
First class Chemistry
1
AQUEOUS SOLUTIONS AND COLLOIDS
Lec.2
By: Dr. Tamathir Abbas 2/12/2015
TYPES OF SOLUTIONS
--A solution as a homogeneous mixture of the molecules, atoms, or ions
of two
or more different substances.
--The substances that make up a solution are called its components. There
is usually more of one component than the other components in the
solution.
--The other components are called the solutes.
-- In a solution of sugar in water, water is the solvent and sugar is the
solute.
-- The three states of matter can combine in nine different ways to form
solutions containing two components. These are listed in Table 8-1.
-- Solutions that contain liquids as solvents are the types of solutions most
familiar to us.
-- Numerous examples of solutions containing solids in liquids, gases in
liquids, and liquids in liquids are available from everyday experience.
Less familiar as solutions are those with solids as solvents, yet alloys
amalgams are important in many commercial products.

First class Chemistry
2
--A given solution has a particular composition, but the composition can
be varied by adding more of either component. For example, the
sweetness solution of sugar and water varies with the amount of sugar
dissolved.
SOLUBILITY
--There is usually a limit to the amount of solute that can be dissolved in
a solvent at a particular temperature. When this limit is reached, no more
solute will dissolve in the solvent. When this happens, we say that the
solvent is saturated with solute. We call such a solution a saturated
solution
Solubility:
is defined as the amount of solute dissolves in a given
quantity of solvent to form a saturated solution. --solubility of a solute in
