1
Glaucoma
Lecture no. 17 & 18
2015-2016
Baghdad medical college
Anatomy:
The trabecular meshwork:
It is a sieve-like structure at the angle of the anterior chamber through which
90% of the aqueous humor leaves the eye.
Microscpical picture of trabecular meshwork
Schlemm's canal:
Is a circumferential channel in the peri limbal sclera. The inner wall of the
canal (which is attached to trabecular meshwork) is lined by endothelial cells,
which contain giant vacuoles. The outer wall of the canal lined by flat cells
and contains the openings of the collector channels. These collector channels
connected either directly or indirectly with episcleral veins. This is the
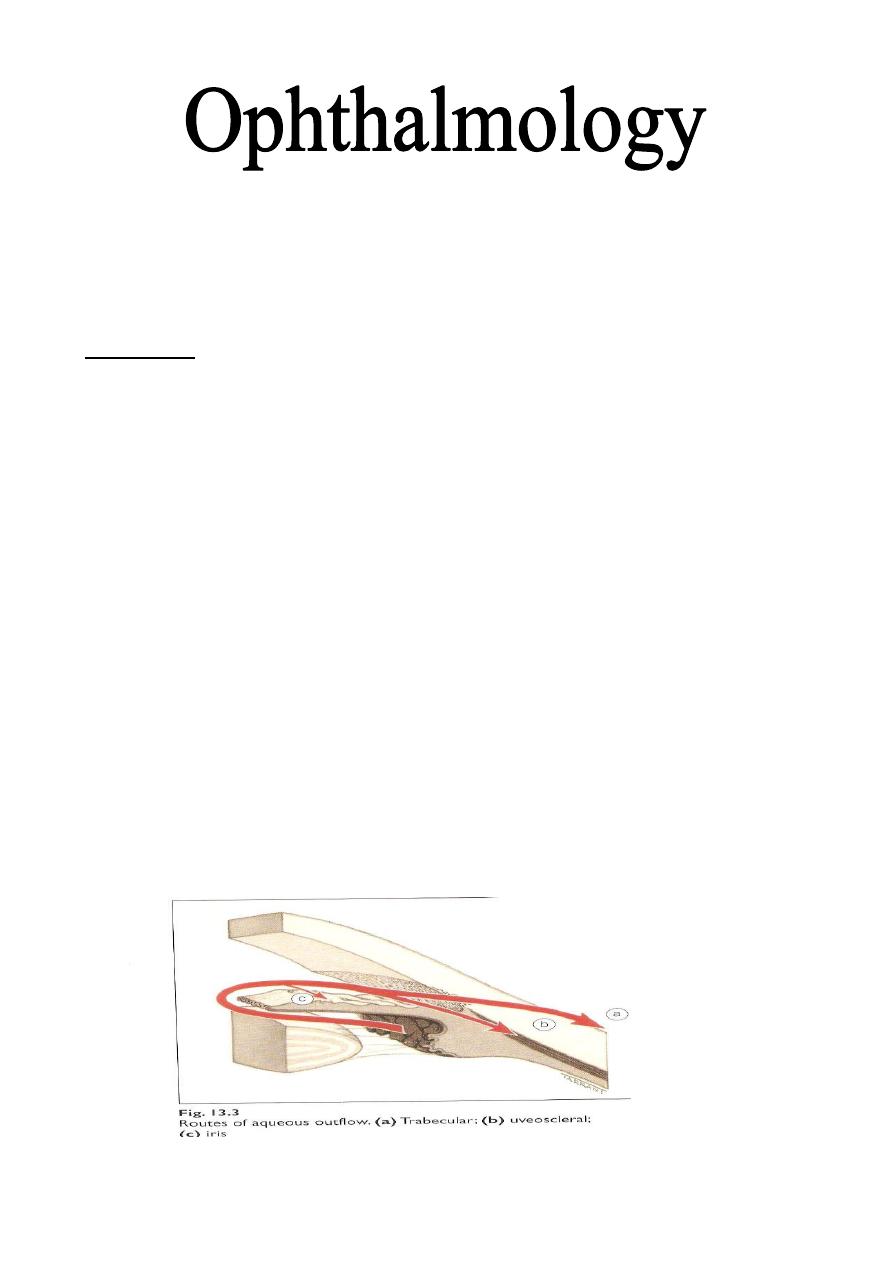
conventional drainage (outflow or pathway). There is also the non-
conventional (uveoscleral) outflow, where the aqueous pass to anterior part of
the Ciliary body and pass between Ciliary body and sclera.
