
AFTER MID
LEC: 4
DR. KHUDAIR
Oncology
Management of Breast Cancer
TOTAL LEC: 4
Dr. Khudair


Breast Cancer Surgery: Can I still keep my breast?
By
Dr. Khudair Al-Rawaq
History of breast surgery
•
1894 – Radical mastectomy by William Halsted
•
1967 – Modified Radical Mastectomy
•
1981 – Breast conservation surgery (lumpectomy and removal of
axillary lymph nodes)
•
Studies have shown that there is no difference in the outcome in
all these three types of surgery
Breast cancer management
1 Staging
2 Surgery
3 Radiation therapy
Indications for radiation
Types of radiotherapy
Side effects of radiation therapy
4 Systemic therapy
- Chemotherapy
- Hormonal Receptor Status
- Targeted therapy
- Immunotherapy
- Chemoimmunotherapy

- Thermochemotherapy
- Alternative treatments
5 Gene expression profiling
6 Treatment response assessment
Blood test
7 Managing side effects
Insomnia
Hot flashes
8 Reoccurrence monitoring
Why is there no difference whatever type of surgery is done?
•
Even when a breast cancer is 1 cm, cancer cells can go into the
blood and lymphatic vessels and be carried to any part of the body
•
Hence surgery alone usually cannot cure the patient
•
Systemic therapy such as chemotherapy or hormone therapy will
also be required
•
However surgery is important to get rid of all obvious gross cancer
Survival after BCS and Mastectomy
Trial
Endpoint
Overall Survival
CS&RT Mastect
Disease-free Survival
CS&RT Mastect
NCI Milan
18 yrs
65% 65%
N/A
Institut Gustav
Roussy
15 yrs
73% 65%
N/A
NSABP B-06
12 yrs
63% 59%
50% 49%
NCI USA
10 yrs
77% 75%
72% 69%
EORTC
8 yrs
54% 61%
N/A
Danish Breast
Cancer Group
6 yrs
79% 82%
70% 66%
Local recurrence rates after lumpectomy +RT, lumpectomy alone and
mastectomy
Trial
Follow-
up
Lumpectomy
And RT
Lumpectomy
alone
Mastectomy
NSABP-B06
8 yrs
10%
39%
8%
EORTC
8 yrs
15%
NA
9%
Jacobsen etal
10yrs
17%
NA
9%
European
EORTC/DBCG
10 yrs
10%
NA
9%

Radiotherapy
•
After lumpectomy, radiotherapy is essential, otherwise the local
recurrence rate is unacceptably high
•
Without radiotherapy, the local recurrence can be as high as 40%
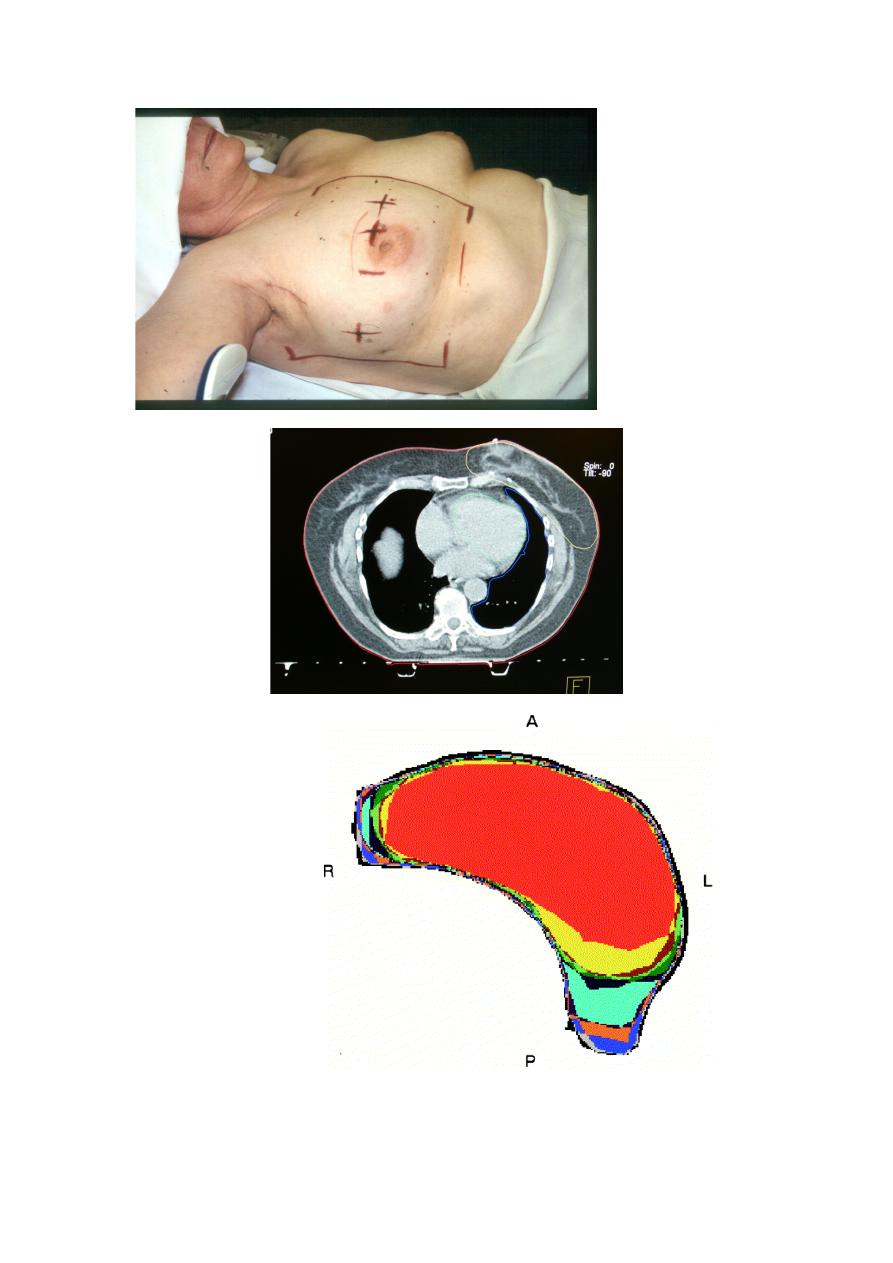
Radiation Oncology/Breast/RT technique
1- Anatomy: Regional LNs
2- Multifield breast technique
3- RT Prescription
4- Target Delineation
5- Accelerated Partial Breast Irradiation
Seroma cavity delineation
6- Whole Breast RT
IMRT
Active Breath Hold
7- Axilla
8- SCV field
9-PAB field
10- IMN irradiation
11- Postmastectomy IMRT
12-Proton Therapy
Clinical
Dosimetry
13- Effect of surgery to radiotherapy interval (SRI)
Boost dose may overcome
Effect of margins
14- Irradiation after breast augmentation
15- Irradiation after breast reconstruction
16- RT Utilization
When can we try to save your breast?