a particular solvent depends on a number of factors:
1- The kind of solvent
2- The kind of solute.
3- The temperature of solvent.
4- The pressure above the solvent.
---------------

First class Chemistry
3
1- The kind of solvent
The results of our experiences in the world have led to the very
general rule that "like dissolves like." By this we mean that a polar
solvent such as water is a good solvent for ionic compounds such as
sodium chloride.
** Gasoline, a mixture of nonpolar organic compounds, is a good solvent
for other nonpolar organic compounds such as greases and oils. It follows
from this general rule that polar and nonpolar substances will not form
solutions. An example is gasoline and water.
2- The kind of solute.
--Sometimes there is no limit to the amount of one substance that can
dissolve in another. This is particularly true for solutions of a liquid in a
liquid.
---Some liquids are infinitely soluble in another liquid: any amount of one
liquid will dissolve in any amount of another liquid.
--Ethyl alcohol and water provide an example of two liquids that are
infinitely soluble in each other. Such a pair of liquids is said to be
completely miscible.
--Other liquids are only slightly soluble in each other. Such liquids are
said to be partially miscible.
-- Liquids that are insoluble in each other are said to be immiscible. Thus,
gasoline is immiscible with water.
3- The temperature of solvent.
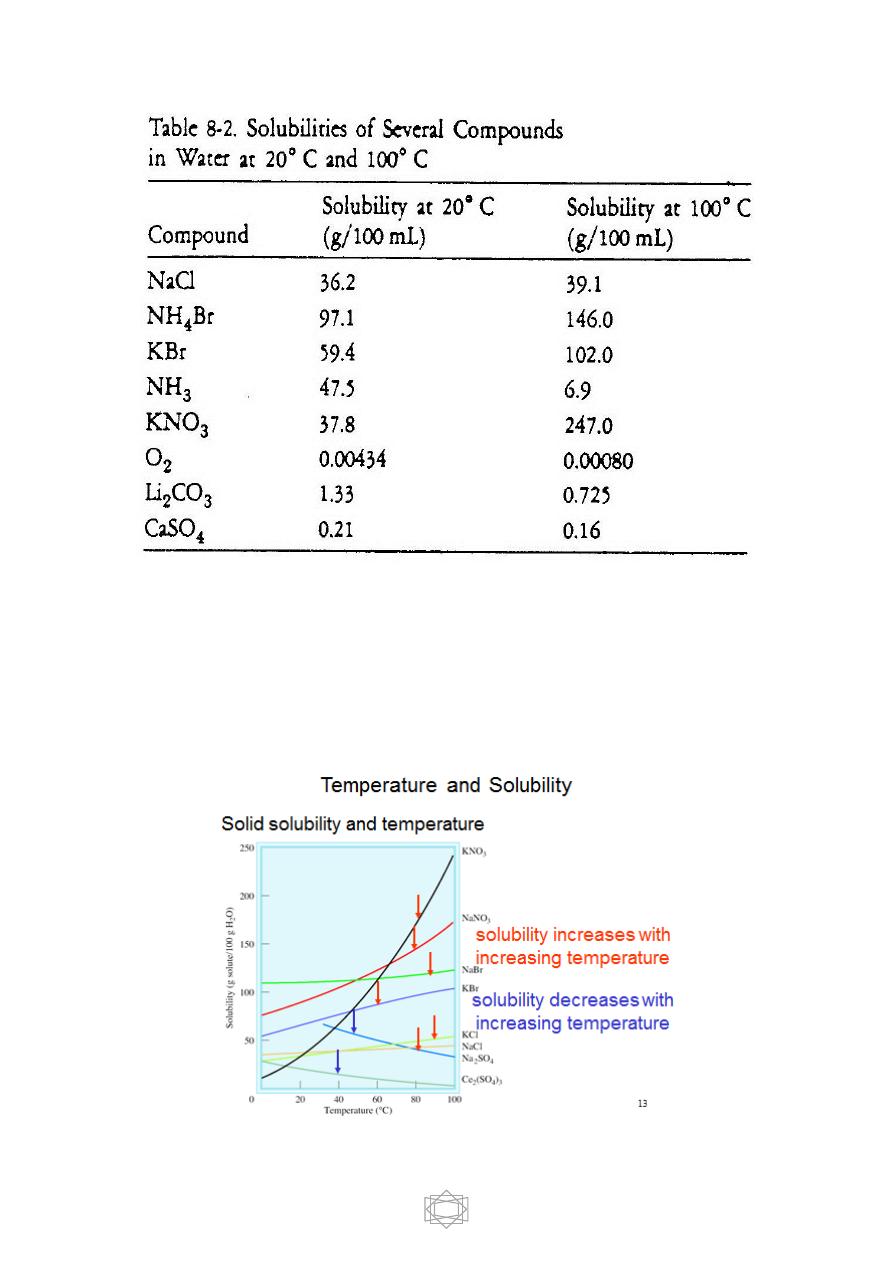
--The temperature of the solvent affects the solubility of a solute. In
general, solutes are more soluble in hot than cold solvents. This is shown
by the solubilities of several solutes in water at 20° C and 100° C listed in
