8-11-2015 Parasitology د.اكرام الحسو
Lec: 1&2Blood and tissue Protozoa (Haemo-Flagellates)
Two genera within hemoflagellates infect human which are:• Genus Leishmania
• Genus Trypanosoma
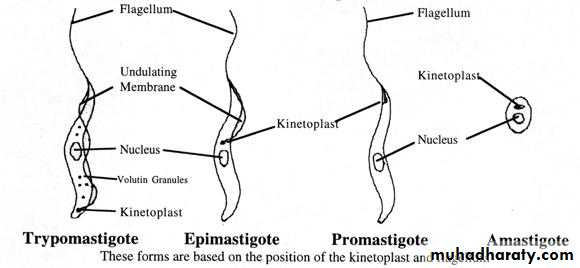
Morphological forms of hemoflagellates
1-Amastigote (Leishmania) formRound or oval in shape, 2-5 microns in diameter, surrounded by delicate cell membrane, have single nucleus with large central karyosome, the kinetoplast (which consists from dot-like blepharoplast and parabasal body beside it) lies at right angle to the nucleus. This form (amastigote) has no flagellum.
2-Promastigote (leptomonad) form
Elongated (spindle in shape) measuring 15-20 microns X 1-2 microns, have centrally located nucleus and the kinetoplast situated at the anterior end. From blepharoplast, single free flagellum projects from the anterior end, equal or longer than the body length.This form has no undulating membrane.
3-Epimastigote form
Elongated form, 15-20 microns long and slightly wider than promastigote, nucleus near middle, kinetoplast is anterior to the nucleus. From blepharoplast flagellum arises forming the undulating membrane extending half of the body length, and project from the anterior end as a free flagellum.
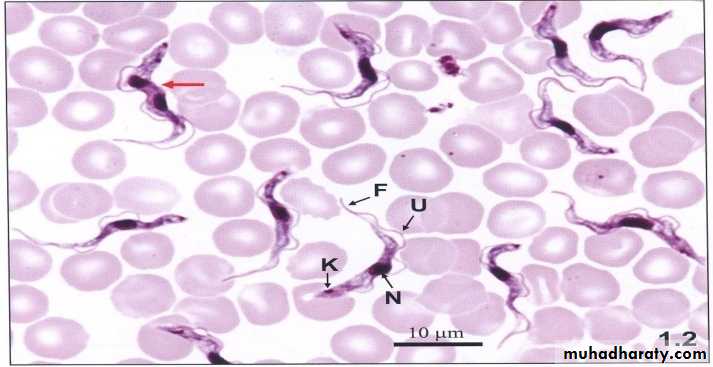
4-Trypomastigote (Trypanosome) form
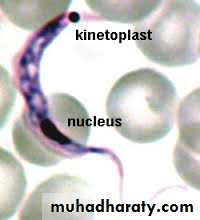
Elongated form (15micron X 2-4micron) .In stained blood film, Trypanosoma cruzi appears as C or U shape. Nucleus near middle, kinetoplast is at the posterior end, the flagellum and undulating membrane pass anteriorly along entire body length and free flagellum extends from anterior end.
Genus Leishmania
Include 4 major speciesLeishmania donovani
Leishmania tropica
Leishmania Mexicana
Leishmania braziliensis
General characters of genus Leishmania
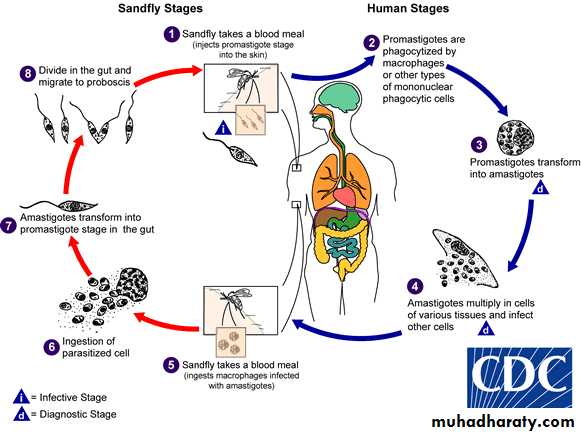
1- Life cycle completed in two hosts, vertebrate (human) as a final host and invertebrate; blood sucking insect (female of sand fly) as an intermediate host (vector).