Water and larger
molecules from the anterior chamber can pass into and through the Ciliary muscle
via its anterior face, and from there, into the suprachoroidal space to be carried
away, some perhaps by the choroidal vessels, but most actually through the scleral
blood vessels into the orbital blood vessels.
Dr. Najah
2
The anterior part (pars plicata) of the Ciliary body bears 70 radially oriented
Ciliary processes which project into the posterior chamber.
In similar way to the inner side of the Ciliary body, the Ciliary processes is
lined by a pigmented epithelia layer continuous with the retinal pigmented
epithelium (RPE) of retina and more inner to it by a non-pigmented epithelial
layer continuous with the neuro-retina (sensory retina).
Each ciliary process has a central arteriole ending in a rich capillary network.
Tight junctions between adjacent non-pigmented epithelial cells constitute
part of blood-aqueous barrier and the other part constitute by the tight junctions
of endothelial cells lining the iris blood vessels, so that the proteins cannot pass
to the posterior chamber and anterior chamber respectively.
Physiology of aqueous production:
Aqueous humor is actively secreted by the non-pigmented epithelium,
depending on several enzyme systems especially the Na
+
/k
+
ATPase.
80-90% of aqueous humor is secreted by active pump mechanism, and the
other 10-20% is secreted by: Hydrostatic pressure (depends on difference
between intravascular and extravascular pressures in ciliary body) & Oncotic
pressure (depends on osmolarity).
The rate of secretion equals the rate of drainage, which estimated about
2.5μL/min of aqueous that pass through the pupil.
Ocular hypertension:
In the general population the mean IOP is 16 mm Hg; two standard deviations on
either side of this gives a 'normal' IOP range of 11-21 mm Hg. It is estimated that
about 7% of the population over age of 40 years have IOPs > 21 mm Hg without
glaucomatous damage on standard clinical tests. These individuals are referred to as
ocular hypertensives or glaucoma suspects.
The IOP is affected by corneal thickness, so the thicker cornea associated with high
IOP {overestimation} and vice versa. For this reason, measurement of corneal
thickness is important in all patients with glaucoma or glaucoma suspect. The normal
central corneal thickness average is 520
μm with minimum variation.
Untreated patients with ocular hypertension have only a 9.5% cumulative risk of
developing primary open angle glaucoma (POAG) after 5 years, therefore do not
require treatment and only those at risk should be treated in order to delay or prevent
the development of POAG.
It should be remembered that once treatment is
instituted, it is continued throughout the patient's lifetime and may have
significant side-effects.
Risky patients are those with:
1- IOP 30 mm Hg or more.
2- C/D ratio 0.4 or more.
3- Family history of glaucoma in a first degree relative.
3
Glaucoma
It is an optic neuropathy with characteristic appearance of the optic disc and
specific pattern of visual field defect that is associated frequently but not
invariably with raised intraocular pressure (IOP).
Classification of glaucoma:
1- Congenital (developmental).
2- Acquired: divided into:
Two major forms of glaucoma exist: open-angle glaucoma, in which aqueous humor
has free access to the trabecular meshwork, and angle-closure glaucoma, in which
access of the aqueous humor to the trabecular meshwork is obstructed. Both forms of
glaucoma demonstrate a progressive optic neuropathy with visual field loss and
characteristic structural changes, including thinning of the retinal nerve fiber layer
and excavation of the optic nerve head.
IOP does not define glaucoma, and many with
glaucoma have IOP measurements that are also found in individuals without
glaucoma.
a- Open angle: this is either primary (raised IOP not associated with other
ocular disorders) or secondary.
b- Angle closure: also either primary or secondary.
Gonioscopy, by using slit lamp and Goniolens is use to determine whether the
angle is open or closed. Visualization of trabecular meshwork means open
angle and absence of trabecular meshwork patially or completely indicate
narrow or closed angle respectively. * The structures that are seen normally by
gonioscopy (using goniolens) in the angle are; (anterio-posteriorly) schwalbe's
line, trabecular meshwork, scleral spur and anterior face of ciliary body.