•
Size is the most important criteria. The lump must be small
enough to be excised with a good margin of normal breast tissue
•
The tumour must be a single lump with no disease elsewhere in
the breast – mammogram before surgery is essential to rule out
multifocal disease
•
The patient must agree to radiotherapy and have no other
diseases which make radiotherapy impossible
When can we try to save your breast?
•
Counseling is very important
•
Decision-making should be a shared decision ie the patient and
the doctor together will decide what is best for the patient
ﻻ ﺗدك ﺑﺎب ﺟﻧت ﺗﻔﺗﺣﮫ
وﺗﻔوت
وﻻﺗﻣر ﺑﺑﺷر ﻣﯾرﯾد ﻣﻠﻛﺎك
:D
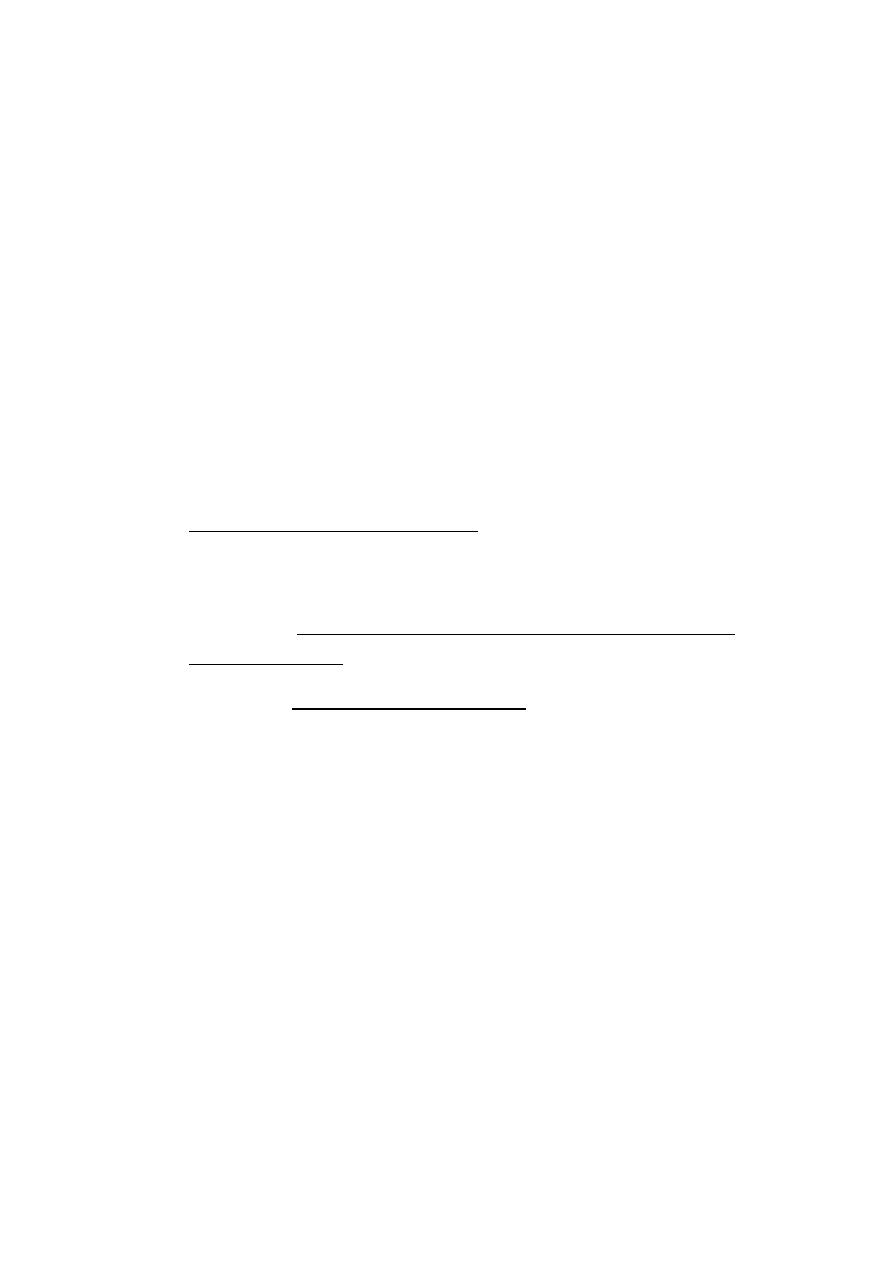
Mastectomy
•
No physical handicap
•
The degree of emotional handicap depends on the patient
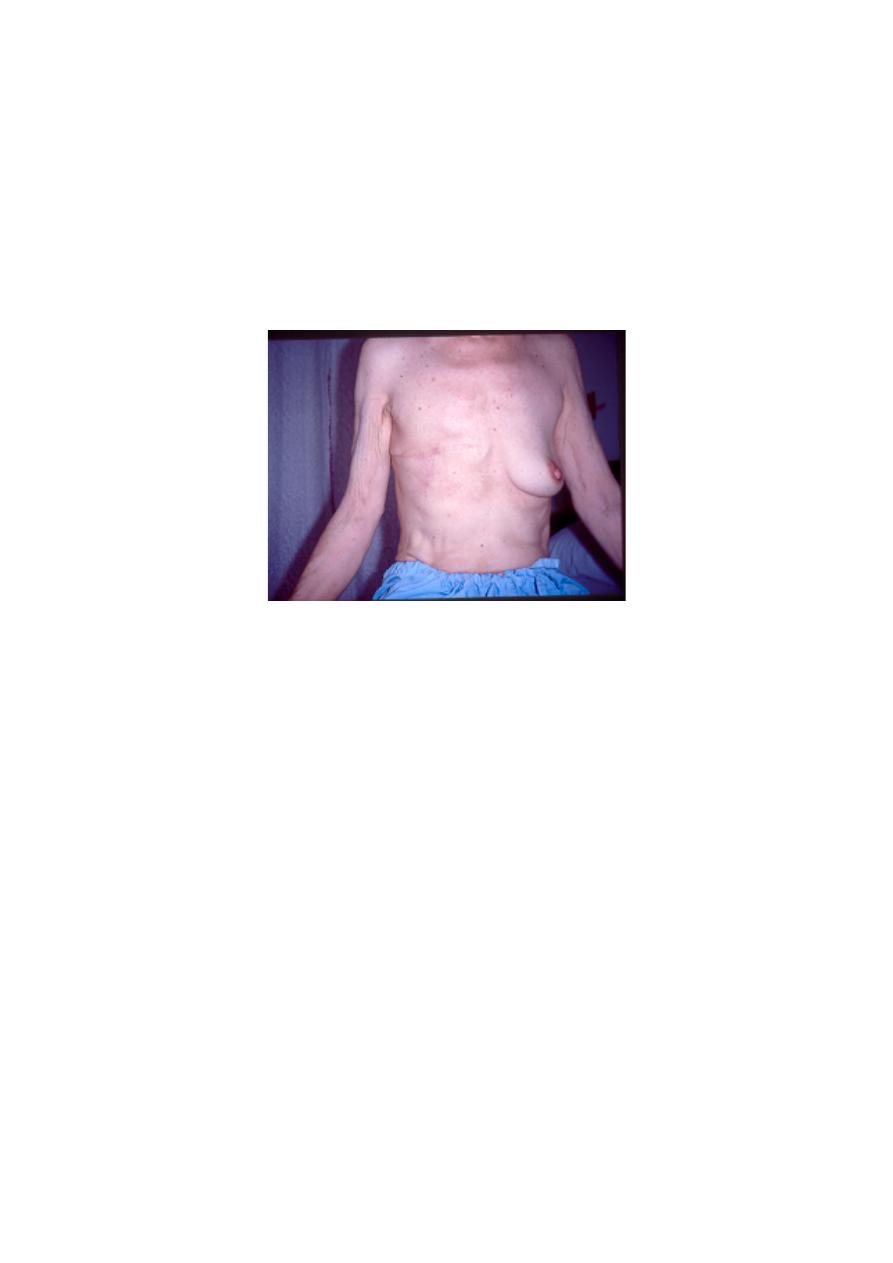
Breast conservation surgery
•
Breast contour is preserved
•
Requires radiotherapy
•
Generally less depression and better body image
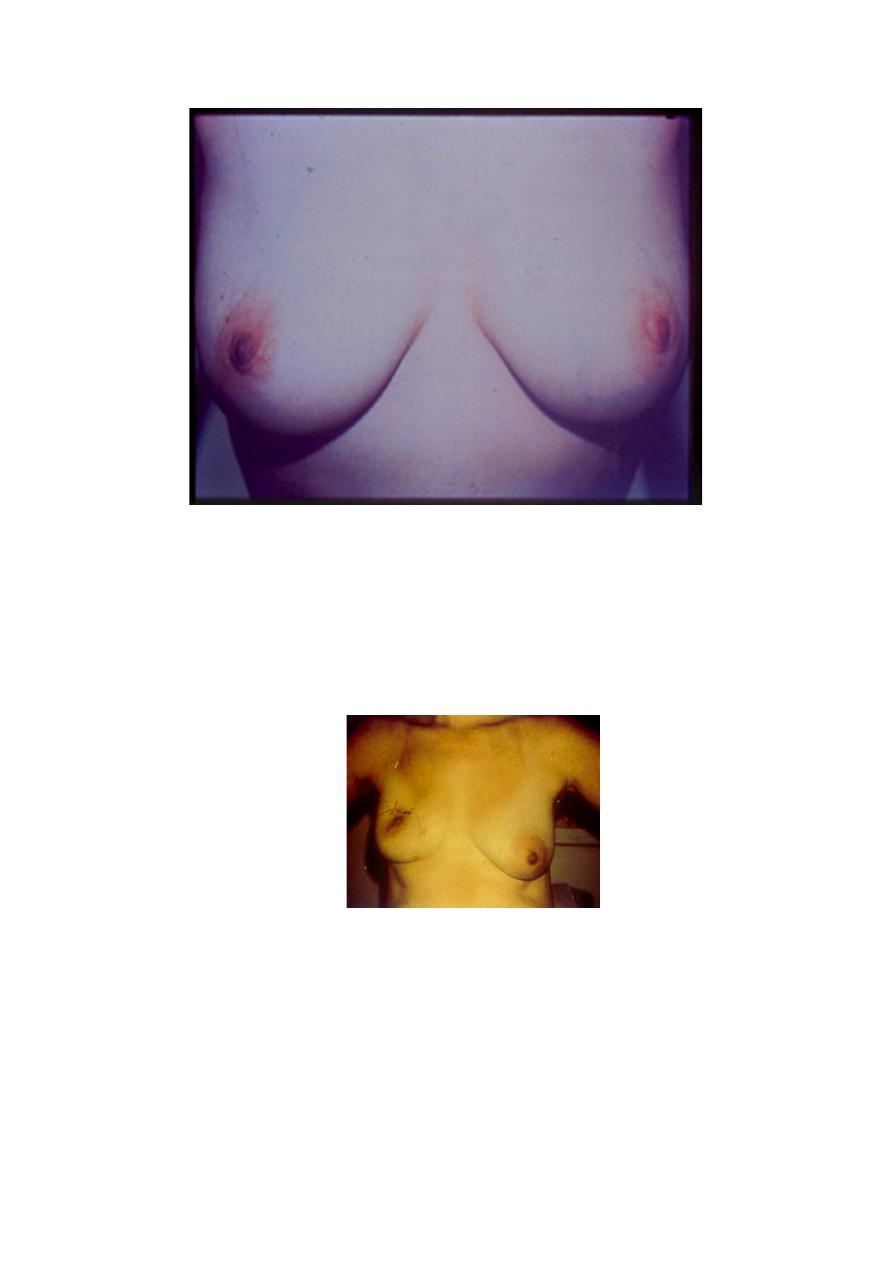
Breast conservation surgery
•
Occasionally may cause a lot of distortion if the lump is large or
too close to the nipple
•
In such cases, may require plastic surgery or a mastectomy is
necessary
What if I cannot save my breast?