2- Two developmental forms are found, amastigote(in the final host) and promastigote(in the vector sand fly).
3- The vector is sand fly of genus Phlebotomus in Old World and genus Lutzomyia in New World.
4- Promastigote is the infective stage to final host (man).
5- The parasite multiplies by binary fission (asexual).
Leishmania donovani
L. donovani is the cause of kala-azar (visceral leishmaniasis,black fever).
life cycle
The life cycle involves the sandfly as the vector and a variety of mammals such as dogs, foxes, and rodents as reservoirs. Only female flies are vectors because only they take blood meals (a requirement for egg maturation). When the sandfly sucks blood from an infected host, it ingests macrophages containing amastigotes. After dissolution of the macrophages, the freed amastigotes differentiate into promastigotes in the gut. They multiply and then migrate to the pharynx, where they can be transmitted during the next bite. The cycle in the sandfly takes approximately 10 days.
Shortly after an infected sandfly bites a human, the promastigotes are engulfed by macrophages, where they transform into amastigotes. The infected cells die and release amastigotes that infect other macrophages and reticuloendothelial cells. The cycle is completed when the fly ingests macrophages containing the amastigotes.
Pathogenesis
In visceral leishmaniasis (kala-azar), the organs of the reticuloendothelial system (liver, spleen, and bone marrow) are the most severely affected. Reduced bone marrow activity with cellular destruction in the spleen, results in anemia, leukopenia, and thrombocytopenia. This leads to secondary infections and a tendency to bleed. The striking enlargement of the spleen is due to a combination of proliferating macrophages and sequestered blood cells.
Epidemiology
Kala-azar occurs in the Mediterranean area, the Middle East, southern Russia, and parts of China, Africa, India and and Kenya.
Clinical Findings
Symptoms begin with intermittent fever, weakness, and weight loss. Massive enlargement of the spleen is characteristic. Hyperpigmentation of the skin (kala-azar means black sickness). The course of the disease runs for months to years. As anemia, leukopenia, and thrombocytopenia become more profound, weakness, infection, and gastrointestinal bleeding occur. Untreated severe disease is nearly always fatal as a result of secondary infection.
Post Kala-azar dermal leishmaniasis (PKDL) (Dermal-Leishmaniod)
It is a dermatotropic form of leishmaniasis, it is found in areas where L. donovani is the causative agent of VL and develops after 2-10 years from cure of VL.
Three clinical type of PKDL are encountered:
1- Depigmented macules.
2- Erythmatous pathches.
3- Nodular lesions.
Diagnosis of kala-azar
1- Microscopic detection of amastigotes (LD bodies:Leishman-Donovan) in Giemsa stained smear of bone marrow, spleen and lymph node or liver.
2- Cultivation of aspirates in specific culture medium as NNN (Novy-MacNeal-Nicolle) medium → promastigotes seen.
3- Serological method (detection of specific anti-leishmanial antibodies):
IFAT (indirect immunofluorescent antibody test)
DAT (Direct agglutination test)
ELISA (enzyme linked immunosorbent assay)
4- Molecular method (PCR polymerase chain reaction) the most sensitive and specific diagnostic method.
.
Leishmania donovani—Amastigotes. (nonflagellated form) in cytoplasm of bone marrow cell.
promastigotes in NNN medium
Treatment:
bed rest, high protein &vitamen diet, antibiotics in secondary bacterial infections, blood transfusion in case of anemia. Sodium stibogluconate (Petnostam), Amphotericin B. The mortality rate is reduced to near 5%. Recovery results in permanent immunity.Prevention
Prevention involves protection from sandfly bites (use of netting, protective clothing, and insect repellents) and insecticide spraying.
Leishmania tropica , Leishmania mexicana & Leishmania braziliensis
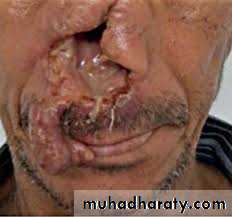
DiseaseL. tropica and L. mexicana both cause cutaneous leishmaniasis; L. tropica is found in the Old World(casing Baghdad boil, oriental sore, Delhi boil, Aleppo boil). whereas L. mexicana is found only in the Americas )new world).