4
The optic nerve head:
It represents the collection of 1.2 million ganglion cell axons as they pass
across the retina to enter the scleral canal to form the optic nerve head (optic
disc), there is no photoreceptors or bipolar or gangalion cells (blind spot).
The lamina cribrosa:
It is a part of posterior pole of Sclera and consists of series of collagenous
connective tissue which are stretch across the scleral canal. It is perforated by
200-400 openings (pores) containing bundles of retinal nerve fibers.
The optic cup:
Is a pale depression in the center of the optic nerve head which is not
occupied by neural tissue, but it is occupied by glial tissue made mainly of
astrocytes.
The cup:disc ratio (C/D ratio):
Indicates the diameter of the cup expressed as fraction of the diameter of the
disc and should be measured in both vertical (which is more important) and
horizontal meridian.
* Most normal eyes have a vertical C/D ratio of 0.3 or less (<30%). So C/D
ratio more than 0.3 or difference of 0.2 (20%) between the two discs should be
regarded with suspicion.
* About 2% of population have C/D ratio more than 0.3 and may even reach 0.7
and they are really normal, and to consider it glaucoma, the patient must have
either raised IOP or optic neuropathy (Two of three of the diagnostic criteria of
glaucoma to say this is glaucoma).
C/D ratio assessed and calculated by:
1- Clinical fundal examination and fundus photograph: it is a crude, rough
method to assess C/D ratio.
2- Heidelberg retinal tomography (HRT): is a very sophisticated method of
imaging technique which is used in diagnosis and follows up of patients
with glaucoma.
3- Ocular coherent tomography (OCT) which is work in the similar way of
ultrasonography but instead of using reflected sound, reflected light is
used. It give accurate measurement of nerve fibers layer changes and C\D
ratio assessment.
Primary Open Angle Glaucoma (POAG)
Definition: a bilateral disease (in general) although not necessarily a
symmetrical disease, characterized by:
1- Adult onset.
2- IOP >21 mmHg.
5
3- An open angle of normal appearance.
4- Glaucomatous optic nerve damage.
5- Visual field loss typical of glaucoma.
* In POAG, the trabecular meshwork is exposed (angle is open) but the pores
are occluded (sclerosis of trabecular meshwork for unknown etiology).
Pathogenesis of optic disc cupping:
1- Ischemic theory (indirect): compromised microvasculature of optic nerve
axons duo to rising IOP.
2- Direct mechanical theory: chronically elevated IOP directly damages optic
head nerve fibers at lamina cribrosa duo to mechanical stretching of nerve
fibers.
Risk factors and associations:
1- Age: older people, after age of 65 years due to aging process and sclerosis
of trabecular meshwork.
2- Race: more common and more severe in Blacks and Asians.
3- Family history and inheritance.
Clinical features: (of POAG)
Insidious (gradual), asymptomatic until late in the course of the disease due
to significant loss of visual field (i.e. it presents with advanced optic nerve
damage and visual field impairment, but with preserved visual acuity as it is not
involved in the course of disease except in very late stages).
Premature presbyopia (usually presbyopia seen clinically at age of 45-46
years, but here occurs earlier at about 38-40 years and its symptoms are
difficulty in reading and visualization of near objects.
Rapid increase in hypermetropia (same mechanism of presbyopia, and also
it is early and rapid) in patients over the age of 40 years.
Rarely: eye ache, headache and halos around light ( occurs duo to transient
corneal epithelial edema that associated with raised IOP).
Signs of POAG:
1- Raised IOP.
2- Fluctuation in IOP (normally there is diurnal variation of IOP according to
circadian rhythm of hormones but not more than 5 mmHg). In glaucomatous
patient, the fluctuation is more than 5 mm Hg.
3- Optic disc changes, increased C/D ratio more than 0.3 or asymmetry of it
(difference more than 0.2 between both eyes).
4- Glaucomatous field change.
5- Gonioscopy, show open angle with no any abnormality (e.g. no new blood
vessels or inflammatory cells occluding the angle)
6
Visual field changes:
1- Generalize constriction of peripheral VF: is the earliest field defect but it is
not specific sign for POAG and can occurs in many other diseases e.g. dense
cataract, miosis of the pupil, retinitis pigmentosa and panretinal
photocoagulation in diabetic patients.