•
If the lump is too big to be safely removed with a margin of
normal tissue, or there are multiple cancers in the breast, and
mastectomy is required, immediate breast reconstruction is
possible and has been shown to be safe
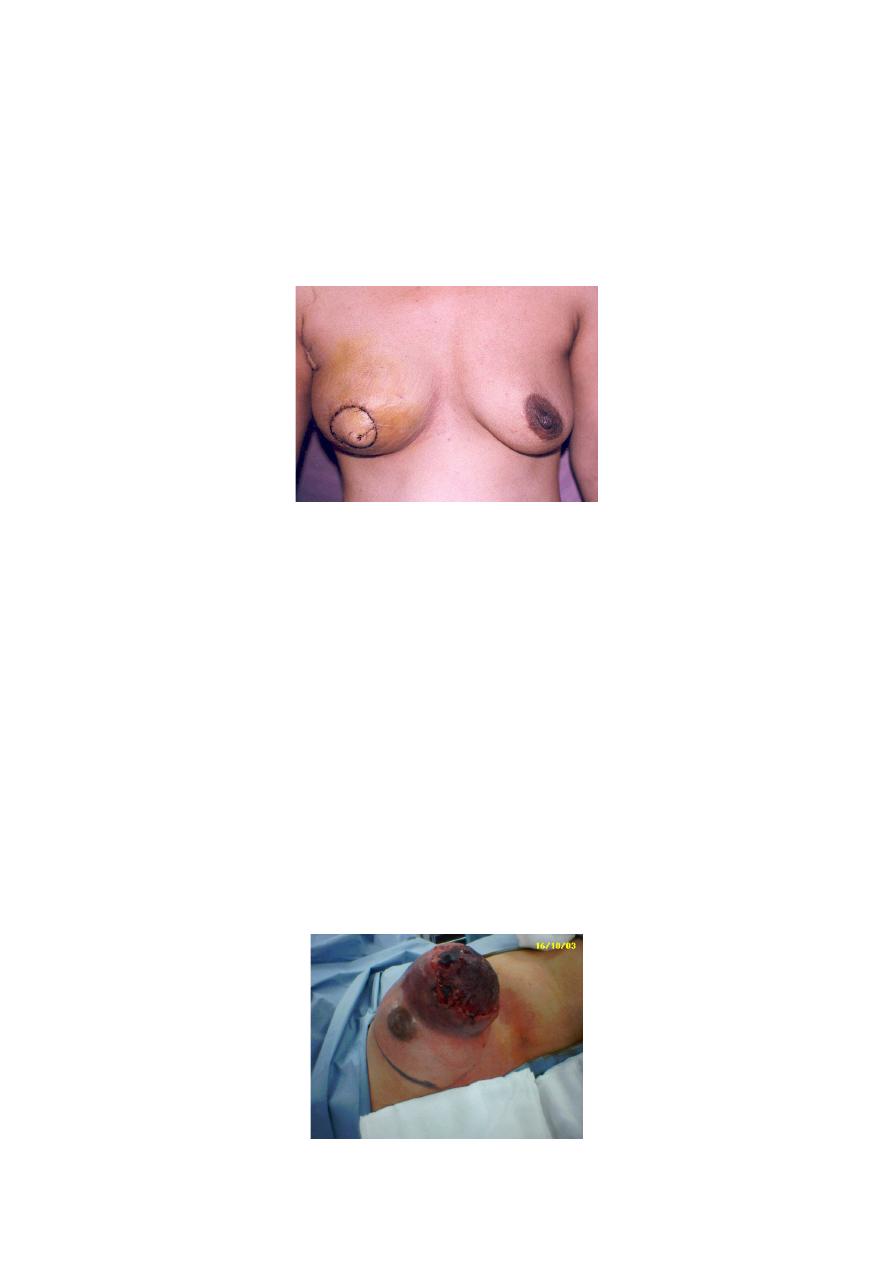
Immediate breast reconstruction
•
Takes longer operating time
•
Own body tissues can be used eg abdomen
•
Psychologically less depression
Is there a way of saving my breast even if I have a big tumor?
•
Primary chemotherapy may be able to shrink the tumour so that
BCS can be done
•
Not standard practice, but can be safely done if the patient wants
BCS and is not willing to have a mastectomy
•
Not advisable in Stage 3 locally advanced breast cancer
What is Stage 3 locally advanced breast cancer?
•
Cancer involving the skin or the whole breast
•
Chemotherapy can be given first to shrink it
•
Mastectomy after chemotherapy
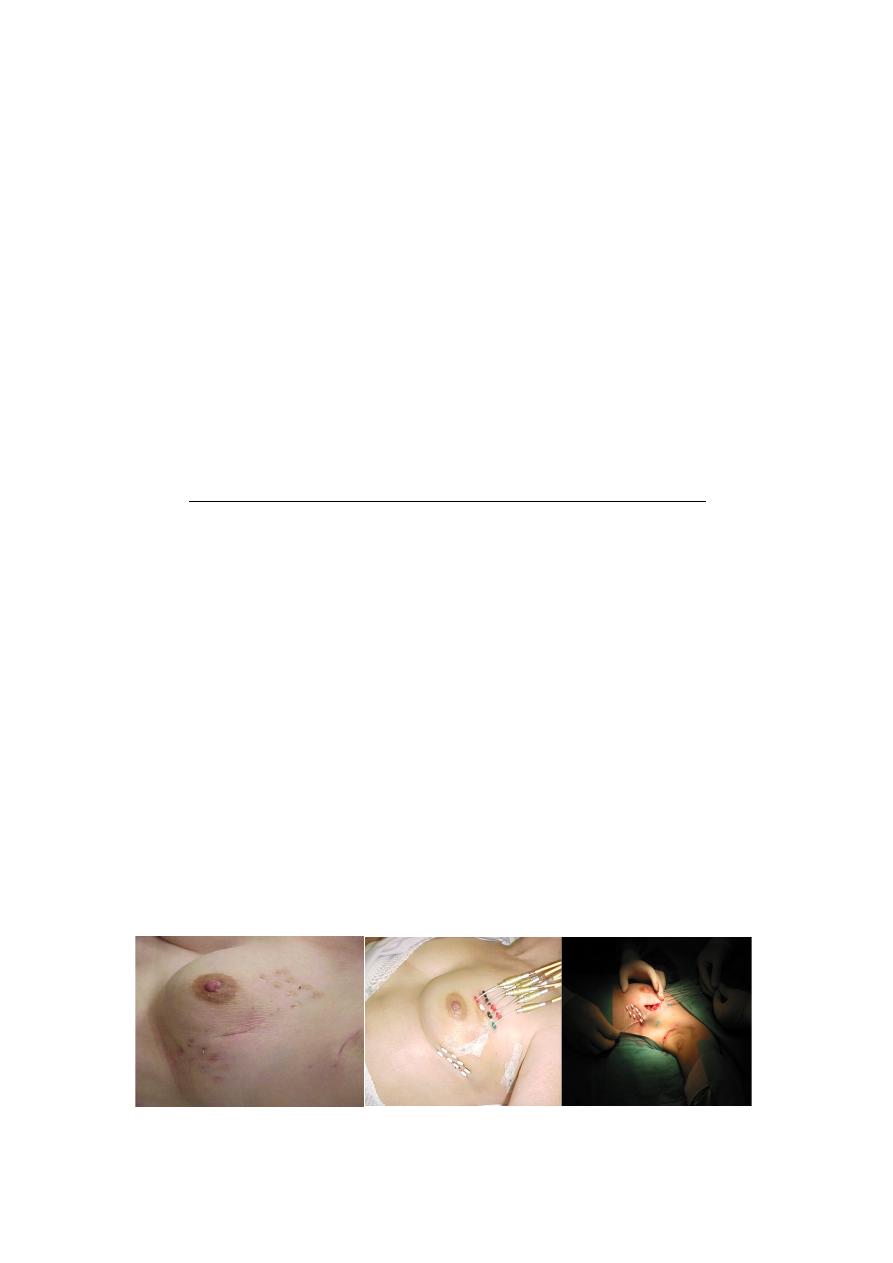
Is breast conservation surgery commonly carried out?