Table 8-2. The solubilities of several solids increase greatly with
increasing temperature. Others increase only slightly, and some actually
decrease.
First class Chemistry
4
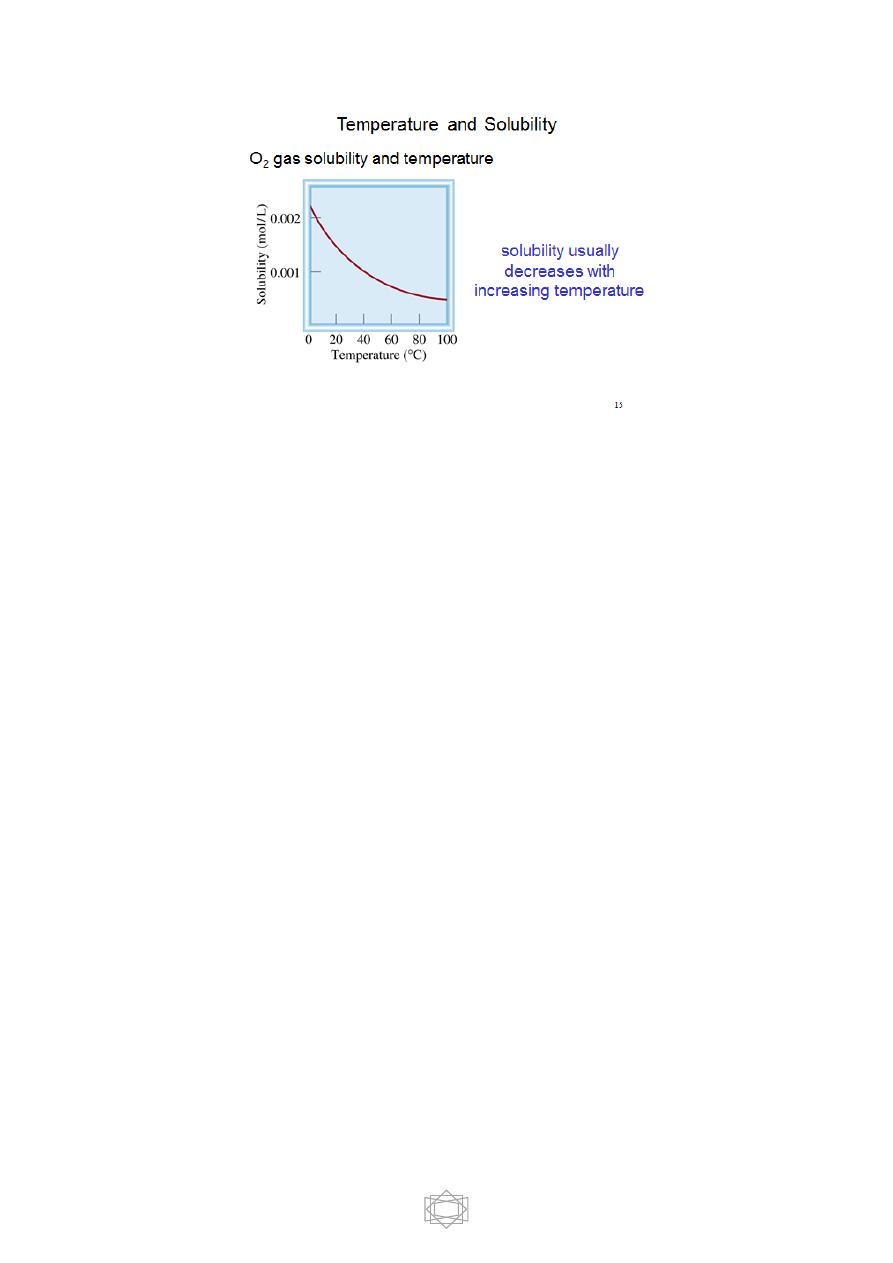
-- Gases are other compounds whose solubilities in water decrease with
increasing temperature.
--A familiar example is boiling water. The bubbles that form when water
is heated are air escaping from solution because dissolved air is less
soluble in water at higher temperatures. Boiled water has a characteristic
flat taste that is due to the absence of dissolved gases.
First class Chemistry
5
4- The pressure above the solvent.
--The solubility of a gas is greatly affected by the pressure of that gas
above the solution. In general, the solubility of any gas increases as the
partial pressure of the gas above the solution is increased. Examples are
the solubilities of oxygen and carbon dioxide in blood.
--pressure of the gas above the solution is increased. Examples are the
solubilities of oxygen and carbon dioxide in blood. Carbonated beverages
are another example. These beverages contain the gas carbon dioxide
dissolved in water and arc bottled under high pressure.
--However, in the laboratory and in clinical work it is necessary to
specify exactly the amount of solute in a solution.
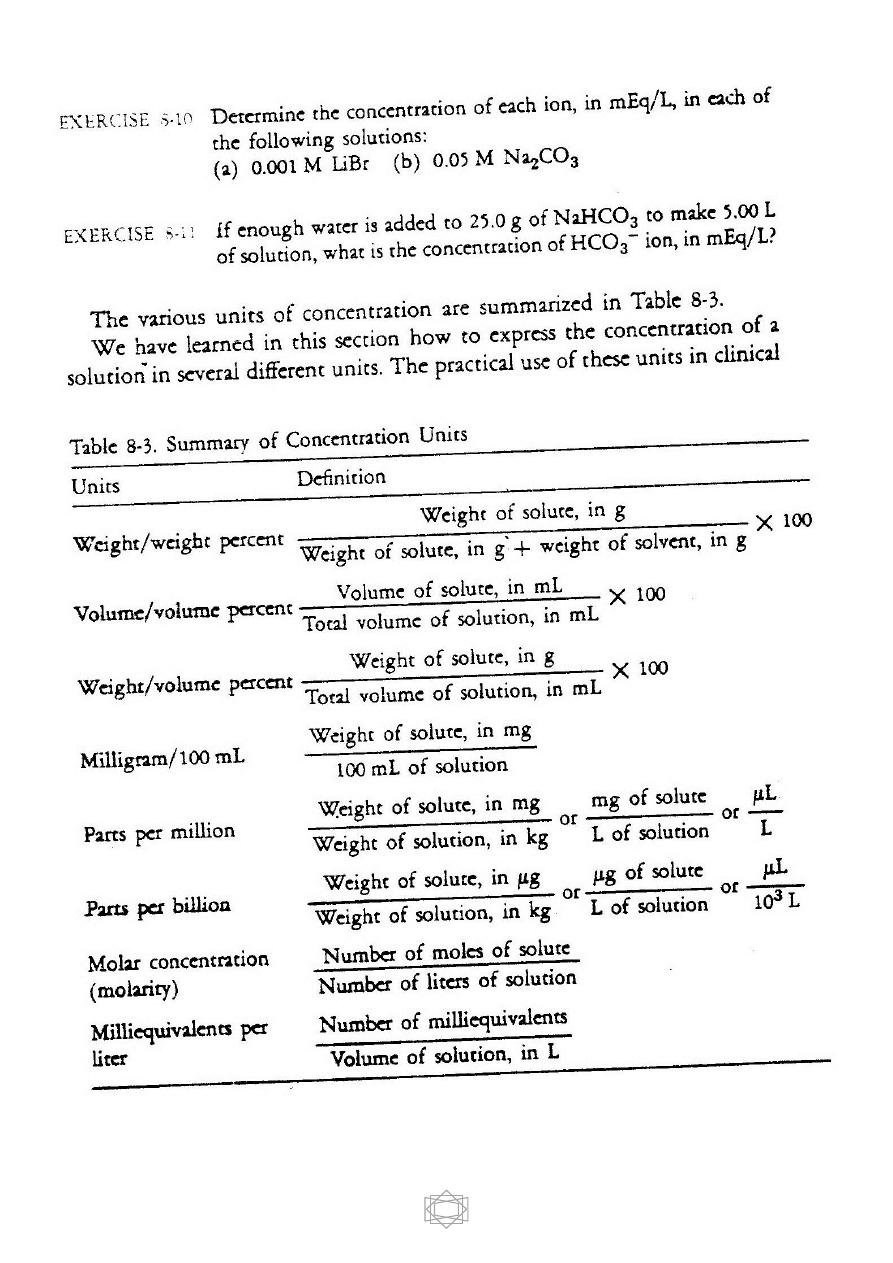
CONCENTRATIONS OF SOLUTIONS
We describe the relative amounts of solute and solvent in a solution by
means of units of concentration. There are several such units, and we will
examine the most commonly used ones.
Weight / Weight Percent
One way to specify the concentration of a solute in a solution is as a
percent by weight. The concentration of the solute is given by the