2- Paracentral scotoma: (central VF change which is specific sign).
3- Arcuate scotoma (central VF change).
4- Temporal (crescent) or central island of vision only (both are end-
stage glaucoma).
Management of POAG:
Treatment Aim: to preserve visual function by controlling IOP and preventing
or retarding further optic nerve damage, but there is no improvement.
Treatment goals:
1- Target pressure: it is assumed that the pre-treatment level of IOP has damaged the
optic nerve and will continue to do so. An IOP level below which further damage is
considered unlikely called target pressure.
2- Monitoring: is performed to the optic disc and visual field.
In the event of further damage the target IOP is reset at a lower level. Although, there
is no safe level, progression is uncommon if the IOP <16 mm Hg in mild to moderate
glaucoma and <12 mm Hg is severe cases.
Medical treatment
:
1- Any chosen drug should be used in its lower concentration and as infrequently as
possible consistent with the desired therapeutic effect.
2-
Ideally, the drug with the fewest potential side-effects should be used.
3-
Initial treatment is usually with one drug, usually a beta-blocker or prostaglandin
analogue.
4-
Follow up after 4 weeks should be performed after initiation of treatment and fall
in IOP of >4 mm Hg is usually considered significant.
5-
If the response is satisfactory subsequent assessment is after 2 months and at 3-4
monthly intervals thereafter for long life.
6-
If the response is unsatisfactory the initial drug is withdrawn and another
substituted.
7-
If the response is still unsatisfactory yet another drug is added or a combined
preparation (e.g. Timolol & Dorzolamide) substituted.
8-
Perimetry: assessment of VF annually is sufficient if the IOP is satisfactory and
the appearance of optic disc is stable.
9-
Gonioscopy: should also perform annually because the anterior chamber
gradually shallows with age.

7
Drugs used in treatment of POAG:
* Latanoprost: is a prostaglandin agonist, and acts by increasing drainage
through uveoscleral outflow. It is regarded now a day as fist choice of
treatment. Dose: once daily before sleeping.
* Beta blockers: e.g. Timolol 0.25 or o.50%, are taken twice daily, they act by
decreasing the secretion, they are contraindicated for patients with heart
problems or asthmatics, but betaxolol is cardio-selective and can be used for
asthmatics.
* Dorzolamide: is a carbonic anhydrase inhibitor and acts by decreasing
secretion. 3 times / day.
* Brimonidine: is an alpha-2 agonist, and acts by decreasing secretion and has
slight effect to increase drainage. Used 3 times / day.
* Parasympathomimetics: (e.g. Pilocarbine) act on ciliary and specifically on
its longitudinal muscles which are inserted in scleral spur. Contraction of
these muscles leads to stretching of the trabecular meshwork and the size of its
pores. Thus, will increase leading to increment of drainage through the
conventional way.
Surgical treatment:
Indications:
a- Failure of medical treatment.
b- Poor compliance.
c- Primary treatment (some doctors consider it primarily).
d- Unavailability of drugs or their expensiveness.
Types:
a- Laser trabeculoplasty (frequency doubled Nd:YAG laser 532 nm): this
type of laser is used to produce a thermal effect on the trabecular meshwork
and not to perforate it. The thermal effect will lead to fibrosis and shrinkage
of the treated sites and stretching of the pores in between.
b- Cyclo-destructive: done by using diode or YAG laser therapy or
cyclocryotherapy. The destruction of the ciliary process leads to decrease
production of aqueous humour.
