
0
Mustafa Hatim Kadhim
Baghdad University
Al-kindy college of medicine
Third Stage
2013 - 2014
1
List of contents
Lab
number
Lab name
Page
number
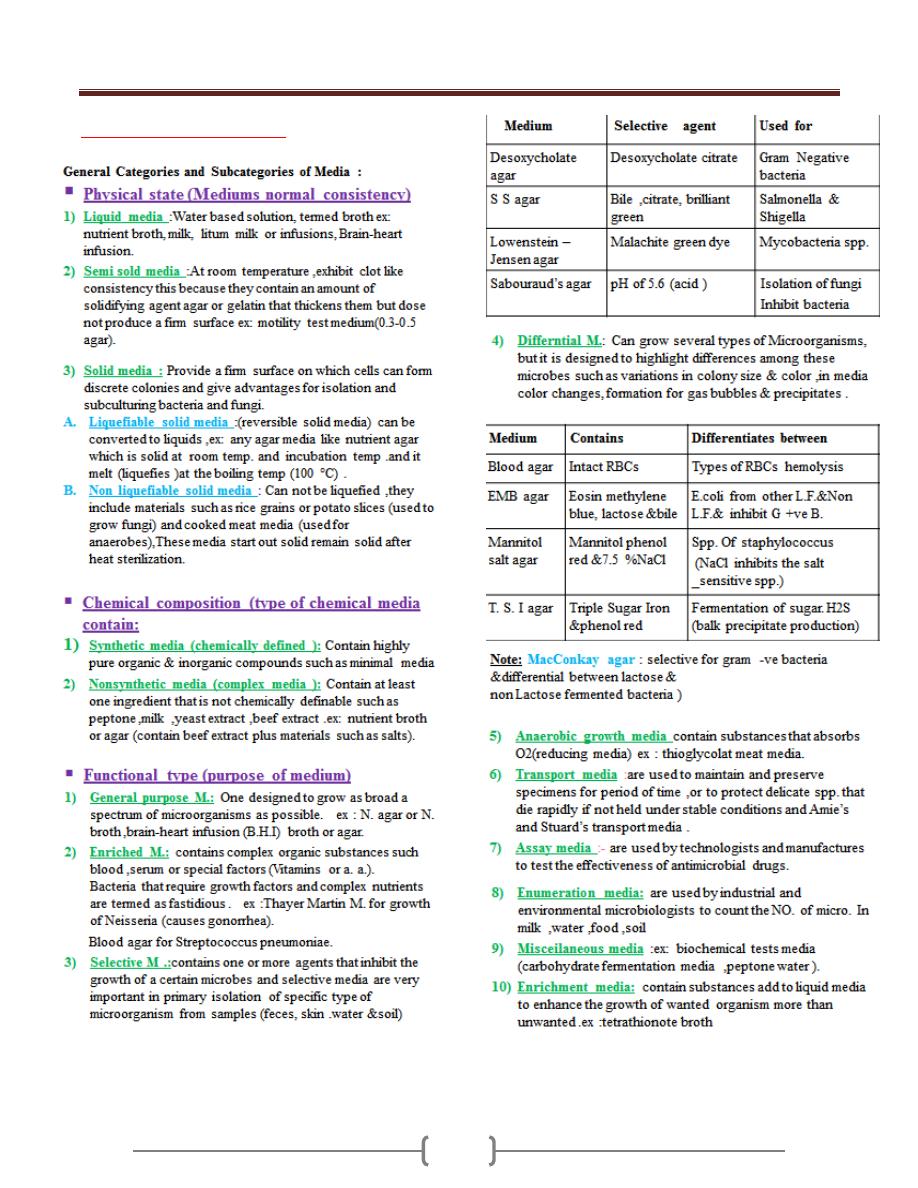
Unit 1: General Microbiology (3 – 14)
1
Sterilization & Disinfection
4 - 5
2
Culture Media
6
3
Preparation of Smear
7 - 8
4+5
Sample
9 - 11
6
Molecular diagnostics (Polymerase chain
reaction)
12 - 14
Unit 2: Immunology (15 – 21)
1+2+3
Practical Immunology
16 - 21
Unit 3: Bacteriology (22 – 54)
Gram Positive Bacteria (23 – 38)
1
Staphylococcus + Streptococcus
24 - 28
2
Bacillus & Neisseria
29 - 32
3
Clostridium & Mycobacterium
33 - 35
4
Corynebacteria
36 - 38
Gram Negative Bacteria (39 – 54)
1+2+3
Enteric Gram Negative rods
40 - 47
4
Vibrio & Heamophilus
48 - 49
5
Brucella, Campylobacter &
Helicobacter
50 - 51
6
spirochetes & Chromagar
52 - 54
2
Unit 4: Mycology (55 – 63)
1+2
Practical Mycology
56 - 63
Unit 5: Virology (64 – 69)
1+2
Laboratory Diagnosis of Viruses
65 - 69

3
Unit 1: General Microbiology
4
Lab1 – Sterilization & Disinfection
Instructions in the lab
1) Lab coat should be worn before the entry to the lab over
street clothes and remove before leaving
2) In typical way, laboratory workers should wear goggles,
mask and gloves.
3) Do not eat, drink or smoke inside the lab, these should be
done in isolated room or rest room.
4) Do not bite the nail or put the pen into the mouth.
5) Do not mouth pipette, rubber teat or mechanical pipette
should be used.
6) Assume that patients are infectious for HIV or blood
borne disease.
7) Wash the hands thoroughly after removing the gloves or if
there is any contamination
Sterilization: The processes by which all forms of
microbial life, including vegetative and spore forming
bacteria are destroyed or killed.
Disinfection: The processes that result in the destruction
of only the vegetative forms of microbial life (pathogenic
organisms) but not spores
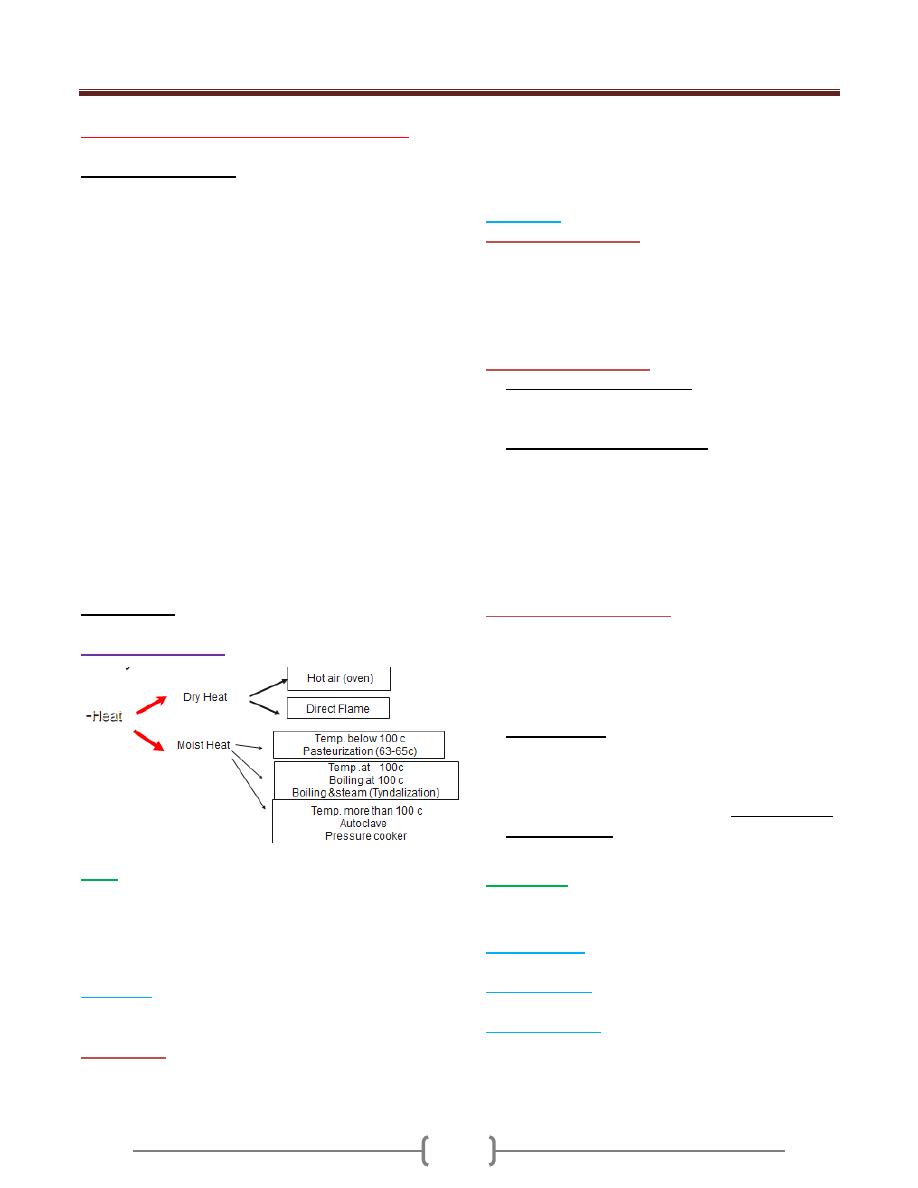
Sterilization
Physical methods
Heat
Is most practical and dependable method of sterilization,
this method depend on the effective oxidation of
microorganisms by carbonization or coagulation of the
protoplasm.
a)
Dry Heat:
E.g. heating of platinum loop, points of forceps, needles
and mouth of culture tubes ect.
Hot air oven:
this type requires longer exposure times
(1.5-3 Hours) and the temperature is higher than moist
heat (160-180 C) .Hot air oven is the best method for
sterilizing dry glass wares, oil petrolatum, liquid paraffin
powders,…
The oven should be set to 160 C for 2 hours, 170 C for 1
hour, 180 C for 30 Min.
b) Moist heat
1) At a temp. below 100 C:
For example pasteurization of milk, the temp. employed either
63-65 c for 30 min. or 71 c for at least 15 seconds. This will
kill all non-spore forming pathogens such as Mycobacterium
tuberculosis, Brucella abortus and various Salmonellae, so that
this method can be used to kill food pathogen
2) At a temperature of 100 c
a) Boiling at 100 c for 5-10 min.
This is sufficient to kill all non-sporing & many but not all
spore-forming microorganisms, used to sterile water.
b) Steaming at 100 c .for example:
Tyndallization which involves steaming for 30 min. on
each 3 successive days.
After heating, the medium is incubated to allow spores
to germinate and vegetative organisms to grow which
will be killed by the second steaming; the 3
rd
heating is
a safety precaution .used for culture media containing
sugar or gelatin.
3) At a temperature above 100c
Steam under increased pressure, this is called
(autoclaving). This is the usual method of sterilization
bacteriological media ,surgical instruments, towels,
dressing ,gloves ,………ect
The 2 common sterilization temp. either
a) 121c for 15 min for sterilizing items such as media,
liquid and medical instruments.
b) Infectious waste such as unused portion of patients’
specimens, patients’ culture, sharp disposable sharp
instruments such as scalpels, needles sterilized at 132
C for 30-60 Min.
Radiation:
Act on nucleic acid, used in commercial purpose to sterile
high quantity of pre packed material
a) Direct sun light
kills vegetative organisms rapidly, but
spores are much more resistant.
b) Ultra violet light
: is commonly used to sterilize the air in
the surfaces (used in Bacteriological hood)
c) Ionizing radiation
includes high speed electrons .X-rays
.It has effect on under surfaces. This method is used for
sterilizing disposal such as gloves catheter, plastic syringe
and Petri dishes.
Unit 1: General Microbiology
5
d) Infrared radiation
: by electrically heat element, a
temp180 C can be attained .used for glassware.
Filtration
To render fluids, including bacterial culture, free from
bacteria by passing them through special filters e.g.seitz
filter. This method is used in sterilizing liquids that
would be damaged by heat such as serum, vaccines
,antibiotic solutions, sugars, toxins ………etc.
Chemical methods
It act on lipid contents of the cell membrane denaturation
of protein, and on nucleic acid, it either kill or stop the
growing of microorganism.
a) Potent: disinfectant:
It is toxic and corrosive for living tissue, it’s used for non-
living
1) Phenol group
: contain benzene ring e.g.bathrooms,
hospital, floor ……ect.
2) Chlorine
is most often used in form of sodium
hypochloride (house hold bleach) in dilution (1:10)
treatment of swimming pools etc.
3) Strong alkaline and acids
e.g. NAOH
Used for treating sputum for detecting TB and decrease
viscosity
b) Mild: Antiseptics:
It is less toxic and can be applied to living tissue like
skin. eg.
1) Detole
2) Chlorhexiden (Hibitane):
very good as skin antiseptics
used to treat surgical wounds because it work on both
gram-positive and gram-negative bacteria
3) Iodine:
used in surgery to sterile skin pre-operation.
4) H2O2(hydrogen peroxide )
can be used for sterilization
of deep wound or gangrene infected by anaerobic bacteria
5) Soap and detergent.
6) Alcohol
at conc.70% because it can penetrate tissue easily
at this concentration better than 99%(absolute)
Unit 1: General Microbiology
6
Lab 2 - Culture Media

Unit 1: General Microbiology
7
Lab 3 - Preparation of Smear

Unit 1: General Microbiology
8

Unit 1: General Microbiology
9
Lab 4+5 - Sample

Unit 1: General Microbiology
10

Unit 1: General Microbiology
11

Unit 1: General Microbiology
12
Lab 6 - Molecular diagnostics
(Polymerase chain reaction)
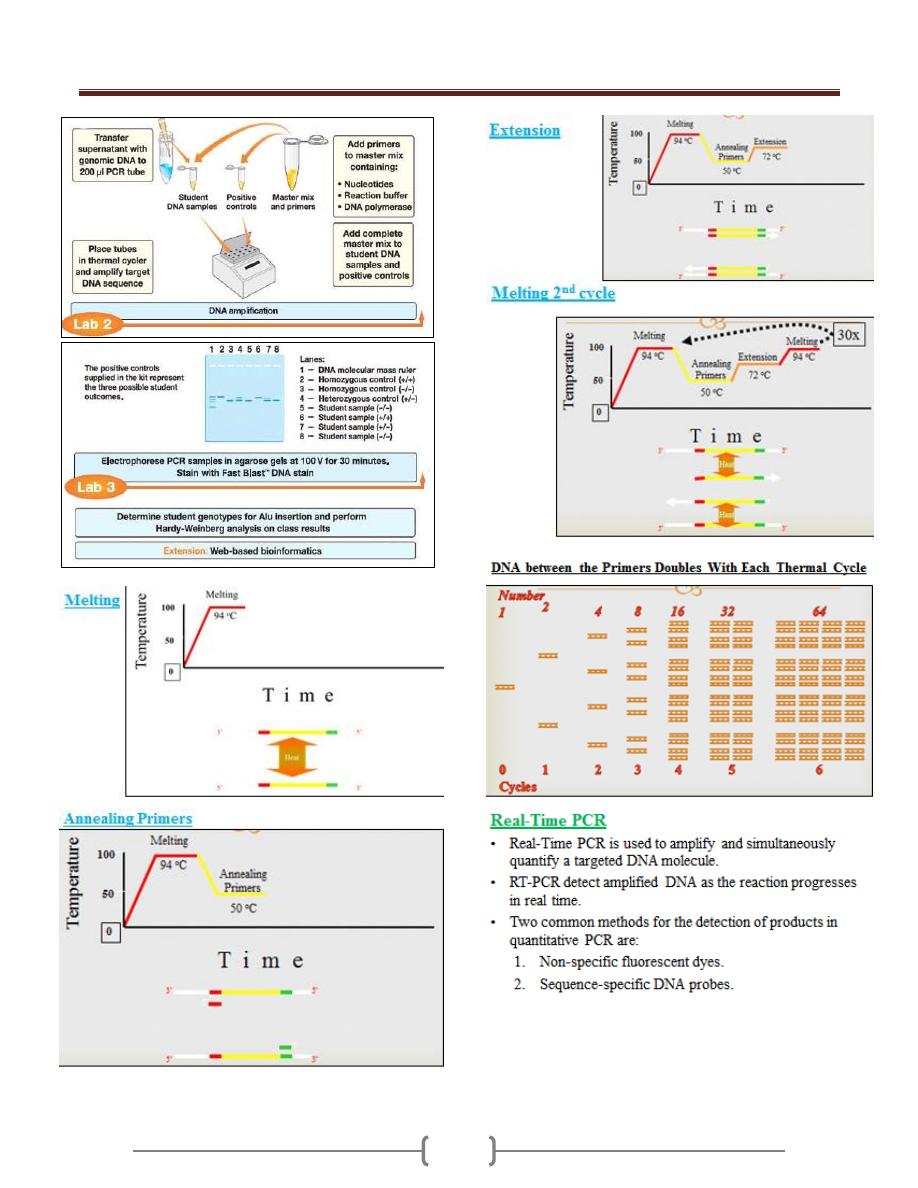
Unit 1: General Microbiology
13

