
1
Analgesics
The terms analgesics and analgetic drugs are often used interchangeably to
describe a diverse group of pain medications such as opioids, nonsteroidal
anti-inflammatory drugs (NSAIDs), and triptans, each with very different
mechanisms of action for relieving pains of a wide array of causes.
Opioids- drugs that produce morphine-like effects that can be blocked by
opioid antagonists. Opioids do not necessarily have to resemble morphine
structurally.
Opioid Receptors
Pharmacological studies have shown that opioids produce their effects
by binding to specific receptors.
These receptors are located on the membranes of certain cells in the
CNS, on nerve terminals in the periphery, and on cells of the GI tract.
The opioid receptor types are:
Mu (
) receptors - Binding produces:
Analgesia
Euphoria
respiratory depression
miosis
reduced GI tract motility
Kappa (
) receptors - binding produces:
Analgesia
dysphoria
sedation
less intense miosis and respiratory depression than
-receptors

2
Delta (
) receptors - binding produces:
dysphoria
psychotomimetic effects (hallucinations, etc.)
Therapeutic Uses
The major uses of opioids include:
Analgesia - for pain control
Cough - antitussive properties
Antidiarrheal
Endogenous Opioid Peptides
While opioid drugs do stimulate the opioid receptors, it was though that
these were not the endogenous ligands for these receptors. The first
endogenous peptide was termed enkephalin, which was found to be a
mixture of the two pentapeptides that only differ in their terminal amino
acid.
Tyr-Gly-Gly-Phe-Met (met-enkephalin)
Tyr-Gly-Gly-Phe-Leu (leu-enkephalin)
Phe = phenylalanine; Met = methionine
The transient nature of the enkephalins’ actions correlated with the rapid
degradation of the enkephalin Tyr-Gly bond by aminopeptidases. Much
synthetic work has been done in an attempt to increase the duration of action
of the opioid peptides and maintain their analgesic effect.

3
SARs of Enkephalins
TYR
1
Most changes to this amino acid, either by substituting with other amino
acids or masking the phenolic hydroxyl (OH) or amino function produce an
inactive or weakly active peptide.
