Supervised by CHEMICAL lect.NO.: ( )
Dr.Basma Date:23/3/2015
stereoisomers of carbon compounds.
Isomers are different compounds that have the same number and kinds
of atoms. But there are different kinds of isomers. we have already
identified several of them .Structural isomers : differ in their structural
formulas.
Geometric isomers : Differ in their arrangements of their atoms in
space. Actually ,geometric isomers are specific examples of a larger
class of isomers called stereolismers.in this chapter ,we will learn about
other kinds of stereoisomers .Let us start by learning
about leucine , a chiral compound.
Chiral compounds: one of the characteristics of isomers is
that they have different physical properties. This is shown in Table
14_1
Physical constants
Type of isomer compound
M.P B.P Density
C˚ C˚ g/mL
Structural
Benzyl bromide
- 3.9 201 1.438
C
7
H
7
Br O-Bromo toluene -26 181 1.4222
m –Bromo toluene - 40 184 1.4019
P-Bromo toluene 28 184 1.3898
Geometric Trans-3-Hexene -113 67 0.6770
3-Hexene cis-3-Hexene -135 67 0.6796
Isomers
Structural stereo
Optical geomet.
Supervised by CHEMICAL lect.NO.: ( )
Dr.Basma Date:23/3/2015
Stereoisomers of carbon compounds: Ch.14 P357