Unit 1: General Microbiology
14

15

Unit 2 - Immunology
16
Lab 1+2+3 – Practical Immunology
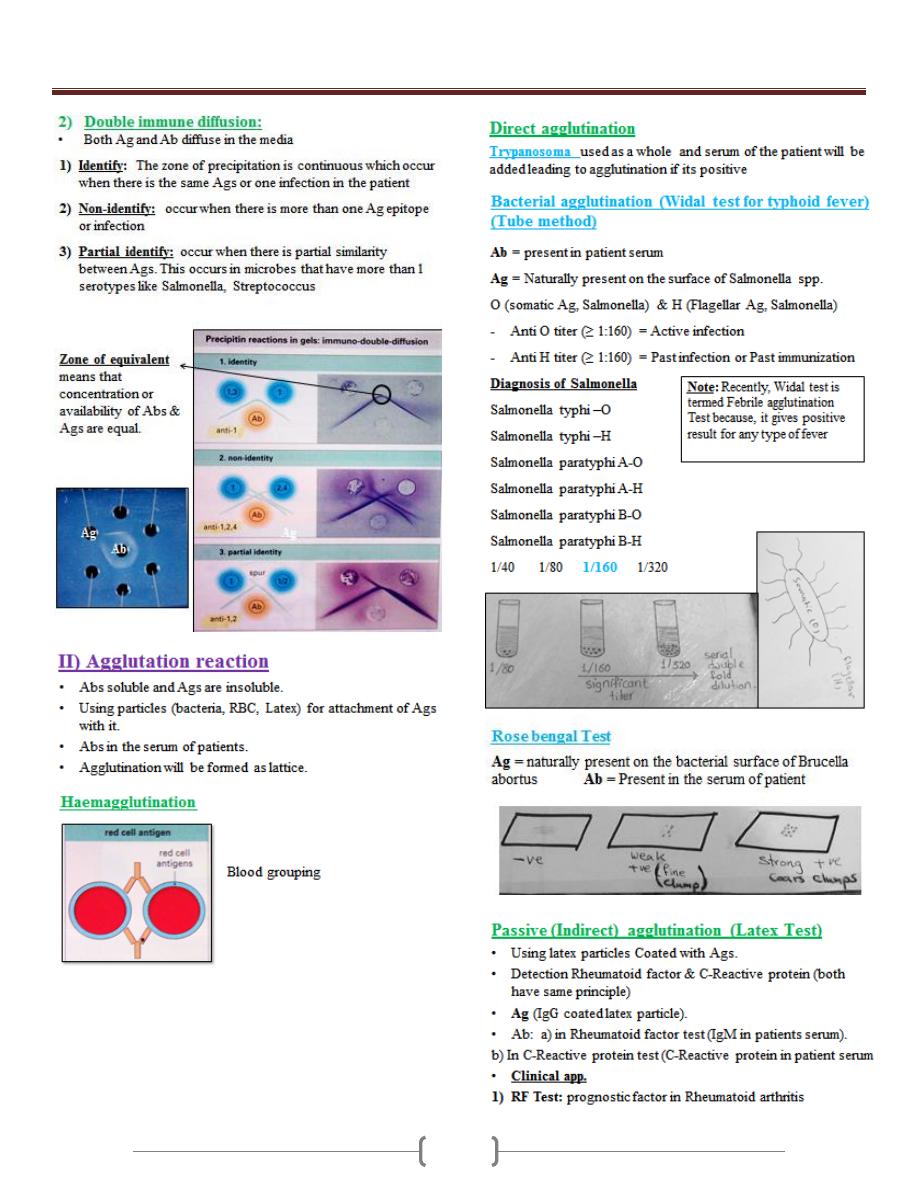
Unit 2 - Immunology
17
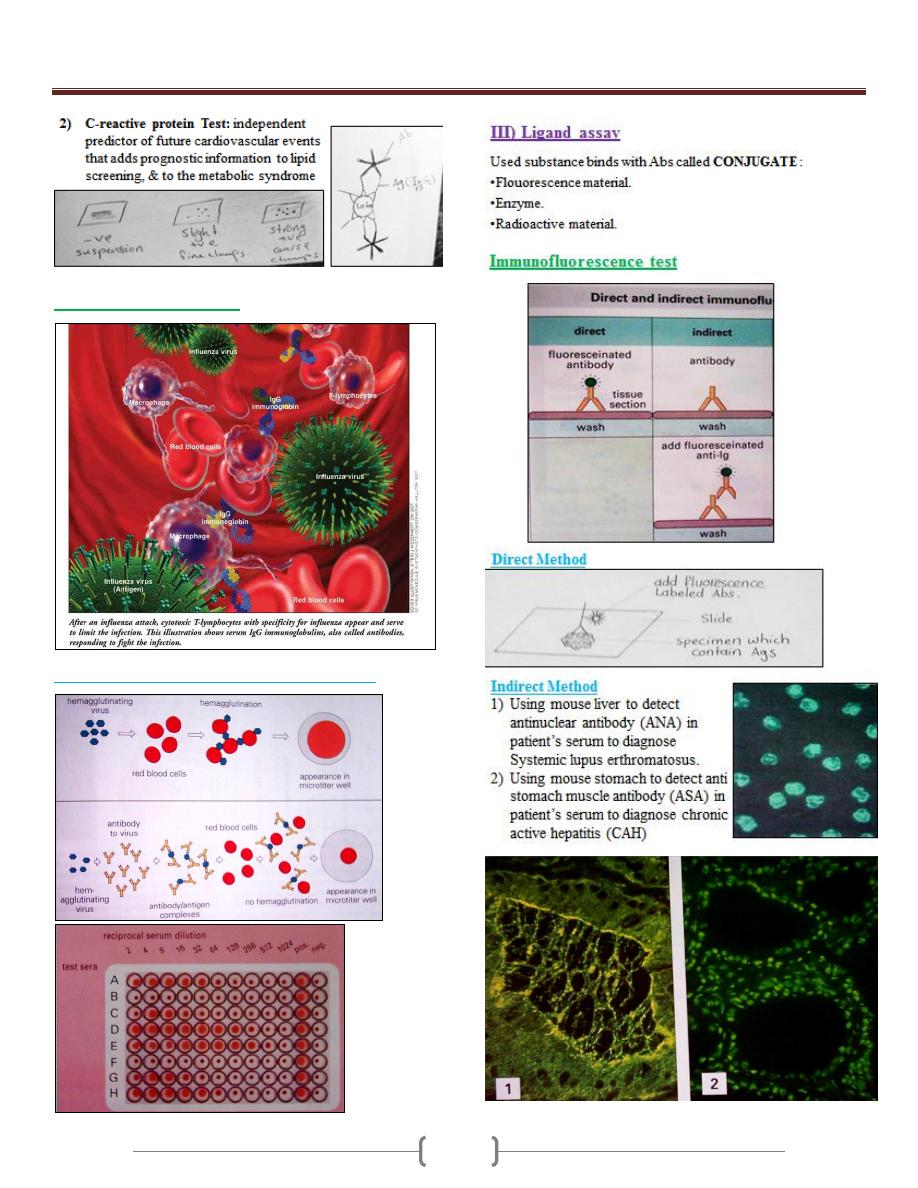
Unit 2 - Immunology
18
Agglutination inhibition
Hemagglutination inhibition (Influenza virus)
Indirect immune
fluorescence test
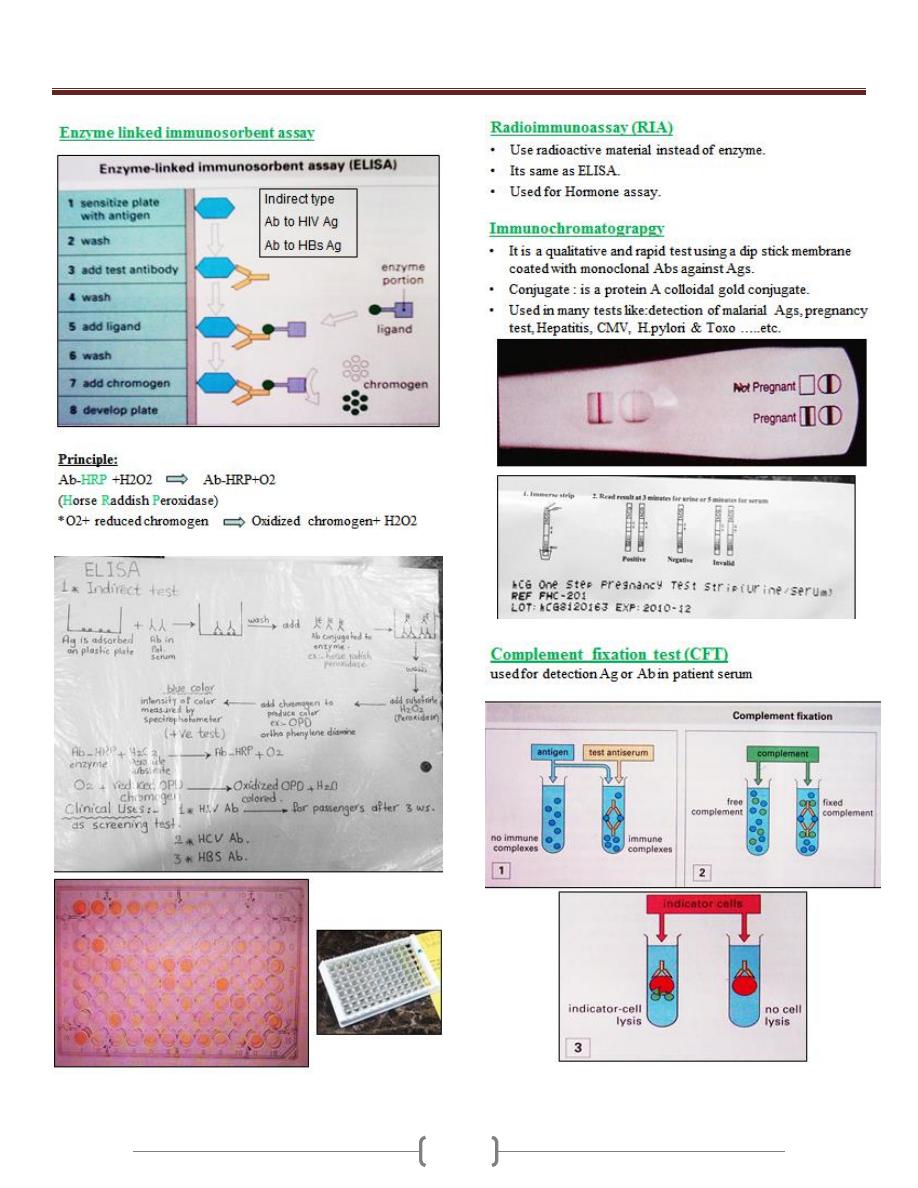
Unit 2 - Immunology
19
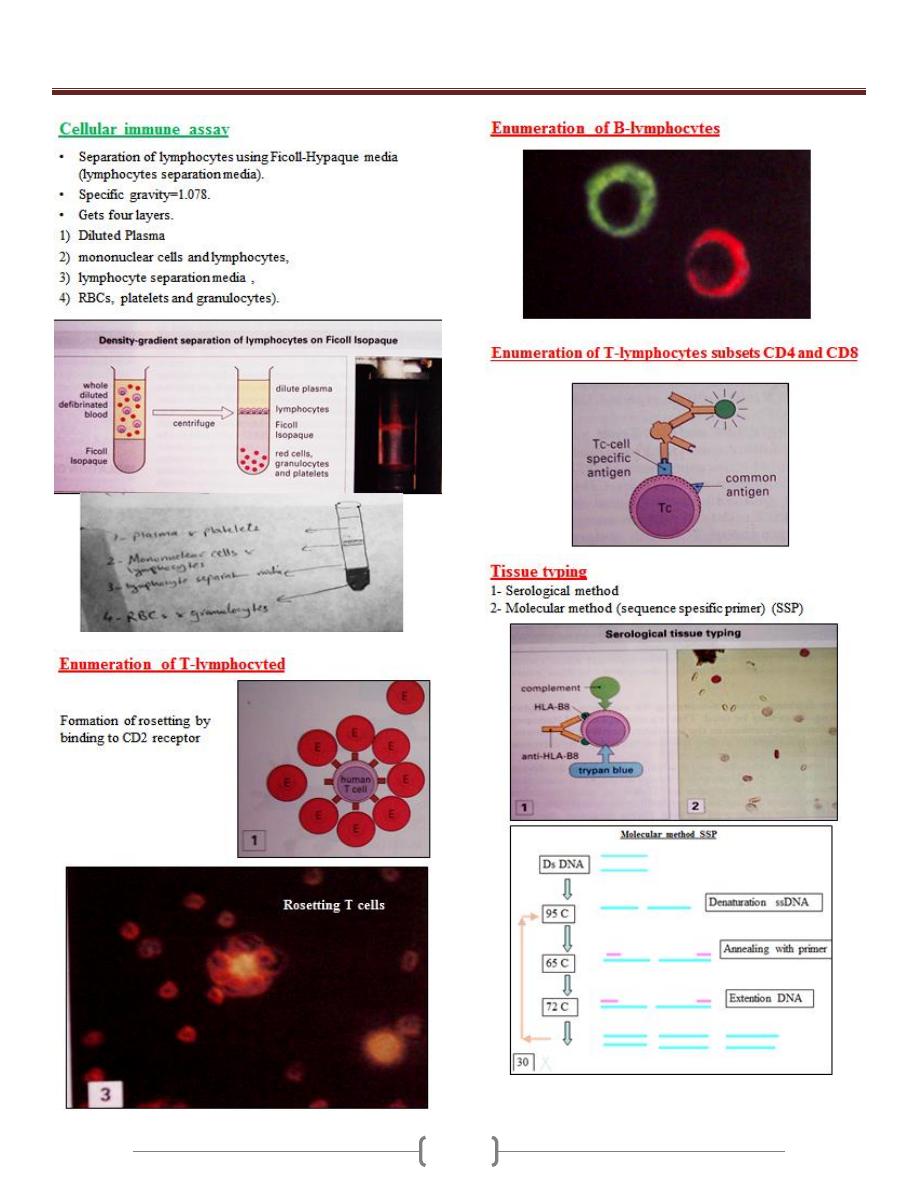
Unit 2 - Immunology
20

Unit 2 - Immunology
21

22
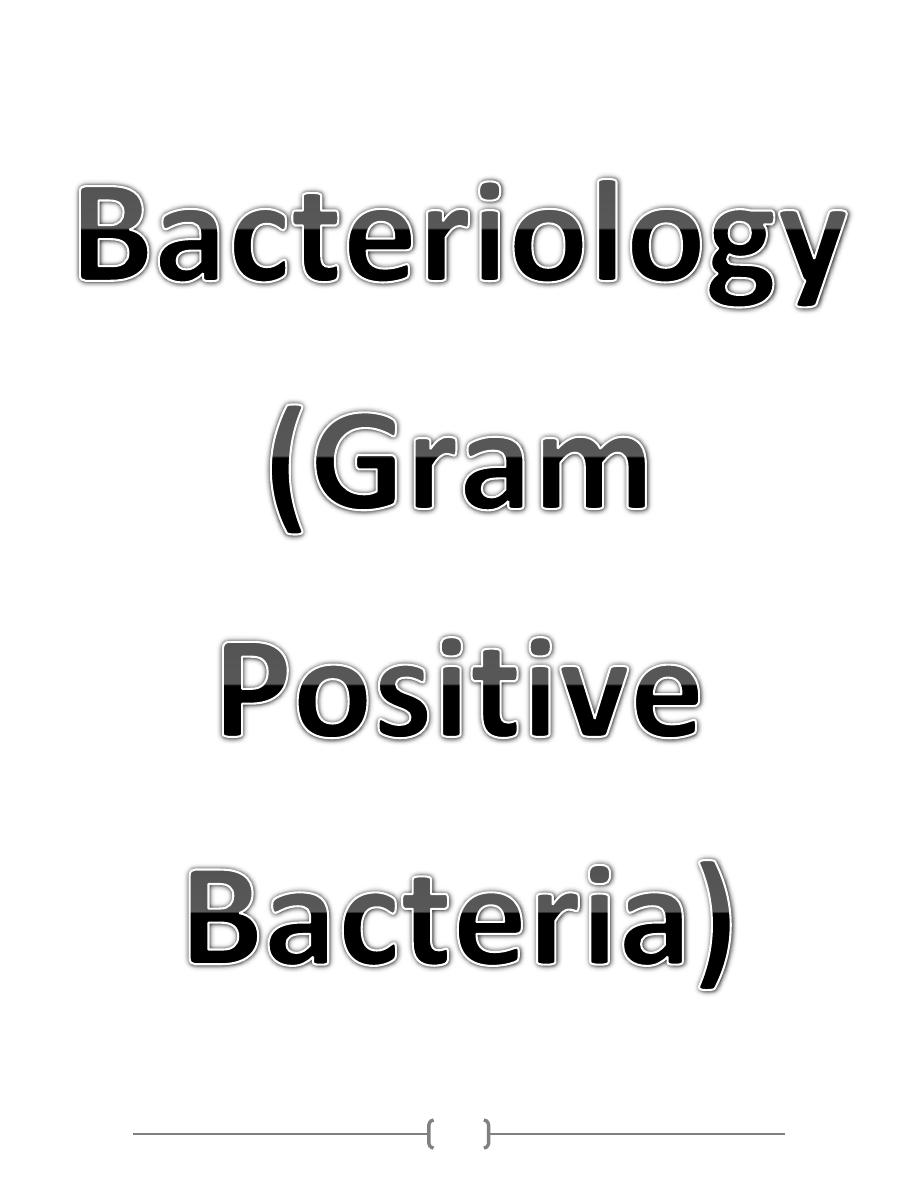
23
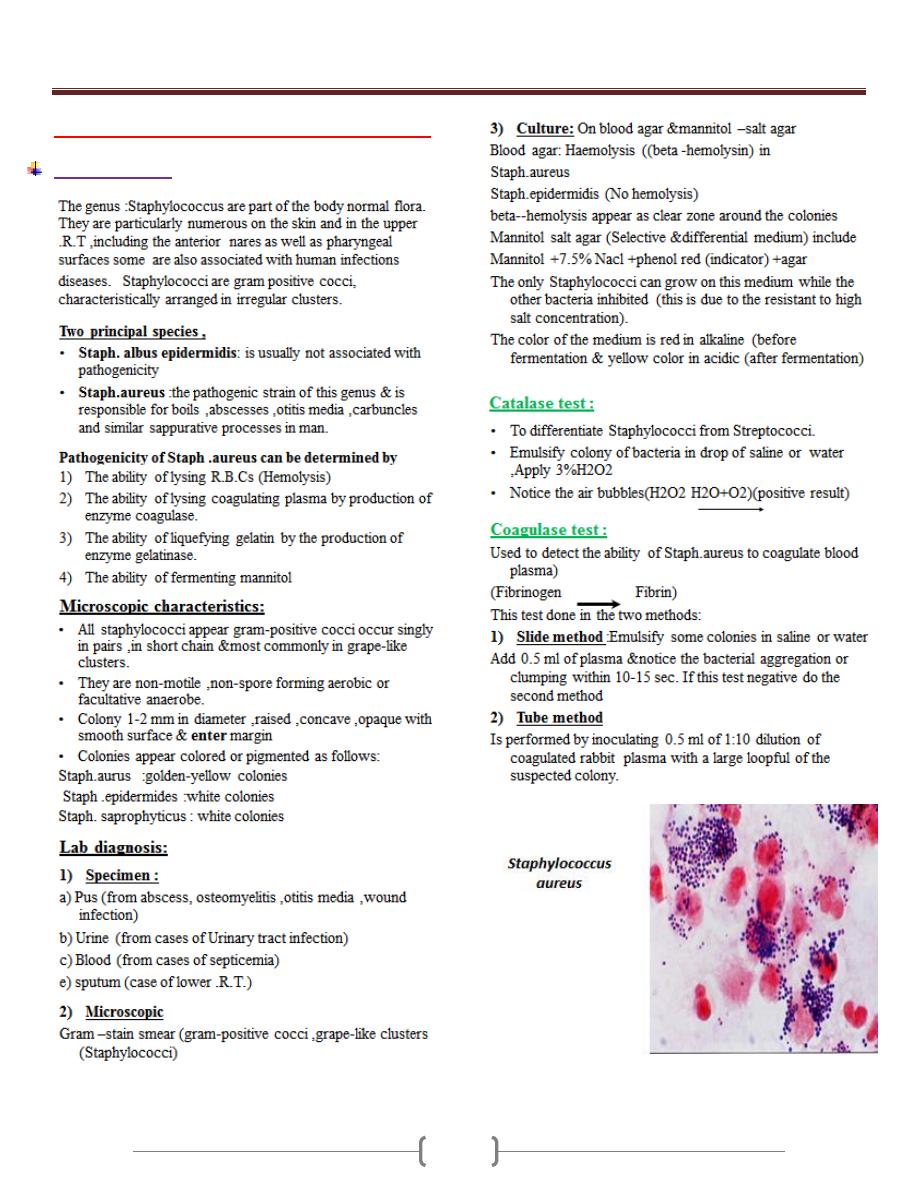
Unit 3 - Bacteriology
24
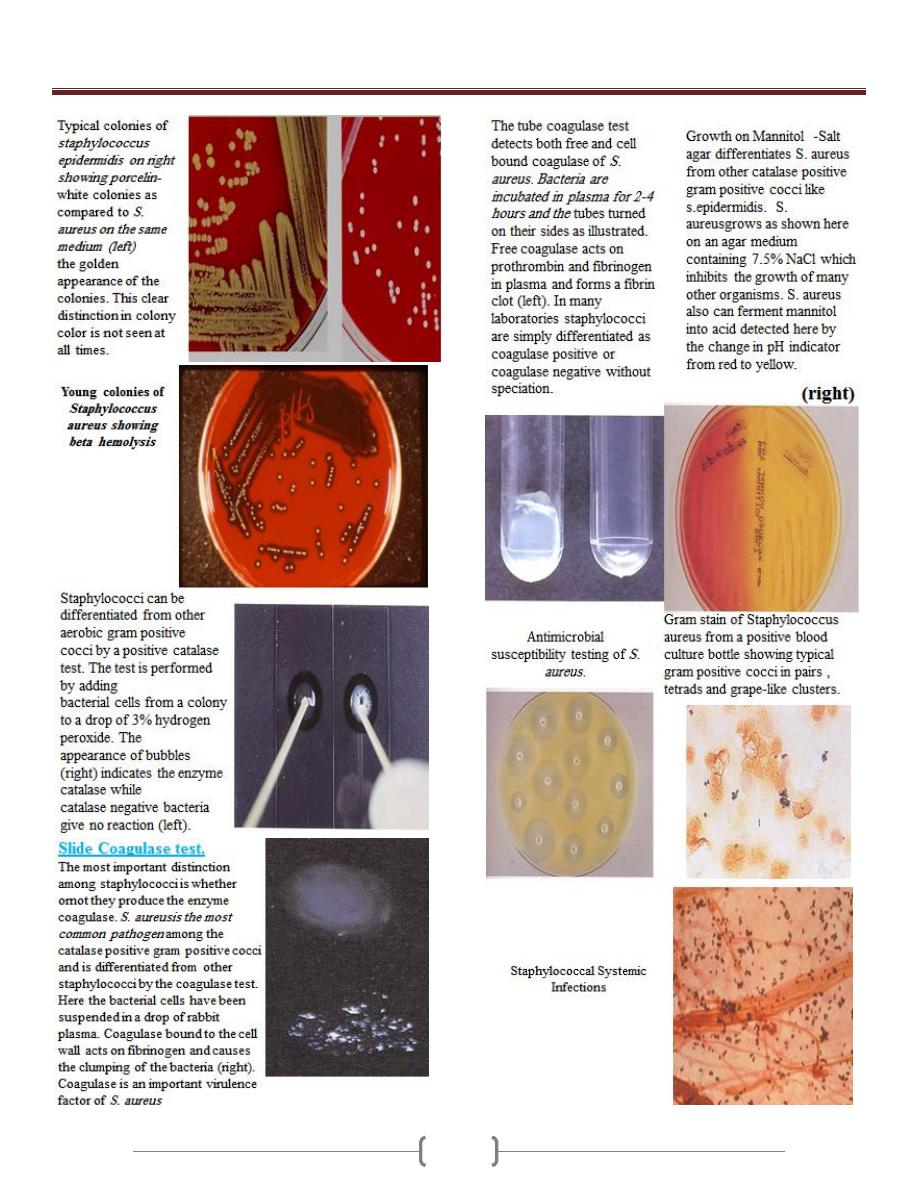
Lab 1 – Staphylococcus + Streptococcus
Staphylococcus
Unit 3 - Bacteriology
25
Unit 3 - Bacteriology
26
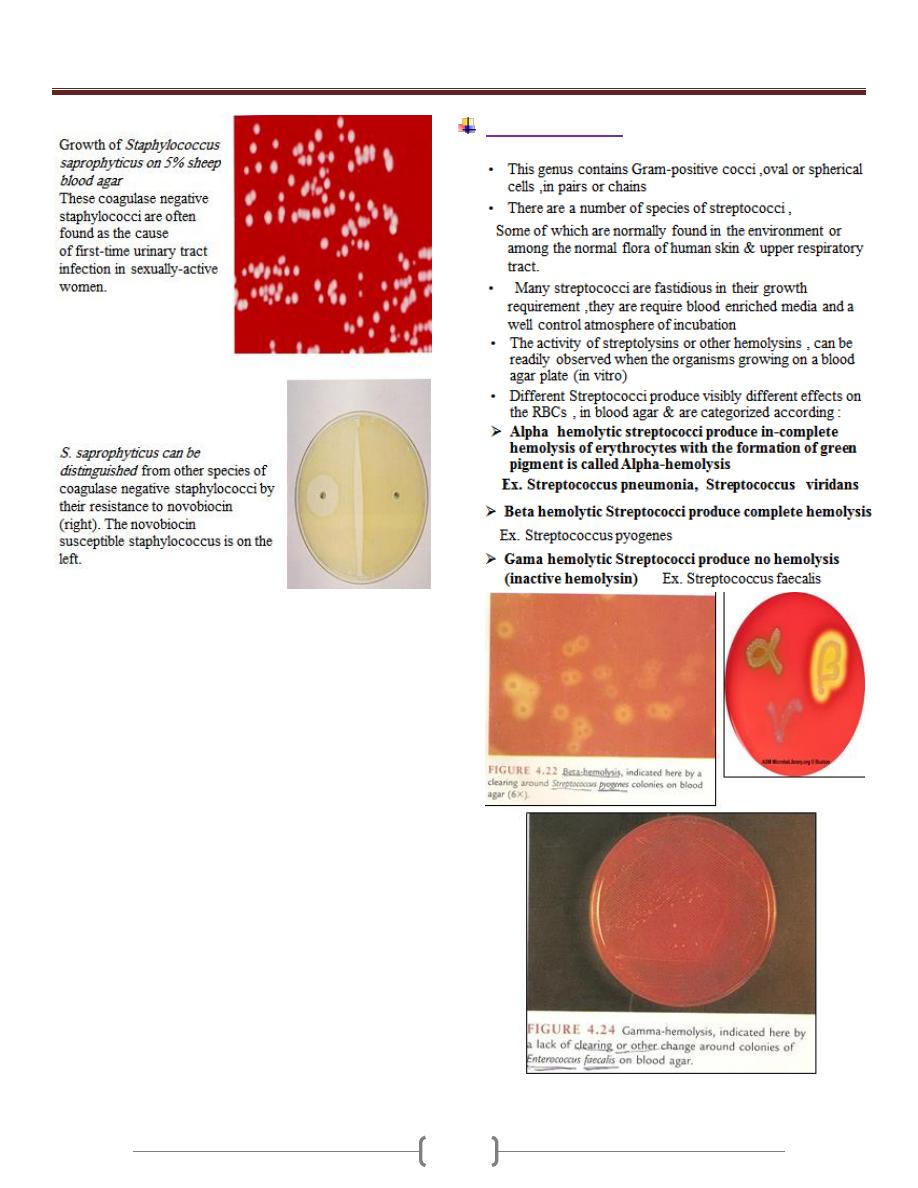
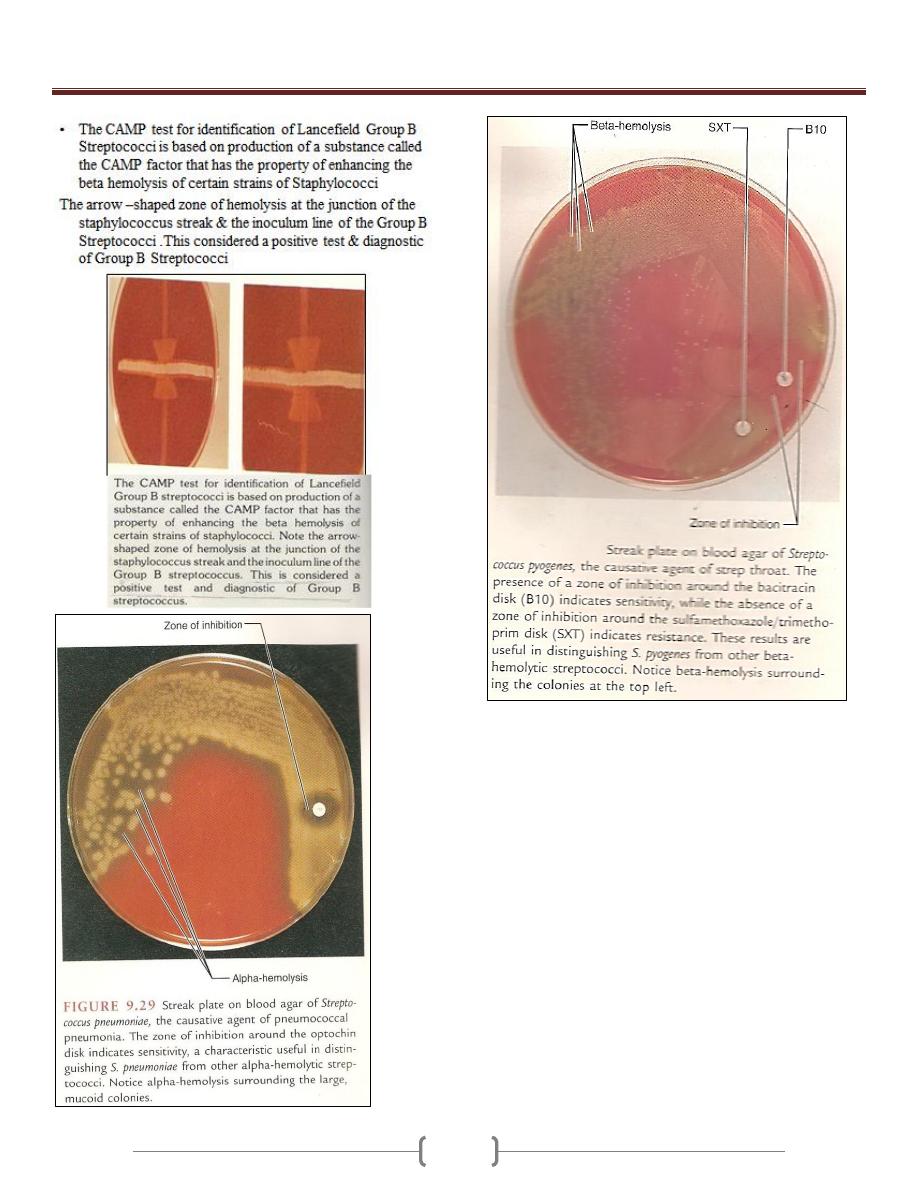
The streptococci