GLY
2
Replacing the naturally occurring L-Gly with various D-amino acids
produces a peptide that is resistant to peptide cleavage by aminopeptidases.
GLY
3
Almost all changes to this amino acid result in a drop in potency, unless they
are also accompanied by another change such as replacing the Gly
2
with D-
Ala
2
as described above.
PHE
4
The aromatic nature of the fourth residue is required for high activity. When
combined with the D-Ala
2
replacement, the addition of an electron
withdrawing substituent (e.g., NO
2
, Cl, and Br) in the para position of Phe
4
greatly increases activity. Para substitutions with electron donating groups
(e.g., NH
2
and OH) abolish activity.
MET
5
/LEU
5
Position 5 appears to tolerate more residue changes than the other positions.
Even the loss of the fifth residue to yield the tetrapeptide Tyr
1
-Gly
2
-Gly
3
-
Phe
4
maintains weak activity.
4
Structure-Activity Relationships of the Opioid Agonists
They possess within their structure tertiary nitrogen with the group on N
being relatively small (usually methyl).
A central quaternary carbon (a carbon with no attached hydrogens - 4
substituents).
A phenyl group (or phenyl isostere) connected to (or one carbon removed
from) the quaternary carbon.
A 2-carbon chain separating the quaternary carbon from the tertiary
nitrogen.
The size and shape of the molecule is also very important (for example large
groups on the nitrogen lead to antagonist properties).
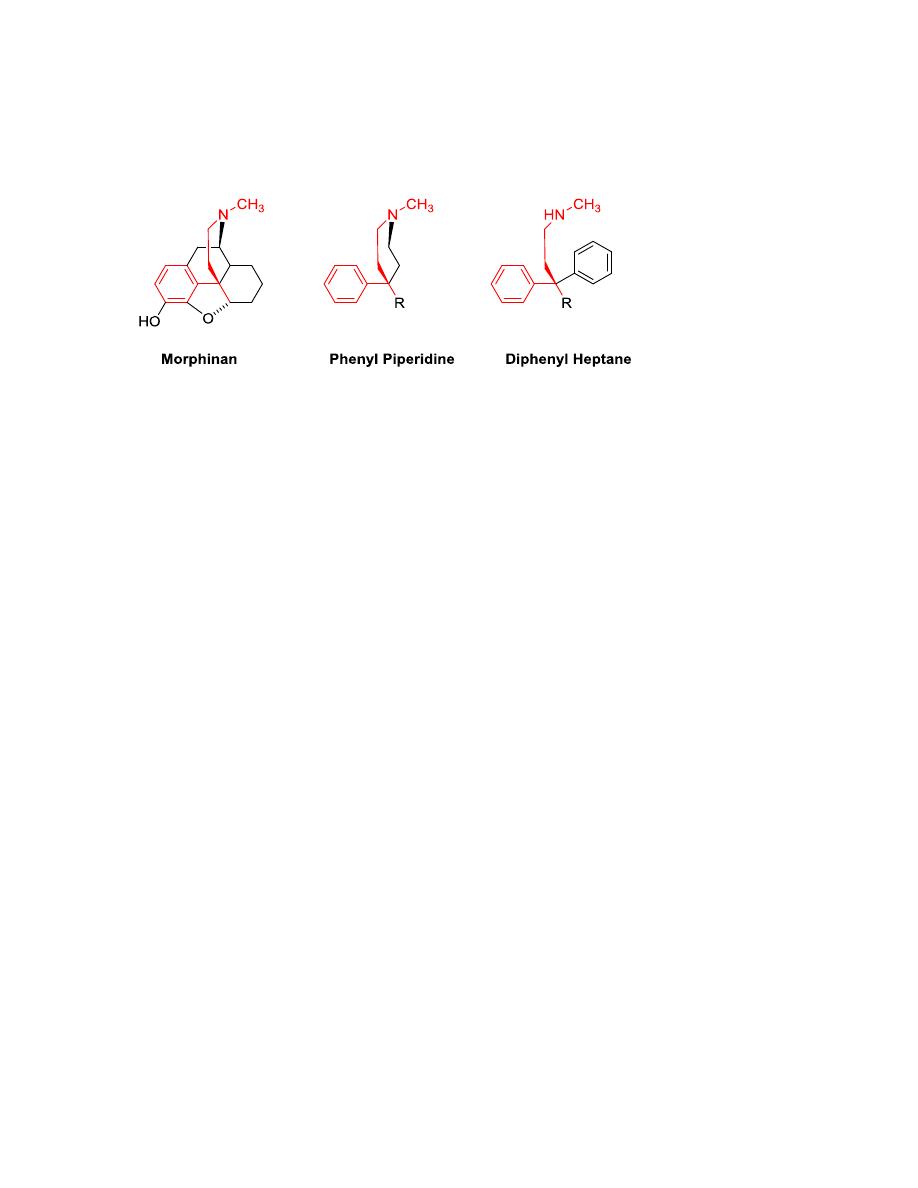
Opioid drugs
Opioids can be classified according to their chemical structure into:
a- 4,5-epoxymorphinan
b- morphinan
c- benzomorphan
d- 4-phenyl/4-anilido piperidines
e- Diphenylheptanes
5
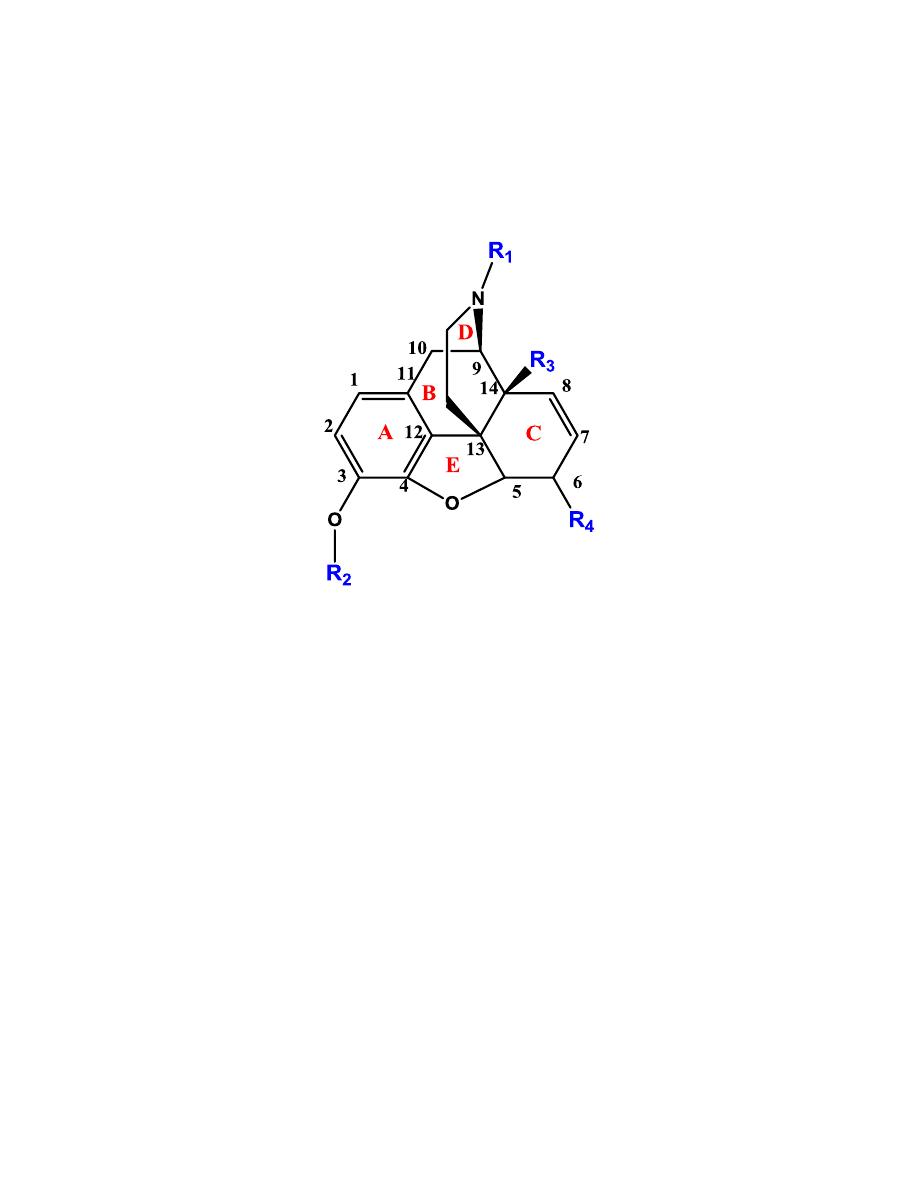
a- 4,5-epoxymorphinan
Ring E is not necessary for activity, but lends rigidity to the overall
structure when present.
R
1
cannot be H (the amine must be tertiary). This nitrogen needs to
be charged to interact with the receptors. Methyl gives agonist
activity. R
1
≥ 3 carbons (cyclopropylmethyl
or cyclobutylmethyl or
-CH
2
-CH=CH
2
) shifts the activity to antagonism at the μ-receptor.
