Treatment and Assessment of R.A
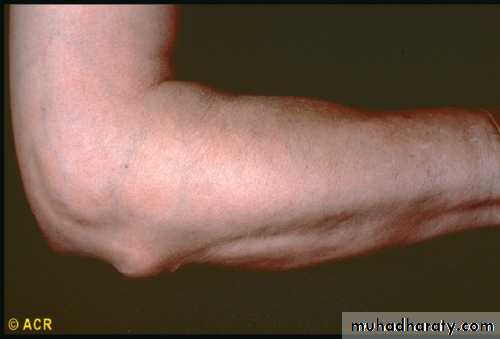
In most cases, rheumatoid arthritis is a chronic progressive disease that, if left untreated, can cause joint damage and disability. Factors that predict poor outcome include severity of disease, seropositivity, low socioeconomic and educational status, and poor functional status.Physical findings are most notable for joint-centered swelling, deformities, and painful or reduced join motion.
Extra-articular disease occurs in seropositive patients and includes rheumatoid nodules, Sjögren’s syndrome, interstitial lung disease, and vasculitis.
Laboratory tests that support a diagnosis of rheumatoid arthritis include elevated erythrocyte sedimentation rate and C-reactive protein, positive rheumatoid factor, positive anti-cyclic citrullinated peptide (CCP) antibody. Further evidence of chronic inflammation includes anemia and hypoalbuminemia.
Radiographs may reveal periarticular osteoporosis, joint space narrowing, erosions, and deformities. Magnetic resonance imaging and ultrasound may be more sensitive in early disease.
Typical sites of osseous erosion of a rheumatoid wrist shown here include triquetrum, pisiform, scaphoid,ulna and radius.
, Boutonnière deformity.
The onset of disease can occur at any age but peak incidence occurs within the fourth and fifth decades of life. The average annual incidence of RA in the United States is 0.5 per 1000 persons per year .The overall prevalence of RA is 1% to 2%, and it steadily increases to 5% in women by the age of 70 (It is important to remember that RA is a systemic disease and individuals may therefore present with symptoms such as fever, weight loss, and fatigue;) however, joint symptoms are usually the most prominent.
Most commonly, the onset of symptoms of joint pain and swelling is insidious, occurring over weeks to months. However, a minority of patients may present with an abrupt explosive onset polyarthritis.
Still others may present with transient self-limited episodes of mono- or polyarthritis lasting days to weeks. This presentation is known as palindromic rheumatism. Approximately 50% of patients with palindromic rheumatism will go on to develop (i.e., fulfill criteria for) RA, and only 15% remain symptom-free after 5 years.
Occasionally RA may present as a monoarthritis; however, infectious and crystalline etiologies should always be ruled out first when inflammation affects a single joint. Rheumatoid arthritis is the most common form of inflammatory arthritis that affects diarthrodial joints.
In early disease,the wrists ,metacarpophalangeal (MCP) joints, proximal interphalangeal (PIP) joints of the fingers, interphalangeal joints of the thumbs, and metatarsalphalangeal (MTP) joints are most commonly affected. As the disease progresses, larger joints such as the ankles, knees, elbows, and shoulders frequently become affected.
Extra-Articular manifastations
Skin :Rheumatoid nodules (25%–50%)Hematologic :Normocytic normochromic anemia (25%–30%), thrombocytosis, thrombocytopenia,a lymphadenopathya Felty’s syndrome: (Splenomegaly with neutropenia, large granular lymphocytes, thrombocytopenia)
Hepatic :Nonspecific transaminitis
Pulmonary :Pleural thickening, pleural effusions,pulmonary nodules, diffuse interstitial lung disease, BOOP, Caplan’s syndrome, cricoarytenoid arthritis (pulmonary arteritis, PAH, shrinking lunga)
Cardiac :Pericarditis, accelerated atherosclerotic disease, valvulitis
Ophthalmologic :Keratoconjunctivits sicca (10%–15%),episcleritis, scleritis, uveitis,ulcerative Keratitis
Neurologic :Peripheral entrapment neuropathy, cervical myelopathy due to cervical spine Subluxation
Muscular :Muscle atrophy, inflammatory myositis.
Renal :Low grade membranous glomerularnephropathy, reactive amyloid
Vascular: Small vessel vasculitis, systemic vasculitis
LABORATORY FINDINGS
The most commonly used inflammatory biomarkers in clinical practice are the ESR and CRP. These markers are usually, but not always, elevated in RA patients with active disease and decline with treatment. Thus, the two inflammatory markers can be followed along with the patients’ symptoms and joint examination to monitor disease activity over time. High ESR and CRP at the onset of disease are predictive of more aggressive disease and potentially worse prognosis.The treatment goal in RA is early and effective control of synovitis to prevent joint damage, disability, and secondary consequences of chronic inflammation such as cardiovascular disease.
