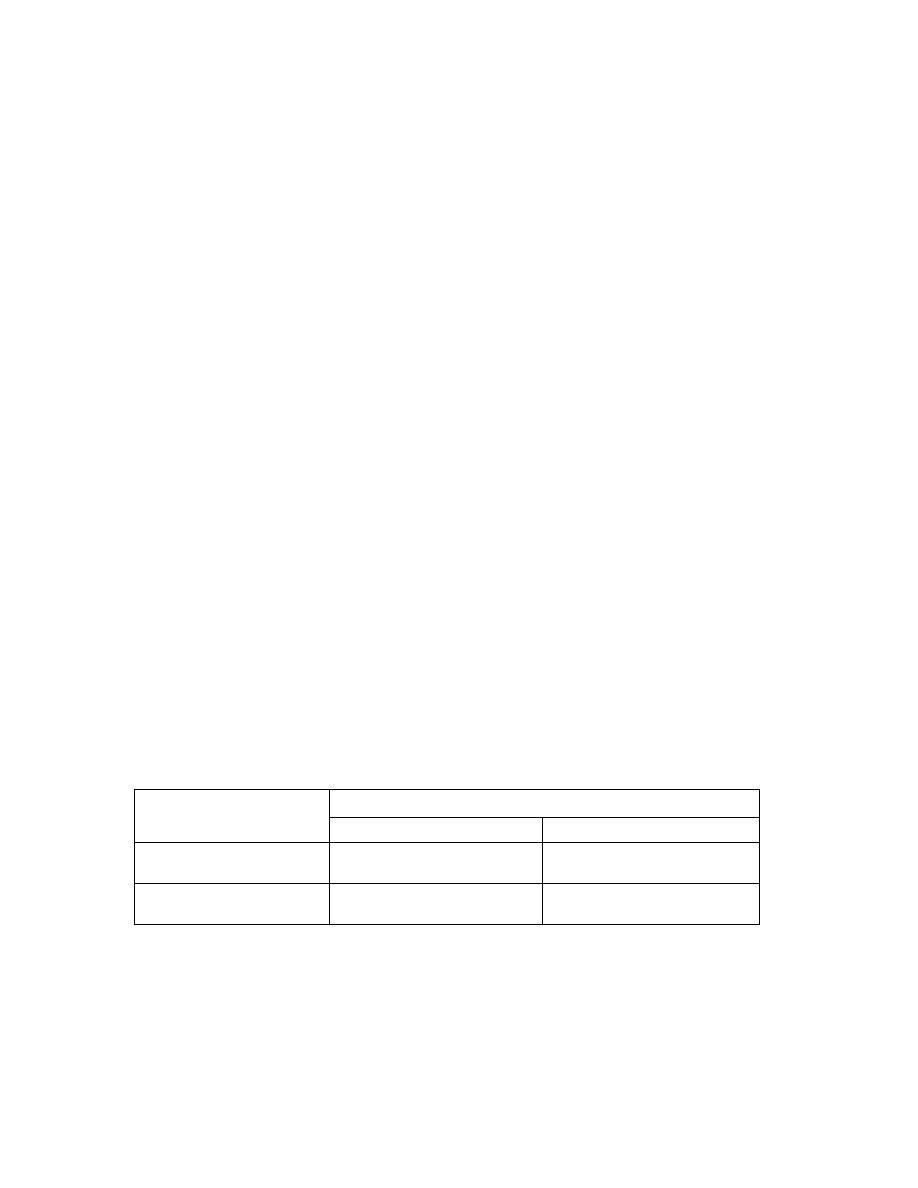
Treatment
:
• All persons diagnosed with African Trypanosomiasis should receive treatment.
• The choice of length and dose of treatment course depends on the type of infection (
gambiense or rhodesiense) and the disease stage (i.e. whether the central nervous
system has been invaded by the parasite).
• After treatment patients need to have serial examinations of their cerebrospinal fluid
for 2 years to detect relapse.
Causative organism
Clinical stage
I( normal CSF)
II(abnormal CS)
T.b.gambiense( west
African disease)
Pentamedine
Eflornithine
T.b.rhodesiense (East
African disease)
Suramin
Melarsoprol
College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3rd 2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 24/11/2021 No.4
Pag
e
6
Prevention and control:
• There is no vaccine or drug for prophylaxis against African trypanosomiasis.
• Preventive measures are to minimize contact with tsetse flies:
1. Avoid Local known heavily infested areas.
2. Wear long-sleeved shirts and clothes of medium-weight material in neutral colors.
3. Inspect vehicles before entering. The flies are attracted to the motion and dust from
moving vehicles.
4. Use insect repellent (Permethrin-impregnated clothing may help).
5. Controlling the tsetse fly vector: Tsetse fly traps and screens in combination with
insecticides and odors that attract the flies.
6. Reducing the reservoir of infection is more difficult for T. b. rhodesiense, since there
are a variety of animal hosts.
Life cycle of T. gambiense (3)
Summary:
• Sleeping sickness is a disease caused by T. brucei rhodesiense and T. brucei gambiense
• The causative parasites are transmitted by the bite Tsetse fly
• Clinical picture has two sages according to presence of parasite in the body
• smears showing trypomastigotes form fastest diagnostic methods
• Treatment depends on stage and species of causative agent
• Prevention and control is difficult but stay the main action to decrease rate of infection
College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3rd 2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 24/11/2021 No.4
Pag
e
7
References
1.
https://www.itg.be/E/Article/hidden-parasite-
hampers-elimination-of-sleeping-sickness
2.
Tsetse.org)
3. CDC.gov
4. BURTON J. Bogitsh,Clint E. Carter, and Thomas N. Oeltmann, 2013, Human parasitology
4
th
edition, USA and UK, Elsevier.
Encyclopedia of Microbiology (Third Edition)
, 2009
6. Paniker J CK , 2018, Paniker
,
s textbook of medical parasitology, 8
th
edition, Newdelhi,
London , Panama, JAYPEE.
7. World health organisation. Photos on leishmaniasis
8.
Zeibig A. Elizabeth, 2013, Clinical parasitology, USA, Elsevier
9. Hayden Randall , Wolk Donna, Caroll Karen and Tang Yi. DIAGNOSTIC
MICROBIOLOGY IMMUNOCOMPROMISED HOST . , N.W., Washington, DC ,
USA. “nd edition.