R
2
is either H or CH
3
. Binding at μ- and κ-receptors requires R
2
to be
H (a phenol), thus the 3-methoxy compounds (like codeine) have
weaker analgesic activity.
R
3
is either H or OH. OH gives enhanced analgesic activity.
R
4
is OH or =O (ketone). Ketone derivatives have stronger analgesic
activity.
6
3,6 diacetylated form of morphine (Heroin) shows greater activity.
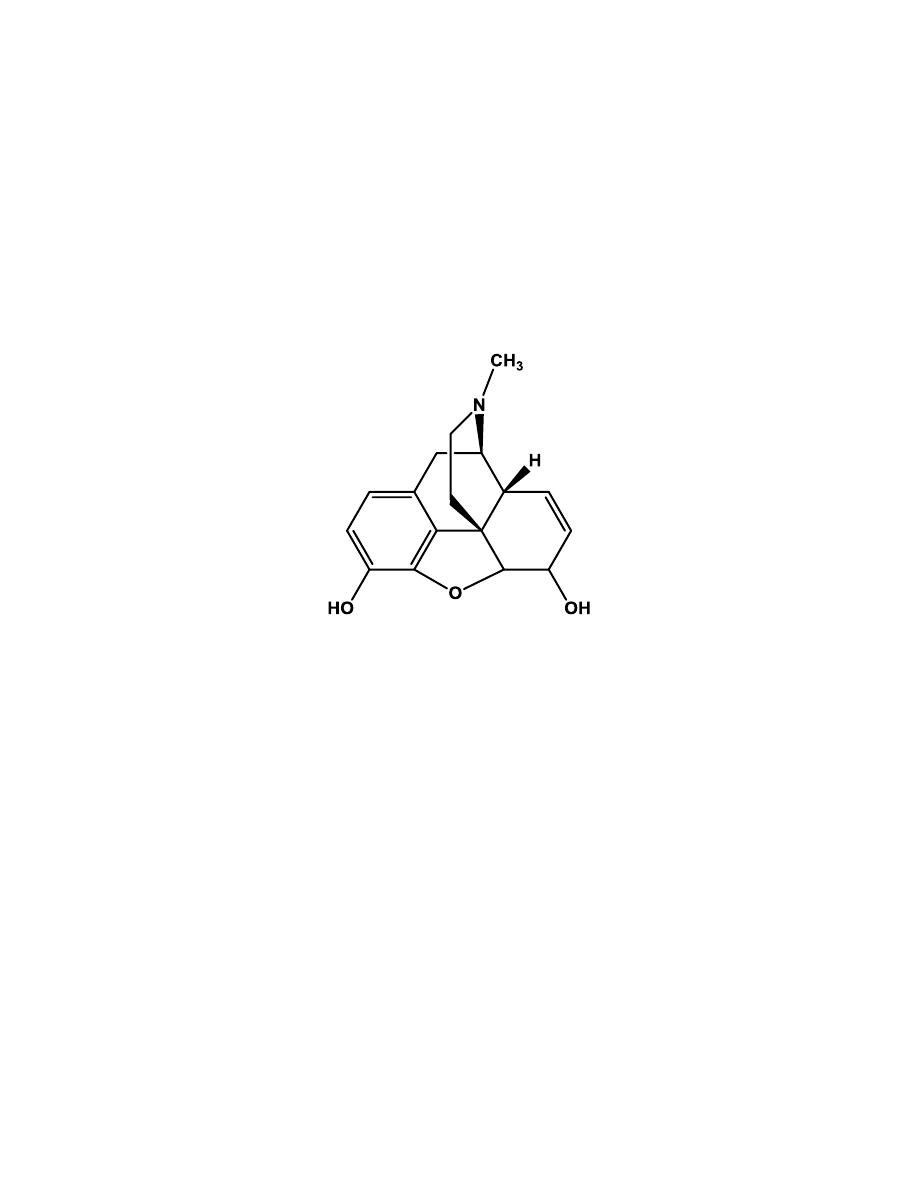
Morphine
Morphine is the prototype µ-receptor agonist; it is the drug to which all other
µ-agonists are compared.
Morphine contains 5 chiral centers and has 16 optical isomers (not 32
because of the restriction of C-9 to C-13 ethanamino bridge). The naturally
occurring, active form of morphine is the levorotatory enantiomorph.
The x-ray determined conformation of morphine is a “T” shape with the A,
B, and E rings forming the vertical portion, and the C and D ring forming
the top.
7
Morphine is extensively metabolized via phase II conjugation to morphine-
3-glucuronide morphine-6-glucuronide and, to a lesser extent, the N-
demethylated metabolite.
The endogenous synthesis of morphine in human has been described. The
genes and enzymes involved in morphine biosynthesis may become targets
for novel drugs used to treat pain.