L. braziliensis causes mucocutaneous leishmaniasis)Espundia(, which occurs only in Central and South America.
Important Properties
Sandflies are the vectors for these three organisms, and rodents are their main reservoirs. life cycle of these parasites is the same as that of L. donovani.
Pathogenesis
The lesions are confined to the skin in cutaneous leishmaniasis and to the mucous membranes, cartilage, and skin in mucocutaneous leishmaniasis. A granulomatous response occurs, and a necrotic ulcer forms at the bite site. The lesions tend to become superinfected with bacteria.Epidemiology
Old World cutaneous leishmaniasis (Oriental sore, Baghdad boil, Delhi boil), caused by L. tropica, is endemic in the Middle East (Iraq, Syria, Palestine, Iran, and Jordan), Africa, and India.
New World cutaneous leishmaniasis (bay sore), caused by L. mexicana, is found in Central and South America. Mucocutaneous leishmaniasis (espundia), caused by L. braziliensis, occurs mostly in Brazil and Central America.
Clinical Findings
The initial lesion of cutaneous leishmaniasis is a red papule at the bite site, usually on an exposed extremity. This enlarges slowly to form multiple satellite nodules that coalesce and ulcerate. There is usually a single lesion that heals spontaneously in patients with a competent immune system. However, in certain individuals, if cell-mediated immunity does not develop, the lesions can spread to involve large areas of skin. Mucocutaneous leishmaniasis)Espundia( begins with a papule at the bite site, but then metastatic lesions form, usually at the mucocutaneous junction of the nose and mouth. Disfiguring granulomatous, ulcerating lesions destroy nasal cartilage but not adjacent bone. These lesions heal slowly. Death can occur from secondary infection.
Diagnosis
1- Usually made in endemic areas on clinical grounds
2- Microscopic detection of amastigotes (L.D. bodies) within large monocytic cells in Giemsa stained smear obtained by aspiration of fluid from beneath the ulcer bed, especially its active borders. Scraping taken from the ulcer surface does not reveal the organisms, which are destroyed in areas secondarily infected with bacteria.
3- Culture: on NNN media will demonstrate promastigote forms.
4- Animal inoculation: aspirate or biopsy material may be inoculated subsequently into the nose of a hamster and the animal watched for nasal inflammation.
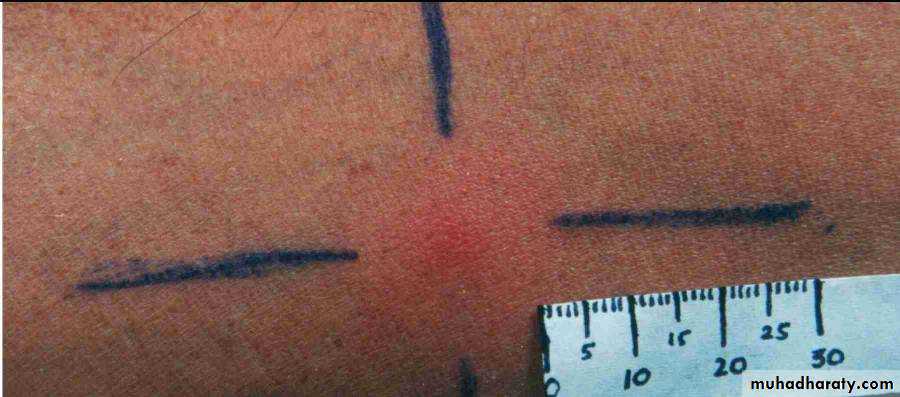
5- Leishmanin skin test (Montenegro test): involves the forearm intradermal injection of 0.1 ml suspension of killed promasitigote, this test is used to measure delayed hypersensitivity. Positive result is indicated by an induration of 5mm or more in 48-72 hours. In cutaneous leishmaniasis this test is positive.
6- Immunological test (serology): has limited role in diagnosis because patient shows no detectable level of circulating antibodies.