Unit 3 - Bacteriology
27
Unit 3 - Bacteriology
28
Unit 3 - Bacteriology
29
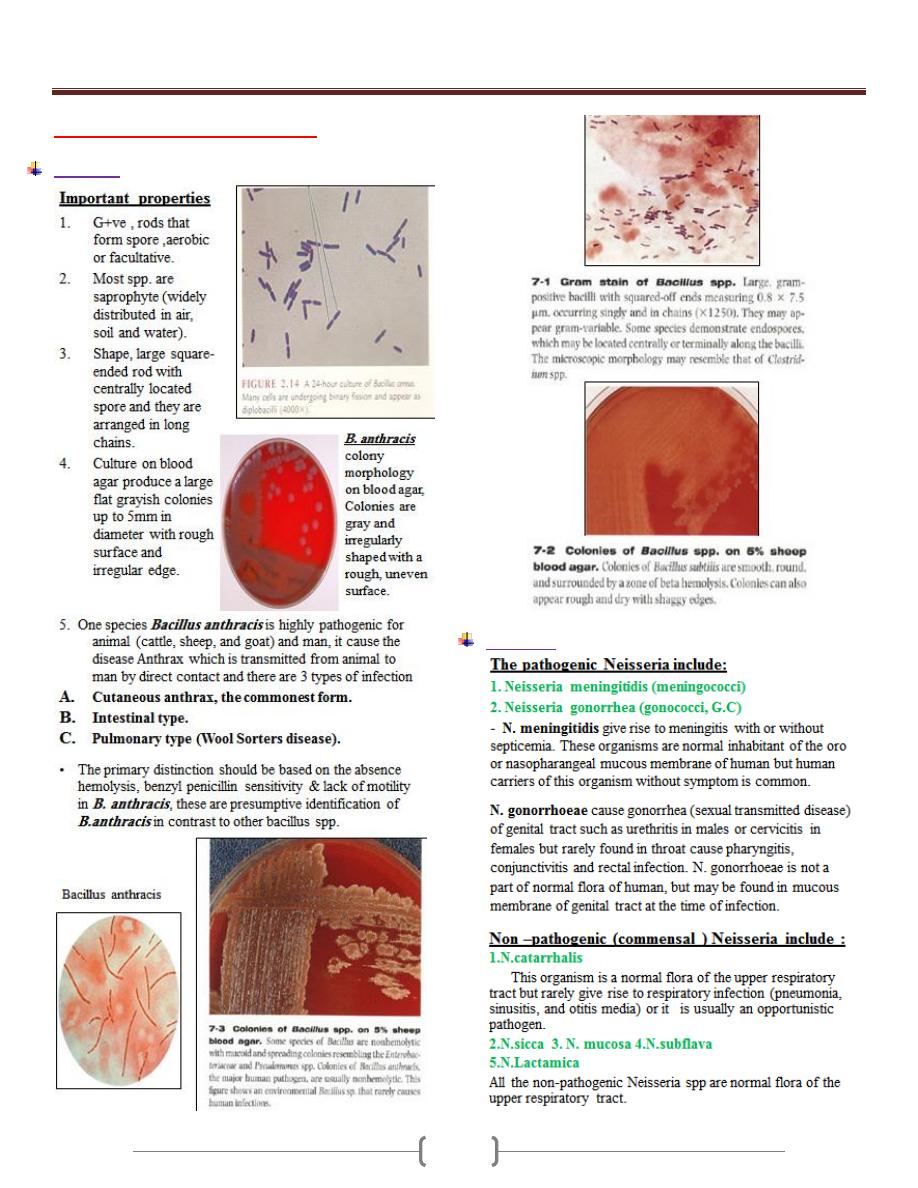
Lab 2 – Bacillus & Neisseria
Bacillus
Neisseria
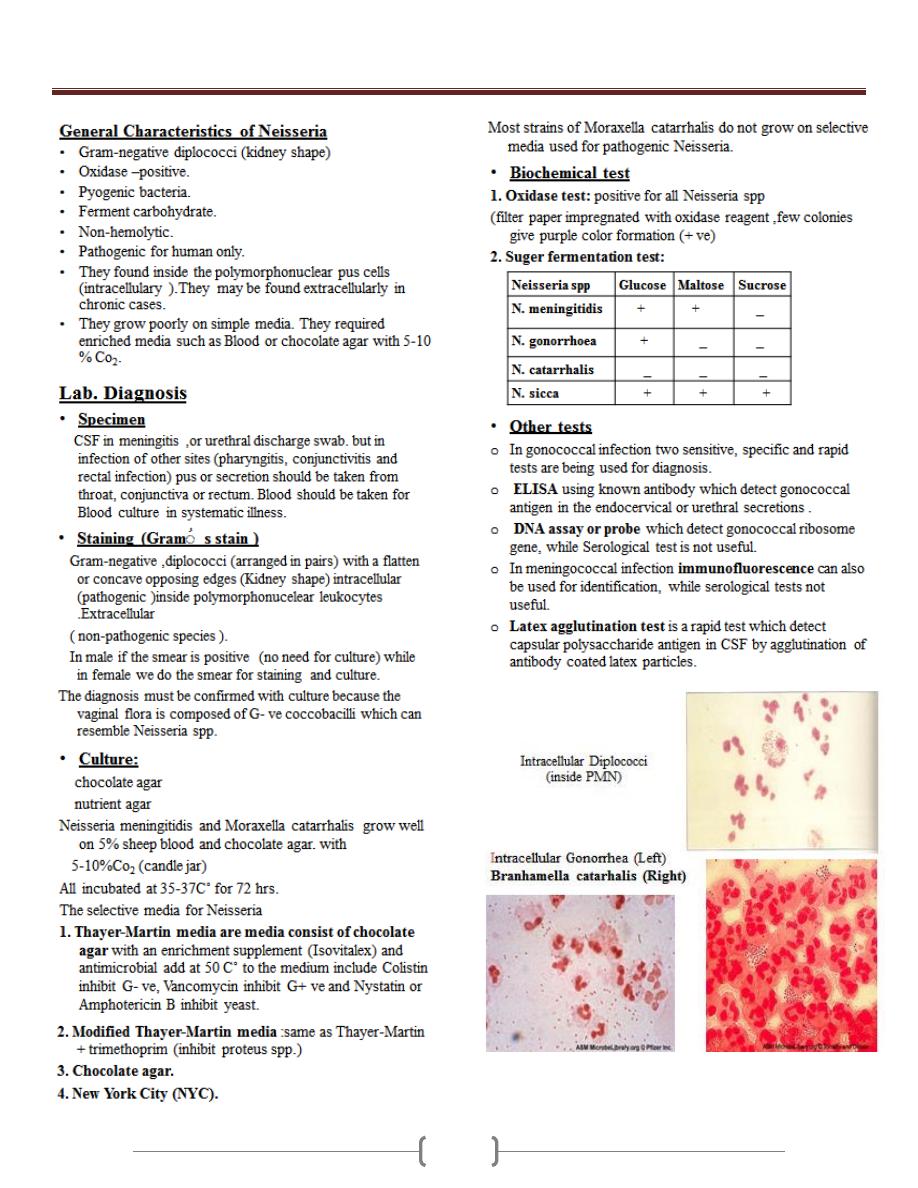
Unit 3 - Bacteriology
30
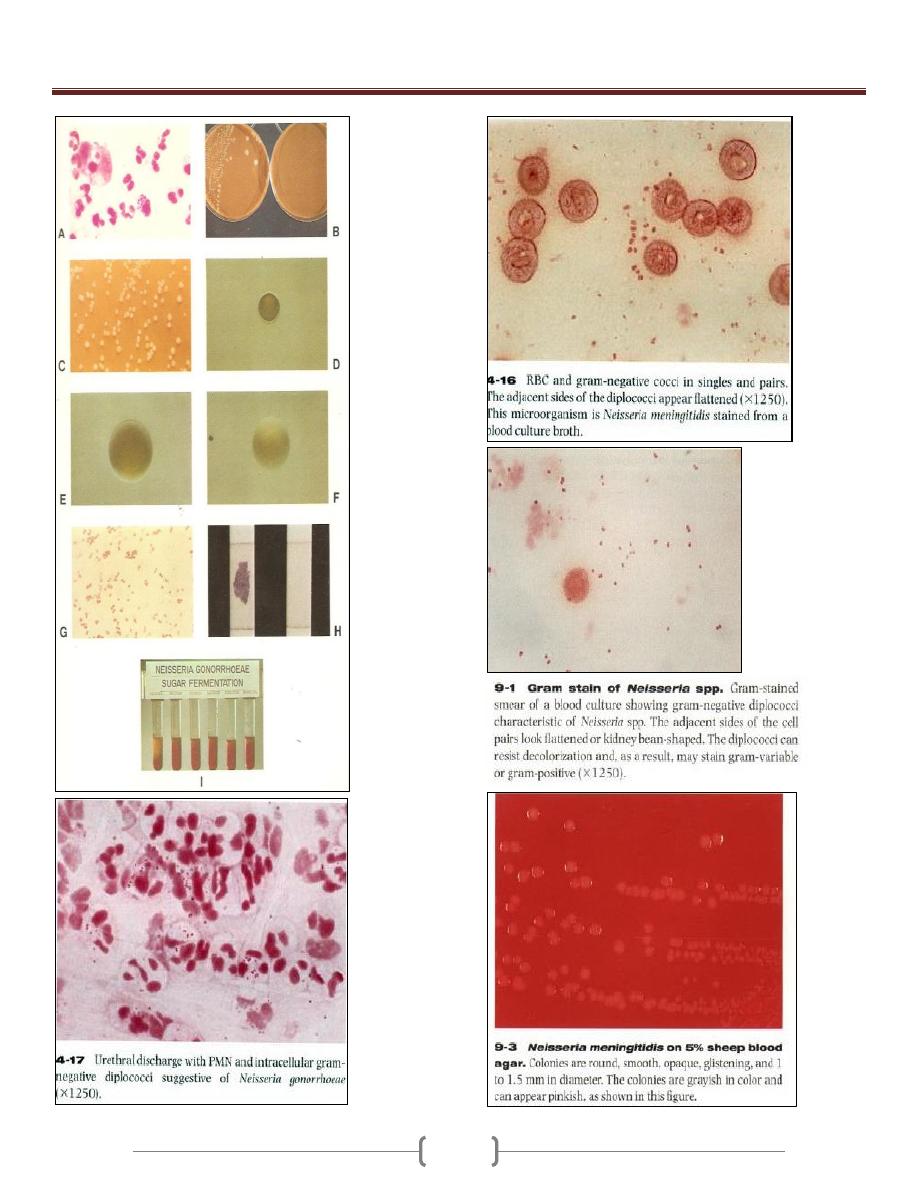
Unit 3 - Bacteriology
31