CHIRAL COMPOUNDS: Example : leucine:
The chemical formula of leucine is C
6
H
13
O
2
N
,
and it has the following
structural formula:
CH3 CH3
CH
CH2
H C CO2H
NH2
Leucine
The three-dimentional structure of leucine is shown in Fig.14-1 p.359
NH
2
CO
2
H C CO
2
H H C NH
2
CH
2
CH(CH
3
)
2
CH
2
CH(CH
3
)
2
-solid line between atoms indicates that the bond is in the plane of
the page
……-A dashed line means that the bond projects below the plane
of the page .
Supervised by CHEMICAL lect.NO.: ( )
Dr.Basma Date:23/3/2015
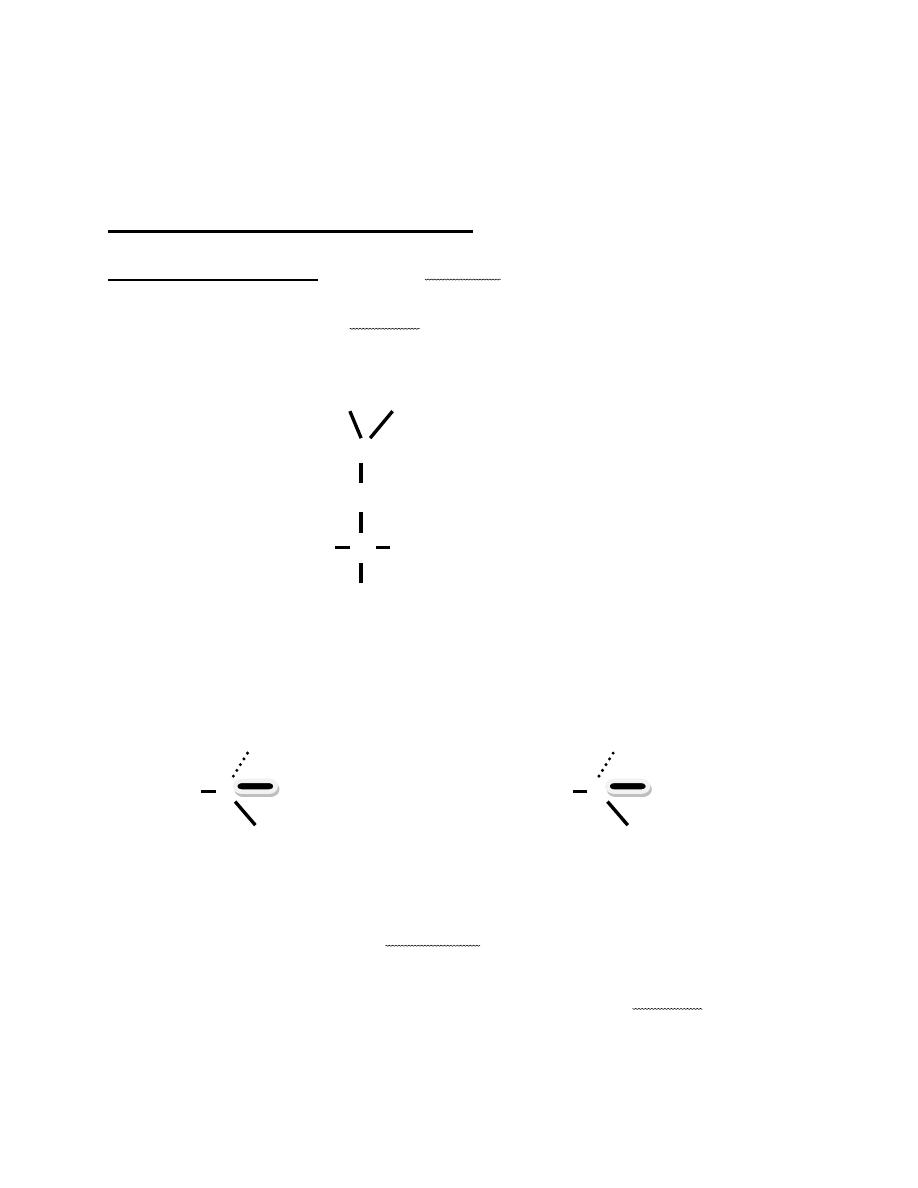
-A solid wedge indicates that the bond projects above the page.
There two structures represent the two isomers of leucine.
They are mirror images of each other ,as shown in fig 14-2,
these mirror images cannot be superimposed.
Mirror
NH
2
H
2
N
H C CO
2
H HO
2
C C H
CH
2
CH(CH
3
)
2
(CH
3
)
2
CH CH
2
Enantiomers: Stereoisomers that are mirror images but cannot be
superimposed .
Not superimposed
R CO
2
H
CO
2
H
Superimposed
H C R Not Superimopsed
H C
NH
2
NH
2
Superimposed R= ـــCH
2
CH(CH
3
)
2
Chiral carbon atom :A carbon atom bonded to four different atoms or
groups.
Chiral: compounds that are not identical to their mirror images.