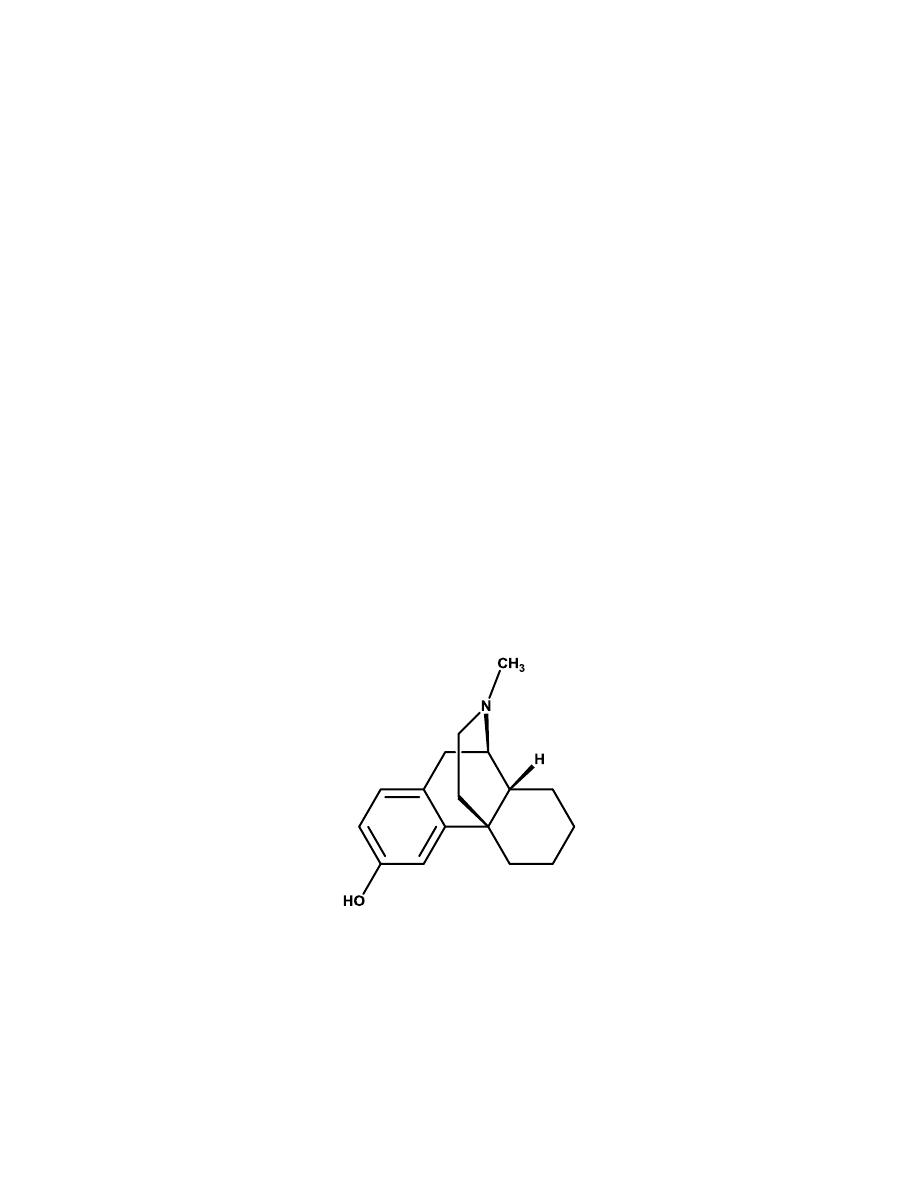
Codeine
8
The general pharmacological action of codeine is similar to that of
morphine, but it does not possess the same analgesic potency. The decreased
potency also leads to a lower addiction potential compared with morphine.
Approximately 5% of codeine is metabolized to morphine via O-
demethylation.
The analgesic component of codeine has long been assumed to be the O-
demethylated metabolite, morphine. If codeine has no analgesic potency
itself, then patients who lack this enzyme should have no analgesic effect to
administered codeine. This has been shown not to be the case, so codeine
itself may posses analgesic activity, or codeine-6-glucuronide may be the
active analgesic.
Codeine’s role as an effective antitussive agent has been questioned. Some
literatures show that codeine is no more effective than placebo for acute
cough in children or adults.
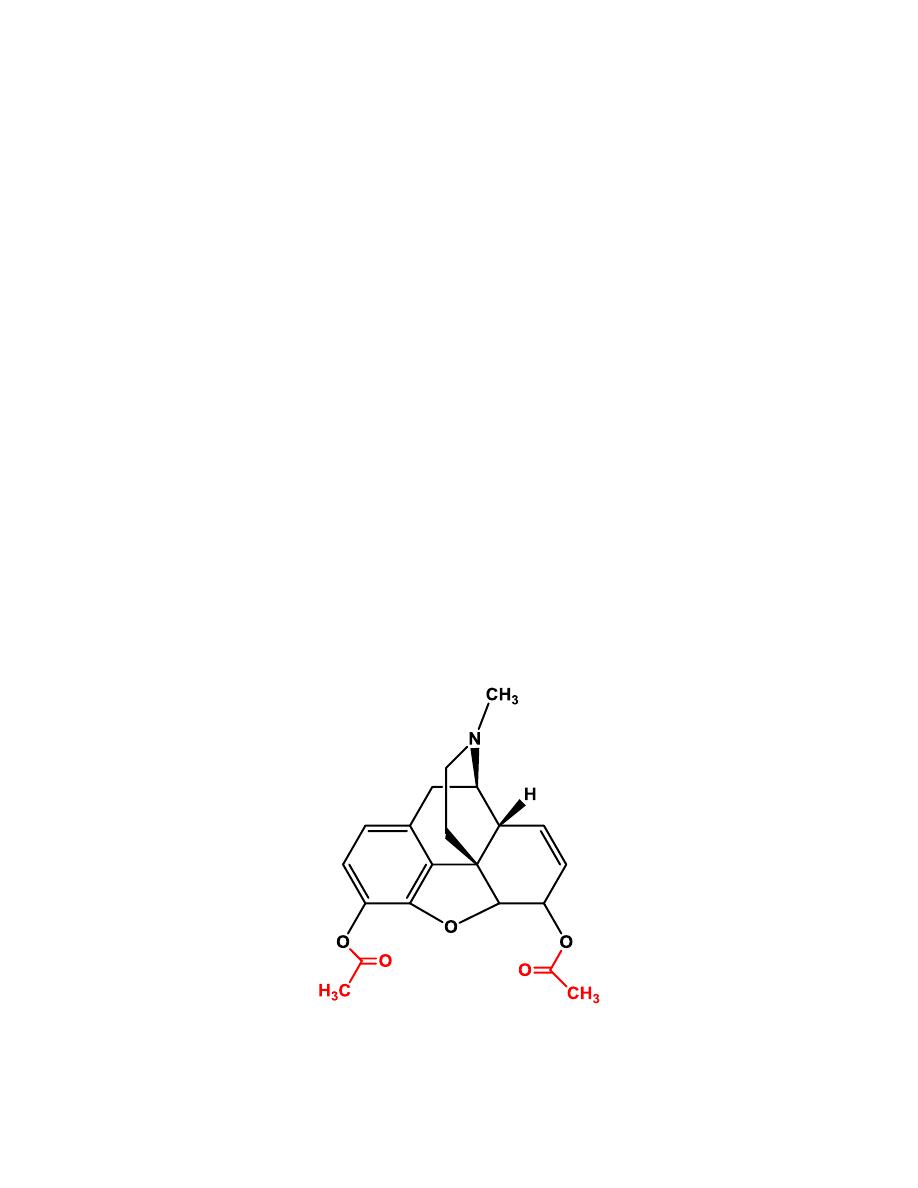
Heroin
Heroin is the 3,6 diacetylated form of morphine. It was believed that heroin
would be an effective analgesic with no addictive properties. This was
9
unfortunately not the case. With both OH groups protected as an ester,
heroin can pass through the blood-brain barrier quicker than morphine and
lead to the euphoric “rush” that becomes so addictive to addicts.