Treatment
1- Sodium stibogluconate (Pentostam)
2- Pentamidine (isothionate) 3.Amphotericine B
Prevention
Prevention involves protection from sandfly bites by using netting, window screens, protective clothing, and insect repellents.
cutaneous leishmaniasis mucocutaneous leishmaniasis
Leishmanin skin test (Montenegro test)
Genus Trypanosoma
Includes three major pathogens:
Trypanosoma cruziTrypanosoma gambiense
Trypanosoma Rhodesiense
Trypanosoma cruzi (American trypanosomiasis, Chagas’ disease)
Life cycle
The life cycle involves the reduviid bug (Triatoma or kissing bug) as the vector, and both humans and animals(domestic cats and dogs and wild species such as raccoon, and rat) as reservoir hosts. The cycle in the reduviid bug begins with ingestion of trypomastigotes in the blood of the reservoir host. In the insect gut, they multiply and differentiate first into epimastigotes and then into trypomastigotes.When the bug bites again, the site is contaminated with feces containing trypomastigotes, which enter the blood and form nonflagellated amastigotes within host cells. Many cells can be affected, but myocardial, glial, and reticuloendothelial cells are the most frequent sites. To complete the cycle, amastigotes differentiate into trypomastigotes, which enter the blood and are taken up by the reduviid bug
Modes of transmission
Bite of reduviid bug (commonest mode).
Blood transfusion in endemic areas.
Congenital transmission.
Epidemiology
Chagas’ disease occurs primarily in rural Central and South America (temperature&humidity). It bites preferentially around the mouth or eyes, hence the name “kissing bug.”
Pathogenesis
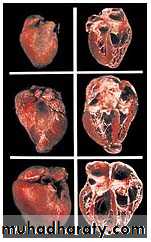
The amastigotes can cause inflammation, consisting mainly of mononuclear cells. Cardiac muscle is the most frequently and severely affected tissue. In addition, neuronal damage leads to cardiac arrhythmias and loss of tone in the colon (megacolon) and esophagus (megaesophagus). During the acute phase, there are both trypomastigotes in the blood and amastigotes intracellularly in the tissues. In the chronic phase, the organism persists in the amastigote form.
Clinical Findings
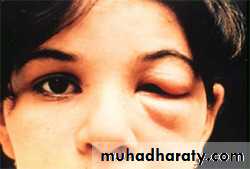
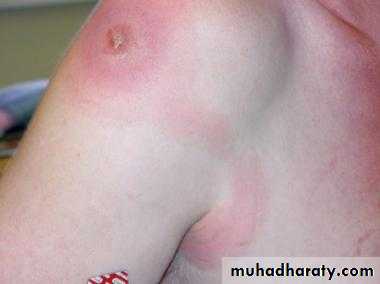
The acute phase of Chagas’ disease consists of facial edema and a nodule (chagoma) near the bite, coupled with fever, lymphadenopathy, and hepatosplenomegaly. A bite around the eye can result in unilateral palpebral swelling & conjuctuvitis ( Romana’s sign). The acute phase resolves in about 2 months. Most individuals then remain asymptomatic, but some progress to the chronic form with myocarditis and megacolon.
Death from chronic Chagas’ disease is usually due to cardiac arrhythmias or congestive heart failure.
Diagnosis
1- Microscopic detection of trypomastigotes in peripheral blood:
a) Wet mount preparation
b) Fixed preparation by Giemsa stained smear: the parasites are typically C or U shaped.
2- Culture: such as on NNN medium.
3- Xenodiagnosis:
4- Biopsy examination: lymph node or skeletal muscle biopsy is examined for amastigotes.
5- Serological diagnosis: IFAT , RIA (radioimmunoassay) , ELISA.
6- PCR (polymerase chain reaction).
In blood C – shape trypomastigotes Amastigotes from biopsy of cardiac muscle
Treatment
The drug of choice for the acute phase is nitrofuran. Benznidazole is an alternative drug. There is no effective drug against the chronic form.
