
0
Mustafa Hatim Kadhim
Baghdad University
Al-kindy college of medicine
Third Stage
2013 - 2014

1
List of contents
Lecture
number
Lecture name
Doctor name
Page number
Unit 1: Immunology (3 – 57)
1
Introduction
دكتورة
بتول
4 - 6
2
Antibodies & Cytokines
7 - 12
3
Cells of the immune system, receptors,
mechanisms of action
13 - 16
4
Major Histocompatibility Complex (MHC) &
Complement system
17 - 20
5
Hypersensitivity
21 - 25
6
Tolerance & Autoimmunity
26 - 28
7
Transplantation
29 - 31
8+9+10
Immune response to infectious diseases
دكتورة
ايمان
32 - 43
11+12+13
Immunodeficiency
44 - 50
14
Tumor Immunology
51 - 53
15
Vaccines
54 - 57
Unit 2: Bacteriology (58 – 135)
General Bacteriology (59 – 76)
1
General Microbiology
دكتور
نجاح
60 - 62
2
The Morphology & Fine structure of bacteria
63 - 65
3
The Physiology of Metabolism and Growth in
Bacteria
66 - 69
4
The Molecular Basis of Bacterial Genetics
70 - 72
5
The Principles of antibacterial therapy
73 - 76
Gram Positive Bacteria (77 – 103)
1
Staphylococcus
دكتور
نجاح
78 - 79
2
Streptococcus and Enterococcus
80 - 84
3
Pathogenic Neisseria, Moraxella and Acinetobacter
85 - 87
4
Aerobic Spore-Former Bacteria (Bacillus)
88 - 90
5
Anaerobic Spore-Former Bacteria (Pathogenic
Clostridia)
91 - 94
6
Corynebacterium & Diphtheroid
95 - 97
7
Mycobacteria
98 - 101

2
8
Nocardia & Listeria monocytogenes
102 - 103
Gram Negative Bacteria (104 – 135)
1+2+3
Enteric gramnegetive rods (enterobacteriaceae)
Enteric bacteria or coliform
دكتورة
جميلة
105 - 109
4
Pseudomonads, Acinetobacter & Uncommon gram
negative bacteria
110 - 111
5
Vibrios (vibrio spp.) & associated bacteria
112 - 113
6
Campylobacters & Helicobacter
114 - 115
7
Haemophilus
116 - 117
8
Bordetellae
118 - 119
9
Legionellae, Bartonella & unusual bacterial
pathogens
120 - 122
10+11
zoonotic gram negative rods
123 - 125
12+13
Spirochetes
126 - 130
14
Mycoplasma, Chlamydia & Rickettsiae
131 - 133
15
Normal microbial flora
134 - 135
Unit 3 – Mycology (136 – 150)
1
Introduction to mycology
دكتورة
اروى
137 - 139
2+3+4+5
Fungal diseases in humans (mycoses)
140 - 148
6
Antifungal Chemotherapy
149 - 150
Unit – Virology (151 – 192)
1+2+3
Introduction
دكتور
حيدر
152 - 156
4
Antiviral Drugs
157 - 159
5+6+ Half 7
RNA Enveloped viruses
160 - 167
Half 7+8
RNA non enveloped viruses
168 - 170
9+10
Retroviruses
171 - 175
11+12
DNA enveloped viruses
176 - 180
13
DNA Non-enveloped Virus
181 - 182
14+15+16
Viral Hepatitis
183 - 188
17
Human cancer viruses
189 - 190
18
Slow Viruses & Prions
191
19
Arbovirus
192

3

Unit 1 - Immunology
4
Lecture 1 - Introduction
Immunity: is resistance to disease
Immune system: collection of cells, tissues, molecules
that mediate response.
Immune response: Coordinated reaction of cells and
molecules to infectious disease.
Immunology: Is the science that study immune system
and its response to pathogens
Function of the immune system:
Prevent infection
Eradicate established infection
Types of immunity
1) Innate immunity:Exterior defense mechanism
Characteristics of innate immunity
1) Preexist 2) Not specific 3) No memory
Types of innate immunity:
1. Anatomic barrier( skin, mucous membrane)
2. Physiologic barriers( tempretire, PH, oxygen)
3. Chemical barriers( Lysozyme, Defensin, Interferon,
complement)
4. Cellular barrier: Neutrophils, Macrophage, Natural
killer cells and dendritic cells.
5. Toll-like receptors:It presents on phagocytic cells that
recognize broad molecules on pathogens enhancing
phagocytosis. It is called pattern recognition receptors
or pathogen associated molecular patterns.
2) Adaptive immunity
Characteristics of Adaptive immunity
1) Specificity 2) Diversity 3) Memory
4) Self non self-recognition 5) Clonal expansion
Central cell in the adaptive immune response
Lymphocytes
1- T lymphocytes-----cell mediated immunity
2- B lymphocytes-------humoral immunity
Antigens (Ag): any molecule that can be specifically
recognized by the adaptive immune system
Epitope: is a restricted part of Ag (short sequence of
sugars, a.a., that bind with antibodies (Abs).
Hapten: a small molecule had a low molecular weight
that cannot initiate an immune response unless its coupled
with a large carrier molecule
Super antigen: certain bacterial toxin (Staphylococcal
enterotoxins can bind to multiple T cells by binding to
T-cell receptor variable beta regions to alpha chain of
class II major histocompatibility complex on antigen
presenting cells.

Unit 1 - Immunology
5
Factors determining antigenicity
1. Degree of foreignness
2. Molecular size:100000 dalton
3. Chemical compositions: proteins-polysaccharide-lipid-
nucleic acids
4. Susceptibility to antigen processing
5. Genotype
6. Dose of immunogens
7. Route of adminstration
8. Adjuvants:substanc mix with Ag to enhance
immunogenicity(Alum-Aluminum potassium sulfate)
Generation of mature lymph. first occurs in the embryo in
-yolk sac
-fetal liver
-fetal bone marrow and continues throughout life
in birds /lymphoid organs called Bursa of fibricius
(primary site of B-cell maturation)
In humans –BM and other lymphoid tissue serve as Bursa
equivalent
Lymphocytes:
B lymphocytes (B cells)
T lymphocytes (T cells)
Important for the adaptive immune response.
B cells:
Surface receptor = Immunoglobulin (Ig).
Humoral Immunity.

Unit 1 - Immunology
6
Thymus
Its flat, bilobed organ situated above the heart. Each lobe
is surrounded by a capsule divide into lobule and
separated from each other by a trabeculae.Each lobule is
organized into cortex and medulla.
Hormones (thymosin, thymulin )and (enzymes like
adenosine deaminase)
Progenitor T cells in hematopoiesis in bone marrow then
ente thymus and acquire differentiation markers during
development calld CD markers (CD) markers.
Immature double negative (CD3+ CD4- CD8-) then
immature double positive thymocytes (CD3+ CD4+
CD8+) then mature single positive single negative
thymocytes (CD3+ CD4+ CD8-)(CD3+ CD4 –CD8+)
Thymic education and selection
The property of mature T cells is recognized only
foreign Ag (non self)+self MHC molecule. This can be
achived by selection process
Negative selection.
Positive selection.
The two processes called lymphocytes teachings.
Negative selection
Any lymph. Acquire receptors with high affinity for self
Ags will be die by a programmed cell death (apoptosis).
This occurs in the medulla by macrophages and dendretic
cells. (95-99%)
Positive selection
Any lymph. Acquire receptors recognize foreign Ags+
self MHC molecule will allow to mature and expand and
survive (1-5%). This occurs in the cortex of the thymus by
epithelial cells.
Lymphocytes homing
Lymphocytes leave thymus to sec. lymphoid organs (LN)
and to sites of inflammations through high endothelial
venules (HEV) by binding to specific receptors on lym.
and cell adhesion molecules on HEV after that lym.
homing to different tissues (GALT, MALT, skin dermal
endothelial venules ) by a cascade of interactions between
adhesions molecules on lym. And other cells.
Lymph nodes
Bean shaped, encapsulated ,containing a reticular network
packed with lymp., macrophages and dendretic cells .
Consist of 3 layers: Cortex, Paracortex & Medulla.
Unit 1 - Immunology
7
Lecture 2 – Antibodies & Cytokines
Immunoglobulin Antibody (Ab)
1) Blood from an individual and put it in a plain tube
without anticoagulant and left it for half an hour.
2) Blood will coagulate and you will get serum.
Protein electrophoresis: Gamma globulin fraction of
protein has antibody activity.
Antibody
Is a specific glycoprotein developed for a specific
antigen.
Synthesized by:
B-Cells armed on its surface and act as a surface
molecule bind an antigen
plasma cell that secrets free specific antibodies
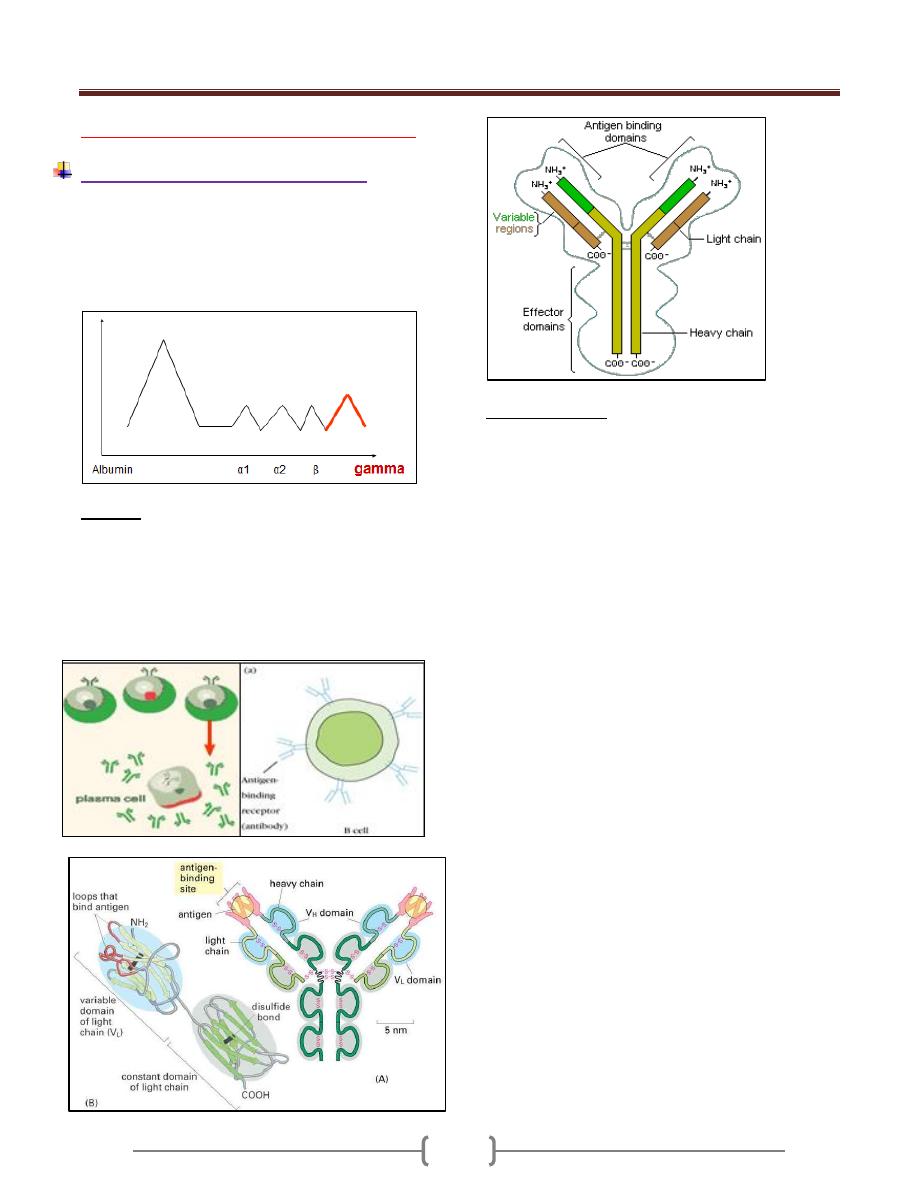
Structure of Ab
Each antibody is made up of two identical heavy
polypeptide chains and two identical light polypeptide
chains, shaped to form a Y Linked covalently bind by a
disulfide bounds
Heavy chain (H) has a molecular weight twice that of
light chain (L), so called heavy and light.
Each polypeptide chain is not linear but folded to form
domes or loops by intrachain disulfide bonds (-s-s) and
called domains.
Light chain had one VL and CL domain
Heavy chain had one VH an CH 1,2,3,
Hinge region: area of heavy chain between CH1 and CH2
domains where the disulfide bond is present. It is a
flexible area permits the movement of Ab binding
fragment (Fab) from 30-180
o
.
Each chain has two regions:
1) The Variable Region: makes up the tips of the Y's arms,
represent the amino terminal of polypeptide chain, varies
greatly in shape from one antibody to another.
This variation is due to change in aa sequence for this
reason called the variable region. it has unique shape
that "match" antigen to antibody., such as a lock
matches a key
2) The Constant Region: The stem of the Y activates the
complement system and encourages phagocytosis
Its amino acid content and sequence is relatively
constant and identical in all antibodies of the same
class and it's called the constant region. It represents
the carboxy terminal of polypeptide chain. This region
of the antibody molecule is called the Fc
region because it can be crystallized.

Unit 1 - Immunology
8
In this variable region (heavy and light) , there is a three
area called a hypervariable region in that area the aa
sequence is highly variable and called the
Complemantarity Determining Region (CDR). This binds
epitope of Ag.
The regions between the complementarity determining
regions are called the framework regions
Paratope: It is a small region (of 15–22 amino acids) of
the antibody's Fv region and contains parts of the
antibody's heavy and light chains
Affinity: Strength of interaction between single epitope
and single paratope.
Functions of Igs
1) Activation of complement
2) Opsonization
3) Ab dependent cell mediated cytotoxicity
4) (ADCC)
5) 4- Neutralization of toxins
6) 5- Agglutination of RBC
7) 6- Blocking the reaction

Unit 1 - Immunology
9
Immunoglobulin Classes (ISOTYPES)
Immunoglobulin classes
The immunoglobulins can be divided into five different
classes, based on differences in the amino acid
sequences in the constant region of the heavy chains.
1. IgG - Gamma heavy chains
2. IgM - Mu heavy chains
3. IgA - Alpha heavy chains
4. IgD - Delta heavy chains
5. IgE - Epsilon heavy chains
Immunoglobulin Subclasses
The classes of immunoglobulins can be divided into
subclasses based on small differences in the amino acid
sequences in the constant region of the heavy chains. All
immunoglobulins within a subclass will have very similar
heavy chain constant region amino acid sequences.
1. IgG Subclasses
a) IgG1 - Gamma 1 heavy chains
b) IgG2 - Gamma 2 heavy chains
c) IgG3 - Gamma 3 heavy chains
d) IgG4 - Gamma 4 heavy chains
2. IgA Subclasses
a) IgA1 - Alpha 1 heavy chains
b) IgA2 - Alpha 2 heavy chains
Immunoglobulin Types
Immunoglobulins can also be classified by the type of light
chain that they have. This based on differences in the amino
acid sequence in the constant region of the light chain
1-Kappa light chains
2- Lambda light chains
Immunoglobulin Subtypes
The light chains can also be divided into subtypes based
on differences in the amino acid sequences in the constant
region of the light chain.
1- Lambda 1 2- Lambda 2 3- Lambda 3 4- Lambda 4
Nomenclature
Immunoglobulins are named based on the class, or
subclass of the heavy chain & type or subtype of light
chain.
IgG = Immunoglobulin Gamma .
IgM - Immunoglobulin Mu
IgA - Immunoglobulin Alpha
IgD - Immunoglobulin Delta
IgE - Immunoglobulin Epsilon
IgG
Structure
The structures of the IgG are made up of two identical
heavy chains and two identical light chains.
All IgG's are monomer. MW=150 000 d.
called so because of its gamma heavy chain
The subclasses (IgG1, IgG2, IgG3, IgG4) differ in the
number of disulfide bonds and length of the hinge region.
Properties:
a) IgG is the major Ig in serum - 75% of serum Ig
b) IgG is the major Ig in extra vascular spaces
c) Placental transfer - IgG is the only class of Ig that crosses
the placenta. IgG2 does not cross well.
d) Fixes complement - Not all subclasses fix equally well;
IgG4 does not fix complement
e) Binding to cells - Macrophages, PMN IgG2 and IgG4 do
not bind to Fc receptors. A consequence of binding to the
Fc receptors on PMNs, monocytes and macrophages. The
antibody has prepared the antigen for eating by the
phagocytic cells. The term opsonin is used to describe
substances that enhance phagocytosis.
f) main Ig in the secondary immune response
IgA
Structure
1- Serum IgA is a monomer
2- IgA found in secretions is a dimer
When IgA exits as a dimer, a J chain is associated with it &
another protein associated with it called the secretory piece
J chain: small glycoprotein that are covalently linked to
the carboxy terminal portions of heavy chains.
Secretary component: is a polypeptide chain synthesized
by exocrine epithelial cells that enable IgA to pass
through mucosal tissues into secretions and protect IgA
from protease enzymes.
Properties
a) IgA is the 2nd most common serum Ig.
b) IgA is the major class of Ig in secretions - tears, saliva,
colostrum, mucus called secretory IgA
c) IgA activates the alternative pathway of complement
d) IgA can bind to some cells - PMN's and some
lymphocytes.
e) E) MW=150 000- 600 000 d
f) F) constitutes 10-15 % of serum Ig

Unit 1 - Immunology
10
g) It is called so because of its alpha heavy chain
components and of two subclasses:
1- Alpha 1-----IgA1
2- alpha 2 -----IgA2
IgM
Structure
1) IgM normally exists as a pentamer but it can also exist as
a monomer on B cell. In the pentameric form all heavy
chains are identical and all light chains are identical.
Thus, the valence is theoretically 10 times
2) IgM did not has a hing region and replaced by an extra
domain on the mu chain (CH4) , so it has 4 constant
heavy domains
3) It has another protein covalently bound via a S-S bond
called the J chain. This chain functions in polymerization
of the molecule into a pentamer.
Properties:
a. IgM is the third most common serum Ig. Constitute 5-
10% of total serum Ig .
MW=900 000 dalton
b. IgM is the first Ig to be made by the fetus and the first Ig
to be made by a virgin B cells as an Ag receptor.
c. As a consequence of its pentameric structure, IgM is a
good complement fixing
d. As a consequence of its structure, IgM is also a good
hemagglutinating Ig
e. IgM binds to some cells via Fc receptors.
f. Called so because of Mu heavy chain
IgE
Structure
IgE exists as a monomer and has an extra domain in the
constant region had four CH domains.
Properties
a) IgE is the least common serum Ig since it binds very
tightly to Fc receptors on basophils and mast cells
b) Involved in allergic reactions - Binding of the allergen to
the IgE on the cells results in the release of various
pharmacological mediators that result in allergic
symptoms.
It is called homocytotropic (bind cell) & called reagenic Ab
c) IgE also plays a role in parasitic helminth diseases. Since
serum IgE levels rise in parasitic diseases, measuring IgE
levels is helpful in diagnosing parasitic infections.
Eosinophils have Fc receptors for IgE and binding of
eosinophils to IgE-coated helminths results in killing of
the parasite.
d) IgE does not fix complement.
e) MW=190 000 d
f) constitutes about 0.002% of total serum Ig
g) called IgE because of its epsilon ε heavy chain
components
IgD
Structure
IgD exists only as a monomer.
Properties
a) IgD is found in low levels in serum; constitutes about
0.2% of total serum Ig
its role in serum uncertain.
MW=150 000 D
b) IgD is primarily found on B cell surfaces where it
functions as a receptor for antigen.
c) IgD does not bind complement
d) D) called IgD because of its delta δ heavy chain
components.
Variation of Igs
1) Isotypes: All classes and subclasses of Ig that are present
in normal individuals (IgG,IgM,IgA,IgE,IgD)
2) Allotype: That there is a single aa ifference in the
peptide chain in CH and CL chain
3) Idiotype: represents the antigen binding specificities of
Igs. The unique aa sequence of VH and VL can function
as antigenic determinants.

Unit 1 - Immunology
11
Immune response
primary humoral immune response. The first contact of
an exogenous Ag with an individual leads to generation a
Characteristics:
1- longer lag phase: during this period , the naive B cells
undergo clonal selection, clonal expansion and
differentiation into memory and plasma cells
2- Log phase (logarithmic): increase in IgM
concentration.
Secondary immune response
Second contact with same exogenous antigen, generates
secondary humoral immune response.
Characterization:
1-shorter lag phase
2-Rapid reaches a greater magnitude of IgG and last for
longer time. This is because of memory B-cells specific
for this Ag is existed. The processes of affinity maturation
and class switching are responsible for higher affinity to
Ag and different isotype
Vaccination (immunization)
Used to provoke a positive immune response by an
individual to various pathogenic microorganisms to
confer protection.
Polyclonal antibody
Most Ags possess multiple epitopes and each one of them
induce different B cells to proliferate into many clones of
cells that recognize different epitopes, these B cells secret
Abs, resulting into a mixture of Abs called polyclonal Abs
Monoclonal antibody
A clone of single B-cells that recognize a single epitope
that secret Abs spesific to a single epitope so it’s called
monoclonal Abs. It’s used for diagnostic and theraputic
purposes.

Unit 1 - Immunology
12
Cytokines
Are regulatory proteins or glycoproteins of low molecular
weight secreted by white blood cells and other cells in
response to a number of stimuli.
Function as intercellular messenger that evoke particular
biological activity after binding to a specific receptor.
Nomenclature
Lymphokines: cytokines secreted by lymphocytes.
Monokines: cytokines secreted by monocytes and
macrophages.
Interlukines
: cytokines are secreted by some leukocytes
and act upon other leukocytes.
IL-1
o Secreted by macrophages
o Act on lymphocytes
o Induce lymphocytes maturation , activation and clonal
expansion
o Acts on hypothalamus inducing fever
IL-2
o Secreted from Th1
o Acts on Ag specific T-cell supporting its growth
o Acts on NK cell increasing activity
o Acts on Tc cell increasing cytotoxicity
o Leads to development cell mediated immunity
o Suppress cytokines secreted from Th2 cells.
IL-3
o Secreted from Th2
o Supports growth and differentiation of hematopoietic cells
IL4 and IL-5
o Secreted from TH2
o Its up-regulate classII MHC expression
o Stimulate growth of mast cell
o Stimulate proliferation of activated B-cell
o Stimulate Abs secretions from plasma cell
o Stimulates humoral immune response
o Down regulates Th1
o IL-4 promotes class switch to IgE
o IL-5 promotes Eosinophil activation and generation
IL-6
o Secreted by macrophages and endothelial cells.
o Effect liver induces acute phase protein synthesis and
proliferation and antibody secretion of B-cells.
IL-10
o Secreted from Th2
o Antagonizes generation of Th1 subsets and cytokines
production by TH cell
o Mediate regulation of the immune system
Interferon (IFN)
o IFN α:secreted from leukocytes and inhibit viral
replication
o IFN β:secreted from fibroblasts and inhibit viral
replication
o IFN γ:secreted from Th1, Tc, NK cell and inhibit viral
replication,
Enhance activity of macrophages,
Increase MHC class-II expression,
Inhibits Th2 proliferation
Tumor necrosis factor (TNF)
o TNF :secreted from macrophages and act on tumor cells
o Had direct cytotoxic effect on tumor cells and tumor
undergoes visible hemorrhagic necrosis and regression by
inhibition angiogenesis, thereby decreasing the flow of
blood that is necessary for progressive tumor growth.
o Causes extensive loss weight (cachexia) by suppression
lipogenetic metabolism.

Unit 1: Immunology
13
Lecture 3 - cells of the immune system,
receptors, mechanisms of action

Unit 1: Immunology
14

Unit 1: Immunology
15

Unit 1: Immunology
16

Unit 1: Immunology
17
Lecture 4 - Major Histocompatibility
Complex (MHC) & Complement system

Unit 1: Immunology
18

Unit 1: Immunology
19

Unit 1: Immunology
20
Unit 1: Immunology
21
Lecture 5 - Hypersensitivity

Unit 1: Immunology
22

Unit 1: Immunology
23

Unit 1: Immunology
24
Unit 1: Immunology
25

Unit 1: Immunology
26
Lecture 6 - Tolerance & Autoimmunity

Unit 1: Immunology
27

Unit 1: Immunology
28

Unit 1: Immunology
29
Lecture 7 - Transplantation

Unit 1: Immunology
30

Unit 1: Immunology
31
Unit 1: Immunology
32
Lecture 8+9+10 - Immune response to
infectious diseases
(Viral infection)
Definition of a Virus
Sub microscopic organism consisting of a single nucleic acid
surrounded by a protein coat and capable of replication only
within the living cells of bacteria, human, animals or plants.
It is obligate Intracellular Parasite
Virus Replication
A. Virus attachment and entry
B. Uncoating of virion
C. Migration of genomic nucleic acid to nucleus, and
Transcrirption
D. Genomic replication
E. Translation of viral mRNA
F and G. Viroin assembly
H. Release of new virus particles
Transmission of Viruses
Respiratory transmission
Influenza A virus
Faecal-oral transmission
Enterovirus
Blood-borne transmission
Hepatitis B virus
Sexual Transmission
HIV
Animal or insect vectors
Rabies virus
Virus Tissue Tropism
Targeting of the virus to specific tissue and cell types
Receptor Recognition
CD4+ cells infected by HIV
CD155 acts as the receptor for poliovirus
In vivo Disease Processes
Cell destruction
Virus-induced changes to gene expression
Immunopathogenic disease
Acute Virus Infections
Localised to specific site of body
Development of viraemia with widespread infection of tissues
Immunity against Viral Infections
Within viruses life cycle they have a relatively short
extracellular period, prior to infecting the cells, and a longer
intracellular period during which they undergo replication.
The immune system has mechanisms which can attack the
virus in both these phases of its life cycle, and which involve
both innate and adaptive effectors mechanisms.
Immunological reactions are thus of two kinds:
Those directed against the virion are predominantly humoral
Those that act upon the virus infected cell are T-cell-mediated.
1) Innate Immunity:
Involves skin, mucous membrane, HCL, enzymes in tears and
secretions, toll-like receptors, but primarily through the
induction of type I interferons (α,β) and activation of NK
cells.
Toll-like receptors (TLR):
TLR allow cells to “see” molecules, it signifying presence of
microbes outside the cell e.g. TLR2, TLR4 and found in
variety of cell types
Toll-like receptor 9 (TLR9) Intracellular sensing in
monocytes, dendritic cells and lymphocytes
NOD proteins:
Are dipeptides binding intracellular protein, signaling via NF-
κb. These receptors recognize bacterial cell wall components
within cytoplasm

Unit 1: Immunology
33
RIG-I:
Intracellular sensing of RNA viruses e.g. Hepatitis C.
It induces type I interferons
A. Induction of type I interferons (IFN-α and IFN-β).
Are one of the first lines of defense against viral infections. ds
RNA produced during the viral life cycle can induce the
expression of IFN- α and IFN- β by the infected cells leading
to induction of "antiviral response or resistant to viral
replication” by binding to IFN- α / β receptor. They activate
the (JAK-STAT) transcription pathway, which in turn induces
the activation of several genes. One of these genes encodes the
enzyme known oligo-adenylate synthetase which activates a
ribonuclease (RNAse) that degrades viral RNA. and also
blocking viral replication.
B. Natural Killer Cells (NK):
NK cells possess the ability to recognize and lyses virally
infected cells and certain tumor cells.
During the initial stages of infection, NK cells undergo non-
specific proliferation mediated by IFN- α and IFN- β and IL-
12. There is no "lag" phase of clone expansion for NK cells to
be active as effectors, as there is with antigen-specific T and B
lymphocytes. Thus NK cells may be effective within 2 days of
viral infection, and may limit the spread of infection during
this early stage.
2) Adaptive Immunity:
A. Viral neutralization by humoral antibodies
Antibodies specific for viral surface antigens are crucial in
preventing the spreading of the virus during the acute infection
and in protecting against reinfection.
Secretary IgA (sIgA): blocking viral attachment to mucosal
cells.
IgG, IgM, IgA: blocks fusion of viral envelop to host cell
plasma membrane.
IgG, IgM: enhance phagocytosis by opsonization.
IgM: agglutinates viral particles.
Complement activated by Ag-Ab immune complex leading to
lysis of virus by membrane attack complex and lysis of
enveloped viral particles.
B. Cell mediated immune response mediated by cytotoxic T
lymphocytes (CD8+) that kill virus infected cell, after
induction by activated Th1 cells which produce a number of
cytokines including IL-2, IFN-γ and TNF-α that defends
against viruses either directly or indirectly.
Mechanism of killing of MHC class I- restricted CD8+ CTLs
cells specific for the virus elimination through the release of
perforin and granzymes or through Fas-FasL interaction. CTL
activity arises within 3-4 days, peak by 7-10 days and then
decline.
But in case of persistent virus infection (e.g. hepatitis B
virus), CTLs release IFN-γ and TNF, resulting in clearance
of the virus without death of the cell.
Viral evasion of host- defense mechanism
1)
Some viruses developed strategies to evade the action of IFN-
α/β. These include hepatitis C virus, which binds to IFN
receptor & blocking or inhibiting the action of protein kinase
(PKR).
2)
Herpes Simplex Viruses (HSV) inhibiting antigen
presentation by infected host cells.
HSV -1 and HSV-2 both express an immediate- early protein
that synthesized shortly after viral replication, they effectively
inhibits the human transporter protein TAP needed for
antigen processing, and thus blocks peptide association with
the class I MHC and effectively shut down a CD8+ T- cell
responses to HSV- infected cells.

Unit 1: Immunology
34
Other viruses used this strategies are: Adenoviruses and
Cytomegalovirus (CMV)
How can viruses interfere with endogenous Ag processing
as part of the active mechanism of evading immunity?
Herpes zoster virus can remain inactive or in latent state
inside the cell (latency virus genome in sensory ganglion) and
undergoing periodic cycles of activation and replication. The
viral genome remains within the host cell but no expression of
viral antigens occurs. When the host defense is upset perhaps
by other infections, the virus may be activated (it is the most
effective mechanism).
3)
CMV, Measles virus and HIV, have been shown to down
regulation of class II MHC on the cell surface, thus blocking
the function of antigen- specific anti- viral helper T cells.
HIV
4)
Vaccina virus is the prototypic member of the poxvirus
family of cytoplasmic DNA viruses have strategies for
evading complement- mediated destruction, by secretion a
protein that binds to the C4b complement component thus
inhibiting the classical pathway.
HSV have glycoprotein component that binds to C3b
complement components, and inhibiting both the classical
and alternative pathways.
5)
Large number of viruses causing generalized
immunosuppresion.
Either by direct infection of B lymphocytes (e.g. Epestien
Bar Virus), or macrophages (e.g. Measles virus) resulting in
direct lysis of immune cells or alter their function. HIV
destroy CD4+ T cells and macrophages
Some causes cytokine imbalance, (e.g. EBV) inhibits T
lymphocytes by producing a protein termed BCRF1 that is
homologous to IL-10 and thus suppress the cytokine
production by the Th 1 subset, resulting in decreased levels of
IL-2, TNF, and IFN-γ.
Measles Virus binds the complement regulatory protein CD46
(is a human cell receptor for measles virus ) on macrophages.
Example: paramyxoviruses that cause mumps, the measles
virus, epstein- barr virus (EBV), CMV and HIV.
6)
Some viruses escape immune attack by constantly changing
their antigens, as in the Influenza virus, Rhinoviruses, the
causative agent of the common cold, and HIV results in the
frequent emergence of new infectious strains due to mutation
and antigenic variation.
Influenza virus
Properties of the virus
o Myxovirus
o Enveloped virus with a segmented RNA genome
o Infects a wide range of animals other than humans
o Undergoes extensive antigenic variation
o Major cause of respiratory infections

Unit 1: Immunology
35
o Influenza viral particles or virions are spherical in shape
surrounded by an outer envelope, a lipid bilayer. Two
glycoproteins particles inserted into the envelope,
hemagglutinin (HA) and neuraminidase (NA), which form
radiating projections.
Hemagglutinin are responsible for the attachment of the
virus to host cells.
Neuraminidase facilitates viral budding from the infected
host cell.
Each virus strain is defined by its animal host of origin or
human, strain number, year of isolation, antigenic
description of HA and NA
o Two different mechanisms generate antigenic variation in HA
and NA:
1. Antigenic drift involves a series of spontaneous point
mutations that occur gradually, resulting in minor changes in
HA and NA.
2. Antigenic shift results in the sudden emergence of a new
subtype of influenza whose HA and possibly also NA are
different from that of the virus present in a preceding
epidemic.
Generation of Novel Influenza A Viruses
o Host response to influenza infection
Humoral antibody specific for the HA molecule is produced
during an influenza infection.
The antibody protects against influenza infection, but its
specificity is strain- specific and is readily bypassed by
antigenic drift.
CTLs can play a role in immune response to influenza.
Unit 1 - Immunology
36
Bacterial infection
Unit 1 - Immunology
37

Unit 1 - Immunology
38

Unit 1 - Immunology
39
Parasitic Infection
Immunity against parasites
Parasites of major medical important successfully adapted
to innate & acquired immune responses of host.
Parasites can cause direct damage to host by:
Competing for nutrients (e.g. tapeworms).
Disrupting tissues (e.g. Hydatid cyst) or destroying
cells (e.g. malaria, hookworm, schistosomiasis; feeding
on or causing destruction of cells causing anaemia).
Mechanical blockage (e.g. Ascaris in intestine).
However, severe disease often has a specific immune or
inflammatory component.
Protozoan Diseases
Protozoans are unicellular eukaryotic organisms. They are
responsible for several serious diseases in humans,
including amoebiasis, African sleeping sickness,
malaria, leishmaniasis, and toxoplasmosis.
Parasitic protozoa may live:
In the gut (e.g. amoebae)
In the blood (e.g. African trypanosomes)
Within erythrocytes (e.g. Plasmodium spp.)
In macrophages (e.g. leishmania spp., Trypanosomes)
In liver and spleen (e.g. leishmania spp.)
Immune responses against protozoan infection
The types of immune response that develop depend on
the location of the parasite within the host.
Many protozoans have life-cycle stages in which they are
free within the blood stream , the humoral antibody is
most effective (e.g. T. brucei)

Unit 1 - Immunology
40
Many of these same pathogens are also capable of
intracellular growth, during these stages, cell mediated
immune reaction are more effective in host defense.
(e.g. Plasmodium malariae (liver and blood stages),
T. cruzi and Leishmamia ( inside macrophages)
Role of Abs in protozoan infection
Antibody responses.
Extracellular protozoa are eliminated by:
Opsonisation and enhance phagocytosis.
Has direct damage, lysis of protozoa by Ag-Ab
immune complex and complement activation.
Intracellular protozoa are
Prevented from entering the host cells by a process of
neutralizing attachment sites, e.g. neutralising
antibody against malaria sporozoites, blocks cell
receptor for entry into liver cells.
Prevents escape from lysosomal vacuoles
Malaria (Plasmodium Species)
It is caused by various spp. of genus Plasmodium, of
which the P. falciparum is the most
virulent. Human infection begins when
sporozoites are introduced into
individual's blood stream as an infected
mosquito takes a blood meal. Then they
migrate to the liver, after that the released
merozoites infect RBCs initiating the symptoms and
pathology of malaria.
Host Response to Plasmodium Infection
In regions where malaria is endemic, the immune
response to plasmodium infection is poor with low
antibody titer.
The type of T cells responsible for controlling an infection
varies with the stage of infection, and depends upon the
kinds of cytokine they produce.
B cells mediate immunity against blood stage
CD8 T cells protect against the liver stage
The action of CD8+ T cells is two folds:
1) They secrete IFN-γ which inhibits the multiplication of
parasites within hepatocytes.
2) They are able to kill infected hepatocytes, but not
infected erythrocytes.
Evasion strategies of Plasmodium
Plasmodium needs time in host to complete complex
development, to sexually reproduce & to ensure vector
transmission.
Chronic infections (from a few months to many years) are
normal; therefore parasite needs to avoid immune
elimination.
Plasmodium has evolved a way of overcoming the
immune response by sloughing off the surface CS-
antigen coat, thus rendering the antibodies ineffective
(immunosupression)

Unit 1 - Immunology
41
The maturational changes from sporoziote to merozoite
to gametocyte allow the organism to keep changing its
surface molecule resulting in continual changes in the
antigen seen by the immune system.
African sleeping sickness (Trypanosoma
Species)
Two spp. of African trypanosomes (Trypanosoma brucei,
Trypanosoma cruzi), which are flagellated protozoan,
can cause sleeping sickness, it transmitted to humans and
cattle by the bite of tsetse fly.
The disease beginning with an early (systemic) stage in
which trypanosomes multiply in the blood and
progressing to a neurologic stage in which the parasite
infects the CNS causing meningeoecephalitis.
Host Response to Trypanosoma Infection
Humoral antibody
African Trypanosomes have one surface glycoprotein that
covers the parasite. This protein is immunodominant for
antibody responses .
The glycoprotein coat, called variant surface
glycoprotein (VSG).
These Abs eliminate most of the parasites from the blood
stream both by complement- mediated lysis and by
opsonization and subsequent phagocytosis.
However about 1% of the organisms which bear an
antigenically different VSG escape the initial Abs
response.
Evasion strategies of Trypanosoma
1) Antigenic variation
Trypanosoma brucei have “gene cassettes” of variant
surface glycoproteins (VSG’s) which allow them to
switch to different VSG.
Several unusual genetic processes generate the
extensive variation in trypanosomal VSG that enable
the organism to escape the immunological clearance.
VSG gene is switched regularly. The effect of this is
that host mounts immune response to current VSG
Abs but parasite is already switching VSG to another
type which is not recognised by the host. A parasite
expressing the new VSG will escape antibody
detection and replicate to continue the infection.
This allows the parasite to survive for months or years.
Up to 2000 genes involved in this process.
This type of antigenic variation is known as
phenotypic variation and is in contrast to genotypic
variation in the case of influenza virus in which a new
strain periodically results
2) Suppression of the immune response, e.g.
Trypanosoma cruzi produces molecules that either inhibit
the formation or accelerate the decay of C3 convertase, so
blocking complement activation on the parasite surface.
Leishmaniasis:
For Intra cellular protozoa
Leishmania major is a protozoan that
lives in the phagosomes of
macrophages.
Resistance to the infection correlates
with the cytokines secreted from TH1
and activation of Macrophages by
IFN-γ and TNF-α and killing by
Nitrous oxide and O2 metabolites

Unit 1 - Immunology
42
Immune evasion mechanisms of Intracellular protozoa
There are three main ways in which protozoa can evade or
modify the host's immunological attack:
1. Antigenic modulation
2. Resistance to macrophages killing
3. Suppression of the immune response
Protozoan immune evasion strategies
1. Leishmania can evade the immune surveillance by
Antigenic modulation, which can rapidly change their
surface coat (cap off) within minutes of exposure to
antibodies, so becoming refractory to the effects of
antibodies and complement.
2. Toxoplasma has evolved mechanisms which prevent
fusion of phagocytic vacuoles with lysosomes and resist
to macrophages killing.
3. Leishmania produce anti-oxidases to counter products of
macrophage oxidative burst, resist lysosomal enzymes
and suppressed the immune responses
Parasitic Worms (Helminthes)
Too large for phagocytosis BUT Immune response can
activate inflammation which results in expulsion of
worms.
Anti-worm IgE can activate degranulation of mast cells
and eosinophils leads to Type I hypersensitivity like
responses.
Initiation of response is poorly understood. Unusual
carbohydrates can be recognized by innate and adaptive
(antibody) responses. These responses are regulated by
the TH2 subsets of CD4 T lymphocytes.
The immunological responses in helminthes diseases
1) High titer of IgE that induced by a substance released
from the parasite acting as B cell mitogens.
2) Accumulation of mast cell and degranulation of these
cells releasing Eosinophils chemotactic factor (ECF),
Neutrophil chemotactic factor (NCF)
3) cytokines secretion by Th2 :
IL-4 induces B-cells to class switching to IgE
production ,
IL-5 induces bone marrow precursors to differentiate
into Eosinophils ,
IL-13 stimulates growth of mast cells
4) Ab- Ag complex activates complement ending in cell lysis
5) The eosinophils express Fc receptors for IgE and IgG and
bind to the Ab- coated parasite. Once bound to the
parasite, an eosinophil can participate in Ab- dependent
cell- mediated cytotoxicity (ADCC), releasing mediators
from its granules called Major basic protein (MBP) that
is toxic to helminthes causing small holes in the surface of
the helminth.
6) Neutrophils and macrophages act by releasing toxic O2
and N2 metabolites
7) In case of Nematodes, there is proliferation and
stimulation of goblet cells, increased mucus secretion
leads to expulsion of worm

Unit 1 - Immunology
43
Helminth immune evasion strategies
1) Antigenic disguise: (e.g. Adult Schistosoma)
Decrease expression of Ag on its outer surface.
Enclosed itself in a glycolipid and a glycoprotein coat
derived from the host , masking its own Ags Like
ABO blood group Ags
2) Suppression of T- & B- cell responses: young schistosomes
actively protect themselves by releasing peptidases that
cleave bound immunoglobulin & other factors that inhibit
both T-cell proliferation 7 release of IFN-γ or the mast cell
signal required for eosinophil activation.
3) Location inside the lumen of the gut like nematodes or
encysted inside protective cyst like Trichenella spirallis.
4) Presence of a thick extracellular cuticle like tegument
of Schistosomes which protect them from the immune
system
5) Molecules production that interfere with host immune
function e.g. Filarial worms secrete a protease inhibitor
Filarial worm evasion of immune responses
Compare between immune response to protozoa
and helminthes
Fungal Infection
Fungi are eukaryotes with a rigid cell wall enriched in
complex polysaccharides such as chitin, glucans. Among
the 70000 species of fungi, only a small number are
pathogenic for humans.
Fungal infections are regularly seen in:
* Patients with untreated AIDS.
* Patients with cancer and undergoing chemotherapy.
* Patients with transplants on immunosuppressive agents.
* Some patients taking long-term corticosteroids.
Degree of fungal infection can range from cutaneous to
deep and systemic

Unit 1 - Immunology
44
Lecture 4+5+6 - Immunodeficiencies
Definition
It is a condition in which the immune system is failed to
protect the host from disease-causing agents or from
malignant cells.
Immunodeficiency disease results from the absence or
failure of normal function of one or more elements of the
immune system.
The immunodeficiencies should be suspected in every
patient, irrespective of age, who has recurrent, persistent,
sever or unusual infections.
Classification
A. Primary immunodeficiency: a condition results from a
genetic or developmental defect in immune system
(intrinsic defect). In such a condition, the defect is present
at birth although it may manifest itself later in life.
B. Secondary immunodeficiency or (acquired
immunodeficiency): is the loss of immune function and
results from exposure to various agents (disease or therapy).
The most common one is acquired immune-deficiency
syndrome or AIDS, which results from infection with the
human immunodeficiency virus 1 (HIV-1).
1) Primary immunodeficiency
Primary immunodeficiencies may affect either adaptive or
innate immune functions.
Specific immunodeficiency diseases involve
abnormalities of T or B cells, the cells of the adaptive
immune system.
Non-specific immunodeficiency diseases involve
abnormalities of elements such as complement proteins or
phagocytes, which act non-specifically in immunity..
Immunodeficiency diseases cause increased susceptibility
to infection in patients. The infections encountered in
immunodeficient patients fall into two categories:
Patients with defects in immunoglobulins, complement
proteins or phagocytes are very susceptible to recurrent
infections with encapsulated bacteria such as
Haemophilus influenzae, Streptococcus pneumoniae and
Staphylococcus aureus. These are called pyogenic
infections, because the bacteria give rise to pus formation.
On the other hand, patients with defects in cell-
mediated immunity, in T cells, are susceptible to
overwhelming, even lethal; infections with opportunistic
microorganisms include yeast and common viruses such
as chickenpox.
Defects in the lymphoid Lineage
A. Primary B-cell deficiencies include:
Patients with common defects in B-cell function have
recurrent pyogenic infections such as pneumonia, otitis
media and sinusitis
1) X- Linked Agammaglobulinemia (XLA) early
B- cell maturation fails
Affected males have few or no B cells in their blood or
lymphoid tissue; their lymph nodes are very small and
their tonsils are absent. Their serum usually contains no
IgA, IgM, IgD or IgE, and only small amounts of IgG
(less than 1 md/dl). Infants for the first 6 – 12 months of
life are protected from infection by the maternal IgG. As
this supply of IgG is exhausted, affected male develop
recurrent pyogenic infections.
The gene that is defective in X-LA is a B-cell cytoplasmic
tyrosine kinase (btk) belonging to the src oncogene family.
It encoded B- cell signal transduction molecule called
Burton’s tyrosin kinase is obviously vital for the process of
B-cell maturation. Bone marrow of males with X-LA
contains normal numbers of pre-B cells but, as a result of
mutations in the btk gene, they cannot mature to B cells.
Treatment by periodic Intravenous administration of Igs

Unit 1 - Immunology
45
2) X- Linked Hyper IgM Syndrome (XHM)
In XHIgM the B cells cannot make the switch from IgM
to IgG, IgA and IgE synthesis that normally occurs in B-
cell maturation.
As a result, patients have decreased levels of serum IgG
and IgA and elevated levels of IgM some times as high as
10 mg/ml (normal Igm concentration is 1.5 mg/ ml).
It results from a variety of genetic defects that affect the
interaction between T-lymphocytes and B-lymphocytes.
It is inherited as an X- linked recessive disorder.
In normal B cells, this switch to IgE is induced by two
factors:
IL-4 must bind to the B-cell receptor for IL-4, and
The CD40 molecule on the B-cell surface must bind to
the CD40 ligand on activated T cells.
70% is due to defect in the gene encoding the CD40
ligand (CD40L) on the membrane of TH cells
Children in first two years suffer recurrent infections,
especially respiratory infections caused by opportunistic
pathogens.
Treatment by administration of intravenous Ig.
3) Common variable immunodeficiency (CVID)
There are defect in T cell signaling to B cells.
Individuals with CVID have acquired
agammaglobulinaemia in the second or third decade of
life, or later. Both males and females are equally affected
and the cause is generally not known, but may follow
infection with viruses such as Epstein – Barr virus (EBV).
Patients with CVID, like males with X-LA, are very
susceptible to pyogenic organisms.
Most patients (80%) with CVID have B cells that do not
function properly and are immature. The B cells are not
defective; instead, they fail to receive proper signals from
the T cells.
Patients with CVID should be treated with intravenous
gammaglobulin.
4) Selective IgA deficiency
B. Primary T-cell deficiencies include:
1) Severe Combined Immunodeficiency (SCID):
Infants with SCID have very few lymphocytes in their
blood (fewer than 3000/ml). Their lymphoid tissue also
contains few or no lymphocytes. The thymus has a fetal
appearance.
Patients with no T cells, or poor T-cell function, are
susceptible to opportunistic infections. Since B-cell
function in humans is largely T-cell dependent, T-cell
deficiency also results in humoral immunodeficiency.
The infants have prolonged diarrhea due to rotavirus or
bacterial infection of the GIT, and develop pneumonia
usually due to protozoal infection.
The common yeast organism Candida albicans grows in
the mouth or on the skin of the patients with SCID.
If the patients with SCID are vaccinated with live
organisms such as poliovirus or BCG they die from
progressive infection with these organisms
Unit 1 - Immunology
46
1. Over 50% of cases are caused by a gene defect on the X
chromosome. SCID is more common in males than
females infants (3:1)
Genetic defect in γ-chain of the IL-2R also shard
receptors for other cytokines IL-4, 7, 11, and 15.
2. The remaining cases of SCID are due to recessive genes
on other chromosomes of these, half have a genetic
deficiency of adenosine deaminase (ADA) or purine
nucleoside phosphorylase (PNP), resulting in the
accumulation of metabolites that are toxic to lymphoid
stem cells. These metabolites inhibit the enzyme
ribonucleotide reductase, which is required for DNA
synthesis and for all replication.
3. Other autosomal recessive form of SCID results from a
mutation in either of the recombinase- activating genes
encoding RAG-1 or RAG-2.
These two genes are absolutely required for cleaving
double- stranded DNA before recombination of DNA
to form the immunoglobulin genes and the genes
encoding the lymphocyte cell receptor that
characterized mature B and T cells.
If these gene rearrangements do not occur, B and T
cells do not develop.
The optimal treatment: is a bone marrow transplant from a
completely histocompatible donor, usually normal sibling,
or the affected infants die within the first 2 years of life.
Also gene therapy of RAG
2) The DiGeorge Syndrome (Congenital Thymic
Aplasia)
The thymic epithelium is derived from the third and
fourth pharyngeal by the sixth week of human gestation.
Defect is associated with the deletion in the embryo of a
region on chromosome 22
The T-cell deficiency is variable, depending on how badly
the thymus is affected. Affected infants have distinctive
facial features in that their eyes are widely separated.
They also have congenital malformations of the heart or
aortic arch and neonatal tetany from the hypoplasia or
aplasia of the parathyroid glands.
Treatment is by supportive therapy, or thymic
epithelial transplant.
3) Wiskott- Aldrich Syndrome (WAS):
WAS is an X-linked immunodeficiency disease.
Affected males have small and abnormal platelets, which
are also few in numbers (thrombocytopenia) which may
lead to fatal bleeding. Boys with WAS develop severe
eczema as well as pyogenic and opportunistic infections.
Their serum contains increased amounts of IgA & IgE,
normal levels of IgG and decreased amounts of IgM.
Their T cells are defective in function. This fails to
occur in the WAS, with the result that collaboration
among immune cell is faulty.

Unit 1 - Immunology
47
C. Genetic Defect in Complement Proteins
Deficiencies of the classical pathway components, C1q,
C1r, C1s, C4, or C2 results in susceptibility to develop
immune complex disease such as systemic lupus
erythematosus (SLE). This correlates with the known
function of the classical pathway in the dissolution of
immune complexes.
Deficiencies of C3, and the alternative pathway
components, factor H, or factor I result in increased
susceptibility to pyogenic infection; this correlates with
the important role of C3 in opsonization of pyogenic
bacteria.
Deficiencies of the terminal components C5-8, and of
the alternative pathway components, factor D and
properdin results in remarkable susceptibility to
infection with two pathogenic spp. Of Neisseria,
gonorrhoeae, and meningitides.
All these genetic complement component deficiencies
are inherited.
Treatment usually maintained with antibiotics.
Hereditary angioneurotic edema (HAE) is
due to C1 inhibitor deficiency
It is well-known disease resulted due to deficiency of the
complement system C1 inhibitor. This molecule is
responsible for dissociation of activated C1, by binding to
C1r2 and C1s2.
This disease is inherited as an autosomal dominant
trait
C1 inhibitor deficiency may be acquired later in life. In
some cases an autoantibody to C1inhibitor is found.
Patients with HAE have recurrent episodes of swelling of
various parts of the body (angioedema). When the edema
involves the intestine, abdominal pains ad cramps results,
with severe vomiting.
When the edema involves the upper airway, the patients
may choke to death from respiratory obstruction.
Angioedema of the upper airway therefore presents a
medical emergency, which requires rapid action to restore
normal breathing.
D. Defects in phagocytes
Phagocytic cells – polymorphonuclear leucocytes and
cells of the monocyte /macrophage lineage – are
important in host defense against pyogenic bacteria and
other intracellular microorganisms. A severe deficiency
of polymorphonuclear leucocytes (neutropenia) can result
in overwhelming bacterial infection.
Two genetic defects of phagocytes are clinically
important in that they result in susceptibility to severe
infections and are often fatal includes:
1. Chronic granulomatous disease
2. Leucocyte adhesion deficiency.
1) Chronic granulomatous disease (CGD) is due
to a defect in the oxygen reduction pathway
Is a genetic disease, 70% is an X- linked form
Defect in the ability of macrophages and PMNs to kill
phagocytosed organisms
Decrease in the ability of macrophages to serve as APCs.
Patients with CGD have defective NADPH oxidase
which catalyzes the reduction of O
2
to •O
2
by the
reaction:
NADPH + 2O
2
→ NADP
+
+ 2•O
2-
+ H
+
Thus, they are incapable of forming superoxide anions
(•O
2
) and hydrogen peroxide in their phagocytes,
following ingestion of microorganisms and so cannot
readily kill ingested bacteria or fungi organisms.
As a result, microorganisms remain alive in phagocytes of
patients with CGD. This gives rise to a cell-mediated
response to persistent intracellular microbial antigens, and
granulomas form. Children with CGD develop
pneumonia, infections the lymph nodes (lymphadenitis),
and abscesses in the skin, liver and other viscera.
Treatment with antibiotics
2) Leukocyte Adhesion Deficiency (LAD)
The receptor in the phagocyte membrane that binds to
C3b on opsonized microorganisms is critical for the
ingestion of bacteria by phagocytes. This receptor, an
integrin called complement receptor 3 (CR3), is
deficient in patients with LAD and consequently they

Unit 1 - Immunology
48
develop severe bacterial infections, particularly of the
mouth and GIT.
CR3 is composed of two polypeptide chains: an α chain
and β chain. In LAD, there is a genetic defect of the β
chain of CR3, encoded by a gene on chromosome 21.
Other integrin proteins share the same β chain, namely
lymphocyte function associated antigen (LFA-1) .
Genetic defect of LFA-1 leading to impairment of
adhesion of leukocytes to vascular endothelium and limits
recruitment of cells to sites of inflammation.
LAD varies in its severity; some affected individuals die
within a few years, whereas others may survive into their
forties.
2) Secondary or Acquired
Immunodeficiency
It results from exposure to a number of chemical &
biological
agents that induce immunodeficient state.
A. Drugs:
Corticosteroids: commonly used for treatment of
autoimmune disorders interfere with the immune response
in order to relief the disease symptoms
Immunosuppressive drugs, such as cyclosporine- A
used in transplantation patients which block the immune
attack to transplanted organ.
Cytotoxic drugs or radiation treatments given to treat
various forms of cancer frequently damage the dividing
cells in the body (depression hematopoiesis)
B. Nutrient Deficiency
Lymphoid tissues are very vulnerable to the damaging
effects of malnutrition.
Numerous enzymes with key roles in immune processes
required zinc, iron, vitamin B6, and other
micronutrients including selenium, and copper.
Lymphoid atrophy is a prominent morphological feature
of malnutrition (T selective deficiencies)
C. Infection:
Acquired Immune Deficiency Syndrome (AIDS):
The most significant global cause of immunodeficiency is
HIV infection. Over 25 million people have died from
AIDS since the first cases were described in 1981. As the
end of 2004, WHO estimate that, approximately 40
million people are living with HIV infection worldwide,
with approximately 5 million new infections and 3 million
deaths each year.
Acquired Immune Deficiency Syndrome (AIDS)
AIDS: Is caused by Human Immunodeficiency Virus
(HIV) a retrovirus, which is found in all cases of the
disease.
The primary targets of HIV are activated CD4+ T helper
lymphocytes but the virus can also infect several other
cell types including macrophages.
Infection leads to loss of T4 helper lymphocytes and
immunosuppression in the patient and the consequent
fatal opportunistic infections.
HIV is a lentivirus, a class of retrovirus.
The name lentivirus means slow virus, so called because
these viruses take a long time to cause overt disease.
Most lentiviruses target cells of the immune system and
thus disease is often manifested as immunodeficiency
Unit 1 - Immunology
49
There are two types of HIV: HIV-1 and HIV-2. These
cause clinically indistinguishable disease, although the
time to disease onset is longer for HIV-2.
The worldwide epidemic of HIV and AIDS is caused
by HIV-1 while HIV-2 is mostly restricted to west Africa
CD4 antigen is the main receptor for the virus entry, and
is present on CD4+ T lymphocytes and monocytes.
The binding of the viral envelope glycoprotein gp120 to
CD4 antigen results in conformational changes in gp120
that expose binding sites for chemokine receptors, which
serve as co-receptors for viral entry, these includes CCR5
and CXCR4
The disease is appeared at the first time in 1981, as clusters
of cases of Kaposi's sarcoma were reported in young
patients in San Francisco and New York. This was an
unusual occurrence since, in the United States, Kaposi's
sarcoma was a rare disease that normally occurred in
elderly men of Jewish or Mediterranean ancestry. however,
these new clusters of patients were all young male
homosexuals and the disease was much more aggressive
Immune Responses against HIV infection
Cell-mediated and humoral anti-HIV immune defense:
Cytotoxic T and B lymphocytes mount a strong defense
and virus largely disappears from the circulation.
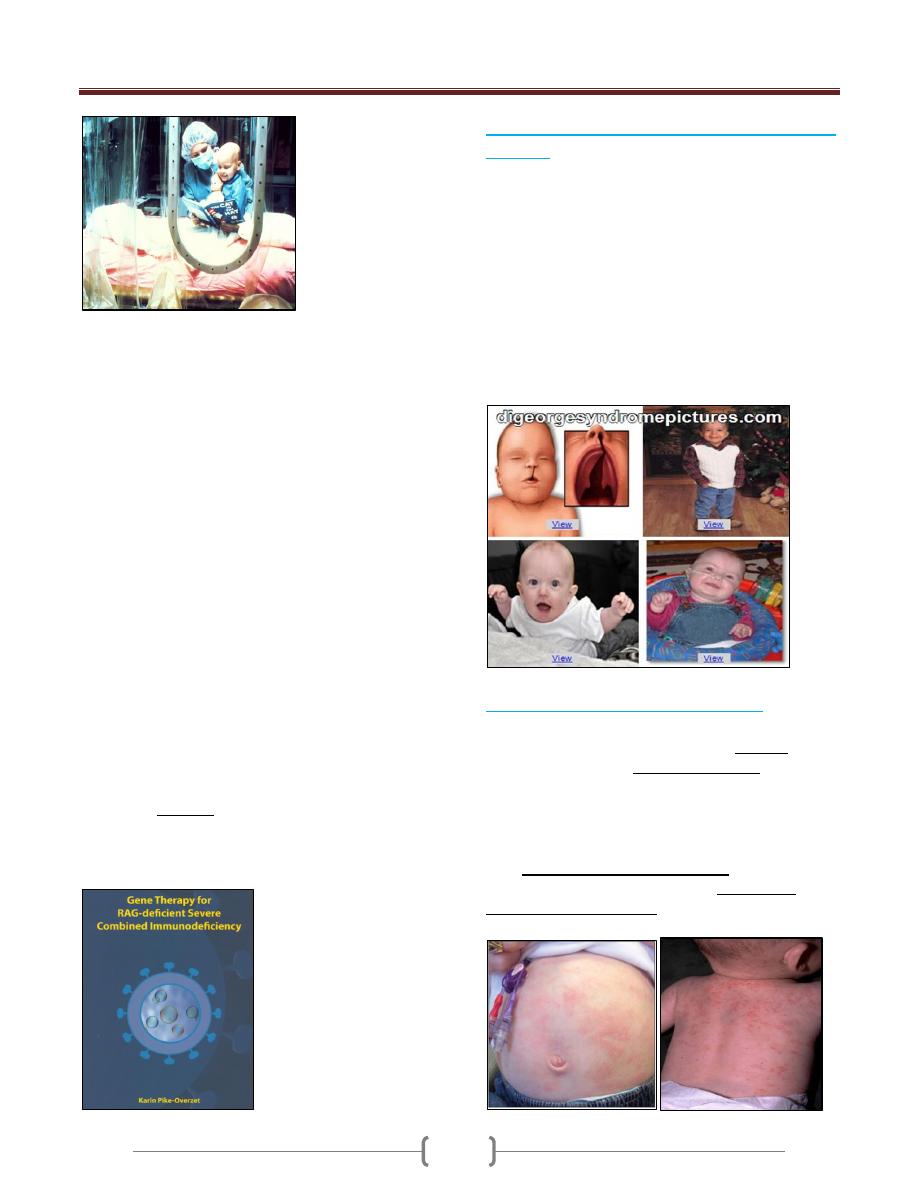
Virus titer, CD4T cells and anti-gp120 titer during the
HIV infection
After the increased cell-mediated immune response, there
is a rise in antibodies in the serum of infected individuals
2-3 weeks after infection, but though these lack the ability
to inhibit viral infection. During this period, more than 10
billion new HIV particles are produced each day. They are
rapidly cleared by the immune system especially anti-
HIV antibody (gp120). So neutralizing antibodies play a
role in controlling HIV viremia.
Despite the presence of high numbers of HIV specific
CTLs in the peripheral blood, but like antibody
responses unable to eliminate infection.
At this stage, most of this virus is coming from
recently infected proliferating CD4
+
cells. Thus, the
virus is destroying the cells that are proliferating.
The infected cells that are producing this virus are
destroyed either by the immune system or by the virus
and have a half-life about 1 day.
Although activated, proliferating CD4+ cells are
destroyed by the immune system, a small fraction of the
infected cells survive long enough to revert back to the
resting memory state (as do non-infected CD4
+
memory cells).
The resting memory cells do not express viral antigens but
do carry a copy of the HIV genome which remains latent
until the cells are reactivated by antigen. These memory
cells may survive many years and constitute a
reservoir that is very important in drug-based
therapy.