•
In UMMC, 30% of breast surgery is breast conservation surgery
while the rest are mastectomy
•
In USA, figures of BCS are more than 70%
•
Early detection is the most important factor in determining
whether your breast can be saved
Follow-up after breast conservation surgery
•
Mammogram at 6 months after radiotherapy
•
Mammogram yearly afterwards
•
If local recurrence detected, mastectomy must be carried out
Conclusion
•
Breast conservation surgery gives the same outcome as
mastectomy
•
Selection of patients important
•
Education and counseling of patients is important
•
Awareness programmes should emphasize that with early
detection, YOU CAN STILL KEEP YOUR BREAST
BREAST CONSERVATION
Interstitial Brachytherapy (iBT)
Most data in the literature are based on iBT !
BREAST CONSERVATION
BREAST CONSERVATION
Brachytherapy-Ballon (Mammosite ®)
In USA very frequent !
TECHNIQUE / RT APPLICATION
BREAST CONSERVATION
Planning-CT and 3D-Planning
BREAST CONSERVATION
IMRT
Open“ homogeneous
Intensity modulated beam (IMB)
beam (OB)
IMRT
n=306
R
Standard 2D 3D IMRT
5yrs – Differences in breast appearence (Photos)
60%
48%
p=0.06
(QoL no difference)

Side effects of radiation therapy
•
muscle stiffness.
•
mild swelling.
•
tenderness in the area.
•
Lymphedema.
•
Pulmonary peumonitis.
•
Cardiac toxicity.
The End
Done by :Hussein Sadun Al-Nuaimy
Management of Breast Cancer
Dr. Khudair El-Rawaq
Frame
l
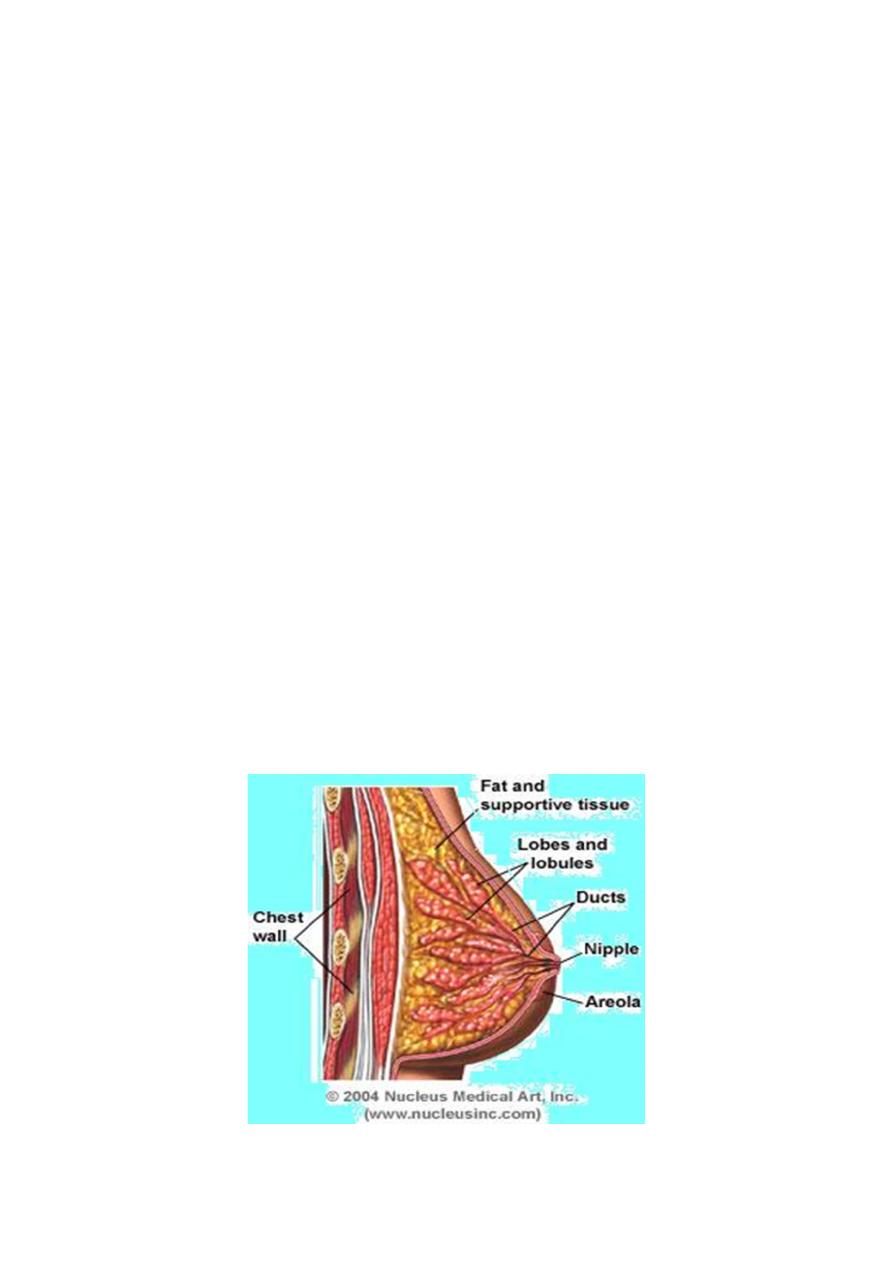
Breast anatomy
l
Epidemiology
l
Risk factors
l
Staging
l
Diagnostic Work-up
l
PROGNOSTIC FACTORS
l
Management
A. Management of Early stages
B. Management of Late stages