Disease-modifying drugs, such as methotrexate, should be initiated within the first 3 to 6 months of disease and, in most cases, effective control of disease activity will require more than one medication.
If RA cannot be controlled with one or more conventional therapies, biologic treatments such as anti– tumor necrosis factor (TNF) drugs should be used.
2010 ACR / EULAR Rheumatoid Arthritis Classification Criteria
Joint involvement, designating the metacarpophalangeal joints, proximal interphalangeal joints, the interphalangeal joint of the thumb, second through fifth metatarsophalangeal joint and wrist as small joints, and shoulders, elbows, hip joints, knees, and ankles as large joints: Involvement of 1 large joint gives 0 pointsInvolvement of 2-10 large joints gives 1 point
Involvement of 1-3 small joints (with or without involvement of large joints) gives 2 points
Involvement of 4-10 small joints (with or without involvement of large joints) gives 3 points
Involvement of more than 10 joints (with involvement of at least 1 small joint) gives 5 points
serological parameters – including the rheumatoid factor as well as ACPA – "ACPA" stands for "anti-citrullinated protein antibody": Negative RF and negative ACPA gives 0 points
Low-positive RF or low-positive ACPA gives 2 points
High-positive RF or high-positive ACPA gives 3 points
acute phase reactants: 1 point for elevated erythrocyte sedimentation rate, ESR, or elevated CRP value (c-reactive protein) duration of arthritis: 1 point for symptoms lasting six weeks or longer
Disease Activity Indices A formula incorporating selected clinical and laboratory variables has been derived to produce a disease activity score (DAS28), which is calculated from the number of tender and swollen joints (28-joint count),
patient self-assessment of disease activity (visual-analog scale), and ESR or serum CRP level.
This formula has been applied in clinical practice to monitor disease activity and guide treatment decisions and is increasingly being used as an endpoint in clinical trials.
There has been a growing emphasis on diagnosing and treating RA early and intensively due to the recognition that disability and damage rapidly accoure during the first several years of the disease.
DMARDs are a diverse group of therapeutic agents that reduce the signs and symptoms of RA as well as retard radiographic progression of joint damage. This class of drugs is central to the control of RA, and is part of nearly every patient’s treatment regimen.
The ability of a drug to slow disease progression or produce a disease-modifying effect is that property which defines it as a DMARD. The biologics are structurally engineered versions of natural molecules (e.g., monoclonal antibodies) designed to specifically target pathogenic mediators of joint inflammation and damage.
Disease-ModifyingAntirheumatic Drugs
The initiation of DMARD therapy within the first 3 to 6 months of disease onset is now the standard of care for RA. The most common DMARD of choice in this setting is methotrexate (MTX) because of its proven clinical benefits and well-understood long-term efficacy and toxicity profile.Moreover, MTX may be combined effectively with most other DMARDs, making it a highly adaptable drug. Alternatively, sulfasalazine (SSZ) and hydroxychloroquine (HCQ) may be employed for the treatment of patients with milder forms of RA.
In early disease, corticosteroids may be used to provide rapid control of the signs and symptoms of RA and serve as a bridge between the initiation of DMARD therapy and its onset of action, which is often delayed by a few months.
METHOTREXATE
the mainstay of DMARD therapy for RA is methotrexate (MTX). MTX inhibits dihydrofolate reductase, an enzyme needed for DNA synthesis.Its therapeutic action was originally thought to be due to suppression of lymphocyte proliferation.
However, MTX’s mechanism of action is most likely due to its anti-inflammatory effects, although the specific mechanisms remain unclear. Inside cells, MTX is converted to a polyglutamated form that inhibits the enzyme 5- aminoimidazole-4-carboxamidoribonucleotide (AICAR) transformylase.
MTX also has been reported to inhibit neovascularization, neutrophil activity and adherence, interleukin (IL) 1 and IL-8 production by stimulated peripheral blood mononuclear cells, and TNF production by stimulated peripheral T cells.
Methotrexate can be taken orally or by subcutaneous injection. Generally, the oral form of MTX is initiated for convenience, but may be switched to the subcutaneous route to improve gastrointestinal tolerability as well as bioavailability.