Unit 3 - Bacteriology
32
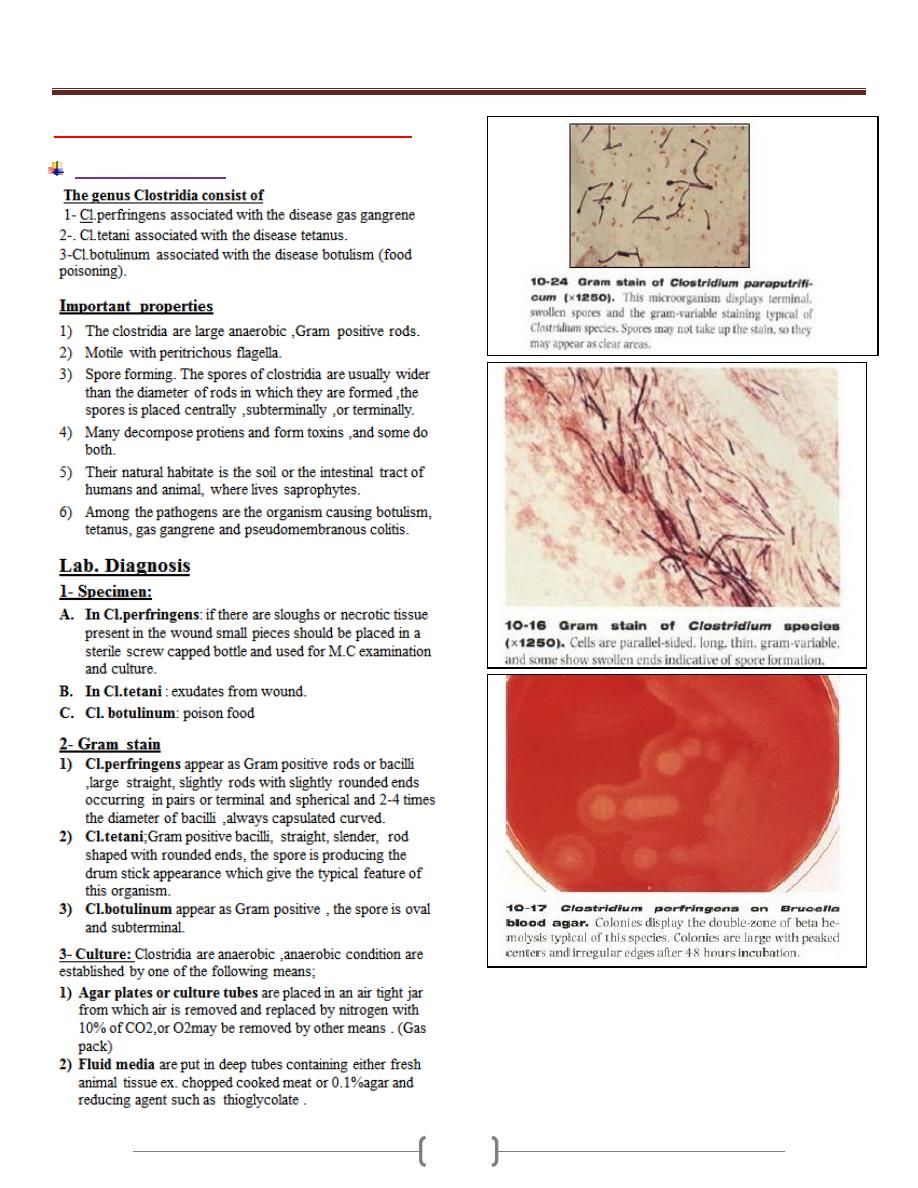
Unit 3 - Bacteriology
33
Lab 3 - Clostridium & Mycobacterium
Clostridium species
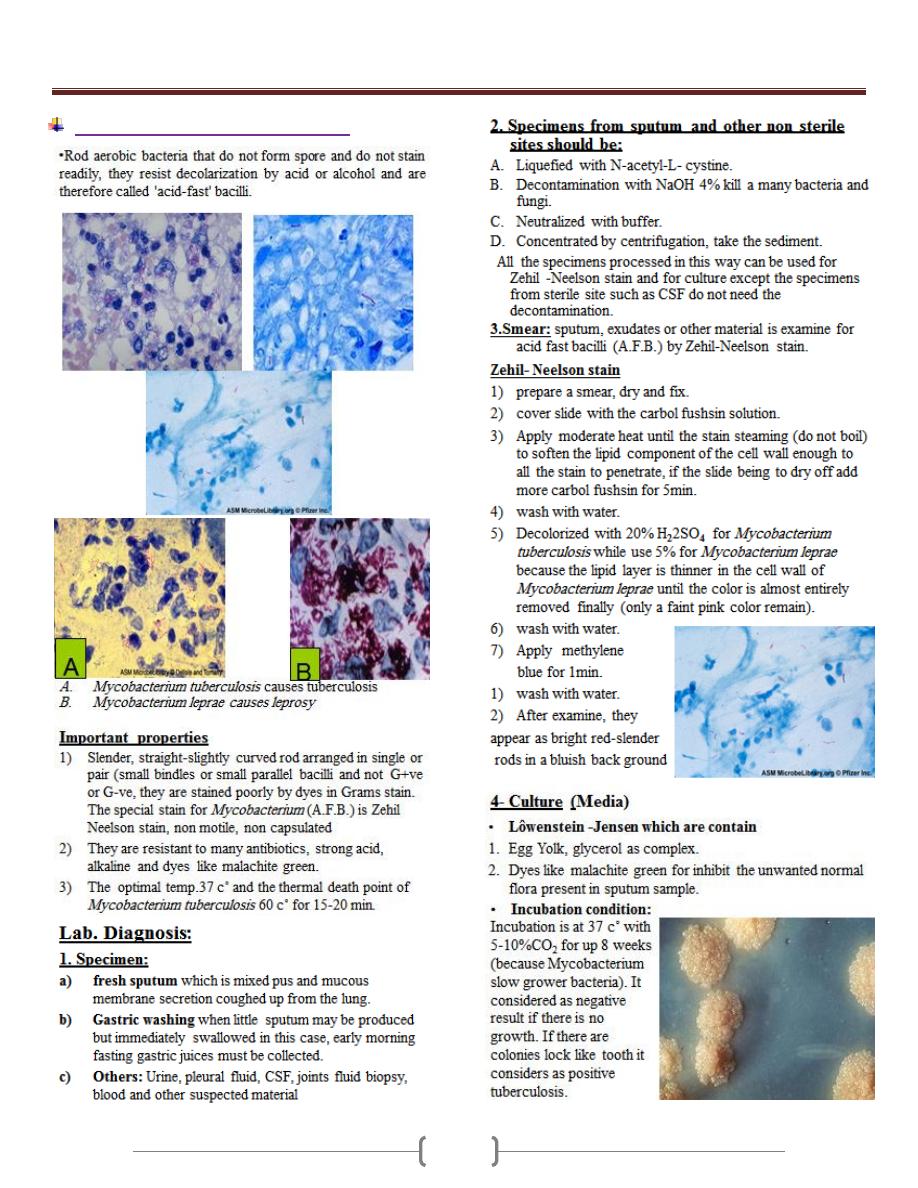
Unit 3 - Bacteriology
34
Mycobacteria (Mycobacterium spp.)
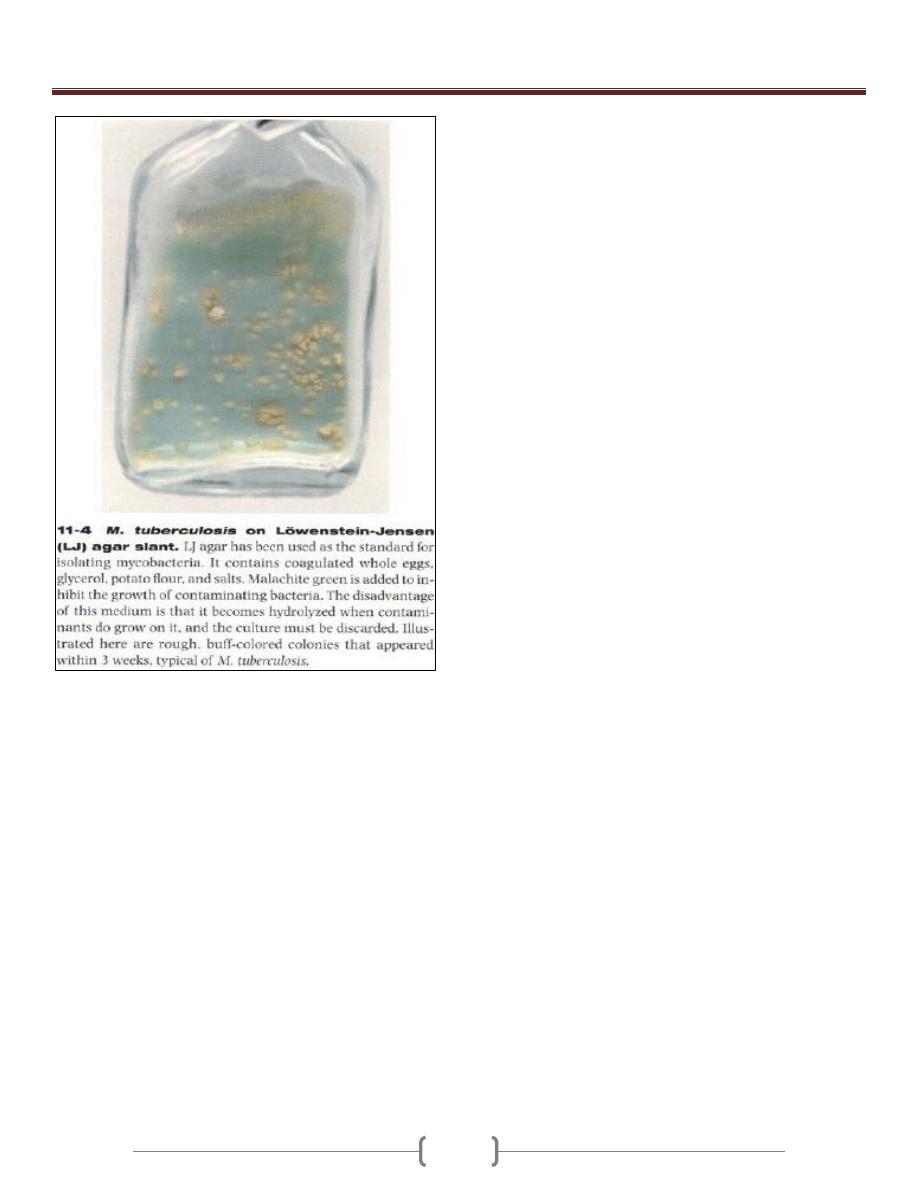
Unit 3 - Bacteriology
35
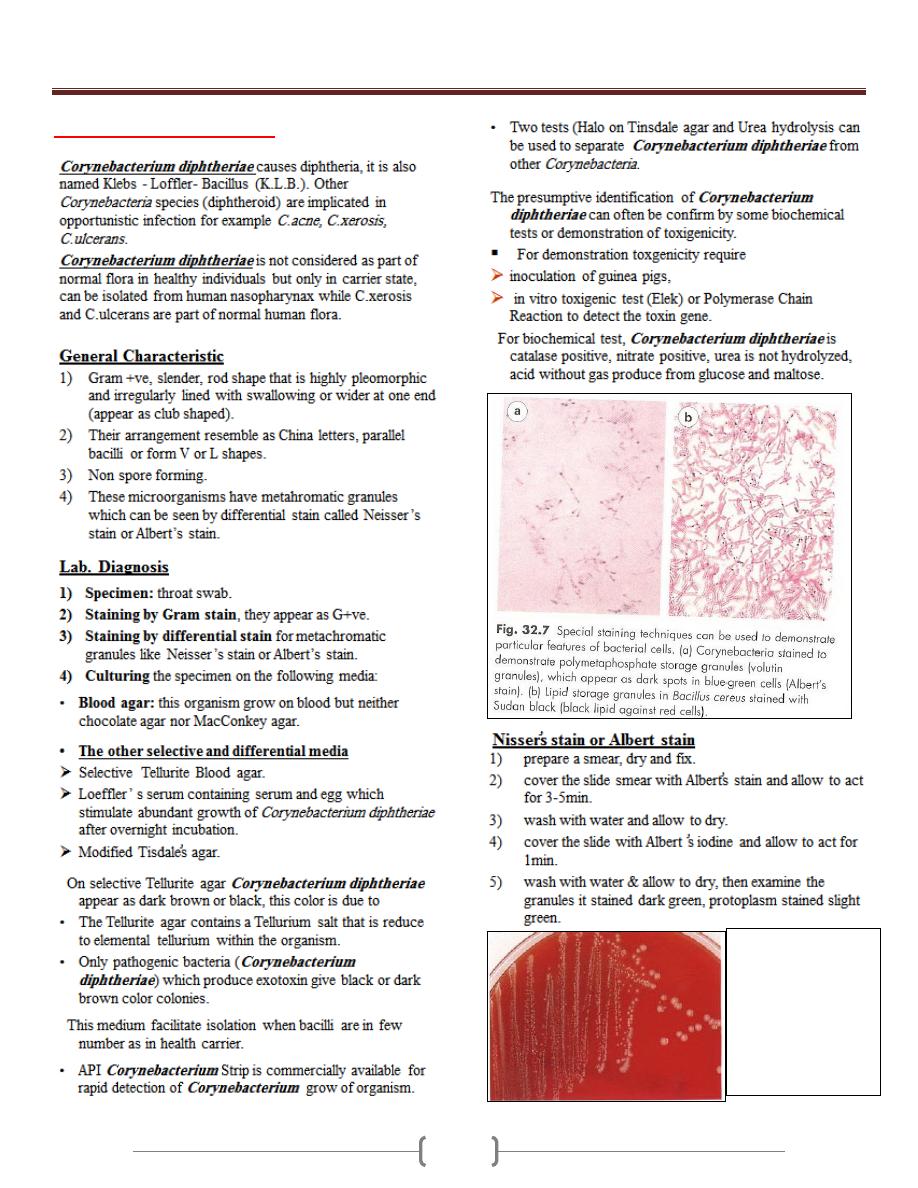
Unit 3 - Bacteriology
36
Lab 4 – Corynebacteria
7-10 colonies of C.
duphtheriae on 5%
sheep blood agar.
Corynebacterium
diphtheriae grow
well on 5% sheep
blood agar as
whitish, opaque
colonies
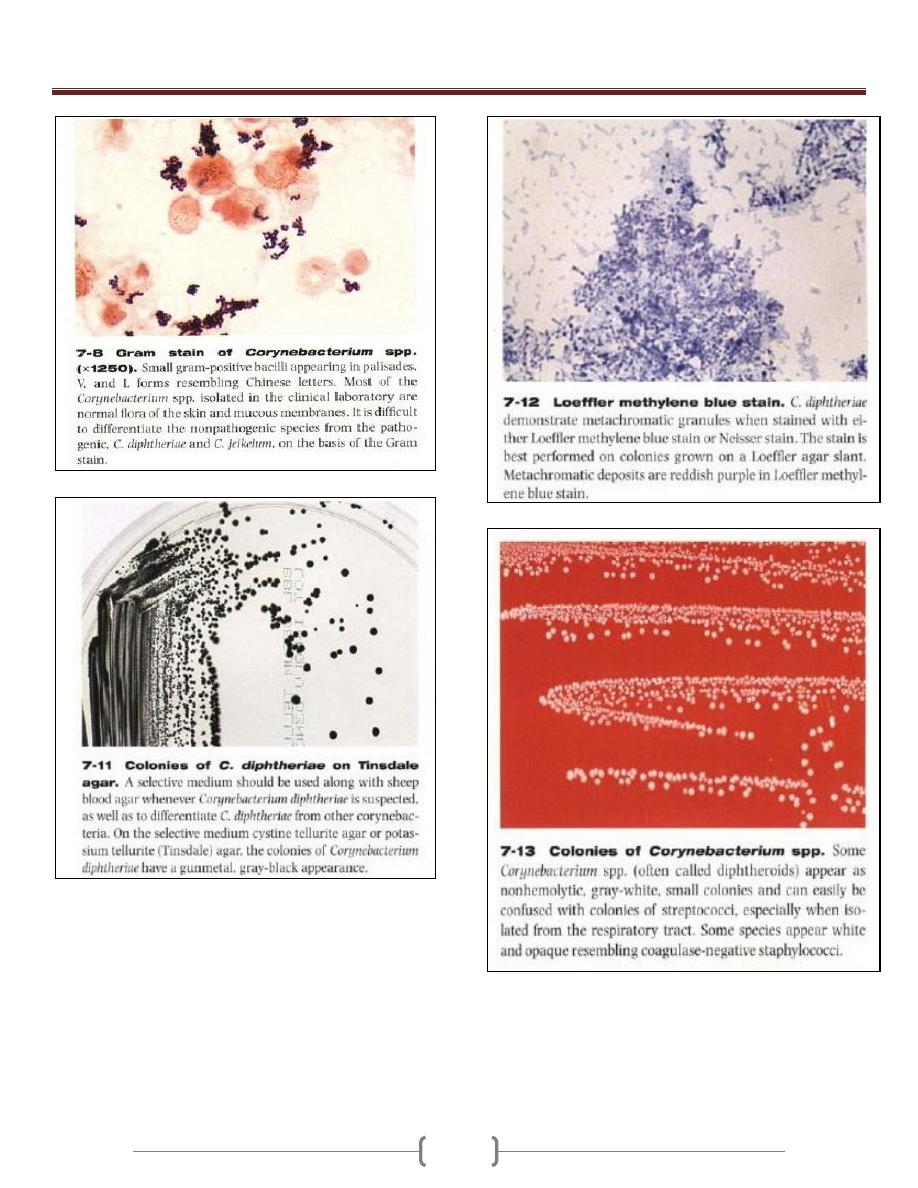
Unit 3 - Bacteriology
37
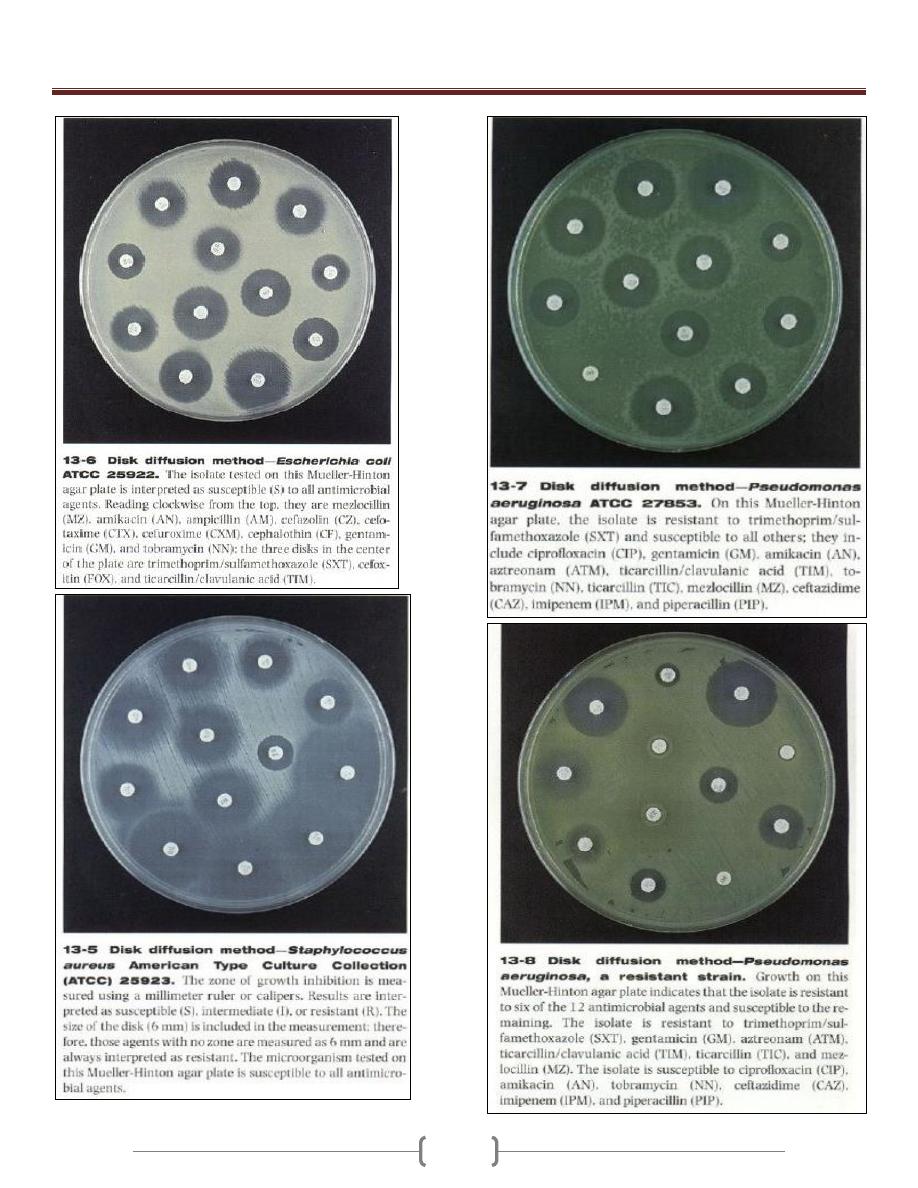
Unit 3 - Bacteriology
38

