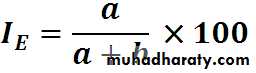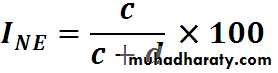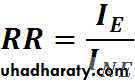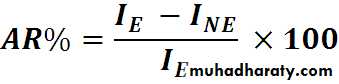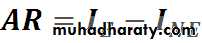Analytic Epidemiological Designs
Basic Question in Analytic EpidemiologyE
D
Exposure
Disease
Cohort Study
Cohort is an ancient Roman military unit of 300 – 600 men.
A group of soldiers marching forward in battleIn epidemiology, cohort is a group of people who share a common characteristic or experience within a defined period (e.g., are born, are exposed to a drug or a vaccine, etc.).
Thus a group of people who were born on a day or in a particular period, say 1980, form a birth cohort.
A study design that follows over time one or more populations (called cohorts) to determine which patient characteristics (risk factors) are associated with the development of a disease or outcome.
Key Point:
Presence or absence of risk factor is determined before outcome occurs.General consideration while selection of cohorts
Both the cohorts are free of the disease.Both the groups should equally susceptible to disease
Both the groups should be comparable
Diagnostic and eligibility criteria for the disease should be defined well in advance.
ANALYSIS
Calculation of incidence rates among exposed and non exposed groupsEstimation of risk
Incidence rates of outcomeN
d
c
b
a
Yes
No
Disease Status
Yes
No
Exposure
Status
a+b
c+d
b+d
a+c
Total
ExposedNon Exposed
Relative Risk
• = 1 No association• < 1 Negative association
•
Risk estimation
AR: Attributable RiskExcess risk attributed to exposure
AR%: Attributable Risk percent
Express the gain or the benefit if the exposure removed
Incidence
I. among
exposed
A R%
A RBase-line risk
I. among non exposed• Smoking
• Lung cancer• Total
• YES• NO
• YES
• 70
• 6930
• 7000
• NO
• 3
• 2997
• 3000
• 73
• 9927
• 10000
Find out RR and AR for above data
Incidence of lung cancer among smokers
70/7000 = 10 per 1000
Incidence of lung cancer among non-smokers
3/3000 = 1 per thousand
RR = 10 / 1 = 10
(lung cancer is 10 times more common among smokers than non smokers)
AR = 10 – 1 / 10 X 100
= 90 %
(90% of the cases of lung cancer among smokers are attributed to their habit of smoking)
Timeframe of Studies
Prospective Study - Outcomes have not yet occurred as study begins. looks forward, looks to the future, examines future events, follows a condition, concern or disease into the futuretime
Study begins hereTimeframe of Studies
Retrospective Study - Outcomes have already occurred as the study begins. “to look back”, looks back in time to study events that have already occurredtime
Study begins here
Strengths
We can find out incidence rate and riskMore than one disease related to single exposure
can establish cause - effect
good when exposure is rare
minimizes selection and information bias
Weaknesses
losses to follow-upoften requires large sample
Ineffective for rare diseases
long time to complete
Expensive
Ethical issues
…... several famous large cohort studies continue to provide important information …..
Framingham Heart Study
Case Control study
Why case-control study?In a cohort study, you need a large number of the subjects to obtain a sufficient number of case, especially if you are interested in a rare disease.
Gastric cancer incidence in Japanese male:
128.5 / 100,000 person year
A case-control study is more efficient in terms of study operation, time, and cost.
Case ControlDefinition….
The case-control study is an analytic epidemiologic research design in which the study population consists of 2 groups who either have (cases) or do not have a particular health problem or outcome (controls).The investigator looks back in time to measure exposure of the study subjects. The exposure is then compared among cases and controls to determine if the exposure could account for the health condition of the cases.
Case Control
Case-Control Studies
Cases: DiseaseControls: No disease
Case Control
Case-control study - Sequence of determining exposure and outcome status
Step1: Determine and select cases of your research interestStep2: Selection of appropriate controls
Step3: Determine exposure status in both cases and controls
Case ControlDesign of Case Control Study
• cases with the diseaseShould have clear case definition i.e. clear criteria for defining the disease of interest
May be taken from clinics, hospitals, disease registries
Preferred newly diagnosed
Case Control
Design of Case Control Study• Appropriate controls without the disease
Should comes from the same study base or population as cases
Can come from geographical sample, medical inistitution, neighbors, friends,….
Can have multiple control groups
May be matched
•
Case Control
1)a population-based case-control study
Both cases and controls are recruited from the population.2)a case-control study nested in a cohort
Both case and controls are members of the cohort.3)a hospital-based case-control study
Both case and controls are patients who are hospitalized or outpatients.Controls with diseases associated with the exposure of interest should be avoided.