Initial doses of MTX range from 7.5to 15 mg weekly and may be escalated to a maximum dose of 25 mg weekly to yield maximal disease control. Weekly MTX therapy has been shown in randomized, controlled trials to reduce the signs and symptoms of RA and slow its rate of radiologic progression.
Importantly, women of childbearing age must use appropriate contraceptive measures because of the known teratogenic effects of MTX. Because MTX is partially eliminated through the kidney, this DMARD is generally avoided in patients with a serum creatinine of greater than 2.0 mg/dL.
Suppression of bone marrow occurs more commonly if renal insufficiency is present. In addition, MTX may cause an increase in serum transaminases and, rarely, liver fibrosis. Periodic laboratory monitoring of complete blood counts and liver enzymes are recommended in all patients taking MTX .and it should be used with folic acid.
LEFLUNOMIDE
represents an alternative oral agent to MTX. It inhibits an enzyme involved in pyrimidine synthesis, orotic acid dehydrogenase.
Leflunomide is taken once a day orally, in doses of 10 or 20 mg. Leflunomide’s active metabolite has a long half-life of 15 to 18 days, which is a notable feature of its pharmacokinetics. Its proven to reduce structural damage.
Its use is limited to some extent by gastrointestinal side effects and potential for teratogenicity. Similar to MTX, leflunomide therapy has been associated with elevated serum transaminases and should be monitored by regular liver enzyme testing.
HYDROXYCHLOROQUINE AND SULFASALAZINE
They are typically used to treat milder forms of RA and in combination with other DMARDs.The mechanism of action of HCQ is not well understood but may, in part, be due to the fact that it concentrates inside cells, principally within acidic cytoplasmic vesicles.
In lysosomes, accumulation of HCQ raises the intravesical pH and may thereby interfere with the processing of autoantigenic peptides .
The clinical efficacy of HCQ therapy has been shown in a randomized, controlled trial of patients with relatively mild disease of less than 5 years’ duration . To date, no studies have shown that HCQ alone can decrease the rate of structural damage in RA.
Sulfasalazine was initially designed as a drug that linked an antibiotic, sulfapyridine, with an anti-inflammatory agent, 5-aminosalicyclic acid (5-ASA), which was based on a belief many decades ago that RA was an infectious disease.
Approximately 30% of SSZ is absorbed from the gastrointestinal (GI) tract. The remainder is degraded in the gut to sulfapyridine and 5-ASA. Whereas the bulk of the sulfapyridine is absorbed from the gut, most 5-ASA is excreted in the feces.
SSZ suppresses various lymphocyte and leukocyte functions and, like MTX, inhibits AICAR transformylase, resulting in extracellular adenosine release
Steroid (systemic) is not indicated in patients wit R.A except in specific situations .
Pulse steroid is used in active disease to control te disease rapidly.Local steroid injection is indicated to control one joint .
OTHER ANTIRHEUMATICMEDICATIONS
Several well-designed controlled trials attest to the clinical efficacy of minocycline and doxycyline for the treatment of RA, but they appear to be suited primarily for mild disease.Large trials have not been performed using these agents, and they are not approved drugs for the treatment of RA.
The mechanisms by which tetracyclines exert their ameliorating effects are unknown, but they have been shown in vitro to inhibit collagenase activity and nitric oxide production. In addition, minocycline upregulates the synthesis of IL-10, an antiinflammatory cytokine. Minocycline and doxycycline have been shown to decrease the signs and symptoms of RA,
Gold compounds are seldom used now because of their frequent toxicity and the availability of other agents with better tolerability.
There are two parenteral gold formulations, gold sodium malate and myochrysine, and an oral compound, auranofin.
Treatment with injectable gold and methotrexate produce similar response rates in clinical trials but gold therapy has higher rates of drug discontinuation due to toxicity .
Auranofin has fewer side effects than gold injections, but has had limited use in clinical practice due to slow onset of action, lack of sustained clinical efficacy, and poor gastrointestinal tolerability.
In clinical trials, cyclosporine has been shown to reduce the signs and symptoms of RA, as well as slow the development of joint erosions. Cyclosporine has been shown to produce incremental clinical benefit in combination with MTX therapy .
The microemulsion- based formulation of cyclosporine (NeoralTM) has higher oral bioavailablitiy and more predictable absorption than the standard form.
Cyclosporine’s effectiveness may be due to its biologic activities of inhibiting IL-2 production and the proliferation of activated T cells. Its renal side effects have been a major limiting factor in long-term use.