39
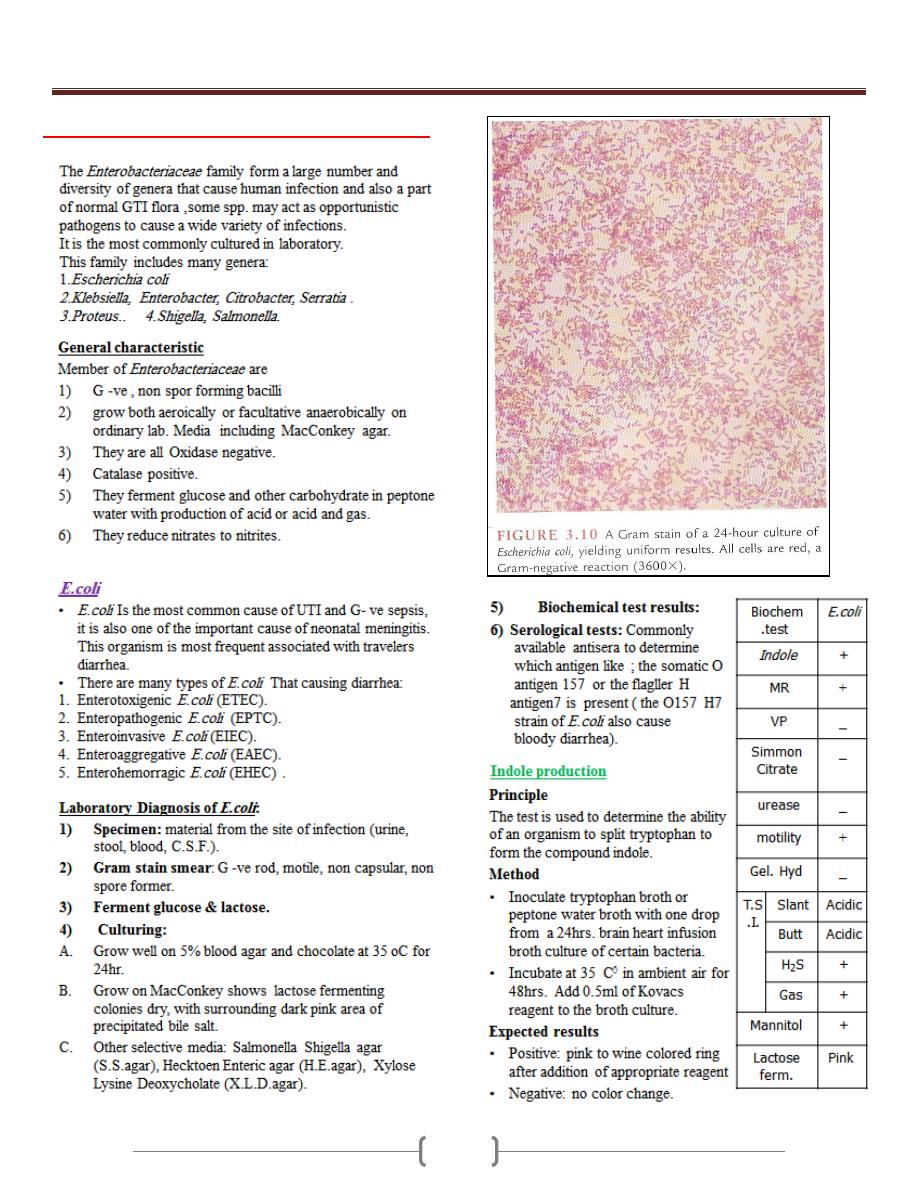
Unit 3 - Bacteriology
40
Lab 1+2+3 – Enteric Gram Negative rods

Unit 3 - Bacteriology
41

Unit 3 - Bacteriology
42
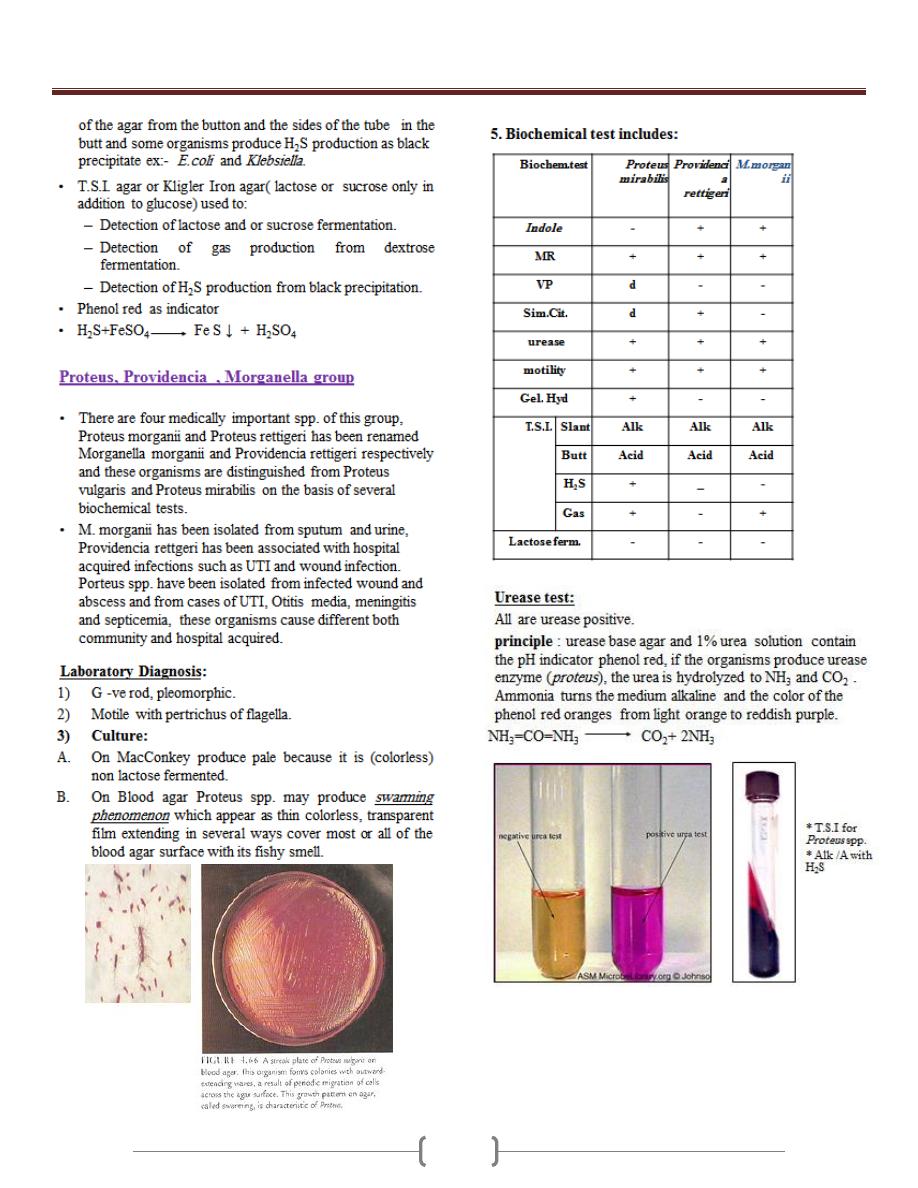
Unit 3 - Bacteriology
43
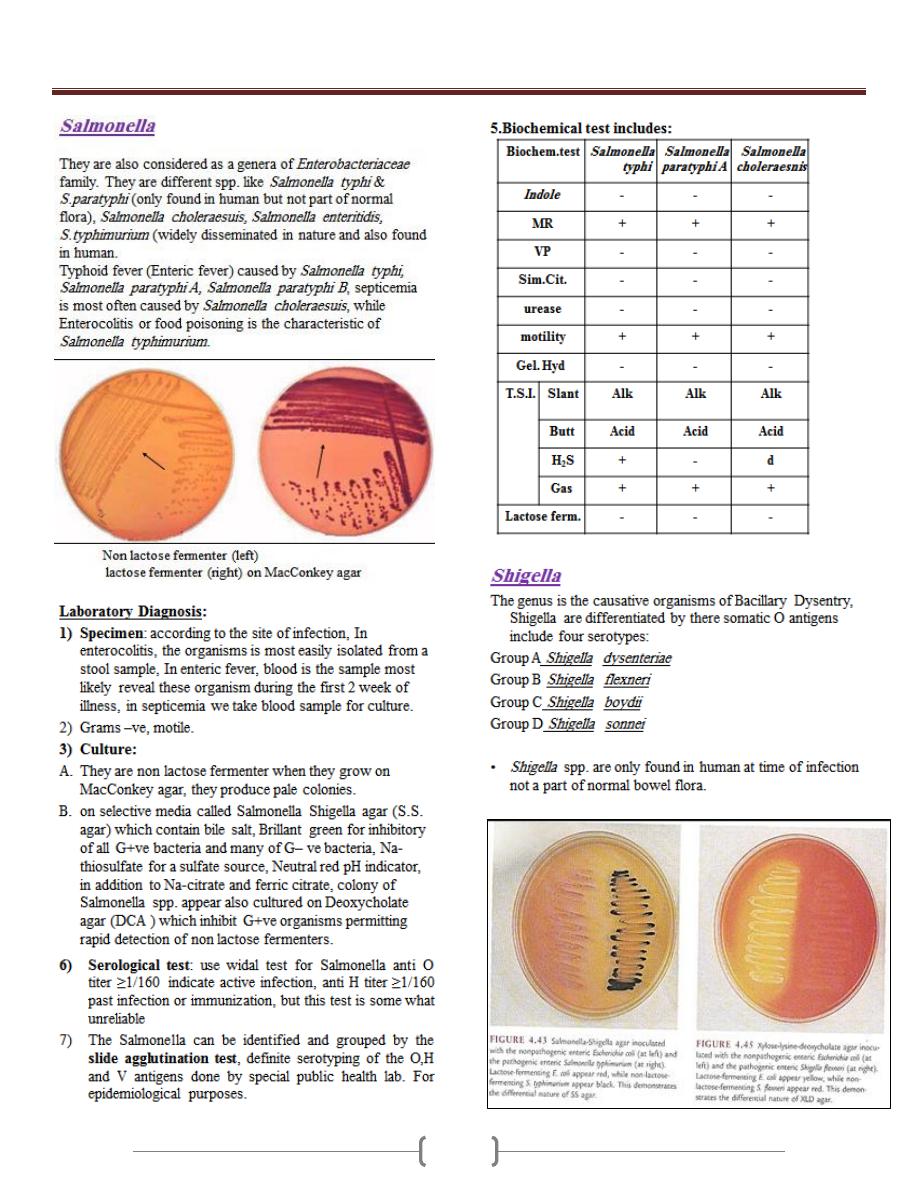
Unit 3 - Bacteriology
44
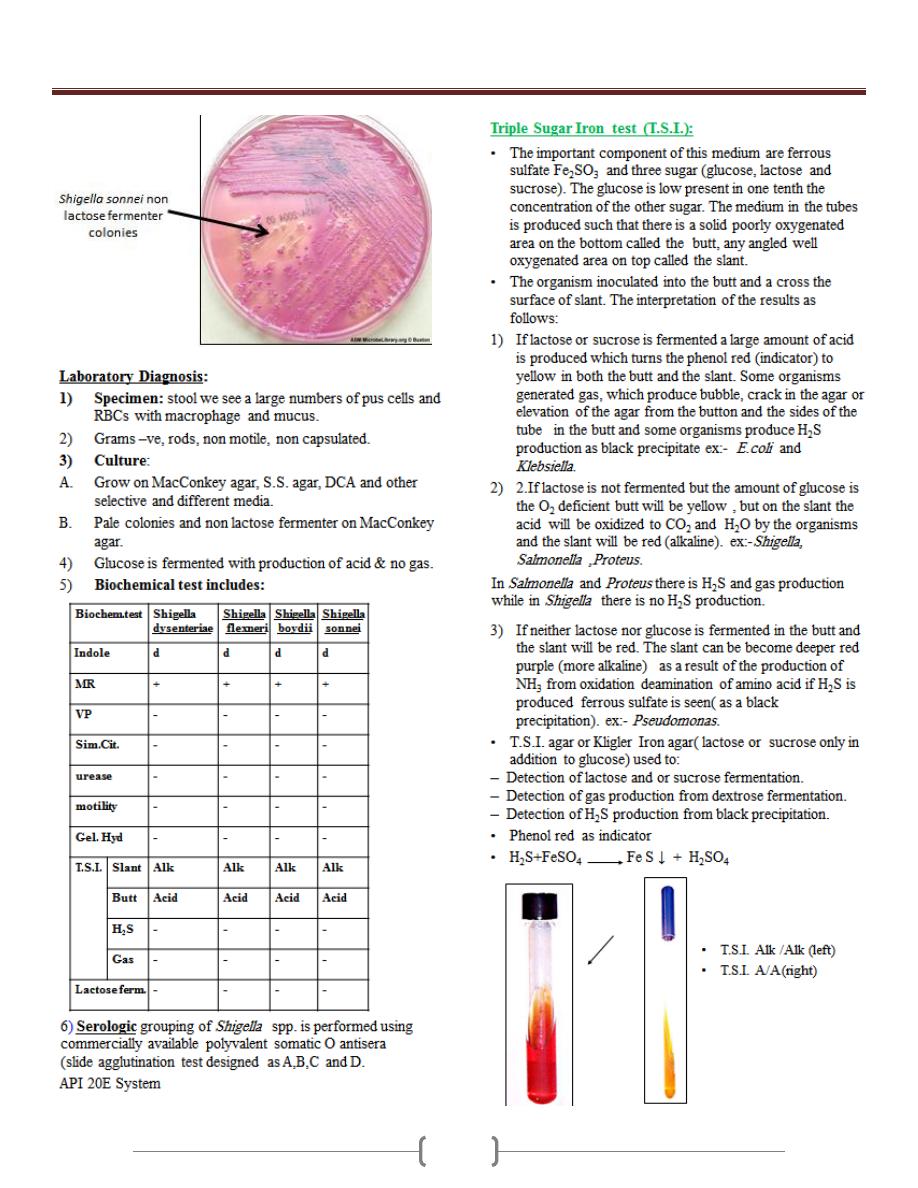
Unit 3 - Bacteriology
45
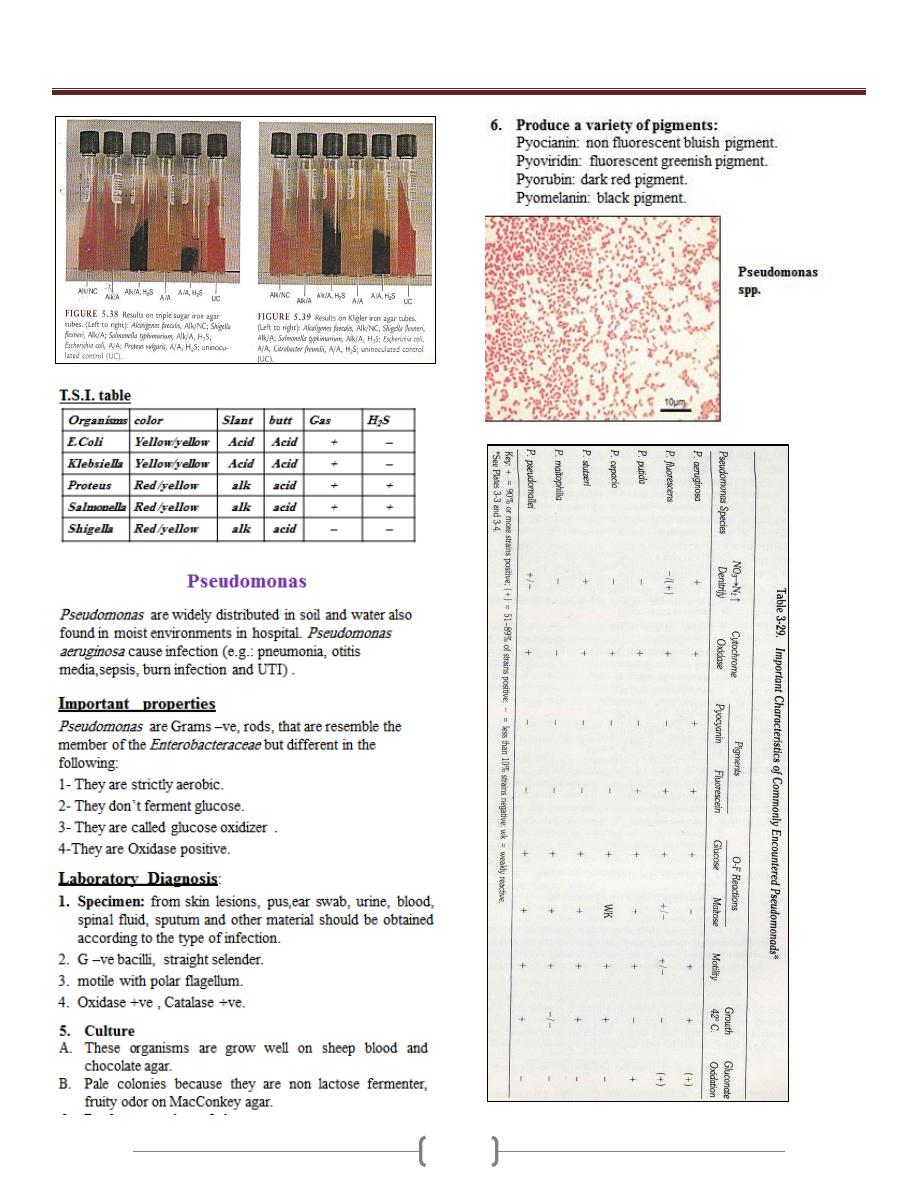
Unit 3 - Bacteriology
46

Unit 3 - Bacteriology
47
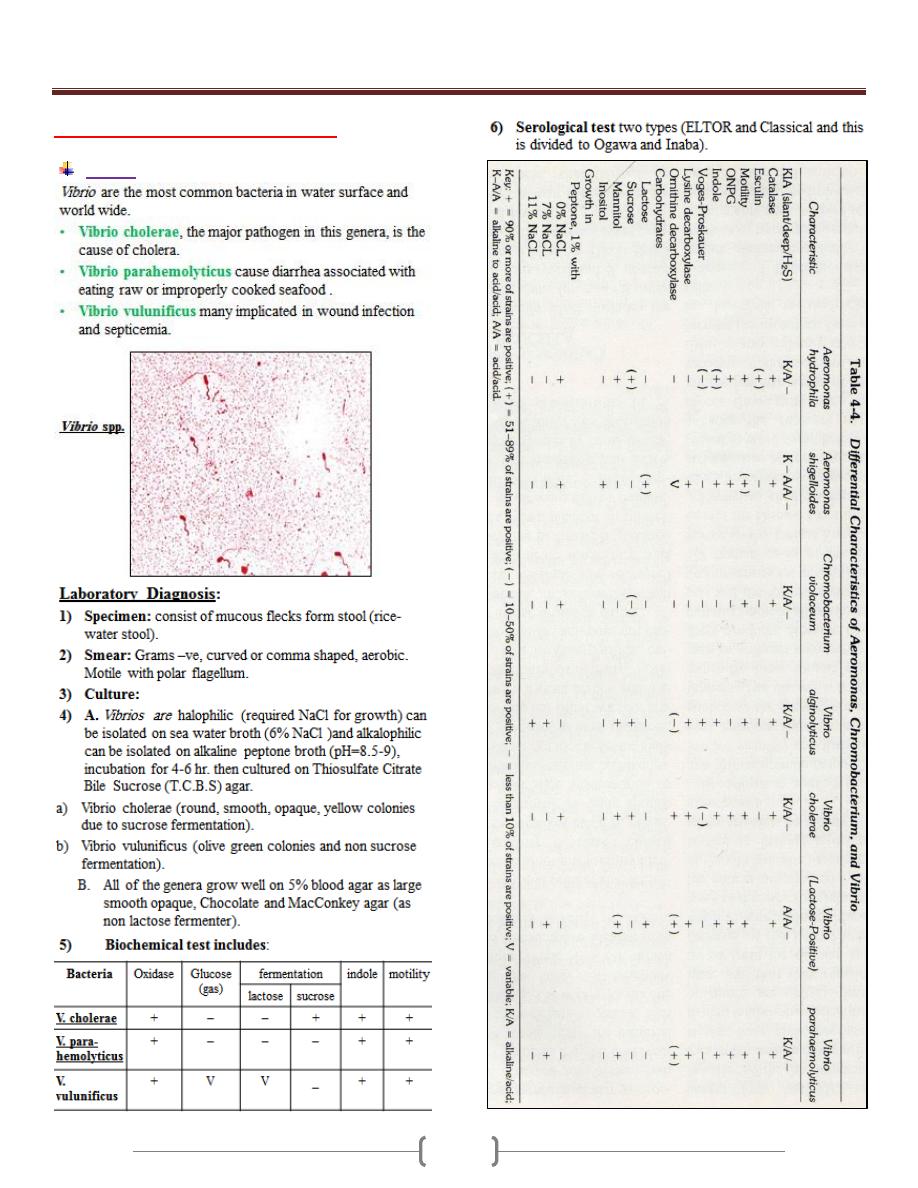
Unit 3 - Bacteriology
48
Lab 4 - Vibrio & Heamophilus
Vibrio