Unit 1 - Immunology
50
During this period, the virus disseminates to other regions
including to lymphoid and nervous tissue. This is the most
infectious phase of the disease.
Loss of CD4
+
cells & collapse of the immune
response
During the course of infection, there is a profound loss of
the specific immune response to HIV because:
Responding CD4+ cells become infected. Thus, there is
clonal deletion leading to tolerance and escape of HIV
from the immune surveillance.
Activated CD4+ T cells are susceptible to apoptosis.
Spontaneous apoptosis of uninfected CD4
+
and CD8
+
T
cells occurs in HIV-infected patients.
Also there appears to be selective apoptosis of HIV-
specific CD8
+
cells
the number of follicular dendritic cells falls over time,
resulting in diminished capacity to stimulate CD4+ cells
More severe infections are associated with a low
CD+4 T cell count
It is the phase of the disease that lacks the neoplasms and
opportunistic infections that are the definition of AIDS
Patients at this stage of the disease show weight loss and
fatigue together with fungal infections of the mouth,
finger and toe nails especially with Candida
Orofacial
granulomatos
is with cobble
stone mucosa
in AIDS
Facial
sarcoidosis
in AIDS
Opportunistic
infections that
are the definition
of AIDS

Unit 1 - Immunology
51
Lecture 7 – Tumor Immunology

Unit 1 - Immunology
52

Unit 1 - Immunology
53

Unit 1 - Immunology
54
Lecture 8 – Vaccines

Unit 1 - Immunology
55

Unit 1 - Immunology
56
Making of DNA Vaccine against West Nile Virus

Unit 1 - Immunology
57

58

59

Unit 2: Bacteriology
06
Lecture 1 – General Microbiology
Microbiology in Medicine & Host – Parasite
Relationship
Microbiology: is the study of microorganisms, which are
tiny organisms that live around us & inside our body. An
infection is caused by the infiltration of a disease-causing
microorganism known as a Pathogenic microorganism.
Some pathogenic microorganisms infect humans, but not
other animals & plants. Some pathogenic microorganisms
that infect animals or plants also infect humans. Not all
microorganisms are pathogens. In fact many
microorganisms help to maintain homeostasis in our
bodies and are used in the production of food in our
intestines that assist in the digestion of food & play a
critical role in the formation of vitamins such as vitamin
B & vitamin K. They help by breaking down large
molecules into smaller ones.
The History of Infectious Diseases
The Past
Infectious diseases have been known for thousands of
years, although accurate information on their etiology has
only been available for about a century. In the medical
teachings of Hippocrates, the cause of infections
occurring frequently in a certain locality or during a
certain period (epidemics) was sought in “changes” in the
air according to the theory of miasmas. This concept, still
reflected in terms such as “swamp fever” or “malaria,”
was the predominant academic opinion until the end of
the 19th century, despite the fact that the Dutch cloth
merchant A. van Leeuwenhoek had seen and described
bacteria as early as the 17th century, using a microscope
he built himself with a single convex lens and a very short
focal length. At the time, general acceptance of the notion
of “spontaneous generation”—creation of life from dead
organic material—stood in the way of implicating the
bacteria found in the corpses of infection victims as the
cause of the deadly diseases. It was not until Pasteur
disproved the doctrine of spontaneous generation in the
second half of the 19th century that a new way of thinking
became possible. By the end of that century,
microorganisms had been identified as the causal agents
in many familiar diseases by applying the Henle-Koch
postulates formulated by R. Koch in 1890.
The Henle–Koch Postulates
The postulates can be freely formulated as follows:
The microorganism must be found under conditions
corresponding to the pathological changes and clinical
course of the disease in question.
It must be possible to cause an identical (human) or
similar (animal) disease with pure cultures of the
pathogen.
The pathogen must not occur within the framework of
other diseases as an “accidental parasite.”
These postulates are still used today to confirm the cause
of an infectious disease.
However, the fact that these conditions are not met does
not necessarily exclude a contribution to disease etiology
by a pathogen found in context. In particular, many
infections caused by subcellular entities do not fulfill the
postulates in their classic form.
The Present
The frequency and deadliness of infectious diseases
throughout thousands of years of human history have kept
them at the focus of medical science. The development of
effective preventive and therapeutic measures in recent
decades has diminished, and sometimes eliminated
entirely, the grim epidemics of smallpox, plague, spotted
fever, diphtheria, and other such contagions. Today we
have specific drug treatments for many infectious
diseases. As a result of these developments, the attention
of medical researchers was diverted to other fields: it
seemed we had tamed the infectious diseases. Recent
years have proved this assumption false. Previously
unknown pathogens causing new diseases are being found
and familiar organisms have demonstrated an ability to
evolve new forms and reassert themselves. The origins of
this reversal are many and complex: human behavior has
changed, particularly in terms of mobility and nutrition.
Further contributory factors were the introduction of
invasive and aggressive medical therapies, neglect of
established methods of infection control and, of course,
the ability of pathogens to make full use of their specific
genetic variability to adapt to changing conditions.The
upshot is that physicians in particular, as well as other
medical professionals and staff, urgently require a basic
knowledge of the pathogens involved and the genesis of
infectious diseases if they are to respond effectively to
this dynamism in the field of infectiology. The aim of this
textbook is to impart these essentials to them.

Unit 2: Bacteriology
06
Pathogens: Prokaryotic and Eukaryotic
Microorganisms
According to a proposal by Woese that has been gaining
general acceptance inrecent years, the world of living
things is classified in the three domains: bacteria,
archaea, and eucarya. In this system, each domain is
subdivided into
kingdoms. Pathogenic microorganisms
are found in the domains bacteria and eucarya.
Bacteria. This domain includes the kingdom of the
heterotrophic eubacteria and includes all human pathogen
bacteria. The other kingdoms, for instance that of the
photosynthetic cyanobacteria, are not pathogenic. It is
estimated that bacterial species on Earth number in the
hundreds of thousands, of which only about 5500 have
been discovered and described in detail.
Archaea. This domain includes forms that live under
extreme environmental conditions, including thermophilic,
hyperthermophilic, halophilic,and methanogenic
microorganisms. The earlier term for the archaea was
archaebacteria (ancient bacteria) , and they are indeed a
kind of living fossil. Thermophilic archaea thrive mainly in
warm, moist biotopes such as the hot springs at the top of
geothermal vents. The hyperthermophilic archaea, a more
recent discovery, live near deep-sea volcanic plumes at
temperatures exceeding 100 °C.
Eucarya. This domain includes all life forms with cells
possessing a genuine nucleus.The plant and animal
kingdoms (animales and plantales) are all eukaryotic life
forms. Pathogenic eukaryotic microorganisms include
fungal and protozoan species.
Classic bacteria.
These organisms reproduce asexually by binary transverse
fission. They do not possess the nucleus typical of
eucarya. The cell walls of these organisms are rigid (with
some exceptions, e.g., the mycoplasma).
Chlamydiae. These organisms are obligate intracellular
parasites that are able to reproduce in certain human cells
only and are found in two stages: the infectious,
nonreproductive particles called elementary bodies (0.3
µm) and the noninfectious, intracytoplasmic, reproductive
forms known as initial (or reticulate) bodies (1 µm).
Rickettsiae. These organisms are obligate intracellular
parasites, rod-shaped to coccoid, that reproduce by binary
transverse fission. The diameter of the individual cell is
from 0.3–1 µm.
Mycoplasmas. Mycoplasmas are bacteria without rigid
cellwalls. They are found in a wide variety of forms, the
most common being the coccoid cell (0.3–0.8
µ
m).
Threadlike forms also occur in various lengths.
Fungi and Protozoa
Fungi. Fungi (Mycophyta) are nonmotile eukaryotes with
rigid cell walls and a classic cell nucleus. They contain no
photosynthetic pigments and are carbon heterotrophic,
that is, they utilize various organic nutrient substrates (in
contrast to carbon autotrophic plants). Of more than 50
000 fungal species, only about 300 are known to be
human pathogens. Most fungal infections occur as a result
of weakened host immune defenses.
Protozoa. Protozoa are microorganisms in various sizes
and forms that may be free-living or parasitic. They
possess a nucleus containing chromosomes and organelles
such as mitochondria (lacking in some cases), an en-
doplasmic reticulum, pseudopods, flagella, cilia,
kinetoplasts, etc. Many parasitic protozoa are transmitted
by arthropods, whereby multiplication and transformation
into the infectious stage take place in the vector.
Animals
Helminths. Parasitic worms belong to the animal
kingdom. These are metazoan organisms with highly
differentiated structures. Medically significant groups
include the trematodes (flukes or flatworms), cestodes
(tapeworms), and nematodes (roundworms).
Subcellular Infectious Entities
Prions (proteinaceous infectious particles). The evidence
indicates that prions are protein molecules that cause
degenerative central nervous system (CNS) diseases such
as Creutzfeldt-Jakob disease, kuru, scrapie in sheep, and
bovine spongiform encephalopathy (BSE) (general term:
transmissible spongiform encephalopathies [TSE]).
Viruses. Ultramicroscopic, obligate intracellular parasites
that:
contain only one type of nucleic acid, either DNA or RNA
possess no enzymatic energy-producing system and no
protein-synthesizing apparatus, and
Force infected host cells to synthesize virus particles.
Arthropods. These animals are characterized by an external
chitin skeleton, segmented bodies, jointed legs, special
mouthparts, and other specific features. Their role as direct
causative agents of diseases is a minor one (mites, for
instance, cause scabies) as compared to their role as vectors
transmitting viruses, bacteria, protozoa , & helminths.

Unit 2: Bacteriology
06
Basic Terminology of Infectiology
The terms pathogenicity and virulence are not clearly
defined in their relevance to microorganisms. They are
sometimes even used synonymously. It has been proposed
that pathogenicity be used to characterize a particular
species and that virulence be used to describe the sum of
the disease-causing properties of a population (strain) of a
pathogenic species.
Pathogenicity and virulence in the microorganism
correspond to susceptibility in a host species and
disposition in a specific host organism, whereby an
individual may be anywhere from highly disposed to
resistant.
Host–Pathogen Interactions
The factors determining the genesis, clinical picture and
outcome of an infection include complex relationships
between the host and invading organisms that differ
widely depending on the pathogen involved. Despite this
variability, a number of general principles apply to the
interactions between the invading pathogen with its
aggression factors and the host with its defenses. Since
the pathogenesis of bacterial infectious diseases has been
researched very thoroughly, the following summary is
based on the host–invader interactions seen in this type of
infection. The determinants of bacterial pathogenicity
and virulence can be outlined as follows:
Adhesion to host cells (adhesins).
Breaching of host anatomical barriers (invasins) and
colonization of tissues (aggressins).
Strategies to overcome nonspecific defenses, especially
antiphagocytic mechanisms (impedins).
Strategies to overcome specific immunity, the most
important of which is production of IgA proteases
(impedins), molecular mimicry, and immunogen
variability.Damage to host tissues due to direct bacterial
cytotoxicity, exotoxins, and exoenzymes (aggressins).
Damage due to inflammatory reactions in the
macroorganism: activation of complement and
phagocytosis; induction of cytokine production
(modulins).
The above bacterial pathogenicity factors are
confronted by the following host defense mechanisms:
Nonspecific defenses including mechanical, humoral, and
cellular systems. Phagocy- tosis is the most important
process in this context.
Specific immune responses based on antibodies and
specific reactions of T lymphocytes.
The response of these defenses to infection thus involves
the correlation of a number of different mechanisms.
Defective defenses make it easier for an infection to take
hold. Primary, innate defects are rare, whereas acquired,
secondary immune defects occur frequently, paving the
way for infections by microorganisms known as
“facultative pathogens” (opportunists).
Determinants of Bacterial Pathogenicity and Virulence
Relatively little is known about the factors determining
the pathogenicity and virulence of microorganisms, and
most of what we do know concerns the disease -causing
mechanisms of bacteria.
There are five groups of potential bacterial contributors to
the pathogenesis of infectious diseases:
1) Adhesins. They facilitate adhesion to specific target cells.
2) Invasins. They are responsible for active invasion of the
cells of the acroorganism.
3) Impedins. These components disable host immune
defenses in some cases.
4) Aggressins. These substances include toxins and tissue-
damaging enzymes.
5) Modulins. Substances that induce excess cytokine
production (i.e., lipopolysaccharides of Gram-negative
bacteria, superantigens, murein fragments).

Unit 2: Bacteriology
06
Lecture 2 – The Morphology & Fine
structure of bacteria
The main characteristics that distinguish
prokaryotes from eukaryotes are the
following:
1) Eukaryotic cells are generally more complex than
prokaryotic cells.
2) DNA is enclosed in a nuclear membrane and is associated
with histones and other proteins only in eukaryotes.
3) Organelles are membrane-bound in eukaryotes.
4) Prokaryotes divide by binary fission whereas eukaryotes
divide by mitosis.
5) Some structures are absent in prokaryotes: for example,
Golgi complex, endoplasmic reticulum, mitochondria, and
chloroplasts.
There are more other differences between prokaryotes
and eukaryotes you can find them in reference text books.
The Morphology of Bacteria
Bacteria differ from other single-cell microorganisms in
both their cell structure and size, which varies from 0.3–5
µm. Magnifications of 500–1000X—close to the
resolution limits of light microscopy—are required to
obtain useful images of bacteria. Another problem is that
the structures of objects the size of bacteria offers little
visual contrast. Techniques like phase contrast and dark
field microscopy, both of which allow for live cell
observation, are used to overcome this difficulty.
Chemical-staining techniques are also used, but the
prepared specimens are dead. Bacteria have three basic
forms: cocci, straight rods, and curved or spiral rods.
Cocci are spherical bacteria. Those found in grapelike
clusters as in this picture are staphylococci (Scanning
electron microscopy (SEM)).
The straight rod bacteria with rounded ends
Shown here are coli bacteria (SEM).
Spirilla, in this case borrelia are spiral
bacteria (light microscopy (LM), Giemsa stain).
The Structures of Bacterial cell
The Nucleoid and plasmids
The prokaryote
Nucleoid ( the equivalent of the
eukaryotic Nucleus)
consists of a very thin, long,
circular DNA molecular double strand that is not
surrounded by a membrane and localized in the
cytoplasm. In E. coli (and probably in all bacteria), it
takes the form of a single circular molecule of DNA. The
genome of E. coli comprises 4.63 X106 base pairs (bp)
that code for 4288 different proteins. The genomic
sequence of many bacteria is known. The plasmids are
nonessential genetic structures. These circular, twisted
DNA molecules are 100–1000X smaller than the nucleoid
genome structure and reproduce autonomously .The
plasmids of human pathogen bacteria often bear important
genes determining the phenotype of their cells (resistance
genes, virulence genes).

Unit 2: Bacteriology
06
The nucleoid (nucleus equivalent) of bacteria consists of a
tangled circular DNA molecule without a nuclear
membrane.
Transmission electron microscopy (TEM) image of
staphylococci
A) Open circular form (OC). The result of a rupture in
one of the two nucleic acid strands. B) Twisted (CCC =
covalently closed circular), native form (TEM image).
Cytoplasm
The cytoplasm contains a large number of solute (low-
and high-molecularweight) substances, RNA and
ribosomes (approximately 20 000 per cell). Bacteria have
70S ribosomes comprising 30S and 50S subunits.
Bacterial ribosomes function as the organelles for protein
synthesis. The cytoplasm is also frequently used to store
reserve substances (glycogen depots, polymerized
metaphosphates, lipids).
The Cytoplasmic Membrane
This elementary membrane, also known as the plasma
membrane, is
a 40–80 A ˚-thick semipermeable
membrane
. It is basically a double layer of phospholipids
with numerous proteins integrated into its structure. The
most important of these membrane proteins are
permeases, enzymes for the biosynthesis of the cellwall,
transfer proteins for secretion of extracellular proteins,
sensor or signal proteins, and respiratory chain enzymes.
In electron microscopic images of Gram-positive bacteria,
the mesosomes appear as structures bound to the
membrane. How they function and what role they play
remain to be clarified.
Cell Wall
The tasks of the complex bacterial cellwall are to protect
the protoplasts from external noxae, to withstand and
maintain the osmotic pressure gradient between the cell
interior and the extracellular environment (with internal
pressures as high as 500–2000 kPa), to give the cell its
outer form and to facilitate communication with its
surroundings.
Peptidoglycan (syn. murein).
The most important
structural element of the wall is peptidoglycan, a netlike
polymer material surrounding the entire cell (sacculus). It
is made up of polysaccharide chains crosslinked by
peptides.
The cell wall of Gram-positive bacteria
. The
murein sacculus may consist of as many as 40 layers (15–
80 nm thick) and account for as much as 30% of the dry
mass of the cell wall. The membrane lipoteichoic acids
are anchored in the cytoplasmic membrane, whereas the
cell wall teichoic acids are covalently coupled to the
murein. The physiological role of the teichoic acids is not
known in detail; possibly they regulate the activity of the
autolysins that steer growth and transverse fission
processes in the cell.
The cell wall of Gram-negative bacteria
. Here, the
murein is only about 2 nm thick and contributes up to 10%
of the dry cell wall mass. The outer membrane is the salient
structural element. It contains numerous proteins (50% by
mass) as well as the medically critical lipopolysaccharide.
Outer membrane proteins
—
OmpA (outer membrane protein A) and the murein
lipoprotein form a
bond between outer membrane and murein.
—
Porins, proteins that form pores in the outer
membrane, allow passage of hydrophilic, low-molecular-
weight substances into the periplasmic space.
—
Outer membrane-associated proteins constitute
specific structures that enable bacteria to attach to host
cell receptors.
A
B

Unit 2: Bacteriology
06
—
A number of Omps are transport proteins.
Examples include the LamB proteins for maltose transport
and FepA for transport of the siderophore ferric (Fe3+)
enterochelin in E. coli .
Lipopolysaccharide (LPS).
This molecular complex, also known as endotoxin, is
comprised of the lipoid A, the core polysaccharide, and
the O-specific polysaccharide chain.
—
Lipoid A is responsible for the toxic effect.
—
The O-specific polysaccharide chain is the so-called
O antigen.
L-forms (L = Lister Institute). The L-forms are bacteria
with murein defects, e.g., resulting from the effects of
betalactam antibiotics. L-forms are highly unstable when
subjected to osmotic influences.
Capsule
Many pathogenic bacteria make use of extracellular
enzymes to synthesize a polymer that forms a layer
around the cell: the capsule. The capsule protects bacterial
cells from phagocytosis. The capsule of most bacteria
consists of a polysaccharide. The bacteria of a single
species can be classified in different capsular serovars (or
serotypes) based on the fine chemical structure of this
polysaccharide.
Flagella
Flagella give bacteria the ability to move about actively.
The flagella (singular flagellum) are made up of a class of
linear proteins called flagellins. Flagellated bacteria
display various flagellar arrangements ranging from
monotrichous (polar flagellum; e.g., Vibrio coma),
lophotrichous (bundle of flagella at one end of the cell;
e.g., Spirillum volutans), to peritrichous (several flagella
distributed around the cell; e.g., Escherichia coli)
.
Together with the O antigens, they are used to classify
bacteria in serovars.
a
Flagellated bacterial cell (SEM, 13 000!). b Helical
structure of bacterial flagella (SEM, 77 000X).
Attachment Pili (Fimbriae), Conjugation Pili
Many Gram-negative bacteria possess thin microfibrils
made of proteins (0.1–1.5 nm thick, 4–8 nm long), the
attachment pili. They are anchored in the outer membrane
of the cell wall and extend radially from the surface.
Using these structures, bacteria are capable of specific
attachment to host cell receptors (ligand—receptor, key—
keyhole). The conjugation pili (syn. sex pili) in Gram-
negative bacteria are required for the process of
conjugation and thus for transfer of conjugative plasmids.
Biofilm
A bacterial biofilm is a structured community of bacterial
cells embedded in a self-produced polymer matrix and
attached to either an inert surface or living tissue. Such
films can develop considerable thickness (mm) . Foreign
body infections are caused by bacteria that form a biofilm
on inert surfaces. The bacteria located deep within such a
biofilm structure are effectively isolated from immune
system cells, antibodies, and antibiotics. The polymers
they secrete are frequently glycosides, from which the
term glycocalyx (glycoside cup) for the matrix is derived.
Bacterial Spores
Bacterial spores (endospores) are purely dormant life
forms. Their development from bacterial cells in a
“vegetative” state does not involve assimilation of
additional external nutrients. They are spherical to oval in
shape and are characterized by a thick spore wall & a high
level of resistance to chemical & physical noxae. Among
human pathogen bacteria, only the genera Clostridium
and Bacillus produce spores. The heat resistance of these
spores is their most important quality from a medical
point of view, since heat sterilization procedures require
very high temperatures to kill them effectively.

Unit 2: Bacteriology
00
Lecture 3 - The Physiology of
Metabolism and Growth in Bacteria
Fine structure of Both: The Cell Wall of Gram-Positive Bacteria
(left) and the
Cell Wall
of Gram-Negative Bacteria (right)

Unit 2: Bacteriology
06
Types of Metabolism
Metabolism is the totality of chemical reactions occurring
in bacterial cells. They can be subdivided into anabolic
(synthetic) reactions that consume energy and catabolic
reactions that supply energy. In the anabolic, endergonic
reactions, the energy requirement is consumed in the form
of light or chemical energy—by photosynthetic or
chemosynthetic bacteria, respectively. Catabolic reactions
supply both energy and the basic structural elements for
synthesis of specific bacterial molecules. Bacteria that
feed on inorganic nutrients are said to be lithotrophic,
those that feed on organic nutrients are organotrophic.
Human pathogenic bacteria are always chemosynthetic,
organotrophic bacteria (or chemo-organotrophs).
Catabolic Reactions
Organic nutrient substrates are catabolized in a wide
variety of enzymatic processes that can be schematically
divided into four phases:
Digestion
. Bacterial exoenzymes split up the nutrient
substrates into smaller molecules outside the cell. The
exoenzymes represent important pathogenicity factors in
some cases.
Uptake
. Nutrients can be taken up by means of passive
diffusion or, more frequently, specifically by active
transport through the membrane (s). Cytoplasmic
membrane permeases play an important role in these
processes.
Preparation for oxidation
. Splitting off of carboxyl
and amino groups, phosphorylation, etc.
Oxidation.
This process is defined as the removal of
electrons and H+ ions. The substance to which the H2
atoms are transferred is called the hydrogen acceptor.
The two basic forms of oxidation are defined by
the final hydrogen acceptor.
o Respiration. Here oxygen is the hydrogen acceptor. In
anaerobic respiration, the O2 that serves as the hydrogen
acceptor is a component of an inorganic salt.
o Fermentation. Here an organic compound serves as the
hydrogen acceptor. The main difference between
fermentation and respiration is the energy yield, which
can be greater from respiration than from fermentation for
a given nutrient substrate by as much as a factor of 10.
Fermentation processes involving microorganisms are
designated by the final product, e.g., alcoholic
fermentation, butyric acid fermentation, etc.
The energy released by oxidation is stored as chemical
energy in the form of a thioester (e.g., acetyl-CoA) or
organic phosphates (e.g., ATP).
The role of oxygen. Oxygen is activated in one of
three ways:
o Transfer of 4e
–
to O
2
, resulting in 2 oxygen ions (2 O2
–
).
o Transfer of 2e
–
to O
2
resulting in 1 peroxide anion(1 O2
2–
o Transfer of 1e– to O2, resulting in one superoxide anion
(1 O2 –).
Hydrogen peroxide and the highly reactive superoxide
anion are toxic and therefore must undergo further
conversion immediately.
Bacteria are categorized as the following
according to their O2-related behavior:
o Facultative anaerobes. These bacteria can oxidize
nutrient substrates by means of both respiration and
fermentation.
o Obligate aerobes. These bacteria can only reproduce in
the presence of O2.
o Obligate anaerobes. These bacteria die in the presence of
O2. Their metabolism is adapted to a low redox potential
and vital enzymes are inhibited by O2.
o Aerotolerant anaerobes. These bacteria oxidize nutrient
substrates without using elemental oxygen although,
unlike obligate anaerobes, they can tolerate it.
Basic mechanisms of catabolic metabolism.
The principle of the biochemical unity of life asserts that
all life on earth is, in essence, the same. Thus, the
catabolic intermediary metabolism of bacteria is, for the
most part, equivalent to what takes place in eukaryotic
cells. The reader is referred to textbooks of general
microbiology for exhaustive treatment of the pathways of
intermediary bacterial metabolism.
Anabolic Reactions
It is not possible to go into all of the biosynthetic feats of
bacteria here. Suffice it to say that they are, on the whole,
quite astounding. Some bacteria (E. coli) are capable of
synthesizing all of the complex organic molecules that
they are comprised of, from the simplest nutrients in a
very short time. These capacities are utilized in the field
of microbiological engineering. Antibiotics, amino acids,
and vitamins are produced with the help of bacteria. Some
bacteria are even capable of using aliphatic hydrocarbon
compounds as an energy source. Such bacteria can “feed”
on paraffin or even raw petroleum. Culturing of bacteria

Unit 2: Bacteriology
06
in nutrient mediums based on methanol is an approach
being used to produce biomas in addition to many other
ones.
Metabolic Regulation: Bacteria are highly efficient
metabolic regulators, coordinating each individual
reaction with other cell activities and with the available
nutrients as economically and rationally as possible.
Growth and Culturing of Bacteria
Nutrients
The term bacterial culture refers to proliferation of
bacteria with a suitable nutrient substrate. A nutrient
medium in which chemoorganotrophs are to be
cultivated must have organic energy sources (H2
donors) and H2 acceptors. Other necessities include
sources of carbon and nitrogen for synthesis of specific
bacterial compounds as well as minerals such as
sulfur, phosphorus, calcium, magnesium, and trace
elements as enzyme activators. Some bacteria also
require “growth factors,” i.e., organic compounds they
are unable to synthesize themselves. Depending on the
bacterial species involved, the nutrient medium must
contain certain amounts of O2 and CO2 and have
certain pH and osmotic pressure levels. Growth
factors are required in small amounts by cells because
they fulfill specific roles in biosynthesis. The need for a
growth factor results from either a blocked or missing
metabolic pathway in the cells. Growth factors are
organized into three categories:
1. Purines and pyrimidines: required for synthesis of
nucleic acids (DNA and RNA).
2. Amino acids: required for the synthesis of proteins.
3. Vitamins: needed as coenzymes and functional groups
of certain enzymes.
Some bacteria (e.g E. coli) do not require any growth
factors: they can synthesize all essential purines,
pyrimidines, amino acids and vitamins, starting with their
carbon source, as part of their own intermediary
metabolism. Certain other bacteria (e.g. Lactobacillus)
require purines, pyrimidines, vitamins and several amino
acids in order to grow. These compounds must be added
in advance to culture media that are used to grow these
bacteria. The growth factors are not metabolized directly
as sources of carbon or energy, rather they are assimilated
by cells to fulfill their specific role in metabolism. Mutant
strains of bacteria that require some growth factor not
needed by the wild type (parent) strain are referred to as
auxotrophs. Thus, a strain of E. coli that requires the
amino acid tryptophan in order to grow would be called a
tryptophan auxotroph and would be designated E. colitrp-.
The function(s) of these vitamins in essential enzymatic
reactions gives a clue why, if the cell cannot make the
vitamin, it must be provided exogenously in order for
growth to occur.
Growth and Cell Death
Bacteria reproduce asexually by means of simple
transverse binary fission. Their numbers (n) increase
logarithmically (n = 2G). The time required for a
reproduction cycle (G) is called the generation time (g)
and can vary greatly from species to species. Fast-
growing bacteria cultivated in vitro have a generation
time of 15–30
minutes. The same bacteria may take
hours to reproduce in vivo. Obligate anaerobes grow
much more slowly than aerobes; this is true in vitro as
well. Tuberculosis bacteria have an in-vitro generation
time of 12–24 hours. Of course the generation time also
depends on the nutrient content of the medium.
The so-called normal growth curve for bacteria is
obtained by inoculating a nutrient broth with bacteria the
metabolism of which is initially quiescent, counting them
at intervals and entering the results in a semilog
coordinate system . The lag phase is characterized by an
increase in bacterial mass per unit of volume, but no
increase in cell count. During this phase, the metabolism
of the bacteria adapts to the conditions of the nutrient
medium. In the following log (or exponential) phase , the
cell count increases logarithmically up to about 109/ml.
This is followed by growth deceleration and transition to
the stationary phase due to exhaustion of the nutrients and
the increasing concentration of toxic metabolites. Finally,
death phase processes begin. The generation time can
only be determined during log phase, either graphically or
by determining the cell count (n) at two different times.
Normal Growth Curve of a Bacterial Culture

Unit 2: Bacteriology
06
The summary:
Human pathogenic bacteria are
chemosynthetic and organotrophic (chemo-
organotrophic). They derive energy from the breakdown
of organic nutrients and use this chemical energy both for
resynthesis and secondary activities. Bacteria oxidize
nutrient substrates by means of either respiration or
fermentation. In respiration, O2 is the electron and proton
acceptor, in fermentation an organic molecule performs
this function. Human pathogenic bacteria are classified in
terms of their O2 requirements and tolerance as
facultative anaerobes, obligate aerobes, obligate
anaerobes, or aerotolerant anaerobes. Nutrient broth or
agar is used to cultivate bacteria. Nutrient agar contains
the inert substrate agarose, which liquefies at 100°C and
gels at 45°C. Selective and indicator mediums are used
frequently in diagnostic bacteriology. Bacteria reproduce
by means of simple transverse binary fission. The time
required for complete cell division is called generation
time. The in-vitro generation time of rapidly proliferating
species is 15–30 minutes. This time is much longer in
vivo. The growth curve for proliferation in nutrient broth
is normally characterized by the phases lag, log (or
exponential) growth, stationary growth, and death.

Unit 2: Bacteriology
66
Lecture 4 - The Molecular Basis of
Bacterial Genetics
The Structure of Bacterial DNA
A bacterium’s genetic information is stored in its
chromosome and plasmids. Each of these structures is
made of a single DNA double helix twisted to the right,
then additionally twisted to the left about its helical axis
(supercoiled), Plasmids consisting of linear DNA also
occur, although this is rare.
Chromosome.
The chromosome corresponds to the nucleoid . The gene
sequence is colinear with the expressed genetic products.
The noncoding interposed sequences (introns) normally
seen in eukaryotic genes are very rare.
Plasmids
The plasmids are autonomous DNA molecules of varying
size (3 X 103 to 4.5 X 105 bp) localized in the cytoplasm.
Large plasmids are usually present in one to two copies
per cell, whereas small ones may be present in 10, 40, or
100 copies. Plasmids are not essential to a cell’s survival.
Many of them carry genes that code for certain
phenotypic characteristics of the host cell. The following
plasmid types are medically relevant:
▪
Virulence plasmids.
Carry determinants of bacterial
virulence, e.g., enterotoxin genes or hemolysin genes.
▪
Resistance plasmids. Carry genetic information bearing
on resistance to anti-infective agents. R plasmids may
carry several R genes at once . Plasmids have also been
described that carry both virulence and resistance genes.
DNA Replication
The identical duplication process of DNA is termed
semiconservative because the double strand of DNA is
opened up during replication, where upon each strand
serves as the matrix for synthesis of a complementary
strand. Thus each of the two new double strands
“conserves” one old strand. The doubling of each DNA
molecule (replicon) begins at a given starting point, the
so-called origin of replication. This process continues
throughout the entire fission cycle.
Transcription and Translation
Transcription
. Copying of the sense strand of the DNA
into mRNA. The continuous genetic nucleotide sequence
is transcribed “colinearly” into mRNA. This principle of
colinearity applies with very few exceptions. The
transcription process can be broken down into the three
phases promoter recognition, elongation, and
termination. The promoter region is the site where the
RNA polymerase begins reading the DNA sequence. A
sigma factor is required for binding to the promoter.
Sigma factors are proteins that associate temporarily with
the RNA polymerase (core enzyme) to form a
holoenzyme, then dissociate themselves once the
transcription process has begun, making them available to
associate once again. The mRNA synthesized by the
transcription of an operon is polycistronic, i.e., it contains
the information sequences of several genes.
Translation
. Transformation of the nucleotide sequence
carried by the mRNA into the polypeptide amino acid
sequence at the 70S ribosomes. In principle, bacterial and
eukaryotic translation is the same. The enzymes and other
factors involved do, however, differ structurally and can
therefore be selectively blocked by antibiotics
.
The Genetic Variability of Bacteria
Changes in bacterial DNA are the result of spontaneous
mutations in individual genes as well as recombination
processes resulting in new genes or genetic combinations.
Based on the molecular mechanisms involved, bacterial
recombinations are classified as homologous, site-
specific, and transpositional.
The latter two in particular reflect the high level of
mobility of many genes and have made essential
contributions to the evolution of bacteria. Although sexual
heredity is unknown in bacteria, they do make use of the
mechanisms of intercellular transfer of genomic material
known as parasexual processes.
Transformation designates transfer of DNA that is
essentially chemically pure from a donor into a receptor
cell. In transduction, bacteriophages serve as the vehicles
for DNA transport.

Unit 2: Bacteriology
66
Conjugation is the transfer of DNA by means of cell-to-
cell contact. This process, made possible by conjugative
plasmids and transposons, can be a high-frequency one
and may even occur between partners of different species,
genera, or families. The transfer primarily involves the
conjugative elements themselves. Conjugative structures
carrying resistance or virulence genes are of
considerable medical significance.
The processes of restriction and modification are
important factors limiting genetic exchange among
different taxa. Restriction is based on the effects of
restriction endonucleases capable of specific excision of
foreign DNA sequences. These enzymes have become
invaluable tools in the field of genetic engineering.
Molecular Mechanisms of Genetic Variability
Spontaneous Mutation
In the year 1943, Luria and Delbru¨ ck used the so-
called fluctuation test to demonstrate that changes in the
characteristics of bacterial populations were the results of
rare, random mutations in the genes of individual cells,
which then were selected. Such mutations may involve
substitution of a single nucleotide, frame-shifts,
deletions, inversions, or insertions. The frequency of
mutations is expressed as the mutation rate, which is
defined as the probability of mutation per gene per cell
division. The rate varies depending on the gene involved
and is approximately 10-6 to 10-10. Mutation rates may
increase drastically due to mutagenic factors such as
radioactivity, UV radiation, alkylating chemicals, etc.
Recombination
The term recombination designates processes that lead
to the restructuring of DNA, formation of new genes or
genetic combinations.They may be Homologous
(generalized) recombination, Site-specific
recombination,or Transposition.
Intercellular Mechanisms of Genetic Variability
Although bacteria have no sexual heredity in the strict
sense, they do have mechanisms that allow for
intercellular DNA transfer. These mechanisms, which
involve a unilateral transfer of genetic information from a
donor cell to a receptor cell, are subsumed under the term
parasexuality.
Transformation.
Transfer of “naked” DNA.
Transduction.
Transfer of DNA from a donor to a
receptor with the help of transport bacteriophage
.Bacteriophages are viruses that infect bacteria.
Bacterial gene-transfer mechanism
T bacteriophage
Conjugation.
Conjugation is the transfer of DNA from a
donor to a receptor in a conjugal process involving cell-
to-cell contact. Conjugation is made possible by two
genetic elements: the conjugative plasmids and the
conjugative transposons.

Unit 2: Bacteriology
66
Schematic drawing of bacterial conjugation.
Conjugation diagram 1-Donor cell produces
. 2-
Pilus attaches to recipient cell and brings the two cells
together. 3- The mobile plasmid is nicked and a single
strand of DNA is then transferred to the recipient cell. 4-
Both cells synthesize a complementary strand to produce
a double stranded circular plasmid and also reproduce
pili; both cells are now viable donors.
The F-factor in Escherichia coli
. This is the prototype of
a conjugative plasmid. This factor contains the so-called
tra (transfer) genes responsible both for Conjugative
resistance and virulence plasmids. Conjugative
plasmids that carry determinants coding for antibiotic
resistance and/or virulence in addition to the tra genes
and repA are of considerable medical importance.
Restriction, Modification, and Gene Cloning .
A number of control mechanisms limit these genetic
exchange processes. Among the most important are
restriction and modification. Restriction endonucleases
can destroy foreign DNA that bears no “fingerprint”
(modification) signifying “self.” These modifications take
the form of methylation of the DNA bases by
modification enzymes. Bacterial restriction endonucleases
are invaluable tools in modern gene cloning techniques.
The process is termed gene “cloning” because it involves
replication of DNA that has been manipulated in vitro in a
suitable host cell so as to produce identical copies of this
DNA: molecular clones or gene clones The technique
simplifies the replication of DNA, making experimental
manipulations easier. On the other hand, the bacteria can
also be used to synthesize gene products of the foreign
genes. Such foreign proteins are called recombinant
proteins. Bacterial plasmids often function in the role of
vectors into which the sequences to be cloned are
inserted.
Summary:
Bacteria possess two genetic structures: the chromosome
and the plasmid. Both of these structures consist of a
single circular DNA double helix twisted
counterclockwise about its helical axis. Replication of this
DNA molecule always starts at a certain point (the origin
of replication) and is “semiconservative,” that is, one
strand in each of the two resulting double strands is
conserved. Most bacterial genes code for proteins
(polypeptides). Noncoding
interposed sequences (introns), like those seen in
eukaryotes, are the exception. Certain bacterial genes
have a mosaic structure. The phases of transcription are
promoter recognition, elongation, and termination. Many
bacterial mRNAs are polycistronic, meaning they contain
the genetic information for several polypeptides.
Translation takes place on the 70S ribosomes. Special
mRNA codons mark the start and stop of polypeptide
synthesis. Many genes that code for functionally related
polypeptides are grouped together in chromosome or
plasmid segments known as operons. The most important
regulatory
mechanism is the positive or negative control of
transcription initiation. This control function may be
exercised by individual localized genes, the genes of an
operon or genes in a regulon.

Unit 2: Bacteriology
66
Lecture 5 - The Principles of
antibacterial therapy
Definitions
Most microbiologists distinguish 2 groups of antibacterial
agents used in the treatment of infectious disease
: antibiotics, which are natural substances produced by
certain groups of microorganisms (fungi or bacteria),
and chemotherapeutic agents, which are chemically
synthesized substances. A hybrid substance is
a semisynthetic antibiotic, wherein a molecular version
produced by the microbe is subsequently modified by the
chemist to achieve desired properties. One feature of
antibactrial pharmaceuticals is “selective toxicity,” that
is, they act upon bacteria at very low concentration levels
without causing damage to the macroorganism. The most
important group of anti-infective agents is the antibiotics.
The term “antibiotic” is often used in medical contexts to
refer to all antibacterial pharmaceuticals, not just to
antibiotics in this narrower sense. the relations between an
anti-infective agent, the host organism & a bacterial
pathogen
Characteristics of Antibiotics
Antibiotics may have a cidal (killing) effect or a static
(inhibitory) effect on a range of microbes. The range of
bacteria or other microorganisms that is affected by a
certain antibiotic is expressed as its spectrum of action.
Antibiotics effective against procaryotes that kill or
inhibit a wide range of Gram-positive and Gram-negative
bacteria are said to be broad spectrum. If effective
mainly against Gram-positive or Gram-negative bacteria,
they are narrow spectrum. If effective against a single
organism or disease, they are referred to as limited
spectrum.
A clinically-useful antibiotic should have as many of
these characteristics as possible:-
It should have a wide spectrum of activity with the ability
to destroy or inhibit many different species of pathogenic
organisms.
It should be nontoxic to the host and without undesirable
side effects.
It should be nonallergenic to the host.
It should not eliminate the normal flora of the host.
It should be able to reach the part of the human body
where the infection is occurring.
It should be inexpensive and easy to produce.
It should be chemically-stable (have a long shelf-life).
Microbial resistance is uncommon and unlikely to develop.
Fundamental ways that antibacterial
antibiotics work as therapeutic agents:
The target of an antibiotic should be unique to the
bacterium and not found, or not accessible, to the patient.
These are the most important targets in bacteria that have
been exploited so far.
1) Attack bacterial cell wall synthesis
. Bacteria have
murein in their cell walls, not found in the host, and
murein (peptidoglycan) is essential to the viability of the
bacterium.
Beta lactam antibiotics represented by the
penicillins and cephalosporins, are example of these
antibiotics which contain a 4-membered beta lactam ring,
they are the products of two genera of fungi,
Penicillium and Cephalosporium.
2) Interfere with protein synthesis
. Attack is almost
always at the level of translation using 70S ribosomes in
the translation machinery.
The most important antibiotics
with this mode of action are
the tetracyclines, chloramphenicol, the macrolides (e.g.
erythromycin) and the aminoglycosides (e.g.
streptomycin).
3) Interference with nucleic acid synthesis
(RNA and
DNA), which exploits differences between RNA
polymerases and DNA replication strategies in bacteria
and eucaryotes.
Two nucleic acid synthesis inhibitors
which have selective activity against procaryotes and
some medical utility are the quinolones and rifamycins.
4) Inhibition of an essential metabolic pathway
that
exists in the bacterium but does not exist in the host. This
is usually brought about through the use of competitive
chemical analogs for bacterial enzymatic reactions.
The
sulfonamides (e.g. Gantrisin and Trimethoprim) are
inhibitors of the bacterial enzymes required for the
synthesis of tetrahydofolic acid (THF), the vitamin form
of folic acid essential for 1-carbon transfer reactions.
5) Membrane inhibition or disruption
doesn't work
too well because of the similarities between eucaryotic
and bacterial membranes. However, the outer membrane
of Gram-negative bacteria is a reasonable point of attack
and some membrane inhibitors are included in the
discussion below.
The only antibacterial antibiotics of
clinical importance that act by this mechanism are
the polymyxins, produced by Bacillus polymyxa .

Unit 2: Bacteriology
66
Spectrum of Action
Each anti-infective agent has a certain spectrum of action,
which is a range of bacterial species showing natural
sensitivity to the substance. Some antiinfective agents
have a narrow spectrum of action (e.g., vancomycin).
Most, however, have broad spectra like tetracyclines,
which affect all eubacteria.
Efficacy (syn. kinetics of action)
The efficacy of an anti-infective agent defines the way it
affects a bacterial population. Two basic effects are
differentiated: bacteriostasis, i.e., reversible inhibition of
growth, and irreversible bactericidal activity. Many
substances can develop both forms of efficacy depending
on their concentration, the type of organism, and the
growth phase. Many of these drugs also have a
postantibiotic effect (PAE) reflecting the damage inflicted
on a bacterial population. After the anti-infective agent is
no longer present, the bacterial cells not killed require a
recovery phase before they can reproduce again. The PAE
may last several hours.
Pharmacokinetics
Pharmacokinetics covers the principles of absorption,
distribution, and elimination of pharmacons by the
macroorganism. You could refere to standard textbooks of
pharmacology for details. The dosage and dosage interval
recommendations for antibacterial therapy take into
account the widely differing pharmacokinetic parameters
of the different anti-infective agents.
Side Effects
Treatment with anti-infective agents can cause side
effects, resulting either from noncompliance with
important therapeutic principles or specific patient
reactivity. On the whole, such side effects are of minor
significance.
Toxic effects
.
These effects arise from direct cell and
tissue damage in the macroorganism. Blood
concentrations of some substances must therefore be
monitored during therapy if there is a risk of cumulation
due to inefficient elimination (examples:
aminoglycosides, vancomycin).
Allergic reactions
.
For possible mechanisms (example:
penicillin allergy).
Biological side effects
.
Example: change in or
elimination of normal flora, interfering with its function
as a beneficial colonizer.
The Problem of Resistance
.
There are many features
of resistance of bacteria:-
o Clinical resistance. Resistance of bacteria to the
concentration of anti-infective agents maintained at the
infection site in the macroorganism.
o Natural resistance. Resistance characteristic of a
bacterial species, genus, or family.
o Acquired resistance. Strains of sensitive taxa can acquire
resistance by way of changes in their genetic material.
o Biochemical resistance. A biochemically detectable
resistance observed in strains of sensitive taxa. The
biochemical resistance often corresponds to the clinically
relevant resistance. Biochemically resistant strains
sometimes show low levels of resistance below the
clinically defined boundary separating resistant and
sensitive strains. Such strains may be medically
susceptible.
Significance
Problematic bacteria
. Strains with acquired resistance
are encountered frequently among Enterobacteriaceae,
pseudomonads, staphylococci, and enterococci. Specific
infection therapy directed at these pathogens is often
fraught with difficulties, which explains the label
problematic bacteria. They are responsible for most
nosocomial infections. Usually harmless in otherwise
healthy persons, they may cause life-threatening
infections in highly susceptible, so-called problematic
patients. Problematic bacteria are often characterized by
multiple resistances. Resistance to anti-infective agents is
observed less frequently in nonhospital bacteria.
Genetic variability
. The basic cause of the high
incidence of antibiotic resistance experienced with
problematic bacteria is the pronounced genetic variability
of these organisms induced by different mechanisms.
Most important are the mechanisms of horizontal transfer
of resistance determinants responsible for the efficient
distribution of resistance markers among these bacteria.
Selection
. The origin and distribution of resistant strains
is based to a significant extent on selection of resistance
variants. The more often anti-infective substances are
administered therapeutically, the greater the number of
strains that will develop acquired resistance. Each hospital
has a characteristic flora reflecting its prescription
practice. A physician must be familiar with the resistance
characteristics of this hospital flora so that the right anti-
infective agents for a “calculated antibiotic therapy” can
be selected even before the resistance test results are in.

Unit 2: Bacteriology
66
Such therapies take into account the frequency of
infections by certain bacterial species (pathogen
epidemiology) as well as current resistance levels among
these bacteria (resistance epidemiology).
Resistance Tests
Two standard test systems are used to determine the in-
vitro resistance levels of bacteria. The dilution series
tests, in which the minimum inhibitory concentration
(MIC) of an anti-infective agent required to inhibit
proliferation of a bacterial population is determined, and
the agar dilution test, in which the nutrient agar plates
containing antibiotic are inoculated (“spotted”) with the
test organisms. In the microbroth dilution test, the final
volume is usually 100 µl per microplate well. This test
type can also be automated. The final volume in a
macrobroth dilution test is 2 ml per tube.
Due to the complexity and time-consuming nature of
the above test types, routine laboratories often use the
agar diffusion test. This involves diffuse inoculation of
the nutrient agar plate with the test strain. Then disks of
filter paper containing the anti-infective agents are placed
on the agar. After the plates thus prepared are incubated,
the inhibition zones around the disks (i.e., whether or not
they develop and their size) provide information on the
resistance of the microorganisms tested. This is possible
because of the linear relation between the log2 MIC and
the diameter of the inhibition zones. To interpret the
results, the MICs or inhibition zones are brought into
relation with the substance concentrations present at a site
of infection at standard dosage levels. This calculation is
based on known averages for various pharmacokinetic
parameters (serum concentration, half-life) and
pharmacodynamics parameters (bactericidal activity or
not, postantibiotic effect, etc.). The interpretation also
takes into account clinical experience gained from therapy
of infections with pathogens of given suceptibility. Such
data are used to establish general guideline values
defining the boundary between susceptible and resistant
bacteria.The minimum bactericidal concentration
(MBC) is the smallest concentration of a substance
required to kill 99.9% of the cells in an inoculum. The
MBC is determined using quantitative subcultures from
the macroscopically unclouded tubes or (microplate)
wells of an MIC dilution series.
Agar Diffusion Test: This method, also known as the
“disk test,” is
used to test the resistance of a bacterial culture to
various anti-infective agents. The method provides a
basis for classification of a bacterial strain as
“susceptible,” “resistant,” or “intermediate”
according to the dimension of the inhibition zone.
Combination Therapy
Combination therapy is the term for concurrent
administration of two or more anti-infective agents. Some
galenic preparations combine two components in a fixed
ratio (example: cotrimoxazole). Normally, however, the
dividual substances in a combination therapy are
administered separately.
Several different objectives can be pursued with
combination therapy:
Broadening of the spectrum of action.
In mixed
infections with pathogens of varying resistance; in
calculated therapy of infections with unknown, or not yet
known, pathogenic flora and resistance characteristics.
Delay of resistance development.
In therapy of
tuberculosis; when using anti-infective agents against
which bacteria quickly develop resistance.
Potentiation of efficacy.
In severe infections requiring
bactericidal activity at the site of infection. Best-known
example: penicillin plus gentamicin in treatment of
endocarditis caused by enterococci or streptococci.
Combining the effects of anti-infective drugs can have
several different effects:
No difference. The combination is no more efficacious
than the more active of the two components alone.
Addition. Summation of the effects.
Synergism. Potentiation of the effects.
Antagonism
. The combination is less efficacious than
one of the twocomponents alone.
Rule of thumb
:
combinations of bacteriostatics with
substances that are bactericidal in the cell division phase
only often result in antagonism, e.g., penicillin plus
tetracycline in therapy of pneumococcal pneumonia.

Unit 2: Bacteriology
60
In-vitro investigations of the mechanism of action of a
combination when used against a pathogen usually
employ the so-called “checkerboard titration” technique,
in which the combinatory effects of substances A and B
are compared using a checkerboard-like pattern.
Chemoprophylaxis
One of the most controversial antibiotic uses is
prophylactic antibiosis. There are no clear-cut solutions
here. There are certain situations in which
chemoprophylaxis is clearly indicated and others in which
it is clearly contraindicated. The matter must be decided
on a case-by-case basis by weighing potential benefits
against potential harm (side effects, superinfections with
highly virulent and resistant pathogens, selection of
resistant bacteria).
Chemoprophylaxis is considered useful in, rheumatic
fever, pulmonary cystic fibrosis, recurring pyelonephritis,
following intensive contact with meningococci carriers,
before surgery involving massive bacterial contamination,
in heavily immunocompromised patients, in cardiac
surgery or in femoral amputations due to circulatory
problems. Chemoprophylaxis aimed at preventing a
postsurgical infection should begin a few hours before the
operation and never be continued for longer than 24–72
hours.
Immunomodulators
Despite the generally good efficacy of anti-infective
agents, therapeutic success cannot be guaranteed.
Complete elimination of bacterial pathogens also requires
a functioning immune defense system. In view of the fact
that the number of patients with severe
immunodeficiencies is on the rise, immunomodulators are
used as a supportive adjunct to specific antibiotic therapy
in such patients. Many of these “cytokines” produced by
the cells of the immune system can now be produced as
“recombinant proteins.” Myelopoietic growth factors have
now been successfully used in patients suffering from
neutropenia. Additional immunomodulators are also
available, e.g., interferon gamma (IFNc) and interleukin 2
(IL-2).
Summary:
Specific antibacterial therapy refers to treatment of
infections with antiinfective agents directed against the
infecting pathogen. The most important group of anti-
infective agents are the antibiotics, which are products of
fungi and bacteria (Streptomycetes). Anti-infective agents
are categorized as having a broad, narrow, or medium
spectrum of action. The efficacy, or effectiveness, of a
substance refers to its bactericidal or bacteriostatic effect.
Anti-infective agents have many different mechanisms of
action. Under the influence of sulfonamides and
trimethoprim, bacteria do not synthesize sufficient
amounts of tetrahydrofolic acid. All betalactam antibiotics
irreversibly block the biosynthesis of murein. Rifamycin
inhibits the DNA-dependent RNA polymerase
(transcription). Aminoglycosides, tetracyclines, and
macrolides block translation. All 4-quinolones damage
cellular DNA topology by inhibiting bacterial
topoisomerases. Due to their genetic variability, bacteria
may develop resistance to specific anti-infective agents.
The most important resistance mechanisms are:
inactivating enzymes, resistant target molecules, reduced
influx, increased efflux. Resistant strains (problematic
bacteria) occur frequently among hospital flora, mainly
Enterobacteriaceae, pseudomonads, staphylococci, and
enterococci. Laboratory resistance testing is required for
specific antibiotic therapy. Dilutions series tests are
quantitative resistance tests used to determine the
minimum inhibitory concentration (MIC). The disk test is
a semiquantitative test used to classify the test bacteria as
resistant or susceptible. In combination therapies it must
be remembered that the interactions of two or more
antibiotics can give rise to an antagonistic effect. Surgical
chemoprophylaxis must be administered as a short-term
antimicrobial treatment only.

77

Unit 2: Bacteriology
78
Lecture 1 - Staphylococcus
Staphylococci are Gram-positive cocci occurring in
clusters. They can be cultured on normal nutrient
mediums both aerobically and anaerobically. The most
important species from the viewpoint of human medicine
is S. aureus. A number of extracellular enzymes and
exotoxins such as coagulase, alphatoxin, leukocidin,
exfoliatins, enterotoxins, and toxic shock toxin are
responsible for the clinical symptoms of infections by this
pathogen, which are observed in the three types invasive
infections, pure toxicoses, and mixed forms. The
antibiotics of choice for therapy of these infections are
penicillinase-resistant penicillins. Laboratory diagnosis
involves identification of the pathogen by means of
microscopy and culturing. S. aureus is a frequent
pathogen in nosocomial infections and limited outbreaks
in hospitals. Hand washing by medical staff is the most
important prophylactic measure in hospitals.
Coagulase-negative staphylococci are classic
opportunists. S. epidermidis and other species are frequent
agents in foreign body infections due to their ability to
form biofilms on the surfaces of inert objects. S.
saprophyticus is responsible for between 10 and 20% of
acute urinary tract infections in young women.
Staphylococci are small spherical cells (1 µm) found in
grapelike clusters.Staphylococci are nonmotile, catalase-
producing bacteria. The genus Staphylococcus includes
over 30 species and subspecies. S. aureus (and E. coli) are
among the most frequent causal organisms in human
bacterial infections.
Staphylococcus aureus
Morphology and culturing
. This is a facultative
anaerobe that is readily cultured on normal nutrient
mediums at 37 ˚C. Hemolytic zones are frequently
observed around the colonies.
Fine structure
. The cellwall consists of a thick layer of
murein. Linear teichoic acids and polysaccharides are
covalently coupled to the murein polysaccharide. The
lipoteichoic acids permeating the entire murein layer are
anchored in the cell membrane. Teichoic and lipoteichoic
acids can trigger activation of complement by the
alternative pathway and stimulate macrophages to secrete
cytokines. Cell wall-associated proteins are bound to the
peptide components of the murein. Clumping factor,
fibronectin-binding protein, and collagen-binding protein
bind specifically to fibrinogen, fibronectin, and collagen,
respectively, and are instrumental in adhesion to tissues
and foreign bodies covered with the appropriate matrix
protein. Protein A binds to the Fc portion of
immunoglobulins (IgG). It is assumed that “false” binding
of immunoglobulins by protein A prevents “correct”
binding of opsonizing antibodies, thus hindering
phagocytosis.
Extracellular toxins and enzymes
.
S. aureus secretes numerous enzymes and toxins that
determine, together with the fine structures described
above, the pathogenesis of the attendant infections. The
most important are:
1) Plasma coagulase is an enzyme that functions like
thrombin to convert fibrinogen into fibrin. Tissue
microcolonies surrounded by fibrin walls are difficult to
phagocytose.
2) a-toxin can have lethal CNS effects, damages membranes
(resulting in, among other things, hemolysis), and is
responsible for a form of dermonecrosis.
3) Leukocidin damages microphages and macrophages by
degranulation.
4) Exfoliatins are responsible for a form of epidermolysis.
5) Food poisoning symptoms can be caused by eight
serologically differentiated enterotoxins (A-E, H, G, and
I). These proteins (MW: 35 kDa) are not inactivated by
heating to 100 ˚C for 15–30 minutes. Staphylococcus
enterotoxins are superantigens.
6) Toxic shock syndrome toxin-1 (TSST-1) is produced by
about 1% of Staphylococcus strains. TSST-1 is a
superantigen that induces clonal expansion of many T
lymphocyte types (about 10%), leading to massive
production of cytokines, which then give rise to the
clinical symptoms of toxic shock.
Pathogenesis and clinical pictures
.
The pathogenesis and symptoms of S. aureus infections
take one of three distinct courses:
1) Invasive infections. In this type of infection, the
pathogens tend to remain in situ after penetrating through
the derma or mucosa and to cause local infections
characterized by purulence. Examples include furuncles
,carbuncles,wound infections, sinusitis, otitis media, and
mastitis puerperalis. Other kinds of invasive infection
include postoperative or posttraumatic
ostitis/osteomyelitis, endocarditis following heart surgery
(especially valve replacement), postinfluenza pneumonia,
and sepsis in immunocompromised patients. S. aureus and
E. coli are responsible for approximately equal shares of
nearly half of all cases of inpatient sepsis.
Inert foreign bodies can be colonized by S. aureus.
Colonization begins with specific binding of the

Unit 2: Bacteriology
79
staphylococci, by means of cell wall-associated adhesion
proteins, to fibrinogen or fibronectin covering the foreign
body, resulting in a biofilm that may function as a focus
of infection.
2) Toxicoses. Food poisoning results from ingestion of food
contaminated with enterotoxins. The onset a few hours
after ingestion takes the form of nausea, vomiting, and
massive diarrhea.
3) Mixed forms. Dermatitis exfoliativa (staphylococcal
scalded skin syndrome, Ritter disease), pemphigus
neonatorum, and bullous impetigo are caused by
exfoliatin-producing strains that infect the skin surface.
Toxic shock syndrome (TSS) is caused by strains that
produce TSST-1. These strains can cause invasive
infections, but may also only colonize mucosa. The main
symptoms are hypotension, fever & a scarlatiniform rash.
Diagnosis.
This requires microscopic and culture-based pathogen
identification. Differentiating S. aureus from the
coagulase-negative species is achieved by detection of the
plasma coagulase and/or the clumping factor. The
enterotoxins and TSST-1 can be detected by means of
immunological and molecular biological methods (special
laboratories).
Plasma Coagulase and Clumping Factor Test
To detect plasma coagulase, suspend several colonies in
0.5 ml of rabbit plasma, incubate the inoculated plasma
for one, four, and 24 hours and record the levels of
coagulation.
For the clumping factor test, suspend colony material in a
drop of rabbit plasma on a slide. Macroscopically visible
clumping confirms the presence of the factor.
Therapy
.
Aside from surgical measures, therapy is based on
dministration of antibiotics. The agents of choice for severe
infections are penicillinase resistant penicillins, since 70–
80% of all strains produce penicillinase. These penicillins
are, however, ineffective against methicillin-resistant
strains, & this resistance applies to all betalactams.
Epidemiology and prevention.
S. aureus is a frequent colonizer of skin and mucosa. High
carrier rates (up to 80%) are the rules among hospital
patients and staff. The principle localization of
colonization in these persons is the anterior nasal mucosa
area, from where the bacteria can spread to hands or with
dust into the air and be transmitted to susceptible
persons.S. aureus is frequently the causal pathogen in
nosocomial infections. Certain strains are known to cause
hospital epidemics. Identification of the epidemic strain
requires differentiation of relevant infection isolates from
other ubiquitous strains. Lysotyping can be used for this
purpose, although use of molecular methods to identify
genomic DNA “fingerprints”is now becoming more
common.
The most important preventive measure in hospitals is
washing the hands thoroughly before medical and nursing
procedures. Intranasal application of antibiotics
(mupirocin) is a method of reducing bacterial counts in
carriers.
Coagulase-Negative Staphylococci (CNS)
CNS is an element in the normal flora of human skin and
mucosa. They are classic opportunists that only cause
infections given a certain host disposition.
S. epidermidis. This is the pathogen most frequently
encountered in CNS infections (70–80% of cases). CNS
cause mainly foreign body infections. Examples of the
foreign bodies involved are intravasal catheters,
continuous ambulant peritoneal dialysis (CAPD)
catheters, endopro- stheses, metal plates and screws in
osteosynthesis, cardiac pacemakers, artificial heart valves,
and shunt valves. These infections frequently develop
when foreign bodies in the macroorganism are covered by
matrix proteins (e.g., fibrinogen, fibronectin) to which the
staphylococci can bind using specific cell wall proteins.
They then proliferate on the surface and produce a
polymeric substance—the basis of the developing biofilm.
The staphylococci within the biofilm are protected from
antibiotics and the immune system to a great extent. Such
biofilms can become infection foci from which the CNS
enter the bloodstream and cause sepsis like illnesses.
Removal of the foreign body is often necessary.
S. saprophyticus is responsible for 10–20% of acute
urinary tract infections, in particular dysuria in young
women, and for a small proportion of cases of nonspecific
urethritis in sexually active men.
Antibiotic treatment of CNS infections is often
problematic due to the multiple resistances often
encountered in these staphylococci, especially S.
hemolyticus.
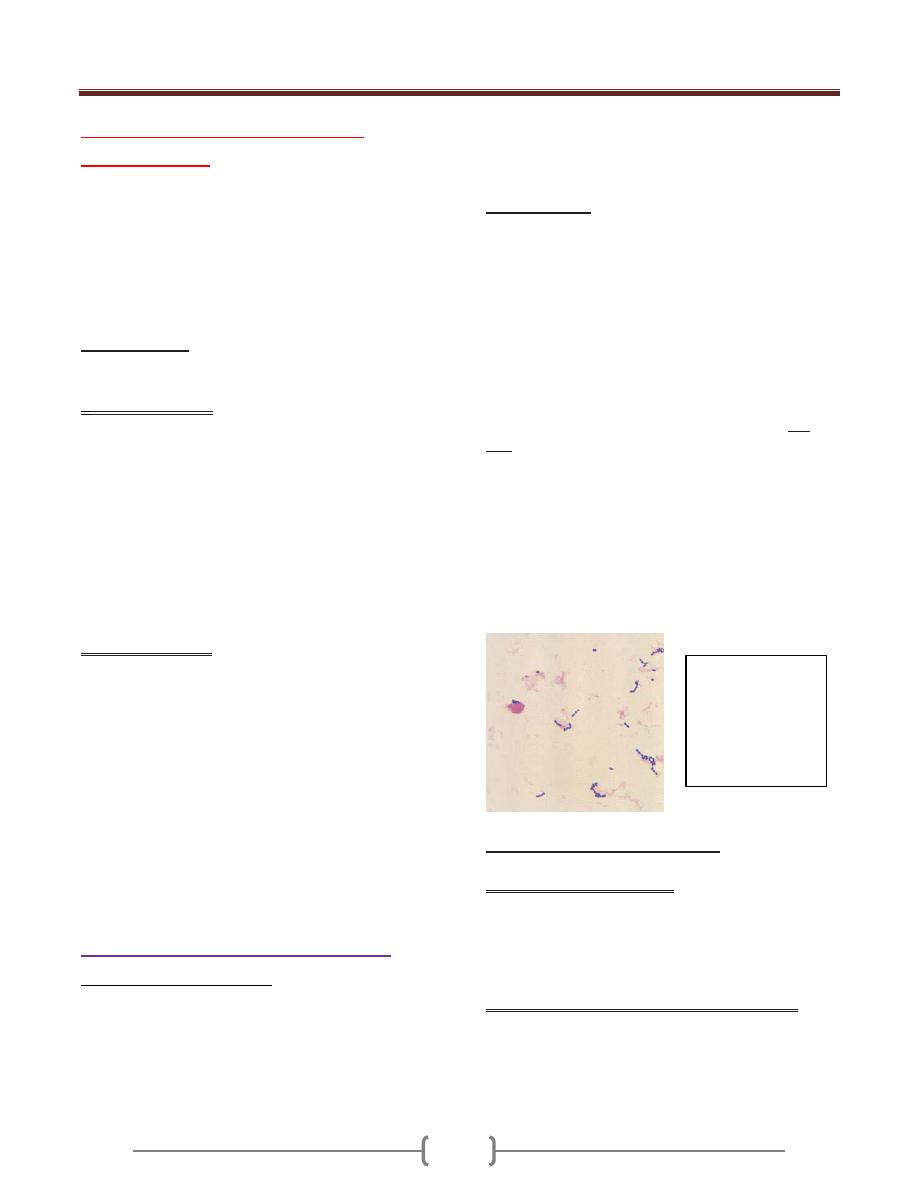
Unit 2: Bacteriology
80
Lecture 2 - Streptococcus and
Enterococcus
Streptococci are round to oval, Gram-positive,
nonmotile, nonsporing bacteria that form winding chains
(streptos [greek] = twisted) or diplococci. They do not
produce catalase. Most are components of the normal
flora of the mucosa. Some can cause infections in humans
and animals.
Classification.
The genera Streptococcus and Enterococcus comprise a
large number of species.
α
-, β-, ƴ-hemolysis.
Alpha-hemolysis
(
α-hemolysis). Colonies on blood agar
are surrounded by a green zone. This “greening” is
caused by H2O2, which converts hemoglobin into
methemoglobin.
Beta-hemolysis (β-hemolysis). Colonies on blood agar
are surrounded by a large, yellowish hemolytic zone in
which no more intact erythrocytes are present and the
hemoglobin is decomposed.
Nonhemolytic colonies have been termed gamma-
hemolytic
(
ƴ-hemolysis) .This (illogical) term indicates
the absence of macroscopically visible hemolytic zones.
Lancefield groups.
Many streptococci and enterococci have a polymeric
carbohydrate (C substance) in their cell walls called the
Lancefield antigen.They are classified in Lancefield
groups A-V based on variations in the antigenicity of this
antigen. Group A streptococci are nearly always beta-
hemolytic; related Group B can manifest alpha, beta or
gamma hemolysis. Most strains of S. pneumoniae are
alpha-hemolytic but can cause ß-hemolysis during
anaerobic incubation. Most of the oral streptococci and
enterococci are non-hemolytic. The property of hemolysis
is not very reliable for the absolute identification of
streptococci, but it is widely used in rapid screens for
identification of S. pyogenes and S. pneumoniae.
Streptococcus pyogenes (A Streptococci)
Morphology and culturing.
Gram-positive, round-to-ovoid cocci, 0.6-1.0
micrometer in diameter. Streptococci divide in one plane
and thus occur in pairs or (especially in liquid media or
clinical material ) in chains of varying lengths. The
metabolism of S. pyogenes is fermentative; the organism
is a catalase-negative aerotolerant anaerobe (facultative
anaerobe), and requires enriched medium containing
blood in order to grow. Group A streptococci typically
have a capsule composed of hyaluronic acid and exhibit
beta (clear) hemolysis on blood agar.
Fine structure.
The murein layer of the cell wall is followed by the
serogroup A carbohydrate layer, which consists of C
substance and is covalently bound to the murein. Long,
twisted protein threads that extend outward are anchored
in the cell wall murein: the M protein. A streptococci are
classified in serovars with characteristic M protein
chemistry. Like the hyaluronic acid capsules seen in some
strains, the M protein has an antiphagocytic effect.
The
surface of Streptococcus pyogenes is incredibly complex
and chemically-diverse. Antigenic components
include capsular polysaccharide (C-substance), cell
wall peptidoglycan and lipoteichoic acid (LTA), and a
variety of surface proteins, including M protein, fimbrial
proteins, fibronectin-binding proteins, (e.g. Protein F)
and cell-bound streptokinase.
The cytoplasmic
membrane of S. pyogenes contains some antigens similar
to those of human cardiac, skeletal, and smooth muscle,
heart valve fibroblasts, and neuronal tissues, resulting
in molecular mimicry and a tolerant or suppressed
immune response by the host.
Extracellular toxins and enzymes
.
The most important in the context of pathogenicity are:
1) Streptolysin O, streptolysin S. Destroy the membranes
of erythrocytes and other cells.
Streptolysin S is an
oxygen-stable leukocidin; Streptolysin O is an oxygen-
labile leukocidin. Streptolysin O acts as an antigen. Past
infections can be detected by measuring the antibodies to
this toxin (antistreptolysin titer).
2) Pyrogenic streptococcal exotoxins (PSE) A, B, C.
Responsible for fever, scarlet fever exanthem and
enanthem, sepsis, and septic shock. The pyrogenic
exotoxins are superantigens and therefore induce
production of large amounts of cytokines
Streptococcus
pyogenes is a
gram-positive
bacterium that
usually grows in
pairs or chains.

