
College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3
rd
2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 29/11/2021 No.5
Pag
e
1
Trypanosoma cruzi
Objectives
• Introduction
• Biology: morphology, host, habitat,mode of transmission, life cycle.
• Clinical presentation
• Diagnosis
• Treatment
• Prevention and control
Introduction:
• It causes Chagas disease,
• Zoonotic disease
• Tansmitted to humans by blood-sucking Triatomine reduviid bugs.
Biology:
Morphology
Amastigote stage:
• They are oval bodies measuring 2-4 μm in diameter having a nucleus and kinetoplast .
• Flagellum is absent.
• Morphologically, it resembles the amastigote of Leishmania sp.
• Multiplication of the parasite occurs in this stage.
Structure of amastigote (1)
Trypomastigote stage:
• Trypomastigotes are non multiplying forms and are taken up by the insect vectors.
• They appear as long, thin flagellates about 15-20 μm long .
• In stained blood smears, they are shaped-like alphabet "C''; "U''; or rarely "S';
• They have an undulating membrane, and a flagellum running along the undulating
membrane, leaving the body at the anterior end.
• A typical trypomastigote has a large, subterminal or terminal kinetoplast
• Centrally located nucleus

College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3
rd
2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 29/11/2021 No.5
Pag
e
2
• Motile circulating trypomastigotes are readily seen on slides of fresh anticoagulated
blood in acute infection but are rarely detectable in chronic infection.
• Trypomastigotes may be seen in cerebrospinal fluid (CSF) in central nervous system
infections.
T. Cruzi Trypomastigote (2)
Epimastigote stage:
• Epimastigote forms are the culture form of T. cruzi.
• It has a kinetoplast adjacent to the nucleus.
• An undulating membrane runs along the anterior half of the parasite.
• Epimastigotes divide by binary fission in midgut of the vector.
Note:
• Kineloplast consists of
A deeply staining parabasal body
+
Adjacent dot Like blepharoplast.
Host:
1. Definitive host: Man.
2 . Intermediate host (vector): Reduviid bug (triatomine bugs):
• Triatoma infestans
• Rhodnius prolixus
• Panstrongylus megistus.
Epimastigot of T.cruzi (3)

College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3
rd
2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 29/11/2021 No.5
Pag
e
3
They are large (up to 3 cm long) night-biting bugs, which typically defecate while feeding.
The feces of infected bugs contain the metacyclic trypomastigote.
3. Reservoir host: Armadillo, cats, dogs and pigs.
Infective form:
Metacyclic trypomastigotes forms are the infective forms found in feces of reduviid bugs.
Habitat
In humans, T cruzi exists in both amastigote and trypomastigote forms:
• Amastigotes are the intracellular parasites. They are found in muscular tissue, nervous
tissue and reticuloendothelial system
• Trypomastigotes are found in the peripheral blood.
In reduviid bugs, epimastigote forms are found in the midgut and metacyclic
trypomastigote forms are present in hindgut and feces.
Mode of transmission:
• Transmission of infection to man and other reservoir hosts takes place when mucus
membranes, conjunctiva, or wound on the surface of the skin is contaminated by feces
of the bug containing metacyclic trypomastigotes( posterior station transmission).
• T. cruzi can also be transmitted by:
• Blood transfusion
• Organ transplantation
• Congenital transmission
• Very rarely by ingestion of contaminated food or drink with presence of mouth ulcers.
Three T. cruzi trypomastigotes in a thin blood smear stained with Giemsa (5).