C. Palliative Management
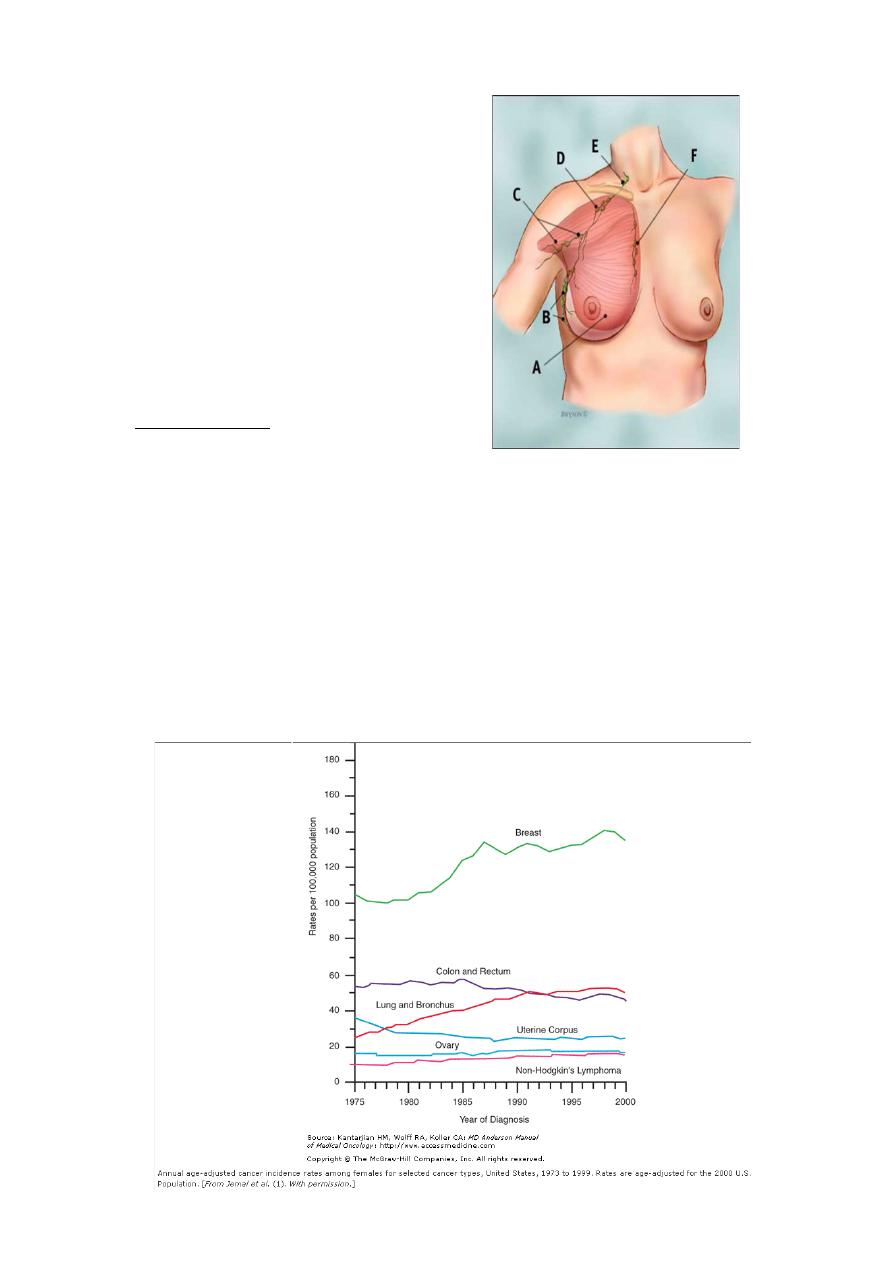
Br Lymphatics
A :PM muscle
B : level I
C : level II
D : level III
E :SCV
F: IMN
Epidemiology
:
Incidence (In the year of 2008)
l
Breast cancer is the second most common cause of death for
women .
l
the most common cause of death for women aged 45 to 55..
l
it is predicted that 215,990 American women will be diagnosed
with breast cancer and that 40,110 women will die from this
disease.
Incidence per 100,000 in USA

Epidemiology
l
Breast cancer incidence has long varied in different regions of the
world. Incidence is highest in Northern Europe and North America
and lowest in Asia and Africa .
l
Mortality rates declined by 1.4% per year from 1989 to 1995 and
thereafter by 3.2% per year. This is thought to be due in part to
increased use of mammography, resulting in earlier diagnosis, and
the use of effective treatments.
Risk factors
(Trentham-Dietz A; Cancer Causes Control 2000 Jul;11(6):533-42).
l
A large meta-analysis → a small but significant increase in relative
risk of breast cancer (RR =1.24) in current OCP users
l
Age >50
l
Alcohol increases risk of breast cancer
l
Due to ↑ levels of estrogens, particularly free estradiol
l
Early menarche- late menopause
l
Exposure to ionizing radiation
l
First child > 30 yrs
l
Jewish- black
l
Lactation (longer time) (Lancet 2002:360:187-195)
l
More than 10 yrs : risk x2 in >55 yrs (Van Hoften et al. Int J Cancer
2000)
l
Nulliparity
l
Oral contraceptive agents
l
Personal or family Hx
l
The relationship between high BMI and ↑ BC risk is seen for
postmenopausal F
Lancet 1996 Jun 22;347(9017):1713-27.