following equation :

First class Chemistry
6
Example 8-1 : What is the percent by weight of sugar in a solution
made by dissolving 10 g of sugar in 90 g of water?
Solution:
Volume/Volume Percent
A convenient way of expressing the concentration of a liquid solute
dissolved in a liquid is as a percent by volume. This unit of concentration
is similar to percent by weight except that volumes in milliliters are used
instead of weights in grams. The equation is as follows:
Example 8-2 : What is the percent by volume of ethyl alcohol in a
solution made by diluting 10 mL of ethyl alcohol to 100 mL with water?
Solution:

First class Chemistry
7
EXERCISE 8-2: Determine the percent by volume of the solute in each
of the following solutions:
(a) 5.0 mL of rubbing alcohol diluted to 150 ml with water
(b) 15 mL of ethyl alcohol diluted to 500 mL with water
Weight / Volume Percent
This widely used method of expressing concentrations is a
combination of weight and volume. The weight is usually that of the solid
solute and the volume is that of the total solution. This unit is defined as
follows:
Example 8-3 ; What is the percent by weight/volume of sodium chloride
in a solution made by diluting 1.5 g of sodium chloride to 100 mL with
water?
Solution:
Low concentrations of solute are often expressed in milligrams per
100 mL. This weight/volume percent unit is defined as follows:

First class Chemistry
8
The unit mg/100 mL is sometimes called mg percent.
This unit is often used to express the concentrations of solute in
blood and urea, as shown in the following example:
Example 8-4 A 1-mL sample of blood plasma is found to contain 3.3
mg of sodium ions. Express this concentration in mg/100 mL.