Unit 3 - Bacteriology
49
Haemophilus
A: Identification of H.influenzae by X & V strips
B: H.influenzae satelliting around staphylococcus aureus

Unit 3 - Bacteriology
50
Lab 5 – Brucella, Campylobacter &
Helicobacter
Brucella
Campylobacter
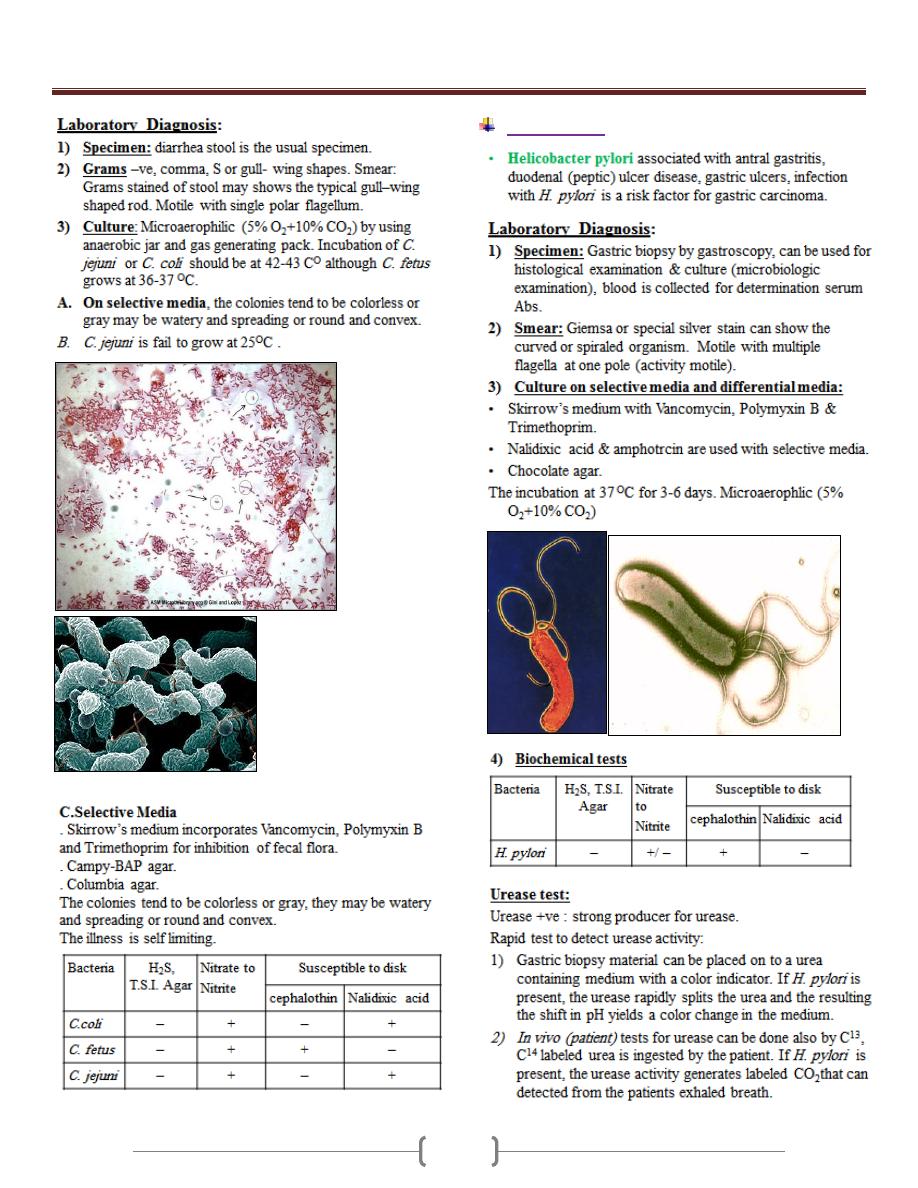
Unit 3 - Bacteriology
51
Helicobacter
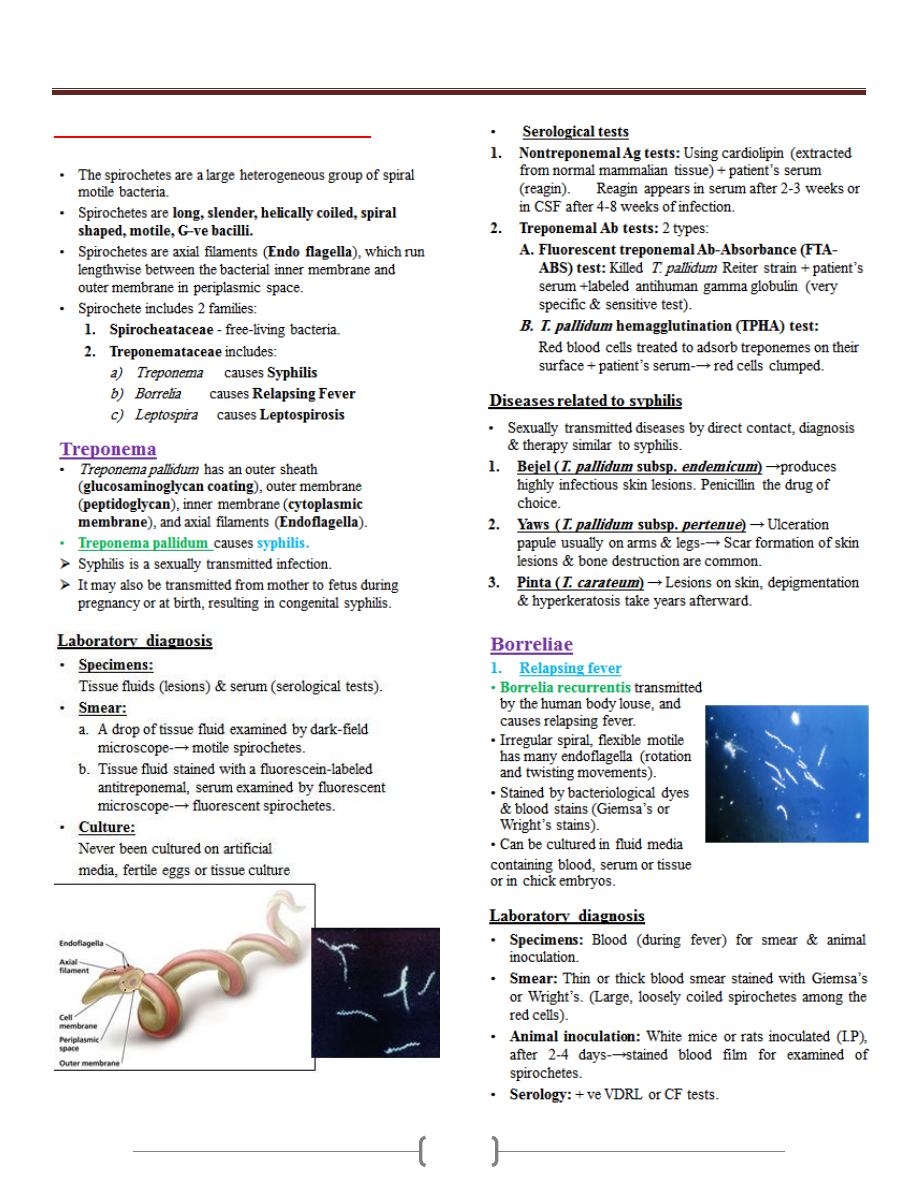
Unit 3 - Bacteriology
52
Lab 6 – spirochetes & Chromagar
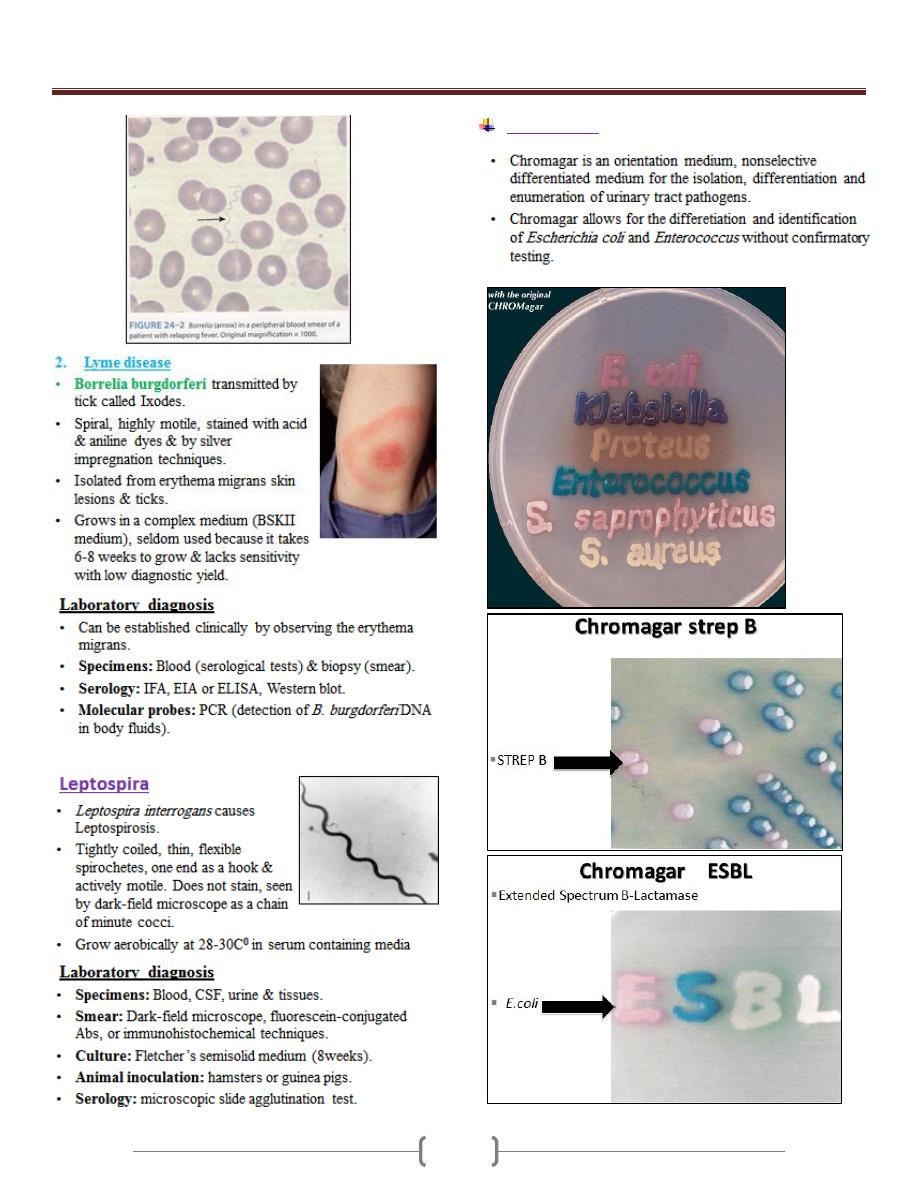
Unit 3 - Bacteriology
53
Chromagar
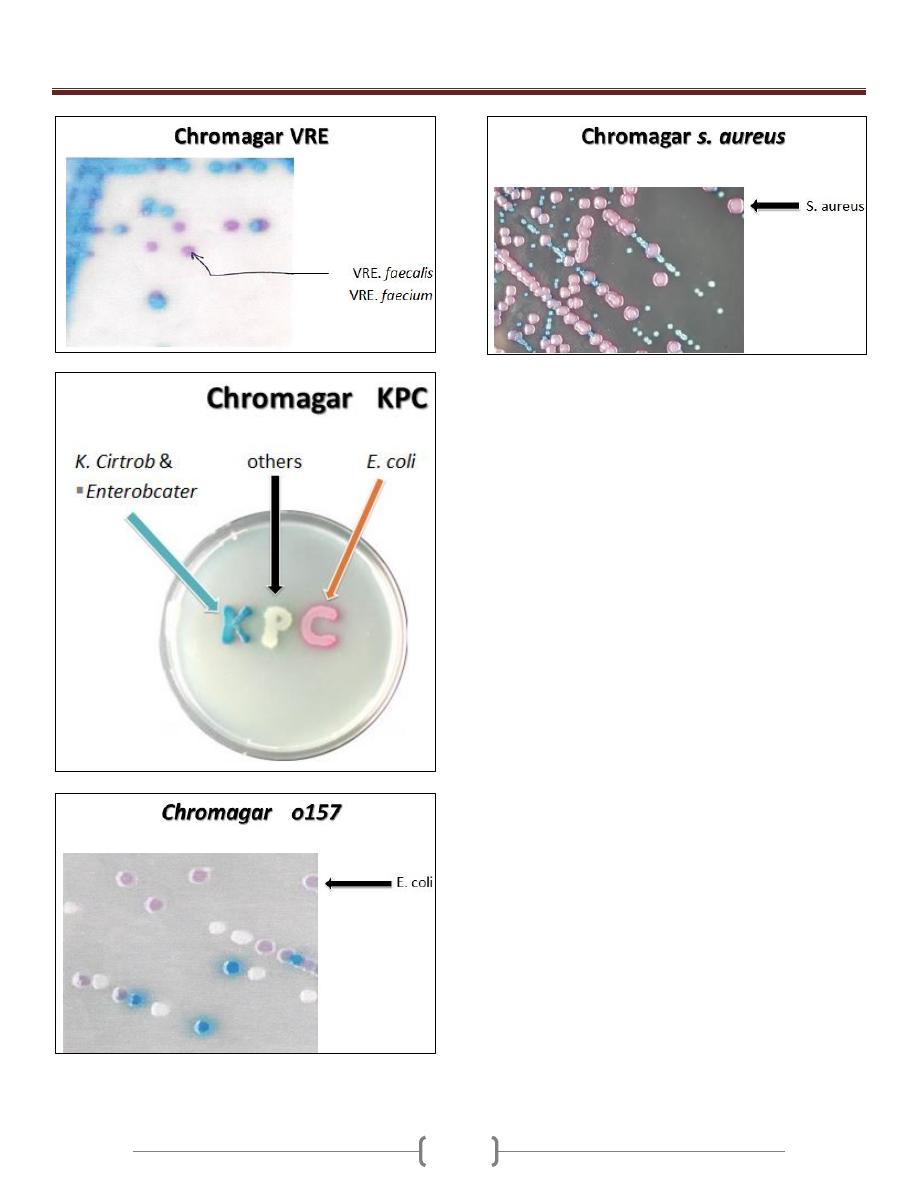
Unit 3 - Bacteriology
54

55

Unit 4 - Mycology
56
Lab 1+2 - Practical Mycology
Medical Mycology is the science that deals with
medically important fungi (fungus), fungi belong to the
kingdom Fungi.
General features of the fungi:
Fungi seen in the clinical laboratory can generally be
separated into two groups based on the appearance of the
colonies formed. The yeasts are produced as moist,
creamy opaque or pasty colonies on media, while the
filamentous fungi or molds produce a fluffy cottony
woody or powdery colony.
Several pathogenic spp. of fungi that exhibit either a yeast
or filamentous appearance are called dimorphic.
Yeasts
Yeasts are unicellular structures that are round to oval in
shape. The microscopical morphology features usually
similar among genera and difficult to differentiate
however, the size the presence or absence of capsule or
narrow necked budding are features that can be helpful to
differentiate between Cryptcoccus spp. and from Candida
spp.
Molds
The basic structural units of the molds are tube like
projection known as hyphae , as the hyphae grow, they
become intertwined to form loose network called
mycelium, which penetrate the substrate from which the
molds obtain the necessary nutrients for growth, this is
called vegetative mycelium. The portion extend above the
substrate surface, is known as aerial mycelium that are
responsible for the fluffy filamentous nature of the mold.
Three types of hyphae exist in medically important
fungi these include
1) The sparsely septate (a septate hyphae) ,an example of
zygomycetes
2) The dark pigmented septate hyphae an example of
dermatiaceous fungi.
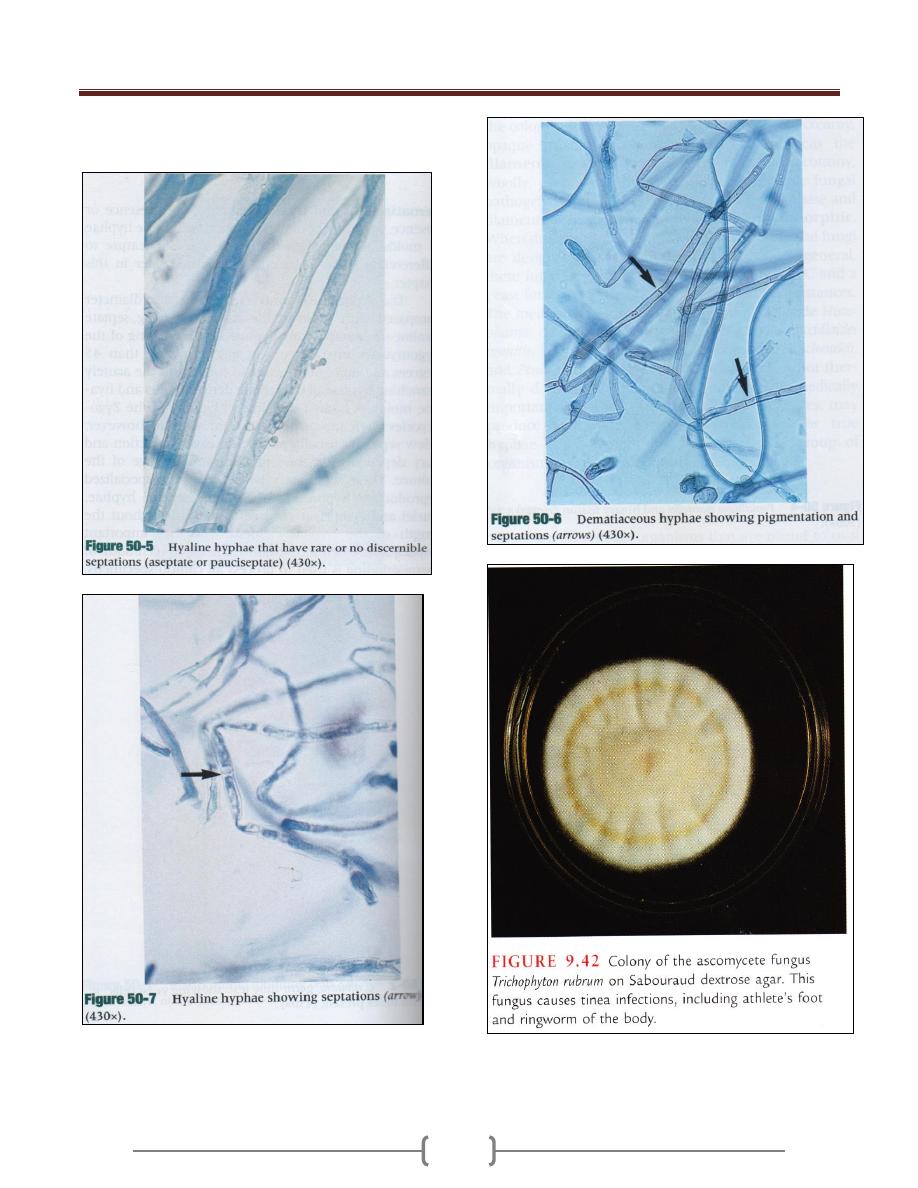
Unit 4 - Mycology
57
3) The septate non pigmented hyphae an example of hyaline
molds.