Unit 2: Bacteriology
81
3) Streptokinase. Dissolves fibrin; facilitates spread of
streptococci in tissues.
4) Hyaluronidase. Breaks down a substance that cements
tissues together.
5) DNases. Breakdown of DNA, producing runny pus.
NADase is leukotoxic.
6) Streptodornases A-D possess deoxyribonuclease
activity; Streptodornases B & D possess ribonuclease
activity as well
7) Protease activity similar to that inStaphylococcus
aureus has been shown in strains causing soft tissue
necrosis ortoxic shock syndrome. This large repertoire of
products is important in the pathogenesis of S.
pyogenes infections. Even so, antibodies to these products
are relatively insignificant in protection of the host.
Cell surface structure of Streptococcus pyogenes and
secreted products involved in virulence.
Pathogenesis and clinical pictures.
Streptococcus pyogenes is one of the most frequent
pathogens of humans. It is estimated that between 5-15%
of normal individuals harbor the bacterium, usually in the
respiratory tract, without signs of disease. As normal
flora, S. pyogenes can infect when defenses are
compromised or when the organisms are able to penetrate
the constitutive defenses. When the bacteria are
introduced or transmitted to vulnerable tissues, a variety
of types of suppurative infections can occur.
Streptococcal diseases can be classified as either acute,
invasive infections or sequelae to them.
Acute
diseases associated with Streptococcus pyogenes occur
chiefly in the respiratory tract, bloodstream, or
the skin. Streptococcal disease is most often a respiratory
infection (pharyngitis or tonsillitis) or a skin infection
(pyoderma). Some strains of streptococci show a
predilection for the respiratory tract; others, for the skin.
Generally, streptococcal isolates from the pharynx and
respiratory tract do not cause skin infections. S.
pyogenes is the leading cause of uncomplicated
bacterial pharyngitis and tonsillitis commonly referred to
a strep throat. Other respiratory infections
include sinusitis, otitis,and pneumonia. Infections of the
skin can be superficial (impetigo) or deep (cellulitis).
Invasive infections. Invasive streptococci cause joint or
bone infections, destructive wound
infections (necrotizing fasciitis)
and myositis, meningitis and endocarditis . The pathogens
enter through traumas or microtraumas in the skin or
mucosa and cause invasive local or generalized infections.
The rare cases of severe septic infection and necrotizing
fasciitis occur in persons with a high-risk MHC II allotype.
In these patients, the PSE superantigens (especially
PSEA) induce large amounts of cytokine by binding at the
same time to the MHC II complex and the b chain of the
T cell receptor. The excess cytokines thus produced are the
cause of the symptoms.
Sequelae. Two post streptococcal sequelae, rheumatic
fever and glomerulonephritis, may follow streptococcal
disease, and occur in 1-3% of untreated infections. These
conditions and their pathology are not attributable to
dissemination of bacteria, but to aberrent immunological
reactions to Group A streptococcal antigens. Scarlet
fever and streptococcal toxic shock syndrome are
systemic responses to circulating bacterial toxins.
Glomerulonephritis is an immune complex disease and
acute rheumatic fever may be a type II immune disease.
Fig: Pathogenesis of Streptococcus pyogenes infections

Unit 2: Bacteriology
82
Diagnosis
What is involved in diagnosis is detection of the pathogen
by means of microscopy and culturing. Group A antigen
can be detected using particles coated with antibodies that
precipitate agglutination (latex agglutination,
coagglutination). Using these methods, direct detection
of A streptococci in tonsillitis is feasible in the medical
practice. However, this direct detection method is not as
sensitive as the culture. Differentiation of A streptococci
from other β-hemolytic streptococci can be realized in the
laboratory with the bacitracin disk test, because A
streptococci are more sensitive to bacitracin than the other
types.
Therapy.
The agents of choice are penicillin G or V. Resistance is
unknown. Alternatives are oral cephalosporins or
macrolide antibiotics, although resistance to the latter can
be expected. In treatment of septic shock, a polyvalent
immunoglobulin is used to inactivate the PSE.
Epidemiology and prophylaxis.
Infection frequency varies according to geographical area,
season, and age. Humans are the only pathogen reservoir
for S. pyogenes. Transmission is by direct contact
(smear infection) or droplets. The incubation period is
one to three days. The incidence of carriers among
children is 10–20%, but can be much higher depending
on the epidemiological situation. Carriers and infected
persons are no longer contagious 24 hours after the start
of antibiotic therapy. Microbiological follow-up checks of
patients and first-degree contacts are not necessary
(exception: rheumatic history). In persons with recurring
infections or with acute rheumatic fever in their medical
histories, continuous penicillin prophylaxis with a long-
term penicillin is appropriate (e.g., 1.2 million IU
benzathine penicillin per month).
Streptococcus pneumoniae (Pneumococci)
Morphology and culturing
Pneumococci are Gram-positive, oval to lancet-shaped
cocci that usually occur in pairs or short chains. The cells
are surrounded by a thick capsule. When cultured on
blood agar, S. pneumoniae develop a-hemolytic colonies
with a mucoid (smooth, shiny) appearance (hence “S”
form). Mutants without capsules produce colonies with a
rough surface (“R” form). Antigen structure.
Pneumococci are classified in 90 different serovars based
on the fine chemical structure of the capsule
polysaccharides acting as antigens. This capsule antigen
can be identified using specific antisera in a reaction
known as capsular swelling.
Streptococcus pneumoniae (also called Diplococcus
pneumoniae) bacteria may occur singly, in pairs or
diplococci form, and in chains . Capsule stain light
micrograph at a magnification of X1000.
Pathogenesis and clinical pictures
The capsule protects the pathogens from phagocytosis
and is the most important determinant of pneumococcal
virulence. Unencapsulated variants are not capable of
causing disease. Other potential virulence factors include
pneumolysin with its effects on membranes and an IgA1
protease. The natural habitat of pneumococci is provided
by the mucosa of the upper respiratory tract. About
40–70% of healthy adults are carriers. Pneumococcal
infections usually arise from this normal flora
(endogenous infections). Predisposing factors include
primary cardiopulmonary diseases, previous infections
(e.g., influenza), and extirpation of the spleen or
complement system defects. The most important
pneumococcal infections are lobar pneumonia and
bronchopneumonia. Other infections include acute
exacerbation of chronic bronchitis, otitis media,
sinusitis, meningitis, and corneal ulcer. Severe
pneumococcal infections frequently involve sepsis.
Diagnosis
The laboratory diagnosis includes detection of the
pathogen in appropriate test samples by means of
microscopy and culturing. Pneumococcus can be
differentiated from other a-hemolytic streptococci based
on their greater sensitivity to optochin (ethyl hydrocuprein
hydrochloride) in the disk test or their bile solubility. Bile
salts increase autolysis in pneumococci.
Therapy
Penicillin is still the antibiotic of choice. There have been
reports of high-frequency occurrence of strains resistant to
penicillin (South Africa, Spain, Hungary, USA). These
Unit 2: Bacteriology
83
strains are still relatively rare in Germany, Switzerland,
and Austria (5–10%). Macrolide antibiotics are an
alternative to penicillins, but resistance to them is also
possible. Penicillin resistance is not due to penicillinase,
but rather to modified penicillin-binding proteins
(PBPs) to which penicillins have a lower level of affinity.
PBPs are required for murein biosynthesis.
Biochemically, penicillin resistance extends to
cephalosporins as well. However, certain cephalosporins
(e.g., ceftriaxone) can be used against penicillin-resistant
pneumococci due to their higher levels of activity.
Epidemiology and prophylaxis
Pneumococcal infections are endemic and occur in all
seasons, more frequently in the elderly. Humans are the
natural pathogen reservoir. The vaccine (product
Pneumovax®) is available for immunization purposes, it
contains 25mg of the purified capsule polysaccharides of
each of 23 of the most frequent serovars. Eighty to ninety
percent of all isolated pneumococci have antigens
contained in this vaccine, which is primarily indicated in
persons with predisposing primary diseases. There is also
a seven-valent conjugate vaccine that is effective in
children under two years of age . Exposure prophylaxis is
not necessary.
Streptococcus agalactiae (B Streptococci)
B streptococci occasionally cause infections of the skin
and connective tissues, sepsis, urinary tract infections,
pneumonia, and peritonitis in immunocompromised
individuals. About one in 1000 neonates suffers from a
sepsis with or without meningitis. These infections
manifest in the first days of life (early onset type) or in
the first weeks of life (late onset type). In the early onset
form, the infection is caused intrapartum by B
streptococci colonizing the vagina. Potential
predisposing factors include birth complications,
premature birth, and a lack of antibodies to the capsule in
mother and neonate.
Oral Streptococci
Most of the oral streptococci of the type often known as
the viridans group have no group antigen. They usually
cause α-hemolysis, some c-hemolysis as well. Oral
streptococci are responsible for 50–70% of all cases of
bacterial endocarditis, overall incidence of which is one to
two cases per 100 000 annually. The origins of
endocarditis lie in invasion of the vascular system through
lesions in the oral mucosa. A transitory bacteremia
results. The heart valves are colonized and a biofilm is
formed by the organism. Predisposing factors include
congenital heart defects, acute rheumatic fever, cardiac
surgery, and scarred heart valves. Laboratory diagnosis of
endocarditis involves isolation of the pathogen from
blood cultures. Drug therapy of endocarditis is carried
out with either penicillin G alone or combined with an
aminoglycoside (mostly gentamicin). Bactericidal
activity is the decisive parameter. S. mutans, S. sanguis,
and S. mitis are, besides Actinomyces viscosus and A.
naeslundii, responsible for dental caries .These
streptococci can attach to the proteins covering the tooth
enamel, where they then convert sucrose into extracellular
polysaccharides (mutan, dextran, levan). These sticky
substances, in which the original bacterial layer along
with secondary bacterial colonizers are embedded, form
dental plaque. The final metabolites of the numerous
plaque bacteria are organic acids that breach the
enamel,allowing the different caries bacteria to begin
destroying the dentin.
Enterococcus (Enterococci)
Enterococci are a widespread bacterial genus normally
found in the intestines of humans and other animals. They
are nonmotile, catalase-negative, and characterized by
group antigen D. They are able to proliferate at 45 °C, in
the presence of 6.5% NaCl and at pH 9, qualities that
differentiate them from streptococci. As classic
opportunists, enterococci show only low levels of
pathogenicity. However, they are frequently isolated as
components of a mixed flora in nosocomial infections .
Ninety percent of such isolates are identified as E.
faecalis, 5–10% as E. faecium. Among the most
dangerous enterococcal infections is endocarditis, which
must be treated with a combination of an aminopenicillin
and streptomycin or gentamicin. Therapeutic success
depends on the bactericidal efficacy of the combination
used. The efficacy level will be insufficient in the
presence of high levels of resistance to either
streptomycin (MIC >1000 mg/l) or gentamicin (MIC
>500 mg/l) or resistance to the aminopenicillin.
Enterococci frequently develop resistance to antibiotics.
Strains manifesting multiple resistance are found mainly
in hospitals, in keeping with the classic opportunistic
character of these pathogens. Recently observed
epidemics on intensive care wards involved strains that

Unit 2: Bacteriology
84
were resistant to all standard anti-infective agents
including the glycopeptides vancomycin and teicoplanin.
Gram-Positive, Anaerobic Cocci
Gram-positive, strictly anaerobic cocci are included in the
genera Peptococcus and Peptostreptococcus. The only
species in the first genus is Peptococcus niger, whereas
the latter comprises a number of species. The anaerobic
cocci are commonly observed in normal human flora. In a
pathogenic context they are usually only encountered as
components of mixed florae together with other anaerobes
or facultative anaerobes. These bacteria invade tissues
through dermal or mucosal injuries and cause subacute
purulent infections. Such infections are either localized
in the head area (cerebral abscess, otitis media,
mastoiditis, sinusitis) or lower respiratory tract
(necrotizing pneumonia, pulmonary abscess,
empyema). They are also known to occur in the abdomen
(appendicitis, peritonitis, hepatic abscess) and female
genitals (salpingitis, endometriosis, tubo-ovarian
abscess). Gram-positive anaerobic cocci may also
contribute to soft-tissue infections and postoperative
wound infections.
Summary:
Streptococci are Gram-positive, nonmotile, catalase-
negative, facultatively anaerobic cocci that occur in
chains or pairs. They are classified based on their
hemolytic capacity (α-, β-, ƴ-hemolysis) and the
antigenicity of a carbohydrate occurring in their cell walls
(Lancefield antigen).b-hemolytic group A streptococci
(S. pyogenes) cause infections of the upper respiratory
tract and invasive infections of the skin and subcutaneous
connective tissue. Depending on the status of the immune
defenses and the genetic disposition, this may lead to
scarlet fever and severe infections such as necrotizing
fasciitis, sepsis, or septic shock. Sequelae such as acute
rheumatic fever and glomerulonephritis have an
autoimmune pathogenesis. The α-hemolytic pneumococci
(S. pneumoniae) cause infections of the respiratory tract.
Penicillins are the antibiotics of choice. Resistance to
penicillins is known among pneumococci, and is
increasing. Laboratory diagnosis involves pathogen
detection in the appropriate material. Persons at high risk
can be protected from pneumococcal infections with an
active prophylactic vaccine containing purified capsular
polysaccharides. Certain oral streptococci are responsible
for dental caries. Oral streptococci also cause half of all
cases of endocarditis. Although enterococci show only
low levels of pathogenicity, they frequently cause
nosocomial infections in immunocompromised patients
(usually as elements of a mixed flora).

Unit 2: Bacteriology
85
Lecture 3 - Pathogenic Neisseria,
Moraxella and Acinetobacter
The family Neisseriaceae consists of Gram-negative
aerobic bacteria from 14 genera, including Neisseria,
Chromobacterium, Kingella,and Aquaspirillum. The
genus Neisseria contains two important human
pathogens, N. gonorrhoeae and N. meningitidis.
Neisseria gonorrheae
Morphology and culture
Gonococci are Gram-negative, coffee-bean-shaped cocci
that are usually paired and have a diameter of
approximately 1 µm. Attachment pili on the bacterial cell
surface are responsible for their adhesion to mucosal cells.
Gonococci can be grown on moist culture mediums
enriched with protein (blood). The atmosphere for
primary culturing must contain 5–10% CO2.
Neisseria gonorrhoeae
Pathogenesis and clinical picture.
Gonorrhea is a sexually transmitted disease. The
pathogens penetrate into the urogenital mucosa, causing a
local purulent infection. In men, the prostate and
epididymis can also become infected.
Uncomplicated
gonorrhea in the adult male is an inflammatory and
pyogenic infection of the mucous membranes of the
anterior urethra. The most common symptom is a
discharge that may range from a scanty, clear or cloudy
fluid to one that is copious and purulent. Dysuria
(difficulty in urination)is often present. Inflammation of
the urethral tissues results in the characteristic redness,
swelling, heat, and pain in the region. There is intense
burning and pain upon urination. In women, the
gonococci can also cause salpingitis, oophoritis, or even
peritonitis.
Endocervical infection is the most common form of
uncomplicated gonorrhea in women. Such infections are
usually characterized by vaginal discharge and sometimes
by dysuria. About 50% of women with cervical infections
are asymptomatic. Asymptomatic infections occur in
males, as well. Males with asymptomatic urethritis are an
important reservoir for transmission and are at increased
risk for developing complications. Asymptomatic males
and females are a major problem as unrecognized carriers
of the disease, which occurs in the U.S. at an estimated
rate of over 700,000 cases per year. Gonococci reaching
the conjunctival membrane may cause a purulent
conjunctivitis, seen mainly in newborn children.
Gonococci can also infect the rectal or pharyngeal
mucosa. Hematogenously disseminated gonococci may
also cause arthritis or even endocarditis.
Determinants of the Pathogenicity of Gonococci.
Attachment pili on the surface and the outer
membrane protein Opa are responsible for adhesion to
cells of the urogenital tract. Opa also directs the invasion
process by means of endocytosis. Immune defenses
against granulocytes are based on the outer membrane
porin Por that prevents the phagosome from fusing with
lysosomes, resulting in the survival—and
proliferation—of phagocytosed gonococci in
granulocytes. The lipo-oligosaccharide (LOS) in the
outer membrane is responsible for resistance to
complement (serum resistance) as well as for the
inflammatory tissue reaction in a manner analogous to
the more complexly structured LPS of enterobacteria.
Gonococci can capture iron from the siderophilic
proteins lactoferrin and transferrin, accumulating it inside
the bacterial cells to facilitate their rapid proliferation.
An IgA1 protease produced by the gonococci hydrolyzes
secretory antibodies in the mucosal secretions. The
pronounced antigen variability of the attachment pili
and the Opa protein make it possible for gonococci to
thwart specific immune defense mechanisms repeatedly.
Diagnosis
The method of choice is detection of the pathogens by
means of methylene blue and gram staining and
culturing. Gonococci are sensitive in cultures and the
material must be used immediately after they are obtained
to inoculate. Thayer-Martin blood agar with antibiotics
added to eliminate accompanying flora, on which medium
the cultures are then transported to the laboratory. The
identification procedure involves both morphology and
biochemical characteristics. Techniques developed
recently utilize immunofluorescence or coagglutination
methods utilizing monoclonal antibodies to the main
protein of the outer membrane, Por.
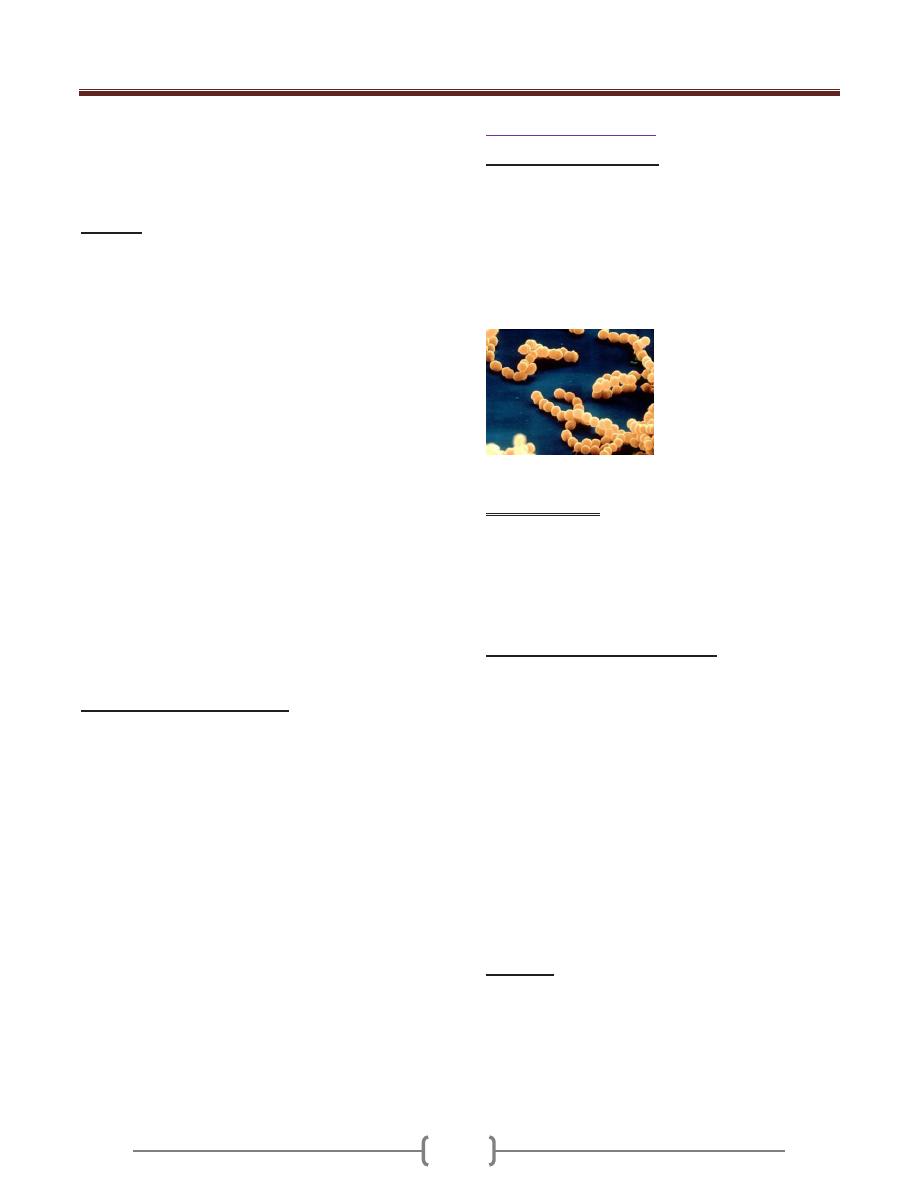
Unit 2: Bacteriology
86
Direct detection in pus and secretion samples is possible
using an enzymatic immunosorbence test or detection of
gonococcus-specific DNA sequences coding for rRNA
using a gene probe.
Therapy
The agent of choice used to be penicillin G. In recent
years, however, the percentage of penicillinase-producing
strains has increased considerably all over the world. For
this reason, third-generation cephalosporins are now
used to treat uncomplicated cases of gonorrhea. They are
applied in a single dose (e.g., ceftriaxone, 250–500mg
i.m.). Good results have also been reported with single-
dose oral application of fluorinated 4-quinolones (e.g.,
0.5 g ciprofloxacin or 0.4 g ofloxacin).
Penicillin Resistance in Gonococci : The determinants of
high-level penicillin resistance in gonococci are small,
nonconjugative plasmids, which are mobilized by a
conjugative helper plasmid for transmission from one
gonococcal cell to another. The penicillin resistance
plasmids code for the TEM betalactamase that occurs
frequently in Enterobacteriaceae. It is therefore assumed
that the penicillinase gene in gonococci derived from the
Enterobacteriaceae gene pool. Low-level, inherent
resistance to penicillin is based on chromosomal genes (
penA, penB) that code for penicillin-binding proteins
with reduced affinity to penicillin. These genes are
products of mutations.
Epidemiology and prevention
Gonorrhea is a worldwide sexually transmitted disease
that occurs only in humans. Its level of annual incidence
in developed countries is estimated at 12 cases per 1000
inhabitants. The actual figures are likely to be much
higher due to large numbers of unreported cases. A
reduction in incidence seen in recent years may be due
to AIDS prophylaxis. Protective immunization for
high-risk persons is not feasible due to the antigen
variability of the organism as described above. Stopping
the spread of gonorrhea involves mainly rapid recognition
of infections and treatment accordingly. One hundred
percent prevention of ophthalmia neonatorum is possible
with a single parenteral dose of 125mg ceftriaxone. Local
prophylaxis is also practiced using a 1% solution of
silver nitrate or eye ointments containing 1%
tetracycline or 0.5% erythromycin.
Neisseria meningitidis
Morphology and culture
Meningococci are Gram-negative, coffee-bean shaped
cocci that are frequently pleomorphic and have a
diameter of 1 µm. They are nonmotile and feature a
polysaccharide capsule.
Growing meningococci in cultures requires mediums
containing blood. A concentration of 5–10% CO2
encourages proliferation.
Neisseria meningitidis scanning EM
Antigen structure. Serogroups A, B, C, D, etc. (a total
of 12) are differentiated based on the capsule chemistry.
Epidemics are caused mainly by strains of serogroup A,
sometimes by B strains as well and, more rarely, by group
C strains. Serogroups are divided into serovars based on
differences in the outer membrane protein antigens.
Pathogenesis and clinical picture
Meningococci are parasites of the nasopharynx. These
microorganisms are carried by 5–10% of the population.
If virulent meningococci colonize the nasopharyngeal
mucosa of a host lacking the antibodies, pathogen
invasion of the mucosa by means of “parasite directed
endocytosis” becomes possible. The CNS is doubtless the
preferred compartment for secondary infections,
although hematogenously disseminated pathogens can
also infect the lungs, the endocardium, or major joints.
Onset of the meningitis is usually sudden, after an
incubation period of two to three days, with severe
headache, fever, neck stiffness, and severe malaise.
Severe hemorrhagic sepsis sometimes develops
(Waterhouse-Friedrichsen syndrome).
Diagnosis
Diagnosis requires detection of the pathogen in
cerebrospinal fluid or blood by means of microscopy
and culturing techniques. For success in culturing, the
material must be used to inoculate blood agar without
delay. Identification of the pathogen is based on
identification of metabolic properties. The slide
Unit 2: Bacteriology
87
agglutination test is used to determine the serogroup.
Latex agglutination or coagglutination can be used for
direct antigen detection in cerebrospinal fluid.
Therapy
The antibiotic of choice is penicillin G. Very good results
have also been obtained with third-generation
cephalosporins, e.g., cefotaxime or ceftriaxone. It is
important to start treatment as quickly as possible to
prevent delayed damage. The advantage of
cephalosporins is that they are also effective against other
meningitis pathogens due to their broad spectrum of
action (with the exception of Listeria monocytogenes).
Epidemiology and prevention
Meningococcal infections are more frequent in the winter
and spring months. Transmission of meningococci is by
droplet infection. Humans are the only pathogen
reservoir. Sources of infection include both carriers and
infected persons with manifest disease. In developed
countries, meningitis occurs sporadically or in the form
of minor epidemics in more or less isolated collectives
(work camps, recruiting camps, school camping
facilities). The incidence level is approximately 12 cases
per 100 000 inhabitants per year. In parts of the
developing world (African meningitis belt) the level is
higher. Lethality runs to 85% if the disease is left
untreated, but is reduced to less than 1% if treatment is
begun early enough. Prophylactic antibiosis is indicated
for those in close contact with diseased persons (e.g., in
the same family). Prophylactic measures also include
treatment of carriers to eliminate this reservoir, whereby
minocylin or rifampicin must be used instead of
penicillin G. Prophylactic immunization can be
achieved with a vaccine made from the purified capsule
polysaccharides A, C, Y, and W-135. There is no
serogroup B vaccine, since the capsule in serogroup B
consists of polyneuraminic acid, which the immune
system does not recognize as a foreign substance.
Moraxella and Acinetobacter
The taxonomic definitions of these genera are still
inconclusive. Bergey’s Manual of Systematic
Bacteriology groups both under the family
Moraxellaceae.These bacteria are short, rounded rods,
often coccoid, sometimes also diplococcoid. Their
natural habitat is either human mucosa (Moraxella) or
the natural environment (Acinetobacter).
Moraxella.
The genus comprises two medically important species:
Moraxella catarrhalis
. Component of the normal flora of
the upper respiratory tract. May be responsible for:
pneumonia, acute exacerbation of chronic bronchitis,
otitis media (up to 20% in children), and sinusitis. About
90% of all strains produce one of the so-called BRO
penicillinases, so that therapy with a penicillinase-stable
betalactam antibiotic is indicated.
Moraxella lacunata
. Formerly Diplobacterium Morax-
Axenfeld. Can cause conjunctivitis and keratitis. The
reason why this organism is now rarely found as a
pathogen in these eye infections is unknown.
Acinetobacter
In immunodeficient persons, A. baumannii, A.
calcoaceticus, and other species can cause nosocomial
infections (urinary tract infections, pneumonias,
wound infections, sepsis). Clinical strains of these
species often show multiresistance to antibiotics, so that
treatment of these infections may prove difficult.
Summary:
Neisseria are Gram-negative, aerobic cocci that are often
arranged in pairs.They are typical mucosal parasites that die
rapidly outside the human organism. Culturing on enriched
nutrient mediums is readily feasible.
Neisseria gonorrheae is the pathogen responsible for
gonorrhea (“clap”).
Infection results from sexual intercourse. The organisms
adhere to cells of the urogenital tract by means of attachment
pili and the protein Opa, penetrate into the organism using
parasite-directed endocytosis and cause a pyogenic infection,
mainly of the urogenital epithelium. An infection is
diagnosed mainly by means of microscopy and culturing of
purulent secretions. The therapeutic of choice is penicillin G.
Alternatives for use against penicillinase-positive gonococci
include third-generation cephalosporins and 4-quinolones. N.
meningitidis is a parasite of the nasopharyngeal mucosa.
These meningococci cause meningitis and sepsis. Diagnosis
involves detection of the pathogens in cerebrospinal fluid
and blood. The disease occurs sporadically or in the form of
minor epidemics in children, youths, and young adults. The
antibiotics of choice are penicillin G and third-generation
cephalosporins.
The family Neisseriaceae includes aerobic, Gram-
negative cocci and rods, the most important of which are the
human pathogens N. gonorrheae and N. meningitidis. Other
species in the genus Neisseria are elements of the normal
mucosal flora.

Unit 2: Bacteriology
88
Lecture 4 - Aerobic Spore-Former
Bacteria (Bacillus)
Bacillus anthracis
Introduction
The anthrax bacillus, Bacillus anthracis, was the first
bacterium shown to be the cause of a disease. In
1877, Robert Koch grew the organism in pure culture,
demonstrated its ability to form endospores, and
produced experimental anthrax by injecting it into
animals. Anthrax occurs primarily in animals, especially
herbivores. The pathogens are ingested with feed and
cause a severe clinical sepsis that is often lethal.
Morphology and culturing
Bacillus anthrac is very large, Gram-positive,
sporeforming rod, 1 - 1.2µm in width x 3 - 5µm in length,
with a capsule made of a glutamic acid polypeptide. The
bacterium can be cultivated in ordinary nutrient medium
under aerobic (or anaerobic) conditions. Genotypically
and phenotypically it is very similar to Bacillus
cereus, which is found in soil habitats around the world,
and to Bacillus thuringiensis, the pathogen for larvae
of Lepidoptera. The three species have the same cellular
size and morphology and form oval spores located
centrally in a nonswollen sporangium.The bacterium is
readily grown in an aerobic milieu.
Bacillus anthracis. Gram stain. 1500X. The cells have
characteristic squared ends. The endospores are ellipsoidal
shaped and located centrally in the sporangium. The spores
are highly refractile to light and resistant to staining.
Pathogenesis and clinical picture
The pathogenicity of B. anthracis results from its
antiphagocytic capsule as well as from a toxin that causes
edemas and tissue necrosis. Human infections are
contracted from diseased animals or contaminated animal
products. Anthrax is recognized as an occupational
disease. Dermal, primary inhalational, and intestinal
anthrax are differentiated based on the pathogen’s portal
of entry. In dermal anthrax, which accounts for 90–95%
of human B. anthracis infections) the pathogens enter
through injuries in the skin. A local infection focus
similar to a carbuncle develops within two to three days.
A sepsis with a foudroyant (highly acute) course may then
develop from this primary focus. Inhalational anthrax
(bioterrorist anthrax), with its unfavorable prognosis,
results from inhalation of dust containing the pathogen.
Ingestion of contaminated foods can result in intestinal
anthrax with vomiting and bloody diarrheas.
Anthrax
Anthrax is primarily a disease of domesticated and wild
animals, particularly herbivorous animals, such as cattle,
sheep, horses, mules and goats. Humans become infected
incidentally when brought into contact with diseased
animals, which includes their flesh, bones, hides, hair and
excrement. The natural history of Bacillus anthracis is
obscure. Although the spores have been found naturally in
soil samples from around the world, the organisms cannot
be regularly cultivated from soils where there is an
absence of endemic anthrax. In the United States, the
incidence of naturally-acquired anthrax is extremely rare
(1-2 cases of cutaneous disease per year). Worldwide, the
incidence is unknown, although B. anthracis is present in
most of the world. Unreliable reporting makes it difficult
to estimate the true incidence of human anthrax
worldwide.
The most common form of the disease in humans
is cutaneous anthrax, which is usually acquired via
injured skin or mucous membranes. A minor scratch or
abrasion, usually on an exposed area of the face or neck
or arms, is inoculated by spores from the soil or a
contaminated animal or carcass.The spores germinate,
vegetative cells multiply, and a characteristic gelatinous
edema develops at the site. This develops into
papule within 12-36 hours after infection. The papule
changes rapidly to a vesicle, then a pustule (malignant
pustule), and finally into a necrotic ulcer from which
infection may disseminate, giving rise to septicemia.
Lymphatic swelling also occurs within seven days. In
severe cases, where the blood stream is eventually
invaded, the disease is frequently fatal.
Another form of the disease, inhalation
anthrax (woolsorters' disease), results most commonly
from inhalation of spore-containing dust where animal
hair or hides are being handled. The disease begins
abruptly with high fever and chest pain. It progresses
rapidly to a systemic hemorrhagic pathology and is often

Unit 2: Bacteriology
89
fatal if treatment cannot stop the invasive aspect of the
infection.
Gastrointestinal anthrax is analogous to cutaneous
anthrax but occurs on the intestinal mucosa. As in
cutaneous anthrax, the organisms probably invade the
mucosa through a preexisting lesion. The bacteria spread
from the mucosal lesion to the lymphatic system.
Intestinal anthrax results from the ingestion of
poorly cooked meat from infected animals.
Gastrointestinal anthrax is rare but may occur as
explosive outbreaks associated with ingestion of infected
animals. Intestinal anthrax has an extremely high
mortality rate.
Meningitis due to B. anthracis is a very rare complication
that may result from a primary infection elsewhere.
Pathogenicity of Bacillus anthracis
Bacillus anthracis clearly owes its pathogenicity to two
major-determinants of virulence: the formation of a poly-
D-glutamyl capsule, which mediates the invasive stage
of the infection, and the production of the
multicomponent anthrax toxin which mediates the
toxigenic stage.
Bacillus anthracis forms a single
antigenic type of capsule consisting of a poly-D-
glutamate polypeptide. All virulent strains of B.
anthracis form this capsule.
The poly-D-glutamyl capsule
is itself nontoxic, but functions to protect the organism
against complement and the bactericidal components of
serum and phagocytes, and against phagocytic engulfment
and destruction. Production of capsular material is
associated with the formation of a characteristic mucoid
or "smooth" colony type. "Smooth" (S) to "rough" (R)
colonial variants occur, which is correlated with ability to
produce the capsule. R variants are relatively avirulent
.
One component of the anthrax toxin has a lethal mode
of the action . Death is apparently due to oxygen
depletion, secondary shock, increased vascular
permeability, respiratory failure and cardiac failure. Death
from anthrax in humans or animals frequently occurs
suddenly and unexpectedly. The level of the lethal toxin
in the circulation increases rapidly quite late in the
disease, and it closely parallels the concentration of
organisms in the blood.
Production of the anthrax toxin is mediated by a
temperature-sensitive plasmid, pX01, of 110
megadaltons. The toxin consists of three distinct antigenic
components. Each component of the toxin is a
thermolabile protein with a mw of approximately 80kDa.
I. Factor I is the edema factor (EF) which is necessary for
the edema producing activity of the toxin. EF is known to
be an inherent adenylate cyclase, similar to
the Bordetella pertussis adenylate cyclase toxin.
II. Factor II is the protective antigen (PA), because it
induces protective antitoxic antibodies in guinea pigs. PA
is the binding (B) domain of the anthrax toxin which has
two active (A) domains, EF (above) and LF (below).
III. Factor III is known as the lethal factor (LF) because it
is essential for the lethal effects of the anthrax toxin.
Apart from their antigenicity, each of the three factors
exhibits no significant biological activity in an animal.
However, combinations of two or three of the toxin
components yield the following results in experimental
animals.
PA+LF combine to produce lethal activity
EF+PA produce edema
EF+LF is inactive
PA+LF+EF produces edema and necrosis and is lethal
Diagnosis.
The diagnostic procedure involves detection of the
pathogen in dermal lesions, sputum, and/or blood
cultures using microscopic and culturing methods.
Several nonselective and selective media for the detection
and isolation of Bacillus anthracis have been described,
as well as a rapid screening test for the bacterium based
on the morphology of microcolonies . The capsular
material can be detected by the McFadyean reaction
which involves staining with polychrome methylene
blue. Blue rods in a background of purple/pink-stained
capsular material is a positive test. Neither B.
cereus nor B. thuringiensis synthesizes this capsular
polymer, so the detection of capsular material can be used
to distinguish B. anthracis from its closest relatives .The
Table-1 bellow provides the differential characteristics
that are used to distinguish Bacillus anthracis from most
strains of Bacillus
The Table-1 show Differential Characteristics of B. anthracis
B. cereus and B. thuringiensis
Characteristic
B.
anthracis
B.cereus & B.
thuringiensis
growth requirement for thiamin
+
-
hemolysis on sheep blood agar
-
+
glutamyl-polypeptide capsule
+
-
lysis by gamma phage
+
-
Motility
-
+
growth on chloral hydrate agar
-
+
string-of-pearls test
+
-
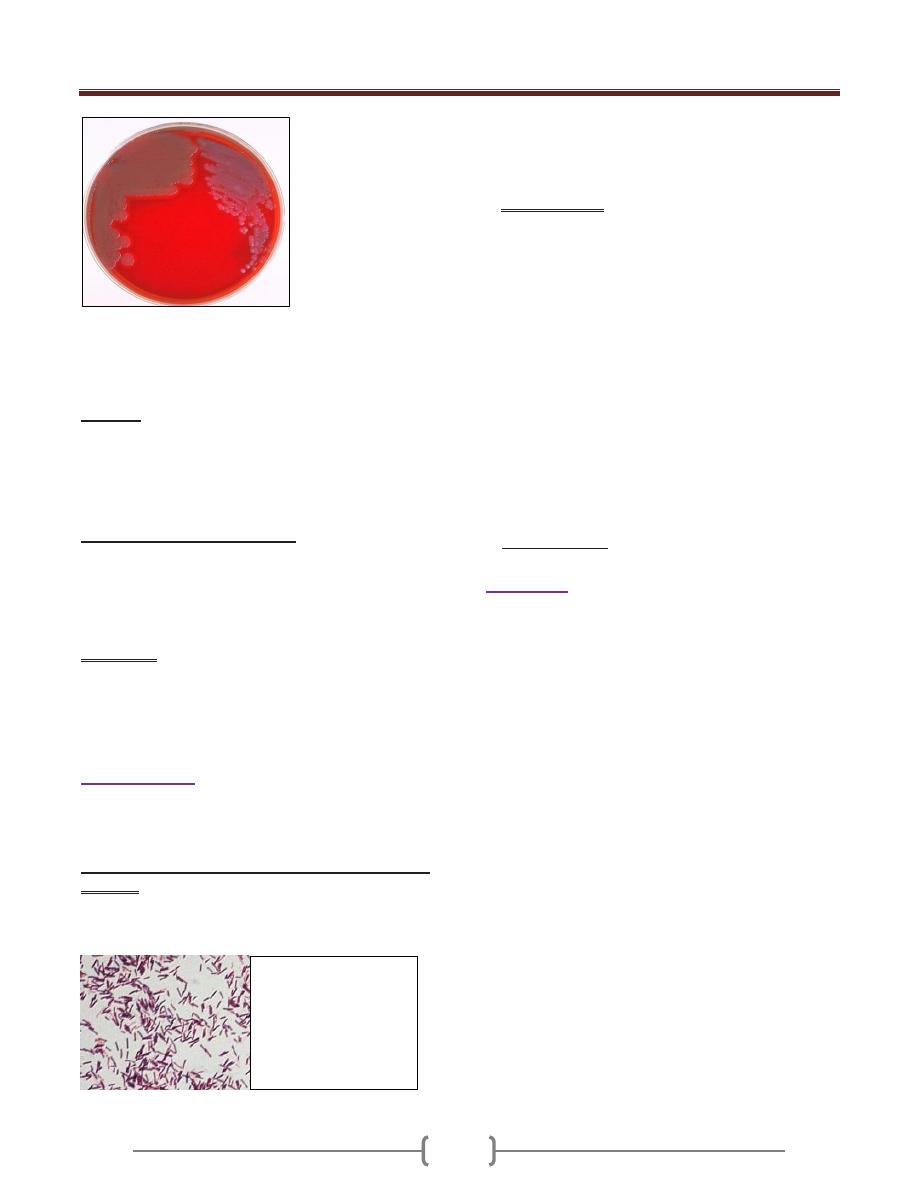
Unit 2: Bacteriology
90
Figure 1. Colonies of Bacillus cereus on the left; colonies
of Bacillus anthracis on the right. B. cereus colonies are
larger, more mucoid, and this strain exhibits a slight zone of
hemolysis on blood agar.
Therapy
The antimicrobial agent of choice is penicillin G.
Doxycycline (a tetracycline) or ciprofloxacin (a
fluoroquinolone) are possible alternatives. Surgery is
contraindicated in cases of dermal anthrax.
Epidemiology and prophylaxis
Anthrax occurs mainly in southern Europe and South
America, where economic damage due to farm animal
infections is considerable. Humans catch the disease from
infected animals or contaminated animal products.
Anthrax is a classic zoonosis.
Prophylaxis involves mainly exposure prevention
measures such as avoiding contact with diseased animals
and disinfection of contaminated products. A cell-free
vaccine obtained from a culture filtrate can be used for
vaccine prophylaxis in high-risk persons.
Bacillus cereus
Bacillus cereus has been recognized as an agent of food
poisoning since 1955. It is not a reportable disease, and
usually goes undiagnosed.
B. cereus causes two types of food-borne illnesses.
One type is characterized by nausea and vomiting and
abdominal cramps and has an incubation period of 1 to 6
hours. It resembles Staphylococcus aureus
(staph) food poisoning in its symptoms and incubation
period. This is the "short-incubation" or emetic form of
the disease.
The second type is manifested primarily by abdominal
cramps and diarrhea following an incubation period of 8
to 16 hours. Diarrhea may be a small volume or profuse
and watery. This type is referred to as the "long-
incubation" or diarrheal form of the disease, and it
resembles food poisoning caused by Clostridium
perfringens. In either type, the illness usually lasts less
than 24 hours after onset. In a few patients symptoms
may last longer. The short-incubation form is caused by a
preformed, heat-stable emetic toxin, ETE. The
mechanism and site of action of this toxin are unknown,
although the small molecule forms ion channels and holes
in membranes. The long-incubation form of illness is
mediated by the heat-labile diarrheagenic enterotoxin
Nhe and/or hemolytic enterotoxin HBL, which cause
intestinal fluid secretion, probably by several
mechanisms, including pore formation and activation
of adenylate cyclase enzymes.
Summary:
The natural habitat of Bacillus anthracis, a Gram-positive,
sporing, obligate aerobic rod bacterium, is the soil. The
organism causes anthrax infections in animals. Human
infections result from contact with sick animals or animal
products contaminated with the spores. Infections are
classified according to the portal of entry as dermal
anthrax (95% of cases), primary inhalational anthrax, and
intestinal anthrax. Sepsis can develop from the primary
infection focus. Laboratory diagnosis includes
microscopic and cultural detection of the pathogen in
relevant materials and blood cultures. The therapeutic
agent of choice is penicillin G. The genera Bacillus and
Clostridium belong to the Bacillaceae family of sporing
bacteria. There are numerous species in the genus Bacillus
(e.g., B. cereus, B. subtilis, etc.) that normally live in the
soil. The organism in the group that is of veterinary and
human medical interest is Bacillus anthracis.
Bacillus cereus. Gram
stain. 450X. Bacilli are
large bacteria, so that
they are readily
observed with the
microscope's "high dry
objective".

Unit 2: Bacteriology
91
Lecture 5 - Anaerobic Spore-Former
Bacteria (Pathogenic Clostridia)
The genus Clostridium consists of relatively large,
Gram-positive, rod-shaped bacteria. All species
form endospores and have a strictly fermentative type
of metabolism. Most clostridia will not grow under
aerobic conditions and vegetative cells are killed by
exposure to O
2
, but their spores are able to survive long
periods of exposure to air.
Stained pus from a mixed anaerobic infection. At least
three different clostridia are apparent
Clostridium perfringens
:
Clostridium perfringens (formerly known as
Clostridium welchii) is a Gram-positive, rod-shaped,
anaerobic, spore-forming bacterium of the genus
Clostridium. C. perfringens is ubiquitous in nature and
can be found as a normal component of decaying
vegetation, marine sediment, the intestinal tract of humans
and other vertebrates, insects, and soil. Virtually every
soil sample ever examined, with the exception of the
sands of the Sahara, has contained C. perfringens.
Photomicrograph of large, Gram-positive, rod-shaped
Clostridium perfringens bacilli.
Morphology and Colony characteristics
On blood agar plates, C. perfringens grown anaerobically
produces β-haemolytic, flat, spreading, rough, translucent
colonies with irregular margins. A Nagler agar plate,
containing 5-10% egg yolk, is used to presumptively
identify strains which produce α-toxin, a diffusible
lecithinase which interacts with the lipids in egg yolk to
produce a characteristic precipitate around the colonies.
One half of the plate is inoculated with antitoxin to act as
a control in the identification.
Pathogenesis and Clinical Finding:
Clostridium perfringens, which produces a huge array of
invasins and exotoxins, causes wound and surgical
infections that lead to gas gangrene, in addition to severe
uterine infections. Clostridial hemolysins and
extracellular enzymes such as proteases, lipases,
collagenase and hyaluronidase, contribute to the
invasive process. Clostridium perfringens also produces
an enterotoxin and is an important cause of food
poisoning. Usually the organism is encountered in
improperly sterilized (canned) foods in which endospores
have germinated. C. perfringens is commonly
encountered in infections as a benign component of the
normal flora. In this case, its role in disease is minor.
Infections due to C. perfringens show evidence of tissue
necrosis, bacteremia, emphysematous cholecystitis, and
gas gangrene, which is also known as clostridial
myonecrosis. The toxin involved in gas gangrene is
known as α-toxin, which inserts into the plasma
membrane of cells, producing gaps in the membrane
which disrupt normal cellular function. The action of
C. perfringens on dead bodies is known to mortuary
workers as tissue gas and can only be halted by
embalming.
Gas gangrene:
Is a bacterial infection that produces gas within tissues in
gangrene. It is a deadly form of gangrene usually caused
by Clostridium bacteria. It is a medical emergency. Gas
gangrene generally occurs at the site of trauma or a recent
surgical wound. The onset of gas gangrene is sudden and
dramatic. About a third of cases occur on their own.
Patients who develop this disease in this manner often
have underlying blood vessel disease (atherosclerosis or
hardening of the arteries), diabetes, or colon cancer.
Clostridium perfringens produces many different toxins,
four of which (alpha, beta, epsilon, iota) can cause
potentially deadly syndromes. The toxins cause damage to
tissues, blood cells, and blood vessels. Gas gangrene is
marked by a high fever, brownish pus, gas bubbles
under the skin, skin discoloration, and a foul odor.
Pathophysiology of Gas gangrene is caused by exotoxin-
producing Clostridial species (most often Clostridium

Unit 2: Bacteriology
92
perfringens, but less commonly C. septicum or
C. ramnosum ), which are mostly found in soil but also
found as normal gut flora, and other anaerobes (e.g.
Bacteroides and anaerobic streptococci). The exotoxin is
commonly found in C. perfringens type A strain and is
known as alpha toxin. These environmental bacteria may
enter the muscle through a wound and go on to
proliferate in necrotic tissue and secrete powerful
toxins. These toxins destroy nearby tissue, generating gas
at the same time. Other organisms may rarely cause gas
gangrene (for example, Klebsiella pneumoniae in the
context of diabetes).
Treatment of gas gangrene
Treatment of gas gangrene is usually by
amputation
if necessary in many cases.
alone are not effective because they do not
penetrate ischaemic
However,
penicillin
is given as an adjuvant treatment to
surgery
. In addition to surgery and antibiotics,
(HBOT) is used and acts to
inhibit the growth of and kill the anaerobic C.
perfringens.
Food poisoning
Some strains of C. perfringens produce toxins (which
cause gas gangrene) if ingested. In the United Kingdom
and United States they are the third most common cause
of food-borne illness, with poorly prepared meat and
poultry the main culprits in harboring the bacterium. The
clostridial enterotoxin mediating the disease is often heat-
resistant and can be detected in contaminated food and
feces. Incubation time is between 6 and 24 (commonly
10-12) hours after ingestion of contaminated food.
Manifestions typically include abdominal cramping and
diarrhea - vomiting and fever are unusual. The whole
course usually resolves within 24 hours. Very rare, fatal
cases of clostridial necrotizing enteritis have been
known to involve "Type C" strains of the organism,
which produce a potently ulcerative β-toxin. It is likely
that many cases of C. perfringens food poisoning remain
subclinical, as antibodies to the toxin are common
amongst the population.
Treating food poisoning:
In most cases, food poisoning
can be treated at home without seeking medical advice. It
is very important that the patient do not become
dehydrated because it will make him feel worse and slow
down his recovery. Oral rehydration salts (ORSs) are
recommended for people vulnerable to the effects of
dehydration, such as the elderly and those with a pre-
existing health condition.
Clostridium tetani
Clostridium tetani is a rod-shaped, anaerobic bacterium
of the genus Clostridium. Like other Clostridium species,
it is Gram-positive, and its appearance on a gram stain
resembles tennis rackets or drumsticks. C. tetani is
found as spores in soil or as parasites in the
gastrointestinal tract of animals. C. tetani produces a
potent biological toxin, tetanospasmin, and is the
causative agent of tetanus.
Clostridium tetani with characteristic 'tennis racket'
appearance.
Characteristics
C. tetani is a rod-shaped, obligate anaerobe which stains
Gram positive in fresh cultures; established cultures may
stain Gram negative. During vegetative growth, the
organism cannot survive in the presence of oxygen, is
sensitive to heat and has flagella which provide limited
mobility. As the bacterium matures, it develops a
terminal spore, which gives the organism its
characteristic appearance. C. tetani spores are extremely
hardy and are resistant to heat and most antiseptics.
The spores are distributed widely in manure-treated soils,
and can also be found on human skin and in contaminated
heroin .
Toxicity
C. tetani usually enters a host through a wound to the skin
and then it replicates. Once an infection is established, C.
tetani produces two exotoxins, tetanolysin and
tetanospasmin. Eleven strains of C. tetani have been
identified, which differ primarily in flagellar antigens
and in their ability to produce tetanospasmin. The genes
that produce toxin are encoded on a plasmid which is
present in all toxigenic strains, and all strains that are

Unit 2: Bacteriology
93
capable of producing toxin produce identical toxins.
Tetanolysin serves no known function to C. tetani, and the
reason the bacteria produce it is not known with certainty.
Tetanospasmin is a neurotoxin and causes the clinical
manifestations of tetanus. Tetanus toxin is generated in
living bacteria, and is released when the bacteria lyses,
such as during spore germination or during vegetative
growth. A minimal amount of spore germination and
vegetative cell growth are required for toxin production.
On the basis of weight, tetanospasmin is one of the most
potent toxins known. The estimated minimum human
lethal dose is 2.5 nanograms per kilogram of body
weight, or 175 nanograms in a 70 kg (154 lb) human. The
only toxins more lethal to humans are botulinum toxin,
produced by close relative Clostridium botulinum and the
exotoxin produced by Corynebacterium diphtheriae, the
causative agent of diphtheria.
Toxin Action: Tetanospasmin is distributed in the blood
and lymphatic system of the host. The toxin acts at
several sites within the central nervous system,
including peripheral nerve terminals, the spinal cord,
and brain, and within the sympathetic nervous system.
The toxin is taken up into within the nerve axon and
transported across synaptic junctions, until it reaches the
central nervous system, where it is rapidly fixed to
gangliosides at the presynaptic junctions of inhibitory
motor nerve endings. The clinical manifestations of
tetanus are caused when tetanus toxin blocks inhibitory
impulses, by interfering with the release of
neurotransmitters, including glycine and gamma-
aminobutyric acid. This leads to unopposed muscle
contraction and spasm. Characteristic features are Risus
Sardonicus (a rigid smile), Trismus (commonly known
as lock-jaw), and Opisthotonus (rigid, arched back).
Seizures may occur, and the autonomic nervous system
may also be affected. Tetanospasmin appears to prevent
the release of neurotransmitters by selectively cleaving
a component of synaptic vesicles called synaptobrevin
II.
Treatment:
When a tetanus infection becomes established, treatment
usually focuses on controlling muscle spasms, stopping
toxin production, and neutralizing the effects of the
toxin. Treatment includes administration of tetanus
immune globulin (TIG), which comprises antibodies
that inhibit tetanus toxin (also known as tetanus
antitoxins), by binding to and removing unbound
tetanus toxin from the body. Binding of the toxin to the
nerve endings appears to be an irreversible event, and
TIG is ineffective at removing bound toxin. Large doses
of antibiotic drugs (such as metronidazole or
intramuscular penicillin G) are also given once tetanus
infection is suspected, to halt toxin production.
Prevention of tetanus includes vaccination, and
cleaning the primary wound. Prophylaxis is effective,
in the form of a tetanus toxoid vaccine, which is given
with or without passive immunization with tetanus
immune globulin. Very few cases of tetanus have
occurred in individuals with up-to-date tetanus
vaccinations. DPT vaccine (diphtheria-pertussis-tetanus)
may provide protection from tetanus for many years (
about 10 years), and every 10 years thereafter, a booster
shot of tetanus vaccine is recommended. Tetanus is not
contagious from person to person, and is the only
vaccine-preventable disease that is infectious but not
contagious. A C.tetani infection does not result in
tetanus immunity, and tetanus vaccination should be
given as soon as the patient has stabilized.