Solution:

First class Chemistry
9
Exercise 8-4 Determine the concentration of solute in each of the
following solutions in mg/100 mL.
(a) 32.0 mg of sugar diluted to 10.0 mL with water
(b) 5.00 mL of solution that contains 1.00 g of sodium ion
Parts Per Million and Parts Per Billion
**These units of concentration are widely used to report very small
amounts of solute in a solution. The concentration of pollutants in water
and air are usually reported in these units.
--One part per million, abbreviated ppm, contains 1 part of solute per 1
million (10
6
) parts of solution. By parts we mean any unit of measure
such as grams, liters, or anything else we choose.
**For example, the concentration of solid pollutants in solid food is given
in ppm expressed as mg of pollutant (the solute) in 1 million mg of solid
food (the solution). Because 1 million mg is equivalent to 1 kg, ppm is
usually defined as follows:
To express the concentrations of small quantities of solid solutes in
water, the unit ppm is usually defined as mg of solute per liter of
solution. This change from weight to volume of solvent can be made
because 1 million mg (1 kg) of water occupies approximately 1 L. This
definition of ppm is also frequently used even though the solution may
weigh somewhat more or less than 1 kg.

First class Chemistry
11
**Air pollution is measured in ppm on the basis of measurements of
volume rather than weight. Thus, 1 ppm means that there is 1 µL of
pollutant (the solute) per 1 million (10
6
) µL (1 L) of air (the solution).
** The sensitivity of analytical methods has improved so much that parts
per billion, abbreviated ppb, has become a common unit of
concentration.
**Its use and definition are similar to those of ppm. Thus, 1 ppb contains
1 part of solute per 1 billion (10
9
) parts of solution. Again, the parts refer
to weight or volume, depending on whether the solution is a gas, liquid,
or solid.
Example 8-5 The maximum Food and Drug Administration (FDA)
