First stage
BiologyLec-1
7/12/2015
د.انعام
Biology is a natural science Concerned with the study of life and living organisms, including their structure, function, growth ,evolution, distribution, and taxonomy.
How to Define Life
There are some characteristics that distinguish living and non-living things .
Organization: Being structurally composed of one or more cells — the basic units of life. Organisms are organized from atoms up to cells. The matter is structured in an ordered way .Atoms are arranged into molecules, then into macromolecules, which make up organelles, which work together to form cells. Beyond this, cells are organized in higher levels to form entire multicellular organisms.
Homeostasis: Regulation of the internal environment to maintain a constant state. Stable internal conditions of pH, temperature, water balance, etc. for example, sweating to reduce temperature.
Metabolism: Refers to the sum of the total chemical processes that occur in a cell or organism that are necessary for life. These processes can
be classified into anabolic and catabolic processes.
Growth and Development : Growth means that organism increases in size and number. Development refers to all changes that occur during life.
Adaptation: The ability to change over time in response to the environment. This ability is fundamental to the process of evolution and is determined by the organism's heredity.
Response to stimuli: Organisms respond to stimuli (Temperature, Water, Food Supplies, etc.) in order to survive & reproduce.
Reproduction: The ability to produce new individual organisms, either asexually from a single parent organism, or sexually from two parent organisms.
(1)
Water is essential for life.
Water is the most abundant molecule in cells, accounting for 70% or more of total cell mass. The total amount of water in our body is found in three main locations: within our cells (two-thirds of the water), in the space between our cells and in our blood (one-third of the water).
Water serves a number of essential functions in the Body
Water is the primary building block of cells.
It regulates our internal body temperature by sweating and respiration.
The carbohydrates and proteins that our bodies use as food are metabolized and transported by water in the bloodstream.
Water is used to flush waste and toxins from the body via urine.
Forms saliva .
Lubricates joints.
Water has a number of Important properties essential for life.
Water (H2O) is a polar molecule because of a slightly negative charge at the oxygen end and a slightly positive charge at the hydrogen end. Water molecules can form hydrogen bonds with each other. Polar substances are hydrophilic (water loving). Nonpolar substances are hydrophobic (water hating) and are repelled by water.
Solvent- It is a very good solvent. Molecules such as salts, sugars, amino acids dissolve readily in water (once dissolved they can be transported e.g. glucose in the blood).
Water has a high specific heat capacity .This means that water does not change temperature easily. This minimises fluctuations in temperature inside cells.
uires a great amount of heat to change to a gas
Latent heat of vaporization- Water requires a lot of energy to change state from a liquid to a gas, providing a cooling mechanism (sweating) .As water evaporates it extracts heats from the surrounding area, cooling the organism.
Density- The solid state of water (ice) is less dense than the liquid
(2)
state. As the air temperature cools, bodies of water freeze from the surface, forming a layer of ice with the liquid beneath. This allows aquatic ecosystem to exist in low temperatures.
Cohesion and adhesion Water molecules due to hydrogen bonds stick together, and to other biologically important polar molecules.
Acids and Basses
Acids are molecules that dissociate in water , releasing hydrogen ions (H+) ,so called proton donor.For example, Hydrochloric acid dissolved in water forms (H+) and ( Cl-) ions.
HCl ---> H+ + CL-
Bases are molecules that either take up hydrogen ions (H+) or release hydroxide ions (OH-) ( accept proton) . For example, Sodium hydroxide, when dissolved in water, forms Na+ and OH- ions.
NaOH ---> Na+ + OH-
pH scaleA scale ranging from 0 to 14 pH units, reflecting the concentration of hydrogen ions in solution.
A solution with a pH of 7.0 is neutral. Solutions with a lower pH value (< 7.0) are increasingly acidic, and those with a higher pH value (>7.0) are increasingly alkaline.
(3)
pH in living systems
CompartmentpH
Gastric acid
1
Lysosomes
4.5
Human skin
5.5Urine
6.0
Cytosol
7.2
Cerebrospinal fluid (CSF)
7.5
Blood
7.34–7.45
Mitochondrial matrix
7.5
Pancreas secretions
8.1
Buffers
A buffer is a substance that helps minimize the change in the pH of a solution when acids or bases are added. This is important because, most of the chemical processes that occur in living organisms are highly sensitive to pH, and drastic changes in pH can cause some serious trouble.
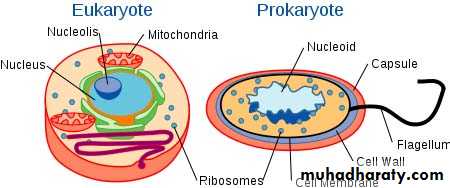
Prokaryotic and Eukaryotic Cells
Cells are of two types, eukaryotic, which contain a nucleus, and prokaryotic, which do not. Prokaryotes are single-celled organisms, while eukaryotes can be either single-celled or multicellular.The distinction between prokaryotes and eukaryotes is considered to be the most important distinction among groups of organisms. Eukaryotic cells contain membrane-bound organelles, such as the nucleus, while prokaryotic cells do not.
(4)
Comparison between prokaryotic and eukaryotic cells.
Similarities:They both have DNA as their genetic material.
They are both membrane bound.
They both have ribosomes .
They have similar basic metabolism .
Differences:
1. Eukaryotes have a nucleus, while prokaryotes do not.2. Eukaryotes have membrane-bound organelles, while prokaryotes do not. 3. Eukaryotic cells are, on average, ten times the size of prokaryotic cells.4. The DNA of eukaryotes is much more complex and therefore much more extensive than the DNA of prokaryotes.5. Prokaryotes have a cell wall composed of peptidoglycan, a single large polymer of amino acids and sugar . Many types of eukaryotic cells also have cell walls, but none made of peptidoglycan.6. The DNA of prokaryotes floats freely around the cell; the DNA of eukaryotes is held within its nucleus and associated with histones (proteins).7. Eukaryotes undergo mitosis; prokaryotes divide by binary fission (simple cell division).
(5)
Comparison of features of prokaryotic and eukaryotic cells
ProkaryotesEukaryotes
Typical organisms
bacteria
, plants, animals
Typical size
~ 1–5 µm[11]
~ 10–100 µm[11]
Type of nucleus
nucleoid region; no true nucleus
true nucleus with double membrane
DNA
circular (usually)
linear molecules (chromosomes) with histone proteins
RNA/protein synthesis
coupled in the cytoplasm
RNA synthesis in the nucleusprotein synthesis in the cytoplasm
Ribosomes
50S and 30S
60S and 40S
Cytoplasmic structure
very few structures
highly structured by endomembranes and a cytoskeleton
Cell movement
flagella made of flagellin
flagella and cilia containing microtubules; lamellipodia and filopodia containing actin
Mitochondria
none
one to several thousand
Chloroplasts
none
in algae and plants
Organization
usually single cells
single cells, higher multicellular organisms with specialized cells
Cell division
binary fission (simple division)
mitosis and meiosis
Chromosomes
single chromosome
more than one chromosome
(6)