Unit 2: Bacteriology
94
Clostridium botulinum
Clostridium botulinum is a Gram-positive, rod shaped
bacterium that produces the neurotoxin botulin, which
causes the flaccid muscular paralysis seen in botulism.
It is also the main paralytic agent in botox.
C. botulinum stained with gentian violet
It is an anaerobic spore-former, which produces oval,
subterminal endospores and is commonly found in soil.
C. botulinum is arod-shaped microorganism. It is an
obligate anaerobe, meaning that oxygen is poisonous to
the cells. However, they tolerate very small traces of
oxygen due to an enzyme called superoxide dismutase
(SOD) which is an important antioxidant defense in
nearly all cells exposed to oxygen. Under unfavorable
circumstances they are able to form endospores that
allow them to survive in a dormant state until exposed to
conditions that can support their growth. In laboratory the
microorganism is usually isolated in Tryptose Sulfite
Cycloserine (TSC) growth media, always in an anaerobic
environment with less than 2% of Oxygen. This can be
achieved by several commercial kits that use a chemical
reaction to replace O
2
with CO
2
(E.J. GasPak System). C.
botulinum is lipase negative microorganism, it grows
between pH values of 4.8 and 7 and it can't use lactose
as a primary carbon source, characteristics important
during biochemical identification. C. botulinum strains
that do not produce a botulin toxin are referred to as
Clostridium sporogenes. The complete genome of C.
botulinum has now been sequenced.
Phenotypic types
The current nomenclature for C. botulinum recognises
four physiological groups (I-IV). This is mostly based
on the ability of the organism to digest complex proteins.
Most outbreaks of human botulism are caused by group I
(proteolytic) or II (non-proteolytic) C. botulinum.
Group III organisms mainly cause diseases in animals.
There has been no record of Group IV C. botulinum
causing human or animal disease.
Clostridium difficile
This bacterium is a very important cause of diarrhea and
more severe intestinal disease in people, and is also
possibly an important cause of diarrhea in some animals
(dogs and cats). Clostridium difficile toxin A is a toxin
generated by Clostridium difficile. It is usually described
as a enterotoxin, but it also has some activity as a
cytotoxin. C. difficile occurs in the fecal flora of 1–4%
of healthy adults and in 30–50% of children during the
first year of life. The factors that lead to development of
the disease are not known with certainty. Cases of
pseudomembranous colitis are observed frequently
under treatment with clindamycin, aminopenicillins, and
cephalosporins (hence the designation antibiotic-
associated colitis), but also occur in persons not taking
antibiotics. Occasional outbreaks are seen in hospitals.
The pathological mechanism is based on formation of two
toxins. Toxin A is an enterotoxin that causes a
dysfunction characterized by increased secretion of
electrolytes and fluids. Toxin B is a cytotoxin that
damages the mucosa of the colon.
The clinical course
includes fever, diarrhea, and spasmodic abdominal
pains. Coloscopy reveals edematous changes in the colon
mucosa, which is also covered with yellowish-whitish
matter. Laboratory diagnosis involves culturing the
pathogen from patient stool and detection of the
cytotoxin in bacteria-free stool filtrates on the basis of a
cytopathic effect (CPE) observed in cell cultures, which
CPE is then no longer observed after neutralization with
an antiserum. Toxins A and B can also be detected with
immunological test kits (ELISA tests). A specific therapy
is not required in many cases. Antibiotic treatment is
indicated in severe cases. The agent of choice is currently
metronidazole.

Unit 2: Bacteriology
95
Lecture 6 – Corynebacterium &
Diphtheroid
Corynebacteria are Gram-positive, aerobic, nonmotile,
rod-shaped bacteria classified as Actinobacteria.
Corynebacteria are related phylogenetically to
mycobacteria and actinomycetes. They do not form
spores or branch as do the actinomycetes, but they have
the characteristic of forming irregular, club-shaped or
V-shaped arrangements in normal growth. They undergo
snapping movements just after cell division, which
brings them into characteristic forms resembling Chinese
letters or palisades. The genus Corynebacterium consists
of a diverse group of bacteria including animal and
plant pathogens, as well as saprophytes. Some
corynebacteria are part of the normal flora of humans,
finding a suitable niche in virtually every anatomic site,
especially the skin and nares. The best known and most
widely studied species is Corynebacterium diphtheriae,
the causal agent of the disease diphtheria.
Corynebacterium diphtheriae:
Morphology and culturing
Diphtheria bacteria are Gram-positive, pleomorphic, often
club-shaped rods. The individual cells tend to group in
V, Y, or palisade arrangements. Neisser staining
reveals the polar bodies (polyphosphates stored at one
end of the rod). Lo¨ffler nutrient medium, which
consists of coagulated serum and nutrient broth, is still
used for the primary cultures. Selective indicator
mediums containing tellurite are used in selective
culturing. K tellurite is used to inhibit the accompanying
flora. The K tellurite is also reduced to tellurium,
coloring the colonies a brownish black.
Stained Corynebacterium cells. The "barred"
appearance and Note the characteristic "Chinese-
letter" arrangement of cells.
Three strains of Corynebacterium diphtheriae
are
recognized, gravis, intermedius and mitis. They are
listed here by falling order of the severity of the disease
that they produce in humans. All strains produce the
identical toxin and are capable of colonizing the throat.
The differences in virulence between the three strains can
be explained by their differing abilities to produce the
toxin in rate and quantity, and by their differing growth
rates.
Extracellular toxin
Diphtheria toxin consists of two functionally distinct
fragments, A and B, whereby B stands for binding to
receptors of target cells and A stands for toxic activity.
Fragment A irreversibly blocks protein synthesis
translation in the target cells, which then die. The toxin
gene is always a prophage genome component.
Pathogenesis and Clinical Picture
Local infection. Infection of the mucosa of tonsils,
pharynx, nose, and conjunctiva. Wounds and skin lesions
can also be infected. The pathogens invade the host
through these portals, reproduce, and produce
toxin, resulting in local cell damage. The inflammatory
reaction leads to collection of a grayish-white exudate, the
matrix of the “diphtherial pseudomembrane” consisting
of fibrin, dead granulocytes, and necrotic epithelial cells.
This coating adheres quite strongly to the mucosa. It may
extend into the larynx, thus eventually hindering
respiration. Regional lymph nodes are highly swollen.
Diphtheria is an upper respiratory tract illness
characterized by sore throat, low fever, and an adherent
membrane (called a pseudomembrane on the tonsils,
pharynx, and/or nasal cavity. Diphtheria toxin produced
by C. diphtheriae, can cause myocarditis, polyneuritis,
and other systemic toxic effects. A milder form of
diphtheria can be restricted to the skin. Diphtheria is a
contagious disease spread by direct physical contact or
breathing aerosolized secretions of infected individuals.
Once quite common, diphtheria has largely been
eradicated in developed nations through wide-spread use
of the DPT vaccine.Diphtheria is a serious disease, with
fatality rates between 5% and 10%. In children under 5
years and adults over 40 years, the fatality rate may be
as much as 20%. Outbreaks, although very rare, still
occur worldwide, even in developed nations.
Systemic intoxication. Parenchymal degeneration in the
cardiac muscle, liver, kidneys, and adrenal glands.
Motor cranial nerve paralysis. Late sequel damage due to

Unit 2: Bacteriology
96
the intoxication is frequently seen after the acute infection
has subsided.
Toxin-negative strains of C. diphtheriae are occasionally
observed as pathogens in endocarditis or dermal
infections. The pathogenicity of such strains corresponds
to that of commensal corynebacteria .
Diagnosis
The method of choice is detection and identification of the
pathogen in cultures from local infection foci. The
culture smear, which arrives at the laboratory in
transport medium, is plated out on Lo¨ffler medium
and a selective indicator medium. Identification is based
on both morphological and physiological characteristics.
The toxin is detected by the Elek-Ouchterlony
immunodiffusion test. A molecular method is now also
being used to identify the toxin gene. Toxin detection is
necessary for a laboratory diagnosis of diphtheria because
of the occurrence of toxin-negative strains.
Therapy
Antitoxic serum therapy is the primary treatment and it
must commence as soon as possible if diphtheria is
suspected. This treatment is supplemented by
administration of penicillin or erythromycin.
Epidemiology and prevention
Humans are the sole pathogen reservoir for diphtheria.
Infection sources include infected persons and carriers
(rare). The disease is usually transmitted by droplet
infection, or less frequently indirectly via contaminated
objects. The incubation period is two to five days.
Incidence levels in central Europe are low. From 1975 to
1984, only 113 cases were reported in Germany.
Incidence levels are higher in other countries (Russia).
Protective immunization with diphtheria toxoid is the
most important preventive measure . Exposure
prophylaxis involves isolation of infected persons until
two cultures from specimens taken at least 24 hours apart
are negative.
Actinomyces
Actinomycetes are Gram-positive bacteria that tend to
grow in the form of branched filaments. The resulting
mycelial masses are, however, not observed in older
cultures, which strongly resemble those of corynebacteria
in their morphology.
Occurrence
.
Actinomycetes are part of the normal mucosal flora in
humans and animals. They colonize mainly the oral
cavity, and an actinomycosis infection is therefore always
endogenous. Ninety percent of actinomycetes infections
in humans are caused by A. israelii, with far fewer cases
caused by A.naeslundii and other species.
Actinomyces israelii
Morphology and culture
Actinomycetes are Gram-positive, pleomorphic rod
bacteria that sometimes also show genuine branching.
The yellowish sulfur granules, measuring 1–2 mm, can
be observed macroscopically in actinomycetes pus.
These particles are conglomerates of small Actinomyces
colonies surrounded by a wall of leukocytes. Mycelial
filaments extend radially from the colonies (actinium =
Greek for raylike). Culturing the organism requires
enriched mediums and an anaerobic milieu containing
5–10% CO2. Mycelial microcolonies form only during
the first days. Whitish macrocolonies, often with a
rough surface, begin to appear after two weeks.
Pathogenesis and clinical picture
The pathogens breach mucosa (perhaps normal dermis
as well) and are able to establish themselves in tissue in
the presence of a low redox potential. The factors
responsible for these conditions include poor blood
perfusion and, above all, contributing bacterial
pathogens. Genuine actinomycoses are actually always
polymicrobial. The mixed flora found includes mainly

Unit 2: Bacteriology
97
the anaerobes of the oral cavity. Actinobacillus
actinomycetemcomitans is frequently isolated along with
various species of Bacteroidaceae. Facultative anaerobes
such as staphylococci, streptococci, and
Enterobacteriaceae are, however, also found among the
contributing flora.
Cervicofacial actinomycosis. This is the most frequent
form of actinomycetes infection (>90%). The abscesses
are hard and tumor-like at first, then they necrotize.
They may also break through to the dermal surface to
create fistulae.
Thoracic actinomycosis. This rare form results from
aspiration of saliva; sometimes this type also develops
from an actinomycosis in the throat or hematogenous
spread.
Abdominal actinomycosis. This type results from
injuries to the intestine or female genitals.
Genital actinomycosis. May result from use of
intrauterine contraceptive devices.
Canaliculitis. An inflammation of the lacrimal canaliculi
caused by any of several Actinomyces species.
Caries. The Actinomyces species involved in caries
development are A. viscosus, A. naeslundii, and A.
odontolyticus . A possible contribution to periodontitis is
also under discussion.
Diagnosis
Involves identification of the pathogen by microscopy and
culturing in pus, fistula secretion, granulation tissue, or
bronchial secretion. The samples must not be
contaminated with other patient flora, in particular from
the oral cavity and must be transported to the laboratory
in special anaerobe containers. Microscopic detection of
branched rods suffices for a tentative diagnosis. Detection
of mycelial microcolonies on enriched nutrient mediums
after one to two weeks further consolidates this diagnosis.
Final identification by means of direct
immunofluorescence, cell wall analysis, and metabolic
analysis requires several weeks.
Therapy
: Treatment includes both surgical and
antibiotic measures. The antibiotic of choice is an
aminopenicillin. Antibiosis that also covers the
contributing bacterial pathogens is important.
Epidemiology and prevention
. Actinomycoses occur
sporadically worldwide. Average morbidity (incidence)
levels are between 2.5 and five cases per 100 000
inhabitants per year. Men are infected twice as often as
women. Prophylactic considerations are irrelevant due to
the endogenous nature of actinomycetes infections.
Diphtheroid
Lists of gram-positive rod bacteria that are rarely
involved in infections and normally infect only persons
with defective immune defenses. Recent years have seen
considerable changes in their classification and
nomenclature—still an ongoing process. Some of these
bacteria are designated by collective terms such as
“diphtheroid rods” or “coryneform bacteria.” Many of
these bacteria are part of the normal dermal and mucosal
flora. They are frequently found in sampled materials as
contaminants, but also occasionally cause infections.
Summary:
Corynebacterium (Diphtheria bacteria) are pleomorphic,
club-shaped rod bacteria that often have polar bodies and
group in V, Y, or palisade forms. They can be grown on
enriched nutrient media. Their pathogenicity derives from
diphtheria toxin, which binds to receptors of sensitive cells
with the B fragment. Once the binding process is completed,
the active A fragment invades the cell. This substance
irreversibly blocks translation in the protein biosynthesis
chain. The toxin gene is a component of the β-prophage.
Local and systemic intoxications are differentiated when
evaluating the clinical picture. Local infection usually affects
the tonsils, on which the diphtherial pseudomembrane
develops. Systemic intoxications affect mainly the liver,
kidneys, adrenal glands, cardiac muscle, and cranial nerves.
Laboratory diagnosis is based on pathogen identification.
The most important treatment is antitoxin therapy.
Diphtheria occurs only in humans. Thanks to extensive
diphtheria toxoid vaccination programs, it is now rare.
Actinomycetes are part of the normal mucosal flora.
These are Gram-positive rods that often occur in the form of
branched filaments in young cultures. Conglomerates of
microcolonies in pus form so-called sulfur granules.
Actinomycetes are obligate anaerobes. The pathogens enter
body tissues through mucosa defects. Monoinfections are
rare, the most frequent case being actinomycetes-dominated
endogenous polyinfections. Cervicofacial actinomycosis,
caused by oral cavity colonizer A. israelii, is the most
frequent form of actinomycosis. Treatment includes surgical
procedures and antibiosis with aminopenicillins.
The group of Gram-positive, irregular (pleomorphic),
nonsporing rod bacteria includes many different genera that
are normal components of the skin and mucosal flora .
Pathogens in this group cause two characteristic
diseases: diphtheria, caused by Corynebacterium diphtheriae
and actinomycosis, caused mainly by Actinomyces israelii.

Unit 2: Bacteriology
98
Lecture 7 – Mycobacteria
Tuberculosis Bacteria (TB)
Tuberculosis is unquestionably among the most
intensively studied of all human diseases. In view of the
fact that tuberculosis can infect practically any organ in
the body.
Tuberculosis (TB) is the leading cause of death
in the world from a bacterial infectious disease. The
disease affects 1.8 billion people/year which is equal to
one-third of the entire world population.
Mycobacterium tuberculosis is the etiologic agent of
tuberculosis in humans. Humans are the only reservoir
for the bacterium. Mycobacterium bovis is the etiologic
agent of TB in cows and rarely in humans. Both cows
and humans can serve as reservoirs. Humans can also be
infected by the consumption of unpasteurized milk.
This route of transmission can lead to the development of
extrapulmonary TB, exemplified in history by bone
infections that led to hunched backs.
Other human pathogens belonging to the
Mycobacterium genus include
Mycobacterium avium
which causes a TB-like disease especially prevalent in
AIDS patients, and Mycobacterium leprae, the causative
agent of leprosy.
Mycobacterium tuberculosis (Ziehl Neilson Stain)
Mycobacterium tuberculosis
Morphology and culturing
TB are slender, acid-fast rods, 0.4 µm wide, and 3–4 µm
long, nonsporing and nonmotile. They can be stained with
special agents (Ziehl-Neelsen, Kinyoun, fluorescence).
Mycobacterium tuberculosis (MTB) is distantly related
to the Actinomycetes. Many nonpathogenic mycobacteria
are components of the normal flora of humans, found
most often in dry and oily locales. Mycobacterium
tuberculosis is an obligate aerobe. For this reason, in the
classic case of tuberculosis, MTB complexes are always
found in the well-aerated upper lobes of the lungs. The
bacterium is a facultative intracellular parasite, usually
of macrophages, and has a slow generation time, 15-20
hours, a physiological characteristic that may contribute
to its virulence.
Two media are used to grow MTB Middlebrook's
medium which is an agar based medium and
Lowenstein-Jensen medium which is an egg based
medium. MTB colonies are small and buff colored when
grown on either medium. Both types of media contain
inhibitors to keep contaminants from out-growing MT. It
takes 4-6 weeks to get visual colonies on either type of
media. TB are obligate aerobes. Their reproduction is
enhanced by the presence of 5–10% CO2 in the
atmosphere. Cultures must be incubated for three to six or
eight weeks at 37 ºC until proliferation becomes
macroscopically visible.
Cell wall.
Many of the special characteristics of TB are
ascribed to the chemistry of their cell wall, which features
a murein layer as well as numerous lipids, the most
important being the glycolipids (e.g.,
lipoarabinogalactan), the mycolic acids, mycosides, and
wax D . For example
Glycolipids and wax D are
r
esponsible for resistance to chemical and physical
noxae. Also they have adjuvant effect (wax D), i.e.,
enhancement of antigen immunogenicity. Tuberculin is
partially purified tuberculin contains a mixture of small
proteins (10 kDa). Tuberculin is used to test for TB
exposure (Delayed allergic reaction).
Pathogenesis and clinical picture
It is necessary to differentiate between primary and
secondary tuberculosis (reactivation or postprimary
tuberculosis) . The clinical symptoms are based on
reactions of the cellular immune system with TB antigens.
Primary tuberculosis.
In the majority of cases, the pathogens enter the lung in
droplets, where they are phagocytosed by alveolar
macrophages. TB bacteria are able to reproduce in these
macrophages due to their ability to inhibit formation of
the phagolysosome. Within 10–14 days an inflammatory
focus develops the so-called
primary focus
from which
the TB bacteria move into the regional lymph nodes,
where they reproduce and stimulate a cellular immune
response, which in turn results in clonal expansion of
specific T lymphocytes and attendant lymph node
swelling. The primary complex (Ghon’s complex)

Unit 2: Bacteriology
99
develops between six and 14 weeks after infection. At the
same time,
granulomas
form at the primary infection
site and in the affected lymph nodes, and macrophages
are activated by the cytokine
MAF
(
macrophage
activating factor
).
A tuberculin allergy
also develops in
the macroorganism The further course of the disease
depends on the outcome of the battle between the TB and
the specific cellular immune defenses.
Postprimary
dissemination foci
are sometimes observed as well, i.e.,
development of
local tissue defect foci
at
other
localizations
, typically the
apices
of the lungs.
Mycobacteria may also be transported to other organs
via the lymph vessels or bloodstream and produce
dissemination foci there. The host eventually prevails in
over 90% of cases: the granulomas and foci fibrose,
scar, and calcify, and the infection remains clinically
silent.
Secondary tuberculosis.
In about 10% of infected persons the primary
tuberculosis reactivates to become an
organ tuberculosis
,
either within months (5 %) or after a number of years (5
%). Exogenous reinfection is rare in the populations of
developed countries.
Reactivation
begins with a
caseation necrosis
in the center of the granulomas (also
called
tubercles
) that may progress to
cavitation
(formation of caverns). Tissue destruction is caused by
cytokines, among which tumor necrosis factor a (TNFa)
appears to play an important role. This cytokine is also
responsible for the
cachexia
associated with tuberculosis.
Reactivation frequently stems from old foci in the lung
apices.The body’s immune defenses have a hard time
containing necrotic tissue lesions in which large numbers
of TB cells occur (e.g., up to 109 bacteria and more per
cavern); the resulting lymphogenous or hematogenous
dissemination may result in infection foci in other organs.
Virtually all types of organs and tissues are at risk for this
kind of secondary TB infection. Such infection courses
are subsumed under the term
extrapulmonary
tuberculosis
.
Predisposing factors for TB infection include:
Close contact with large populations of people, i.e.,
schools, nursing homes, dormitories, prisons, etc.
Poor nutrition
IV drug use
Alcoholism
HIV infection is the number 1 predisposing factor for
MTB infection. 10 percent of all HIV-positive individuals
harbor MTB. This is 400-times the rate associated with
the general public. Only 3-4% of infected individuals will
develop active disease upon initial infection, 5-10%
within one year. These percentages are much higher if the
individual is HIV+.
Immunity
Humans show a considerable degree of genetically
determined resistance to TB. Besides this inherited
faculty, an organism acquires an (incomplete) specific
immunity during initial exposure (first infection). This
acquired immunity is characterized by localization of the
TB at an old or new infection focus with limited
dissemination (Koch’s phenomenon). This immunity is
solely a function of the T lymphocytes. The level of
immunity is high while the body is fending off the
disease, but falls off rapidly afterwards.It is therefore
speculated that resistance lasts only as long as TB or the
immunogens remain in the organism (= infection
immunity).
Tuberculin reaction.
Parallel to this specific immunity,
an organism infected with TB shows an altered reaction
mechanism, the tuberculin allergy, which also develops
in the cellular immune system only. The tuberculin
reaction, positive six to 14 weeks after infection, confirms
the allergy. The tuberculin proteins are isolated as purified
tuberculin (PPD = purified protein derivative). Five
tuberculin units (TU) are applied intracutaneously in the
tuberculin test. If the reaction is negative, the dose is
sequentially increased to 250 TU. A positive reaction
appears within 48 to 72 hours as an inflammatory
reaction (induration) at least 10mm in diameter at the
site of antigen application. A positive reaction means that
the person has either been infected with TB or
vaccinated with BCG. It is important to understand that a
positive test is not an indicator for an active infection or
immune status. While a positive test person can be
assumed to have a certain level of specific immunity, it
will by no means be complete. One-half of the clinically
manifest cases of tuberculosis in the population are
secondary reactivation tuberculoses that develop in
tuberculin positive persons.
Diagnosis
R
equires microscopic and cultural identification of the
pathogen or pathogen-specific DNA.
Traditional method
Workup of test material, for example with N-acetyl-L-
cysteine-NaOH (NALC-NaOH method) to liquefy
viscous mucus and eliminate rapidly proliferating

Unit 2: Bacteriology
100
accompanying flora, followed by centrifugation to enrich
the concentration.
Microscopy. Ziehl-Neelsen and/or auramine fluorescent
staining . This method produces rapid results but has a
low level of sensitivity (>104–105/ml) and specificity
(acid-fast rods only).
Culture on special solid and in special liquid mediums.
Time requirement: four to eight weeks.
Identification. Biochemical tests with pure culture if
necessary. Time requirement: one to three weeks.
Resistance test with pure culture.
Time requirement: three
weeks.
Rapid methods.
A number of different rapid TB diagnostic methods have
been introduced in recent years that require less time than
the traditional methods.
Culture.
Early-stage growth detection in liquid mediums
involving identification of TB metabolic products with
highly sensitive, semi-automated equipment. Time
requirement: one to three weeks. Tentative diagnosis.
Identification.
Analysis of cellular fatty acids by means of
gas chromatography and of mycolic acids by means of
HPLC. Time requirement: 12 days with a pure culture.
DNA probes.
Used to identify M. tuberculosis complex
and other mycobacteria. Time requirement: several hours
with a pure culture.
Resistance test.
Use of semi-automated equipment .
Proliferation/ nonproliferation determination in liquid
mediums containing standard antituberculotic agents.
Time requirement: 7–10 days.
Direct identification in patient material.
Molecular methods used for direct detection of the M.
tuberculosis complex in (uncultured) test material. These
methods involve amplification of the search sequence.
Therapy
The previous method of long-term therapy in sanatoriums
has been replaced by a standardized chemotherapy often
on an outpatient basis.
Epidemiology and prevention
Tuberculosis is endemic worldwide. The disease has
become much less frequent in developed countries in
recent decades. Tuberculosis is still a major medical
problem. It is estimated that every year approximately 15
million persons contract tuberculosis and that three
million die of the disease. The main source of infection is
the human carrier. There are no healthy carriers. Diseased
cattle are not a significant source of infection in the
developed world. Transmission of the disease is generally
direct, in most cases by droplet infection. Indirect
transmission via dust or milk (udder tuberculosis in cattle)
is the exception rather than the rule. The incubation
period is four to 12 weeks.
Exposure prophylaxis. Patients with open tuberculosis
must be isolated during the secretory phase. Secretions
containing TB must be disinfected. Tuberculous cattle
must be eliminated.
Disposition prophylaxis. An active vaccine is available
that reduces the risk of contracting the disease by about
one-half. It contains the live vaccine BCG (lyophilized
bovine TB of the Calmette-Gue´rin type). Vaccination of
tuberculin-negative persons induces allergy &
(incomplete) immunity that persist for about five to 10
years.
Leprosy Bacteria (LB)
Morphology and culture
Mycobacterium leprae (Hansen, 1873) is the causative
pathogen of leprosy. In morphological terms, these acid-
fast rods are identical to tuberculosis bacteria. They differ,
however, in that they cannot be grown on nutrient
mediums or in cell cultures.
Pathogenesis
The pathomechanisms of LB are identical to those of TB.
The host organism attempts to localize and isolate
infection foci by forming granulomas. Leprous
granulomas are histopathologically identical to
tuberculous granulomas. High counts of leprosy bacteria
are often found in the macrophages of the granulomas.
Immunity
The immune defenses mobilized against a leprosy
infection are strictly of the cellular type. The lepromin
skin test can detect a postinfection allergy.
This test is not, however, very specific (i.e., positive
reactions in cases in which no leprosy infection is
present). The clinically differentiated infection course
forms observed are probably due to individual immune
response variants.
Clinical picture
Leprosy is manifested mainly on the skin, mucosa, and
peripheral nerves. A clinical differentiation is made
between tuberculoid leprosy and lepromatous leprosy
(LL). There are many intermediate forms. TL is the
benign, nonprogressive form characterized by spotty
Unit 2: Bacteriology
101
dermal lesions. The LL form, on the other hand, is
characterized by a malignant, progressive course with
nodular skin lesions and cordlike nerve thickenings
that finally lead to neuroparalysis. The inflammatory
foci contain large numbers of leprosy bacteria.
Diagnosis
Detection of the pathogens in skin or nasal mucosa
scrapings under the microscope using Ziehl-Neelsen
staining . Molecular confirmation of DNA sequences
specific to leprosy bacteria in a polymerase chain reaction
is possible.
Therapy
Paucibacillary forms are treated with dapson plus
rifampicin for six months. Multibacillary forms require
treatment with dapson, rifampicin, and clofazimine over a
period of at least two years.
Epidemiology and prevention
Leprosy is now rare in socially developed countries,
although still frequent in developing countries. There are
an estimated 11 million victims worldwide. Infected
humans are the only source of infection. The details of the
transmission pathways are unknown. Discussion of the
topic is considering transmission by direct contact with
skin or mucosa injuries and aerogenic transmission.
The incubation period is 2–5–20 years.
Isolation of patients under treatment is no longer required.
An effective epidemiological reaction requires early
recognition of the disease in contact persons by means of
periodical examinations every six to 12 months up to five
years following contact.
Nontuberculous Mycobacteria (NTM)
Mycobacteria that are neither tuberculosis nor leprosy
bacteria are categorized as atypical mycobacteria (old
designation), nontuberculous mycobacteria (NTM) or
MOTT (mycobacteria other than tubercle bacilli).
Morphology and culture
In their morphology and staining behavior, NTM are
generally indistinguishable from tuberculosis bacteria.
With the exception of the rapidly growing NTM, their
culturing characteristics are also similar to TB. Some
species proliferate only at 30 °C. NTM are frequent
inhabitants of the natural environment (water, soil) and
also contribute to human and animal mucosal flora. Most
of these species show resistance to the antituberculoid
agents in common use.
Clinical pictures and diagnosis
Some NTM species are apathogenic, others can cause
mycobacterioses in humans that usually follow a chronic
course
NTM infections are generally rare. Their occurrence is
encouraged by compromised cellular immunity. Frequent
occurrence is observed together with certain
malignancies, in immunosuppressed patients and in
AIDS patients, whereby the NTM isolated in 80% of
cases are M. avium or M. intercellulare. As a rule, NTM
infections are indistinguishable from tuberculous lesions
in clinical, radiological, and histological terms. Diagnosis
therefore requires culturing and positive identification.
The clinical significance of a positive result is difficult to
determine due to the ubiquitous occurrence of these
pathogens. They are frequent culture contaminants. Only
about 10% of all persons in whom NTM are detected
actually turn out to have a mycobacteriosis.
Therapy
Surgical removal of the infection focus is often
indicated. Chemotherapy depends on the pathogen
species, for instance a triple combination (e.g., INH,
ethambutol, rifampicin) or, for resistant strains, a
combination of four or five antituberculoid agents.

Unit 2: Bacteriology
102
Lecture 8 - Nocardia & Listeria
monocytogenes
Nocardia
Nocardia is
a genus of weakly staining Gram-positive,
catalase-positive, rod-shaped bacteria. It forms partially
acid-fast beaded branching filaments (acting as fungi, but
being truly bacteria). It has a total of 85 species. Some
species are non-pathogenic while others are responsible
for nocardiosis. Nocardia are found worldwide in soil that
is rich with organic matter. In addition, Nocardia are oral
microflora found in healthy gingiva as well as periodontal
pockets. Most Nocardia infections are acquired by
inhalation of the bacteria or through traumatic
introduction.
The genus Nocardia includes species with morphology
similar to that of the actinomycetes, differing from them
in that the natural habitat of these obligate aerobes is the
soil and damp biotopes. The pathogens known for
involvement in Nocardiosis, a generally very rare type
of infection caused by N. asteroides and other species
include N. brasiliensis, N. farcinia, N. nova, and N.
otitidiscaviarum.
N. asteroids Direct Partial Acid Fast Stain
Morphology and culture.
Nocardia are Gram-positive, fine, pleomorphic rods that
sometimes show branching. They can be cultured on
standard nutrient mediums and proliferate particularly
well at 30 °C.
Pathogenesis and clinical picture.
Nocardia penetrate from the environment into the
macroorganism via the respiratory tract or dermal
wounds. An infection develops only in patients with
predisposing primary 5diseases directly affecting the
immune defenses. Monoinfections are the rule. There are
no typical clinical symptoms. Most cases of infection
involve pyogenic inflammations with central necroses.
The following types have been described: pulmonary
nocardioses (bronchial pneumonia, pulmonary abscess),
systemic nocardioses (sepsis, cerebral abscess, abscesses
in the kidneys and musculature), and surface nocardioses
(cutaneous and subcutaneous abscesses, lymphocutaneous
syndrome).
Actinomycetomas are tumorlike processes affecting the
extremities, including bone. An example of such an
infection is Madura foot, caused by Nocardia species, the
related species Actinomadura madurae, and
Streptomyces somaliensis. Fungi can also be a causal
factor in this clinical picture.
Diagnosis
Detection of the pathogen by means of microscopy and
culturing techniques is required in materials varying with
the specific disease. Due to the long generation time of
these species, cultures have to be incubated for at least
one week. Precise identification to differentiate
pathogenic and apathogenic species is desirable, but
difficult.
Therapy
The anti-infective agents of choice are sulfonamides and
cotrimoxazole. Surgery may be required.
Epidemiology and prevention
Nocardioses are rare infections. Annual incidence levels
range from about 0.5 to 1 case per 1 000 000 inhabitants.
The pathogens, which are present in the natural
environment, are carried by dust to susceptible patients.
There are no practicable prophylactic measures
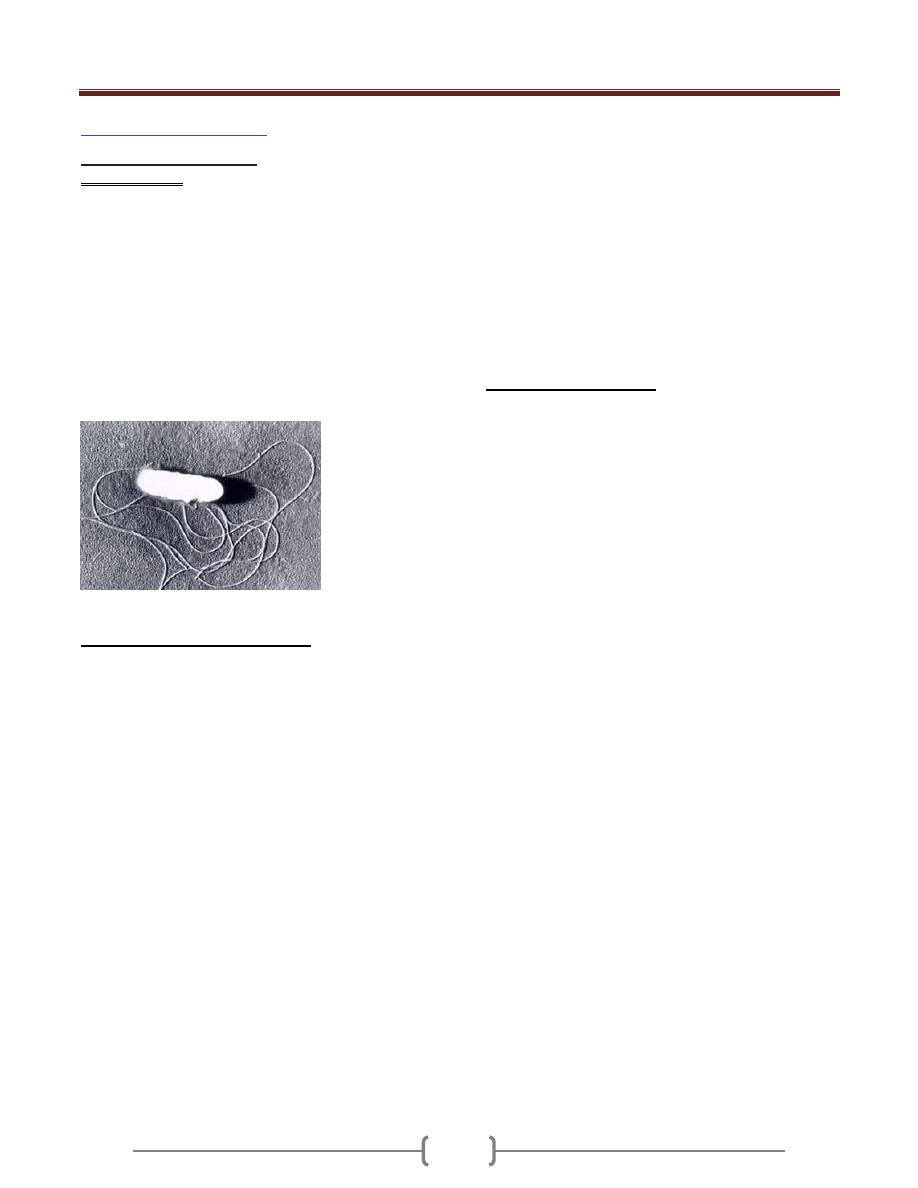
Unit 2: Bacteriology
103
Listeria monocytogenes
Morphology and culture.
Microscopically, Listeria species appear as small, Gram-
positive rods, which are sometimes arranged in short
chains, nonsporeforming and catalase-positive. In direct
smears they may be coccoid, so they can be mistaken for
streptococci. Longer cells may resemble corynebacteria.
Flagella are produced at room temperature but not at
37°C. Hemolytic activity on blood agar has been used as a
marker to distinguish Listeria monocytogenes among
other Listeria species, but it is not an absolutely definitive
criterion. Further biochemical characterization may be
necessary to distinguish between the
different Listeria species.
Listeria monocytogenes Scanning Electron Micrograph
Pathogenesis and clinical picture
Listeria monocytogenes is presumably ingested with raw,
contaminated food especialy that food stored in the
refrigerator for a long period of time. An invasin secreted
by the pathogenic bacteria enables the listeriae to
penetrate host cells of the epithelial lining gastrointestinal
system. The term listeriosis encompasses a wide variety
of disease symptoms that are similar in animals and
humans. Listeria monocytogenes causes listeriosis in
animals and humans. The true incidence of listeriosis in
humans is not known, because in the average healthy
adult, infections are usually asymptomatic, or at most
produce a mild influenza-like disease. Clinical features
range from mild influenza-like symptoms to meningitis
and/or meningoencephalitis. Illness is most likely to occur
in pregnant women, neonates, the elderly and
immunocompromised individuals, but apparently healthy
individuals may also be affected. In the serious (overt)
form of the disease, meningitis frequently accompanied
by septicemia, is the most commonly encountered disease
manifestation. In pregnant women, however, even though
the most usual symptom is a mild influenza-like illness
without meningitis, infection of the fetus is extremely
common and can lead to abortion, stillbirth, or delivery of
an acutely ill infant. Overt listeriosis following infection
with L. monocytogenes is usually sporadic, but outbreaks
of epidemic proportions have occurred. After engulfment
by macrophage, the bacterium may escape from the
phagosome before phagolysosome fusion occurs mediated
by a toxin, which also acts as a hemolysin, listeriolysin O
(LLO). This toxin is one of the so-called SH-activated
hemolysins, which are produced by a number of other
Gram-positive bacteria, such as group A streptococci
(streptolysin O), pneumococci (pneumolysin), and
Clostridium perfringens. The hemolysin gene is located
on the chromosome.
Treatment and Prevention
If diagnosed early enough, antibiotic treatment of
pregnant women or immunocompromised individuals
can prevent serious consequences of the disease.
Antibiotics effective against Listeria species include
ampicillin, vancomycin, ciprofloxacin, linezolid and
azithromycin. Because pregnant women, older adults,
and people with weakened immune systems are at higher
risk for listeriosis, CDC recommends specific certain
measures for these persons.

104

Unit 2: Bacteriology
105
Lecture 1+2+3 - Enteric gram-
negetive rods (enterobacteriaceae)
Enteric bacteria or coliform
General Characteristics:
A large heterogeneous group of G-ve rods (non-spore
forming), natural habitat is the G.I.T. of humans and
animals, motile with peritrichous flagella or non-motile,
aerobes and facultative anaerobes, ferment not oxidize
carbohydrate, catalase +ve, oxidase-ve.
It most common cultured in laboratory, includes more
than 25genera & 110 spp., only 20-25 spp. are clinically
significant. the most common are :
1) Escherichia coli (part of intestinal normal flora) cause
disease incidentally.
2) Klebsiella-Enterobacter-Serratia group.
3) Proteus-Morganella-Providencia group.
4) Citrobacter (2,3,4,are as intestinal normal flora and
incidentally cause disease but less than E.coli).
5) Shigella
6) Salmonella (Both Shigella & Salmonella are regularly
pathogenic for humans)
7) Other Enterobac.:Yersinia, Edwardsiella, Ewingella,
Hafnia, Cedecea, Kluyvera
Enteric bacteria produce a variety of toxins and
other virulence factors and enzymes, include:
1) LPS (endotoxin) have pathophysiological effects: fever,
leukopenia, hypotension, hypoglycemia, activation the
complement cascade, and disseminated intravascular
coagulation (DIC).
2) Most of G-ve rods produce exotoxins such as
enterotoxins and these toxins, has 2 types:
A. heat-labile exotoxin (LT Exotoxin): under genetic
control transmissible plasmid .
LT exo. Contains 2 subunits (A&B): subunit B binds
toGmI ganglioside at the brush border of epithelial cells
of the small intestine and facilitates the entry of subunit
A, which activates adenylyl cyclase → increase the
concentration of cAMP and → hypersecration of
sodium and lead to the diarrhea.
B. Heat-stable enterotoxin (ST Enterotoxin): activates
guanylyl cyclase in enteric epithelial cells and stimulate
fluid secretion and lead to the diarrhea.
3) R-factor (R plasmid) & colonization or adherence
factors.
4) Bactriocins (Colicins): Virus-like bactericidal substances
are produced by certain strains of bacteria against other
strains of the same or closely related spp.; their production
is controlled by plasmid . It can be used for typing of
bacteria because bacteriocin-producing strains are
resistant to their own bacteriocin .
Antigenic structure
1) antigen (Somatic Ag):
•
Side chain of the cell wall LPS, consist of
polysacchride heat-stable.
•
Eneric bac. are classified by more than 150 O–
Ags. Antibodies to these Ags. are IgM.
2) K–Ag. (Capsular Ag): external to O–Ags. on some
bacteria, heat-labile polysacchride or proteins , more than
100 K-Ags. Associated with virulence, Salmonella typhi
→ capsular Ag.(Vi Ag.).
3) H- Ag. (Flagellar Ag): a protein heat-labile or alcohols,
more than 50 H – Ags., Abs. to H-Ags. are IgG.
Diseases caused by enteric bacteria:
Generally as normal flora in intestine or upper
R.T.,pathogenic only when they reach tissues (U.T.,biliary
T., other abdominal sites,lungs,bone, meninges,prostatic
G.) ,and may cause bacteremia.
Either hospital- or community- acquired infection
1) Escherichia coli
General Characteristics:
• Lactose fermented (pink colonies→ MacConkey`s agar),
green metallic sheen colonies on EMB agar.
• Fermentative for mannitol & glucose with gas production.
• Hemolysis on blood agar, only when isolated from urine (UTIs)
Pathogenesis:
Depends on the site of infection, cannot differentiated by
symptoms from other bacteria.
-The main infections are:

Unit 2: Bacteriology
106
1) UTIs (urinary tract infections):
E.coli is the most common of UTIs.(90% in young
women).The symptoms includes: urinary frequency,
dysuria , hematuria , pyuria , & flank pain with upper UTIs.
UTIs can result in bacteremia with clinical sings of sepsis.
Nephropathogenic E.coli
(have specific O-Ags types &
produce hemolysin).
Pyelonephritis (have a specific types of pilus, P pilus).
2) Diarrheal disease:
Classified according to their virulence factors to :
A. EPEC (Enteropathogenic E.coli): diarrhea in infants &
outbreaks diarrhea in nurseries in developing countries .
Virulence factors: chromosomally mediated factors cause
tight adherence of EPEC to the mucosal cells of the small
intestine, entry to these cells →watery diarrhea (self-
limited or chronic), can treated by antibiotics. EPEC have
specific serotypes of O&H Ags.
B. ETEC (Enterotoxigenic E.coli): traveler`s diarrhea &
infants diarrhea in developing countries.
Virulence factors: colonization factors adherence it to
epithelial cells of small intestine, some strains produce LT
exotoxin others ST enterotoxin & some produce both of
them.
C. STEC (Shiga toxin producing E.coli) or EHEC
(Enterohemorrhagic E.coli): has been associated with-
1. hemorrhagic colitis (a severe form of diarrhea)
2. hemolytic uremic syndrome (a disease resulting in
acute renal failure, microangiopathic hemolytic
anemia, and thrombocytopenia)
• (E.coli O157:H7 strain).There are at least 2 antigenic
forms of the shiga-like toxin (shiga-like toxin -1 & -2).
D. EIEC (Enteroinvasive E.coli):
diarrhea in children in developing countries & traveler`s
to these countries (invading intestinal mucosal cells).
E. EAEC (Enteroaggregative E.coli):
acute & chronic diarrhea in developing countries & as a
food – borne illness in industrialized countries .Suggested
that it adheres to the intestinal mucosa & elaborates
enterotoxin & cytotoxin →mucosal damage →secretion
mucous & secretory diarrhea .
3) Sepsis:
When host defenses are inadequate, E.coli may reach the
bloodstream & cause sepsis in newborns & as a secondary
to UTI in adults.
4) Meningitis: E.coli & group B streptococci are the leading
causes of meningitis in infant (neonatal meningitis).75% of
E.coli from meningitis cases have K1 Ag.
*NOTE: the presence of E.coli in the water (colony count
above 4/dLin drinking water) unacceptable feacal
contamination, killed by chlorination of water.
2- Klebsiella-Enterobacter-Serratia Group
General Characteristics:
K.: lactose Fermentaion rapidly, viscous (mucoid)
colonies because it have a large capsule, non-motile.
E.: lactose F. rapidly, raised colonies (small capsule), motile
S.: lactose F. slowly, may be pigmented colonies ,motile.
Pathogenesis:
K. pneumoniae
: in 5% of normal persons (in R. T. &
feces). It cause 1% of bacterial pneumonia (extensive
hemorrhagic necrotizing consolidation of the lung),
occasionally cause UTI & bacteremia with focal lesions in
debilitated patients .
K. pneumoniae subsp. Ozaenae
: hospital-acquired
infection (upper R.T.) ,isolated from the nasal mucosa, a
fetid, progressive atrophy of mucous membrans.
K. pneumoniae subsp. rhinoscleroderma
: cause
rhinoscleroma (destructive granulomatous disease of the
nose & pharynx ).
K. granulomatis
(formerly Calymmatobacterium
granulomatis) causes genital ulcerative disease.
E. aerogenes
: free-living in GIT , opportunistic cause
UTIs & sepsis .
S. marcescens
: a common opportunistic pathogen in
hospitalized patients, cause pneumonia, bacteremia &
endocarditis .Can be treated by 3ed–generation of
cephalosporins .
3- Proteus-Morganella-Providencia Group
General characteristics:
• Proteus: non-lactose F.,very active motile (peritrichous
flagella)→swarming on blood agar ,urease +, susceptible
to antimicrobial drugs (penicillins).
• Morganella: non-lactose F., motile, urease + .
• Providencia: lactose F. slowly or not , urease .
Pathogenesis:
Proteus:
UTIs, bacteremia, pneumonia & focal lesions in
debilitated patients .
P. mirabilis
: UTIs & other infections .
P.vulgaris
: nosocomial infection .
The rapid motility of Proteus help to it invasion of the
U.T., & production of urease resulting rapid hydrolysis of
urea with liberation of ammonia (urine become alkaline)&
promoting stone formation.
Diagnosis by Weil-Felix test .
Morganella morganii:
nosocomial pathogen .
Providencia
(P. rettgeri ,P. alcalifaciens ,P.stuartii):
normal intestinal flora ,all cause UTIs & other infections ,
resistant to antimicrobial therapy .

Unit 2: Bacteriology
107
4- Citrobacter:
Lactose F. very slowly or not, motile, cause UTIs.
Diagnostic test for Enteric bacteria
• Specimens: urine, blood, pus, C.S.F., sputum, others.
• Culture: on both blood agar & differential media.
• Serological tests: agglutination with specific antisera .
• Variation in bacterial susceptibility is great, so antibiotic
sensitivity are essential .No single drug is available .
• Sulfonamides, ampicillin, cephalosporins, fluoroquinolons
& aminoglycosides .
Prevention & control
Depends on hand washing , rigorous asepsis , sterilization
of equipments , disinfections , strict precautions in I.V.
therapy & keeping U.T. catheters sterile .
Prophylaxis: using ciprofluxacin or trimethprim-
sulfamethaxzole .
Prevention of traveler`s diarrhea, daily ingestion of
bismuth subsalicylate suspension .
EPEC serotypes controlled by orally vaccines (a virulent
mutant strain ) or injection of killed bacterial suspension
Salmonella-Arizona group
Pathogenic by the oral route, transmitted from animals &
animals products to humans & cause enteritis
(enterocolitis), systemic infection & enteric fever.
Morphology:
• Vary in length, motile with peritrichous flagellae. They
survive freezing in water for long periods & resistant to
certain chemicals (brilliant green, Na-tetrathionate & Na-
deoxycholate) that inhibit other enteric bacteria, so such
compounds useful for isolation salmonellae from feces.
• Aerobic or facultative anaerobic, grow in pH (6-8) & 15-
41 C° → produce large, smooth & circular colonies (2-3
mm in diameter).
• On MacConkey’s & deoxycholate-citrate agars → Pale
colonies (non-lactose fermented). Ferment glucose,
mannitol, mannose (with acid & with or without gas);
produce H
2
S (Black precipitate on TSI agar).
Antigenic Structure
1) H (flagellar) Ag: heat-labile protein & highly antigenic.
2) O (somatic) Ag: heat-stable polysaccharide (integral part
of LPS).
3) Vi (capsular or surface) Ag: heat-labile, related to virulence
Variation may occurs by lose H Ag (become non-motile),
lose of O Ag (change from smooth to rough colony form)
& lose of Vi Ag partially or completely.
Classification
• 4 serotypes(group1)causes enteric fever (primarily
infective for humans):
Salmonella Paratyphi A(serogroup A)
S. Paratyphi B (serogroup B)
S. Typhi and S. Enteritidis (serogroup D)
S. Choleraesuis (serogroup C
1
)
•
Currently, the genus Salmonella is devided into 2 species:
1. S.entterica (5 subsp.):
1.= = subsp.enterica (subsp.I)
2.= = subsp.salamae (subsp.II)
3.= = subsp.arizonae (subsp.IIIa),and
subsp.diarizonae (subsp.IIIb)
4.= = subsp.houtenae (subsp.IV)
5.= = subsp.indica (subsp.VI)
2. S.bongori (subsp.V)
Most human illness is caused by subsp.I strains, rarely by
IIIa, IIIb, & others, which found in cold-blooded animals.
Pathogenesis & Clinical Findings
The majorities of salmonellae are pathogenic in animals
(poultry, pigs, rodents, cattle & others)- → the reservoir for
human infection. The bacteria enter via the oral route by
contaminated food or drink → produce 3 main types of
diseases (enteric or typhoid fever, bacteremia or septicemia
or systemic infection & enteritis or enterocolitis).
In typhoid fever: ingested S.Typhi reach the small
intestine →------ enter the lymphatics & bloodstream, the
blood carries them to many organs including the intestine
→------ bacteria multiply in intestinal lymphoid tissue &
excreted in stools. The lesions are hyperplasia & necrosis
of lymphoid tissues (e.g., Peyer’s patches), hepatitis, focal
necrosis of liver & inflammation of the gall bladder,
periosteum, lungs & other organs.

Unit 2: Bacteriology
108
Diagnostic Lab. Tests:
• Specimens: blood & stool (urine rare).
• Culture:
1) Differential media (MacConkey’s, EMB, Deoxycholate,
Bismuth sulfite agar).
2) Selective media (SS, XLD, Hektoen enteric agar).
3) Enrichment media usually for stool (Selenite F broth or
tetrathionate broth, incubation (1-2days) →- plated on
differential & selective media.
4) Biochemical reaction patterns (TSI agar→------ black
precipitate).
• Serological tests:
1) Agglutination test: serotyping for unknown culture +
commercial kit, known sera (anti-O Ags for serogroups
salmonellae A, B, C
1
, C
2
, D & E).
2) Widal test (tube dilution agglutination): determination of
antibody titer in patient serum. The result as following:
a) High titer O (≥ 1:160)- → Active infection.
b) High titer H (≥ 1:160)- → Passive infection or past
immunization.
c) High titer Vi (in some carriers).
Immunity:
• Secretory IgA may prevent attachment of salmonellae to
intestinal epithelium.
• Circulating Abs to O & Vi are related to resistance of
infection →-relapses may occur in 2-3 weeks after recovery
in spite of Abs-→ reinfection milder than the 1
st
infection.
Treatment:
• Enteric fever & bacteremia require antimicrobial therapy
but enterocolitis do not, because the clinical symptoms &
excretion of the salmonellae may be prolonged by
antimicrobial therapy.
• In severe diarrhea, replacement of fluids & electrolytes is
essential.
• Therapy: Ampicillin, trimethoprim-sulfomethaxzole or
3ed-generation cephalosporine. In most carriers, the
organisms persist in the gall bladder (if gallstones are
present) &in the biliary tract. Chronic carriers cured by
ampicillin, but in most cases cholecystectomy must be +
drug treatment.
Epidemiology:
• Carriers: After manifest or subclinical infection, some
individuals continue to harbor salmonellae in their tissues
for variable length of time (convalescent carriers or
healthy permanent carriers). 3% of survivors of typhoid
become permanent carriers, harboring the organisms in
the gallbladder, biliary tract, or rarely, the intestine or
urinary tract.
• The feces of persons who have unsuspected subclinical
disease or carriers are a more important source, So food
handlers are shedding organisms. The contamination of
the following sources is important:
1) Water.
2) Milk & other dairy products (ice cream, cheese & custard
3) Shellfish.
4) Dried or frozen eggs.
5) Meat & meat products (poultry) or contaminated with
feces by rodents or humans.
6) Recreational drugs (Marijuana).
7) Household pest (dogs, cats, turtles, etc.).
8) Animal dyes (carmine) used in drugs, food & cosmetics.
Prevention & Control:
1) Sanitary measures must be taken to prevent contamination
of food & water.
2) Infected poultry, meats & eggs must be thoroughly
cooked
3) Carriers must not be allowed to work as food handlers.
4) Strict hygienic precautions.
Unit 2: Bacteriology
109
5) Vaccination:
a) 2 injections of acetone-killed S. Typhi followed by a
booster injection some months later→------ partial
resistance.
b) Oral administration of a live avirulent mutant S.Typhi
strain.
Shigellae (shigella)
Natural habitat is the intestinal tract of humans and
primates, causes bacillary dysentery.
Morphology & identification:
• Slender G-ve rods, coccobacillary in young culture,
facultative anaerobes, non-motile.
• Convex, circular, transparent colonies, non-lactose
F.(except S.sonnei), mannitol fermenters(except S.
dysenteriae).
• Antigenic structure: somatic O-Ag (LPS), more than 40
serotypes (share with other enteric bacilli).
•
Classification: on biochemical & antigenic structure,
pathogenic spp. are:
S.dysenteriae, S.flexneri, S.boydii,
S.sonnei.
Pathogenesis:
The infective dose is 10
3
organisms(10
5
-10
8
for
salmonellae & vibrios), it is limited to the GIT, invasion
of the mucosal epithelial cells by inducing phagocytosis,
escape from the phagocytic vacuole, multiplication &
spread within the cytoplasm then passage to adjacent
cells. Bloodstream invasion is rare. Microabscesses
formation in the wall of the large intestine & terminal
ileum lead to necrosis, superficial ulceration, bleeding, &
formation of pseudomembrane (consists of fibrin,
leucocytes, cell debris, a necrotic mucous membrane, &
bacteria). Granulation tissue fills the ulcers and scar
tissue forms. Blood & pus found in stools.
Toxins:
1) Endotoxin: Causes irritation of the bowel wall.
2) Shiga toxin (exotoxin): produced by S.dysenteriae type
1(Shiga bacillus) heat-labile, antigenic protein acting as
verotoxin of E.coli → inhibit sugars & amino acids
absorption in the small intestine & acting as neurotoxin →
----- CNS reactions, meningismus & comma → -----
severity & fatal infection of S.dysenteriae .
Clinical Findings:
After short incubation period (1-2 days)→ sudden
abdominal pain, fever & watery diarrhea. When infection
involves the ileum & colon → the No. of stools increases
(less liquid but contain mucus & blood).
In children & elderly, loss of water & electrolytes-→
dehydration, acidosis & death.
More than 50% of adult cases, fever & diarrhea subside
spontaneously in 2-5days, few patients-→chronic
intestinal carrier & recurrent of diseases.
Diagnostic Lab. Test:
Specimens: Fresh stool, mucus flecks & rectal swabs→
smear & culture.
Smear: Direct microscopic exam. Of stool-→ large No.
of leukocytes & RBC’s.
Culture: Using differential media (MacConkey’s agar,
EMB agar) & selective media (SS agar, Hektoen enteric
agar).
• Serology: Normal persons have Abs against Shigella spp.
→ not used for diagnosis.
Immunity:
Serum IgM (anti-O shigellae Ags) → not protect against
shigellae infection.
Treatment:
• Multiple drug resistance can be transmitted by R-factor so
resistant infections are wide spread.
• Ciprofloxacin, Ampicillin, Tetracycline, Trim.-
sulfamethasone & Chloramphenicol.
Epidemiology:
S. dysenteriae spread widely.
-Shigellae infections occur in children under 10 years,
transmitted from person-person by food, fingers, feces &
flies.
Prevention & Control:
Eliminating shigellae from reservoirs by:
1) Sanitary control of water, foods & milk; sewage disposal
&fly control.
2) Isolation of patients & disinfecting of excreta.
3) Detection of subclinical cases & carriers especially food
handlers.
Antibiotic treatment of infected individuals.

Unit 2: Bacteriology
110
Lecture 4 – Pseudomonads,
Acinetobacter & Uncommon gram
negative bacteria
Pseudomonads
General characteristics:
Gram negative, motile, aerobic rods, some of which
produce water-soluble pigments. Habitat: soil, water, plant,
and animals. Small No. found in the normal intestinal flora
and on the skin of humans.
Medically important Pseudomonas:
1. Pseudomonas aeruginosa
Widely distributed and is present in moist environments in
hospitals and in the normal intestinal flora.. An important
nosocomial pathogen.
Morphology:
Gram negative rods, motile with polar flagellum, as a
single, pairs and short chains.
Three looks at Pseudomonas, the head of the Gram-negative
aerobic rods. A. Electron micrograph, negative stain. B.
Scanning electron micrograph. C. Gram stain.
Culture:
Grows on many types of media, obligate aerobe, sometimes
producing a sweet or grape-like odor. Some strains
hemolysis blood.
It forms smooth round colonies with:
a fluorescent greenish pigment (Pyoverdin)
or non-fluorescent bluish pigment (Pyocyanin) or dark
red pigment (Pyomelanin).
Grows well at 37-42 C
˚,
42 C˚
helps in differentiation
P.aeroginosa from other spp., oxidase + , catalase + , dose
not ferment carbohydrates, but many strains oxidize
glucose, dose not ferment lactose → pale colonies on
MacConkey’s agar.
Antigenic Structure & Toxins:
1) Pili (attachment to host epithelial cells).
2) Polysaccharide capsule (mucoid colonies in culture from
patients with cystic fibrosis).
3) LPS (endotoxin).
4) Pyocin (bacteriocin).
5) Extracellular enzymes (elastases, proteases, and 2 type of
hemolysins: a heat-labile phospholipase C and a heat-staple
glycolipid).
6) Exotoxin A (causes tissue necrosis by blokes protein
synthesis).
Pathogenesis:
It is pathogenic only when there is abnormal host defenses
(mucous membranes & skin are disrupted by direct damage,
I.V. or urinary catheters are used or neutropenia as in
cancer therapy).The bacterium attaches & colonizes the
mucous membrane, invades locally and produces systemic
disease. These processes are promoted by the pili, enzymes,
& toxins described above.
LPS (endotoxin) causing fever, shock, oliguria,
leukocytosis, leukopenia & DIC & adult respiratory distress
syndrome. Pseudomonas are resistance to many
antimicrobial agents→ important when the normal flora are
suppressed.
aeruginosa infections are:
1) Wounds & burns infection (with blue green pus).
2) Meningitis (contamination due to lumbar puncture).
3) UTIs (by catheters).
4) RTIs (by respirators).
5) Otitis externa (in swimmers) & malignant otitis externa (in
diabetic patients).
6) Eye infection (after injury or surgical procedures).
7) Fatal sepsis (in infants or debilitated persons).
The symptoms are nonspecific & related to the organ
involved
Veroglobin: a breakdown product of hemoglobin, a
fluorescence pigment can be detected in wounds and burns
or urine.
Ecthyma gangrenosum: a hemorrhagic necrosis lesion of
skin occurs in sepsis due to P. aeruginosa are surrounds by
erythema without pus.

Unit 2: Bacteriology
111
Diagnostic Lab. Test:
Specimens: skin lesions, pus, urine, blood, c.s.f., sputum &
other materials.
Culture: the specific test for diagnosis of P. aeruginosa
infections.
Treatment:
Should not be treated with single-drug, because success rate
is low and the bacteria can rapidly develop resistance when
single drugs are used.
penicillin combination with aminoglycosides.
others: aztreonam, imipenem, ciprofloxacin, newer
cephalosporines
2. Burkholderia pseudomallei:
Causes melioidosis, an endemic glanders-like disease of
animals and humans, as acute, subacute, or chronic
infection. A localized suppurative infection can occur at the
inoculation site such as break in the skin, this may lead to
acute septicemic infection with involvement of many
organs.
The most commons of melioidosis is pulmonary infection
(pneumonitis).
May develop chronic suppurative infection with abscesses
in skin, brain, lung, myocardium, liver, bone and other
sites.
Treatment: Susceptible to tetracycline, sulfonamides,
chloramphenicol, amoxicillin & 3ed. Generation
cephalosporines.
3. Burkholderia mallei:
Cause glanders, a disease of horses and donkeys
transmissible to humans, may be fatal. Begins as ulcer of
the skin or mucous membranes followed by lymphangitis
(lymphatic thickening with nodules), & sepsis.
Inhalation of organisms may lead to primary pneumonia,
can be treated with tetracyclines plus aminoglycosides.
4. B.cepacia:
slow growth (may take 3 days for colonies are visible),
multidrug-resistant, causes necrotizing pneumonia and
bacteremia in patients with cystic fibrosis. In hospitals, it
has been isolated from a variety of water and environmental
sources from which it can be transmitted to patients and
from one cystic fibrosis patients to another by close contact.
Acinetobacter & Uncommon gram -ve bacteria
Acinetobacter:
coccobacillary or coccal (diplococci forms,
resemble neisseriae in smears, also recovered from female
genital tract has been mistaken for N.gonorhoeae and
recovered from meningitidis and sepsis has been mistaken
for N.meningitidis). Commensal but causes nosocomial
infections & as opportunistic pathogen & cause sepsis
(isolated from blood, sputum, skin, pleural fluid & urine).
-Resistant to antimicrobial agents. Therapy: difficult, but
responded to gentamicin, amikacin, tobramicin & newer
penicillins or cephalosporines.
Actinobacillus
: causes sever periodontal disease in
adolescents, endocarditis, abscesses, osteomyelitis and
others. Treatable with tetracycline or chloramphenicol and
penicillin G, ampicilline or erythromycin.
Alcaligenes
: as normal human flora, isolated from
respirators, nebulizers & renal dialysis systems & from
urine, blood, c.s.f., wounds & abscesses.
Capnocytophaga
: as oral human flora, causes bacteremia
and sever systemic disease in immunocompromised
patients and assotiated with wound infections from dog or
cat bites or scratches.
Cardiobacterium
: normal flora of upper R. T. & bowel
causes endocarditis.
Chromobacterium
: found in subtropical climates in soil &
water. Infects humans through breaks in the skin or via the
gut, cause abscesses, diarrhea, sepsis (many deaths).