c- Trabeculectomy with or without antimetabolites (5-FU, mitomycine):
which is creation of surgical fistula between AC and subconjunctival space for
drainage of excess aqueous humour without or with antimetabolites at site of
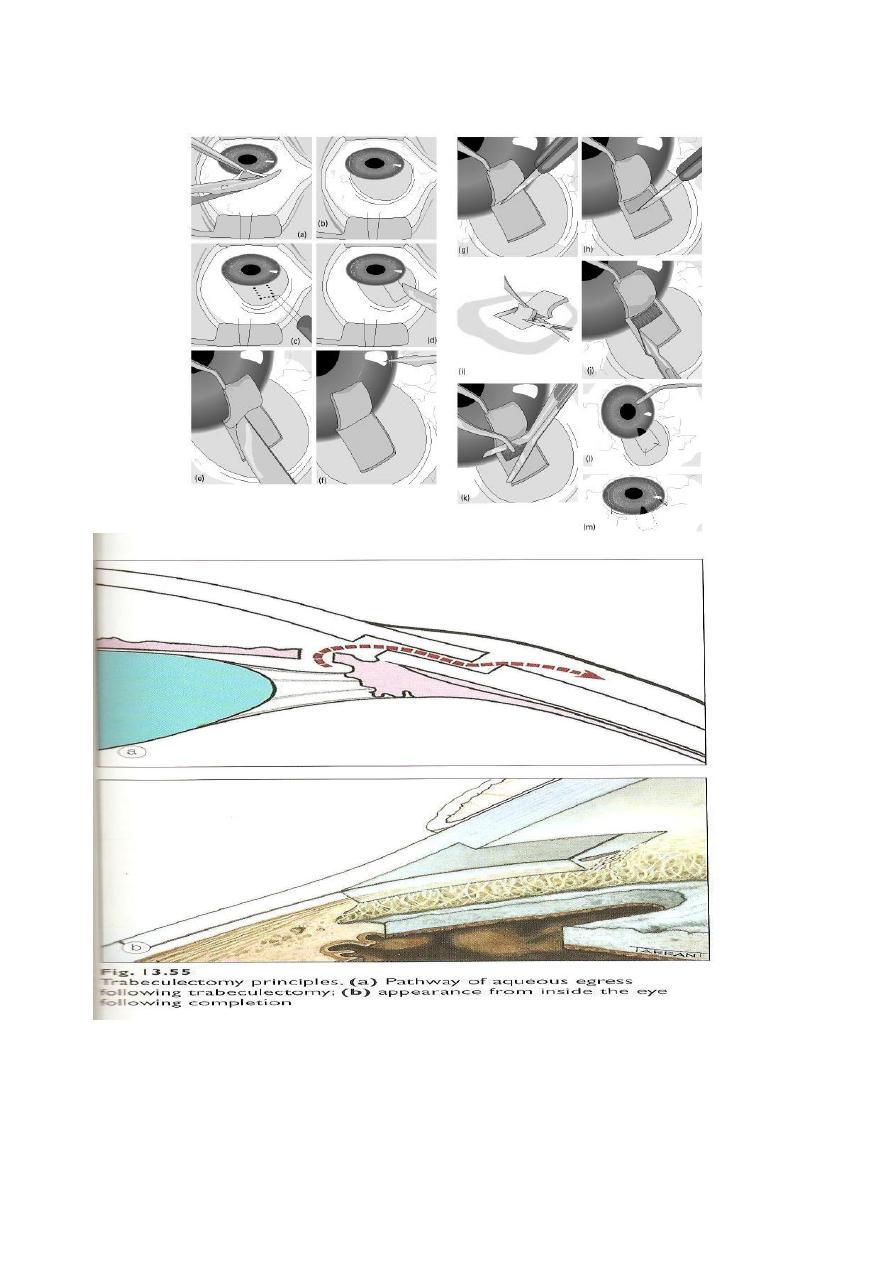
8
surgery to diminish fibroblast activity and preventing risk of occlusion of this
fistula and failure of surgery.
d-Antiglaucoma devices (Molteno, Ahmed): a similar operation to the
previous one, but here a tube connected to a valve system is inserted to
communicate the anterior chamber with the Subconjunctival space. It is used
for resistant cases of trabeculectomy.
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>

9
Corticosteroid responsiveness:
Normal population is divided into 3 groups on the basis of their IOP
response to a six week course of topical betamethasone (potent steroid):
1- High responders: IOP >30mmHg.
2- Moderate responders: IOP 22-30 mmHg.
3- Non responders: no change in IOP.
* Incidence of steroid responsiveness %:
High
Moderate
Non
General population
5
35
60
Patients with POAG
90
10
0
* 100% of patients with POAG are steroid responders in comparison to 40% in
general population. Steroids should be avoided in patients with POAG.
Normal tension glaucoma (NTG)
Definition:
NTG also referred to us low tension glaucoma, is variant of POAG, it is characterized
by:
1- A mean IOP ≤ 21 mm Hg on diurnal testing.
2- Glaucomatous optic disc damage and VF loss.
3- Open drainage angle on Gonioscopy.