tolerance of mercury in fish is 0.5 ppm. A 10-g sample of fish is found to
contain 72 µg of mercury. Does the amount of mercury in the fish exceed
the FDA maximum tolerance?
Solution:
Another solution :

First class Chemistry
11

First class Chemistry
12
Molar Concentrations (Molarity)
Molar concentration, or molarity, which is defined as the number
of moles of solute per liter of solution, is designated by the capital letter
M. This definition is given in the form of an equation, as follows:
H.W :EXERCISE 8-6 Determine the molar concentration of each of the
following
solutions:
(a.) 40.0 g of NaOH made up to 1.0'L with water
(b.) 250 mL of a solution that contains 5.40g of NaCl
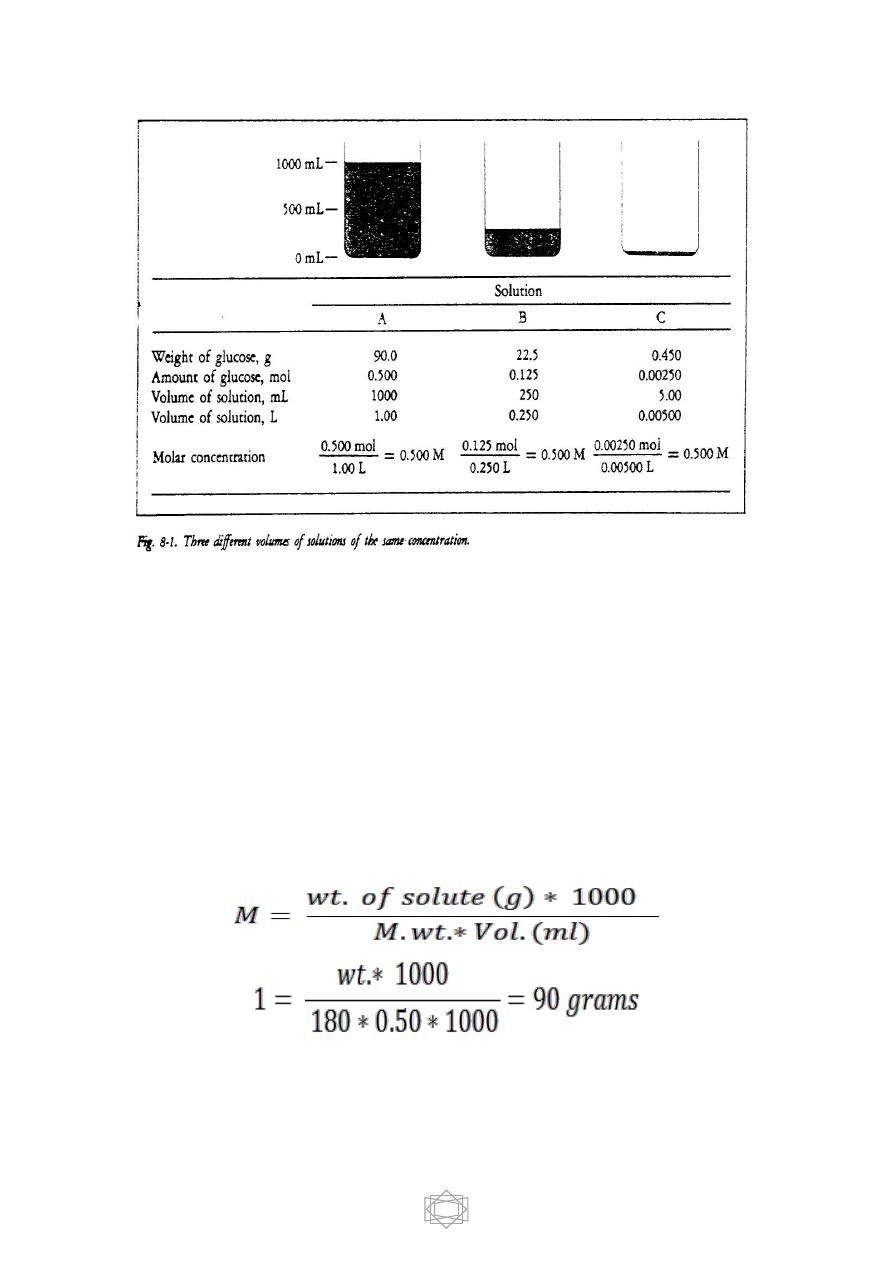
** Molar concentration expresses the ratio of solute to solution. Two
solutions that have the same molar concentrations have the same ratios of
solute to solution even though the total volumes of the two solutions may
be different. We can demonstrate this important fact by means of the
three solutions of glucose in water shown in Figure 8-1.
First class Chemistry
13
**Thus, a bottle labeled 0.500 M glucose may contain 10 L or as little as
1 mL. No matter how much solution there is in the bottle, every drop of it
has a glucose concentration of 0.500 M.
**The importance of molar concentration is that we can determine the
weight of the solute contained in any volume of solution. This fact is in
the following examples.
Example 8-7:A patient is fed intravenously 0.50 L of a 1.0 M glucose
solution. How many grams of glucose has the patient received?
Solution:
H.W. EXERCISE 8-7 ;How many grams are needed to make each of the
following solutions?

First class Chemistry
14
1.00 L of a 0.100 M NaCl solution
a. 250 mL of a 1.50 M glucose solution
b. 500 mL of a 0.150M sucrose (C
12
H
22
O
1:l
) solution
EXERCISE 8-8 ;How many grams of solute are there in each of the
following quantities of solution?