Unit 2: Bacteriology
112
Lecture 5 - Vibrios (vibrio spp.) &
associated bacteria
Found in marine & surface water, curved aerobic rods &
motile with polar flagellum. The medically important
vibrios are:
Vibrio spp.
1. Vibrio cholerae
Causes cholera (profuse watery diarrhea that can lead to
death)
Morphology:
Comma-shaped, curved rod, motile with polar flagellum.
Culture:
Convex, smooth, round colonies, grows at 37C˚ on many
kinds of media. On TCBS (Thiosulfate-Citrate-Bile-
Sucrose) agar→Yellow colonies, Oxidase +, grow at very
high pH (8.5-9.5).
Some vibrios are halophilic (requiring NaCl to grow).
Others are halotolerant (NaCl stimulate their growth).
Vibrios grow on media containing 6% NaCl & susceptible
to compound O/129 (2,4-diamino-6,7-diisopropyl
pteridine phosphate).
Antigenic Structure:
1) H (flagellar) Ag.
2) O Ag of LPS (139 O Ag groups).
V.cholerae strains O group 1& O group 139 causes classic
(epidemic & pandemic) cholera.
Serogroup O1 Ag (including Ogawa & Inaba serotypes).
Non O1/non O139 V.cholerae causes cholera like-disease
(mild diarrhea), rarely extraintestinal infections.
For epidemiological studies, 2 biotypes of epidemic
V.cholerae Classic & ElTor (ElTor biotype cause mild
diarrhea than Classic biotype).
3) Capsule (polysaccharide) Ag: in O139 & non O1
V.cholerae (serogroup O1 dose not).
Pathogenesis:
V.cholerae is pathogenic only for humans.
Ingest as many as 10
10
or more organisms (vehicle is water)
or 10
2
-10
4
(vehicle is food) → infection.
Any medication or condition that decreases stomach acidity
makes a person more susceptible to infection with
V.cholerae.
Cholera is not an invasive infection (the organisms do not
reach the bloodstream but remain within the intestinal
tract).
Virulent V.cholerae attach to the microvilli of the brush
border of epithelial cells, multiply & liberate cholera toxin
& mucinases & endotoxin.
V.cholerae enterotoxin (cholera toxin):
a heat-labile, consist of subunits A & B.Ganglioside G
mI
the
receptor for subunit B, which promotes entry of subunit A
into the cell.Activation of subunit A yields increased levels
of cAMP → hypersecretion of water & electrolytes
(increased sodium chloride secretion & inhibit absorption
of sodium & chloride) →diarrhea (20-30 L/day) →
dehydration, shock, acidosis & death.
Clinical Findings:
The incubation period is 1-4 days (depending upon the size
of the inoculum ingested) →sudden onset of nausea,
vomiting and profuse diarrhea with abdominal cramps.
Stool resembles “ rice water” contain mucus, epithelial cells
& large No. of vibrios.
Rapid loss of fluids & electrolytes→dehydration,
circulatory collapse & anuria.If without treatment, 25-50%
mortality.
Diagnosis:
Specimens: mucus flecks from stools (for culture).
Smear: Dark-field or phase contrast microscopy show
rapidly motile vibrios.
Culture: Peptone agar, blood agar (pH 9) or TCBS agar.
Specific Tests: biochemical tests & slide agglutination
(using anti-O group O1 & O139 antiserum).
Vibrio cholerae has a
single polar
flagellum for
swimming
movement. Electron
Micrograph of Vibrio
cholerae

Unit 2: Bacteriology
113
Immunity:
Duration & degree of immunity are not known. Specific
IgA occur in lumen of the intestine.
Gastric acid provides some protection against vibrios.
Treatment:
Water & electrolytes replacement (to correct the sever
dehydration) & oral tetracycline (V.cholerae tetracycline
resistance by transmissible plasmids).
Epidemiology; Prevention & Control:
Africa (millions of people), rare in North America. Cholera
is endemic in India & Southeast Asia. It is carried along
shipping lanes, trade routes & migration routes.
Cholera is spread by person-person contact, water, food &
flies. True chronic carrier is rare (after 3-4 weeks).
Control rests on education & sanitation of food & water.
Patients should be isolated, and their excreta disinfected &
contacts followed up.
Chemoprophylaxis with antimicrobial drugs.
The WHO vaccination for cholera for 6 months only.
Repeated injection of a vaccine containing either LPSs
extracted from vibrios or dense vibrios suspensions (limited
protection).
2. Vibrio parahaemolyticus:
Causes gastroenteritis
Halophilic (required NaCl for growth), grows on blood agar
& TCBS agar (green colonies), oxidase +.
Causes acute gastroenteritis, following ingestion of
contaminated seafood (raw fish or shellfish). After
incubation period (12-24 hrs), nausea, vomiting &
abdominal cramps, fecal leukocytes are observed. Subside
spontaneously in 1-4 days with no treatment.
3. Vibrio vulnificus:
Causes severe wound infections (in immunocompromised
persons), bacteremia (in alcoholism & liver diseases) &
gastroenteritis (oysters, in warm months).
Wound infections may be mild but proceed rapidly (a few
hrs) with development of bullous skin lesions, cellulitis &
myositis with necrosis.
Diagnosis by TCBS agar (green colonies).
Treatment: tetracycline, ciprofloxacin.
Other Vibrios:
V.mimicus
causes diarrhea (uncooked seafood).
V.hollisae
&
V.fluvialis
causes diarrhea.
V.alginolyticus
causes eye, ear, or wound infection after
exposure to seawater.
V.damsela
causes wound infection.
Aeromonads (aeromonas spp.)
3 spp. Are clinical importance:
Aeromonas hydrophila,
A.caviae & A.veronii biovar sorbria.
(Diarrhea).
Rods, motile, their colony morphology is similar to enteric
G – rods but it is oxidase + .Differentiated from vibrios by
resistance to compound O/129 & lack of growth on media
containing 6% NaCl.
Produce hemolysins (large zone of hemolysis on blood
agar) & enterotoxin (some strains), cytotoxin & invade cells
in tissue culture (but non of these characteristics have been
clearly associated with diarrheal disease in humans).
Susceptible to tetracycline, aminoglycosides &
cephalosporines.
Plesiomonas
Exists in both cold- & warm-blooded animals (isolated
from freshwater fish & many animals), cause diarrhea in
tropical & subtropical areas.
P. shigelloides
is a G – rod with polar flagella, isolated
from stool culture of human with diarrhea.
Plesiomonas grows on the same media for Salmonella &
Shigella (but oxidase +). Some strains cross-react with
Shigella antisera.
P. are DNase +, can distinguish it from Aeromonas by
other biochemical tests.

Unit 2: Bacteriology
114
Lecture 6 – Campylobacters &
Helicobacter
Campylobacters (Campylobacter spp.)
Found in animals (including domesticated) cause both
diarrheal & systemic diseases (wide spread of infections in
the world). The most important Spp.:
1. Campylobacter jejuni: 2. C. coli :
Common human pathogens (as common as Salmonella &
Shigella), are causing enteritis & systemic infection.
Morphology & Identification:
G – rods with comma, S, or gull-wing shapes, motile with
single polar flagellum.
Culture: selective media are needed (Skirrow’s medium &
Campy-Bac medium), in atmosphere 5% O
2
+10% CO
2
(by
using anaerobic jar with gas generating pack), temperature
42 C˚(inhibit most bacteria found in stool.
The colonies colorless or gray, may be watery & spreading
or round & convex (these 2 colonies types may appear on
one plate), oxidase + & catalase +, not ferment or oxidize
carbohydrates, nitrate reduction, H
2
S production. For
further identification of species hippurate test &
antimicrobial susceptibility test can be used.
Antigenic structure:
Have LPS with endotoxic activity.-Cytopathic extracellular
toxins & enterotoxins (not well defined).
Pathogenesis & Clinical Findings:
The infection due the oral route from food, drink, or contact
with infected animals or its products.
C.jejuni is susceptible to gastric acid (10
4
organisms is
necessary).
The organism multiplies in the small intestine, invade the
epithelium & produce inflammation that results in the
appearance of red & white blood cells in the stool (may
invade the bloodstream). Localized tissue invasion with the
toxic activity, responsible for the enteritis.
Acute crampy abdominal pain, diarrhea (may be bloody),
headache, malaise & fever. The illness is self-limited to 5-8
days (may be longer). May resolve without antimicrobial
therapy.
Therapy shortens the duration of fecal shedding of bacteria.
C.jejuni is susceptible to erythromycin.
Diagnostic Lab. Tests:
Specimens: Diarrheal stool.
Smear: Gram-stained smears of stool→ gull-wing shaped.
Dark-field or phase contrast microscopy → darting motility
of this bacterium.
Culture: Selective media is the definitive test.
Epidemiology & Control:
Campylobacter enteritis resembles other acute bacterial
diarrheas (Sh. dysenteriae).The source of infection, food
(milk) or contact with infected animals or humans & their
excreta.
Outbreaks from a common source (unpasteurized milk).
Require puplic health control measures.
3. Campylobacter fetus subspecies fetus:
Opportunistic pathogen cause systemic infection in
immunocompromised patiens.The portal of entry
G.I.T→bacteremia & systemic infection (may be
diarrhea).Have a surface array proteins(S protein),which
form a capsule-like structure on the surface of the
organism, correlated with the ability of the bacteria to
cause bacteremia.
4.
Other campylobacters:
C.lari
(in seagulls) &
C.upsaliensis
(in dogs), both causes
diarrhea in humans.

Unit 2: Bacteriology
115
Helicobacter pylori
Antral gastritis, duodenal (peptic) ulcer disease, gastric
ulcers & gastric carcinoma.
Morphology & Identification:
Spiral-shaped or curved G-rods has multiple flagella at one
pole (actively motile).
Culture: grows in (3-6) days at 37C˚ in microaerophilic as
C.jejuni .
Media:
1) Skirrow’s media
2) Chocolate media.
3) Other selective media with vancomycin, nalidixic acid, &
amphotericin.
The colonies are translucent & small (1-2 mm in diameter),
Oxidase +, Catalase + & strong producer of urease.
Pathogenesis:
H.pylori found deep in the mucus layer near the epithelial
surface (at pH 6-7 because gastric mucus is relatively
impermeable to acid & has buffering capacity, on the lumen
side the pH is low 1-2, while on the epithelial side the pH is
high 6-7) & it is quite motile even in mucus able to find its
way to the epithelial surface. H.pylori produces a protease,
which reduces the ability of acid to diffuse & production of
active urease, which release ammonia & that cause further
buffering of acid.
H.pylori causes gastritis & hypochlorhydria & duodenal
ulceration-→ invade the epithelial cells also toxins & LPS
& ammonia → damage the mucosal cells (antimicrobial
therapy → clearing bacteria & improvement of gastritis &
duodenal ulcer).
Destruction of the epithelium is common, and glandular
atrophy may occur, H.pylori thus may be a major risk factor
for gastric cancer
Clinical findings:
Acute infection of upper gastrointestinal illness with nausea
& pain, vomiting & fever (duration less than 1-2 weeks).
This bacterium may persist for years, decades or even a
lifetime.
90% of patients with duodenal ulcers & 50-60% of gastric
ulcers have H.pylori infection. It may have a role in gastric
carcinoma & lymphoma.
Diagnostic Lab. Tests:
Specimens: Gastric biopsy(gastroscopy) → histological
exam. & culture.
Blood → determination serum antibodies.
Smear: Biopsy stained by Giemsa stain→ curved or
spiraled organisms.
Culture: Selective & differential media.
Serology: The role of Abs tested is limited (serums Abs
can persist even if the infection eradicated).
Detection of H.pylori Ags in stool, a test of cure.
Special rapid test: to detect urease activity in biopsy
material or in vivo by
13
C- or
14
C-labeled urea ingested by
the patient & then detection of labeled CO
2
in the patient’s
exhaled breath.
Immunity:
Patients infected develops IgM, subsequently, IgG & IgA
(persist systemically & at the mucosa in high titer in
chronically infected persons).
Treatment: Triple therapy:
Metronidazole + bismuth subsalicylate or bismuth
subcitrate +amoxicillin or tetracycline for 14 days.
Proton pump inhibitor + amoxicillin & calithromycin or
amoxicillin + metronidazole.
Epidemiology:
Acute epidemic gastritis is a common source for H.pylori.
Transmission of H.pylori by person-person & intrafamilial
culstring of infection.
H.pylori is present on the gastric mucosa of less than 20%
of persons under age 30, 40-60% of persons age 60
(including asymptomatic).
In developing countries, the prevalence of infection may be
80% or higher.
Other Helicobacters
H. fennelliae
and
H. cinaedi
can cause either diarrheal
or extraintestinal disease.
Arcobacter spp
. Are uncommon enteric pathogens.

Unit 2: Bacteriology
116
Lecture 7 - Haemophilus
Small, G- pleomorphic bacteria. Identification of H.
group depends (in part) upon requirement for growth
factors (X & V factors).
X factor: heme
V factor: Nicotinamide-adenine dinucleotide
1. Haemophilus influenzae:
Found on the mucous membranes of upper R.T.
occasionally causes R.T. infections in children & adults.
Morphology:
In specimens: coccoid bacilli, in pairs or short chain.
In young culture (6-8 hours): small coccobacilli & have a
finite capsule. Later become longer rods & very
pleomorphic form.
Culture:
On brain-heart infusion agar with blood (requires X & V
factors), after 24 hours → small, round, convex colonies.
Chocolate agar (36-48 hours) →larger colonies (1 mm,
not hemolytic).
Satellite phenomenon: H. influenzae grow much larger
colonies around staphylococcal or other colony.
Antigenic Structure:
Encapsulated H. influenzae contains capsular
polysaccharides (6 types Ags a-f), H. influenzae type b
an important human pathogen may lose its capsule & the
type specificity. H. influenzae in the normal flora of
upper R.T. are not capsulated.
Somatic Ags: outer membrane proteins.
LPS (endotoxin): share many structures with those of
neisseriae.
Pathogenicity:
The capsule is antiphagocytic, type b capsule (PRP=
polyribose ribitol phosphate) is the major virulence factor
of H. influenzae (causes meningitis, pneumonia &
empyema, epiglottitis, cellulitis, septic arthritis & other
invasive infections). H. influenzae types c-f rarely cause
disease.
Nontypeable H. influenzae causes invasive infections less
than type b (chronic bronchitis, otitis media, sinusitis &
conjunctivitis).
H. influenzae type b enters by way of the R.T →
extend to the sinuses or middle ear, may reach the
bloodstream →to the meninges (meningitis) or
establish in the joints (septic arthritis).
Clinical Findings:
Infant: fulminating obstructive laryngotracheitis with
swollen, cherry-red epiglottis (requires tracheostomy or
intubation as a lifesaving).
Small children & old or debilitated people: Pneumonitis
& epiglottitis may follow upper R.T. infections.
Adults: may have bronchitis or pneumonia.
H. influenzae the most common cause of meningitis in
children (age 5 months-5 years) resembles other forms of
childhood meningitis.

Unit 2: Bacteriology
117
H. influenzae type b & pneumococci are the most
common cause of otitis media & acute sinusitis.
Diagnostic Lab.:
Specimens: nasopharyngeal swab, pus, blood & csf.
Direct identification by immunofluorescence or by
specific rabbit antiserum for a capsule (type b) swelling
test. Commercial kits for immunologic detection of H.
influenzae Ags in csf.
Culture: IsoVital X enriched chocolate agar(24-48 hrs)
→ typical colonies.
Test for X & V factors can be done by placed strips or
disks containing these factors on the surface of agar,
growth of H. influenzae in the area between the strips
indicates requires both factors.
Immunity:
Infants (less than 3 months) have Abs transmitted from
the mother (rare infection). By age 3-5 years, many
children have naturally acquired anti-PRP Abs that
promote complement-dependent bactericidal killing &
phagocytosis.
Immunization of children with H. influenzae type b
vaccine induces the same Abs. Pneumonia or arithritis
due to H. influenzae can develops in adults with such Abs.
Treatment:
Untreated H. influenzae meningitis causes mortality rate
up to 90%. H. influenzae type b susceptible to ampicillin
(25% of strains are resistant by produce ß-lactamase
under control of a transmissible plasmid).More strains are
susceptible to chloramphenicol & all to the newer
cephalosporins (cefotaxime).
Influenzal meningitis may develop a subdural
accumulation of fluid, so requires surgical drainage.
Epidemiology, Prevention & control:
Encapsulated H. influenzae type b is transmissible from
person-person by the respiratory routes.
Infection can be prevented by administration of
haemophilus b conjugate vaccine (H. influenzae type b
with protein carrier either CRM, mutant C. diphtheriae
toxin protein or N. meningitidis outer membrane
complex) to children aged 2 months or older.
Children aged 15 months or older can receive H.
influenzae type b vaccine conjugated with diphtheria
toxoid (it is not immunogenic in younger children). The
vaccine reduces the incidence of meningitis & the carrier
rates for H. influenzae type b.
Contact with patients posses little risk for adults but
not to nonimmune children (less than 4 years), so can
use rifambin for them.
2. H. aegyptius
: Called the Koch-Weeks bacillus (H.
influenzae biotype III) causes conjunctivitis & it is the
cause of Brazilian purpuric fever, a disease of children
characterized by fever, purpura, shock & death.
3. H. aphrophilus (Aggregatibacter aphrophilus):
found as a normal oral & respiratory tract flora
causes infective endocarditis & pneumonia.
4. H. ducreyi
: Causes chancroid (soft chancre), a
sexually transmitted disease, a ragged ulcer on the
genitalia with marked swelling & tenderness, the regional
lymph nodes are enlarged & painful. Treatment with
ceftriaxone (IM), oral trimethoprime-sulfamethoxazole or
oral erythromycin.
5. H. parainfluenzae
: as a normal flora of the R.T.
causes infective endocarditis & urethritis.
6. H. haemolyticus
: as a normal flora of the
nasopharynx & associated with rare R.T. infections of
moderate severity in childhood.

Unit 2: Bacteriology
118
Lecture 8 – Bordetellae
1. Bordetella pertussis:
Causes whooping cough (pertussis).
Morphology:
Minute G-, nonmtile encapsulated coccobacilli
toluidine blue stain → bipolar metachromatic granules
Culture: Strict aerobic requires enriched media:
1) Bordet-Gengou (potato-blood-glycerol) agar + penicillin
G or Regan-Lowe medium.
2) BCYE (buffered charcoal-yeast extract) agar.
Incubation at 35-37Cº for 3-7 days in a moist
environment & identified by IF staining.
Hemolysis of blood is associated with virulent pertussis.
Antigenic Structure
B. pertussis produces many factors that are involved in
the pathogenesis:
1) Pili: adherence of bacteria to the ciliated epithelial cells
of upper R.T.
2) Tracheal cytotoxin: inhibits DNA synthesis in ciliated
cells =
3) LPS: causing damage to the epithelial cells of upper R.T.
4) five virulence factors regulated by vir or bvg (bordetella
virulence gene):
a. Filamentous hemagglutinin: mediates adhesion to
ciliated cells.
b. Pertussis toxin: a protein consists of 2 subunits A & B
has similar action of cholera toxin & promotes
lymphocytosis, sensitization to histamine, enhanced
insulin secretion & has ADP-ribosylating activity.
c. Adenylyl cyclase toxin.
d. Dermonecrotic toxin.
e. Hemolysin.
Pathogenicity:
Transmitted by respiratory route from carriers or early
cases, adheres to & multiplies on the epithelial surface of
trachea & bronchi & interferes with ciliary action (no blood
invasiveness), liberate toxins & substances that irritate
surface cells → coughing & lymphocytosis may be necrosis
& PMNs infiltration peribronchial inflammation &
pneumonia (secondary invaders like staphylococci & H.
influenzae may cause pneumonia) obstruction of smaller
bronchioles by mucous plugs & oxygenation of blood →
causing whooping cough (in infants).
Clinical Findings:
After incubation period (2weeks) →catarrhal stage: mild
coughing, sneezing & large No. of bacteria in droplets of
patient (highly infectious but not very ill) → paroxysmal
stage: explosive cough & characteristic whoop upon
inhalation → rapid exhaustion may associated with
vomiting, cyanosis & convulsions. Rarely become fatal
complication of encephalitis.
WBC`s count high (16000-30000/μL) with lymphocytosis.
Several types of adenovirus & Chlamydia pneumoniae can
produce a similar clinical picture of B. pertussis.

Unit 2: Bacteriology
119
Diagnostic Lab
Specimen: saline nasal wash, nasopharyngeal swabs or
cough droplets (onto a cough plate).
Direct fluorescent Ab (FA) test: FA reagent used to
examine nasopharyngeal swab & to B. pertussis after
culture on solid media.
Culture: Bordet-Gengou medium or BCYE medium →
identified by IF staining or by slide agglutination with
specific antiserum.
PCR test: for B. pertussis & B. parapertussis.
Immunity:
The 1
st
defense against B. pertussis infection is the Ab that
prevents attachment of bacteria on the cilia of the
respiratory epithelium.
After recovery & vaccination 2
nd
infections may occur but
mild. Reinfections may occur years later in adults that may
be sever.
Pertussis vaccine: using toxin-producing phase I cells →
acellular pertussis vaccines contains 3-5 Ags.
Treatment:
Erythromycin during catarrhalstage to elimination of
bacteria, as a prophylactic & prevent paroxysmal stage.
Oxygen inhalation to prevent anoxic damage to the brain.
Epidemiology, Prevention & Control:
Worldwide, the source of infection is the patient in the
catarrhal stage. Most cases in children less than 5 years &
the most deaths occur in the 1
st
year of life. So every infant
should receive 3 injections of pertussis vaccine during the
1
st
year followed by a booster of 5 doses.
Pertussis vaccine administered in combination with toxoid
of diphtheria & tetanus (DTP).
Prophylactic: erythromycin for 5 days benefits to infants &
exposed adults.
2. B. parapertussis:
produce disease similar to whooping cough, it has a silent
copy of the pertussis toxin gene. Grows more rapidly than
B. pertussis produces larger colonies.
3. B. bronchiseptica (bronchicanis):
found in the R.T. of canines, causes diseases in animals &
pertussis- like illness in humans a chronic R.T. infections. It
has a silent copy of the pertussis toxin gene.
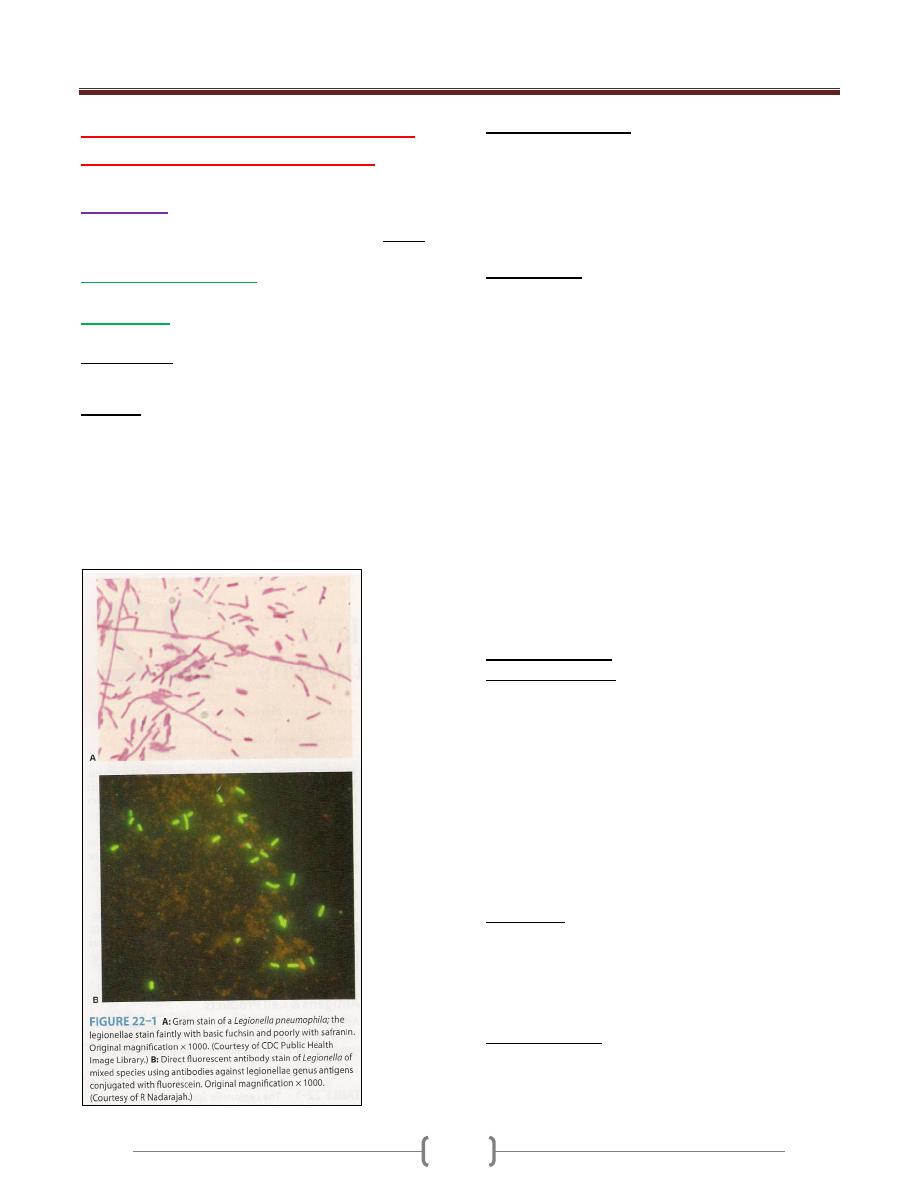
Unit 2: Bacteriology
120
Lecture 9 – Legionellae, Bartonella
& unusual bacterial pathogens
Legionella
Outbreak of pneumonia in attending an American Legion
convention (1976). There are several species of Legionella:
-Legionella pneumophila
: the most important, cause
Legionnaire’s disease & Pontiac fever.
-L. micdadei
: sometimes causes pneumonia.
Morphology:
Fastidious, aerobic G- rods stain poorly by Gram’s method
Culture:
Grown on complex media BCYE + α-ketoglutarate at pH
6.9, 35C˚ & humidity 90% →grow slowly, visible
colonies after 3 days (in blood culture require 2 weeks or
more) → variation in colony, round or flat with entire
edges, vary in color from colorless to iridescent pink or
blue & translucent.
Catalase +, L. pneumophila is oxidase + & others variable
Antigenic Structure:
There is more than 10 serogroups of L. pneumophila,
serogroup 1 the most common (complex antigenic
structure).
Legionellae produce: proteases, phosphatase, lipase,
DNase & RNase.
Pathogenesis:
Legionellae found in warm moist environments &
infection of immunocompromised humans follows
inhalation of the bacteria from aerosols (contaminated air-
conditioning systems, showerheads & similar sources).
L. pneumophila causes a lobar, segmental or patchy
pulmonary infiltration, acute purulent pneumonia
involving the alveoli with dense intra-alveolar exudate of
macrophages, PMNs, RBCs & proteinaceous material.
There is interstitial infiltration & little or no inflammation
of the bronchioles & upper airways.
L. pneumophila enters & grows within human alveolar
macrophages & monocytes (phagocytosed but not killed),
so L. pneumophila in lesions found within phagocytic
cells. Ribosomes, mitochondria & small vesicles
accumulate around phagocytic vacuoles → the cells
destroyed → the bacteria released & infected other
macrophages.
Clinical Findings:
Legionnaire’s disease: asymptomatic infection is common
in all ages, highest in men over 55 years. Risk factors:
smoking, chronic bronchitis & emphysema, steroid &
other immunosuppressive drugs, cancer chemotherapy &
diabetes mellitus.
Infection may nondescript febrile illness of short duration
or sever, rapidly progressive illness with high fever,
chills, malaise, nonproductive cough, hypoxia, diarrhea &
delirium. Chest X-rays reveal patchy & multilobar
consolidation.
There are leukocytosis, hyponatremia, hematuria (even
renal failure) or abnormal liver function.
Pontiac fever: fever, chills, myalgia, malaise, headache
(6-12 hours), dizziness, photophobia, neck stiffness &
confusion. Respiratory symptoms are much less in
Pontiac fever than Legionnaire’s disease & include mild
cough & sore throat.
Diagnostic Lab.:
Specimens: bronchial washings, pleural fluid, lung
biopsy & blood.
Smear: Direct Ab tests (low sensitivity).

Unit 2: Bacteriology
121
Culture: BCYE agar then IF staining.
Specific tests: Legionellae Ags in patient’s urine by
immunologic method.
Serologic tests: detection Abs to Legionellae (outbreaks).
Treatment:
The drug of choice is erythromycin. Rifampin when
treatment delayed.
Ventilation & management of shock is essential.
Epidemiology & Control:
The natural habitats for Legionellae are lakes, streams,
rivers & thermally heated bodies of water & soil (survives
up to 63C˚). Cooling towers & evaporative condensers
heavily contaminated with L. pneumophila.
Contamination of residential water systems
community acquired inf.
Contamination of hospital water systems hospital
acquired or nosocomial infection.
Control of Legionellae in water & air-conditioning
systems by hyperchlorination & superheating of water.
Bartonella
Is a genus of Gram-negative bacteria. Faculative
intracellular parasite, Bartonella species can infect
healthy people but are considered especially important as
opportunistic pathogen. Bartonella are transmitted by
insect vectors such as ticks, fleas, sand
flies&mosquitoes
At least eight Bartonella species or subspecies are known
to infect humans. G- pleomorphic rods, difficult to
isolated but can be seen in infected tissues by Warthin-
Starry silver stain
3 medically important spp.:
1. B. bacilliforms:
2 stages of Bartonellosis or Carrion’s disease:
a) Oroya fever (initial stage):
serious infectious anemia
due to blood cells destruction, hemorrhage into the lymph
nodes & enlargement spleen & liver. Masses of
bartonellae fill the cytoplasm of cells lining the blood
vessels & endothelial swelling → vascular occlusion &
thrombosis → 40% mortality rate.
b) Verruga peruana (eruptive stage):
begins 2-8 weeks
later through verrugae (absence of Oroya fever), vascular
skin lesions no anemia & fatalities, lasts for 1 year.
• Diagnosis by stained smear & blood culture (28C˚ for 10
days or more).
• In Peru, Colombia & Ecuador, transmitted by sandflies
• Control: by insecticides.
• Treatment: penicillin, streptomycin or chloramphenicol.
2. B. henselae
:
Causes bacillary angiomatosis & cat-scratch fever.
Bacillary angiomatosis:
proliferative, vascular lesions in the skin & visceral
organs (immunocompromised &
ADIS patients).
Treated with doxycycline or erythromycin.
Cat-scratch fever:
transmitted by cat scratch or bites &
cat fleas bites, after 3-10 days, skin lesions at the site of
scratch with low-grade fever, headache, sore throat or
conjunctivitis.
2 weeks later → lymphoadenopathy (regional lymph
nodes enlarged, tender & may discharge pus). Self-limited
illness (many weeks or months).
Treated by tetracycline or erythromycin.
3. B. quintana
:
Causes
bacillary angiomatosis
&
trench fever
(World
War I in trenches), transmitted by body lice → sudden fever
for 5-7 days, headache & sever pains in trunk & limbs.

Unit 2: Bacteriology
122
Unusual Bacterial Pathogens
* Calymmatobacterium granulomatis:
G- rods, causes granuloma inguinale, a sexually
transmitted disease → genital ulceration & soft tissue &
bone destruction. Diagnosis by stained smear from the
lesion → Donovan bodies (stained organism within large
macrophages).
-Treated by tetracycline.
* Tropheryma whippelii:
G+ bacilli related to actinomycete, causes Whipple’s
disease: fever, abdominal pain, diarrhea, weight loss &
migratory polyarthralgia. Diagnosis by periodic acid-
Schiff stain.
Gardnerella vaginalis:
Isolated from the normal female genitourinary tract &
associated with vaginosis (nonspecific vaginitis) → no
inflammatory cells & absence of common causes of
vaginitis, but in wet smear there are a clue cells (epithelial
cells covered with many Gram-variable bacilli. Vaginal
discharge has a fishy odor & contains many anaerobes
with G. vaginalis. Treatment: oral metronidazole.
Mobiluncus: motile, curved, Gram-variable or G-,
anaerobic rods isolated from bacterial vaginosis may be
part of the normal vaginal anaerobic flora in women.
Streptobacillus moliniformis:
Aerobic, G- highly pleomorphic forms irregular chains of
bacilli with fusiform enlargements.
Causes:
Rat-bite fever:(normal habitant of this bacteria the throats
of rats, humans infected by rat bites), a septic fever,
blotchy & petechial rashes & very painful polyarthritis.
Diagnosis by cultures of blood, joint fluid or pus & by
serum agglutination test.
Haverhill fever: infection after ingested contaminated
milk with these bacteria. Penicillin & other antibiotics as
effective therapy.

Unit 2: Bacteriology
123
Lecture 10+11 - zoonotic gram
negative rods
Zoonoses: are human diseases caused by organisms that
are acquired from animals (animal sources). There are
bacteria, viral, fungal & parasitic zoonoses. Some
zoonotic organisms are acquired directly from the animal
reservoir, others transmitted by vectors (mosquitoes, fleas
or ticks). There are 4 medically important G- rods (have
animal reservoirs):
Yersinia, Pasteurella , Francisella & Brucella species.
(YERSINIA, FRANCISELLA, & PASTEURELLA)
They are short; pleomorphic G- rods can exhibit bipolar
staining.
Yersinia
Y.pestis, Y. enterocolitica, & Y. pseudotuberculosis
Y.pestis:
Short, pleomorphic G- rods, motile (like safety pin)
Bipolar staining by methylene blue & carbol fuchsin.
Causes plague.
Grow as a facultative anaerobic on media containing
blood or tissue fluids, freshly isolates possess capsule,
incubation at 37C˚ → gray viscous colonies, catalase+,
oxidase -
Antigenic Structure:
LPS (endotoxin activity).
Envelope contains protein = fraction 1 (antiphagocytic).
V & W antigens (proteins encoded by plasmid, lack of
this plasmid → avirulent strain used as vaccine).
Exotoxin (1μg is lethal in mice).
Pesticin= bacteriocin.
Note: There is a cross-reaction between Y.pestis &other yersiniae
Pathogenesis:
2 types of plague: Pneumonic p. & Bubonic p.
Pneumonic p.: results from inhalation of droplets or septic
emboli (contains the bacteria) that reach the lungs.
Bubonic p.:Y.pestis found in bacteremic rodent, blood
meal of flea ingests the bacteria which cause blood clots
in stomach (by bacterial coagulase), the bacteria trapped
in fibrin & proliferate → mass of bacteria block of
flea΄s intestinal tract → regurgitate the bacteria into next
animal or bites a human (the flea become hungrier &
loses its natural host, rodents).
The flea bite a human, inoculated bacteria → spread to
regional lymph nodes → swollen & tender (groin &
axillae) → buboes (bubonic p.).
Clinical Findings of bubonic p.:
Incubation period 2-7 days, high fever,
lymphoadenopathy, vomiting & diarrhea may develop.
DIC (hypotension, renal & cardiac failure),signs of
meningitis may appear.
Endotoxin-related symptoms (DIC & cutaneous
hemorrhages) → black death.
Diagnostic Lab.:
Specimens: blood, sputum, lymph aspirate & csf.
1) Smear: Giemsa’s stain or Wayson΄s stain.
2) Culture: blood agar (is best confirmed by
immunofluorescence).
Serum Ab. Titer ≥ 1:16 is presumptive evidence of
Y.pestis infection.
Control & prevention:
1. Control of flea by insecticide.
2. Control of spreading of rodents.
3. Vaccine (formalin-killed bacteria).
Treatment:
Streptomycin (drug of choice), tetracycline (alternative or
give in combination with streptomycin).

Unit 2: Bacteriology
124
Y.enterocolitica & Y.pseudotuberculosis:
G-rods, urease +, grow best & motile at 25Cº but non-
motile at 37Cº. They are found in the intestinal tract of
animals.Both of them cause bacterimia.
Y.enterocolitica:
isolated from rodents & domestic animals (sheep, cattle,
swine, dogs & cats). Transmitted to humans by
contamination of food, drinks & fomites. Produce a heat-
stable enterotoxin.
Y.pseudotuberculosis:
Found in farm animals & birds which excreted in feces,
human infection → ingestion of materials contaminated
from animals feces.
Pathogenesis & Clinical Findings:
Incubation period = 5-10 days →yersiniae multiply in gut
mucosa of ileum →inflammation & ulceration
→leukocytes appear in feces, the infection extend to
mesenteric lymph nodes → blood (bacteremia).
Fever, diarrhea (watery or bloody because of invasion or
enterotoxin?), abdominal pain the right lower quadrant
suggesting appendicitis.
Diagnostic Lab.:
Specimens: stool & blood for culture.
Serology: agglutination test (cross-reaction with vibrios,
salmonellae & brucellae).
Treatment: Aminoglycosides, Chloramphenicol,
Ttimethoprime-sulfamethoxazole, & Piperacillin.
Pasteurella
Non-motile, G- coccobacilli with bipolar appearance on
stained smears, aerobic or facultative anaerobes, oxidase
+, catalase + & encapsulated bacteria.
The important species is
P.multocida.
Pathogenesis & Clinical Findings:
Part of the normal flora in the mouth of many animals
(domestic animals, cats & dogs), transmitted by biting,
capsule is a virulence factor & endotoxin is present in the
cell wall. No exotoxins.
Rapid onset of cellulitis at the site of cat bite. After cat
bites osteomyelitis developed (because cats teeth sharp
pointed implant bacteria under the periosteum).
Diagnosis Lab.: Culture the sample from wound site.
Treatment: Penicillin G.
Francisella
Francisella tularensis
Small, G- pleomorphic rods, causes tularemia, transmitted
to humans by:
1) Biting arthropods (ticks).
2) Direct contact with infected animal tissue (rabbits &
deer).
3) Inhalation of aerosols.
4) Ingestion of contaminated food or water.
Pathogenesis:
Bacteria on skin abrasions (2-6 days) → an inflammatory
& ulcerating papule develops regional lymph nodes
enlargement.
Inhalation → peribronchial inflammation & pneumonitis.
Droplet or infected finger touches eye (conjunctiva) →
oculograndular tularemia.
Fever, malaise, headache & pain in the regional lymph nodes.
Diagnostic Lab.:
Specimens: Blood.
Agglutination test: titer 1:160 is highly suggestive if the
history or physical findings are compatible with the
diagnosis (cross-reaction with brucellae).
Culture: glucose blood agar or glucose cystine blood agar.
Treatment: Streptomycin or gentamicin for 10 days.

Unit 2: Bacteriology
125
Brucellae
They are cause brucellosis (undulant fever & Maltafever)
is characterized by an acute bacteremic phase then a
chronic stage may extend over many years & involve
many tissues.
4 species (infected humans):
1) Brucella melitensis (goats & sheep)
2) B. abortus (cattle)
3) B. suis (pigs)
4) B. canis (dogs)
Morphology:
G-, short coccobacillary, aerobic, non-capsulated, non-
spore forming & non-motile.
Culture:
Small convex, smooth colonies.
B. abortus requires 5-10 % CO
2
.Brucellae are oxidase,
catalase & urease positive, produce H
2
S & moderately
sensitive to heat & acid (in milk killed by pasteurization).
Antigenic Structure:
LPS: 2 antigens A & M (present in different proportions
in 4 species).
L antigen (resembles of Vi Ag of salmonellae).
Pathogenesis:
Obligate intracellular parasite transmitted from animal to
human (zoonoses).
The common routes of infection in human:
1) Intestinal tract (ingestion of infected & unpasteurized
milk, & cheese).
2) Mucous membrane by droplets.
3) Skin (contact with infected tissues of animals).
After infection, brucellae → lymph duct or channels &
lymph nodes → thoracic duct & bloodstream →
parenchymatous organs → granulomatous tissues
(abscess) in lymphatic tissue, liver, spleen, bone marrow
& other parts of reticuloendothelial systems.
The disease has 2 phases: acute bacteremic phase &
chronic phase.
Placentas & fatal membranes of cattle, swine, sheep &
goats contain erythritol (growth factor for brucellae). The
proliferation of brucellae in pregnant animals →
placentitis & abortion. No erythritol in human (no
abortion)
B. melitensis infections more sever & prolonged than B.
abortus infections which are more self-limited.
Clinical Findings:
After incubation period (1-6 weeks), insidious onset with
malaise, fever (rises in the afternoon & fall during the
night with drenching sweat), weakness, aches & sweats.
There may be gastrointestinal & nervous symptoms,
lymph nodes enlarge & the spleen becomes palpable,
hepatitis may be with jaundice → these generalized
symptoms subside in weeks or months but localized
lesions & symptoms may continue.
Chronic stage may develop (weakness, aches, pains, low-
grade fever, nervousness & other nonspecific with
psychoneurotic symptoms). The diagnosis of this stage is
difficult unless local lesions are present (brucellae cannot be
isolated from the patient but agglutinin titer may be high)
Treatment:
Brucellae susceptible to tetracyclines & ampicillin for few
days or prolonged for best results & combined treatment
(tetracycline + streptomycin or gentamicin).
Epidemiology, Prevention & Control:
Brucellae are zoonotic pathogens, so the infection is more
frequent in men because of occupational contact (farmers,
veterinarians, slaughterhouse workers). The majority of
infections remain asymptomatic (latent).
Eradication of brucellosis in cattle (immunization) &
examined by agglutination test. Active immunization of
humans with avirulent live strain 19.
Control by:
1) Limitation of spread & possible eradication of animals
infection.
2) Pasteurization of milk & milk products.
3) Reduction of occupational hazards wherever possible.

Unit 2: Bacteriology
126
Lecture 12+13 - Spirochetes
Spiral motile bacteria, includes 2 families:
1) Spirocheataceae: (free-living bacteria).
2) Treponemataceae include: Treponema, Borrelia &
Leptospira.
Treponema
T.pallidum subsp. Pallidum
causes syphilis, a long
{0.2X(5-15) mm}, slender, helically coiled, spiral, G-
bacilli
Structural characteristics:
1) Outer sheath (glucosaminoglycan coating).
2) Outer membrane (peptidoglycan).
3) Endoflagella (axial filaments, encapsed by the outer
membrane & wind around the organism).
4) Inner membrane (cytoplasmic membrane).
5) Cytoplasm contains body fibrils (cytoplasmic tubules).
Actively motile & so thin-→cannot be seen unless
immunofluorescin stain or dark-field microscope is
employed.
Never been cultured on artificial media or in fertile eggs
or in tissue culture.
Antigenic Structure:
1) Does not contain LPS.
2) The endoflagella: similar to bacterial flagellin protein.
3) Cardiolipin (an important treponemal antigens).
4) Hyaluronidase (breaks down the hyaluronic acid in tissue
& enhances the invasiveness of this organism, so it is
Virulence Factor).
Human with syphilis develops antibodies (can be used for
staining T.pallidum by direct IF test or complement
fixation test.
Also develops Ab-like substances or non-specific Abs.
called reagin, directed against some Ags. distributed in
normal tissues.
Pathogenesis & Clinical Findings:
1) Acquired syphilis:
T.pallidum transmitted by sexual
contact (the infectious lesion is on the skin or mucous
membrane of genitalia & only 10-20% on intrarectal,
perianal & oral lesions).
• It is penetrate through mucous membrane or a break in the
epidermis, multiply locally-→ spread to lymph nodes-→
bloodstream.
• In 2-10 weeks after infection: primary syphilis or primary
lesion (a papule develops at the site of infection breaks
down to form an ulcer with clean, hard base called
hardchancre), heals spontaneously.
• 2-10 weeks later: secondary syphilis or secondary lesions
(a red maculopapular rash anywhere of the body) &
condylomas (a moist, pale papules in the anogenital
region, axilles & mouth). Also may be appear syphilitic
meningitis, chorioretinitis, hepatitis or periostitis → heals
spontaneously.
• Both primary & secondary lesions are rich in spirochetes
& highly infectious.
• 40% progresses to the tertiary stage (latent syphilis)-→
gummas (a granulomatous lesion in skin, bones, liver &
degenerative changes in CNS or cardiovascular lesions.
This lesion due to hypersensitivity to the organisms).

Unit 2: Bacteriology
127
2) Congenital syphilis:
Pregnant syphilitic women can transmit T.pallidum to the
fetus through the placenta in 10
th
-15
th
weeks of gestation.
Some infected fetus die, others are borne live with sings
of congenital syphilis in childhood (keratitis,
Hutchinson’s teeth, saddlenose, periostitis & variety of
CNS anomalies).
Treatment mother during pregnancy prevents congenital
syphilis.
Diagnostic Lab. Tests:
Specimens: Tissue fluids (lesions) & serum (serological
tests).
Dark-field examination: drop of tissue fluid examined
by dark-field microscope-→ motile spirochetes.
Immunofluorescence test: Tissue fluid stained with a
fluorescein-labeled antitreponemal serum examined by
fluorescent microscope-→ fluorescent spirochetes.
Serological tests:
1) Nontreponemal Ag tests: using cardiolipin (extracted from
normal mammalian tissue) + patient’sserum (reagin).
Reagin appears in serum after 2-3 weeks or in CSF after
4-8 weeks of infection. 2 types:
A. Complement fixation (CF) test or Wassermann-
Kolmer test: rarely used.
B. Flocculation test: VDRL (Venereal Disease Research
Lab.) or RPR (Rapid Plasma Reagin). Cardiolipin
form visible clumps with reagin containing sera.
2) Treponemal Ab tests: 2 types:
A. Fluorescent treponemal Ab-Absorbance (FTA-ABS) test:
Killed T.pallidum Reiter strain + patient’s serum +labeled
antihuman gamma globulin (very specific& sensitive test)
B. T.pallidum hemagglutination (TPHA) test:
-Red blood cells treated to adsorb treponemes on their
surface + patient’sserum-→ red cells clumped
Immunity: after cure-→ patient become susceptible.
Treatment:
Syphilis less than 1 year-→ benzathine penicillin (IM).
Older (latent syphilis) -→ = = (3 times weekly intervals).
Neurosyphilis-→ penicillin (large amount, IV).
Epidemiology:
Transmitted sexually.
An infected person may remain contagious for 3-5 years.
Control:
1) Treatment of all discovered cases.
2) Follow-up on sources of infection & contacts.
3) Safe sex.
Diseases Related to Syphilis
None are sexually transmitted diseases but by direct
contact. Diagnosis & therapy similar to syphilis.
1) Bejel
(T.pallidum subsp. endemicum)
→produces highly
infectious skin lesions. Penicillin the drug of choice.
Among children, in Africa, Middle East & Southeast Asia.

Unit 2: Bacteriology
128
2) Yaws
(T.pallidum subsp. pertenue)
→ Ulceration
papule usually on arms & legs-→ Scar formation of skin
lesions & bone destruction are common.
Among children, in humid, hot tropical countries.
3) Pinta
(T.carateum)
→ Lesions on skin, depigmentation
& hyperkeratosis take years afterward.
In all ages (dark-skinned races), in Mexico, American,
Pacific areas & Philippines.
Borreliae (Borrelia SPP.)
1) Relapsing Fever
(Borrelia recurrentis)
transmitted by the human body
louse. Endemic relapsing fever in western USA
(B.hermsii)
transmitted by ticks (Orinthodoros hermsii).
Irregular spiral {0.3 X (10-30)μm}, flexible motile has
many endoflagella. Stained by bacteriological dyes &
blood stains (Giemsa’sor Wright’sstains).
Can be cultured in fluid media containing blood, serum or
tissue or in chick embryos.
Antigenic Structure:
Have antigenic variation-→ relapsing course due to the
multiplication of antigenic variants against which the host
must then develop new Abs. After 3-10 relapses-→
Ultimate recovery (associated with the presence of Abs
against antigenic variants).
Pathogenesis & Clinical Findings:
After incubation period (3-10 days), sudden chills & fever
(spirochetes found in blood). The fever persists for (3-5
days, febrile period). Then declines leaving the patient
weak but not ill (afebrile period), followed by a 2
nd
attack
of chills, fever,headache & malaise(3-10 such
recurrences)-→ recovery
Diagnostic Lab. Tests:
Specimens: Blood (during fever) for smear & animal
inoculation.
Smear: Thin or thick blood smear stained with Giemsa’s
or Wright’s→ Large, loosely coiled spirochetes among
the red cells.
Animal inoculation: White mice or rats inoculated (I.P),
after 2-4 days-→stained blood film for examined of
spirochetes.
Serology: + ve VDRL or CF tests.
Immunity: Short duration.
Treatment: Tetracycline or erythromycin & penicillin for
single day may be sufficient.
2)
Lyme Disease
(B.burgdorferi)
transmitted by tick
called Ixodes.
Spiral, highly motile, stained with acid & aniline dyes &
by silver impregnation techniques.
Isolated from erythema migrans skin lesions & ticks.
Grows in a complex medium (BSKII medium), seldom
used because it takes 6-8 weeks to grow & lacks
sensitivity with low diagnostic yield.
Antigenic Structure:
Have large No. of lipoproteins (Outer Surface Proteins;
Osp A-F) → help this organism live in the very different
tick & mammalian hosts.

Unit 2: Bacteriology
129
Pathogenesis & Clinical Findings:
Transmitted to human by injection of the organisms in
tick saliva, migrates (from the bite site) → skin lesions
called erythema migrans (a flat reddened area near the
tick bite slowly expands with central clearing, begins 3
days to 4 weeks after bite) →flu-like illness with fever,
chills, myalgia & headache (the initial stage) →
dissemination by lymphatics or blood to the other skin
area & organs (the second stage) occurs weeks to months
later (arthralgia & arithritis, meningitis, cardiac disease).
The third stage begins years later with chronic skin,
nervous system or joint involvement (the organism can be
isolated from all these sites).
Diagnostic Lab. Tests
Can be established clinically by observing the erythema
migrans, if not present→diagnostic lab.T.
Specimens: Blood (serological tests) & biopsy (smear). -
Serology: IFA, EIA or ELISA, Western blot.
Molecular probes: PCR (detection of B.burgdorferi
DNA in body fluids).
Immunity:
4-6 weeks after infection IgG to OspA & OspB.
Treatment:
Amoxicillin or doxycycline (20-30 days).
Arithritis: penicillin + probencide (30 days or longer).
Epidemiology & Control:
In USA, Europe & other areas.
Mice & deer (the main reservoirs), rodents & birds also
infected.
Prevention by avoidance ticks (using insecticides for
control)
OspA vaccine efficient 66-76 % for how lives or works in
endemic areas.
Leptospira & Leptospirosis
Leptospirosis includes 2 worldwide diseases:
1) Infectious jaundice
(L.interrogans serovar. canicola)
(dogs)
2) Weil’sdisease
(L.interrogans serovar.
icetrohaemorrhagia)(rats).
Tightly coiled, thin, flexible spirochetes {(0.1-0.2) X (5-15)
μm}, one end as a hook & actively motile. Does not stain,
seen by dark-field microscope as a chain of minute cocci.
Grow aerobically at 28-30Cº in serum containing media
(Fletcher’sor Stuart’smedia)-→ diffuse zone of growth.
Antigenic Structure:
Have LPS variable from one strain to another (used for
classification & determines the immune response).
Pathogenesis & Clinical Findings:
Infection results by ingestion of water or food
contaminated with leptospirae from urine of dog, rats,
mice, cattle, swine & others (rare through mucous
membranes or breaks in the skin). After incubation period
(1-2weeks), variable febrile onset, flu-like illness
(spirochetes in blood) → establish in parenchymatous
organs (liver & kidneys) → hemorrhage & necrosis of
tissues & dysfunction of organs (initial phase) →second
phase, when IgM titer rises, aseptic meningitis, nephritis,
hepatitis & may be skin, muscle & eye lesions.
Diagnostic Lab. Tests:
Specimens: Blood, CSF, urine & tissues.
Smear: Dark-field microscope or fluorescein-conjugated
Abs or immunohistochemical techniques.
Culture: Fletcher’ssemisolid medium (8weeks). -Animal
inoculation: hamsters or guinea pigs.
Serology: microscopic slide agglutination test.
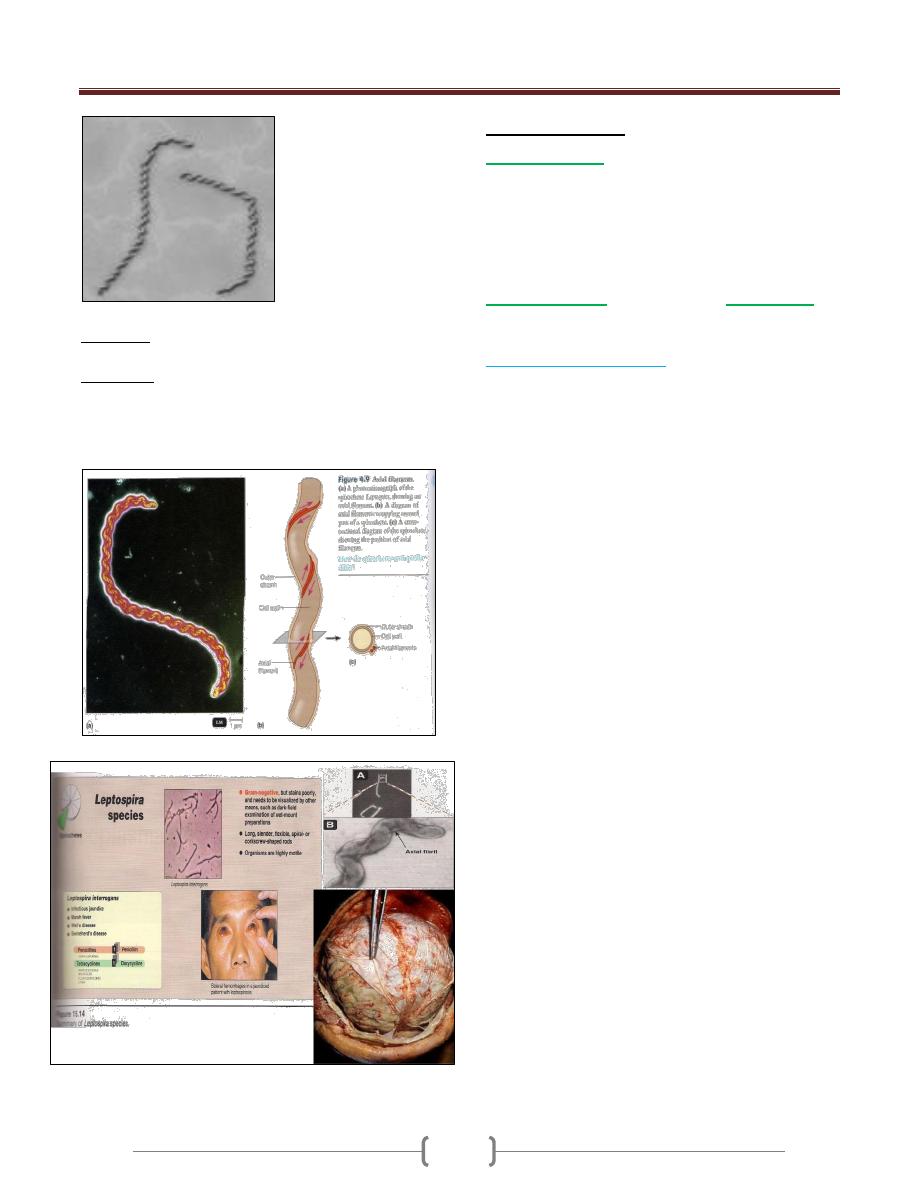
Unit 2: Bacteriology
130
Immunity: serovar specific immunity but reinfection
with different serovar may occur.
Treatment:
Mild disease: doxycycline, ampicillin or amoxicillin
(orally)
Sever = : penicillin or ampicillin (I.V).
Other Spirochetes
Spirillum minor
-→ rat-bite fever (Sodoku disease).
Very small spirochetes (3-5 μm) & rigid spiral.
Infection through the bite of rat-→ local lesion, regional
glands swellings skin rash & fever (relapsing type). Can
be isolated from enlarged lymph nodes material or blood-
→ inoculated to mice or guinea pigs.
Borrelia bucallis
in normal mouth &
B.refringens
in
normal genitalia.
Fusospirochetal Diseases:
The normal spirochetes with anaerobic fusiform bacilli
(fusobacteria), not primary pathogens but after other
infection (HSV), mucous membrane injury or nutritional
deficiency-→ disease:
1) Ulcerative gingivostomatitis (trench mouth, poor oral
hygiene).
2) Vincent’s stomatitis (Vincent’s angina) → ulcerative
tonsillitis & massive tissue involvement.
3) Leg (tropical) ulcers (similar to V. angina).

Unit 2: Bacteriology
131
Lecture 14 – Mycoplasma,
Chlamydia & Rickettsiae
Mycoplasmas (Mycoplasmas spp)
Mycoplasmas are groups of small, wall-less organisms.
They are the smallest free-living organisms (0.3μm in
diameter).
Important properties:
1) Mycoplasmas stain poorly with Gram stain.
2) The outer surface is a flexible three layer cell membrane;
hence the organisms can assume a variety of shapes.
3) It contain cholesterol in their bacterial membrane.
4) The colony frequently has a characteristic (fried egg)
shape, with a raised center & a thinner outer edge.
In human there are four important species:
1) Mycoplasma pneumoniae 2) M. Hominis
3) M. Genitalium 4) Ureaplasma urealyticum
Pathogenesis:
Pathogenic Mycoplasmas have flask-like or filamentous
shapes & have specialized polar tip structures that
mediate the adherence to host cells (ciliated & non
ciliated cells).
1) M. pneumoniae
Is transmitted from person to person by means of infected
respiratory secretions & cause atypical pneumonia. May
be ranged from asymptomatic infection to serious
pneumonitis.
Incubation period 1-3 weeks.
Symptoms:
Fever, headache, sore throat, & cough which is non-
productive .Complications are uncommon, but sometimes
hemolytic anemia, meningitis & pericarditis may occur
Diagnosis:
Culture: haert infusion peptone broth (with 2% agar &
30% human ascitic fluid or animal serum, pH 7.8).
Complement fixation (cf test).
Cold hemagglutination (at 4C
°
). After 3-4 weeks, titer of
1:128 or higher is an indicative of recent infection.
ELISA.
Treatment: Tetracycline & erythromycin.
2)
Mycoplasma hominis
: causes infections of uterine
tubes (salpingitis) & tubo-ovarian abscesses (in Women).
3)
Mycoplasma genitalium
: Associated with some
infections of chronic nongonococcal urethritis (in men).
4)
Ureaplasma urealiticum
: causes nongonococcal
urethritis in some men (may play role in male infertility).
Lung diseases in premature low birth-weight infants
(acquired during birth).
Cell Wall-defective Bacteria
L phase variants (L forms):
Are wall-defective bacteria that can replicate serially as
non-rigid cells & produce colonies on solid media.
Some of L phase variants are stable; others are unstable &
revert to bacterial parental forms .Wall-defective forms
are not genetically related to Mycoplasma.
Cell wall-defective bacteria result from:
Spotaneous mutation.
Effect of chemicals: penicillin & lysozyme.
They are important for the persistence of bacteria in
tissues & recurrence of infection after antimicrobial
treatment, as in causes of endocarditis.
Types of cell wall-defective bacteria:
1) Protoplast:
forms usually derived from G-positive
bacteria osmotically fragile.
2) Spheroplast
: forms usually derived from G-negative
bacteria (they retain some outer membrane material).
Chlamydiae (Chlamydia spp.)
Chlamydiae: are obligate intracellular bacteria, luck the
ability to produce sufficient energy to grow independently
& therefore can grow only inside host cell. Chlamydiae
have a replicative cycle different from that of all other
bacteria. Within cells site of replication appears as an
inclusion body, which can be stained & visualized
microscopically. These inclusions are useful in the
identification of these organisms in the clinical laboratory.
They have rigid cell wall but they don’t have typical
peptidoglycan. Their cell walls resemble those of G-
negative but luck muramic acid.
Pathogenesis:
Chlamydiae infect primarily epithelial cells of the mucous
membrane and the lungs.
Diseases:
1)
Chlamydia psittaci
: infects the lungs →human
psittacosis, this disease may be asymptomatic or produce
high fever & pneumonia.
2)
C. pneumoniae
→upper & lower respiratory tract
infections especially bronchitis & pneumonia.
3)
C. trachomitis types A, B&C
→trachoma (chronic
conjunctivitis endemic in Africa & Asia).