Unit 4 - Mycology
58
Laboratory diagnosis of fungal infection
The diagnosis of fungal infection depends entirely on the
selection and collection of an appropriate clinical
specimen for culture. The proper collection of the
specimen and their rapid transport to the clinical
laboratory is of a major importance for recovery of fungi.
Type of specimen
Respiratory secretion
Sputum, induced sputum, bronchial washing and
transtrachial aspiration are the most commonly submitted
specimen for fungal culture.
Cerebrospinal fluid (CSF)
CSF collected for culture should be filtered through 0.45
Mm membrane filters attach to a sterile syringe. After
filtration, the filter is removed and is placed onto the
surface of an appropriate culture media & examine daily.
Blood
The concentration blood is incubated onto the surface
appropriate medium and most fungi are detected within
the first 4 days except Histoplasma capsulatum may
require approximately 10-14 days for recovery.
Hair, Skin and Nail Scrapings
o These specimens are usually submitted for
dermatophytes culture and may be contaminated with
bacteria and rapidly growing fungi sample collected
from lesions may be obtained by scraping the skin or
nail with a scraped blade or microscopical slide
o Infected hairs are removed by plucking them with
forceps .Clipping may be taken from the distal border
of the nail with scissor or nail clapper when the
dystrophy extend to the distal end of the nail.
o These specimens should be placed in sterile pertridish
and they should not be refrigerated. The agar contains
chloramphenicol and cyclohexamide for recovery of
dermatophytes, culture should be incubated at 30
O
C
for 30 days. before being reported as negative.
Urine
All urine samples should be centrifuged and the sediment
cultured by loop because urine is often contaminated with
G –ve bacilli, it is necessary to use media containing
antibacterial and antifungal agents.
Tissues, Bone marrow and Sterile body fluids
All tissues should be processed before culturing by
mincing or grinding after processing, at least 1ml of the
specimen should be cultured as soon as possible on
appropriate media and incubated at 30
O
C for 30 days.
Bone marrow may be placed directly to the appropriate
medium, sterile body fluids should be concentrated by
centrifugation before culturing and at least 1ml of the
specimen should be cultured onto the surface of an
appropriate medium.
Culture media and Incubation requirements
For optimal recovery a battery media should be used and
the following points recommended:
1) Media with and without blood enrichment 5% sheep
RBCs blood.
2) Media with and without cycloheximide 0.5 mg/ml.
3) All media should contain antibacterial agent except
specimen sterile body fluids
Sabauroud’s dextrose agar is the most common media
used for recovery of pathogenic fungi. Agar plates or
screw capped agar tubes are satisfactory for the recovery
of fungi but plates are preferred since they provide better
aeriation of culturing, a large surface area for better
isolation of colonies and easily handling for making
microscopical examination.
Direct microscopical examination of clinical specimen
A. Wet mount preparation: This method done by take a
portion of colony from point intermediate between center
and periphery place in a slide and a drop of lactophenol
cotton blue stain is added and then place a cover slide
press firmly then examine microscopically.
B. Gram stain is used for detection of most of fungi that
stain well however others such as Cryptococcus stain
weakly.
C. Calcofluor white is florescent stain for detection of fungi
rapidly because of bright fluorescence and this can be
mixed with KOH.
D. Indian Ink is important of Cryptococcus neoformans to
demonstrate the capsule in CSF when positive in CSF
diagnosis of meningitis (50%of case meningitis give
positive results).
Unit 4 - Mycology
59
E. E-Potassium hydroxide KOH preparation 10-20% used
for cleaning of specimen tissue to make fungi more
reading visible by digest protein and clear keratinized
tissue. If takes 5min. for clearing. If clearing is not
complete add another 5-10mins. This is a rapid method
for detection of fungi element.
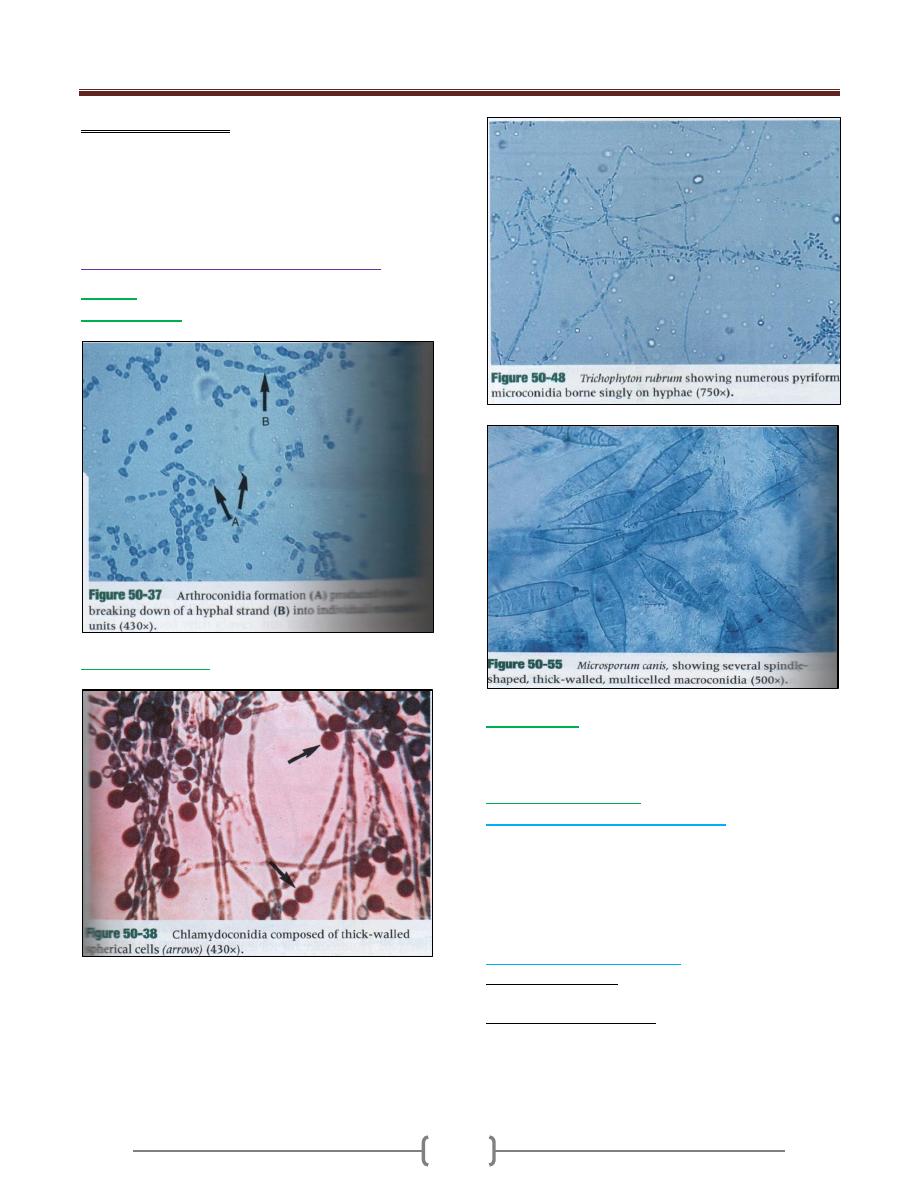
General morphologic features of molds
Conidia
Arthroconidia
Chlamydioconidia
Fungi may produce conidia of two sizes: microconidia:
small unicellular, round elliptic in shape or macroconidia:
large, multiseptated, club or spindle shaped. Microconidia
may be formed on the side of the hyphae on the end of the
hyphae but macroconidia or usually bare on a
conidiophore and may be smooth or roughly walled.
Sporangium:
Sporangiospores: they are spores produced within the
sporangium and released by rupture of the sporangial wall.
Hyaline septate molds
Cutaneous infection (Dermatophytes)
Dermatophytes includes genus Trichophyton that capable
of invading the hair nail and skin. Genus microsporum
involve dry hair and skin, The genus Epidermophyton
involve the skin and nail.
o Direct detection
o Culture and Identification
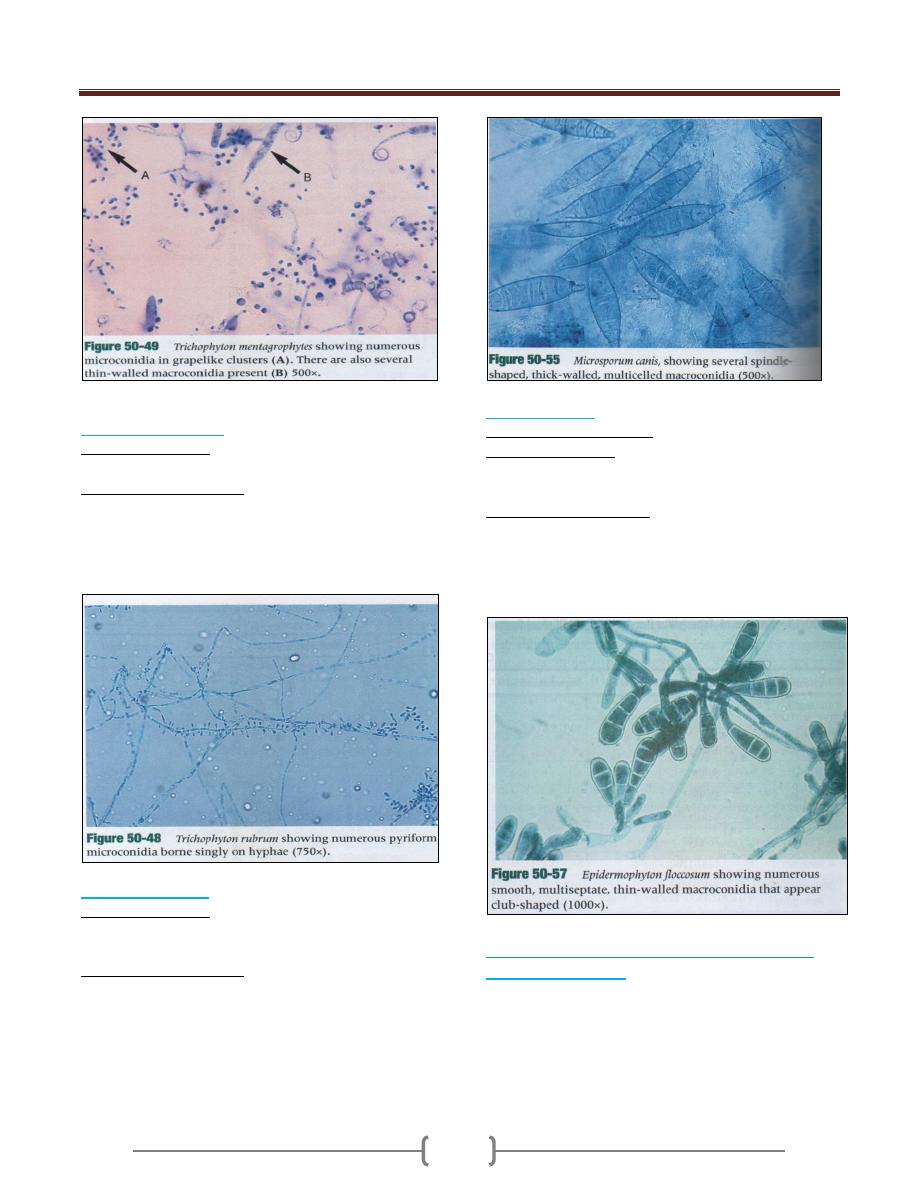
Trichophyton mentagrophytes
o Colonial morphology White to pinkish granular and fluffy
slow growing colonies.
o Microscopical Identification
Many round microconidia most commonly borne on
grape like clusters or laterally along the hyphae:
macroconidia are thin walled, smooth, club shaped and
multiseptate.
Unit 4 - Mycology
60
Trichophyton rubrum
o Colonial morphology
Colonial types vary from white to pink granules
o Microsopical Identification
Microconidia is usually teardrop shaped or club shaped
most commonly borne a long sides of hyphae,
macroconidia is usually absent but when present are thin
or smooth walled.
Microsporium canis
o Colonial morphology
Colonies of Microsporium canis grow rapidly; they are
granular or fluffy.
o Microsopical Identification
Thick walled, spindle shaped, large multisepate rough
walled macroconidia, some with a curved tip,
microconidia rarely seen.
Epidermophyton
Epidermophyton floccosum
o Colonial morphology
Colony grows slowly and the growth appears khaki green
in the center and tends to folded, the periphery is yellow.
o Microsopical Identification
Macroconidia large ,smooth walled numerous,
multiseptate, they are round at top and are borne singly in
conidiophore or in group of two or three. Microconidia
are absence.
Hyaline, septate molds (opportunistic mycosis)
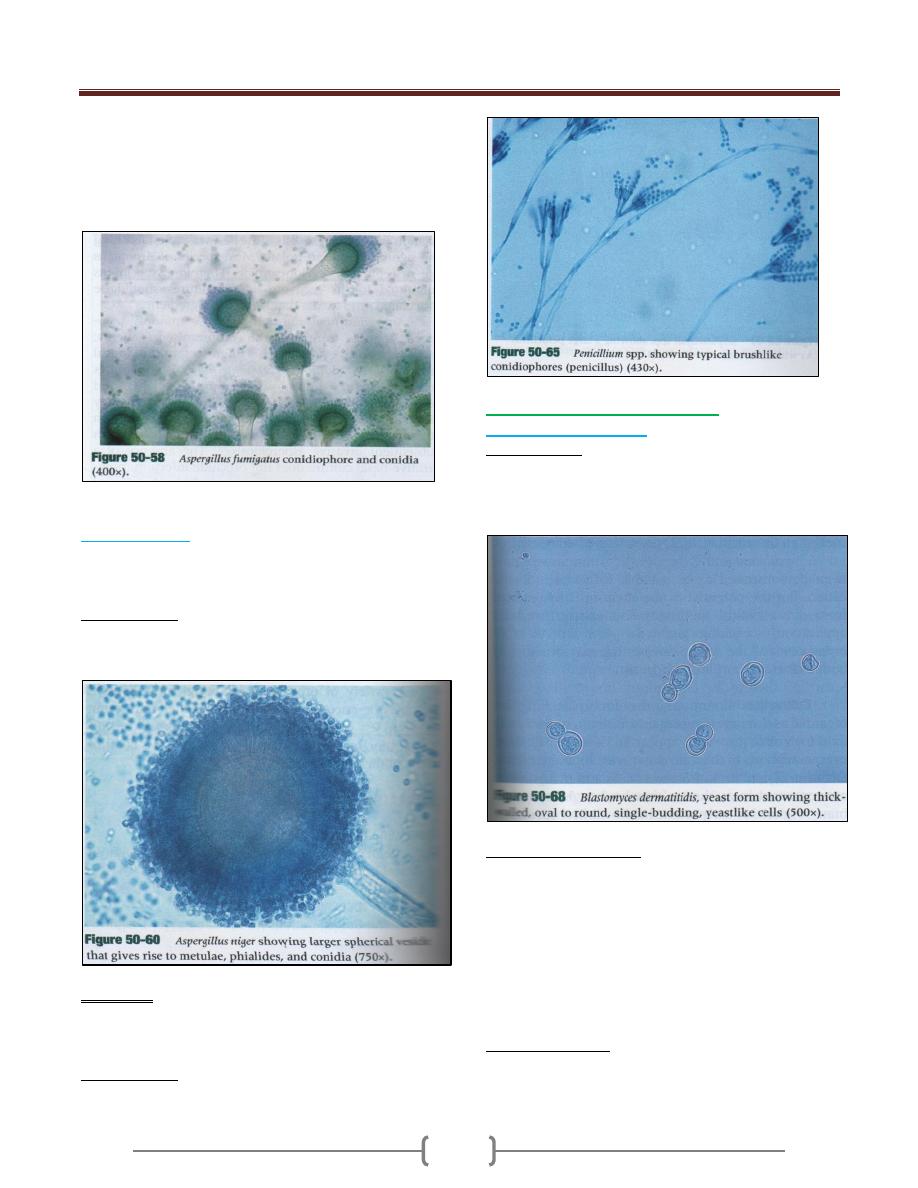
Aspergillus fumigatus
o Is the most common spp. recovered from immune
compromised patients, and it is the most common spp.
seen in clinical lab.
o Aspergillus fumigatus is a rapidly growing mold 2-6 days
that produce a fluffy to granular, white to blue green
colony.
Unit 4 - Mycology
61
o Microscopically is characterized by the presence of
septate hyphae branching with short or long conidiophore.
The tip of conidiophore expand into a large, dome shaped
vesicle that has bottle- shaped covering the upper half of
its surface.
Aspergillus niger
o Produce mature colonies within 2-6 days. Growth begins
as yellow colonies then develop a black, dotted surface as
conidia is produced.
o Microscopically
Aspergillus niger shows septate hyphae, long
conidiophore that support spherical vesicles.
Penicillium
o Penicillium is one of the most common organisms
recovered by clinical lab. Colonies of Penicillium are
green, blue green, pink or white .
o Microscopically the hyphae are hyaline septate and
produce brush like conidiophores (penicillens).
Hyaline septate dimorphic molds
Blastomyces dermatitidis
o Direct detection
B. dermatitides appear as large, spherical, thick walled
yeast cell usually with a single bud that is connected to
the parent cell by broad base
o Culture and Identification
The mold form develops as well as waxy appearing
colonies that become off-white to white color.
Microscopically the hyphae of the mold form are septate,
and single teardrop conidia on long to short conidiophore.
When incubated at 37
O
C colonies of the yeast form
develop in 7 days and appear waxy, and cream to tan in
color. Microscopically large, thick walled yeast cell with
bud attached by a broad base.
o Coccidiodes immitis produce spherules which contain non
budding endospore microscopically.
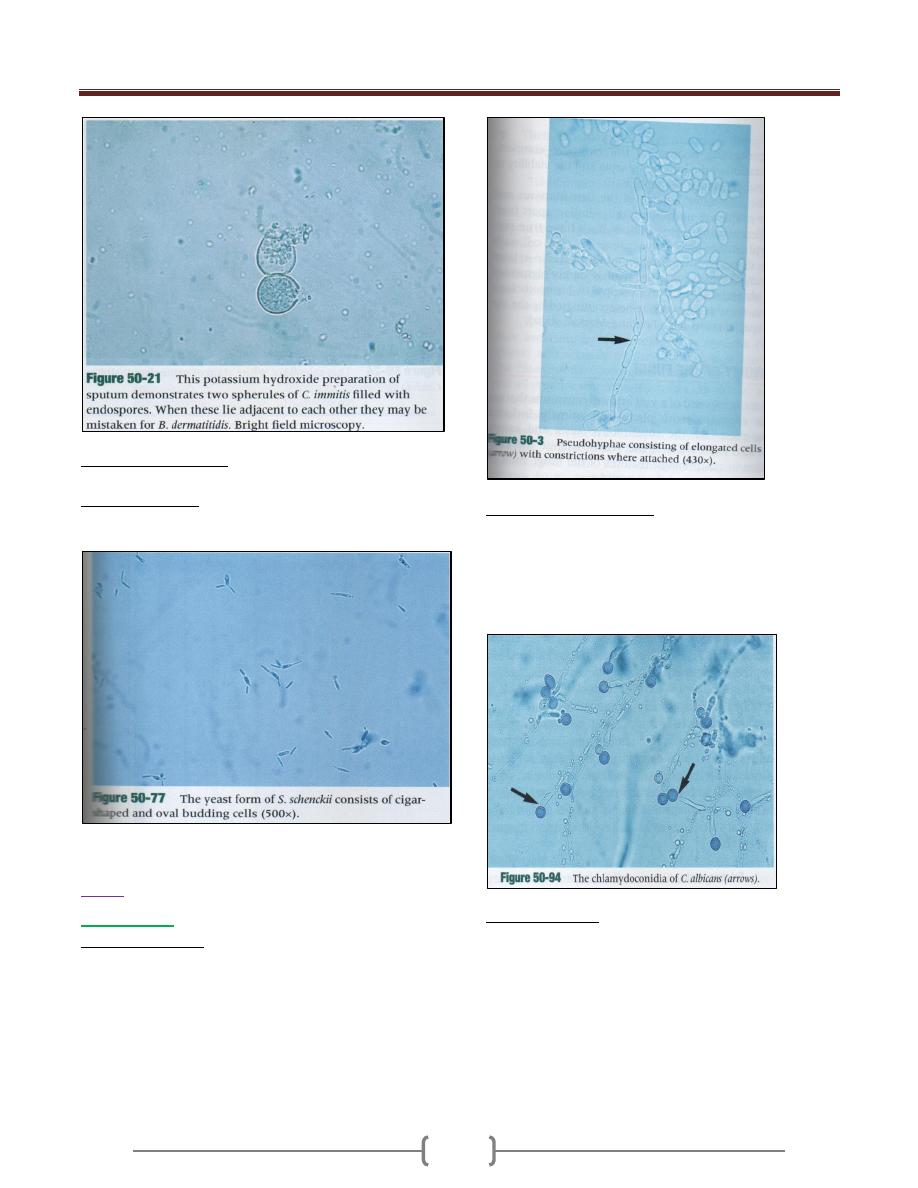
Unit 4 - Mycology
62
o Histoplasma capsulatum produce small, budding yeast
cell within the macrophage seen microscpically.
o Sporothrix schenkii in direct microscope show small
round to oval to cigar shaped yeast cell.
Yeast
Ex. Candidia
Direct examination
The direct examination microscopically of clinical
specimen containing Candidia reveal budding yeast cell
(Blastoconidia) and/ or Pseudohyphae showing regular
point of constriction or true hyphae.
The Blastoconidia, hyphae. Pseudohyuphae are strongly
Grams +ve
Culture and Identification
Colonies of Candidia are creamy, moist, and opaque with
sweaty odor.
Candida albicans may be identified by germ tube
production or production of chlamydospores on corn
meal agar .
o Germ tube method
Suspend a very small inoculum of yeast cell from isolate
colony in 0.5 c.c. of sheep serum then incubate at 37
O
C
for up to 3hr. often incubation a drop of suspension is
removed and place on shade and examine under low
power for the presence of germ tube which is appear as
appendage extended from mother cell that is half the
width and 3-4 times the length of the yeast cell.