Types of case-control studies
Case Control
Case-Control DesignStudy
population
Cases
(disease)
Controls
(no disease)
factor present
factor absent
factor present
factor absent
present
past
time
Study begins hereCase Control
31
Case-control Study – Design
Select subjects on the basis of disease statusCase Control
• Interpretation of (OR) odds ratio
• > 1 means the exposure is a risk factor.• = 1 means the exposure is not associated with the disease.
• < 1 means the exposure is protective
Case Control
Lung cancer Controls
cases
N=100 N=100
Smokers (NOT recently started)
↓ ↓
70 40
An example of unmatched case-control study
CasesControls
smoker
70
40
Non-smoker
30
60
Odds ratio=
Case ControlAdvantages
• Simple, not time consuming (quick) and inexpensive.
• Suitable for rare diseases.
• Can examine multiple exposures for a single disease.
• Support, but not provide causal association.
• Suitable for diseases of long latency period
• Dose response relationship can be assessed
• Small sample size
• No ethical problem
Case Control
Disadvantages
• Recall bias• Selection bias
• Different diagnostic tools so “case groups” may be not homogenous.
• The chosen cases are selective survivors (the history of died cases may be different) thus the cases does not represent a universe of cases.
• The time sequence between the exposure and the disease is not clear.
• Control of confounding factors
• Not suitable for rare exposure
• Only one outcome
Case Control
36
Controlling extraneous variables (confounding)
Exposure of interest may be confounded by a factor that is associated with the exposure and the disease i.e., is an independent risk factor for the disease
A
B
C
Case Control
37How to control for confounding
At the design phase
Randomization
Restriction
Matching
At the analysis phase
Age-adjustment
Stratification
Multivariable adjustment (logistic regression modeling, Cox regression modeling)
Case Control
Matching
• Selection of controls to match specific• characteristics of cases
• Frequency matching
• Select controls to get same distribution of variable as cases (e.g. age group)
• Individual matching
• Select a specific control per case by matching variable (e.g. date of birth)
Case Control
Intervention or Experimental studies
TherapeuticStudy population
Patients with disease
Objectives
Cure patientsDiminish symptoms
Prevent recurrence of disease/risk of death
Preventive
Study Population
Population at risk
Objectives
Reduce the risk of developing disease
• Clinical trials are the most well known experimental design
Such designs are differentiated from observational designs by the fact that there is manipulation of the study factor (exposure), and randomization (random allocation) of subjects to treatment (exposure) groups.
RCT
Why Performed ?
• Provide stronger evidence of the effect (outcome) compared to observational designs, with maximum confidence and assurance• Yield more valid results, as variation is minimized and bias controlled
• Determine whether experimental treatments are safe and effective under “controlled environments” (as opposed to “natural settings” in observational designs), especially
• when the margin of expected benefit is doubtful / narrow (10 - 30%)
RCT
Experimental Studies
A study in which a population is selected for a planned trial of a regimen, whose effects (consequences of some treatment on some outcome) are measured by comparing the outcome of the regimen between 2 groups.The subjects in the study who actually receive the treatment of interest are called the treatment group.
The subjects in the study who receive no treatment or a different treatment are called the comparison group.
RCT
Experimental Design
time
Study begins here (baseline point)Study
population
Intervention
Control
outcome
no outcome
outcome
no outcome
baseline
futureRANDOMIZATION
RCTTypes of trials
RCT
Design - conduct
Different phasesEnrollment (selection of study population)
Allocation of study regimesFollow-up
Maintainence and assessment of adherenceHigh and uniform rates of ascertainment
Analysis and interpretation
RCTPopulation hierarchy for intervention study
Reference populationExperimental population
Exclusion criteriaInformed consent
Excluded
Refused
Study population
Intervention group
Control groupOutcome
Losses to follow-upLosses to follow-up
Random allocation
RCTRCT Advantages (I)
the “gold standard” of research designs. They thus provide the most convincing evidence of relationship between exposure and effect. Example:trials of hormone replacement therapy in menopausal women found no protection for heart disease, contradicting findings of prior observational studies
RCT
RCT Advantages (II)
Best evidence study design
No inclusion bias (using blinding)
Controlling for possible confounders
Comparable Groups (using randomization)
RCT
Disadvantages
Large trials (may affect statistical power)Long term follow-up (possible losses)
Compliance
Expensive
Public health perspective ?
Possible ethical questions
RCT
Blinding
Hiding information about the allocated study regimes from key participants in a trial• Depending on
outcome of interest
Ethics, feasibility, compromise
By Using Placebo which is Inert medication, i.e No effect, intended to give the patient the perception they are receiving treatment
Types:
• Single – blind :Observer or subject are kept ignorant about allocated study regime
• Double blind :Both observer and the subject are kept ignorant about allocated study regime
RCT