Unit 2: Bacteriology
132
Trachoma may recur over many years & may lead to
blindness but without systemic illness.
4)
C. trachomatis types D-K
→genital tract infections, which occasionally transmitted
to the eyes or the respiratory tract.
In men: it common cause of non-gonococcal urethritis,
which may progress to epididymitis, prostatitis or proctitis
In women: cervictitis develops & may progress to
salpingitis & pelvic inflammatory disease →this may
result infertility or ectopic pregnancy.
Infants borne to infected mothers often develop
mucopurulent eye infections (neonatal inclusion
conjunctivitis) 7-12 days after delivery. Some develop
chlamydial pneumonitis 2-12 weeks after birth.
C. trachomitis L1-L3 immunotypes
→lymphogranuloma venereum ,a sexually transmitted
disease with lesions on genitalian & in lymph nodes .
Diagnosis:
Group-specific Ag. (lipopolysaccharide) → complement
fixation test.
Species-specific & immunotype–specific Ag. (Protein)→
Immunofluorescence test .
Chlamydiae form cytoplasmic inclusions →stain with
Giemsa →immunofluorescence test .
Treatment:
Tetracyclines such as doxycycline & macrolides,such as
erythromycin & azithromycin .
Figure 17.2
Structural features of
Chlamydia.
A. Schematic drawing
B. Electron micrograph
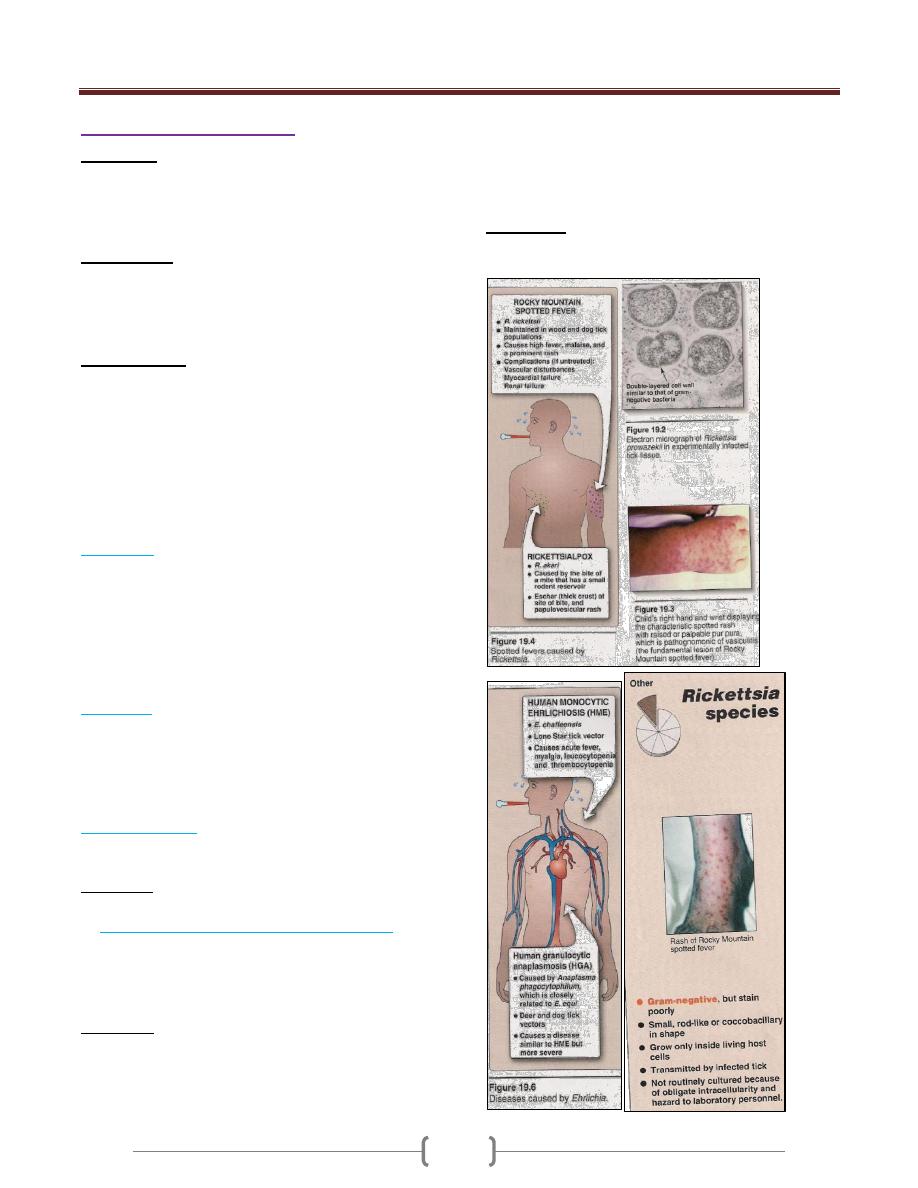
Unit 2: Bacteriology
133
Rickettsiae (Rickettsia spp.)
Rickettsiae: are small obligate intracellular bacteria (they
are unable to produce suffecint energy to replicate
extracellular). They transmitted by the bite of the
arthropods (except for Q fever from cattle, sheep & goats).
Morphology:
Rickettsiae are pleomorphic ,coccobacilli ,gram-negative.
They are visible under light microscope when stained
with Giemsa's stain (purple).
Pathogenesis:
The human pathogens include: Rickettsia, Coxiella,
Orientia,& Ehrlichia.
The typical lesions caused by rickettsiae are a
vasculitis, particularly in the endothelial lining of the
vessel wall where the organism is found. Damage to the
vessels of the skin result →rash & hemorrhage caused by
increased capillary permeability (endotoxin?).
There are four important rickettsial diseases:
A) Typhus:
There are several forms of typhus: epidemic, endemic
(Rickettsia typhi by flea from rodents) & scrub typhus
(Orientia by mite from rodents).
Symptoms: Chills, fever, headache & influenza like
symptom
Louse bite →macular rash on the trunk →spread
peripherally →sever meningoencephalitis.
B) Q fever:
Caused by Coxiella burnetii. The main organ involved in
Q fever is lungs.
Symptoms: fever, severe headache, cough & other
influenza like Symptoms & pneumonia .Combination of
pneumonia & hepatitis should be suggested in Q fever.
C) Spotted fevers:
1) Rocky Mountain spotted fever caused by R. rickettsii
2) Rickettsial pox caused by R. akari
Symptoms: Typical rash, which appears 2-6 days, later
begins with macules & progress to petechia.
D) Human monocyte or granulocyte ehrlichiosis:
Caused by Ehrlichia transmitted by tick from dear, dogs,
mice & other mammals.
Symptoms: Fever, headache and atypical WBC's.
Diagnosis:
Rickettsiae can be growing in cell culture or
emberyonated eggs; this is hazardous procedure that is not
available in all laboratories.
Serological test: Weil-Felix, this test is based on the
cross-reaction of an Ag present in many rickettsiae with
the O Ag polysaccharide found in Proteus vulgaris (OX-
2, OX-19 & OX-K).
Treatment:
Tetracycline &chloramphenicol as a second choice.

Unit 2: Bacteriology
134
Lecture 15 - Normal microbial flora
Do you know that you have 3X as many bacterial cells
as human cells?
If you include fungal and viral cells, only about 25% of
your cells are actually human
The other 75% are MICROBES
The population of microorganisms (bacteria & fungi)
that inhabit the skin & mucous membrane of pharynx,
colon & vagina of healthy normal persons. Viruses &
parasites are not considered as normal flora. Includes 2
groups:
1) The resident flora: consist of fixed types of commensals
of microorganisms reestablishes itself. Play a role in
maintaining health & normal function, in intestine
synthesize vit. K & aid in the absorption of nutrients, in
skin & mucous membrane prevent the colonization of
pathogens through bacterial interference. But normal flora
may produce disease under certain circumstances.
2) The transient flora: consist of nonpathogenic or
potentially pathogens that inhabit skin or mucous
membranes for hours, days or weeks. It is derived from
the environment does not produce disease & does not
establish itself.
N.F of the Skin:
S.epidermidis, S.aureus, Micrococcus, streptococci,
Enterococcus, nonpathogenic N., diphtheroids
(Corynebacterium spp.), Propionibacterium, G -
coliform, fungi & yeast (Candida albicans in skin folds),
nonpathogenic mycobacteria (in external ear & genitalia,
which are rich in sebaceous secretions).
Factors that eliminating nonresident microorganisms from
the skin:
1) Low pH.
2) Lysozyme.
3) Fatty acids in sebaceous secretions.
Anaerobic & aerobic bacteria join to form syngeristic
infections (gangrene, necrotizing fasciitis & cellulitis).
N.F of the Mouth & Upper R.T:
Nose: S.aureus, S.epidermidis, streptococci &
Corynebacteria.
Mucous membrane of mouth & pharynx: at birth
sterile, after 4-12 hrs contaminated from birth canal with
viridans streptococci (as resident flora remain so far life).
Mouth: (early in life) staphylococci, N., Moraxella
catarrhalis, Diphtheroids & lactobacilli.
(When teeth erupt) spirochetes, Fusobacterium spp.,
Prevotella spp., Rothia spp.(G+, pleomorphic aerobes),
Capnocytophaga spp. & lactobacilli.(in adults)
Actinomyces spp.(in tonsillar tissue & gingiva),
yeast(C.albicans) & various protozoa.
Pharynx & trachae: similar flora of mouth.
Bronchi: few bacteria. Small bronchi & alveoli: sterile.
The predominant flora in upper R.T: streptococci,
staphylococci, N., diphtheroids, Hemophilic,
pneumococci, Mycoplasma & Prevotella.
Infections of mouth & upper R.T (by aerobes &
anaerobes): periodontal infection, perioral abscesses &
sinusitis, necrotizing pneumonia, lung abscess &
empyema (by aspiration of saliva containing up to 10
2
of
aerobes & anaerobes).
N.F of the Intestinal Tract:
At birth is sterile.
Newborns (in intensive care): the intestine colonized by
Enterobacteriaceae (K., Citrobacter, Enterobacter).
Breast-feed children: Large No. of lactobacilli, lactic
acid streptococci & Bifidobacterium spp. (G+, nonmotile,
anaerobic bacteria), produce acid from carbohydrates &
tolerate pH 5.
Bottle-feed children: More mixed flora (lactobacilli less
prominent).
Provetella
Fusobacterium
Unit 2: Bacteriology
135
Normal adult:
Esophagus: contains microorganisms arriving with saliva
& food.
Stomach: acidity keeps the No. of microorganisms at
minimum (10
3
-10
5
bacteria/g of contents).Cimetidine (for
peptic ulcer) → increase in microbial flora of the
stomach.
As the pH of intestinal contents becomes alkaline →
resident flora gradually increases. Duodenum (10
3
-10
6
bac./g of contents), Jejunum & Ileum (10
5
-10
8
), Cecum &
transverse colon (10
8
-10
10
), Sigmoid colon & Rectum
(10
11
=10-30% of fecal mass).
Upper Intestine: lactobacilli & enterococci.
Colon: anaerobes (96-99% of resident flora):
Fusobacterium spp., Bifidobacterium, clostridia (10
3
-10
5
bac./g of contents) & anaerobic G+ cocci
(Peptostreptococcus) & facultative aerobes (1-4%)
includes: G- coliform & enterococci & small No. of
Pseudomonas, lactobacilli, Proteus & Candida.
The role of intestinal flora:
1) Synthesis of vitamin K.
2) Conversion of bile pigments & bile acids.
3) Absorption of nutrients & breakdown of products.
4) Antagonism to microbial pathogens, through bacterial
interference.
5) Anaerobic flora of the colon (B.fragilis, clostridia,
Peptostreptococci), plays a main role in abscess formation
after perforation of the bowel. Prevotella spp. cause
abscess formation in the pelvis of female genital organs.
Antimicrobial drugs taken orally suppress the drug-
susceptible flora, replaced by drug-resistant ones
(staphylococci, enterococci, enterbacters, Pseudomonas,
C.difficile & yeasts).By feeding large quantities of
Lactobacillus acidophilus → partial suppression of gut
microbial flora.
N.F of the Urethra:
Anterior urethra of both sexes contains small No. of the
same organisms found on the skin & perineum → appear
in normal urine (10
2
-10
4
org./ml).
N.F of the Vagina:
Soon after birth: aerobic lactobacilli appear & persist as
long as pH acid for several weeks, when pH become
neutral (until puberty) → mixed flora of cocci & bacilli.
At puberty: Aerobic & anaerobic lactobacilli
(maintenance of acidic pH by production of acid from
carbohydrates; glycogen) → preventing the establishment
harmful microorganisms in the vagina.
If lactobacilli suppressed (by drugs) → increase No. other
bacteria & yeasts----- irritation & inflammation.
After menopause: Lactobacilli again increase with a
mixed flora (group B streptococci, peptostreptococci,
clostridia, Prevotella, Gardnerella vaginalis, Ureaplasma
urealyticum, sometimes lesteria or Mobiluncus spp.,
E.coli or coliform).
The cervical mucus has antimicrobial activity & lysozymes
In some women, vagina contains a heavy flora resembling
that of perineum & perianal area → predisposing factor in
recurrent UTIs & at delivery may infect the newborn
(e.g., group B streptococci).
N.F Of The Eye (conjunctiva):
Diphtheroids (Corynebacterium xerosis), S. epidermidis,
neisseriae, nonhemolytic streptococci, G- bacilli
(Haemophilus, Moraxella spp.).
Bacteroides fragilis,

136

Unit 3: Mycology
137
Lecture 1 - Introduction to mycology
Mycology (myco=fungus, logy=study)
Approximately 80,000 known species, less than 400 species
are medically important and less than 50 Species presently
known to be pathogenic for humans and other animals.
Fungi are eukaryotic organisms that do not contain
chlorophyll but have cell wall.
Fungi initially classified with plant kingdom, and then
fungi have transferred to the kingdom fungi.
Importance of Fungi:
Drug manufacturing (usually their waste products are to
our benefit)
Citric acid
Ethanol (yeast)
Antibiotic griseofulvin, penicillin
Cortisone (Rhizopus)
Immunosuppressive agents (cyclosporine)
Classification in mycology:
Fungi are classified on their ability to reproduce sexually,
asexually or by combination of both.
The first criteria are sexual morphological form; the
second set of criteria is based upon a sexual reproductive
structure
1) Ascomycota
– sexual reproduction in a sack called an
Ascus with the production of ascospores.
2) Basidiomycota
– sexual reproduction in a sack called a
Basidium with the production of basediospores.
3) Zygomycota
– A sexual reproduction by gametes while
sexual reproduction with the formation of Zycospores.
4) Fungi imperfecti
– nonrecognizable form of asexual
reproduction most pathogenic fungi.
Structure
Molds
Yeasts
Molds are aerobic,
filamentous fungi
including (mildews,
rusts & smuts)
Molds tend to grow on
surfaces rather than
throughout substrates.
Unicellular /
nonfilamentous
Yeast are fungi which
are: Typically sepherical
or oval & Faculatively
anaerobic
They are often observed
as powdery coatings on
plant material
The fungal cell has typical eukaryotic features including a
nucleus with a nucleolus, nuclear membrane and linear
chromosomes.
The cytoplasm contains organelles such as mitochondria
and the Golgi apparatus fungal cells, which have a rigid cell
wall external to the cytoplasmic membrane, differ from
mammalian cells. The composition of that wall makes fungi
different from bacteria and plants. Another important
difference from mammalians involves the sterol makeup of
the cytoplasmic membrane. In fungi, the dominant sterol is
ergosterol. In mammalian cells, it is cholesterol.
Feature
Fungi
Bacteria
Diameter
Approximately 4
µm (Candida)
Approximately 1µm
(staphylococcus )
Nucleus
Eukaryotic
Prokaryotic
Cytoplasm
Mitochondria and
endoplasmic
reticulum present
Mitochondria and
endoplasmic reticulum
absent
Cell
membrane
Sterol present
sterol absent (except
Mycoplasma)
Cell wall
content
Chitin
peptidoglycan
Spores
Sexual and asexual
reproduction
Endospores for survival,
not for reproduction
Metabolism
Require organic
carbon; no obligate
anaerobes
Many do not require
organic carbon; many
obligate anaerobes
Metabolism
Fungal growth requirements
In contrast to bacteria, fungi tend to grow in places that are:
More acidic
Have higher osmotic pressure
Are lower in moisture
Are lower in nitrogen
Contain coplex carbohydrates

Unit 3: Mycology
138
Reproduction
Fungi may reproduce sexually or asexually. Reproductive
elements produced a sexually are termed conidia. Those
produced sexual are termed spores, spores may be either
sexual or a sexual in origin, sexual spores includes
ascospors, Basidiospores or Zygospores. Sexual
reproduction occurs by the fusion of two haploid nuclei
followed by meiotic division of diploid nucleus. Asexual
spores are produced in sac like cell called sporangia and
called sporangiospores. Asexual reproduction results from
division of nuclei by mitosis. Fungi that do not form
sexual spores called Fungi imperfecti.
Basidiomycetes
Basidiospore. Examples: boletes, puffballs, smuts,
stinkhorns & tooth fungi
.
Culture
In vitro, culture at room
temperature with low pH
& minimal nutrients,
supports the growth of
environmental (mycelial)
phase. Incubation at body
temperature with media
supplemented with blood
& amino acids supports
the growth of the body
(yeast) phase of dimorphic fungi.
Yeast & other filamentous fungi may grow in either
condition
Asexual reproduction
Conidial fungus
Reproduces by means of asexual spores called conidia
Conidia vary greatly in shape, size & color
Most of the common household molds & mildews are
conidial fungi
Asexual spores
Conidiaspore
o Multiple (chains) or single spores formed at the end of
an aerial hypha
o Not enclosed within a sac
o Aspergillus spp.
o Penicillium spp.
Sporangiospores (sporulation)
o Hundreds formed within a sac (sporangium) at the end
of an aerial hypha (hyphal tip)
o Rhizopus spp.
Entry
Fungi infect the body through several portals of entry.
The first exposure to fungi that most humans experience
occurs during birth, when Candida albicans encounter
while passing through the vaginal canal. During this
process the fungus colonizes the buccal cavity and
portions of upper and lower gastrointestinal tract of
newborn and maintains a lifelong as a commensal. Other
fungi, malassezia furfur is common in areas of skin in
sebaceous glands. The mechanism of disease with these
two fungi is called endogenous both M. furfur and C.
albicans are considered part of normal flora. Other fungi
that have implicated in human diseases come from
exognous sources, where exist as saprophytes.
Blastospores:
Another type of conidiophore
A bud coming off the parent cell
Candida albicans

Unit 3: Mycology
139
Diagnosis
Skin scrapings
Suspected to contain dermatophytes or pus from lesion
can be mounted in 10-20 % KOH on a slide (wet
preparation) to dissolve tissue materials leaving the
fungus intact or stained with special fungal stains and
examined directly under the microscope.
Skin test is used be popular as a diagnostic tool, but this is
now discouraged.
Serology
May be helpful when it is applied to a specific, these tests
for the presence of antibodies in the patient’s serum or
CSF which are useful in diagnosing systemic mycosis.
The most common serological test for fungi based on
double immunodiffusion, complement fixation. The
complement fixation test is most frequently used in
suspected cases of Coccidioidomycosis, Histoplasmosis .
If Cryptococcal meningitis, the presence of the
polysaccharide capsular antigen of C.neoformas in CSF
can be detected by Latex Agglutination text.
Direct fluorescence microscopy may be used for fungal
identification, calcofluor white is a fluorescent dye that
binds to fungal wall and useful for identification of fungi
in tissue specimen or cultures.
Biopsy and histopathology:
A biopsy may be very useful for the identification of
tissue invading fungi. Gomori methenamine silver, H&E
stain or Geimsa stains can used.
DNA probe:
This test is rapid (2 hours) and species – specific. Can
identify colonies growing in culture at earlier stage of
growing than can based on visual detection of colonies
DNA probe are available for Coccidioidomycosis,
blastomycosis, Histoplasmosis and cryptococcosis
Culture:
A definite diagnosis requires a culture. Pathogenic fungi
are usually grown on Sabouraud dextrose agar it has a
slightly acidic pH (5.6).
Cycloheximide, penicillin or other inhibitory substances
are often added to prevent bacterial overgrowth. Two
cultures are inoculated and incubated at 25 degree C and
37 degree C to reveal dimorphism, the cultures examined
macroscopically and microscopically, the appearance of
the mycelium and the nature of a sexual spore are
sufficient for identify of the organism.
.

Unit 3: Mycology
140
Lecture 2+3+4+5 - Fungal diseases
in humans (mycoses)
Fungal diseases are also called Mycoses. Mycoses are
classified as:
1) Superficial.
2) Cutaneous.
3) Subcutaneous.
4) Systemic.
5) Opportunistic.
Depending on the usual portal of entry and
initial site of involvement the Table below
shows the types of Mycoses, their causative
fungal agent and Diseases:-
Type of
Mycosis
Causative fungal agent
Mycosis
Superficial
Malassezia ,Hortaea
werneckii Trichosporon
species Piedraia hortae
Pityriases vericolor
Tinea nigra White
piedra Black piedra
Cutaneous
Microsporum species,
trichophyton species, and
Epidermophyton
floccosum
Candida albicans and other
Candida species
Dermatophytosis
candidiasis of skin,
mucosa or nails
Subcutaneous
Sporothrix schenckii
Phialophora verrucosa,
fonsecaea pedrosei,
others
Pseudallescheria boydii,
madurella mycetomatis,
others
Exophiala, bipolaris,
exserohilum, and others
Sorotrichosis
Chromobalstomy
cosis Mycosis
Phaeophomycosis
Endemic
(primary,
systemic)
Cocidioides immitis,
cposadasii Histoplasma
capsulatum Blastomyces
dermatitidis
Paracoccidioides
brasiliensis
Coccidioidomycos
is
Hhistoplasmosis
Blastomycosis
Paracoccidioidom
ycosis
Opportunistic
Candida albicans and
other Candida species
Cryptococcus neoformans
Aspergillus fumigataus
and other aspergillus
species
Species of Rhizoupus,
absidia, Mucor, and other
zygomycetes
Penicillium mameffei
Systemic
Candidiasis
Cryptococcosis
Aspergillosis
Mucormycosis
(zygomyccosis)
penicilliosis
Superficial mycosis
These are some of the most common infections in
humans; superficial infections of the skin and hair
(pityriasis versicolor. Tinea niger. Black and white
piedras) mainly cause cosmetic problems.
Pityriasis versicolor
Pityriasis versicolor is a chronic mild superficial
infection involves only the superficial keratin layer
(stratum corneum) of the skin caused by Malassezia
furfur. the yeast M.furfur is a common skin
inhabitant. Pityriasis (tinea) versicolor is a common
superficial mycosis, which characterized by discrete
hyper or hypopigmented macules of skin of the neck,
shoulder, chest, upper back arm or abdomen. The
lesions are chronic and may enlarged and coalesce to
from scaling plaque, the lesions are not usually itchy
and in some patients resolve spontaneously. it occur
more frequently in hot, humid weather.
Diagnosis
Specimen: Direct microscopical examination of scrapings
of infected skin treated with 10 - 20% KOH or stained
with Calcofluor white short unbranched hyphae and round
yeast dorm, other lesion also fluoresce under wood lamp.
Culture: malassezia furfur is lipophilic yeast (most require
lipid in the medium) for growth but culture is not is not done
Treatment
Daily application of selenium sulfide.
Topical or oral azoles are effective.
Lesion have tendency to recur and a permanent cure is
difficult to achieve.
Tinea nigra (tinea nigra palmris)
A superficial chronic and asymptomatic infection of the
outermost layer of stratum corneum caused by the
dermatiaceous fungus Hortaea (Exophiala weneckii)
which is found in soil and transmitted during injury.
Pityriasis
versicolor
showing
hyperpigmented
lesions in a
Caucasian and
hyphopigmented
lesions

Unit 3: Mycology
141
More prevalence in warm coastal regions (in USA mainly
in southern states) and among young women.
Tinea nigra most typically presents as a dark brown black
sliver nitrate like stain on the palm of the hands or sole of
the foot.
Diagnosis
Microscopical examination of scrapings of the periphery
of the skin lesions will reveal branched, septate hyphae
and budding yeast cells with melaninized cell wall.
Treatment
Response to keratolytic solution such as salicylic acid or
Azole antifungal drugs.
Piedra
Superficial fungal infection involves the cuticle of the
hair shaft and it is endemic in tropical underdevloped
countries.
Black piedra is a nodular infection of the hair shaft due to
piedra hortae which is manifested by a small form black
nodule involve hair shaft, by comparison, white piedra
due to trichosporon species is characterized by a large
soft, friable yellow nodule of the distal ends of hair shaft
Axillary, pubic scalp may be infected.
Treatment
Removal of the infected hair.
Application of topical antifungal agents.
Cutoeus mycoses
Cutaneous mycoses are caused by fungi that infect only
the superficial keratinized (skin, hair and nails).
The most important of these are dermatophytes that are
classified in three genera: Microsporum, Trichphyton and
Epidermophyton which are the main etiological agent of
the dermatophytosis, such cutaneous mycoses are referred
to as tinea (Latin word for ring worm). These infections
may be characterized by another latin noun according to
the area of the body involved eg. Tinea corporis (body),
tinea capitis (scalp and hair), tinea manum (hand and
finger) , tinea pedis (foot), tinea unguium (nail).
The dermatophytosis is characterized by anatomic site
specificity according to genera. For expmple ,
Epidermphyton floccosum infected only skin & nails
whereas microsporum Spp. Infected hair & skin but do not
involve nails. Trichphyton may infect hair, skin & nails.
Dermatophytosis are among the most prevalent infections
in world. Dermatophytosis are chronic infections favored
by heat and humidity eg: athlete’s foot (Tinea pedis) and
Jock itch (Tinea cruris).
Species of dermatophytes are calssified as anthropophilic
Zoophilic or geophilic depending upon their primary
source (humen, animal or soil). the anthropophilic Spp.
Are the most common causes of dermatophyte infection.
Geophilic are uncommon causes of human disease but are
seen in people who have appropriate exposure such as
gardener and cultural. Dermatophytes are acquired by
contact with contaminated soil or with infected animals or
humans
Identification
Dermatophytes are identified by their colonial appearance
and microscopic morphology after growth for a week at
25C on sabouraud’s dextrose agar..
Epidemiology
The incidence is higher in hot humid climates and under
crowded condition.
The source of infection is soil in case of geophilic
dermatophytes and other source is animal in Clinical
findings
Tinea pedis is the most prevalence of all dermatophytosis,
tinea unguinum: nail infection may follow prolonged tinea
pedis, one or more nails of the feet or hands may be
involved after hyphal invasion when the infection occurs
in the area also called jock itch involve hypersensitivity
reaction called kerion caused by tinea capitis, while
anthrophilic spp. May be transmitted by direct contact or
through fomites such as contaminated towels, clothing
and shared shower staff. Human to human transmittion
require close contact with infected human or animal.
Transmission take place within the family.

Unit 3: Mycology
142
Subcutaneous Mycoses
These are chronic, localized infections of the epidermis
and adjacent connective tissue and lymphatics following
the traumatic implantation of the organism. The causative
fungi are all soll saprophytes whose ability to adapt to the
tissue environment and elicit disease is extremely variable
Subcutaneous sporotrichosis, mycetoma, and
chromoblastomycosis are well characterized subcutaneous
mycoses
As you will see, these are clearly more significant than the
superficial and drmatophycoses
Sporotrichosis
Common name
: ‘Rose Gardener’s Disease’
Etiology
:
Sporothrix schenckii
Sporothrix schenckii is the only dimorphic fungus to
cause subcutaneous mycosis
Epidemiology:
Occurs worldwide and in all age groups, although it is
more common in tropical and subtropical areas
More infections occur in men due to occupational
exposure (foresters, gardeners, horticulturists)
Sporothrix schenckii grows frequently as an environmental
saprobe on woody plants & rich organic soll
They are well known to grow on roses
Most cases are traced to rose thorns, splinters or other
plant materials penetrating the skin
Many recent cases have been traced to sphagnum moss
purchased from commercial suppliers
Clinical manifestations
Most cases are cutaneous (relatively local/shallow &
mild) or lymphocutaneous
Primary “fixed” cutaneous lesions at site of injury are
small papules (colored raised area) most often occurring
on an extremity
Lymphocutaneous: lymph nodes become sequentially
infected as organisms are swept along the lymph channels
Lymph nodes become enlarged, firm, and discolored
(buboes)
Draining sinuses may develop from a lymph node and
terminate in the adjacent skin
One rare occasions disease occurs following when conidia
from environment are inhaled (in particular the recent
cases related to sphagnum moss exposure)
The rare pulmonary cases cause symptoms that range
from bronchitis to tuberculosis-like infections
The most common extracutaneous disorder is
osteoarticular in nature-confined to long bones near joints
Dissemination is rare and limited to
immunocompromised patients: many organs involved
Primary “fixed’
lesions of
cutaneous
sporotrichosis
Lymphocutaneous
Sporotrichosis
Sequential
infection of
lymph node

Unit 3: Mycology
143
Endemic mycoses (Primary or
systemic mycosis)
Four fungi causing systemic mycosis Coccidioidomycosis ,
Histoplasmosis, Blastomycosis & Paracoccidoidomycosis
Characteristic of Endemic Mycoses:-
1) Endemic mycoses is geographically restricted to specific
area of endemicity that why called endemic mycosis.
2) The fungi that cause Coccidioidomycosis and
Histoplasmosis exit in nature in dry soil while the agent of
blastomycosis and paracoccidioidomycosis reside in
nature but their habit are not clearly known.
3) All of these mycoses are caused by a thermally dimorphic
fungus.
4) Most of these infections are initiated in lungs following
inhalation of conidia.
5) Most of these infections are asymptomatic and self –
limiting, only few infections lead to disease which involve
dissemination from lungs to other organs.
6) Infected persons do not communicate these diseases to
others ( non – transmissible ).
7) The initial host defense mechanisms are provided by the
alveolar macrophage for all of these infections.
8) Within endemic areas, 90% or more occur in
immunocompetent individuals but persons with impaired
cellular immunity such as patients with AIDS/ HIV have a
new risk of serious infection.
Coccidioidomycosis
The etiological is
coccidioides immitus.
The infection is endemic in the desert southwestern of
US, Maxico Central and South America.
Coccidoicdomycosis is acquired by inhalation of
arthroconidia of C. immitis.
Approximately 60% of the cases are usually
asymptomatic and self – limited respiratory infection.
The infection may become disseminated to meninges,
bone and skin dissemination occurs most frequently in
person with dark skin.
Coccidioides immitis is a dimorphic fungus but instead of
a yeast phase, a large, distinctive, round walled spherule
is produced in the invasive tissue form, spherule are also
be produced in the laboratory by cultivation, this structure
unique among the pathogenic fungi. Development of
spherule and with production of multiple endospores
within each spherule, the spherule eventually rupture,
releasing 200-300 endospores, each of which can
differentiate into another species.
In alkaline soil and in culture, Coccidioides immitis grow
only as a mold regardless the temperature. Growth
become visible in 2-5 days , the hayphae are septated and
produce thick walled barrel shaped arthroconidia which
are the infection unit in nature and highly infection unit in
nature and highly infection in the laboratory. Spherule has
been produced from arthroconidia in vitro under
specialized condition.
Life cycle Coccydioides immitus
The nature cycle take place in desert climates with modest
rainfull. Hyphae differentiate into arthroconidia which
break loose and may be suspended in the air. Soil disruption
and wind facilltate spread and probably of inhalation into
human lungs. In human host environment in vivo
differentiation produce spherules. The spherules releasing
endospores which can be repeat the in vivo cycle.
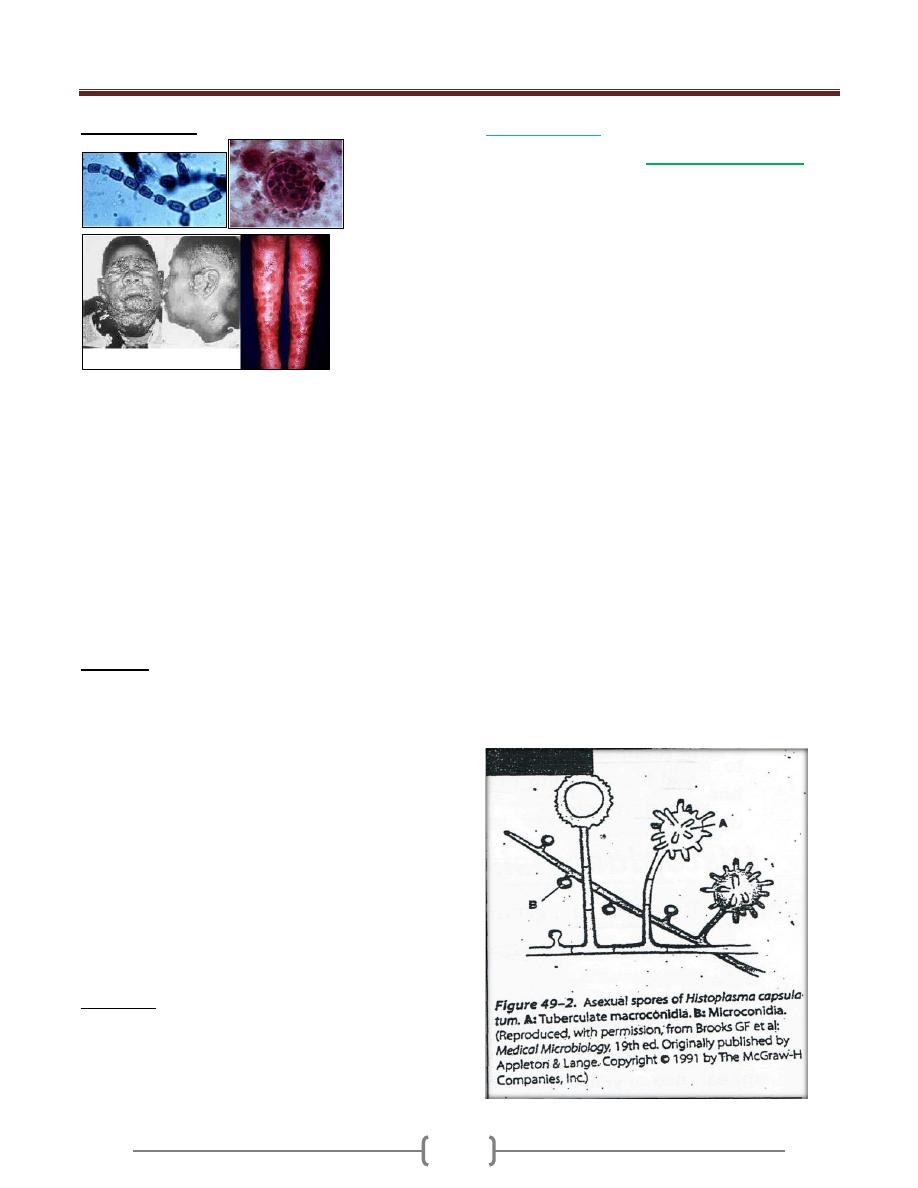
Unit 3: Mycology
144
Clinical findings
Acute primary infection with C.immitis is either a
symptomatic in 60% of cases or present as a complex
called valley fever or desert rheumatism by resident of
endemic areas. Valley fever includes fever, malaise, dry
joint pain & headache after 1-2 weeks. 15% of these
patients may develop rash due to hypersensitivity reaction
There are physical or radiological findings, less than 1%
of persons infected with C. immitis develop secondary or
disseminated Coccidiomycosis ( occur a year after
primary infection) which is ofter a life threatening.
Dissemination disease involve lesions in the bone, joints
or skin. the clinical course is often characterized by
remission and relapses.
Diagnosis
Expectorated sputum, skin & visceral lesions are most
likely to demonstrate spherule by direct examination mixed
with KOH or Calcofluor white while CSF is least likely,
spherules stain well with H& E stain or special stain.
Culture of C. immitis from sputum, visceral lesions or skin
lesions on mold agar, Sabouraud’s dextrose agar, incubated
at room temp. or at 37C Culture must be examined only in a
biological safety cabinet because the culture conidia are
highly infectious. Identification may be confirmed by
animal inoculation use specific DNA probe.
Skin test become positive within 2-4 weeks but is often
negative in patients with disseminated disease.
In serological test 1gM and 1gG precipitin within 2-4
weeks of infection then decline in the subsequent months
and then rise greatly in dissemination occur.
Treatment
In most of person, symptomatic infection is a self –
limited and requires only supportive treatment.
IV Amphteracin B is required in severe disease followed by
several months of oral therapy with itraconazol. Coccidioidal
meningitis have been treated with oral fluconazol.
Histoplasmosis
The causative organism is
histoplasma capsulatum.
Histoplaxmosis is the most prevalence pulmonary
mycotic infection in human and animals.
Hisplasmosis is limited to the endemic area where the
majority of cases are symptomatic or with fever and
cough. H capsulatum received its name from the
appearance of yeast cells in histopathologic section.
In the USA it is endemic in central and eastern states
especially Ohio and Mississippi river.
H. capsulatum a dimorphic fungus that grows in soil and
humid climatic conditions, particularly soil containing
bird or bat droppings. This dimorphic fungi exists as a
mold in soil the mold is hyaline, septate hyphae produce
microconidia and large thick walled macroconidia with
peripheral projections of cell wall material. Microconidia
are small enough to reach the terminal bronchioles and
alveoli and believe to the mode of infection.
Inhaled spores are engulfed by macrophages and develop
into yeast form. In tissue H capsulatum occurs as an oval
budding yeast inside macrophage , the organism spread
widely throughout the body to reticuloendothelial tissues
such as liver, spleen, bone marrow and lymph node but
most of infections remain asymptomatic because of initial
inflammatory reaction as a small granulomatous foci.
Birds themselves do not carry the disease, but their
dropping provides nutrents as a source of nitrogen for
fungus. Bats which have a lower body temperature than
bird carry the fungus, shed it in the feces and probably
infect new soil sites.

Unit 3: Mycology
145
Diagnosis
In tissue biopsies or bone marrow aspirates, oval yeast
cells within macrophages are seen microsocopically after
stain with fungal stain or Geimsa stain for blood bone
marrow smears.
Specimen for culture including sputum, bone marrow
aspirates to be inoculated on sabouraud’s dextrose agar of
inhibitory mold agar at 25 – 30C for 4-12 weeks.
Two serological test are useful for diagnosis; complement
fixation test (CF) and immunodiffusion (ID) test. The ID
test is more specific but less sensitive than the CF test.
Skin test is no useful for diagnosis.
Treatment
Acute pulmonary infection is managed with supportive
therapy and rest.
In mild to moderate infection, itroconazol is the drug of
choice.
Amphotericin B is used for treatment of disseminated
systemic infection.
Prolonged treatment may be needed, relapse may occur.
Blastomycosis
Caused by fungus: blastomyces dermatitidis.
It is a dimorphic fungi with some characteristic similar to
those of H. capsulatum. Growth develop in the yeast
phase in tissue, the yeast are typically larger than those of
H. capsulatum with broad based buds and a thick wall.
The mold phase appears in culture at 25C. Hyphae are
septate and sufficiently similar to the macroconidia
produce by H. capsulatum. Most of the cases occur in the
us and Canada but blastomycosis has been documented in
Africa and Asia.
Clinical findings
Most of clinical features of blastomycosis are similar to
hsitoplasmosis asymptomatic or mild cases are rarely
recognized. Dissemination may result in ulcerated
granuloma on exposed area of the skin, bone or other sites.
Diagnosis
Wet mount of specimen such as sputum,
exudates and biopsies from lesions may
show broady attached buds in thick walled
yeast cells.
Colonies develop on sabouraud’s agar at 30C and
confirmed by detection of B.dermatitids specific antigen
of by specific DNA probe.
Treatment
Amphtericin B are used for sever cases.
Paraconcciioidomycosis
Paracoccidioides braziliensis cause paracoccidiomycosis.
P. braziliensis is a dimorphic fungi that exist as mold in
soil and as a yeast in tissue. The yeasts are larger and have
thinner walls than those of B . dermatiditis and buds are
multiple and attached by narrow connection.
It is endemic in south America
The spores are inhaled and early lesions occur in the
lungs. A symptomatic infection is common alternatively,
oral mucous membrane lesions, lymph node enlargement,
some tissue dissemination may occur.
Diagnosis
In pus or tissue yeast cell with multiple buds are seen
microscopically specimen culture for 2-4 weeks may
grow typical organism. Serology is most useful for
diagnosis, skin test are rarely helpful.
Treatment
Itraconzol is the most effective drug. And trimethoprim
and sulfamethoxazol are also effective.

Unit 3: Mycology
146
Opportunistic Mycosis
Opportunistic Mycosis is a group of fungal infection that
occurs almost exclusively in immune compromised
patients. Patients with compromised host defenses are
susceptible to ubiquitous fungi to which healthy people
are expose but usually resistant many causes type of
fungus and the natural history of the mycotic infection are
determined by the underlying predisposing condition
.Candida and related yeast as member of normal flora are
endogenous opportunists. Other opportunistic mycosis are
caused by exogenous fungi present in soil, water and air.
The type of patient who acquire an opportunistic fungal
infection is one who is compromised by some underlying
disease such as AIDS, Lymphoma, Leukemia, diabetes
also any patients treated with corticosteroid cytotoxic
drugs or other immunosuppressive drugs are exposed to
opportunistic mycosis.
Common etiologic agents are
1) Yeasts
Candida spp. Cryptococcus spp
2) Mycelial or Filamentous Fungi
Aspergillus spp. Zygomycetes, Rhizopus
3) Protozoan-like fungi
Pneumocystis carinii
Candida (Candidiasis)
Candida albicans, the most important sp. of candida
Is an oval yeast with asingle buds, it is a part of the
normal flora in the upper respiratory tract, gastrointestinal
tract, femal genital tracts.
In tissues may appear as yeast or pseudohyphae
Pseudohyphae are elongated yeast that resemble hyphae
but are not true hyphae. Candida species colonize the
mucosal surface of all humans during or soon after birth.
Candidiasis may be caused by:
Candida albicans
C. tropicalis
C. krusei
C. pseudotropicalis
C. albicans var. stellatoida
C. parapsilosis
C. guilliermondii
C. glabrata
Clinical manifestation of Candidiasis
A. Mucocutaneous involvement
1) Oral Candidiasis (thrush)
Appearance of milk curds as they crumble
Common in older people, diet deficiencies. Premature
babies, first sign of clinical AIDS
2) Vaginitis
Predisposing factors
Pregnant women (growth promoted by secretion of
glycogen and progesterone) –obesity –diabetes (high
sugar content in urine) –may be sexually transmitted but
rarely infects the penis (balanitis).
3) Bronchial and pulmonary (not common)
Difficult to diagnose because organism is common in
most chronic lung conditions
4) Alimentary candidiasis
Infection resides in the esophagus, intestine and anus
B. Cutaneous involvement
Often associated with skin that is kept moist and hair
abrasions occur
inter digital between the fingers groin, axillary regions
(under arms),umbilicus, feet and nails• bacteria may be
involved in a secondary invader nail infections
onychomycosis
diaper rash may be caused by a species of Candida
C. Systemic involvement
1) Endocarditis - heart
Predisposing conditions: drug addicts using unclean needles
preexisting valvular disease people treated with antibiotics
intravenous infusion = gets into tubes of machine
2) Urinary Tract
Bladder and kidney included more common in women
than men, yeast can be found in urine with no obvious
infection present

Unit 3: Mycology
147
3) Meningitis relatively rare.
4) Septicemia
In blood and potentially fatal patients often predisposed
through antibiotic therapy or a result of having leukemia
D. Allergic Diseases
Candidiasis- similar to the dermatophytosis reaction
caused by dermatophytes
Eczema- Reddening and itching of skin, may become
crusty and scaly
Gastritis
Cryptococcus
The genus Cryptococcus contain different Spp.,
Cryptococcus neoformans
is considered the most
important human pathogen.Cryptococcus neoformans is
an encapsulated fungus ,this yeast occur widely nature
and found abundantly in dry pigeon feces. C.neoformans
cause cryptococcosis after inhalation of yeast from lung,
these yeast migrate to the CNS but also involve other
organs (skin,eye,prostat).Cryptococcosis is usually
associated with immunocompromized person especially in
AIDS patients.
Morphology and identification
C.neoformans grow at 35C to 37C,. Whitish mucoid is
produced within 2-3 days. The microscopical examination
of colony or clinical materials appears as spherical, single
or multiple budding thicked wall yeast surrounded by
thick unstained capsule. All members of genus are
encapsulated and produce urease by its ability to grow at
37C .There are 5 serotypes of Cneoformans (A-D and
AD), A and D are the most common worldwide.
Pathogenesis
Initial cryptococcal infection begins by inhalation of fungus
into the lung initially followed by hematogenous spread to
the brain meninges .Development of meningitis being the
first indication of the disease. The primary pulmonary
infection may be asymptomatic or mimic flu like influenza
respiratory infection, resolve spontaneously. Involvement
of the eye, skin, prostate are seen. The inflammatory
reaction is minimal & granulomatous.
Diagnosis Lab. test
Specimen: CSF,exudate,sputum,blood and serum. CSF is
centrifuged before microscopical examination & culturing
Microscopical examination: The diagnosis of
Cryptococcus depends on demonstrating the organism or
its capsule in tissue or body fliud The specimen mixed
with India Ink,the yeast cell is seen microscopically by a
wide, unstained capsule .Demontsration of the pathogen
on an India Ink preparation is pathognomonic for
C.neoformans.
Culture: The organism can be cultured from CSF or other
specimen on most fungal media at room temperature or
37C. The diagnosis ofthis organism is confirmed by
urease test
Serology: test for capsular antigen can be performed on
CSF or serum.
The latex slide agglutination test for cryptococcal antigen
is positive in 90% of patients with cryptococcal
meningitis.
Treatment and prevention
Amphotericin B with or without flucytocin is curative in
most of the cases of meningitis or disseminated disease.
There is no specific mean of prevention.Fluconazol is
used in AIDS patients for long term suppression of
cryptococcal meningitis.
Epidemiology
Birds (pigeons) are considered to serve as reservoir of
infection but the birds are not infected .The organisms are
transmitted by respiratory droplets
Aspergillosis
Aspergillosis is a spectrum of human disease that may be
caused by Aspergillus Spp .these species are saprophytic
organism and widely distributed in nature and found
throughout the world .The most common human pathogen
is Aspergillus fumigatus,and to lesser extent A.niger,
A.ilayus and A.terreus.
This mold produce abundant conidia that easily dispersed
into environment Human become infected by inhaling
them. A topic individual may develop severe allergic
reaction to the conidial antigen. The conidia may
germinate to produce hyphae and invade the lung or other
tissue ,these may occur in immunocompromized patients
but also causing invasive lung infection including external
otomycosis ,mycotic keratitis, OnychomycosiS, Sinusitis
and CNS infection.
Morphology and Identification
Aspergillus spp. Exist only as mold,they are not
dimorphic ,these Spp. Are grow rapidly producing aerial
septate hyphae that form V shaped branches and long
conidiophore expand in vesicle and phialides are
produced directly from the vesicle surface and phialides

Unit 3: Mycology
148
produce besipetal chain of conidia .The Spp, Are
identified according to morphologic differences in these
structure including the size, Shape, texture and color of
conidia.
Aspergillus
Moulds
True hyphae
Exogenous, airborne
o Soil
o Water / storage tanks in hospitals etc
o Food
o Compost and decaying vegetation
o Fire proofing materials
o Bedding, pillows
o Ventilation and air conditioning systems
o Computer fans
Portal of entry: nasal passages, respiratory tract
Potential for hospital outbreaks
Pathogenesis
In the lung, alveolar macrophage are able to engulf and
destroy conidia,in immune compromized patients
especially leukemia ,bone marrow transplant the conidia
may smell and germinate to produce hyphae that invade
the preexisting cavities (Aspergilloma or fungal ball) or
blood vessel.
Clinical findings
A. Allergic form A topic individual often develop severe
allergic reaction to conidial antigen elicits an immediate
asthmatic reaction and after subsequent exposure in
another hand the conidia germinate and hyphae colonize
the bronchial tree without invading the lung parenchyma
,this phenomena is characteristic allergic
bronchpulmonary aspergillosis presented with asthma,
chest infiltration ,eosinophilia with type one and type
three skin test hypersensitivity
B. Aspergilloma and Extra pulmonary colonization:
Patients who have already a chronic pulmonary disease
(TB and Sarcoidosis) Fungus ball called aspergilloma
within a preexisting cavity.
C. Invasive Aspergillosis
Following inhalation and germination of conidia,invasive
disease develop as an acute pneumonic process
characterized by fever, cough ,hemoptysis from the lung,
the disease may spread to the GIT ,kidney, liver ,brain or
other organ producing abscess and necrotic lesion even
when hyphae invade the wall of the blood vessel. Patients
at risk are those with lymphoma and leukemia.
Diagnostic Lab. Test
A. Specimen: sputum or other respiratory secretions or lung
biopsy.
B. Microscopical examination: Sputum treated with KOH
or Calcofuor white should examined directly under
microscope show septate,branching hyaline hyphae.
C. Culture: Culture show colonies which differ according to
the morphology of their conidial structure when grow
within few days on media at room temp.
D. Serology:-The Intradermal test for precipitin to
A.fumigatus is positive in over 80% of patients with
Aspergillosis or allergic form of Aspergillosis.
Treatment
Aspergilloma treated with Itraconazol with Amphotericin
B and surgery Invasion Aspergillosis require rapid
adminstration of Amphotericin B,vericonazol,flucytocin
also used.Allergic form treated with corticosteriod or
sodium cromoglycate.
Epidemiology:
Avoid exposure to the conidia in person at risk for allergic
disease leukemic; lemphoma patients with bone marrow
transplant should minimize their risk of exposure to conidia

Unit 3: Mycology
149
Lecture 6 - Antifungal Chemotherapy
Compared with antibacterial agents, relatively few antimicrobics are
available for treatment of fungal mfection, in addition to that mere are
limitations in use of antibiotic such as profound side effects, a
narrow antifungal spectrum, poor penetration of certain tissues and
the selection of resistant fungi.
New azoles (has significantly highest antifungal activity and lowest
toxicity to the host). Fortunately most of fungal infections are self-
limiting and require no treatment .superficial mycosis are often
treated but topical therapy can be used ,thus limited toxicity to the
host .The remain small group of deep or systemic mycosis that are
uncontrolled by the host immune system require prolonged use of
relatively toxic antifungals.
Amphotericin B
The major polyeneantibiotic, Amphtericin B is the most effective
drugs for systemic mycoses, it has a broad spectrum.
Mechanism of action
Amphotericin B binds the ergosterol component of the fungal cell
membrane and alters the selection of the permeability of this
membrane, mammalian cell lack ergosterol and are relatively
resistant to this drug.
Indication
In Gryptococcosis, candidiasis, Histoplasmosis & sporotrichosis.
Amphtericin B is used in combination with flucytocin to treat
Cryptococcosis.
Is fluorinated derivative of cytocin, it penetrate well into all tissue, it
is oral antifungal component
Azoles
The azoles groups of antifungal agents consist of the
imidazol (eg.Ketoconazol
9
Clotrimazol or Miconazol) or
Triazol (eg. Fluconazol, itraconazol, orVoriconazole
Xmidazzol (Miconazol or Clotrimazol) are used topically while others
are used orally or IV.
Clotrimazol and Miconazol
Are available for topical and intravaginal (suppositories or
creams) applications.they are useful in mild case of
dermatophytosis (Tineapedis,Tineacruris ,Tineacorporis)
Tineaversicolor and cutaneous candidiasis vulvovaginal candidiasis
can be treated with suppositories Or creams.
Clotrimazol is effective against oral or esophageal thrush in
immunocomromized patients.
Fluconazol
is given either orally or IV Fluconazol has an
excellent activity against both Candida Spp. and Cneoformans and
oropharyngeal candidiasis in AIDS patients and candedemia in
^immunocompetentpatients.this drug is the best in this group in
penetration of the CNS hence, can be used as maintenance therapy for
Cryptococcal or Cooccidioidal meningitis,
Itraconazol
is now the agent of first choice for
Histoplasmosis, Paracoccidioidomycosis and Aspergillosisalso
effective in Sporotichosis ^Cryptococcosis and Onychomycosis in
immunocompetent and AIDS patientsltraconazol or fluconazol or
terbinafen may be used intermittently in case of long term therapy
of Tineaunguium.
Vericonazol
which can be given either orally or IV and active
against Aspergillosis or Pseudallescheriasis. Side effect
Adverse effect of
Ketoconazol include transient elevation of liver enzymeSjnausea
,irreversiblegynecomastia, decreaselipido and oligospermia in males
and menstrual irregularity.it is the most toxic agent in this group.
Clotrimazol and Miconazol side effect include burn, itching and/or
skin irritation.
Adverse effect of Itraconazol consisting of GIT disturbences,
vestibular disturbences,odema or skin irritation.
Vericonazol causes irreversible visual impairment in about 30% of
patients.
Griseofolvin
Griseofulvin is an antifungal antibiotics produce by Spp. of
pencillium.Griseofulvin is an oral agent poorly absorbed and
concentrate on the stratum corneunvt is inhibit hyphal growth.
Side effect Headache, GIT disturbances ^photosensitivity,
drowsiness and hepatotoxicity.
Indication: Useful for treatment of Dermatophyte infection of the
skin, hail and nail,OraI therapy for weeks or months are required.
Nystatin
It is a polyene antibiotics produced by StreptomyceSjStructurally
related to Amphotericin B»it is not absorbed by GIT, it is too toxic
for parentraladministration.
It is used locally to treat oral or vulvovaginalcandidiasis ,also
suppress esophageal candidiasis and gastrointestinal overgrowth
with no side effect. Other fungal agent
Echinocandins
Echinocandins are a new class of antifungal agents»their mode of
action by disturbing cell wall integrity, the recently licensed drug
Caspofungin has shown efficacy against invasive Aspergilosis and
Systemic candidiasis, Two newly approved
ecynocandms»micafunginand antdulafungJn,have similar activity

Unit 3: Mycology
150
against Spp. of Candida and Aspergillus, also used for
esophageal candidiasis.
Terbinafine
Is an allylamin drug, it is block ergosterol synthesis
Indications: is given orally for treating Dermatophytic infections
of nails and hair.
Side effects are not common but include GI disturbancesj,
headache, and loss of sense of taste.
Tolnaftate and naftiftne
Are topical antifungasl agents used in treatment of many
dermatophyticinfections and Tineaversicolor, available as
creams, powders and sprays Selenium sulfide
Selenium sulfide shampoo effective against Malazzesiafurfur. the
causative agents of Tineaversicolor, Undecylenic acid
Is available in several formulations for treatment of Tineacruris and
Tineapedis.
Hypersensitivity to fungi
Throughout life, the respiratory tract is exposed to airborne conidia
and spores of many saprophytic fungi. These particles often possess
potent surface antigens capable of stimulating strong allergic
reactions. These hypersensitivity responses do not require growth
of inducing fungi.
Depending on the site of deposition of the allergen, a patient may
exhibit rhmitis,bronchialasthma,alveolitis or pneumonitis .A topic
persons are more susceptible.
The diagnosis can be determined by skin testing with fungal
extracts-Management may include avoidance of the offending
allergens, treatment with corticosteroids or attempts to desensitize
patients.
Indoor air exposure to large number of fungal spores has led to the
recognition of a condition described as sick building syndrome
whereby moisture in construction materials such as wood allow
contaminating mold to flourish this resulting in debilitating cases
of systemic allergic or toxic reactions.

151

Unit 4: Virology
251
Lecture 1+2+3 - Introduction
Virus (Virion)
It is the smallest infectious agents and contains one kind
of nucleic acid as their genome (DNA or RNA). Size
range from (20nm-300nm in diameter). Viruses replicates
only inside the living cells of other organisms and can
infect all types of life forms, from animals and plants to
bacteria and archaea
I-Viral mucleic acid
The viral nucleic acid is located internally and can be
either double or single stranded DNA or RNA.
The nucleic acid is either circular or linear.
The DNA is always a single molecule (double or single);
RNA can exist either as a single molecule or in several
pieces.
Almost all viruses contain only a single copy of their
genome (haploid) but retroviruses are the exception (RNA
genome of two copies (Diploid)).
II-Viral capsid
The capsid is the “shell” of virus-coded protein that
encloses the nucleic acid and is more or less closely
associated with it. The combination of these two
components (Capsid and NA) is often termed the
nucleocapsid, especially if they are closely associated as
in the myxoviruses.
The capsid is made up of subunits, the capsomers, the
number of which varies but is specific and constant for
each viral species. These (capsomeres) are spherical or
cylindrical structures composed of several polypeptides.
The capsid protects the nucleic acid from degradation. In
all except enveloped viruses, it is responsible for the
attachment of the viruses to the host cell.
Viral Symmetry
The arrangement of capsomers gives the virus structure
and its geometric symmetry. Viral nucleocapsids have
two forms of symmetry:
1) Icosahedral:
(polyhedrons with 20 equilateral triangular
faces)
in which the capsomers are arrangred in 20
triangles that form a symmetric figure with approximate
outline of the sphere.
2) Helical: in which the capsomers are arranged in a hollow
coil that that appears rod-shaped.
All human viruses that have a helical nucleocapsid are
enclosed by an outer membrane called an envelope while
icosaheral nucleocapsid can be either enveloped or non-
enveloped (naked).
3) Complex symmetry. Complex structural patterns are
found in bacteriophages and the smallpox virus.
III- Viral proteins
Structural proteins of viruses have several important
functions:
1) Facilitate transfer of the viral nucleic acid from one host
cell to another.
2) Protect viral genome against inactivation by nucleases
3) Participate in the attachment of the virus particle to
susceptible cell.
4) Provide the structural symmetry of the virus particle.
5) Determine the antigenic characteristics of the virus.
There are frequently glycoproteins in the form of spike
like projections on the surface, which attach to host cell
receptors during the entry of the virus into the cell.
Another protein is the Matrix protein mediates the
interaction between the capsid protein and the envelope.
Non-Structural proteins
Some viruses carry enzymes inside the virions which are
essential for the initiation of the viral replicative cycle
when the virus enter host cell e.g.: a) RNA polymerase
which is carried by negative sense RNA virus that is
needed to copy the first mRNA. b) reverse transcriptase in
retroviruses that makes a DNA copy of the viral RNA.
Some viruses contain regulatory proteins in the virion in a
structure called the tegument which is located between
nucleocapsid and the envelope. These regulatory proteins
include transcription and translation factors that control
either viral or cellular processes. Herpes simplex and
cytomegalovirus have well characteristics tegument.

Unit 4: Virology
251
IV-Viral envelope
It is a lipoprotein membrane composed of lipid derived
from the host cell membrane and protein that is virus
specific.
Enveloped viruses are more sensitive to heat, ether,
detergents, and dryness. They are transmitted by direct
contact.
Non-enveloped virus (naked) are more stable and
transmitted by indirect contact.
Classification of viruses
The taxonomic system used for viruses is artificial (i.e., it
does not reflect virus evolution) and is based on the
following morphological and biochemical criteria:
1) Genome: DNA or RNA genome (important basic
differentiation of virus types!) as well as configuration of
nucleic acid structure: single-stranded (ss) or double-
stranded (ds); RNA viruses are further subclassified
according to plus and minus polarity.
2) Capsid symmetry: cubic, helical, or complex symmetry.
3) Presence or absence of an envelope.
4) Diameter of the virion, or of the nucleocapsid with
helical symmetry.
Atypical virus –like agents
1) Defective viruses: are viruses composed of viral N.A and
proteins but cannot replicate without a "helper" virus,
which provides the missing function. There is usually a
mutation or deletion of a part of their genetic material
2) Pseudovirions: contain host cell DNA instead of viral
DNA within the capsid.they are formed during infection
with certain viruses when host cell DNA is fragmented
and pieces of it are incorporated within the capsid protein.
3) Viriods: single molecule of circular RNA without a
protein coat or envelope.
4) Prions: are infectious particles that are composed solely
of protein. They are implicated as the cause of certain
slow disease which called transmissible spongiform
encephalopathies.
Viral replication
The growth curve in figure 1 shows that first event is
disappearance of the virus as represented by the solid line
dropping to the x axis. Although the virus particle is no
longer present, the viral nucleic acid continues to function
and begins to accumulate within the cell. The time during
which no virus is found inside the cell is known as the
Eclipse period. It ends with the appearance of the virus
(solid line). The latent period is defined as the time from
the onset of infection to the appearance of the virus
extracellularly. At the end of latent period, alterations of
cell morophology accompanied by marked derangement
of cell function; this is called cytopathic effect (CPE)
which culminates in the lysis and death of cells. These
CPE can be seen under the light microscope.
Figure 1: Viral growth curve.
Specific events during the growth cycle
I-Early events ( attachment, penetration,
&uncoating)
The proteins on the surface of the virion attach to specific
receptor proteins on the cell surface through weak non-
covalent bonding. The specifity of the attachment
determines the host range of the virus.
The virus particle penetrates by being engulfed in a
pinocytic vesicle, within which the process of uncoating
begins.
The receptors for viruses on the cell surface are proteins
that have other functions in the life of the cell.
NOTES:
1- Infectious nucleic acid is purified viral RNA or DNA
(without any protein) that can carry out the entire viral
growth cycle & result in the production of complete virus
particle.
2- All viruses are "infectious" but not all purified genomes
are infectious.
II- Middle events (Gene Expression and Genome
Replication)
The first step in viral gene expression is mRNA synthesis.
It is at this point that viruses follow different pathways
depending on the nature of their nucleic acid and the part
of the cell in which they replicate.