a. 25.0 mL of a 1.00 M LiBr solution
b. 100 mL of a 0.500 M NaOH solution
c. 250 mL of a 0.100 M NaHCO, solution
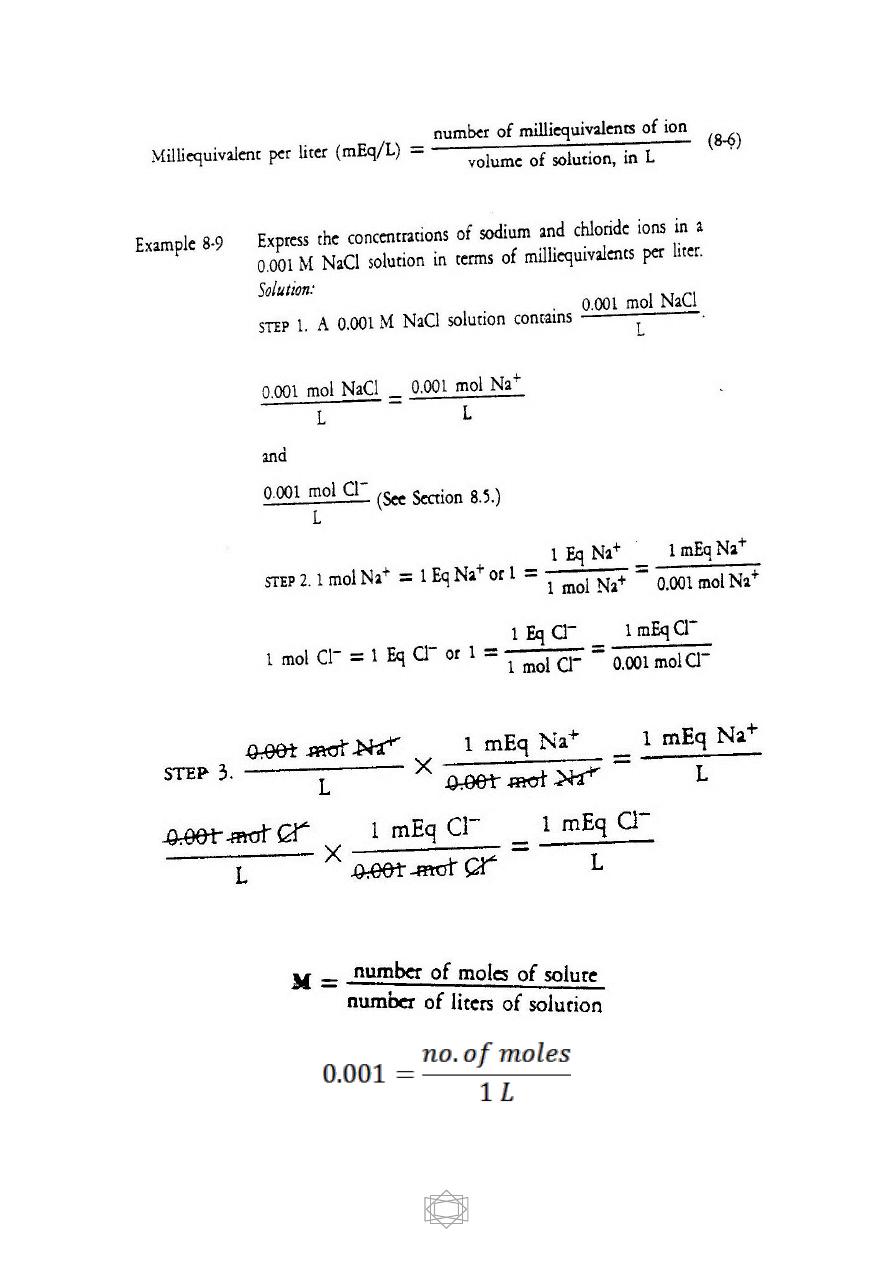
Milliequivalents Per Liter
This unit is used to express low concentrations of ions in body
fluids. To use this unit, we must learn about equivalents.
One
equivalent
of an ion, abbreviated Eq, is defined as 1 mole of that ion
multiplied by the absolute value of its charge.
** For example, 1 mole of sodium ions contains one equivalent of
sodium ions. One mole of chloride ions contains one equivalent of
chloride ions. One mole of magnesium ions contains two equivalents of
magnesium ions.
EXERC1SE 8-9 : How many equivalents are there of each underlined
ion in the following quantities?