Supervised by CHEMICAL lect.NO.: ( )
Dr.Basma Date:23/3/2015
Optical activity :All chiral molecules have ability to rotate
plane _polarized light.
see fig (14-6, 14-7) p 363 and read.
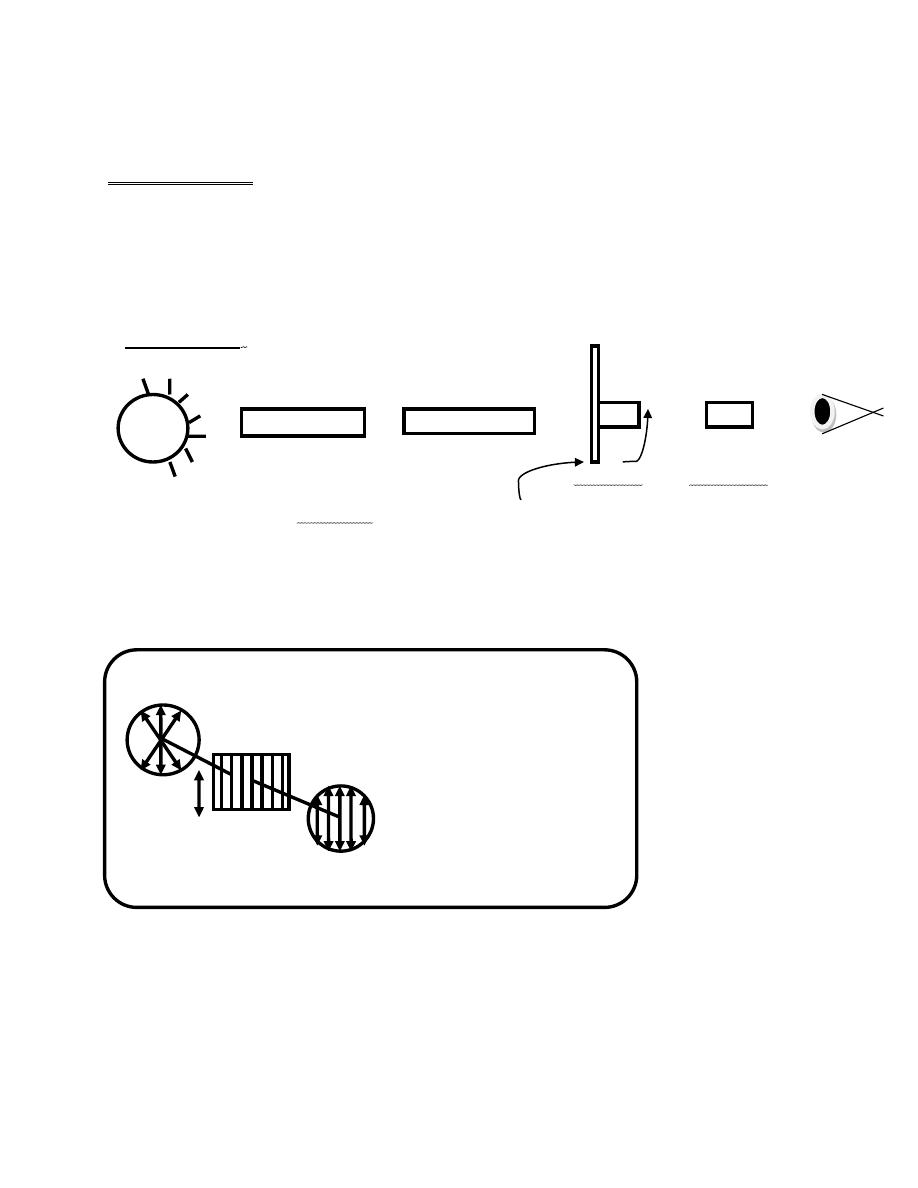
we can detect chiral compounds with an instrument called
a polarimeter.
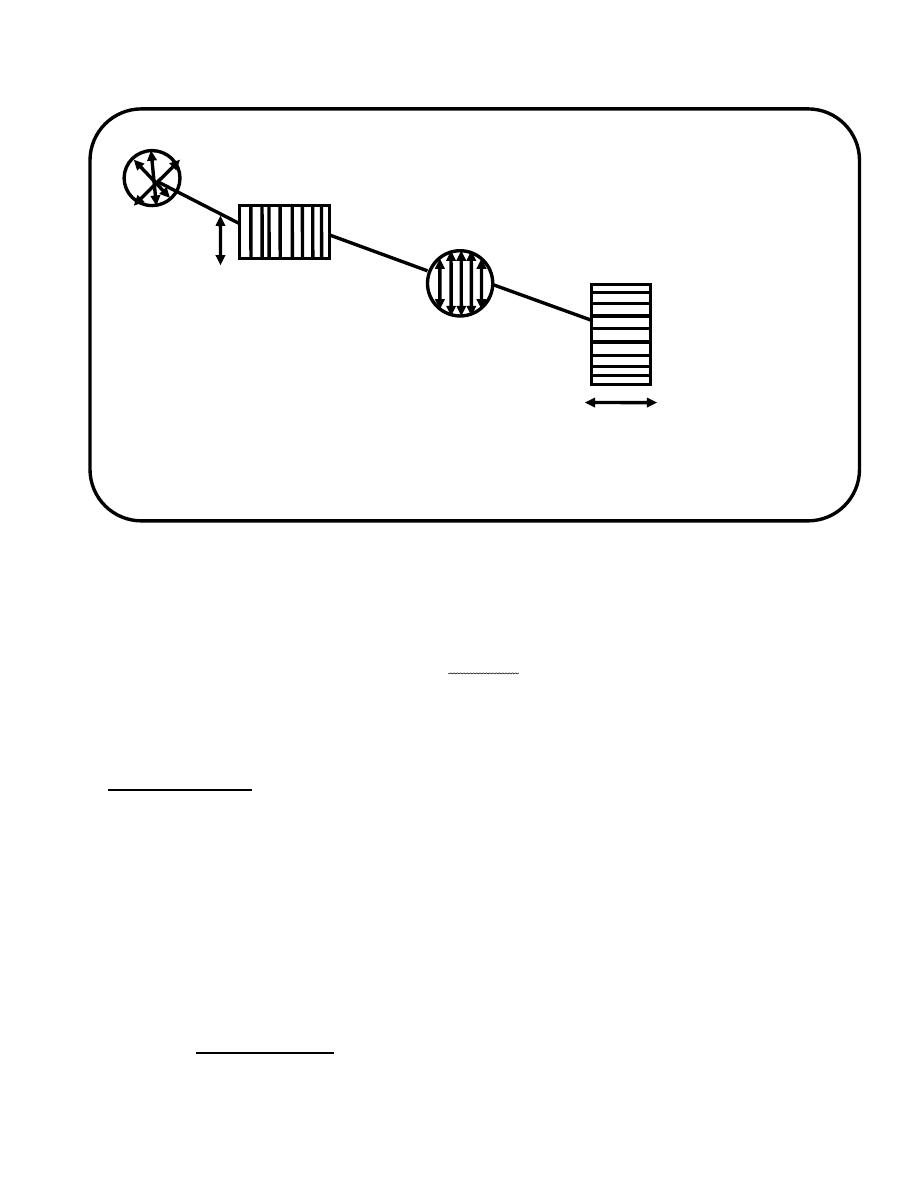
first polarizing
sample tube second polarizing filter Eyepiece Eye
light source filler (Nicol prism) Scale (Nicol prism) , which can
be rotated
fig.14-6.when light passes through a polarizing filter the light become
plane-polarized
Light beam
Polarizing filter
Axis plane-polarized
Of filter light
Supervised by CHEMICAL lect.NO.: ( )
Dr.Basma Date:23/3/2015
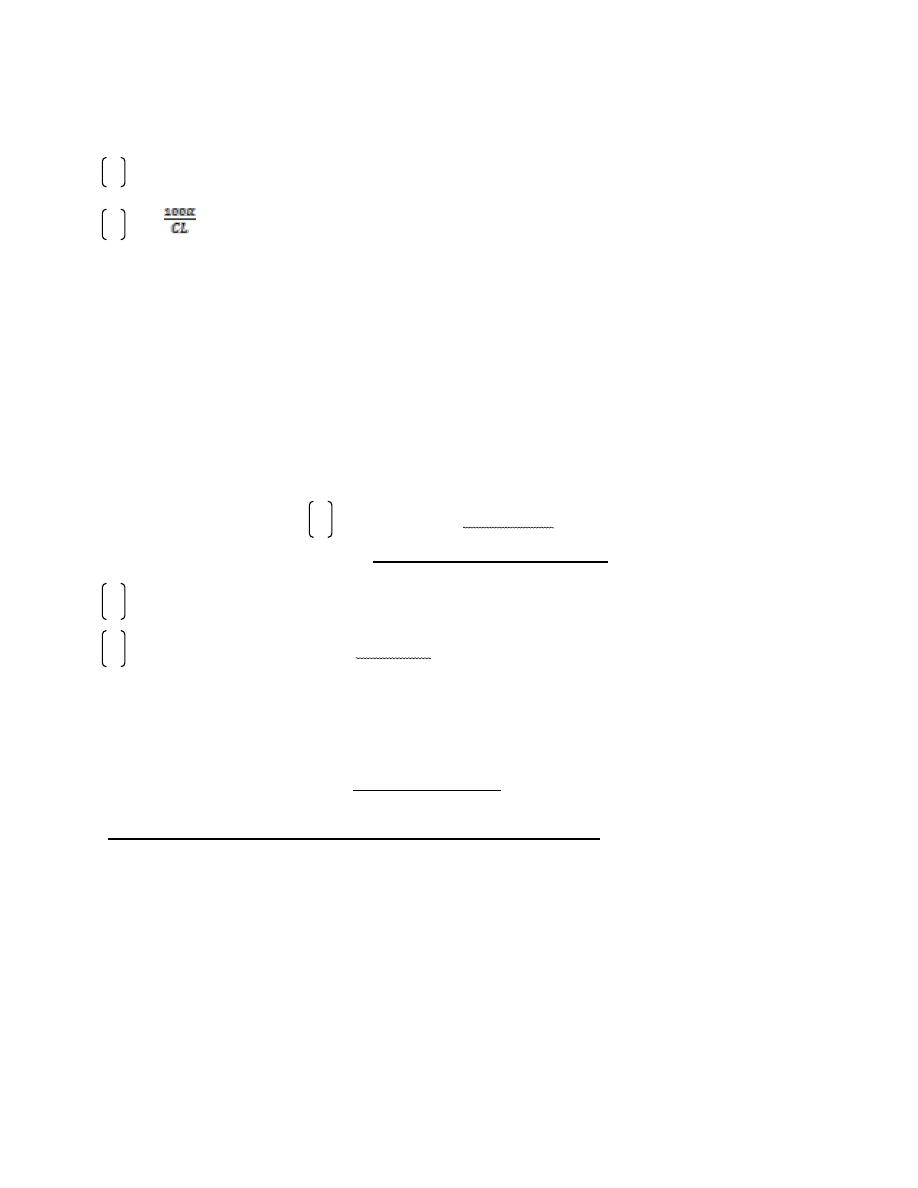
fig14.7 . Effect of two polarizing filters on a light beam. the first
polarizing filter produces abeam of plan-polarized light .when this beam
encounters a second polarizing filter the amount of light that passes
through the filter depends on the relative orientation of the axes of the
two filters .when they are at right angles, no lights gets through.
A compound that rotates plane-polarized light to the right is called
dextrorotatory (+).
if the rotation is to the left the compound is called levorotatory (-).