Prevention
Prevention involves protection from the reduviid bite, improved housing, and insect control. No prophylactic drug or vaccine is available. Blood for transfusion is tested for the presence of antibodies to T. cruzi. Blood containing antibodies should not be used.
myocarditis
Romana’s sign
Trypanosoma brucie (African Trypanosomiasis, Sleeping sickness)
include :T. gambiense ,T. rhodesienseLife cycle of T. brucie
The morphology and life cycle of the two species are similar. The vector for both is the tsetse fly, Glossina, but different species of fly are involved for each. Humans are the reservoir for T. gambiense, whereas T. rhodesiense has reservoirs in both domestic animals (especially cattle) and wild animals.The 3-week life cycle in the tsetse fly begins with ingestion of trypomastigotes in a blood meal from the reservoir host. They multiply in the insect gut and then migrate to the salivary glands, where they transform into epimastigotes, multiply further, and then form metacyclic trypomastigotes, which are transmitted by the tsetsefly bite. The organisms in the saliva are injected into the skin, where they enter the bloodstream, differentiate into blood-form trypomastigotes, and multiply, thereby completing the cycle .these species are rarely found as amastigotes in tissue, in contrast to T. cruzi and Leishmania species, in which amastigotes are commonly found.
Pathogenesis
The trypomastigotes spread from the skin through the blood to the lymph nodes and the brain. The typical somnolence (sleeping sickness) progresses to coma as a result of a demyelinating encephalitis.
In the acute form, a cyclical fever spike (approximately every 2 weeks) occurs that is related to antigenic variation. As antibody-mediated agglutination and lysis of the trypomastigotes occur, the fever subsides. This cycle repeats itself over a long period.
Epidemiology
The disease is endemic in sub-Saharan Africa, the natural habitat of the tsetse fly(temperature&humidity). Both sexes of fly take blood meals and can transmit the disease. The fly is infectious throughout its 2- to 3-month lifetime. T. gambiense is the species that causes the disease in west Africa, whereas T. rhodesiense is found in east Africa. Both species are found in central Africa.
Clinical Findings
Although both species cause sleeping sickness, the progress of the disease differs. T. gambiense–induced disease runs a low-grade chronic course over a few years, whereas T. rhodesiense causes a more acute, rapidly progressive disease that, if untreated, is usually fatal within several months.
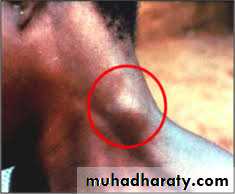
The initial lesion is an indurated skin ulcer (trypanosomal chancre) at the site of the fly bite. After the organisms enter the blood, intermittent weekly fever and lymphadenopathy develop. Enlargement of the posterior cervical lymph nodes (Winterbottom’s sign) is commonly seen. The encephalitis is characterized initially by headache, insomnia, and mood changes, followed by muscle tremors, slurred speech, and apathy that progress to somnolence and coma.
Untreated disease is usually fatal as a result of pneumonia.
Diagnosis of African trypanosomiasis
1-Microscopic detection of trypomastigotes in trypanosomal chancre,Peripheral blood,Bone marrow,Lymph node aspiration or CSF, by:
1)Wet preparation: direct microscopical examination of unstained film.
2)Fixed preparation by Giemsa stained smear.
2-Serological diagnosis:
-IFAT (indirect fluorescent antibody test).
-IHT (indirect haemoagglutination test).
-ELISA.
3-Serum and spinal fluid IgM measurement: are of diagnostic value, because in many cases the total serum IgM exceeds eight (8) times the normal amount.
4-Animal inoculation.
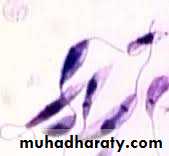
Trypanosoma gambiense or rhodesiense trypomastigote forms in blood smear
TreatmentTreatment must be initiated before the development of encephalitis, because suramin, the most effective drug, does not pass the blood–brain barrier well. Suramin willeffect a cure if given early. Pentamidine is an alternative drug. If central nervous system symptoms are present, suramin (to clear the parasitemia) followed by melarsoprol should be given.
Prevention
The most important preventive measure is protection against the fly bite, using netting and protective clothing. Clearing the forest around villages and using insecticides are helpful measures. No vaccine is available.