a. 1.00 mol NaHCO
3
b. 0.150 mol Na
2
CO
3
c. 2.00 mol LiBr
d. 0.350 mol FeCl
3
e.1.50 mol MgCl
2
d. 0.250 mol Ca
3
(PO
4
)
2
The unit milliequivalent per liter, abbreviated mEq/L, is defined as
follows:
First class Chemistry
15
Another solutions
no. of moles = 0.001

First class Chemistry
16
so
no. of moles of Na
+
= 0.001
no. of mole of Cl
-
= 0.001
no. of Eq of ion = 1 mole of ion * | its charge |
no. of Eq of Na
+
= 0.001 * | 1
+
| = 0.001
no. of Eq of Cl
-
= 0.001 * | 1
-
| = 0.001
no. of mEq of Na
+
= 0.001 * 10
3
= 1 ( 1 mole = 10
3
mmole)
no. of mEq of Cl
-
= 0.001 * 10
3
= 1
First class Chemistry
17
First class Chemistry
18
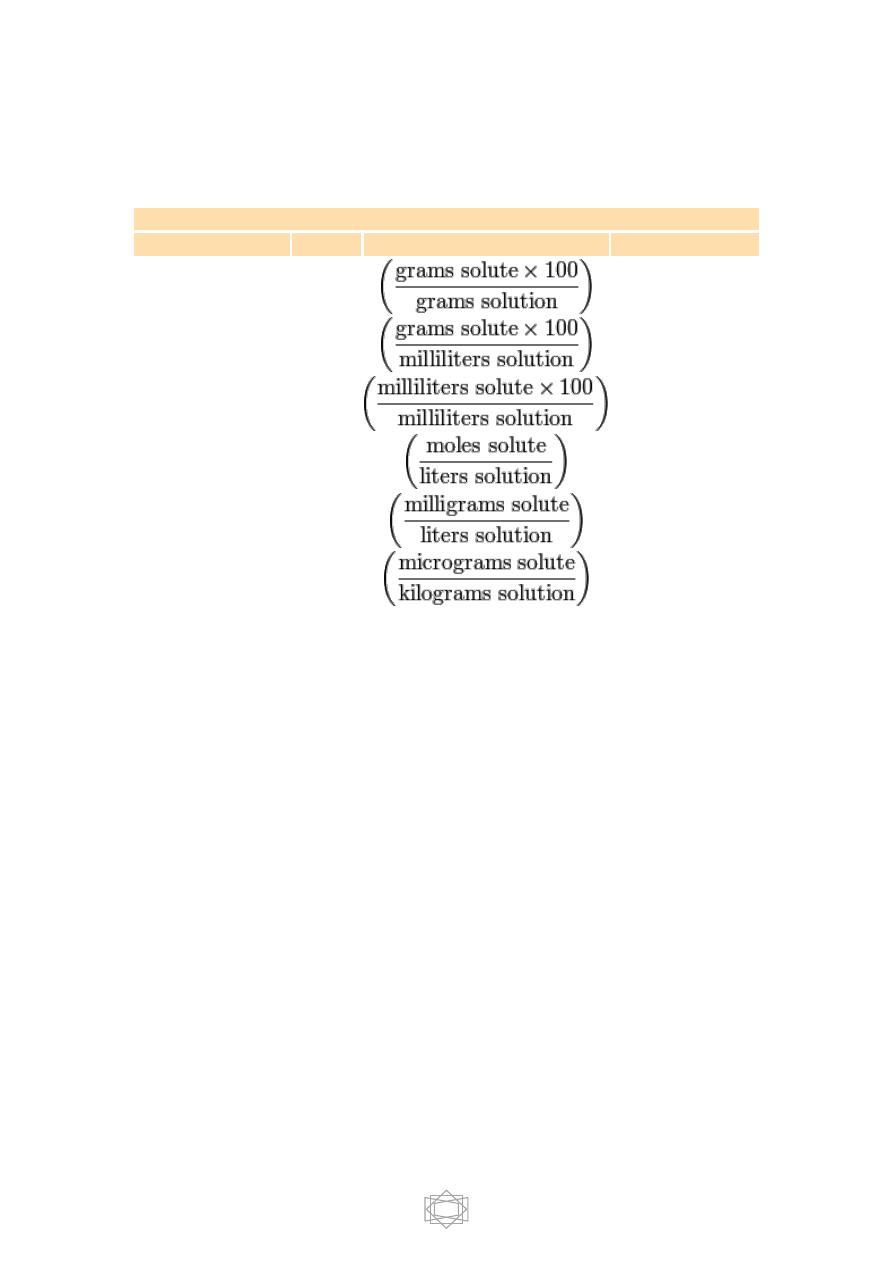
Table of concentration measures
Frequently used standards of concentration
Measurement
Notation
Generic formula
Typical units
Mass percentage
wt%
%
Mass-volume
percentage
-
% though
strictly %g/mL
Volume-volume
percentage
vol%
%
Molarity
M
mol/L (or M or
mol/dm
3
)
Parts per million
ppm
mg/L
Parts per billion
ppb
µg/kg