4- Absence of secondary causes.
Pathogenesis:
The exact cause of NTG has not been conclusively determined although various
mechanisms has been postulated such as:
1- Vascular insufficiency.
2- Decrease optic disc resistance.
3- IOP effects.
4- Optic nerve compression by normal carotid arteries.
Diagnosis and treatment:
Is the same as in POAG except that IOP is normal. We need here to decrease the IOP
even more to reach the target pressure which is safe and causing no more optic disc
damage and no more VF defects.
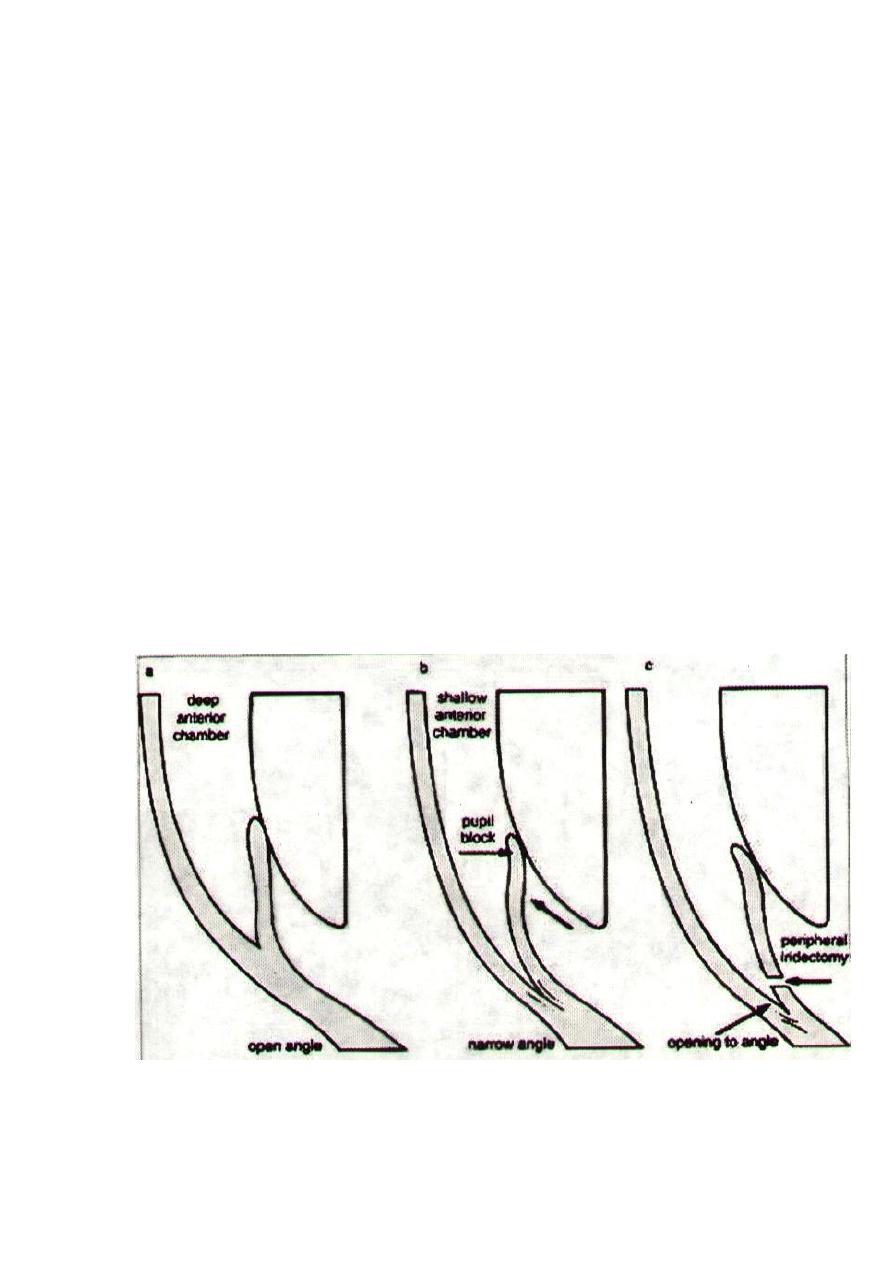
Primary Angle Closure Glaucoma (PACG)

10
Definition: is a condition in which there is obstruction to aqueous outflow due
to partial or complete closure of the angle by peripheral iris.