Biological agents
TUMOR NECROSIS FACTOR (TNF)ANTAGONISTSEtanercept, infliximab, and adalimumab are TNF inhibitors approved for the treatment of RA. These biologic agents have revolutionized the treatment of RA because of their substantial benefits on the signs and symptoms of this disease,
as well as their ability to significantly retard the radiographic progression of joint damage. These drugs were engineered to specifically inhibit TNF, which is a critical mediator of joint inflammation. TNF has been shown to be a pivotal proinflammatory cytokine that regulates the production of other proinflammatory cytokines, such as IL-1 and IL-6.
Etanercept is a soluble receptor fusion protein that binds to soluble TNF, neutralizing its biologic activities. Infliximab is a chimeric monoclonal antibody that binds to both soluble and membrane-bound TNF, whereas adalimumab is a fully human monoclonal antibody with binding properties similar to infliximab.
Etanercept and adalimumab are administered as a subcutaneous injection while infliximab is administered as an intravenous infusion. Clinical trials indicate that all of these TNF blockers, when added to MTX, produce incremental response rates.
Although etanercept and adalimumab can be used as monotherapy, the combination of MTX and a TNF blocker appears to be the most effective regimen for preventing radiographic progression of disease.
While TNF blockers have proven to be clinically efficacious in RA, their use has been associated with side effects, some of which may have serious consequences.
Etanercept and adalimumab have caused injection site reactions but they are rarely severe enough to limit therapy. Infliximab has been associated with infusion reactions, which can range from rash to urticaria and fever, and rarely to anaphylaxis.
Neutralizing antibodies can develop in infliximab-treated individuals that may inhibit the efficacy of the drug and predispose to infusion reactions.
There is also an increased risk of serious bacterial and opportunistic infections, especially reactivation of latent tuberculosis. A long-term study of etanercept therapy for RA showed that the rate for serious infection was 4.2 per 100,000 patient years, which remained relatively stable throughout the time period
reactivated TB have been reported with infliximab therapy. There have also been cases of TB reported with the use of adalimumab.
Use of anti-TNF agents may also confer an increased risk for lymphoproliferative disorders, namely lymphoma. malignancies, including nonmelanoma skin cancers, suggesting a possible relationship between TNF blockers and an increased risk for solid tumors
Other rare side effects of note include demyelinating disorders and drug-induced lupus reactions. The anti-TNF agents should not be used in patients with New York Heart Association (NYHA) class III to V heart failure because these drugs may exacerbate heart failure.
ANAKINRA
Anakinra is a human recombinant anti–IL-1 receptor antagonist that has been approved for the treatment of RA.It is administered as a daily 100 mg subcutaneous injection and has been shown to improve the signs and symptoms of RA. the use of anakinra in RA has been limited to selective patients with refractory disease.
ABATACEPT AND RITUXIMAB
Abatacept and rituximab are among the recent additions to the biologics available for the treatment of moderate-to-severe RAThey are currently approved for patients with active RA who have had an inadequate response to other DMARDs or have failed treatment with an anti-TNF agent.
Abatacept binds to CD80/CD86 on the surface of antigen-presenting cells, thus preventing their binding to CD28 on T cells. Blockade of CD28 binding prevents the so-called second signal of T-cell activation. taking a combination of both abatacept and methotrexate,
rituximab is a chimeric anti-CD20 monoclonal antibody now approved for the treatment of moderateto- severe RA.
Rituximab depletes B cells that have CD20 on their surface. Its mechanism of action is incompletely understood but may involve inhibition of T-cell activation through reduction of antigen presentation by B cells or reduction of B-cell cytokines.
Despite the depletion of peripheral B cells by more than 97%, immunoglobulin levels usually remain within the normal range.
RF may decline; however, clinical improvement often starts before the RF titers decline. Rituximab is infused at a dose of 1000 mg and repeated 2 weeks later.
COMORBIDITIES
Osteoporosis is a major comorbidity in RA and can result from both the disease itself and the use of corticosteroids. Most patients are routinely advised to take calcium and vitamin D to prevent osteoporosis.
Cardiovascular (CV) disease is the number one cause of death in RA patients. Indeed, RA itself is a CV risk factor. It is unclear if intensive treatment of RA influences this risk, though available data suggest that MTX and anti-TNF treatment reduces the rate of CV events. Low dose aspirin should be considered in patients over the age of 50 years as primary prevention for CV disease.
Cholesterol levels should be regularly monitored and cholesterol-lowering medications prescribed as needed. Other CV risk factors, such as hypertension, diabetes, and obesity, should be treated according to usual recommendations.