Triatomine insect vector (“kissing” bug) (4)
College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3
rd
2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 29/11/2021 No.5
Pag
e
4
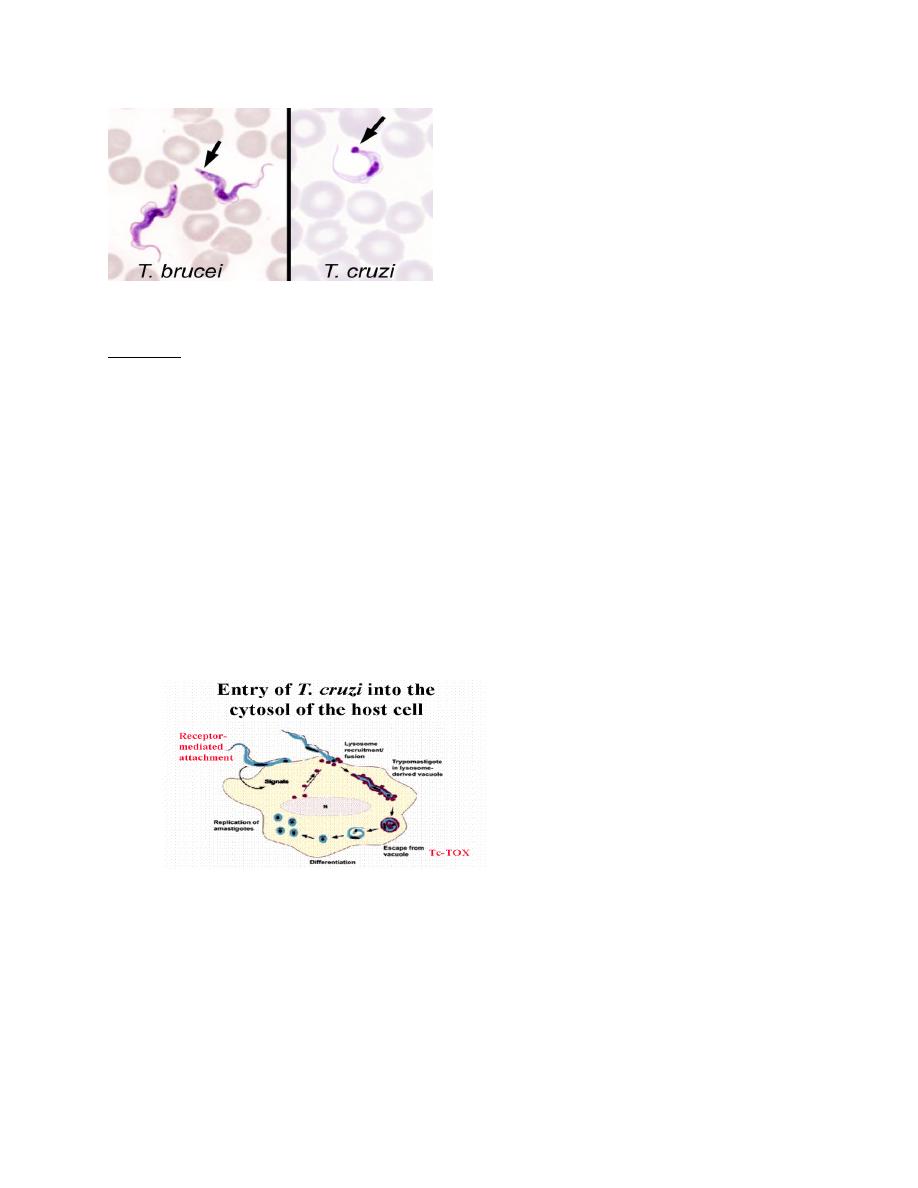
T.brucei vs T.cruzi trypomastigotes (6)
Life cycle:
• An infected Triatomine insect vector (“kissing” bug) takes a blood meal and releases
metacyclic Trypomastigotes in its feces near the site of the bite wound (Posterior
station transmission).
• Trypomastigotes enter the host through the wound or through mucosal membranes,
such as the conjunctiva.
• Inside the host, the trypomastigotes invade cells near the site of inoculation, where they
differentiate into intracellular amastigotes.
• The amastigotes multiply by binary fission
• The amastigotes are released into blood circulation as trypomastigotes.
• Trypomastigotes infect cells from a variety of tissues and transform into intracellular
amastigotes in new infection sites. Clinical manifestations result from this infective
cycle.