Unit 4 - Mycology
63
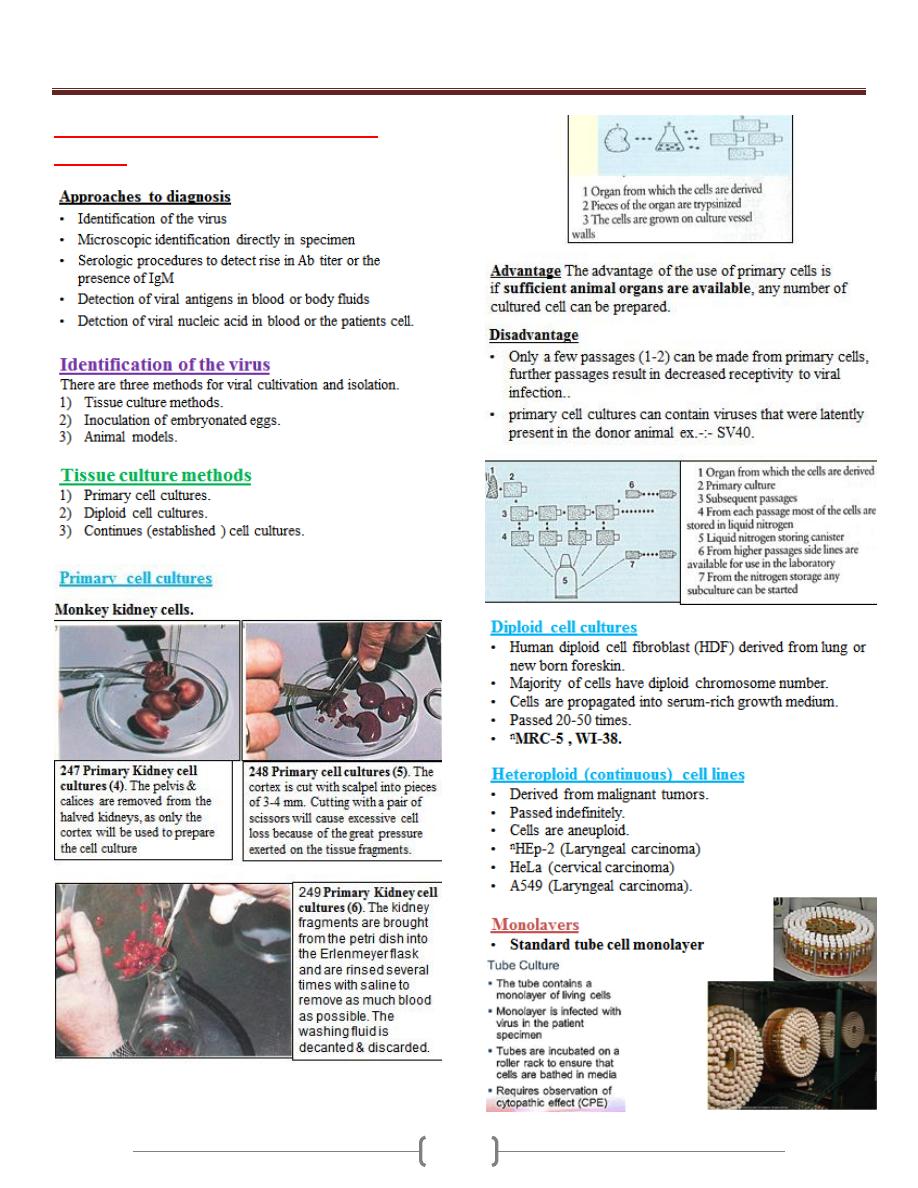
Cryptococcus
Ex. Cryptococcus neoformans
Direct examination
Indian Ink preparation in most widely used method for
rapid detection of Cryptococcus neoformans in clinical
specimen ,Cryptococcus neoformans appear as spherical,
single or multiple budding thick walled yeast like
organism surrounded by wide retractile capsule.
Culture and identification
Colonies of Cryptococcus neoformans usually appear on
culture media within 2-5 days and begin as smooth, white
to tan colony that may become mucoid cream to brown in
color.

64
Unit 5 - Virology
65
Lab 1+2 - Laboratory Diagnosis of
Viruses
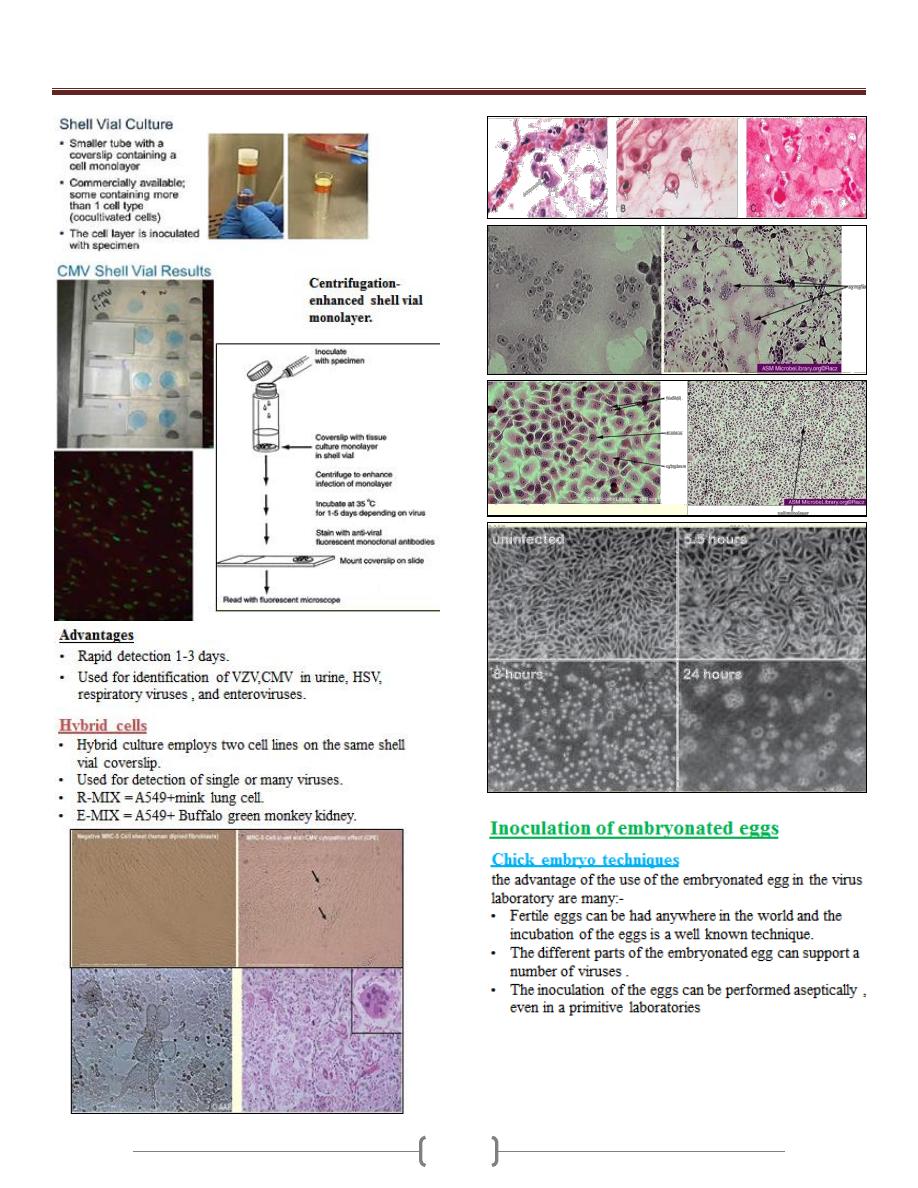
Unit 5 - Virology
66
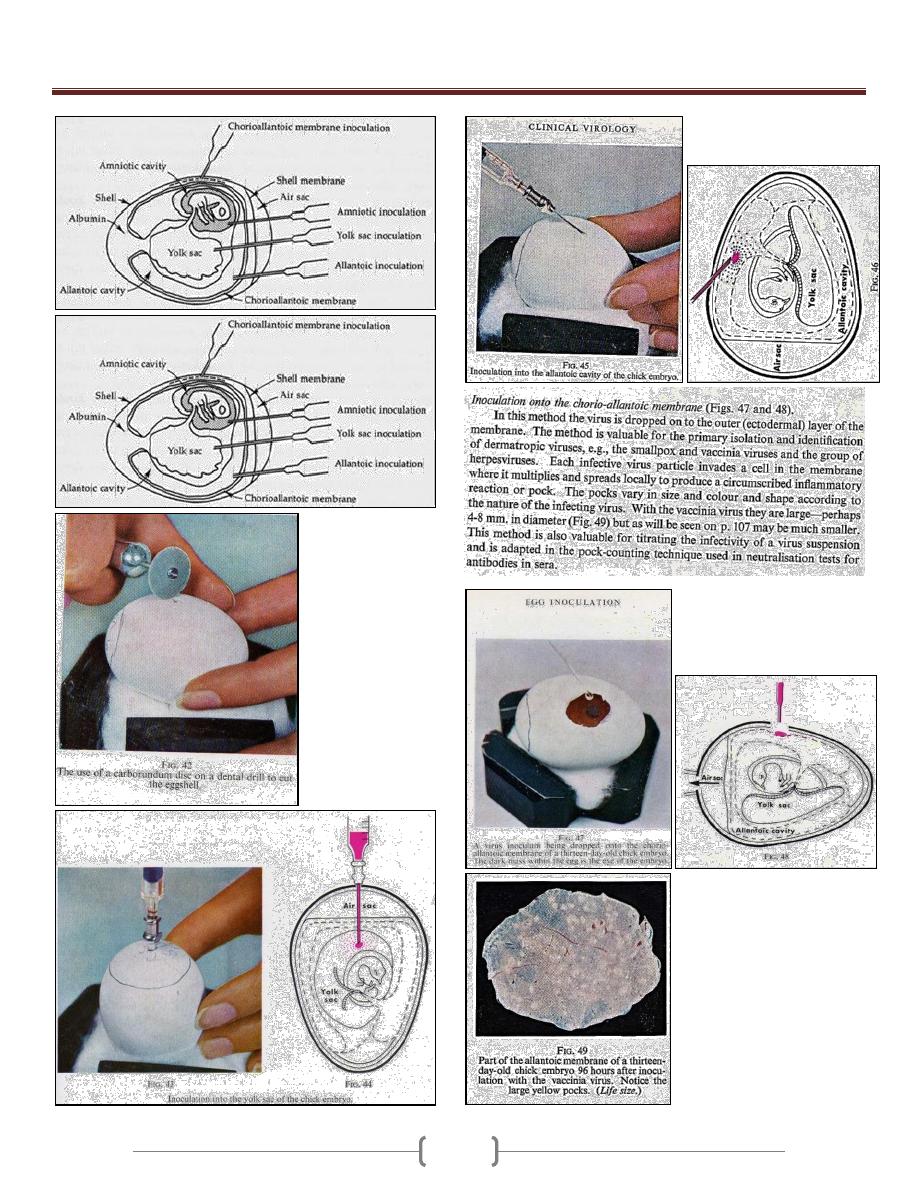
Unit 5 - Virology
67
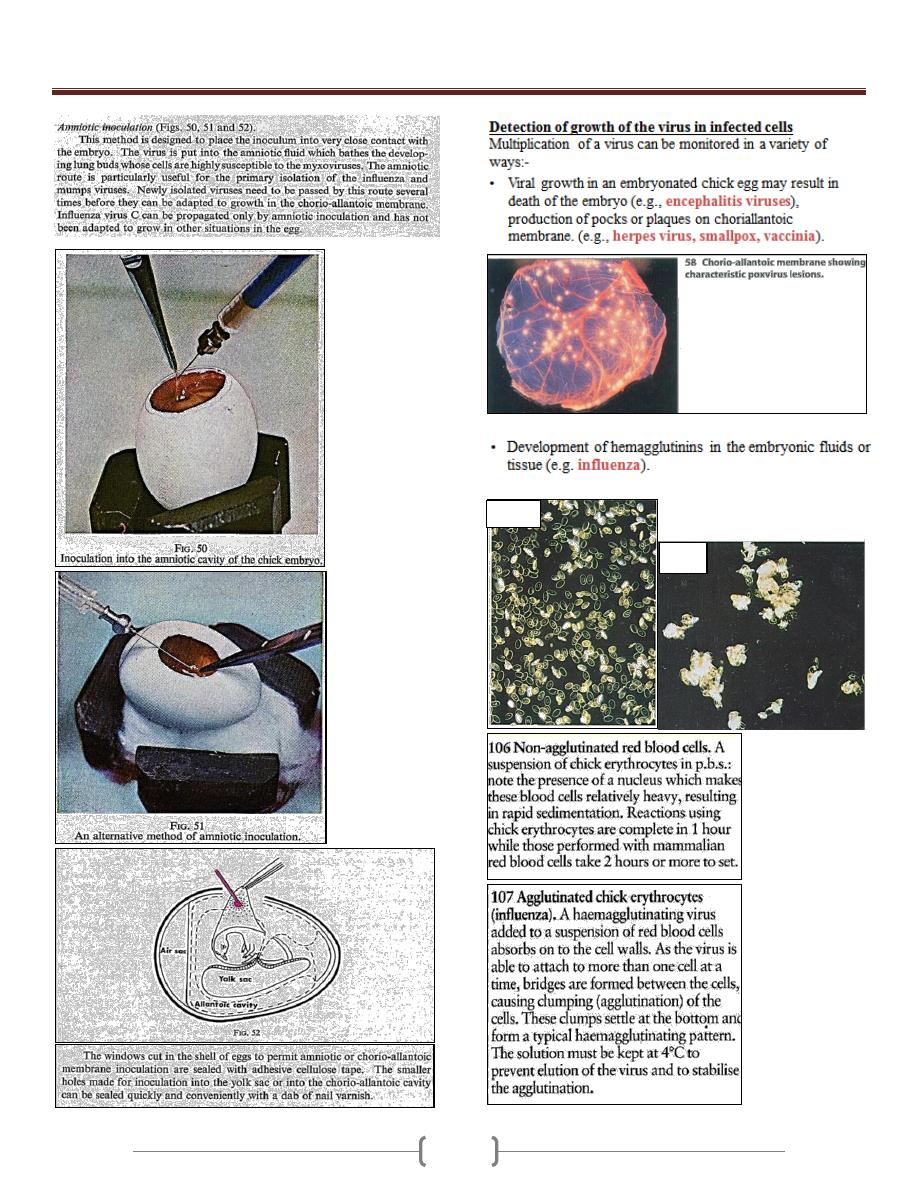
Unit 5 - Virology
68
106
107
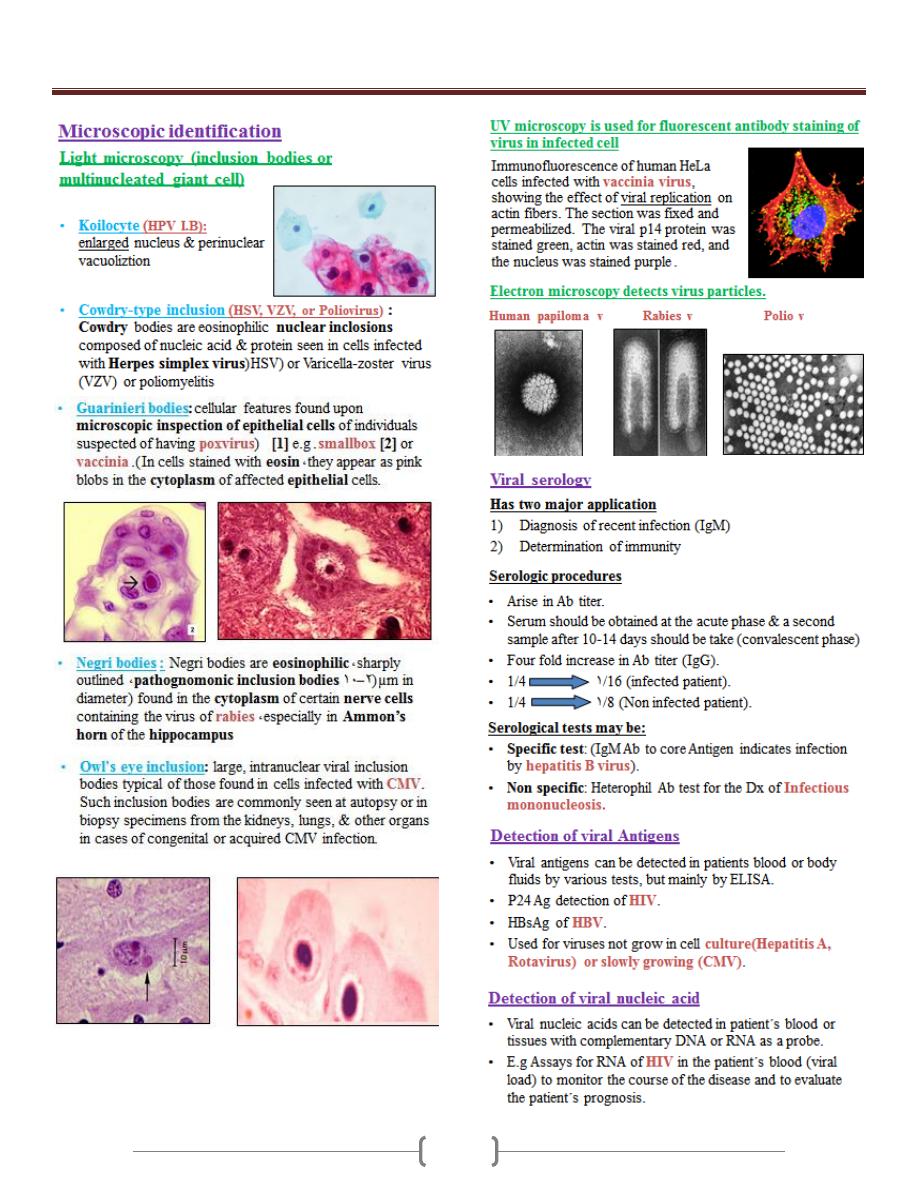
Unit 5 - Virology
69