Staging
l
changes in the AJCC staging criteria from 1988 to 2002 affect
stage-specific outcomes.
l
It has been demonstrated that reclassification will result in
improved outcomes.
l
A recent study examined overall stage-specific survival using both
staging systems for a total of 1350 patients.
l
It was noted that only 55% of patients who were classified as
having stage II disease according to the 1988 system had stage II
according to the 2002 system. However, in direct comparison, the
number of patients with stage III disease increased by 114%.
Diagnostic Work-up for Carcinoma of the Breast
l
History
l
Physical examination
l
Biopsy
l
Radiologic studies
l
Laboratory studies
History
l
with emphasis on
A. presenting symptoms (Br. lump, nipple retraction),
B. menstrual status,
C. parity,
D. family history of cancer,

E. other risk factors.
Physical examination
l
with emphasis on
A. breast, (Lt>Rt, 5 yrs to reach palpable size)
B. axilla(10-40% of T1,T2 have pathologic +ve LNs)
C. supraclavicular area,
D. abdomen
Biopsy
l
core biopsy directed by physical examination, ultrasound, or
mammography as indicated, or needle localization.
l
Complete agreement between the core biopsy and subsequent
histologic sections was reached in 89.7% of lesions and partial
agreement in 9.2%
Radiologic studies
Before biopsy
1. Mammography/ultrasonography
2. Chest radiographs
3. MRI of breast (8 clinical trials The sensitivity of MRI ranged from
71% to 100% J Magn Reson Imaging. 2006 Oct 11)
After positive biopsy:
1. Bone scan (when clinically indicated, for stage II or III disease or
elevated serum alkaline phosphatase levels).
2. Computed tomography of chest, abdomen and pelvis for stage II
or III disease and/or abnormal liver function tests

Laboratory studies
1. Complete blood cell count,
2. blood chemistry
3. Urinalysis
• Other studies
Hormone receptor status (ER, PR)
HER2/neu status,
• Tumor marker level (CD 153 preop level ↑,↑ in bone mets)
• Consider genetic counseling/BRCA testing in selected cases,
mutated P53
PROGNOSTIC FACTORS
Intrinsic factors:
l
The only accepted prognostic markers that provide critical
information necessary for treatment decisions are
–
TNM stage
–
axillary LN status
–
tumor size
–
grade
–
lymphatic or blood vessel invasion
–
hormone receptor status
–
HER-2 neu oncogene
PROGNOSTIC FACTORS Extensive intraductal carcinoma

l
>25% of the primary tumor
l
Associated with higher incidence of breast recurrence in some
studies
l
Does not affect DFS or OAS if –ve margins
(Hurd et al. Ann Surg Oncol 1997)
PROGNOSTIC FACTORS Involvement of axillary LN
l
Direct relation bet + axillary LNs and chest wall recurrence and survival
(Haagensen. IJROBP 1977)
l
Data are insufficient to recommend use of
–
p53 measurements
–
cathepsin D measurements
–
estimates of DNA content or S phase in breast tissue
Extrinsic factors:
l
Age (<45 V >45)
l
Race (black V white)
CHEMOPREVENTION
Breast Cancer Prevention
l
An ASCO working group published an assessment of tamoxifen
use in the setting of breast cancer risk reduction.
l
All women older than 35 years of age with a Gail model risk of >
1.66% (or the risk equivalent to that of women 60 years of age)
should be considered candidates for Breast Cancer Prevention
therapy

Mangement of early stages of Breast cancer
early-stage breast cancer, ie, stages 0 ,I and II disease.
l
Stage 0 breast cancer includes noninvasive breast cancer—
l
lobular carcinoma in situ (LCIS)
l
ductal carcinoma in situ (DCIS)
l
Paget’s disease of the nipple
when there is no associated invasive disease.
DCIS DEFINITION
l
Confined to the ductal system of the breast
l
No evidence of invasion:
–
No disruption of BM
–
No involvement of surrounding breast stroma
l
No risk of mets
l
ALN +(0-5%) ?focus of invasive ca
( )ارﺗﺎﺣو دﻗﯾﻘﺔ وﻛﻣﻠو
:D
MANAGEMENT
l
BREAST CONSERVATION +/- RT
Metaanalysis Local RR at 5 yrs 22.5% vs 8.9%
l
Greatest improvement in local control with RT
–
Necrosis
–
high grade features
–
comedo subtype
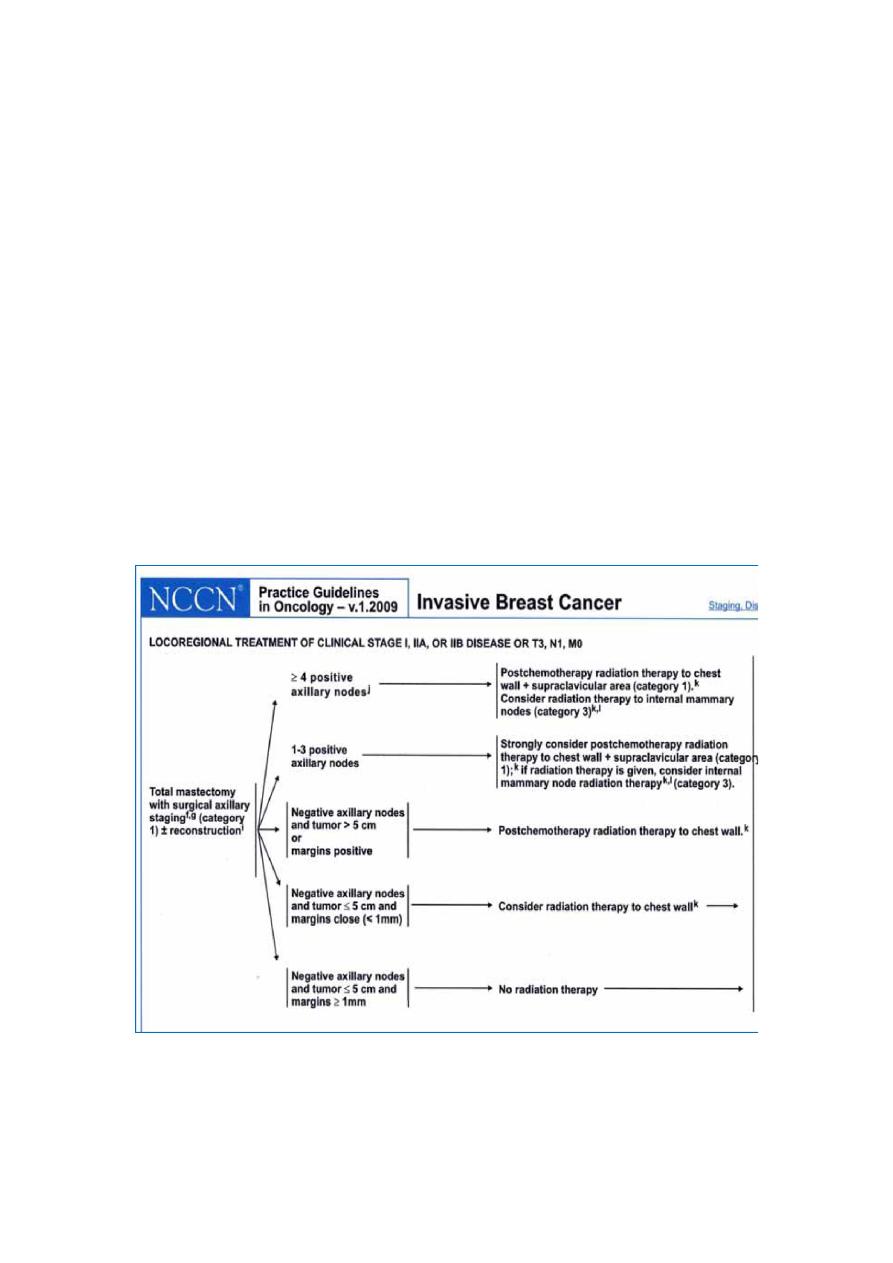
Boyages and colleagues, Cancer 1999
l
Other option is mastectomy
l
Tamoxifen
Stage I and II disease
l
Multiple studies have demonstrated that patients with stage II
breast cancer who are treated with either
A. breast-conservation therapy (lumpectomy and radiation therapy)
or
B. modified radical mastectomy
l
have similar disease-free and overall survival rates.