Heroin is not available as a prescription product in most countries.
b- Morphinans
The morphinans were made by removing the E ring of morphine, the 4,5-
ether bridge, in an attempt to simplify the structure.
Levorphanol
It is the levorotatory isomer and is approximately 7.5 times more potent than
morphine orally. The loss of the 4,5-epoxide and the 7,8-double bond allows
levorphanol greater flexibility and presumable leads to the increased binding
affinity at all opioid receptor subtypes compared with morphine.
c- Benzomorphans
Further structural simplification of the morphine ring system by removing
the C ring of the morphinan structure yields the benzomorphans.
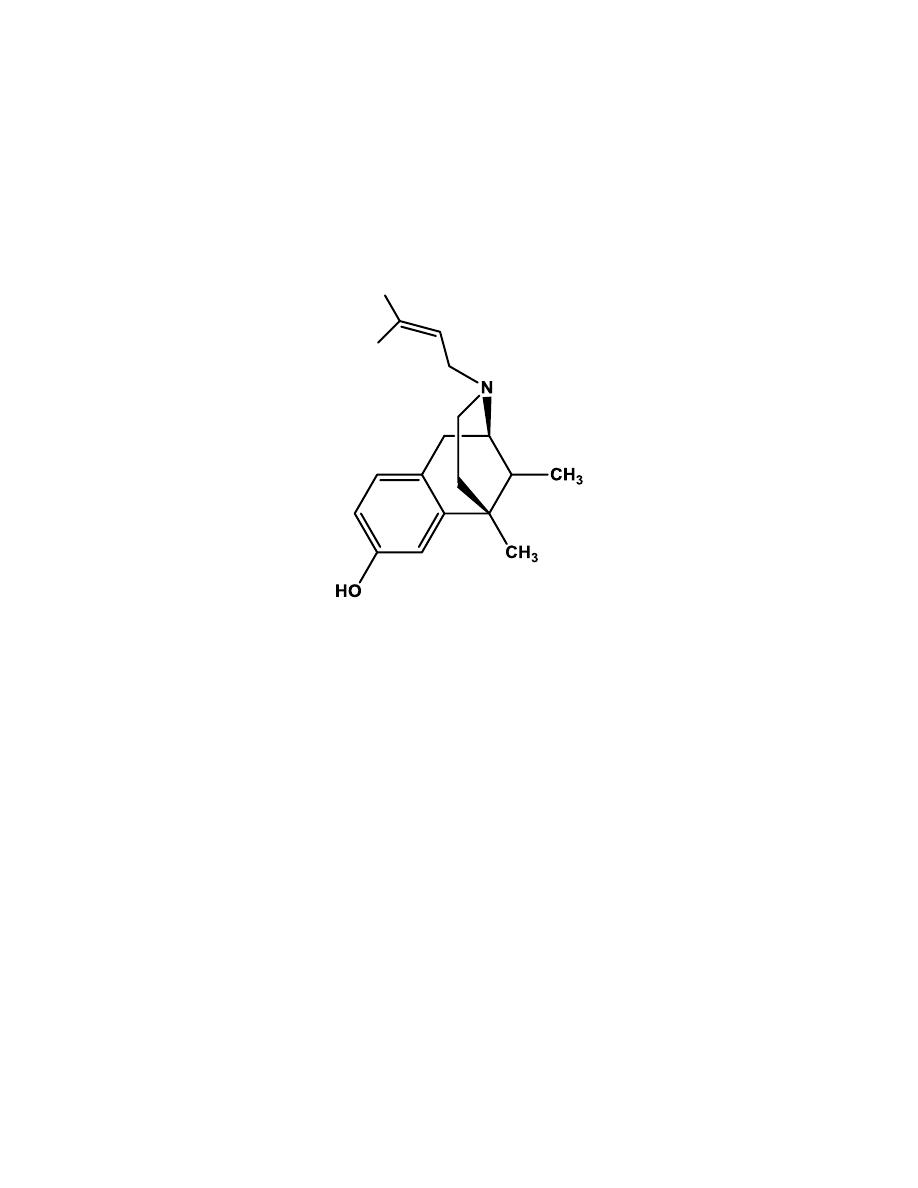
10
Pentazocine
It is the only benzomorphan in clinical use.