Unit 4: Virology
251
DNA viruses: Most have both positive and negative
strand (Ds) (except parvovirus has ssDNA), but only +
strand is read.
All DNA Viruses replicate in the nucleus except
poxvirus which has its own RNA polymerase to replicate
in the cytosol
Retrovirus: Carries reverse transcriptase, which
converts RNA to DNA in the nucleus, inserting DNA
copy of genome into host cell DNA then using host
transcription to mRNA.
Positive ssRNA viruses (e.g. poliovirus): Use RNA
directly as mRNA to begin translation immediately in the
cytosol
Negative ssRNA viruses: Must be transcribed to +ssRNA
before being translated. Uses own RNA-dependent RNA
polymerase to do transcription in the cytosol (except
Influenza in nucleus).
Single RNA and segmented RNA are both in this
category, though segmented RNA viruses may be
ambisense (some -, some +ssRNA)
Double-stranded RNA: Carries its own RNA-dependent
RNA polymerase to translate into mRNA in cytosol (+
strand hydrogen bonded to - strand so cannot be used as
mRNA). Reoviruses
Early proteins are
enzymes
required for replication of
viral genome (e.g. Hepadnavirsus produce reverse
transcriptase to regenerate viral DNA from RNA
intermediate).
Most viruses make a virus-encoded polymerase
(
replicase )to make copies of genome, although some use
host cell polymerases for this
Late proteins
: structural proteins for progeny viruses,
often including precursor polypeptides and virus-coded
proteases to cleave these polypeptides into final
capsomer products (e.g. picornaviruses have single
polypeptide that has intrinsic protease, retroviruses)
III- Late events (Assembly and Release)
The progeny particles are assembled by packing the viral
nucleic acid within the capsid proteins.
Un enveloped viruses are released by cell rupture.
Enveloped viruses are released by budding through the
outer cell membrane Except Herpes virus by budding
from the nuclear membrane.
Lysogeny
Is the process by which viral DNA becomes integrated
into host cell DNA, replication stops and no progeny virus
is made. Later if DNA is damaged by for example by UV
light, viral DNA is excised from the host cell DNA , and
progeny viruses are made. The integrated viral DNA is
called a Prophage. Bacterial cells carrying a prophage
can acquire a new trait, such as the ability to produce
exotoxins such as Diphtheria toxin.
Lysogenic conversion is the term used to indicate that the
cell has acquired a new trait as a result of the integrated
prophage. Lysogenic conversion is mediated by
transduction ( transfer of gene from one bacterium to
another by viruses).
Relationship of lysogeny in bacteria to latency in
human
Members of the Herpesvirus family, such as herpes
simplex virus (HSV), Varicella zoster virus (VZV),
Cytomegalovirus (CMV), and Epstein-Barr virus (EBV),
exhibit a latency – the phenomenon in which no or very
little virus is produced after the initial infection but at
some later time, reactivation and full virus replication
occur.
How Herpesvirus initiate and maintain the latent state?
Shortly after the virus infects neurons, a set of "Latency-
associated transcripts" (LATS) are synthesized. These
are non – coding, regulatory RNAs that suppress viral
replication. The precise mechanism by which they do so
is unclear. Reactivation of viral replication at a later time
occurs when the genes encoding LATS are excised.
CMV employs different mechanisms. The CMV genome
encode microRNAs that inhibit the translation of mRNAs
required for viral replication. Also, the CMV genome
encodes both a protein and an RNA that inhibits
apoptosis. This allows the infected cell to survive.
Virus genetics
Mutation
Mutations are changes in the base sequence of a nucleic
acid (base substitution, deletion and frame shift), resulting
in a more or less radical alteration of the resulting protein.
Medically important are mutant virus with weakened
virulence that have retained their antigenicity and
replication capabilities intact. These are known as
“attenuated” viruses. They are the raw material of live
vaccines.
Interaction between viruses
When two genetically distinct viruses infect a cell, three
different phenomena can ensue:
1) Recombination
Is the exchange of genes between two chromosomes that
is based on crossing over within regions of significant

Unit 4: Virology
255
base sequence homology. Recombination can readily be
demonstrated for viruses with DS DNA as well as by
RNA virus but with a very low frequency.
In case of segmented genome virus,
Reassortment
occurs
when an exchange between segments occur.
2) Complementation :
Refers to interaction of viral gene products in cells
infected with two viruses, one or both of which may be
defective. It results in the replication of one or both under
conditions in which replication would not ordinarily
occur. One of the virus provides a gene product in which
the second is defective, allowing the second virus to grow.
The genotype of the two viruses remain unchanged. if
both mutants are defective in the same gene product, they
will not be able to complement each others growth.
3) Phenotypic mixing
The genome of virus type A can be coated with the
surface proteins of virus type B. This phenotypically virus
can infect cells as determined by its type B protein coat
but the progeny virus from this infection has a type A coat
Pseudotypes, which consist of the nucleocapsid of one
virus and the envelope of another e.g nucleocapsid of
vesicular stomatitis virus and the envelope of HIV are
currently being used in study immune response to HIV.
D- Interference
Infection of either cell cultures or whole animals with two
viruses often leads to an inhibition of multiplication of
one of the viruses.
Several mechanisms as a cause of interference:
1) One virus may inhibit the ability of the second to adsorb
to the cell.
2) One virus may compete for the second for the component
of the replication apparatus.
3) The first virus may cause the infected cell to produce an
inhibitor that prevents replication of the second virus.
Viral pathogenesis
The ability of viruses to cause disease can be viewed on
two distinct levels: (1) the changes that occur within
individual cells (2) the process that takes place in the
infected patient.
The infected cells
Four main effects of virus infection on the cell
1) death
2) Fusion of the cells to form multinucleated giant cells
or inclusion bodies (discrete areas containing viral
proteins or viral particles and may be intracytoplasmic
or intranuclear).
3) Malignant transformation.
4) No apparent morphologic or functional changes.
The infected patient
Pathogenesis in the infected patient involves:
1) Transmission of virus and its entry into the host.
2) Replication of the virus.
3) Spread of the virus to other cells and organs.
4) Immune response.
5) Persistence of the virus.
Transmission of virus and its entry into the host.
Transmission. Viruses can be transmitted horizontally
(within a group of individuals or vertically (from mother
to offspring). Vertical infection is either transovarial or by
infection of the virus in utero (ascending or diaplacental).
Portal of entry. The most important portals of entry for
viruses are themucosa of the respiratory and
gastrointestinal tracts. Intact epidermispresents a barrier to
viruses, which can, however, be overcome through
microtraumata (nearly always present) or mechanical
inoculation (e.g., bloodsucking arthropods).
Viral dissemination in the organism.
There are two forms of infection:
Local infection. In this form of infection, the viruses
spread only from cell to cell. The infection and manifest

Unit 4: Virology
251
disease are thus restricted to the tissues in the immediate
vicinity of the portal of entry. Example: rhinoviruses that
reproduce only in the cells of the upper respiratory tract.
Generalized infection. In this type, the viruses usually
replicate to some extent at the portal of entry and are then
disseminated via the lymph ducts or bloodstream and
reach their target organ either directly or after infecting a
further organ. When the target organ is reached, viral
replication and the resulting cell destruction become so
widespread that clinical symptoms develop. Examples of
such infection courses are seen with enteroviruses that
replicate mainly in the intestinal epithelium, but cause no
symptoms there.
Clinical symptoms in these infections first arise in the
target organs such as the CNS (polioviruses, echoviruses)
or musculature (coxsackie viruses).
Another mode of viral dissemination in the
macroorganism is neurogenic spread along the nerve
tracts, from the portal of entry to the CNS (rabies), or in
the opposite direction from the ganglions where the
viruses persist in a latent state to the target organ (herpes
simplex).
Virus excretion
Excretion of newly produced viruses depends on the
localization of viral replication. For example, viruses that
infect the respiratory tract are excreted in expired air
(droplet infection).
In generalized infections not only the target organ is
involved in excretion, but that primary viral replication at
the portal of entry also contributes to virus excretion (for
example enteroviruses, which replicate primarily in the
intestinal wall and are excreted in feces).
It is important to know, patients are contagious before
they really become ill because excretion of new virus
progeny precede the onset of illness.
Persistent viral infections
In most viral infections, the virus doesn’t remain in the
body for a significant period after clinical recovery, but in
certain instances, the virus persists for long periods either
intact or in the form of subviral component (e.g.: the
genome).
There are three types of persistent viral infections:
1) Chronic carrier infection
: refers to people who produce
virus for long periods of time and can serve as a source of
infection for others.
2) Latent infections
: are those infections that are not
producing virus at the present time but can be reactivated
at subsequent time e.g latent infections that are associated
with herpes simplex virus infection.
3) Slow virus infections
: refer to those diseases with a long
incubation period often in years. Some are caused by
virus (progressive multifocal leukoencephalopathy) ,
whereas others are caused by prions ( Creutzfeldt-Jakob
disease).
Evasion of host defenses:
Viruses have several ways to evade host defense:
1) Some viruses encode the receptors for various mediators
of immunity e,g: Vaccina virus encode protein that bind
to IL-1. Fibroma virus encodes protein that binds to TNF.
These virus encoded proteins called Cytokine decoy.
2) Human Iimmunodeficiency V, Herpes v, CMV reduce
Class I MHC expression.
3) HIV,Epstein Barre V,Adeno v. synthesis RNAs that block
phosphrylation of an initiation factor (eIF2) which reduce
ability of INF to block viral replication
4) CMV encodes a micro RNA binds to mRNA of a cell
surface ligand for NK cell.
5) Measles block IL-12.
6) virus has multiple antigenic type
e.g Rhinovirus (100 serotype).
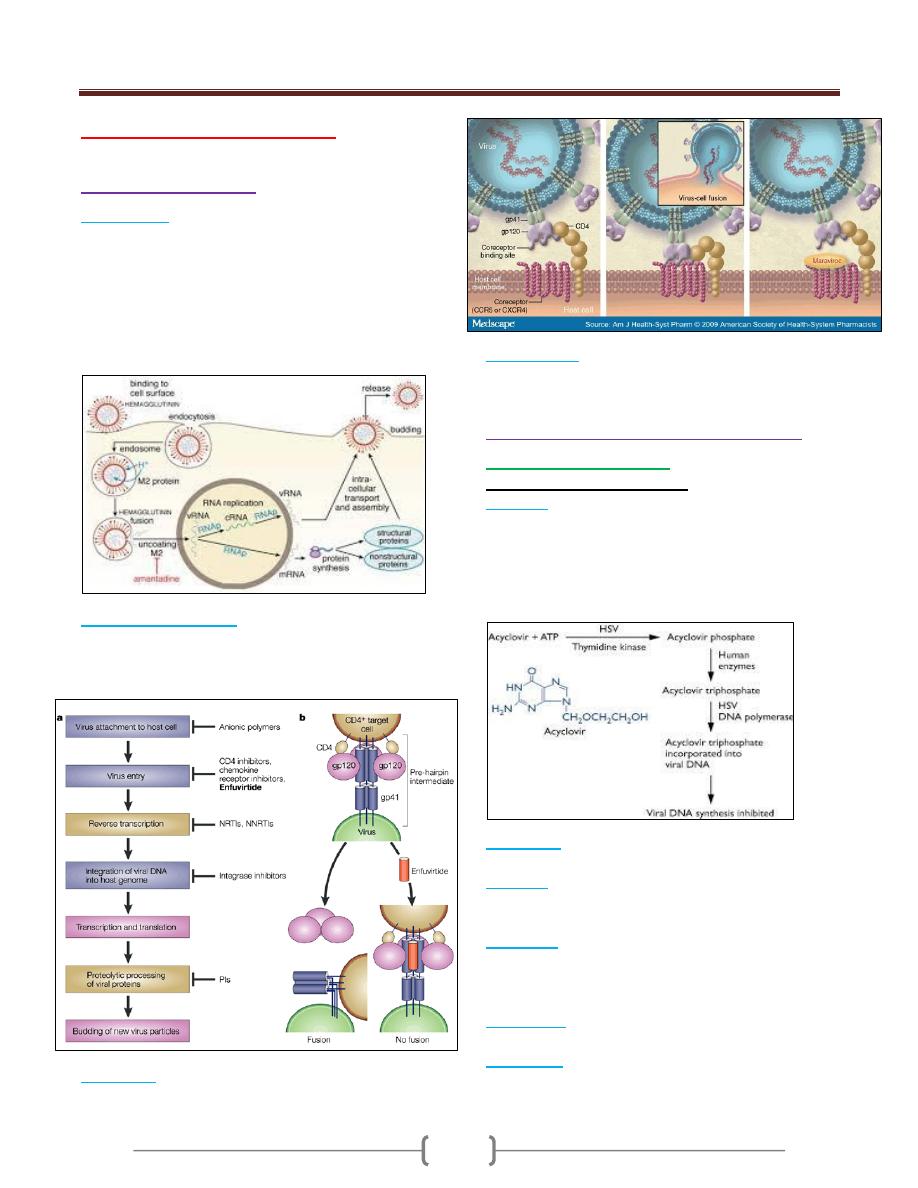
Unit 4: Virology
251
Lecture 4 - Antiviral Drugs
I- Early events inhibitors
1)
Amantidine
:
It inhibits uncoating of the virus by blocking the "ion
channel" activitiy of the matrix protein (M2 protein) in
the virion as shown in the figure below. This drug
specificallyinhibits influenza A but not C and B types. It
has CNS side effects.
Rimantidine is similar to amantidine but with fewer side
effects.
2)
Enfuvirtide ( Fuzeon)
:
It is a synthetic peptide that binds to gb41 on the surface
of HIV, thereby blocking the entry of the virus into the
cell. It is a fusion inhibitor. As shown in the figure below
3)
Maraviroc
it blocks the binding of HIV to CCR-5 as
shown in the figure below.
4)
Palivivumab
:
it is monoclonal antibody directed against
the fusion protein of respiratory syncytial virus (RSV).
II-
II- Inhibitors of Viral nucleic acid inhibitors
A. Inhibitors of herpes virus.
Nucleoside analogue inhibitors:
1) Acyclovir
: it acts against HSV1 and -2 and Varicella
zoster virus (VZV). It is a guanosine analogue. As shown
in the figure below.
Derivatives of acyclovir are (Valacyclovir, penciclovir,
and Famciclovir).
These drugs have no effects on latency.
2) Ganciclovir
it is more active against Cytomegalovirus.it
is a guanosine analogue.
3) Cidofovir
: used in the treatment of retinitis caused by
CMV. It is a nucleoside analogue of cytosine that lacks a
ribose ring.
4) Vidarabine
: treatment of encephalitis and keratitis
caused by HSV-1 but less effective than acyclovir. It is a
nucleoside analogue with arabinose in place of the normal
sugar, ribose.
5) Idoxurudine
: it is effective in the treatment of
keratocnjuctivitis caused by HSV-1.
6) Trifluridine
: it is used topically for treatment of
keratoconjuctivitis because it is too toxic for systemic use.

Unit 4: Virology
251
Nonnucleoside inhibitors.
1) Foscarnet:
it is a pyrophosphate analogue. It binds to
DNA polymerase at the pyropghosphate cleavage site and
prevents removal of the phosphate from nucleoside
triphosphate(dNTP). This inhibits the addition of next
dNTP . the drug inhibits DNA polymerase of all
herpesviruses especially HSV and CMV.
B. Inhibitors of retroviruses.
Nucleoside inhibitors
1) Zidovudine (AZT,Retrovit, azidothymidine):
It does not require a virus-encoded kinase to be
phosporylated, CK phosphorylate the drug, it is active in
both infected and non-infected cells.it cause chain
termination As shown in the figure below.
2)
Others:
didanosine (ddI), lamivudine
(3TC), stavudine (d4T), abacavir and
Emtricitabine (FTC)
. ) .(لالطالع فقط
Nonnucleoside inhibitors (NNRTI):
It doesn’t cause chain termination but it is binds near the
active site of reverse transcriptase and induce
conformational changes that inhibits synthesis of viral
DNA. As shown in the figure below.
E.g., in drug-naïve patients (nevirapine and efavirenz)
In drug-experienced patient (etravirine).
C. inhibitors of hepatitis B virus:
i. Adefovir
: nucleoside analogue of adenosine
monophosphate. it inhibits DNA polymerase of Hepatitis
B virus. it is useful in the treatment of chronic hepatitis B
infection.
ii. Entecavir
: it is a guanosine analogue.
iii. Lamivudine
iv. Telbivudine
: it is a thymidine analogue. it is useful in the
treatment of chronic hepatitis B infection.
D. inhibitors of other viruses.
v. Ribavirin
: it is a nucleoside analogue.
It inhibits the synthesis of guanine nucleotides.
It is used in the treatment of pneumonitis caused by
respiratory syncytial virus in infants and to treat severe
influenza B infection.
It is also used in combination with alpha-interferon for the
treatment of hepatitis C.
III. Inhibition of integrase:
Raltegravir
is an integrase inhibitors used in HIV
infection.as shown in figure below.
IV. Inhibition of cleavage of precursor
polypeptides (protease inhibitors).
A. Inhibition of HIV.
e.g.,
saquinavir followed by indinavir, ritonavir,
lopinavir and tipranavir; more recently, atazanavir,
fosamprenavir and darunavir
have become available.
(االسماء لالطالع فقط
)
B. inhibitors of Hepatitis C virus:
Boceprevir and telaprevir
used in HCV.

Unit 4: Virology
251
V- Inhibitors of viral protein synthesis:
1) Interferons
2) Fomivirsen
: it is an antisense DNA that blocks the
replication of CMV ( antisense is a single –stranded DNA
that has a sequence complemantory to that of viral
mRNA. it is the only antisense that is approved to be used
in treatment of human disease.
3) Methisazone
: it inhibits the protein synthesis of
poxviruses, by blocking the translation of late mRNA.
VI- Inhibition of release of virus:
1- Zanamivir (Relenza) and oseltamivir (Tamiflu)
inhibits the neuraminidase of influenza virus. The drug is
effective against both influenza A and B viruses. These
drugs are effective only against strains of influenza virus
resistant to amantidine.

Unit 4: Virology
211
Lecture 5+6+ Half 7 - RNA
Enveloped viruses
Orthomyxoviruses
Influenza viruses
Influenza viruses are the only members of the
orthomyxovirus family .The orthomyxovirus has a
segmented RNA genome and the replication occurs in the
nucleus of infected cell. .
The term Myxo refers to the observation that these viruses
interact with mucins (glycoprotein on the cell surface).
Important properties
Three immunologic types of influenza viruses are known,
A, B, and C.
Influenza virus particles contain nine different structural
proteins.
The nucleoprotein (NP) associate with the viral RNA,
which is SS, segmented with negative polarity, to form a
ribonucleoprotein (RNP) structure with helical symmetry.
Three large proteins (PB1, PB2, and PA) are bound to
the viral RNP and are responsible for RNA transcription
and replication.
The Matrix (M1) protein is a major compoinent of the
virion.
The outer lipoprotein envelope is covered with two
different spikes, a haemagglutinin (HA) and a
neuraminidase (NA).
The function of the hemagglutinin is to bind to the cell
surface receptor (sialic acid) to initiate infection. In the
laboratory, the Hemaglutinin agglutinate red blood cells,
which is the basis of diagnostic test called the
haemagglutinin inhibition test. The hemagglutinin is also
the target of neutralizing antibody.
HA is a trimer , composed of 3 intertwined HA1 and HA2
dimmers. Cellular protease cleaves HA into HA1 HA2 ,
this cleavage is important for virus infectivity.
The neuraminidase cleaves neuraminic acid ( sialic acid to
release progeny virus from the infected cell. The
hemagglutinins function at the beginning of the infection
whole the neuraminidase at at the end. Neuraminidase
also degrades the protective layer of mucus in the
respiratory tract. NA is tetramer, composed of four
identical monomers.
Influenza viruses have both groups –specific and type-
specific antigens
1) The internal RNP is the group specific antigen that
distinguishes influenza A, B, and C viruses.
2) The hemagglutinin and the neuraminidase are the type-
specific antigens located on the surface. Antibody against
the hemagglutinin neutralize the infectivity, whereas
antibody against the group-specific antigen doesn’t.
Antibody against the NA doesn’t neutralize infectivity but
does reduce disease by decreasing the amount of virus
released from the infected cell and thus reducing spread.
Classification and Nomenclature:
1) Genus influenzavirus A contains human and animal strain
of influenza type A.
2) Genus influenza virus B contains human strain of
influenza type B.
3) Genus influenzavirus C contains human and swine strains
of influenza type C.
The standard nomenclature system for influenza virus
isolates includes the following information: Type, host of
origin, geographic origin, strain no, year of isolation. The
host origin is not indicated for human isolates, e.g.
A/Hong Kong/03/68(H3N2).
There are 15 subtypes of HA; only 4 have been
transferred to humans (H1-H3, H5). Na has nine subtypes,
only 2 affect humans (N1, N2).
Antigenic shift and antigenic drift
Antigenic shift: it is major changes based on
reassortement
*
(between human and animal influenza) of
segments of the genome RNA result in the appearance of
a new subtype. Influenza B and C viruses don’t exhibit
antigenic shift because few related viruses exist in
animals. Antigenic shift is responsible for an epidemic.
Antigenic shift variants appear every 10-40 years.
Antigenic drift: It is a minor change based on mutations
in the genome of RNA (accumulation of point mutation in
the gene, resulting in amino acid changes in the protein).It
occurs in all types of influenza virus.Antigernic drift
variants appear every year.
*
Reassortement: viruses with segmented genome
exchange segments. 0p-
Replicative cycle
1) The virus adsorb to the cell when the viral hemagglutinin
interacts with sialic acid receptors on the cell surface.
2) The virus enters the cell and uncoating .
3) In the nucleus, the viral RNA polymerase transcribes the
eight genome segments into eight mRNA.
4) Most of the mRNA move to the cytoplasm , where they
are translated into viral proteins. Some of the viral mRNA
remains in the nucleus, where they serve as the template

Unit 4: Virology
212
for the synthesis of the –ve strand RNA genomes for the
progeny virions.
5) The newly synthesized NP and matrix protein binds to
progeny RNA genome in the nucleus and that complex is
transported to the cytoplasm.
6) After assembly, the virion is released by budding from the
outer cell membrane at the site where HA and NA are
located.
7) The neuraminidase acts to release the virus by cleaving
neuraminic acid on the cell surface at the site of the
budding progeny virions.
Transmission:
1) Virus is transmitted by airborne respiratory droplets.
2) Influenza occurs most primarily in the winter months.
Pathogenesis
1) After inhalation of the virus, the NA degrades the
protective mucus layer , allowing the virus to gain access
to the cells of upper respiratory tract.
2) Viremia rarely occurs.
3) The systemic symptoms are due to cytokine circulating in
the blood.
4) There is necrosis of the superficial layers of the
respiratory epithelium.
5) Pneumonia as a result of secondary bacterial infection is
interstitial in location.
6) The virulence of the H5N1 strain is greater than H1N1
and H3N2 strains because:
I- H5N1 strain is relative resistance to interferon
II- Increase induction of cytokines, especially TNF, this
mediate the pathogenesis of pneumonia and acute
respiratory distress syndrome (ARDS) seen in H5N1
infection.
Immunity:
Immunity depends on secretary IgA in the respiratiory
tract. IgG is also produced but it is less protecrtive.
Cytotoxic Tcells also play a protective role.
Clinical findings
1) After an incubation period of 24-48 hours , fever,
myalagia, headache, sore throat, and cough develop
suddenly .
2) Vomiting and diarrhea are rare.
3) The symptoms resolve spontaneously in 4-7 days, but
influenzal or bacterial pneumonia may complicate the
course. Staphylococcus aureus is the most common
pathogen for pneumonia.
4) Reye's syndrome which is rare and characterized by
encephalopathy and liver degeneration, life – threatening
complication in children following some viral infections,
particularly influenza and chickenpox. Aspirin given to
reduce fever in viral infection has been implicated in the
pathogenesis of Reye's syndrome.
Laboratory diagnosis:
1) In cases of epidemic, clinical diagnosis is enough.
2) Virus can be detected in specimens such as (nasal or
throat washings, nasal or throat swabs and sputum) by
various techniques such as direct fluorescent antibody,
PCR, or cell culture-based tests.
3) Antibody detection in patient serum.4 fold increase in
antibody titer in paired serum samples taken early in the
illness and 10 days later and detected either by
hemagglutination inhibition or complement fixation test.
Treatment
1) Amantidine in the treatment and prevention of influenza
A .indicated in the prevention in elderly,
immunocompromised persons.
2) Rimantidine is a derivative of amantidine. Drug resistance
observed against the 2 drugs.
3) Zanamivir (Relanza) and oseltamivir (Tamiflu) are also
used for the treatment of influenza. They belong to a class
of neuraminidase inhibitors that act by inhibiting the
release of virus from infected cells. This limits the
infection by reducing the spread of virus from one cell to
another. These drugs are effective against influenza A &B
Prevention
The main mode of prevention is the vaccine, which
consists from two strains of influenza A (H1N1, H3N2)
and one strain of influenza B virus.
There are two types of influenza vaccines available
I- Killed vaccine containing purified protein subunits of the
virus (HA and NA). The virus inactivated with
formaldehyde, then treated with a lipid solvent that
disaggregate the virions. The vaccine given
intramuscularly. Protection lasts only 6 months. It is
indicated in people older than 50 years of age, children 6-
23 months of age, and those with chronic disease.
II- The new vaccine that was approved in 2003 is a live
vaccine containing temperature sensitive mutants of
influenza A and B viruses. These temp.- sensitive mutants
can replicate in the cooler (33c) nasal mucosa where they
induce IgA. This vaccine administered by spraying into
the nose (nasal mist).
Unit 4: Virology
211
Epidemiology:
To date all human pandemic strains have been
reassortement between avian and human influenza
viruses. Pigs serve as mixing vessels for reassortements as
their cells contain receptors recognized by both human
and avian viruses.
Influenza virus occurs worldwide and cause annual
outbreaks of variable intensity.
Influenza outbreaks occur in waves, although there is no
regular periodicity in the occurrence of epidemics.
Influenza A epidemic waves tend to be 2-3 years, for B 3-
6 years. Every 10-40 years, when a new subtype of
influenza A appears, a pandemic results. This happened in
1918 (Spanish flu) (H1N1 the swine like influenza), 1957
(Asian flu) (H2N2), and 1968 (Hong Kong flu) (H3N2).
The H1N1 subtype reemerged in 1977 (Russian flu).
Since 1977, influenza A (H1N1) and (H3N2) viruses and
influenza B have been in a global circulation. In 1997, in
Hong Kong avian influenza A virus (H5N1) occurred.
Paramyxoviruses
The Paramyxovirus family contains four important human
pathogens: measles, mumps, respiratory syncytial virus,
and parainfluenza viruses.
The genome is not segmented, they have a larger
diameter, and their surface spikes are different.
Paramyxoviruses are composed of one piece of SS –ve
polarity RNA, a helical nucleocapsid, and an outer
lipoprotein envelope. The envelope is covered with
spikes, which contain hemagglutinin, neuraminidase, or a
fusion protein that causes cell fusion and, in some cases,
hemolysis.
Measles virus
Important properties
Measles virus has a single serotype, and the
hemagglutinin is the antigen against which neutralizing
antibody is directed. Humans are the natural host.
Replicative cycle:
After adsorption to the cell surface via its hemagglutinin,
the virus penetrates and uncoat.
The virion RNA polymerase transcribes the –ve –strand
genome into mRNA.
Multiple mRNA are synthesized, each of which is
translated into specific viral proteins.
The helical nucleocapsid is assembled, and the virus
released from the cell by budding.
Transmission and epidemiology.
The Measles virus is transmitted via respiratory droplets
produced by coughing and sneezing both during the
prodromal period and for few days after the rash appears.
Measles occur worldwide, usually in outbreaks every 2-3
years, when the number of susceptible children reaches a
high level.
Measles infection is more severe in malnourished children.
Patient with deficient cell –mediated immunity have a
severe, life –threatening disease when they contract measles
Pathogenesis:
After infection of the cells lining the upper respiratory
tract, the virus enters the blood and infects
reticuloendothelial cells, where it replicates again.
It then spreads via the blood to the skin.
The rash is caused by cytotoxic T cells attacking the
measles virus –infected vascular endothelial cells in the
skin. Antibody mediated vasculitis may also play a role.

Unit 4: Virology
211
After the rash appears, the virus can no longer be recovered
and the patient can no longer spread the virus to others.
Multinucleated giant cells, which form as a result of the
fusion protein in the spikes, are characteristic of the lesions
Immunity
Lifelong immunity in individuals who had the disease.
Cell-mediated immunity is more important than humoral
immunity in the recovery and protection.
Maternal antibody passes the placenta, and infants are
protected during the first 6 months of age.
Infection with measles virus can transiently depress cell-
mediated immunity against other intracellular
microorganisms, such as Mycobacterium tuberculosis,
leading to a loss of purified protein derivative (PPD) skin
test reactivity, reactivation of dormant organisms, and a
clinical disease. The proposed mechanism for this
finding is that when measles virus binds to its receptor
(called CD46) on the surface of human macrophages, the
production of IL-12, which is necessary for cell-mediated
immunity to occur, is suppressed.
Clinical findings
1) After an incubation period of 10-14 days, a prodromal
phase characterized by fever, conjunctivitis, running nose,
and coughing occurs.
2) Koplik´ s spots are bright red lesions with a white, central
dot that are located on the buccal mucosa and are
diagnostic.
3) A few days later, a maculopapular rash appears on the
face and proceeds gradually down the body to the lower
extremities, including the palms and soles.
Complications
1) Encephalitis occurs at a rate of 1/1000 cases of measles.
2) Primary measles pneumonia and bacterial pneumonia
occurs.
3) Bacterial otitis media.
4) Subacute sclerosing panencephalitis (SSPE) is a rare, fatal
disease of the central nervous system that occurs several
years after measles.
5) Atypical measles occurs in some people who were given
the killed vaccine and were subsequently infected with
measles virus. It is characterized by an atypical rash
without Koplik s spots.
Laboratory diagnosis
1) Most cases are made on clinical grounds.
2) Viral isolation in cell culture can be done
3) A rise in antibody titer of greater than 4-fold can be
used in the diagnosis.
Prevention
Immunization with live attenuated virus vaccine given at
9 months of age and a second dose combined with mumps
and rubella vaccines at 15 months of age.
The vaccine is contraindicated in immunocompromised
patient and in pregnant women.
Mumps virus
This virus cause Mumps.
Important properties
The virion has two types of envelope spikes, one with
both Hemagglutinin and neuraminidase and the other with
cell-fusing and hemolytic activity. Neutralizing antibody
is directed against the hemagglutinin.
The virus has a single serotype. Humans are the natural host
Replicative cycle
As for measles virus.
Transmission and epidemiology.
Mumps virus is transmitted via respiratory droplets.
Mumps occurs worldwide, with peak incidence in the
winter.
About 30% of children have a subclinical infection, which
confers immunity.
Pathogenesis and immunity
The virus infects the respiratory tract and then spreads
through the blood to infect the parotid glands, testes,
ovaries, pancreas, and in some cases meninges. The virus
may ascend from the buccal mucosa up Stensen´ s duct to
the parotid gland.
Lifelong immunity occurs after the infection.
Maternal antibody passes the placenta and provides
protection during the first 6 months.
Clinical findings
After an incubation period of 18-21 days, a prodromal
stage of fever, malaise, and anorexia is followed by tender
swelling of the parotid glands, either unilateral or
bilateral. The disease resolves spontaneously within 1
week.
Complications
1) Orchitis in postpupertal males, which is if bilateral, results
in sterility because of fibrous tunica albuginea, which
Unit 4: Virology
211
resist expansion, thereby causing pressure necrosis of the
spermatocytes. Unilateral orchitis don’t cause sterility.
2) Meningitis, which is usually benign, self-limited, and
without sequel.
Laboratory diagnosis
1) Clinical diagnosis.
2) Viral isolation in cell culture.
3) A 4 –fold rise in antibody titer in either hemagglutination
inhibition or the complement fixation test is diagnostic.
Treatment
No antiviral therapy.
Prevention
Immunization with live attenuated virus vaccine given at
15 months of age given subcutaneously combined with
measles and rubella vaccines.
The vaccine is contraindicated in immunocompromised
patient and in pregnant women.
Respiratory Syncytial virus
It is the most important cause of pneumonia and
bronchioloitis in infants. It is an important cause of otitis
media in children and of pneumonia in the elderly and in
patients with chronic cardiopulmonary diseases.
Important properties
The surface spikes are fusion proteins, not hemagglutinin
or neuraminidase. Humans are the natural host of RSV.
Two serotypes, A and B.
Replicative cycle
As for measles virus.
Transmission and epidemiology.
1) via respiratory droplets
2) Direct contact of contaminated hands with the nose or mouth
3) RSV causes outbreaks of respiratory infections every winter
4) RSV causes outbreaks of respiratory infections in
hospitalized infants.
Pathogenesis and immunity
RSV infection in infants is more sever and more often
involves the lower respiratory tract than in older children
and adults.
The infection is localized to the respiratory tract; viremia
doesn’t occur.
The sever disease in infants may have an
immunopathogenic mechanism. Maternal antibody passed
to the infant may react with the virus, form immune
complexes, and damage the respiratory tract cells. Trials
with a killed vaccine resulted in more severe disease; an
unexpected finding that supports such a mechanism.
Most individuals have multiple infections caused by RSV.
Laboratory diagnosis
1) Virus detection by immunofluorescence on smears of
respiratory epithelium or by isolation in cell culture.
2) A 4 –fold rise in antibody titer.
3) Reverse transcriptase PCR.
Treatment
Aerosolized ribavirin is recommended for severely ill
hospitalized infants.
Parainfluenza viruses
Diseases
These viruses cause croup, ( acute
laryngotracheobronchitis) in children younger than 5 years
of age (Croup is chartecterized by a harsh cough &
hoarseness), laryngitis, bronchiolitis, & pneumonia in
children & a disease resembling the common cold in adults.
Important properties:
The surface spikes consist of hemagglutinin,
neuraminidase, and fusion protein.
There are four serotypes. The virus is transmitted via
respiratory droplets.
Replicative cycle
As for measles.
Pathogenesis and immunity
These viruses cause lower and upper respiratory tract
disease without viremia.
A large proportion of infections are subclinical.
Parainfluenza 1 & 2 are the major cause of croup.
Parainfluenza 3 is the most commonly isolated from
children with lower respiratory tract infections.
Parainfluenza 4 rarely cause disease except for the
common cold.
Diagnosis:
Most cases are diagnosed clinically.
Treatment:
Neither antiviral therapy nor a vaccine is
available.

Unit 4: Virology
215
Coronaviruses
Diseases
Coronavirus is an important cause of common cold
In 2002, a new disease, an atypical pneumonia called
SARS (severe acute respiratory syndrome) emerged.
Important properties
1) Coronavirus is nonsegmented, SS, +ve polarity RNA
genome, enveloped with helical nucleocapsid.
2) In electron microscope, prominent club-shaped spikes in
the form of a Corona (halo) can be seen.
3) Two serotypes 229E and OC 43.
4) The corona virus recovered in 2002 that cause SARS
(CoV-SARS) is belonging to the second serotype (OC43).
5) The receptor for the SARS coronavirus is angiotensin-
converting enzyme -2.
Replicative cycle
The virus adsorbs to cells via its surface spikes
(hemagglutinin), after which it enters the cytoplasm,
where it is uncoated.
The positive polarity genome is translated into two large
polypeptides, which are self-cleaved by the virus-encoded
protease.
mRNA is synthesized, and then translated into the
structural proteins.
The virus is assembled and obtains its envelope from the
endoplasmic reticulum, not from the plasma membrane.
Transmission and epidemiology.
1) By the respiratory aerosol.
2) SARS originated in china in 2002. Human to human
transmission,
Pathogenesis and immunity
Viral infection is typically limited to the respiratory
mucosa.
50% of infections are asymptomatic
Reinfection can occur.
Pneumonia caused by SARS coronavirus is characterized
by diffuse edema, the binding of the virus to angiotensin
converting enzyme-2 on the surface of respiratory tract
epithelium may contribute to the dysregulation of fluid
balance and edema in the alveolar space.
Clinical findings
The common cold is characterized by coryza (rhinorrhea,
runny nose), scratchy sore throat, and low grade fever.
The illness lasts several days.
SARS is a severe atypical pneumonia characterized by
fever (38C), nonproductive cough, dyspnoea, hypoxia,
chills, rigor, malaise, and headache. Chest X-ray reveals
interstitial (ground-Glass) infiltrates.
Leucopenia and thrombocytopenia.
Laboratory diagnosis
1) Common cold diagnosed clinically.
2) If SARS is suspected, antibody –based and PCR-based
tests can be used.
Treatment and prevention
1) There is no antiviral therapy or vaccine available.
2) A combination of Ribavirin + steroid has been tried in
the treatment of SARS.
Rubella virus
This virus causes rubella (German measles) and
congenital rubella syndrome.
Important properties
1) Rubella virus is a member of togavirus family (not
paramyxovirus).
2) It is composed of one piece of SS RNA, positive polarity
RNA with icosahedral symmetry, and lipoprotein
envelope.
3) Its surface spikes contain hemagglutinin. The virus has a
single antigenic type.
4) Humans are the natural host.
Replicative cycle
The same as for any SS positive sense RNA virus.
Transmission:
1) respiratory droplets
2) From the mother to fetus transplacentally.
Pathogenesis and immunity:
1) Initial replication of the virus occurs in the nasopharynx
and local lymph nodes.
2) From there it spreads via the blood to the internal organs
and skin.
3) The origin of rash is thought to be due to antigen-antibody
mediated vasculitis
4) Natural infection leads to lifelong immunity
5) Antibody crosses the placenta and protects the newborn.

Unit 4: Virology
211
Clinical findings:
A. Rubella
Rubella is milder, shorter disease than measles. After an
incubation period of 14-21 days, a brief prodromal period
with fever , malaise, followed by maculopapular rash,
which starts on the face and progresses downward to
involve the extremities.
Posterior auricular lymphadenopathy is characteristic. The
rash lasts for 3 days.
B. Congenital Rubella Syndrome.
When a non-immune pregnant woman is infected during
the first trimester, especially the first month, significant
congenital malformation (heart leading to patent ductus
arteriosus, the eye leading to cataract, and the brain
leading to deafness and mental retardation) can occur as a
result of maternal viremia and fetal infection. The infected
new born continue to excrete rubella virus for months
following birth. Some shedders are asymptomatic and
without any congenital malformation.
Laboratory diagnosis:
1) Virus isolation by tissue culture.
2) 4 fold increase in antibody titer between acute and
convalescent phase.
3) In pregnant mother, IgM antibody indicates recent
infection.
4) An amniocentesis can reveal whether there is rubella virus
in the amniotic fluid, which indicates definite fetal
infection.
Treatment
No antiviral therapy.
Prevention
Immunization with live attenuated virus vaccine given at
15 months of age given subcutaneously combined with
measles and mumps vaccines. Also the vaccine given to
unimmunized young adult women if they are not pregnant
and will use contraception for the next 3 months.
The vaccine has caused a significant reduction in the
incidence of both rubella and congenital rubella
syndrome. It induces some respiratory IgA, thereby
interrupting the spread of virulent virus by nasal carriage.
Immune serum globulin can be given to pregnant mother
in the first trimester who have been exposed to a known
case of rubella.
Rhabdoviruses
Rabies virus
This virus causes rabies which is an acute infection of the
CNS that is almost always fatal.
Important properties
1) Rabies virus is the only medically important member of
the rhabdovirud family.
2) It is SS, negative polarity RNA virus with bullet-shaped
capsid and an envelope.
3) It has single antigenic type.
4) Rabies virus has a broad host range: it can infect all
mammals, but only certain mammals are important
sources of human infection.
Replicative cycle
1) Rabies virus attaches to the acetylcholine receptor on the
cell surface.
2) After entry into the cell, the virion RNA polymerase
synthesizes five mRNA that code for viral proteins.
3) After replication of the genome viral RNA by a virus-
encoded RNA polymerase, progeny RNA is assembled
with virion proteins to form the nucleocapsid, and the
envelope is acquired as the virion buds through the cell
membrane.
Transmission
1) The virus is transmitted by the bite of rabid animal that
manifests aggressive, bitting behavior induced by viral
encephalitis.
2) In certain developed countries, transmission is usually
from the bite of wild animals such as skunks, raccoons,
and bats.
3) Human rabies has also occurred in certain developed
countries in people who have not been bitten, so called
nonbite exposures, e.g. is the exposure to aerosols of bat
secretions containing rabies virus.
4) Another rare example is transmission in transplants of
corneas taken from patients who died of undiagnosed
rabies.

Unit 4: Virology
211
Pathogenesis:
The virus multiplies locally at the bite site, infects the
sensory neurons, and moves by axonal transport to the
central nervous system (CNS).
During its transport within the nerve, the virus is sheltered
from the immune system, and little, if any, immune
response occurs.
The virus multiplies in the CNS and then travels down the
peripheral nerves to the salivary glands and other organs.
From the salivary glands, it enters the saliva to be
transmitted by the bite.
There is no viremic stage.
Within the CNS, encephalitis develops, with the death of
neurons and demylination.
Infected neurons contain an eosinophilic cytoplasmic
inclusion called a Negri body, which is important in
laboratory diagnosis of rabies.
Clinical findings:
The incubation period varies, according to the location of
the bite, from as short as 2 weeks to 16 weeks or longer. It
is shorter when bites are sustained on the head rather than
on the leg, because the virus has a shorter distance to
travel to reach the CNS.
The patients exhibits a prodrome of non-specific
symptoms such as fever, anorexia, and changes in
sensation at the bite site.
Within a few days, signs such as confusion, lethargy, and
increased salivation develop. Painful spasm of the throat
muscles on swallowing, this result in hydrophobia.
The disease progress to seizure paralysis and coma
Death almost invariably ensues, but with the advent of life
support systems a few individuals have survived.
Laboratory diagnosis:
1) Rabies in humans can be diagnosed by fluorescent-
antibody staining of a biopsy specimen, usually taken
from the skin of the neck at the hairline.
2) Isolation of the virus from sources such as saliva, spinal
fluid, and brain tissue
3) Rise in titer of antibody to the virus.
4) Negri bodies can be demonstrated in corneal scrapings
and in autopsy specimens of the brain.
Treatment
There is no antiviral therapy. Only supportive treatment is
available.
Prevention:
There are two approaches to prevention of rabies in
humans: preexposure and postexposure.
Preexposure immunization:
Vaccine given to high risk individuals: veterinarians,
zookeepers, and travelers to areas of hyperendimic
infection. The schedule consist of three doses given on
days 0, 7, and 21 or 28 .Booster doses given as needed.
Types of rabies vaccine:
Human diploid cell vaccine (HDCV). Inactivated virus
grown in human diploid cells.
Rabies Vaccine, Adsorbed(RVA)
Purified Chick Embryo Cell Vaccine(PCEC)
Nerve Tissue Vaccine : low antigenicity, induce
postvaccinal encephalitis (allergic)
Duck Embryo Vaccine: Low antigenicity.
Live Attenuated Vaccine: for animals but not for humans.
Postexposure immunization:
Both the vaccine and human rabies immune globulin
(RIG) plus immediate cleaning of the wound and tetanus
immunization.
The decision to give immunization depends on many
factors:
1) The type of the animal (all wild animal attacks
demand immunization.
2) Whether an attack by a domestic animal was
provoked, whether the animal was immunized, and
whether the animal is available to be observed.
3) Whether rabies is endemic in the area.
If the decision is to immunize, both HDCV and RIG are
recommended. Five doses of HDCV are given (on days 0,
3, 7, 14, and 28), but RIG is given only once with the first
dose of HDCV (at a different site?).
It is advisable to give RIG as much as possible into the
bite site, and the remainder is given intramuscularly.
If the animal has been captured, it should be observed for
10 days.

Unit 4: Virology
211
Lecture Half 7+8 - RNA non
enveloped viruses
A- Picornaviruses
They are small nonenveloped viruses composed of an
icosahedral nucleocapsid and a SS RNA genome of
positive polarity.
They replicates in the cytoplasm of cells.
The picornavirus family includes 2 groups of medical
importance:
The Enteroviruses and rhinoviruses. Enteroviruses
include: Poliovirus, Coxackie viruses, Echoviruses, and
Hepatitis A virus.
I- Enteroviruses
1- Poliovirus
Disease: poliomyelitis which is an acute infectious
disease that in its serious form affects the central nervous
system.
Important properties
The host range is limited to primates, i.e., humans and
nonhuman such as apes and monkeys. This limitation is
due to the binding of the viral capsid protein to a receptor
found only on primate cell membrane.
There are three serologic (Antigenic) types based on
different antigenic determinants on the outer capsid
protein .There is little cross reaction.
Replicative cycle
1) The virion interact with specific cell receptor on the cell
membrane and then enter the cell
2) After uncoating, the genome RNA functions as mRNA
and is translated into one very large polypeptide called
non capsid viral protein. This polypeptide is cleaved by a
virus –encoded protease in multiple steps to form both the
capsid and non-capsid protein, including the RNA
polymerase that synthesizes the progeny RNA genomes.
3) Replication of the genome occurs by synthesis of a
complementary negative strand, which then serve as the
template for the positive strands. Some of these positive
strands function as mRNA to make more viral proteins,
and the remainder become progeny virion genome RNA.
4) Assembly of the progeny virions occurs by coating of the
genome RNA with caps proteins.
5) Virus released from the cell upon death of the cell.
Transmission, pathogenesis and immunity
Poliovirus is transmitted by feco-oral route. It replicates in
the oropharynx and intestine. The virus is regularly
present in the throat and in stools before onset of illness.
One week after infection there is little virus in the throat,
but virus continues to be excreted in the stools for several
weeks even though high antibody levels are present in the
blood. No permanent carrier state occurs following
infection by poliovirus.
It is believed that the virus first multiply in the tonsils,
lymph nodes of the neck, peyer´s patches, and the small
intestine.
The virus spread through the blood stream or retrograde
along nerve axons to CNS. In CNS, poliovirus
preferentially replicates in the motor neurons located in
the anterior horn of the spinal cord .Death of these cells
results in paralysis of the muscles innervated by those
neurons. The virus also affects the brain stem, leading to
bulbar poliomyelitis.
In infected individuals, the immune response consists of
both intestinal IgA and humoral IgG to specific serotype.
Infection provides lifelong type-specific immunity.
Clinical features
The infection ranges from inapparent infection, to mild
febrile illness, to severe and permanent paralysis.
a) Most infections are subclinical; only about 1% of
infections result in clinical illness. Incubation period is
usually 7-14 days, but it may range from 3 days to 35
days.
b) Mild disease. This is the most common form of the
disease. The patient has only a minor illness,
chartecterized by fever, malaise, drowsiness, headache,
Nausea, vomiting, constipation, and sore throat. Recovery
occurs in a few days.
c) Non paralytic poliomyelitis (Aseptic meningitis): in
addition to the symptoms of mild disease, the patient has
stiffness and pain in the back and neck. The disease lasts
2-10 days, and recovery is rapid and complete.
d) Paralytic poliomyelitis: the predominant complaint is
flaccid paralysis resulting from lower motor neuron
damage. Maximal recovery usually occurs within 6
months, with residual paralysis lasting much longer. The
meninges and brain may be involved in paralytic
poliomyelitis.
e) Progressive Post poliomyelitis Muscle Atrophy: A
recrudescence of paralysis and muscle wasting has been
observed in individuals decades after their experience
with paralytic poliomyelitis. It is not a consequence of
persistent infection but rather a result of physiologic and

Unit 4: Virology
211
aging changes in paralytic patients already burdened by
loss of neuromuscular function.
Laboratory diagnosis
1) Isolation of the virus from the throat, stool, or spinal
fluid by inoculation of cell culture. The virus cause
cytopathic effect (CPE) and can be identified by
neutralization of the CPE with specific antisera.
2) Rise in antibody titer in paired sera.
Treatment
1) There is no antiviral therapy.
2) Treatment is limited to symptomatic relief and
respiratory support, if needed.
3) Physiotherapy for the affected muscles is important.
Prevention
Poliomyelitis can be prevented by both Killed vaccine
(Salk vaccine, inactivated vaccine, IPV) and the live,
attenuated vaccine (Sabin vaccine, oral vaccine, OPV).
Both vaccines induce humoral antibodies, which
neutralize virus entering the blood and hence prevent
central nervous system infection and disease. Both
vaccines contain all three serotypes.
The current version of the inactivated vaccine is called
enhanced polio vaccine, or eIPV .It has a high
seroconversion rate and induces a higher titer of antibody
than the previous IPV. eIPV also induces some mucosal
immunity IgA, making it capable of interrupting
transmission.
Important properties of poliovirus vaccines.
The currently approved vaccine schedule for OPV 2, 4, 6
month age, 18 months & upon entry to school at age 4-6
years.
Passive immunization with immune serum globulin is
available for protection of unimmunized individuals
known to have been exposed.
2- Coxackieviruses
Coxackieviruses are named for the town of Coxackie,
NY, where they were first isolated.
Important properties:
Group classification is based on pathogenicity in mice
Coxackieviruses group A includes 24 serotypes
Coxackieviruses group B includes 6serotypes.
The size and structure of virion and the nature of the
genome RNA are similar to poliovirus but unlike
poliovirus, they can infect mammals other than primates.
Transmission and epidemiology:
Coxackieviruses are transmitted primarily by the fecal-
oral route; but respiratory aerosols also play a role.
They replicate in the oropharynx and the intestinal tract.
Humans are the only natural hosts.
Coxackievirus infection occur worldwide, primarily in the
summer and fall.
Pathogenesis and immunity:
Group A virus has a predilection for skin and mucous
membranes, whereas group B viruses cause disease in
various organs such as the heart, pleura, pancreas, and
liver. Both groups can affect the meninges and the motor
neurons (anterior horn cells) to cause paralysis. From their
original site of replication in the oropharynx and GIT,
they disseminate via blood stream.
Immunity following infection is provided by type –
specific IgG antibodies.
Clinical findings:
1) Group A- specific diseases
Herepangina is characterized by fever, sore throat, and
tender vesicles in the oropharynx.
Foot-and-mouth disease is characterized by a vesicular
rash on the hands and feet and ulcerations in the mouth,
mainly in children.
2) Group-B- Specific diseases:
Pleurodynia (Bronholm disease, epidemic myalgia, devil's
grip) is characterized by fever and severe pleuritic chest
pain.
Myocarditis and pericarditis are characterized by fever,
chest pain, and signs of congestive heart failure.
Diabetes in mice can be caused by pancreatic damage as a
result of infection with coxackievirus B4. This virus is

Unit 4: Virology
211
suspected to have a similar role in juvenile diabetes in
humans.
3) Disease caused by both groups:
Aseptic meningitis, mild paresis, and acute flaccid
paralysis similar to poliomyelitis. Upper respiratory
infections and minor febrile illnesses with or without rash
can occur also.
Laboratory diagnosis:
Virus isolation or serology.
Treatment :
Neither antiviral nor vaccines are available.
3- Echovirus
E= enteric C= cytopathic H= human O= orphan
More than 30 serotypes have been isolated
They are transmitted by the feco-oral route
It causes aseptic meningitis, URTI, febrile illnesses with
or without rash, infantile diarrhea, and hemorrhagic
conjunctivitis.
Neither antiviral nor vaccines are available.
4- Other enteroviruses
Enterovirus 70 cause acute haemorrhagic conjunctivitis
Enterovirus 71 meningitis, encephalitis, and paralysis,
diarrhea, pulmonary hemorrhages, hand –foot-and- mouth
disease, and herpangina.
Enterovirus 72 is hepatitis A virus.
II-Rhinoviruses
They are the main cause of common cold.
Important properties:
1) More than 100 serotypes.
2) They replicate better at 33 C
º
, so they replicate in nose
and conjunctiva.
3) They are acid labile, they are killed by gastric acid
when swallowed.
Transmission and epidemiology:
2 modes of transmission.
1- Direct via respiratory aerosol
2- Indirect, in which respiratory droplets are deposited on
hands or on a surface such as table and then transported
by fingers to the nose or eyes.
A few serotypes of rhinoviruses are prevalent during one
season, only to be replaced by other serotypes during the
following seasons.
Clinical findings
Incubation period of 2-4 days, sneezing, nasal discharge,
and headache are common with chilly sensation. The
illness lasts about 1 week.
Laboratory diagnosis
Viral isolation rarely done.
Treatment
No antiviral drugs are available.
B- Calciviruses
They are small nonenveloped virus with single strand
RNA of positive polarity.
1- Norwalk virus (Norovirus)
It is one of the most common causes of gastroenteritis in
adult in USA and worldwide.
It is transmitted by fecooral route by ingestion of seafood
and contaminated water and diseaee is characterized by
sudden onset of vomiting and diarrhea with low grade
fever and abdominal cramping. The disease last for
several days.
No role for antiviral drugs. Supportive treatment id required.
C- Reovirus (Respiratory Enteric Orphan).
1- Rotavirus:
It is the most common cause of viral gastroenteritis in
young children.
Properties:
It has segmented (11 segments) double strand RNA
genome surrounded by double –layered icosahedral
capsid without an envelope.
The virion has RNA-dependent RNA polymerase.
There are at least six serotype of human rotavirus.
Rota virus attaches to cell surface at the site of ß-
adrenergic receptor.
Laboratory diagnosis:
Detection of rotavirus in the stool by ELISA.
Four fold increase in antibody titer
No role for viral culture in the diagnosis.
Prevention
There are two type of rotavaccine
1) Live attenuated vaccine (Rotarix) which contain single
most common serotype.
2) Live reassortant vaccine ( Rotateq) which contains
five rotavirus strain.