The angle by which the analyzer must be rotated to restore the
maximal intensity of light is called the optical rotation of a compound.
This angle depends on 4 factors :1_ the temperature of the solution 2-
the light source 3- the concentration of the solution ,4- the length of
the sample tube . To take these factors into account, a new quantity
called the specific rotation of a compound,
First polarizing filter
Plane –polarized light
Second polarizing
filter
Axis of filter
5
Supervised by CHEMICAL lect.NO.: ( )
Dr.Basma Date:23/3/2015
α ,is defined an follows:
α
t
ʎ
=
α= observed optical rotation in degree, with sign
t= temperature of the sample c˚.
ʎ = wavelength of light source.
L= length of sample tube in decimeter,
C= concentration of the compound g/100ml solution.
The specific rotation α is a physical constant of a compound , like
boiling and melting points .table 14-2 p 365.
α : Specific rotation of (+ )Leucine : +10.8°
α : Specific rotation of (- )Leucine : -10.8°
االيزوميرات
الضوئية
Enantiomers rotate plane -polarized light by equal amounts
but in opposite direction .
These compounds called optical isomers
Difference between chirality and opticaL activity
Chiral molecule cannot be superimposed on its mirror image, on the
other hand ,an optically active compound rotates plane -polarized light.
Samples at some chiral compounds are not necessarily optically active.
Consider a sample of a compound this is a mixture of equal amounts of
both enantiomers ,the effect of one enantiomer on the plane polarized
light is exactly canceled by the effect of the other enantiomer and the
Supervised by CHEMICAL lect.NO.: ( )
Dr.Basma Date:23/3/2015
sample will be optically inactive(
(
خامل
ضوئيا
As long as there is more of one enantiomer than the other ,the sample
will be optically active.
Racemic mixture :A mixture of equal amounts of the two enantiomers
, (optically inactive ).
*in laboratory ,racemic mixtures are usually formed :
H CH
2
CH
3
CH
2
CH
3
CH
3
CH
2
CH
2
CH
3
+CL
2
Light
C CL + CL C H
Butane CH
3
CH
3
2-chlorobutane formed contains a chiral center ,(sample prepared is
optically inactive) because equal amounts of the dextro & levo isomers
are formed.
*in living system :the chemical reactions usually produce one
enantiomer(this is an important difference between reactions that
occur in the laboratory and those that occur in living systems.