At the µ-receptor, pentazocine is
a partial agonist and a weak antagonist.
Pentazocine is also an agonist at the
κ-receptor.
d- 4-Phenylpiperidines and 4-Anilidopiperidines
Further structural simplification of the benzomorphan ring system, via
removal of the B ring of the benzomorphans yields the 4-substituted
piperidines. The resultant structures are flexible and, without the B ring
locking the A ring in an axial position relative to the piperidine (D) ring, the
A ring can exist in either an axial or an equatorial position.
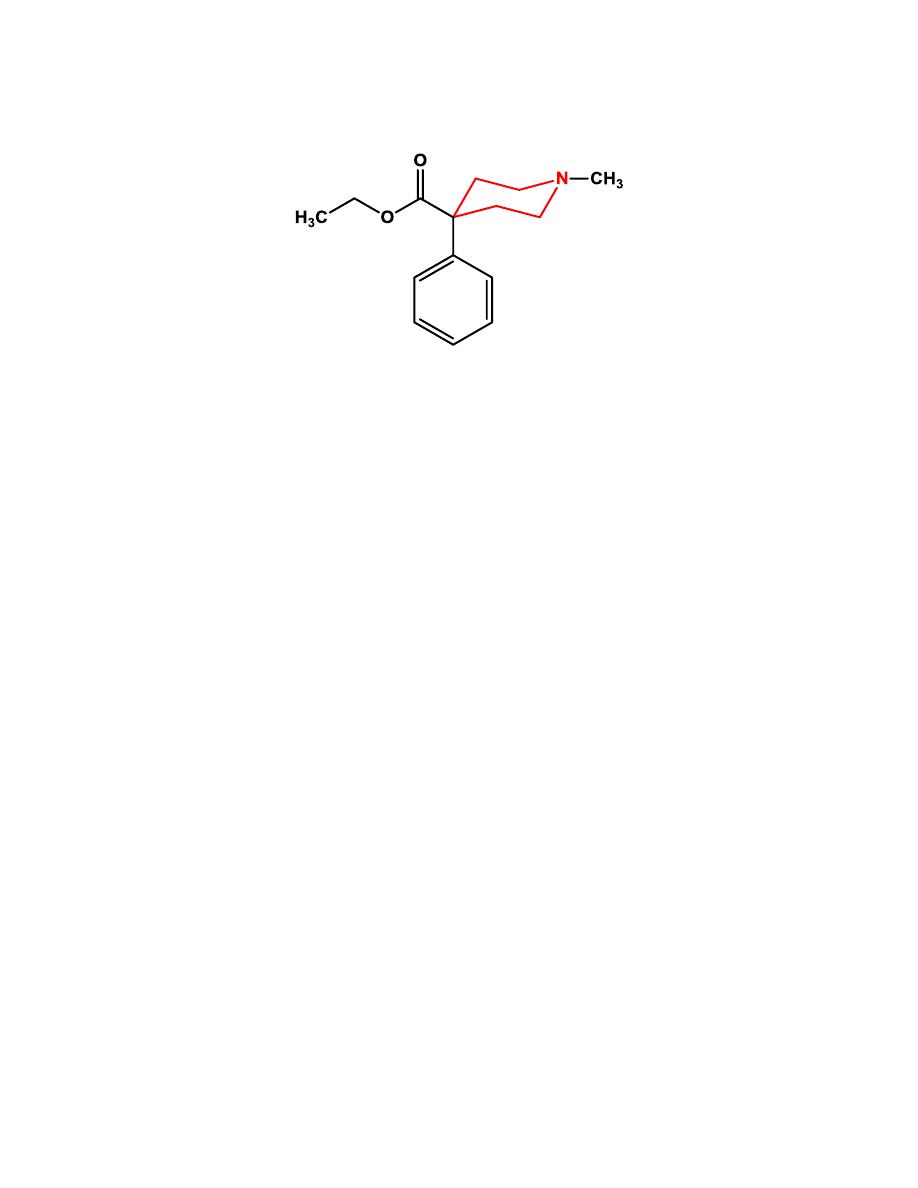
11
Meperidine
Meperidine was found to have low potency at the receptor compared with
morphine but much higher penetration into the brain resulting in a
compound with about 10% of the potency of morphine.
The 4-ethyl ester
was found to be the optimal length for analgesic potency. Increasing or
decreasing the chain length decreased activity.
DIPHENOXYLATE
Diphenoxylate is a weak opioid agonist and is available combined with
atropine (Lomotil) for use as an antidiarrheal agent.
LOPERAMIDE
Loperamide is sufficiently lipophilic to cross the blood-brain barrier, yet it
displays no CNS-opioid effects. The reason for this is that it is actively
pumped out of the brain via the P-glycoprotein pump.
It is used for treatment
of acute and chronic diarrhea.