Unlike POAG, the diagnosis is by examination of anterior segment and
gonioscopy. Presence of a normal optic disc and absence of visual field loss
does not preclude (exclude) diagnosis of PACG (as it is acute so the damage
doesn’t occur unless the condition left without treatment and blindness happen
within few days or even hours). In addition, there are shallow AC and convex
iris-lens diaphragm.
Risk factors:
1- Age: average age 60 years, it does not occur before this age, as the AC is
still deep.
2- Gender: ♀/♂ = 4/1.
3- Race: whites 6% of glaucomas, more common in South East Asians,
Chinese and Eskimos, uncommon in Blacks.
4- Family history: ocular anatomical features are inherited, 1
st
degree relatives
are at increased risk.
Anatomical predisposing factors:
1- Relative anterior location of iris-lens diaphragm.
2- Shallow anterior chamber (AC).
3- Narrow entrance to anterior chamber angle.
Classification of PACG:
1- Latent PACG:
the asymptomatic phase of PACG.
Signs: slit-lamp examination:
a- Shallow AC.
b- Convex-shaped iris-lens diaphragm.
c- Close proximity of iris to cornea.
d- Normal IOP.
11
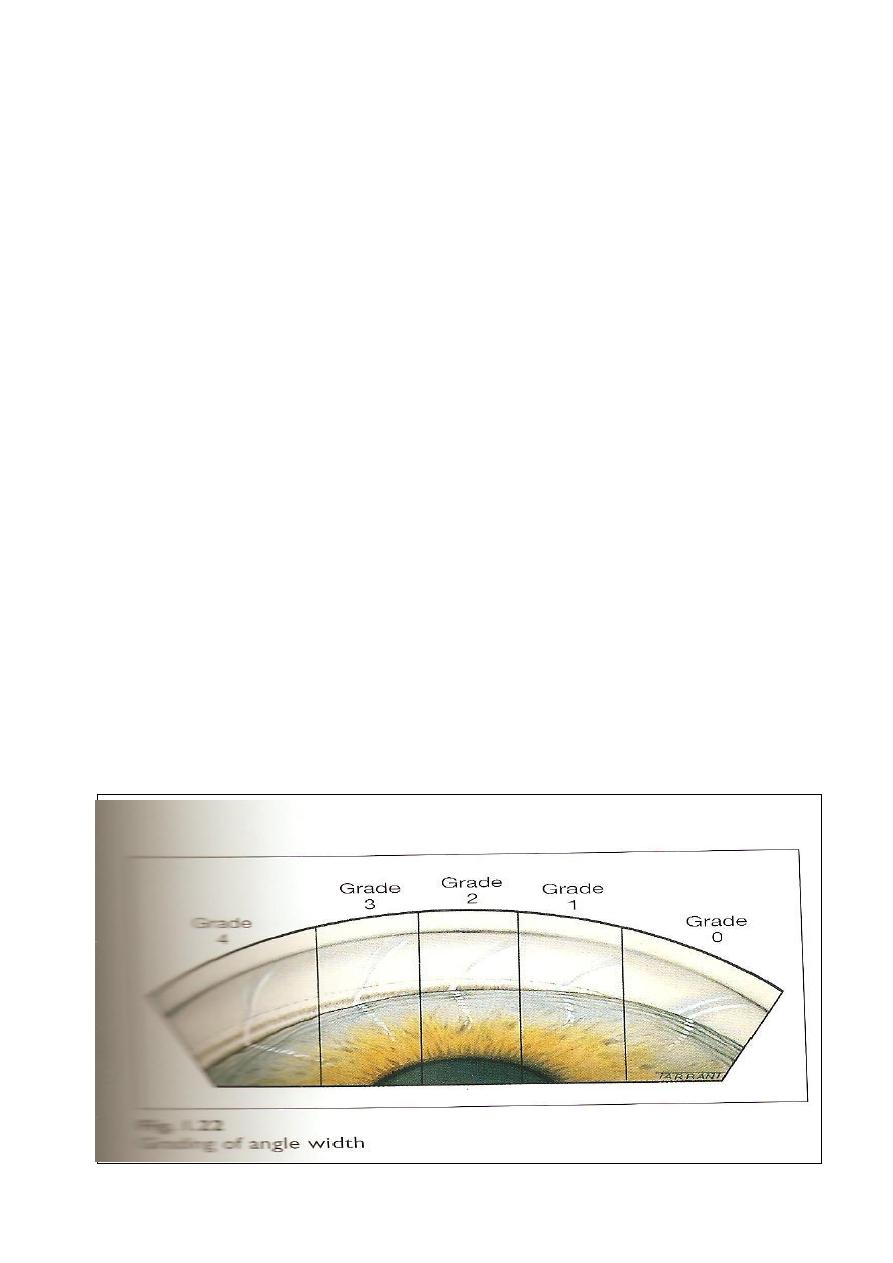
Gonioscopy: shows an occludable angle (grades 2,1 & 0).