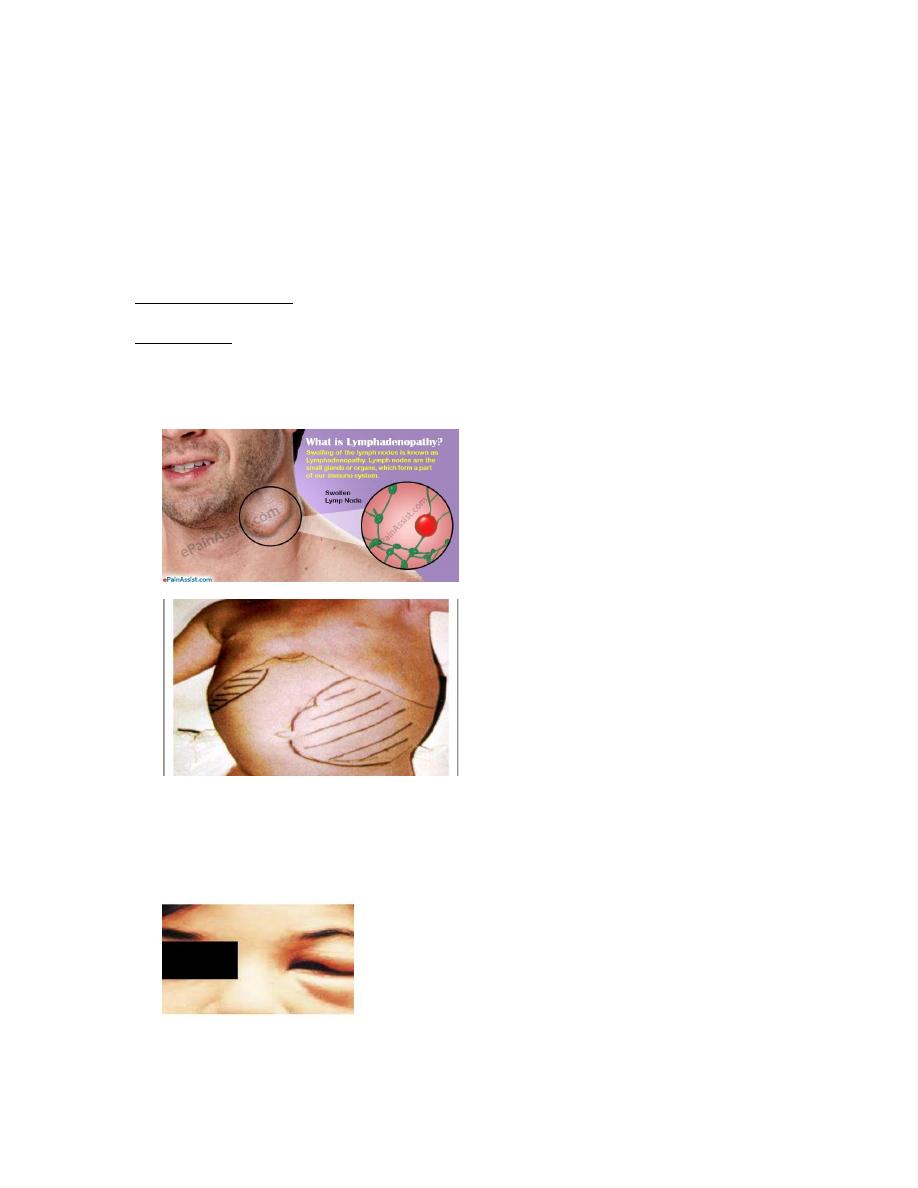
Invasion by T. cruzi to the cytosol (7)
• The bloodstream trypomastigotes do not replicate (different from the African
trypanosomes).
• Replication resumes only when the parasites enter another cell or are ingested by
another vector.
College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3
rd
2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 29/11/2021 No.5
Pag
e
5
• The “kissing” bug becomes infected by feeding on human or animal blood that contains
circulating parasites.
• The ingested trypomastigotes transform into epimastigotes in the vector’s midgut .
• The parasites multiply and differentiate in the midgut .
• They differentiate into infective metacyclic trypomastigotes in the hindgut.