Unit 4: Virology
212
Lecture 9+10 - Retroviruses
The retroviridae family is divided into seven genera .The
genera which are important to human are delta retrovirus
and lentivirus.
Lentivirus
Human immunodeficiency virus (HIV)
Human immunodeficiency virus is a member of lentivrus
which cause slow infection with long incubation period
(AIDS).
The acquired immunodeficiency syndrome (AIDS) was
first recognized in 1981. It is caused by the human
immunodeficiency virus (HIV-1). HIV-2 causes a similar
illness to HIV-1 but is less aggressive and restricted
mainly to western Africa.
HIV is one of the two important human T cell
lymphotropic viruses. It preferentially infects and kills
helper CD-4
+
T cells leading to loss of cell mediated
immunity and high probability of opportunistic infection.
Other cells (macrophage and monocytes) can be infected
also.
General characteristic
HIV has a bar shaped core surrounded by an envelope
contain type specific glycoprotein (gp 120, gp 41).
The genome consists of 2 identical molecules of SS,
positive polarity RNA.
There are three typical retroviral genes gag, pol, and
env, (encode the structural proteins), and six regulatory
genes. Two of the regulatory genes, tat, and rev, are
required for replication, and the other four, nef, vif, vpr,
and vpu, are not required for replication and are termed
accessory genes.
The gag gene encodes the internal corer protein [(p24) (is
the most important used in serology)], and p17 (matrix).
The pol gene encodes:
1) Virion reverse transcriptase.
2) Integrase.
3) Protease
The env gene encodes for gp 160 that is cleaved to gp 120
(attachment to CD4) and gp 41(fusion with host cell).
Regulatory genes:
1) Tat for activation of transcription of viral genes.
2) Rev for transport of late mRNA from nucleus to
cytoplasm.
3) Nef .repress the synthesis of class I MHC proteins,
thereby reducing the ability of cytotoxicT cells to kill HIV
– infected cells.
4) Vif (viral infectivity) inhibit the action of APOBEC3G, an
enzyme that cause hypermutation in retroviral DNA.
5) Vpr transports viral core from cytoplasm into nucleus in
non-dividing cells.
6) Vpu enhances virion release from cell.
Important antigens of HIV:
1) gp 120 and gp 41 are type specific envelop gp. HIV
classified into 3 types (M, N, and O) .M is common types
and contain 10 subtypes (A-J). The gene that encodes gp
120 mutates rapidly, resulting in many antigenic variants.
Antibody against gp 120 neutralizes the infectivity of
HIV, but the rapid appearance of gp 120 variants will
make production of an effective vaccine difficult.
2) The group-specific antigen, p24, is located in the core and
antibody against p24 serves as important serologic
markers of infection.
Origion of HIV
HIV-1 in humans originated from cross-species transmission
of SIV
cpz
(simian immunodeficiency virus of chimpanzee)
while HIV-2 from SIV
sm
(sm= Sooty mangabey)
Disinfection and inactivation
HIV is completely inactivated by treatment for 10 minutes
at room temperature with any of the following: 10%
household bleach, 50% ethanol, 35% isopropanol, 0.5%
paraformaldehyde, 0.3 % H2O2.The virus inactivated by
extreme pH(1,13).If HIV present in clotted or unclotted
blood in a needle or syringe, exposure to undiluted bleach
for at least 30 seconds is necessary for inactivation. HIV
is readily inactivated in liquid or 10% serum by hearting
at 56 c for 10 minutes.
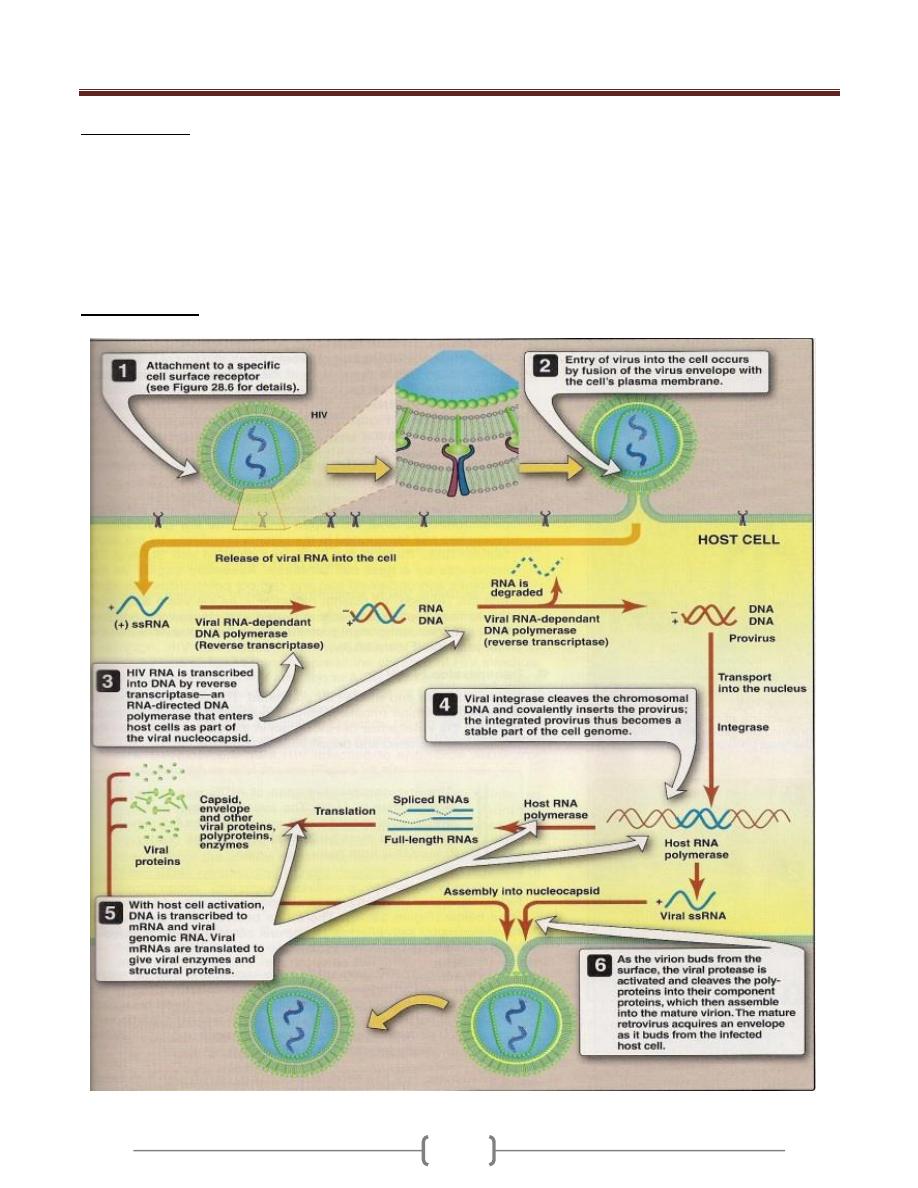
Unit 4: Virology
211
Virus receptors
Lentiviruses use CD4 molecule as a receptor, which is
expressed on macrophages and T lymphocytes. A second
core receptor in addition to CD4 is necessary for HIV-1 to
gain entry to cells (required for fusion of the virus with
cell membrane).The virus first bind to CD4 receptor and
then to core receptor. The core receptors are CXCR4 for
T-tropic strains and CCR5 for macrophage-tropic strains.
Replicative cycle

Unit 4: Virology
211
Transmission
1) Sexual contact
2) Transfer of infected blood
3) From infected mother to neonate.50% of neonatal
infection are acquired at time of birth.25% trancplacental
and 25% via breast feeding.
4) Sharing needles in I.V drug addicts.
5) Transfer through body fluid e.g. saliva, tear.
6) The risk of infection after percutaneous exposure and
needle- stick injuries is about 0.3% .This means that the
infectious dose of HIV is high.
Pathogenesis
HIV infects T
h
cells and kills them, resulting in
suppression of cell-mediated immunity. This predisposes
the host to various opportunistic infections and certain
cancers such as Kaposi ´s sarcoma and lymphoma. HIV
doesn’t directly cause these tumors because HIV genes
arte not found in these cancer cells.
Monocytes and macrophage play a major role in the
dissemination and pathogenesis of HIV infection.
Macrophage tropic strain HIV predominates in the early
infection and these strains responsible for initial
infection.T-tropic strain predominate later. It is believed
that the monocyte and macrophage serve as major
reservoirs for HIV in the body.
The initial infection of the genital tract occurs in dendritic
cells that line the mucosa, after which the local CD-4
+
Th
cells become infected .HIV,
is first found in the blood 4-11
days after infection.
Neurologic abnormalities are common in late stages of
infection and are AIDS-defining condition. The CNS
diseases are HIV encephalopathy, peripheral
neuropathies, and AIDS dementia complex. The
predominant cell types in the brain that are infected with
HIV are monocytes and macrophages.
B-cells abnormalities. Polyclonal activation of B cells is
seen, with resultant high Ig levels. Autoimmune diseases,
such as thrombocytopenia occur.
Mechanisms that explain the death of Th cells in HIV
infection.
1) The immunologic attack of the HIV infected cells by
Tc-CD-8 cells.
2) HIV acts as superantigen which activates many T
h
cells and leads to their death.
A group of HIV infected individuals has lived for
many years without opportunistic infection and
without a reduction in the number of their T
h
CD4 cells. Why?
1) The strain of HIV isolated from these individuals has
mutation in the Nef gene, indicating the importance of this
gene in pathogenesis.
The Nef protein decreases class I MHC protein synthesis,
and the inability of the mutant virus to produce functional
Nef protein allows the cytotoxic T cells to retain their
activity.
2) Production of large amounts of ALPHA- defensins which
has an antiviral activity in addition to its antibacterial
activity.
ALPHA-Defensins interfere with HIV binding to the
CXCR4 receptor and block entry of the virus into the cell.
Immunity
1) The main immune response to HIV infection consists of
cytotoxic CD8-positive lymphocytes. These cells respond
to the initial infection and control it for many years. It is
the ultimate failure of these cytotoxic T cells that results
in the clinical picture of AIDS.Cytotoxic T cells lose their
effectiveness because so many CD4-helper T cells have
died; thus , the supply of lymphokines, such as IL-2,
required to activate the cytotoxic T cells is no longer
sufficient.
2) HIV has three main mechanisms by which it evades the
immune system:
A. Integration of viral DNA into host cell DNA, resulting in
a persistent infection.
B. A high rate of mutation of the env gene.
C. The production of Tat and Nef proteins that downregulate
class I MHC proteins required for cytotoxic T cells to
recognize and kill HIV-infected cells. The ability of HIV
to infect and kill CD4-positive helper T cells further
enhances its capacity to avoid destruction by the immune
system.
Clinical finding
The clinical picture of HIV infection can be divided into
three stages: an early acute stage, a middle latent stage
and a late immunodeficiency stage.
Acute stage:
Begins 2- 4 weeks after infection with mononucleosis –
like picture of fever, lethargy, sore throat, and generalized
lymphadenoppathy occurs .Maculopapular rash on the
trunk, arms, and legs (sparing the palms and soles).
Leukopenia with normal number of CD-4
+
T cells.
Unit 4: Virology
211
High level of viremia and the infection is highly
transmissible.
This stage resolves spontaneously in about 2 weeks
accompanied by a lower level of viremia and a rise in the
number of CD-8
+
T cells directed against HIV.
Abs against HIV typically appears 10-14 days after
infection, and most will have seroconverted by 3-4 weeks
after infection. Window period prior to this period in
which the patient is infected with HIV but antibodies are
not detected leading to false negative results and 87% of
infected patients are asymptomatic.
After initial viremia a virus set point can occur which
represents the amount of virus produced (viral load) and
tend to remain constant for years.
Middle latent stage:
In untreated patient, this period lasts for 7-11 years and
the patient is a symptomatic and viremia is low or absent.
A large amount of HIV is being produced by lymph node
cells but remains sequestered within lymph nodes
A syndrome called AIDS-related complex (ARC) can
occur during this stage and manifested by persistent fever,
weight loss, fatigue and lymphadenopathy.ARC often
progress to AIDS.
Late stage:
The late stage of HIV infection is AIDS , manifested by
decline in the number of CD-4 cells to below 400/ µl
An increase in the frequency and severity of opportunistic
infection. The two most important is pneumocystis
pneumonia and Kaposi's sarcoma in addition to viral,
fungal , and bacterial infections.Neurologic problems also
occur e.g. dementia and neuropathy.
Laboratory diagnosis:
1) The presumptive diagnosis of HIV is made by the
detection of antibodies by ELISA.
2) Because there are some false –positive results with this test,
the definitive diagnosis is made by Western blot analysis in
which the viral proteins are displayed by acrylamide gel
electrophoresis, transferred to nitrocellulose paper and react
with patient´ s serum. If antibodies are present, they will
bind to the viral proteins. Enzymatically labeled Ab to
human IgG is then added .A color reaction reveals the
presence of HIV Ab in the serum.
3) Ora Quick is a rapid screening immunoassay for HIV Ab
detection within 20 minutes that uses a blood sample
obtained by finger prick. Positive Results confirmed by
western blot test.
4) Tissue culture. To assess drug resistance and for the
diagnosis of HIV infection in newborns whose mothers
are HIV
+
.
5) PCR to detect HIV DNA within infected cells and for
detection of viral load.PCR also can be used in the
diagnosis of HIV infection in neonate of HIV
+
mothers.
During the first month after infection, Ab tests may be
negative (window periods).The diagnosis of acute HIV
infection may not be made by using serologic tests. The
presence of HIV can be detected either by viral culture,
p24 antigen test, or PCR assay. Approximately 10-20
days after infection, an increase in HIV RNA can be
detected by PCR assay and by 30 days after infection, an
increase in p24 antigen can be seen in patients whose Ab
test results are negative.
Treatment
Management of HIV involves both treatment of the virus
and prevention of opportunistic infections. The aims of
HIV treatment are to:
reduce the viral load to an undetectable level (< 50
copies/ml) for as long as possible
improve the CD4 count (above 200 cells/mm
3
significant
HIV-related events rarely occur)
increase the quantity and improve the quality of life
without unacceptable drug-related side-effects or lifestyle
alteration
Reduce transmission (mother-to-child & person-to-person).
Treatment with single drugs is liable to develop resistance
to that drug because of high mutation rate in HIV.
The current treatment of choice for advanced disease is a
regimen consisting of two nucleoside (-tide) reverse
transcriptase inhibitors plus a protease inhibitor. This
combination is known as HAART (Highly Active
Antiretroviral Therapy).
In 2003, a new drug emerged, it is fusion inhibitors, and
i.e. they prevent the fusion of the viral envelope with the
cell membrane. The drug is called enfuvertide (Fuzeon).It
is a synthetic peptide that binds to gp 41 on the viral
envelope, thereby blocking the entry of HIV into the cell.
It is given by injection.
Nucleoside (tide) reverse transcriptase inhibitors:
1) Abacavir 2) Didanosine (dideoxyinosine, ddI)
3) Lamivudine 4) Stavudine 5) Tenofovir
6) Zalcitabine 7) Zidovudine (ZDV,AZT)
Non-nucleoside reverse transcriptase inhibitors (NNRTI):
1) Nevirapine 2) Delavirdine 3) Efavirenz
Protease inhibitors:
1- Saquinavir 2- Ritonavir
3- Nelfinavir 4- Indinavir

Unit 4: Virology
215
Human T-cell lymphotropic virus (HTLV-1)
Human T-cell lymphotropic virus -1(HTLV-1) causes 2
distinctly disease:
a) A cancer called Adult T-cell leukemia /lymphoma
(ATL).
b) A neurologic disease called HTLV-associated
myelopathy (HAM) (spastic paraperesis, chronic
progressive myelopathy).
HTLV-2 also appears to cause these diseases, but the
association is less clearly documented.
Important properties
Two copies of SS positive polarity RNA enveloped virus
with reverse transcriptase in the virion.HTLV infects T
cell lymphocyte and cause malignant transformation of
the infected T cell but don’t kill them.
HTLV genome
HTLV genome is more stable than that of HIV.
Three structural genes common to all retroviruses (gag,
pol, env (gp 46, gp21)) plus two regulatory genes, tax
and rex (similar in action to tat and rev).
HTLV don’t possess an oncogene in its genome and does
not integrate its proviral DNA at a specific site near a
cellular oncogene, but it activate transcription of both
cellular and viral mRNA synthesis by the Tax protein that
initiates oncogenesis.The tax protein activates the
synthesis of IL-2 and of the IL-2 receptors.IL-2 promotes
rapid T-cell growth and eventually malignant
transformation of the T-cell.
Replicative cycle
As for HIV.
Transmission and epidemiology
1) Intravenous drug use.
2) Sexual contact.
3) Breast feeding
4) Blood transfusion. This can greatly reduced by screening
of donated blood for antibodies to HTLV and discarding
those that are positive.
5) Transmission by processed blood products, such as
immunglobulins has not occurred.
6) Trancplacental transmission has been rarely documented.
7) Transmission is thought to occur primarily by the transfer
of infected cells rather than free extracellular virus.
8) HTLV is endemic in certain geographic areas, The
Caribbean region including southern fluoride, eastern
South America, western Africa and Southern Japan.
Pathogenesis
ATL in which HTLV infection of CD4-positive T
lymphocytes induce malignant transformation. HTLV
remain latent within malignant cells.
HAM is a demylinating disease of the brain and spinal
cord, especially of the motor neuron in the spinal cord.
HAM is caused either by an autoimmune cross-reaction in
which the immune response against HTLV damage the
neurons or by cytotoxic T cells that kill HTLV-infected
neurons.
Clinical feature
1) ATL
It is characterized by lymphadenopathy,
hepatosplenomegaly, lytic bone lesion, and skin lesions,
hypocalcaemia due to increase osteoclast activity,
opportunistic infections.
2) HAM
Gait disturbance, weakness of the lower limbs, and low
back pain, loss of bowel and bladder control, loss of
motor function is much greater than sensory loss. HAM
occurs primarily in women of middle age. HAM
resembles multiple sclerosis but without the remission
characteristic of multiple sclerosis.
Laboratory diagnosis:
1) ELISA test for Ab detection in patient s serum. Positive
test is confirmed by western blot assay
2) PCR assay for HTLV RNA or DNA within infected cells.
3) ATL is diagnosed by finding malignant T cells in the
lesion.
4) HAM is diagnosed by HTLV Ab in the spinal fluid or
finding HTLV nucleic acid in cells in spinal fluid.
Treatment
There is no specific antiviral treatment for HTLV
infection, and no antiviral drug will cure latent infections
by HTLV.
ATL is treated with anti-cancer chemotherapy regimens
Corticosteroids and danazol have produced improvement
in some patient with HAM.

Unit 4: Virology
211
Lecture 11+12 - DNA enveloped
viruses
Herpesviruses
The herpesvirus family contain six important human
pathogens
1) Herpessimplex virus type 1
2) herpessimoplexvirus type 2
3) varicella zoster virus
4) cytomegalovirus
5) Epstein-Barr virus
6) Human herpes virus 8 (the cause of kaposi´s sarcoma).
All herpesviruses are structurally similar:
1) Enveloped with icosahedral symmetry.
2) Linear DS-DNA.
3) No virion polymerase.
4) They are large in size second in size to pox virus.
5) Replication in nucleus
6) Tegument (play a role in replication) located between
nucleocapsid and the envelope.
They produce latent asymptomatic infection following the
primary acute infection. Some have symptoms of the
latent similar to that of the primary while the other the
symptoms of the latent infection are differ from that of
primary one.
Three of herpesvirus (HSV-1,2 .VZV) cause a vesicular
rash both in primary and in reactivation.
Four herpesvirus induce formation of multinucleated giant
cells ( HSV1,2,VZV,and CMV).
Certain herpes virus cause cancer in man ( e.g: Epestein
Barre virus).
The herpesvirus family can be subdivided into three
categories base on the type of cell most infected and the
site of latency.
Alpha herpesvirus: HSV1,2 VZV) infect epithelial
cells and cause latency in neurons.
Beta herpesviruses: ( CMA,HH6) infect and become
latent in a variety of tissue.
Gamma herpesviruses: Epestein_Barr virus and
HHV8) infect and become latent in lymphoid cells.
Herpesvirus 1&2
They are structurally and morphologically
indistinguishable. They can be distinguished by their
antigenicity and location of lesions.
Replicative cycle (as shown in the figure below)
1) HSV-1 binds first to heparin sulfate on the cell surface
and then to second receptor, nectin.
2) Following fusion of the viral envelope with the cell
membrane, the nucleocapsid and the tegument proteins
are released into the cytoplasm.
3) Viral NC is transported to the nucleus, where it docks to a
nuclear pore and the genome DNA enters the nucleus
along with tegument protein VP 16. the linear genome
DNA now becomes circular.
4) VP 16 interacts with cellular transcription factors to
activatew transcription of viral immediate early (IE) genes
by host cell RNA polymerase.
5) IE mRNA is translated into IE proteins that regulate the
synthesis of early proteins such as DNA polymerase
(repilicate the genome) and thymidine Kinase .
6) Viral DNA polymerase replicate the genome DNA at
which early protein synthesis is shut off and late protein
synthesis begin.
7) The late, structural protein are transported to the nucleus
where virion assembly occurs by budding through nuclear
membrane.
Note: immediate early proteins are proteins whose mRNA
synthesis is activated by a protein brought in by incoming
parental virion whilt early protein do require the synthesis
of new viral regulatory proteins to activate the
transcription of their mRNAs.
Transmission and epidemiology
HSV-1 is transmitted primarily by saliva.
HSV-2 is transmitted by sexual contact.
Pathogenesis and Immunity:
1) Virus replicate in the skin or mucous membrane at the
initial site of infection, then migrates up the neurons by
retrograde axonal flow and becomes latent in the sensory
ganglion cell.
2) HSV-1 becomes latent in trigeminal ganglia
3) HSV-2 becomes latent in the lumbar and sacral ganglia.
4) During latency, most- if not all-viral DNA is located in
the cytoplasm rather than integrated into nuclear DNA.
5) The typical lesion is a vesicle and when vesicle rupture,
virus is librated and can be transmitted to other individual.
6) Multinucleated giant cells are typically found at the base
of herpevirus lesion.
7) Immunity is type specific and incomplete and both
reinfection and reactivation occur in the presence of
circulating IgG.
8) There is viremia in HSV-2 with no viremia in HSV-1.

Unit 4: Virology
211

Unit 4: Virology
211
Clinical findings:
HSV-1 cause the following diseases.
1) Gingivostomatitis (vesicles in the mouth) mainly in
children.
2) Herpes labialis
3) Keratoconjuctivitis.
4) Encephalitis.
5) Herpetic whitlow ( pustular lesion of the skin of the finger
or hanbds) senn in dentists and hospital peresonale.
6) Herpetic gladiatorum (vesicular lesion on the head, neck,
and trunk). Seen on wrestlers bodies.
7) Disseminated infection (oesophagitis and pneumonia).
HSV-2 causes the following:
1) genital herpes
2) Neonatal herpes
3) Aseptic meningitis.
Both HSV-1 and HSV-2 are associated with erythema
multiforme.
Laboratory diagnosis:
1) Isolation of the virus by cell culture
2) A rapid diagnosis from skin lesions can be made by using
the Tzanck smear, in which cells from the base of the
vesicle are stained with Giemsa stain. The presence of
MNG cells suggest herpesvirus infection.
3) Encephalitis diagnosed by PCR for HSV-1 DNA.
4) Serological test such as neutralization test can be used in
the diagnosis of primary infection because of significant
increase in Ab titer, but it is of no benefit in recurrence.
Treatment
1) Acyclovir is the treatment of choice for encephalitis and
systemic disease caused by HSV-1, and treatment of the
primary and recurrent genital herpes; it shortens the
duration of the lesion and reduces the extent of shedding.
2) Foscarnet in mutant strain of HSV-1
3) Trifluridine used in the treatment of eye infection
topically.
4) Penciclovir in the treatment of recurrent orolabial HSV-1.
5) Valacyclovir and famiciclovir in the treatment of genetal
herpes.
Note:
1) Drug is effective in preventing the recurrence after
primary infection.
2) Drugs have no effects on latent state.
Varicella- zoster virus (VZV) Disease
Varicella – chicken pox (primary disease)
Zoster - shingles is the recurrent form.
VZV has a single serotype
Immunity following varicella is lifelong but zoster can
ocurr despite immunity to varicella.
The virus is transmitted by respiratory droplets and by
direct contact with the lesion. Varicella is highly
contagious disease of childhood. It is a mild disease in
children and severe in adult.
VZV infects the mucosa of the URT then spread via the
blood to the skin. The virus infects sensory neurons and is
carried by retrograde axonal flow into the cells of the
dorsal root ganglia where the virus become latent.
Clinical findings:
Varicella:
Incubation period = 14-21 days.
A brief prodromal symptoms of fever, malaise occur
followed by papulovesicular rash in crops on the trunk
and spreads to the head and extremities. The rash evolves
from papules, vesicle pustules and finally crusts.
Zoster
The occurrence of painful vesicles along the course of a
sensory nerve of the head or trunk.
Laboratory diagnosis
It is mainly clinical.
Treatment
No antiviral therapy is required in immunocompetent
patient.
Therapy by acyclovir is indicated in immunocompetent
patient with moderate to severe infection and in
immunocompromised children and adults.
Prevention
There are two live attenuated VZV vaccine:
Varivax against varicella (recommended for children
between 1-12 years)
Zostavax against zoster but does not eradicate the latent
state of VZV (recommended for people older than 60
years and who have had varicella).
In immunocompromised patients prevention is by
acyclovir or Varicella zoster immunoglobulin (VZIG).

Unit 4: Virology
211
Cytomegalovirus (CMV)
Diseases:
Cytomegalic inclusion bodies in neonates. CMV is a very
important of pneumonia and other diseases in
immunocompromised patient. It also causes a heterophil
negative infectious mononucleosis like syndrome.
It has a single serotype.
Replicative cycle:
It is similar to that of HSV but in CMV, some of its
immediate early proteins are translated from mRNA
brought into the infected cell by parental virion rather
than being translated from mRNAs synthesized in the
newly infected cell.
Transmission
Early in life= transplacental, from birth canal, and in
breast milk.
In young children = mainly via saliva
Later in life = sexually; it is present in both semen and
cervical secretions. It can also transmitted by blood
transfusion, and organs transplantation.
About 80% of adults have antibody against the virus.
Pathogenesis
CMV causes cytomegalic inclusion diseases in infants
characterized by MNG cell with prominent intranuclear
inclusions. Many organs are affected. The infection is
acquired if the mother infected during pregnancy and
congenital abnormalities are more common if the
infection is acquired during the first trimester.
Infections of children and adults are usually asymptomatic
and the virus enter a latent state primarily in the
monocytes and can be reactivated when cell mediated
immunity is decreased.
Reactivation of CMV from the latent state in cervical cells
can result in infection of the new born during passage
through the birth canal.
How the virus escape the immune system?
1) In viral infected cell, assembly of the MHC class I viral
peptide complex is unstable, so viral antigens are not
displayed on the cell surface and killing by Tc does not
occur.
2) CMV encodes several microRNAs, one of which binds to
and prevents the translation of the cells mRNA for the
Class I- MHC proteins.
Clinical findings
1) About 20% of infants infected with CMV during gestation
show clinically apparent manifestations of cytomegalic
inclusion disease such as microcephaly, seizure, deafness,
jaundice, & purpura. Hepatosplenomegaly is very common
2) In immunocompetent adults, CMV causes heterophil
negative mononucleosis which is characterized by fever,
lethargy and the presence of abnormal lymphocytes in
peripheral blood smears.
3) In immunocompromised patients, it causes systemic
infection like pneumonitis and hepatitis.it is a common
cause of retinitis in AIDS patient which lead to blindness.
Laboratory diagnosis
1) Culture in shell vial culture coupled with the use of
immunoflourescent antibody which make Diagnosis
within 72 hours.
2) Fluorescent antibody and histologic staining of inclusion
bodies in giant cells in urine and tissue. The inclusion
bodies are intranuclear and have an oval owls-eye shape.
3) A fourfold increase in antibody titer
4) PCR for CMV DNA in tissue or body fluids (spinal or
amniotic).
5) CMV antigenaemia can be measured by detecting pp65
within blood leukocytes using an IF assay. Pp65 is a
protein located in the nucleocapsid of CMV.
Treatment
Ganciclovir is moderately effective in the treatment of
CMV retinitis and pneumonia in AIDS patient.
Valganciclovir is also used for retinitis
Foscarnet
Cidofovir is also useful in the treatment of retinitis.
Fomivirsen is an antisense DNA approved for intraocular
treatment of retinitis.
Prevention
There is no vaccine against CMV
Ganciclovir can suppress retinitis in AIDS patient.
Infants with Cytomegalic inclusion disease who are
shedding virus in their urine should be kept isolated from
other infants.
A high titer of immunoglobulin preparation is used to
prevent disseminated CMV infections in organ transplant
patients.

Unit 4: Virology
211
Epstein-Barr virus (EBV)
Diseases:
EBV causes infectious mononucleosis. It is associated
withy Burkitt's lymphoma, other B-cell lymphomas and
nasopharyngeal carcinoma.
EBV causes hairy leukoplakia.
Properties
Antigens of EBV
1) Viral capsid antigen (VCA) is mostly used in the
diagnosis.
2) Early antigen (EA) are produced prior to viral DNA
synthesis.
3) Nuclear antigen (EBNA)
4) Lymphocyte determined membrane antigen.
5) Viral membrane antigen.
Neutralizing activity is directed against the viral
membrane antigen.
EBV infects mainly lymphoid cells, primarily B-
lymphocytes. It also infects the epithelial cells of the
pharynx.
Transmission and epidemiology
It is transmitted primarily by saliva
Not transmitted by blood transfusion.
Infection in the first few years of life is asymptomatic.
The frequency of clinically apparent IM is highest in
those who are exposed to the virus later in life (e.g.,
college students).
Pathogenesis
The infection first occurs in nasopharynx and then spread
to blood, where it infects B lymphocytes. Cytotoxic T cell
react against the infected B cell. The T cells are the
"Atypical lymphocytes" seen in bloods. The virus
becomes latent within B- lymphocytes.
Immunity
1) First Antibody response is IgM against VCA followed by
IgG and persist for life.
2) Lifetime immunity against second episodes of IM is based
on antibody to the viral membrane antigen.
3) Nonspecific heterophil antibodies are also found.
Heterophil refers to antibodies that are detected by tests
using antigens different from the antigens that induced
them. The heterophil antibodies formed in infectious
mononucleosis agglutinate sheep or horse red blood cells
in laboratory.
Note that these antibodies don’t reacts with any
components of EB!. How?
The answer is that EBV likely modifies a cell membrane
constituent such that it becomes antigenic and induces the
heterophil antibody. These antibodies disappear 6 months
after recovery. These antibodies are not specific for EBV
infection and are also seen in individuals with hepatitis B
and serum sickness.
Clinical findings
Infectious mononucleosis is characterized primarily by
fever, sore throat, lymphadenopathy, and splenomegaly.
Anorexia and lethargy is prominent.
Hepatitis is frequent. Encephalitis could occur.
Spontaneous recovery usually occurs 2-3 weeks. Splenic
rupture is associated with sport activities such as football.
Laboratory diagnosis:
1) Haematologic approach absolute lymphocytosis occurs as
many as 30 % abnormal lymphocytes. These atypical
lymphocytes are enlarged with expanded nucleus and an
abundant often vacuolated cytoplasm. They are cytotoxic
T-cells that are reacting against the EBV-infected B-cell.
2) Immunologic
A. Monospot test for heterophil antibody test.
B. The EBV –specific antibody test are user primarily in
the diagnosis. (VCA,EA,and EBNA).
Treatments
No antiviral test is necessary for uncomplicated IM.
Acyclovir has little activity against EBV, but high doses
may be useful in life threatening EBV infections.
Prevention
There is no EBV vaccine.
Human herpesvirus-8 (Kaposi's sarcoma
associated Herpes virus)
The virus is transmitted sexually and also may be by
organ transplantation.
It causes kaposi's sarcoma by malignant transformation by
the mechanism of inactivation of tumor suppressor gene.
Poxviruses
It includes three viruses:
1) Small pox virus.
2) Vaccine virus.
3)
Mollescum contagiosum virus
.
Unit 4: Virology
212
Lecture 13 - DNA Non-enveloped Virus
I- Adenoviruses
They are non-enveloped with icosahedral symmetry, DS-
DNA. They are the only viruses with a fiber protruding
from each of the 12 vertices of the capsid. The fiber is the
organ of attachment and is a haemagglutinin. The free
fibers are toxic to man.
There are 41 known antigenic types. The fiber protein is
the main type specific antigen. Group specific antigen
located on the hexon protein.
Certain serotypes cause sarcoma at the site of injection in
laboratory rodents such as newborn hamsters. There is no
evidence that adenoviruses cause tumors in human.
Replicative cycle
1) After attachment to the cell surface via its fiber, the virus
penetrates and uncoats, and the viral DNA moves to the
nucleus.
2) Host cell DNA-dependant RNA polymerase transcribes
the early gene resulting in mRNA.
3) Early mRNA is translated into nonstructural proteins in
the cytoplasm.
4) After viral DNA replication in the nucleus, late mRNA is
transcribed and then translated into structural virion
proteins.
5) Viral assembly occurs in the nucleus, and the virus is
released by lysis of the cell, not by budding.
Transmission
1) aerosol droplet
2) fecooral route in young children
3) direct inoculation of conjuctivas by tonometers or fingers
Epidemiology
Serotypes 3,4,7,21 cause respiratory disease.
Types 8 and 19 cause epidemic conjunctivitis
Types 11,21 cause hemorrhagic cystitis.
Type 40, 41 cause infantile gastroenteritis.
Pathogenesis
Adenovirus infects upper and lower respiratory tracts,
gastrointestinal tract, and conjunctivas.
Adenoviruses may become latent in adenoidal and
tonsillar tissues of the throat.
Clinical findings
Upper respiratory tract: pharyngitis, pharyngo-
conjuctival fever, and acute respiratory disease
characterized by fever, sore throat & coryza &
conjunctivitis
Lower respiratory tract: bronchitis & atypical pneumonia
GIT: Non bloody diarrhea
Haematuria and dysuria in hemorrhagic cystitis.
Laboratory diagnosis
Isolation by culture and fourfold increase in antibody titer
Treatment
No antiviral therapy.
Prevention
Three live non attenuated vaccines against serotype 4, 7,
and 21. each f the three vaccine are monovalent. And they
are given separately for each serotype to prevent
interference if given together. The vaccine is delivered by
an enteric coated capsule, which protects the live virus
from inactivation by stomach acid. The virus infects the
GIT, where it causes an asymptomatic infection and
induces immunity to respiratory disease. It is available for
only military.
II- Papilloma virus
Human papilloma virus causes papilloma which is a
benign tumor of squamous cells. Some HPV types
especially type 16,18 cause carcinoma of the cervix, penis
and anus.
Properties
HPV are non-enveloped viruses with DS circular DNA
and an icosahedral nucleocapsid. Two of the early genes
(E6,E7) are implicated in carcinogenesis. They encodes
proteins that inactivate proteins encoded by tumor
suppressor genes in human calls (e.g., p53 and the
retinoblastoma (RB) genes). Inactivation of these two
proteins are important step in the process by which a
normal cell become a cancer cell.
There are at least 100 types of papilloma viruses. Type 1-
4 cause skin warts, type 6and 11 cause genital warts
(condylomata acuminata) .
About 30 types infect the genital tract.
Replicative cycle.
1) the virus cant grown in cell culture
2) in human tissue, infectious viral particles are found in the
terminally differentiated squamous cells rather than in the
basal cells.

Unit 4: Virology
211
3) The HPV initially infects the cells of the basal layer in the
skin, but no virus is produced by those cells. Rather,
infectious virions are produced by squamous cells on the
surface which enhances the likelihood that efficient
transmission will occur.
4) In malignant cells, viral DNA is integrated into the host
cell DNA in the vicinity of cellular proto-oncogenes,& E6
and E7 are over- expressed. But in latently infected non-
malignant cells, the viral DNA is episomal and E6 and E7
are not over-expressed. This differences occurs because
another early gene,E2, control E6 and E7 over-expression.
The E2 gene is functional when the viral DNA is
episomal but it is inactivated when it is integrated.
Transmission and epidemiology
HPV are transmitted by skin to skin contact. Genital warts
are among the most common sexually transmitted disease.
Skin warts are more common among children and young
adults. They tend to regress in older adults.
HPV transmitted from an infected mother to neonate
during child birth causes warts in the mouth and the
respiratory tract especially the larynx of the infant.
The virus infect squamous epithelial cells and induce
characteristic cytoplasmic vacuoles, these cells called
Koilocytes.
Laboratory diagnosis
Infections are usually diagnosed clinically.
A PCR based test using Cobas 4800 system is approved to
detect the presence of DNA of 14 high risk genotypes,
including type 16 and 18.
Diagnostic test based on detection of antibodies in a
patients serum or on isolation of the virus from a patients
tissue are not used.
Treatment and prevention
The usual treatment of genital warts is Podophyllin
Liquid nitrogen is used in the treatment of skin warts
Plantar warts is treated surgically with topical salicylic acid
Cidofovir is used in the treatment of severe HPV infection.
There are two vaccines against HPV
Gardasil contains capsid protein of 6,11, 16,18.
Cervarix is a recombinant vaccine and contain the
protein of types 16 and 18 only.
The role of cesarean section in preventing the transmission
of HPV from the mother to the baby is uncertain
Circumcision reduces the risk of infection by HPV.
III- Parvoviruses
Parvovirus B19 is a SSDNA none enveloped negative
sense with icosahedral symmetry virus. The virus has no
virion polymerase.
There is one serotype only.
It is transmitted by respiratory droplets, transplacental.
Blood transfusion can transmit the virus.
Clinical features.
1) Erythema infectiosum (slapped cheek syndrome, fifth
diseae.)
2) Aplastic anemia especially in patient with sickle cell
anemia, thalassemia and spherocytosis.
3) Fetal infection. If the infection is acquired during the first
trimester (fetal death) or in the second trimester (hydrops
fetalis). The virus is not associated with any congenital
anomalies.
4) Arthritis
5) Chronic B19 infection especially in pt with immunodeficiency.
Laboratory diagnosis
Fifth disease and aplastic anemia is diagnosed by
detecting IgM antibodies. Fetal infection is diagnosed by
PCR analysis of amniotic fluid.
Treatment
No specific treatment of B19 infection
Pooled immunoglobulin may have a beneficial effect on
chronic B19 infection in patient with immunodeficiencies.
IV- Polyomaviruses
2 members of the polyomavirus family that infect human
1)
JC
(causes progressive multifocal
leukoencephalopathy (PMLE)) BK (nephropathy in
renal transplant patients ) viruses.
2)
One member of SV 40
virus that is primarily a
monkey virus but has infected humans when it
contaminated the poliovirus vaccine..
Unit 4: Virology
211
Lecture 14+15+16 - Viral Hepatitis
Viral hepatitis is a systemic disease primarily involving
the liver. Most cases of acute viral hepatitis in children
and adults are caused by one of the following viruses:
HAV, HBV, HCV, HDV the defective virus dependent on
co-infection with HBV, and HEV. There are additional
viruses that can cause sporadic hepatitis: yellow fever
virus, CMV, EBV, herpes virus, rubella virus and
enteroviruses.
Hepatitis viruses produce acute inflammation of the liver,
resulting in clinical illness characterized by fever, GIT
symptoms (Nausea , vomiting and jaundice).
Regardless of the virus type, identical histopathologic
lesions are observed in the liver during acute disease.
Hepatitis A virus
Disease
HAV causes hepatitis A.
Properties
HAV is a typical enterovirus classified in the picorna
virus family. It has single-stranded RNA genome and
non-enveloped icosahedral nucleocapsid and replicate in
the cytoplasm of the cell. Replicative cycle is similar to
that of other enteroviruses.
HAV is stable to treatment with 20% ether , acid (pH 1
for 2 hr),, and heat (60 c for 1 hr) and its infectivity can be
preserved for at least one month after being dried and
stored at 25 c or years at -20c .
The virus is destroyed by boiling in water for 5 min.,
autoclaving ( 121 c for 20 min.), dry heat ( 181 c for 1hr),
UV light (1 min. at 1.1 watt), treatment with formalin
(1:4000 for 3 days at 37 c) or treatment with chlorine (
10-15 ppm for 30 min.).
Heating food to above 85 c for 1 min. and disinfecting the
surfaces with sodium hypochlorite (1:100 dilution of
chlorine bleach) are necessary to inactivate HAV.
Transmission and epidemiology
1) HAV is transmitted by feco-oral route.
2) Humans are the reservoir for HAV.
3) Virus is detected in the stool from about 2 weeks before
the onset of the jaundice up to 2 weeks after.
4) Children are the most frequently infected group, and
outbreaks occur in special living situations such as
summer camps, and boarding school.
5) Common source of outbreak is from fecally contaminated
water or food.
6) HAV rarely transmitted via blood, because the level of
viremia is low and chronic infection doesn't occur.
7) There is no chronic hepatitis and no chronic carrier state.
There is no predisposition to hepatocellular carcinoma.
Clinical findings
The clinical manifestations of hepatitis are virtually the
same regardless of which hepatitis virus is the cause.
Fever, anorexia, nausea, vomiting, and jaundice arte
typical. Dark urine, pale feces, and elevated transaminase
level are seen. Most cases resolve spontaneously in 2-4
weeks.
HAV incubation period is about 3-4 weeks. Most case of
HAV infections are asymptomatic, and are detected solely
and are detected by the presence of IgG antibody.
Laboratory diagnosis
1) IgM specific Anti-HAV in the acute phase. Peaking 2
weeks after elevation of liver enzyme. Anti-HAV IgM
usually decline to non-detectable level within 3-6 months
2) Anti-HAV IgG appears soon after the onset of disease and
persists for decades. Four fold increase in antibody titer
can also be used in the diagnosis.
3) Isolation of the virus from cell culture is possible but not
used for routine clinical work.
Treatment and prevention
No antiviral therapy is available.
1) Active immunization with vaccine containing inactivated
HAV is available. The vaccine is given in 2 doses: an
initial dose and a booster 6-12 months later. No further
booster dose is required.
The vaccine is indicated in travelers to highly endemic
area, and in children age 2-18 months. The vaccine is
effective in post-exposure if it is given within 2 weeks
after the exposure. There is a combination of HAV with
HBV vaccine ( Tminrix).
2) Passive immunization with immune serum globulin prior
to infection or within 14 days after exposure can prevent
or mitigate the disease.
3) Proper hygiene is very important in the preventive
measures.

Unit 4: Virology
211
Hepatitis E virus (HEV)
HEV is a major cause of hepatitis transmitted by the feco-
oral route.
The virus is classified in the virus family, hepeviridae, in
the genus hepevirus. HEV resembles, but is distinct from
calciviruses.
HEV is SS – RNA non-enveloped positive sense virus.
Clinically, the disease resembles HAV with exception of a
high mortality rate in pregnant women (20%).
Chronic liver disease doesn’t occur, and there is no
prolonged carrier state.
In acute infection, IgM antibodies to HEV are positive.
There is no antiviral therapy.
In 2007, a recombinant vaccine against HEV was shown
to be safe and effective and it is available in endemic area.
Hepatitis B virus
Disease
HBV causes hepatitis B.
Properties
HBV is a member of the hepadnavirus family. It is a 42-
nm enveloped virion (known as Dane particle (named for
the scientist who first published electron micrographs of
the virion)), with an icosahedral nucleocapsid core
containing a partially double-stranded circular DNA
genome. The envelope contains a protein called the
surface antigen (HBsAg), which is important for
laboratory diagnosis and immunization (HBsAg was
known as Australia antigen because it was first found in
the serum of an Australian aborigine).
Within the core is a DNA-dependent DNA polymerase.
The genome contains 4 genes that encode five proteins,
the S gene encodes the surface antigen, the C gene
encodes the core Ag and the e Ag, the P gene encodes the
polymerase, and the X gene encodes the X protein. The X
protein is an activator of viral RNA transcription.
The DNA polymerase has both RNA-dependent (reverse
transcriptase) and DNA-dependent activity.
Electron microscopy of a patient’s serum reveals 3
different types of particles:
A few 42-nm virions and many 22-nm spheres and long
filaments 22nm wide, which are composed of surface Ag.
In addition to HBsAg, there are two other important
antigens: the core antigen (HBcAg) forms the
nucleocapsid core of the virion and the "e" antigen
(HBeAg) is secreted from infected cells into the blood.
The e antigen is an important indicator of transmissibility.
For vaccine purposes, HBV has one serotype based on
HBsAg. However, for epidemiologic purposes, there are
four serologic subtypes of HBsAg based on a group
specific antigen, "a" and two sets of manually exclusive
epitopes, d or y and w or r. this lead to four serotypes:
adw,adr,ayw,and ayr.
The specificity of HBV for liver cells is based on two
properties: virus- specific receptors located on
hepatocyte cell membrane (facilitate entry) and
transcription factors found only in the hepatocyte that
enhance viral mRNA synthesis (act postentry).
The stability of HBsAg doesn’t always coincide with that of
the infectious agent. However, both are stable at -20c for
more than 20 years and stable to repeated freezing and
Unit 4: Virology
215
thawing. The virus is stable at 37 c for 60 min. & remains
viable after being dried and stored at 25 c for at least 1 week
HBV (but not HBsAg) is sensitive to higher temperature
(100c for 1 minute) or to longer incubation periods (60 c
for 10 hours).
HBsAg is stable at pH of 2.4 for up to 6 hours, but HBV
infectivity is lost. Sodium hypochlorite, 0.5% (e.g, 1:10
chlorine bleach), destroys antigenicity within 3 minutes at
low protein concentrations, but undiluted serum
specimens require higher concentrations (5%). HBsAg is
not destroyed by UV irradiation of plasma or other blood
products, and viral infectivity may also resist such
treatment.
Replicative cycle
1) After entry of the virion into the cell and its uncoating.
2) Virion DNA polymerase synthesizes the missing portion
of DNA, and a double stranded closed-circular DNA is
formed in the nucleus. This DNA serves as template for
mRNA synthesis by cellular RNA polymerase.
3) After mRNAs are made, a full length positive- strand
transcript is made, which is a template for the minus
strand of the progeny DNA. The minus strand then serves
as the template for the plus strand of the genome DNA.
This RNA-dependent DNA synthesis catalyzed by reverse
transcriptase encoded by HBV takes place within the
newly assembled virion core in the cytoplasm.
The RNA-dependent DNA synthesis that produces the
genome and the DNA-dependent DNA synthesis that fills
in the missing portion of DNA soon after infection of the
next cell are carried out by the same enzyme (i.e., the
HBV genome encodes only one polymerase).
Hepadnaviruses are the only viruses that produce genome
DNA by reverse transcription with mRNA as the
template. (This type of RNA –dependent DNA synthesis
is similar to but different from the process in retroviruses,
in which the genome RNA is transcribed into a DNA
intermediate).
Some of the progeny DNA integrates into the host cell
genome, and this seems likely to be the DNA that
maintains the carrier state. Progeny HBV with its HBsAg
containing envelope is released from the cell by budding
through the cell membrane.
Transmission and epidemiology
Three main methods for transmission:
1) via blood
2) During sexual intercourse.
3) Perinataly from infected mother to newborn. Also by
breast feeding.
4) Needle stick injuries can transmit the virus indicating that
only very small amounts of bloods are necessary.
5) HBsAg can be detected in saliva, nasopharyngeal
washings, semen, menstrual fluid, and vaginal secretions .
6) Transmission from chronic carrier to close contacts by
oral route or by sexual or other intimate exposure occurs.
7) Transmission by feco-oral route has not been
documented.
8) Health care personnel have a higher incidence of hepatitis
and prevalence of detectable HBsAg or Anti-HBs than
those who have no occupational exposure to patients or
blood products.
9) HB infections are more common among patients and staff
of hemodialysis unit. As many as 50% of the renal
dialysis patients who contract HB may become chronic
carrier of HBsAg compared with 2% of the staff group.
Family contacts are also at increased risk.
Pathogenesis and immunity
1) After entering the blood, the virus infects hepatocytes and
the viral antigens are displayed on the surface of the cell.
2) Cytotoxic T-cells mediate an immune attack against the
viral antigens, and inflammation and necrosis occur.
3) The pathogenesis of hepatitis B is probably the result of
this cell mediated immune injury and HBV itself doesn’t
cause a cytopathic effect.
4) Antigen-antibody complexes cause some of the early
symptoms (e.g. arthralgia, arthritis and urtecaria) and
some of the complications in chronic hepatitis (e.g.,
glomerulonephritis, cryoglobulinemia, and vasculitis).
5) About 5% of patients infected with HBV become chronic
carrier and a chronic carrier is defined as patient who has
HBsAg persisting in their blood for at least 6 months. The
chronic carrier state is attributed to a persistent infection
of hepatocytes, which results in prolonged presence of
HBV and HBsAg in the blood. The main determinant for
a person to clear the infection or become a chronic carrier
is the adequacy of the cytotoxic T-cell response.
6) A high rate of hepatocellular carcinoma occurs in chronic
carriers. The HBV has no oncogene, and HCC appears to
be the result of persistent cellular regeneration that
attempts to replace the dead hepatocyte. Alternatively,
malignant transformation could be the result of insertional
mutagenesis, which could occur when the HBV genome
integrates into the hepatocyte DNA. This integration
could lead to activation of cellular oncogene leading to a
loss of growth control.
7) Chronic carrier state is more likely to occur in newborn
(about 90%) than in adults because of less competent
immune system.

Unit 4: Virology
211
8) Lifelong immunity after the natural infection and is
mediated by an antibody against HBsAg which is a
neutralizing and protective antibody while an antibody
against core antigen is not protective.
Clinical finding
Many HBV infections are asymptomatic and are detected
only by the presence of antibody to HBsAg. The mean
incubation period is 10-12 weeks. The clinical appearance
of acute hepatitis B is similar to that of hepatitis A. but in
hepatitis B symptoms tend to be more severe and life
threatening hepatitis can occur. Extrahepatic
manifestations of viral hepatitis include a transient serum
sickness –like prodrome consisting of fever, skin rash, and
polyarthritis, necrotizing vasculitis & glomerulonephritis
Laboratory diagnosis
1) HBsAg is the most important test used for the detection of
early infection. HBsAg appears during the incubation
period and is detectable in most patients during prodrome
and acute disease. It falls to undetectable levels during
convalescence in most cases; its prolonged presence ( > 6
months ) indicates the carrier state and the risk of chronic
hepatitis and hepatic carcinoma. HBsAb is not detectable
in the chronic carrier state. HBsAb is being made but is
not detectable in the laboratory tests because itr is bound
to the large amount of HBsAg present in blood. HBsAb is
also being made during the acute disease but is similarly
undetectable because it is bound in antigen-antibody
complexes.
2) There is a period which is called Window phase in which
the HBsAg has disappeared and the HBsAb is not yet
detectable and at this time, the HBcAb is always positive
and can be used to make the diagnosis. HBcAb is present
in the acute and chronic infection and in patients
recovered from acute infection. Therefore it cant be used
to distinguish between acute and chronic infection. The
IgM form of HBcAb is present during acute infection and
disappears approximately 6 months after infection. The
test for HBcAg is not readily available.
3) HBeAg arises during the incubation period and is present
during the prodrome and early acute disease and in certain
chronic carrier. Its presence indicates a high likelihood of
transmissibility, and , conversely the finding of HBeAb
indicates a lower likelihood, but transmission can still
occur.
4) The detection of viral DNA in the serum is strong
evidence that infectious virions are present.
5) DNA polymerase activity is detectable during the
incubation period and early in the disease, but the assay is
not available in most clinical laboratories.
Treatment
1) No antiviral therapy is typically used in acute hepatitis B.
2) For chronic hepatitis B, pegINF alfa -2a or PegINF alfa-
2b and / or nucleoside analogues can be used.
These drugs reduce hepatic inflammation and lower the
viral load of HBV in patients with chronic active
hepatitis. Neither INF nor the nucleoside analogues cure
the HBV infection. In most patient, when the drug is
stopped, HBV replication resumes.
Prevention
1) The vaccine (Recombivax) contains HBsAg produced in
yeasts by recombinant DNA techniques. The seroconversion
rate is 95% in healthy adults.It is indicated in:
a) Certain health personnel ( medical students, surgeons
and dentists)
b) Patient receiving multiple transfusion or dialysis.
c) Drug abusers
d) Travelers to an endemic area.
The vaccine is given in three doses, and there is no need
for booster dose after the completion of the regime.
2) Hepatitis B immune globulin (HBIG) contains a high titer
of HBsAb. It is used to provide immediate passive
protection to individuals known to be exposed to HBsAg
positive blood. HBIG with HB vaccine can be given to
infant whose mother is HBsAg positive.

Unit 4: Virology
211
Hepatitis C virus (HCV)
Properties
HCV is a member of flavivirus family. It is an enveloped
virion containing a genome of single stranded positive
polarity RNA. It has no virion polymerase.
HCV has at least six genotypes and multiple subgenotype
based on the differences n the genes that encode one of its
two envelope glycoprotein. The genetic variability is due
to high mutation rate in the envelope gene coupled with
the absence of proofreading function in the virion
encoded RNA polymerase. As a result, multiple
subspecies often occur in the blood of an infected
individual at the same time.
Replicative cycle:
The replication of HCV is uncertain because it has not
been grown in cell culture. It follows the replicative cycle
of other flaviviruses, as these viruses replicate in the
cytoplasm and translate their genome RNA into large
polyproteins, from which functional viral proteins are
cleaved by a virion-encoded protease. This protease is the
target of potent Anti-HCV therapy. The replication of
HCV in the liver is enhanced by liver –specific micro-
RNA. This micro-RNA acts by increasing the synthesis of
HCV mRNA.
Transmission and epidemiology
Humans are reservoir of HCV.
It is transmitted primarily via blood.
10-15% of HC cases, source of HCV can’t be detected.
At present injection drug use accounts for almost all new
HCV infections.
Transmission via blood transfusion rarely occurs because
donated blood containing Anti-HCV antibody is
discarded.
Transmission via needle stick injury occurs, but the risk is
lower than that of HBV.
Sexual transmission and transmission from mother to
child occur but are inefficient mode. No risk of
transmission has been associated with breast feeding.
Commercially prepared immuneglobulin preparations are
generally very safe, but several instances of transmission
of HCV have occurred.
WHO in 1997 estimate that about 3% of world population
has been infected, with a prevalence of 10% in African
population and other high prevalence area in Asia and
South America.
Transmission of HCV has been linked to an attempt (from
1950s to 1980s) to treat parasitic disease shistosomiasis
by therapy that involved multiple injections, often with
improperly sterilized or reused needles.
HCV infection has been associated with tattooing.
There was a case in 2009 in which HCV was transmitted
to an organ transplant recipient by the use of a blood
vessel conduit from a HCV-positive donor.
Pathogenesis and immunity
HCV infects hepatocytes primarily, but there is no
evidence for a virus-induced cytopathic effect on the liver
cells. Rather, death of the hepatocytes is probably caused
by immune attack by cytotoxic T cells. HCV infection
strongly predisposes to hepatocellular carcinoma, but
there is no evidence for an oncogene in the viral genome
for insertion of a copy of the viral genome into the DNA
of the cancer cells.
Alcoholism greatly enhances the rate of hepatocellular
carcinoma in HCV-infected individuals.
Antibodies against HCV are made, but approximately
75% of patients are chronically infected and continue to
produce virus for at least 1 year.
Chronic active hepatitis and cirrhosis occur approximately
10% of these patients.
For patients who clear the infection, it is not known
whether re-infection can occur or whether there is life
long immunity.
Clinical findings
HCV acute infection is milder than that of HBV infection.
The average incubation period for HCV is 6-7 weeks. The
average time from exposure to seroconversion is 8-9
weeks, and about 90% of patients are Anti-HCV positive
within 5 months. Many infections with HCV including
both acute and chronic infections are asymptomatic and
are detected by the presence of antibody. HCV can also
lead to significant autoimmune reactions, including,
vasculitis, arthralgia, purpura, and membranoproliferative
glomerulonephritis. HCV is the main cause of essential
mixed cryoglobulinemia.
Laboratory diagnosis
1) Antibody detection to HCV by ELISA. The test doesn’t
distinguish between acute, chronic, or resolved infection.
2) Positive result by ELISA should be confirmed by RIBA
(recombinant immunoblot assay), because of false
positive results of ELISA.

Unit 4: Virology
211
3) If RIBA result is positive, a PCR based test that detects
the presence of viral RNA (viral load) in the serum should
be performed to determine whether active disease exist.
4) A chronic infection is characterized by elevated
transaminase levels, a positive RIBA, and detectable viral
RNA for at least 6 months.
Treatment and prevention
1) Treatment of acute hepatitis C with alpha INF
significantly decreases the number of patients who
become chronic carrier.
2) Treatment of choice for chronic patients is by pegINF and
ribavirin.
3) Genotype 1 is less responsive to the above regimen
(treatment should continue for 1 year) than genotype 2
and 3 (treatment should be for 6 months).
4) Pooled immune serum globulins are not useful for post
exposure prophylaxis.
5) There is no effective regimen for prophylaxis following
needle-stick injury; only monitoring is recommended.
6) Patient with chronic HCV and are alcoholics, should be
advised to eliminate drinking.
7) Patient with chronic HCV should be followed by alpha-
fetoprotein and liver US to detect carcinoma at early
stage.
8) Patients with liver failure due to HCV infection can
receive a liver transplant, but infection of the graft with
HCV typically occurs.
Hepatitis G virus (HGV)
In 1996, HGV was isolated from patients with
posttransfusion hepatitis. HGV is a member of the
flavivirus family. But unlike HCV, HGV don’t cause
acute or chronic hepatitis and don’t predispose to
hepatocellular carcinoma. The role of HGV in the
causation of liver disease has yet to be established, but it
can cause chronic infection lasting for decades.
Approximately 60-70% of those infected clear the virus
and develop antibodies. HGV transmitted via sexual
intercourse and blood. It is carried in the blood of millions
of people worldwide.
Hepatitis D virus (Delta virus)
HDV is a defective virus can replicate only on the cells
also infected with HBV because HDV uses the surface
antigen of HBV as its envelope protein.
HDV is an enveloped SS RNA negative polarity,
covalently closed circle. The RNA genome encodes only
one protein called Delta protein.
HDV has one serotype & there is no animal reservoir for
the virus.
It is transmitted by the same method ad that of HBV.
The patient is either previously infected with hepatitis BV
and then infected with HDV or the infection with both
viruses is acquired at the same time. Hepatitis in patients
co infected with HBV and HDV is more.
The diagnosis can be done by detecting either delta Ag or
IgM antibody to delta antigen in the patient's serum.
There is no specific antiviral therapy but alpha interferon
can mitigate some of the effects of the chronic hepatitis

Unit 4: Virology
211
Lecture 17 - Human cancer viruses
Viruses are etiologic factors in the development of several
types of human tumors, including two great significance
worldwide – cervical and liver cancers.
At least 15-20% of all human tumors worldwide have a
viral cause. The viruses that have been strongly associated
with human viruses include EBV,HCV,HBV,HHV8,and
two retroviral viruses.
Types of tumor viruses
Tumor viruses are of different type: either DNA or RNA
viruses.
DNA tumor viruses encode viral oncoproteins that are
important for viral replication but also affect cellular
growth control pathways.
Most RNA tumor viruses belong to the retrovirus family
which carry an RNA –directed polymerase (reverse
transcriptase) that constructs a DNA copy of the RNA
genome of the virus. The DNA copy becomes integrated
into the DNA of the infected host cell and it is from this
integrated DNA copy that all proteins of the virus are
translated.
RNA tumor viruses are of two general types with respect
to tumor induction:
The highly oncogenic (direct transforming) viruses
carry an oncogene of cellular origin.
The weakly oncogenic viruses don’t contain an
oncogene and induce leukemias after long incubation
periods by indirect mechanism. The two retroviruses
and HCV act indirectly in cancer induction.
Interactions of tumor viruses with their host
a) Persistent infection.
The known tumor viruses establish long term persistent
infections in humans. The chronicity of infection presents
the long term opportunity for a rare event to occur that
allows survival of a cell with growth control mechanisms
that are virus-modified.
b) Host immune responses.
Viruses that establish persistent infections must avoid
detection and recognition by immune system that would
eliminate infection. EBV will restrict expression of viral
genes that makes infected cells nearly invisible to the
host. HPV infect site that are relatively inaccessible to
immune response ( epidermis) HIV cause a mutation in its
antigen that allows escape from antibody and T-cell
recognition, and also causing infection and suppression of
essential immune cells. CMV and adenoviruses cause
modulation of MHC I molecules in infected cells. EBV
causes inhibition of antigen processing.
c) Mechanism of action by human cancer viruses.
There are two general pattern by which tumor viruses
mediate changes in cell behavior. Direct action by
introduction of a new transforming gene into the cell.
Indirect action by altering the expression of a preexisting
cellular gene or genes.
In either case, the cell loses control of normal regulation
of growth process.
d) Cell susceptibility to viral infections & transformation.
Host cells are either permissive or non-permissive for
replication of a given virus.
In DNA viruses, permissive cells are often killed by virus
replication and not transformed unless the viral replicative
cycle that results in death of host cells is blocked in some
way. However, there are situations in which DNA virus
replication doesn’t lyse the host cell and such cells may
be transformed.
RNA viruses are not lethal for cells in which they replicate.
e) Retention of tumor virus nucleic acid in a host cell
The stable genetic change from normal to neoplastic cell
generally require the retention of viral genes in the cell.
With DNA virus, a portion of the DNA of the viral
genome may become integrated into the host cell
chromosome. Sometimes, episomal copies of the viral
genome are maintained in tumor cells. With retroviruses,
the proviral DNA copy of the viral RNA is integrated in
the host cell DNA. Genome RNA copies of hepatitis C
that are not integrated are maintained in tumor cells.
In some cases, viral transformed cells may release growth
factors that affect the phenotype of neighboring
uninfected cells, thereby contributing to tumor formation.
1- Cellular oncogenes
Oncogene is the general term given to genes that are
involved in cancer causation. Normal version of these
transforming genes are present in normal cells and have
been designated proto-oncogen.
Cellular oncogenes are partly responsible for molecular
basis of human cancer. They represent individual
components of complicated pathways responsible for
regulating cell proliferation, division, and differentiation
and for maintaining the integrity of the genome. Incorrect
expression of any component might interrupt that
regulation, resulting in uncontrolled growth of cells.

Unit 4: Virology
211
Two different mechanisms: overexpression and mutation
in proto-oncogene able to convert into oncogene capable
of transforming a cell.
2- Tumor suppressor genes
These are the negative regulators of cell growth. The
inactivation or functional loss of both alleles of such a
gene is required for tumor formation. The example of
tumor suppressor genes are Rb gene by which Rb protein
inhibits entry of cells into S phase by binding to key
transcription factors that regulate expression of S phase
genes.
Another tumor suppressor genes is p35 gene. It also
blocks cell cycle progression; P35 acts as a transcription
factor and regulates the synthesis of a protein that inhibits
the function of certain cell cycle kinases. It also causes
cells with DNA damage to undergo apoptosis.
Human retroviruses
a) Human T-Lymphotropic Viruses (HTLV)
HTLV-1 has been established as the causative agent of
adult T-cell leukemia- lymphoma (ATL) and tropical
spastic paraparesis.
It doesn’t carry an oncogene
Transactivating regulatory genes are believed to be
necessary for viral replication in vivo and may contribute
to oncogenesis by also modulating cellular genes that
regulate cell growth.
b) HIV
The HIV are cytolytic and non-transforming. However,
AIDS patients are at elevated risk of several types of
cancer because of the immune suppression associated
with HIV infection.
Herpesviruses
EBV is etiologically linked to Burkitt lymphoma,
nasopharyngeal carcinoma, popsttransplant lymphomas,
and Hodgkin disease.
EBV encodes a viral oncogene protein (LMP1) that
mimics the activated growth factor receptor.
Several EBV-encoded nuclear antigens are necessary for
immortalization of B cells.
HHV8 is associated with Kaposi sarcoma.
Adenoviruses
They can transform rodent cells but there is no association
with human neoplasia.
HBV and HCV
HBV is a risk factor for causing a liver cancer. It has been
shown that persistent viral infection leads to necrosis,
inflammation, and liver regeneration that over tome
results in cirrhosis. The hepatitis B virus treansactivator
protein , X protein, is a potential viral oncoprotein.
Chronic infection with hepatitis C virus is also considered
to be a causative factor in hepatocellular carcinoma.
How to prove that a virus causes human cancer
1) The geographical distribution of viral infection should
coincide with that of the tumor.
2) The presence of viral markers should be higher in cases
than in controls.
3) The viral infection should precede the tumor.

Unit 4: Virology
212
Lecture 18 – Slow Viruses & Prions
Slow infectious diseases are caused by a heterogenous
group of agents containing both conventional viruses and
unconventional viruses (e.g.,prions).
Prions are protein-containing particles with no decteable
nucleic acid that are highly resistent to inactivation by
heat, formaldehyde, and UV light at doses that will
inactivate viruses.
Prions are inactivated by protein and lipid-disrupting
agents such as phenol, etherr, NaOH and hypochlorite.
The prion protein is encoded by a normal cellular gene
and is thought to function in a signal transduction
pathway in the neurons. The normal prions protein (PRP
c
)
has a significant amount of alpha-helical conformation.
When the alpha-helical conformation changes to a beta-
pleated sheet (PrP
SC
), these abnormal forms aggregate
into filaments, which disrupt neuron function and cause
cell death.
The human prion-mediated diseases are called
transmissible spongiform encephalopathies (TSE). The
term spongiform refers to spongy, Swiss cheese-like holes
seen in the brain parenchyma that are caused by the death
of neurons. The term encephalopathy refers to pathogenic
process in the brain without aigns of inflammation. Prions
can reach the CNS after ingestion by sympathetic nerves
after penetrating the gut mucosa reach the pyre’s patches
then spread to the spleen then to CNS via sympathetic
nerves. It is also possible that the prions reach the CNS
via the blood transfusion and can be transmitted
iatrogenically (via corneal transplant, dura matter grafts,
implanted brain electrodes, and growth hormone extracts
made from human pitutary glands.
Prion caused diseases can be classified into 3
categories:
1) Transmissible (infectious) such as kuru.
2) Hereditary (genetic) such as fatal familial insomnia.
3) Sporadic (neither infectious nor hereditary) such as CJD.
Disease
Pathogenesis
Important features
Kuru
Transmissble
/infectious
Caused by ingestion or
handling brain tissue,
occurred in New Guinea
tribespeople
Creutzfeldt-
Jakob
disease
1- Transmissble
/infectious
Iatrogenic transmission by
corneal transplant, and
growth hormone
2- Hereditary
/genetic
Mutation in germ cells
3- Sporadic
No relationship to any
known cause; possible new
mutation in somatic cells;
most common form
Variant
Creutzfeldt-
Jakob
disease
Transmissble
/infectious
Probably acquired by
eating meat or nervous
tissue from animals with
mad cow disease
Gerstmann
Straussler-
Scheinker
syndrome
Hereditary
/genetic
Mutation in germ cells
Fatal
familial
insomnia
Hereditary
/genetic
Mutation in germ cells
Slow diseases caused by conventional viruses
1) Progressive Multifocal Leukoencephalopathy (PML).
PML is a fatal demyelinating disease of the white matter
and involves multiple areas of the brain. The disease
started with visual field defect, mental status changes, and
weakness and the disease rapidly progress to blindness,
coma and death.
PML is caused by JC virus, a member of polyoma virus
family. It is non-enveloped virus with circular-double
stranded DNA genome. JC infects and kill
oligodendroglia causing demyelination. Neurons are not
affected.
The diagnosis is made typically by PCR assay of the brain
specimen or spinal fluid. There is no antiviral therapy but
cidofovir may be beneficial.
2) Subacute Sclerosing Panencephalitis (SSPE)
SSPE is a slowly progressive disease characterized by
inflammatory lesions in many areas of the brain. It is a
rare disease of children who were infected by measles
virus several years earlier. SSPE is a persistent infection
by a variant of measles virus that cannot complete its
replication.
3) Acquired Immunodeficiency Syndrome (AIDS)

Unit 4: Virology
211
Lecture 19 – Arbovirus
Arbovirus = arthropode borne virus
Transmitted by arthropode (mosquitoes and ticks
It is named either according to the disease cause or due to
place where discovered
Robovirus = rodent born virus e.g : sin nombre virus
(hantavirus) and white water arroyo virus ( an arenavirus).
Togavirus: icosahedral, enveloped, ss, positive polarity
RNA genome
Flavivirus same as above
Bunyavirus: helical nc with an envelope, and segmented
genome (three segment), and negative polarity RNA.
During the life cycle, the virus should be in high level of
viremia in man to be transmitted to the arthropode.
The man is considerd as an dead end host.
The clinical picture usually fits one of the three
caegories:
1- encephalitis
2- haemorrhagic fever.
3- Fever with arthralgia, myalgia and nonhaemorrhagic
rash.
St.louis encephalitis virus
California encephalitis virus
Colorado tick fever virus
West nile virus
Yellow fever
Dengue fever