Breast Conserving Therapy (BCT)
BCT plays an important role in maintaining QOL.
Clinical trials have demonstrated survival equivalent to
mastectomy.
This equivalent was shown using less effective BCT (5-years
LR rates of about 10%) than we have now.
Selection of patients for BCT
Thorough imaging
• U/S, spot compression for densities.
• Magnification views for calcification.
• MRI (only in selected cases).
Establish diagnosis
• Core biopsy (not FNA).
• Excision biopsy if core biopsy not feasible.
Breast-conserving surgery
• Careful evaluation of margins (? > 2-3 mm).
• Post-excision mammogram for residual Ca
++
.
Contraindication
• Multi-centric diseas/diffuse calcification.
• Prior RT.
• Pregnancy.
• Positive margins.
• ? Collagen vascular disease.
BRCA
1
/
2
mutation carriers
- future risk?
Does biologic subtype matter?

Post Mastectomy Radiation
Long history of
clinical
practice and study.
Reduces local regional risk of recurrence by 50-75%
Impact on survival when combined with systemic therapy.
Survival benefit offset somewhat by morbidity of late
toxicity: cardiac and secondary cancers.
Reducing invader tent radiation of normal tissue key to
maximising therapeutic ration.
Management of Late stages of Breast Cancer
This addresses the management of locally advanced, locally
recurrent, and metastatic breast cancer, ie:
Stages IIIB,C and IV disease.
Rates of loco-regional recurrence may vary from < 10% to >
50%, depending on initial disease stage and treatment.
** Neoadjuvant systemic therapy
Can downstage locally advanced disease and render it
operable may allow breast-conservation surgery to be
Performed.
The majority of patients receiving neoadjuvant
chemotherapy, treated with either breast conservation or
mastectomy will require radiation therapy following surgery.
Neoadjuvant (primary) chemotherapy
• Most studies conducted on operable breast cancer (e.g
NSABP B-18, 27)
• Magnitude of benefit from primary CT on survival is
unknown as few studies conducted in this sub-group.
• Anthracycline based chemotherapy: path CR ≈ 15%.
• Taxmen based chemotherapy: path ≈ 30%.
• Perception + Neoadjuvant CT: Path ≈ 40-50%.
Metastatic disease
• Metastatic disease is found at presentation in 5% to 10% of
patients with breast cancer.
THE MOST COMMON SITES OF DISTANT METASTASIS ARE THE LUNGS, LIVER, AND BONE.
Low-risk patients, (elderly)
• Low-risk patients, elderly whose tumor is hormone receptor-
positive (ie, estrogen receptor-positive and/or progesterone
receptor-positive), may be treated with a trial of Hormone
therapy.
• First-line hormonal therapy consists of an aromataseinhibitor
tamoxifen
Hormone-refractory disease can be treated with
• Cytotoxic agents systemic cytotoxic therapy.
• FAC, paclitaxel, TAC (Taxotere[docetaxel],
Adriamycin[doxorubicin], cyclophosphamide), or docetaxel
may be used in this situation.
Radiation Dose for PMR and LABC
PMR
STAGE IIA - IIIA
LABC
IBC
CW Dose
50 GY
50 Gy
50 Gy
CW boost
Optional
60-66 GY
60-66 Gy
SCL + Axilla
45-48GY
50 Gy*
50 Gy*
IMC
45-48 Gy
50 Gy*
50 Gy*
STANDARD FRACTIONATION AT 1.8-2 GY
* Boost 54-60 should be consider for eIIIC disease

Intermediate- or high-risk patients
l
include those with rapidly progressive disease or visceral
involvement, as well as those with disease shown to be refractory
to hormonal manipulation by a prior therapeutic trial. Those
treated by:
1. Cytotoxic agents systemic cytotoxic therapy
2. Monoclonal antibody therapy(Trastuzumab ,Lapatinib) and
3. targeted agents (Avastine)
l
High-dose chemotherapy Patients who present with or
subsequently develop distant metastasis.
l
Adjunctive bisphosphonate therapy
Use of these agents results in a significant reduction in skeleton-
related events, including pathologic fracture, bone pain, and the need
for radiation therapy to bone. Pamidronate and zoledronic acid (Zometa)
ROLE OF RADIATION THERAPY IN METASTATIC DISEASE
l
bone metastases are the most commonly treated metastatic sites
in patients with breast cancer,
l
brain metastases, spinal cord compression, choroidal metastases,
endobronchial lung metastases, and metastatic lesions in other
visceral sites can be effectively palliated with irradiation
Thank you
Done By : Hussein Sadun Al-Nuaimy