Clinical Presentation:
Acute phase:
• The acute phase is usually asymptomatic, but can present with manifestations that
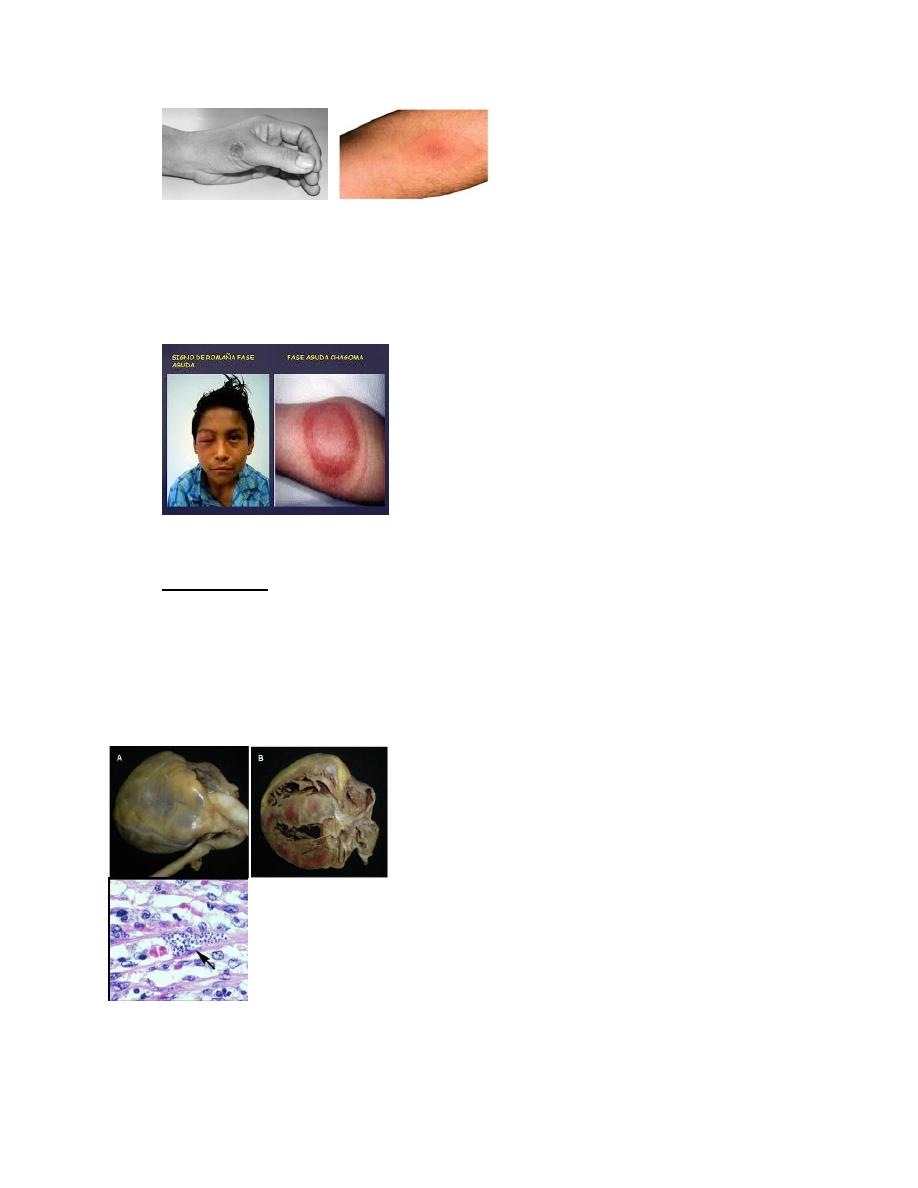
include fever, anorexia, lymphadenopathy, hepatosplenomegaly, and myocarditis.
Hepatosplenomegally and cervical lymphadenopathy (9)
• Romaña’s sign (unilateral palpebral and periocular swelling) may appear as a result of
conjunctival contamination with the vector’s feces.