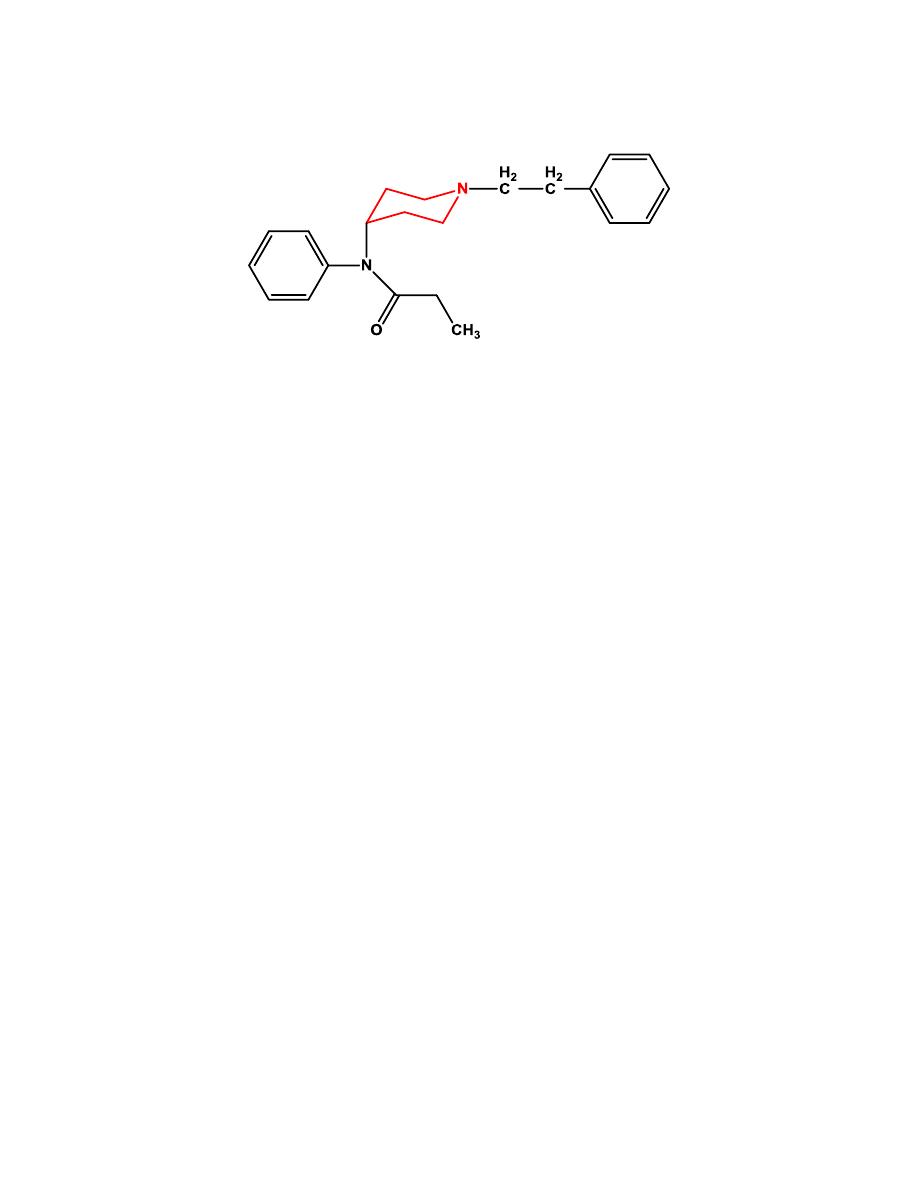
12
Fentanyl
When the 4-phenyl substituent of meperidine was replaced with a 4-aniline
with a nitrogen connection, the potency increased.
Fentanyl was found to be
almost 500 times more potent than meperidine.
The SAR studies of the 4-phenylpiperidine analgesics found that the
propionamide is the optimal chain length. Adding polar groups to the 4-
piperidine carbon increases potency.
e- Diphenylheptanes
Methadone
It is a synthetic opioid approved for analgesic therapy and for the treatment
of opioid addiction.
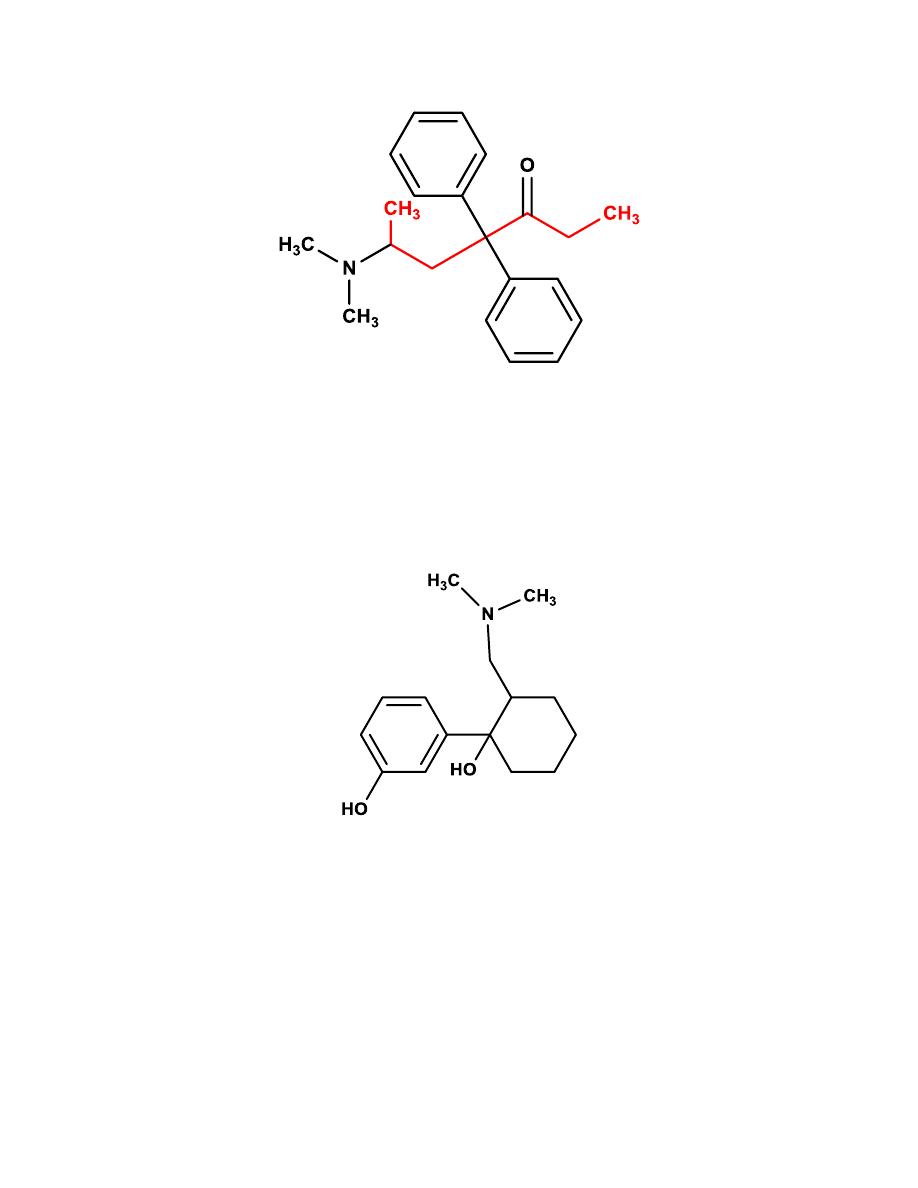
13
f- Miscellaneous
Tramadol
Structurally, tramadol resembles codeine with
the B, D, and E ring removed.
It is a weak µ-agonist with approximately 30% of the analgesic effect
antagonized by the opioid antagonist. It has low abuse potential.
Mixed Agonist/Antagonist
Nalbuphine
It is structurally resembles oxymorphone with a cyclobutyl methyl group on
the nitrogen. Nalbuphine has agonist activity at the κ-receptor and antagonist
activity at the µ-receptor.
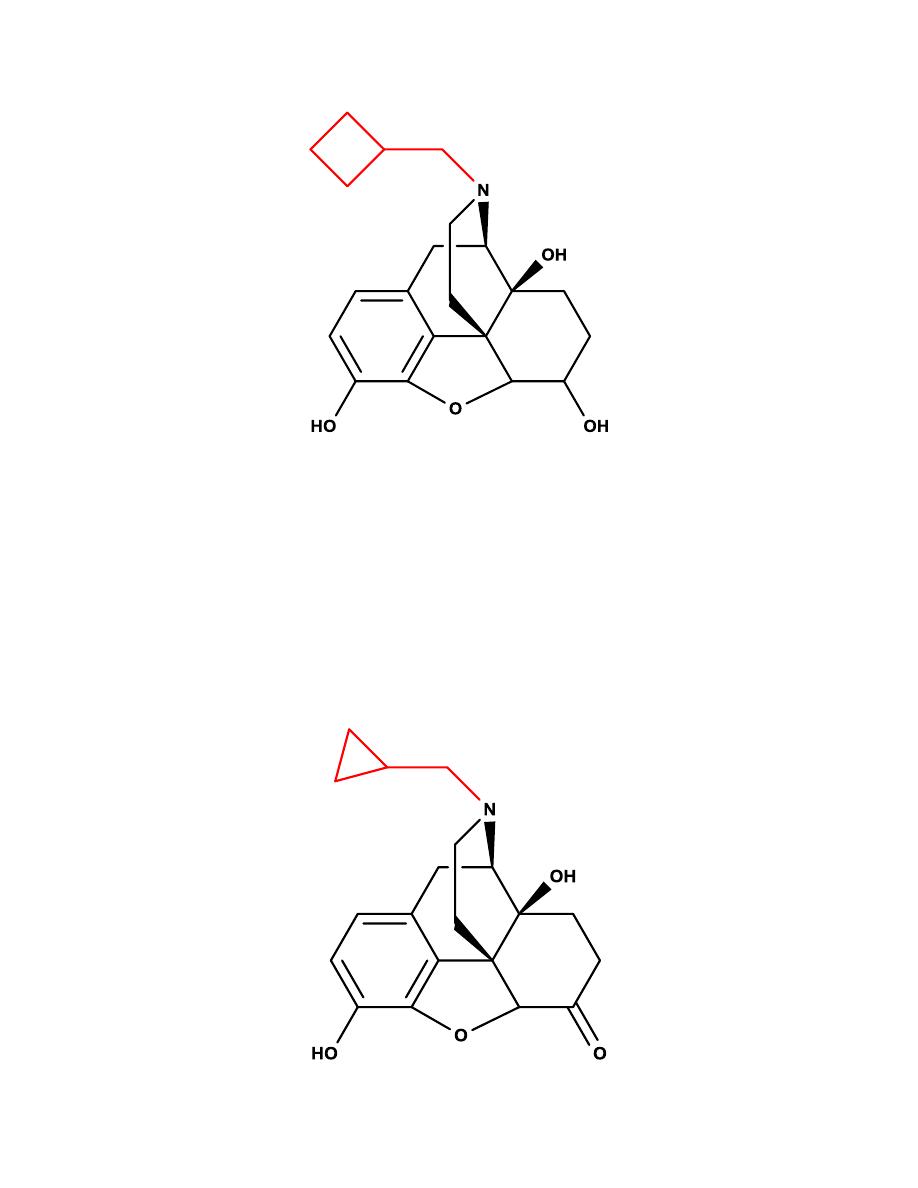
14
Opioid Antagonists
Naltrexone
It is a pure opioid antagonist at all opioid receptor subtypes with the highest
affinity for the µ-receptor. It is used to reverse the effects of opioids after
general anesthesia and in the treatment of overdose.