Romaña’s sign (10)
College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3
rd
2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 29/11/2021 No.5
Pag
e
6
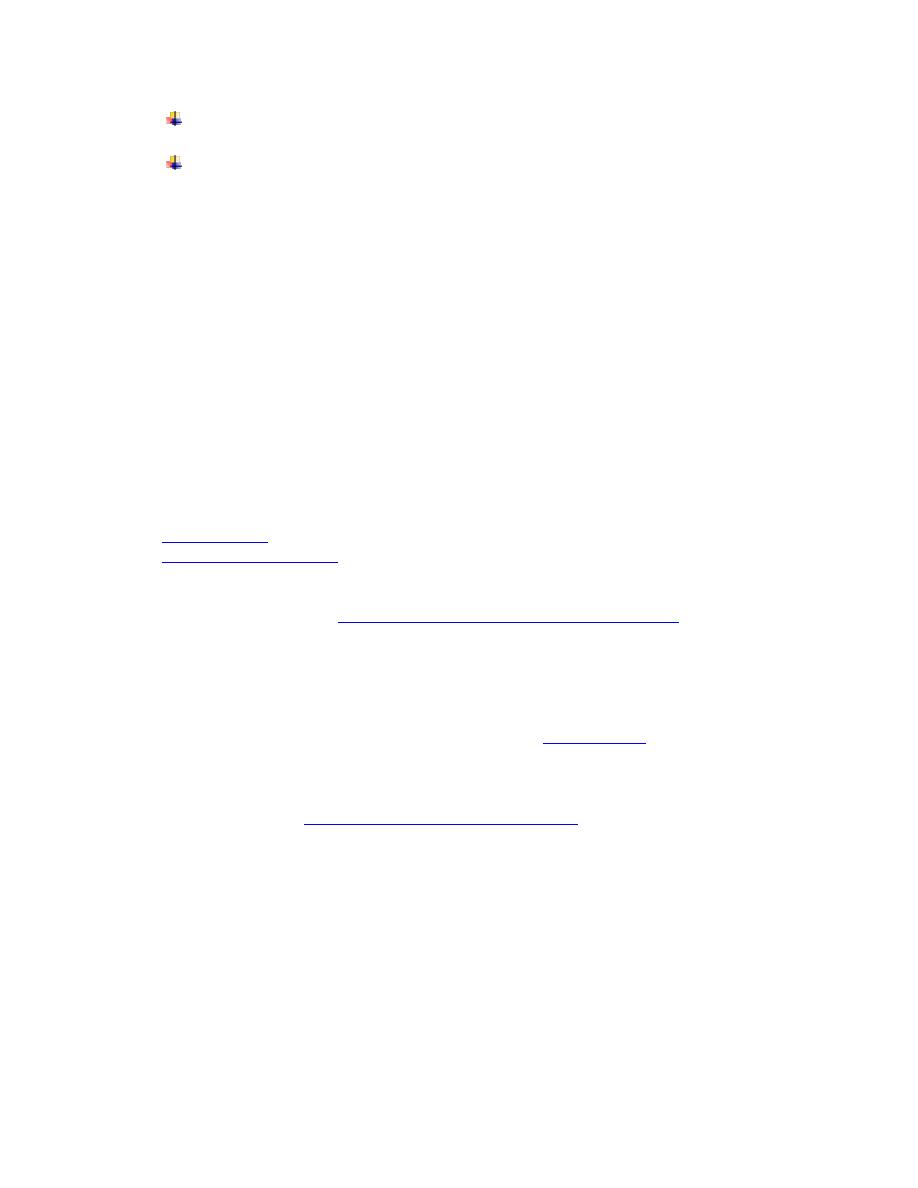
A nodular lesion or furuncle, usually called chagoma, can appear at the site of
inoculation.
Most acute cases resolve over a period of a few weeks or months into an asymptomatic
chronic form of the disease.
Romana sign and chagoma (11)
Chronic phase
• The symptomatic chronic phase may not occur for years or even decades after initial
infection.
• Chronic Chagas disease and its complications can be fatal.
• Its clinical picture includes:
•
Cardiomyopathy (the most serious manifestation): It is caused by auto
immune
reaction: T-cells recognize myosin proteins in the heart. This recognition occurs because
T. cruzi antigen B13 and heart myosin proteins are molecularly homologous
.
A and B Chagas cardiomyopathy
C. Trypnaosoma amastigotes nests in
heart tissue (12)

College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3
rd
2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 29/11/2021 No.5
Pag
e
7
Mega esophagous(10) Mega colon (10)
• Pathologies of the digestive tract such as megaesophagus and megacolon; due to an
inflammatory invasion and degeneration of the nervous plexuses.
Congenital infection:
• Congenital transmission is possible in both acute and chronic phase of the disease
causing myocardial and neurological damage in the fetus.
Diagnosis:
Laboratory Diagnosis
A. Microscopy:
• The diagnosis of acute disease requires detection of parasites.
• Microscopic examination of fresh anticoagulated blood or the buffy coat is the simplest
way to see motile organisms.
• In wet mount, trypomastigotes are faintly visible but their snake-like motion against red
blood cells (RBCs) makes their presence apparent.
• Trypomastigotes can also be seen in thick and thin peripheral blood smear, stained with
Giemsa stain.
Blood film for Trypanosoma cruzi (2,8)
Note: Serologic testing has no role in diagnosing acute Chagas disease
B. Culture: Novy, McNeal and Nicolle(NNN) medium or its modifications are used for
growing T. cruzi. This medium is inoculated with blood and other specimens and
incubated at 22-24°C.
• The fluid taken from the culture is examined microscopically by 4th day and then every
week for 6 weeks. Epimastigotes are found in the culture.
• Cell culture (amastigotes and trypomastigotes seen)
• Culture is more sensitive than smear microscopy.
• C. Animal inoculation AND Xenodiagnosis (insect inoculation)

College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3
rd
2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 29/11/2021 No.5
Pag
e
8
• D. Histopathology: Biopsy examination of lymph nodes and skeletal muscles and
aspirate from chagoma may reveal amastigotes of T. cruzi.
E. Serology:
Antigen detection: T. cruzi antigen can be detected in urine and sera in patients with
chronic Chagas disease.
Antibody detection: Antibodies (IgG) against T. cruzi may be detected by the following
tests:
• Direct agglutination test (DAT)
• Indirect hemagglutination(IHA)
• ELISA
• Indirect immunofluorescence (IIF)
• Radioimmune precipitation assay (RIPA)
• The disadvantage of the antibody based tests is that they may be false positive with
other disease like leishmaniasis and syphilis.
• Intradermal test: The antigen "cruzin" is prepared from T. cruzi culture and used for the
intradermal test. A delayed hypersensitivity reaction is seen.
• F. Molecular diagnosis: PCR is available that detects specific primers.
• Other tests:
• ECG, X-ray :Cardiomyopathy is seen in chronic Chagas disease.
• Endoscopy helps in visualization of megaesophagus in Chagas disease.
Positive IFA result with T. cruzi antigen (magnification 400x) (14).
Treatment:
• No effective specific treatment is available for treating Chagas disease.
• Nifurtimox and benznidazole have been used with some success in both acute and
chronic Chagas diseases.
• These drugs kill only the extracellular trypanosomes but not the intracellular forms.
Prevention and control:
There is no vaccine to prevent Chagas disease.
However, the following prevention and control tools are useful:
• Spraying of insecticides
• Home maintanance to prevent vector infestation.
• Good hygiene practices in food preparation, transportation, storage and consumption.
• Personal preventive measures such as:
Bed nets
Screening of blood, organ, tissue and cell donors and receivers.
College of Medicine University of Mosul /Department of: Microbiology
Subject: Parasitology Stage: 3
rd
2021-2022
Lecturer: Dr. Ahmed Alharbi Date: 29/11/2021 No.5
Pag
e
9
Observance of the standard safety protocols (wearing laboratory coats, gloves, face
masks, caps and glasses) for laboratory accidents prevention.
Screening of infected pregnant women to prevent congenital transmission control.
Summary:
• T cruzi is a kinetoplastid
• Transmitted by blood-sucking Triatomine reduviid bugs.
• Has amastigote, epimastigote and trypomastigote forms
• Causes Chagas disease
• Easiest method of diagnosis is microscopy
• Treatment not affect intracellar stages
• No vaccine against parasite and people shoulds take measures to protect themselves
against bite of the insect.
References
1. Study and score.com
2. Wikipedia
3. P W Pappas and S. M War drop
4.
5.
6. Parasite wonders.blogspot.com
7. Burleigh, B.A. and Woolsey, A.M. (2002), Cell signalling and Trypanosoma cruzi invasion. Cellular
Microbiology, 4: 701-711.
https://doi.org/10.1046/j.1462-5822.2002.00226.x
8. CDC.gov
9. Pain assist.com
10. Hurwitz I, Fieck A, Klein N and et al.,(2012). A Paratransgenic Strategy for the Control of Chagas
Disease. doi:10.1155/2012/178930
11. Study blue.com
12. Gonz C and Mantilla J(2012). Chagas heart disease. DOI:
13. Machado S. Fabiana etal., 2012
14. de Moraes, M.H., Guarneri, A.A., Girardi, F.P. et al. Different serological cross-reactivity
of Trypanosoma rangeli forms in Trypanosoma cruzi-infected patients sera. Parasites
Vectors 1, 20 (2008).
https://doi.org/10.1186/1756-3305-1-20
15. Paniker J CK , 2018, Paniker
,
s textbook of medical parasitology, 8
th
edition, Newdelhi, London ,
Panama, JAYPEE.