Management:
An eye that has latent PACG (which is detected by routine
examination ± family history) needs bilateral YAG laser PI
(peripheral iridotomy) as prophylactic treatment.
If no treatment done the following may happen:
a- Remain normal for long life.
b- Acute or intermittent PACG.
c- Develop chronic PACG without passing through acute or sub-
acute phase.
2- Intermittent (sub-acute) PACG:
In patient with anatomical predisposing factors of PACG and an
occludable angle, the mid-dilated position of the pupil is critical
position as pupillary block can happen due to direct contact between
the iris and lens at the pupillary margin. Pupillary block leads to
increase IOP in the PC more than AC and anterior bowing of iris
periphery (iris bombe) toward corneal periphery till it occlude the
angle of the AC which causing sudden rising of IOP. Then the
pupillary block is spontaneously broken duo to exposure to bright
light, the pupil is miosed, angle opens and IOP drops.

11
Attack could be precipitated by:
a- Physiological mydriasis (e.g. watching TV in dark room,
emotional stress).
b- Physiological shallowing of AC (prone or semi-prone position;
as reading or sewing), as the lens moves forwards.
c- Iatrogenic; by using mydriatic drugs. These drugs should be
avoided in patients with shallow AC and Narrow angles.
Clinical features:
- Transient obscuration of vision.
- Halos around light due to corneal edema.
- Eye ache (due to rapid elevation of IOP).
- Frontal headache.
- Recurrent attacks, broken after 1-2 hours by physiological miosis
(exposure to bright sunlight or sleep).
Treatment:
YAG laser PI to the affected eye and prophylactic PI to the fellow
eye. Without treatment, acute or chronic PACG will develop.
3- Acute congestive angle-closure glaucoma:
This condition is caused by a sudden total closure of the angle.
Clinical features:
Symptoms: a- Rapidly progressive impairment of visual acuity,
due to corneal oedema.
b- Periocular pain + congestion.
c- Nausea and vomiting in severe cases.
Signs seen with slit-lamp:
a- Ciliary flush due to injection of limbal and conjunctival blood
vessels.
b- IOP severely elevated (>50mmHg).
c- Corneal oedema with epithelial vesicles.
d- Shallow AC + peripheral iridocorneal touch.
e- Aqueous humour flare (protein) + cells.
f- Pupil vertically oval + fixed in mid-dilated position, not reactive
to light and accommodation.
Treatment
is surgical●
but initially we start medical treatment to control elevated IOP
which is by:
a- IV acetazolamide: 500mg followed by 250mg qid orally.
b- Hyperosmotic agents: - IV Mannitol 1g/kg of 20%.
Or Oral glycerol 1g/kg of 50% with orange juice.

11
c- Topical therapy:
i- Pilocarpine 2% x4 to affected eye, it causes miosis and pulls
iris periphery away from cornea.
ii- Pilocarpine 1% x4 to unaffected eye (as prophylaxis).
iii- Beta-blocker x2, only for the affected eye.
iv- Topical steroid: to avoid permanent adhesion between the
periphery of iris and the cornea (peripheral anterior synechia)(
PAS).
Surgical treatment:
a- We do bilateral YAG PI, then we stop pilocarpine to fellow eye
(unaffected) and we decrease medication of affected eye, 1
st
we
stop IV mannitol, then oral acetazolamide, if IOP is elevated again
(due to peripheral anterior synechiae (PAS) formation) go to:
b- Trabeculectomy is indicated, which means that 50% of the
angle is already closed by permanent anterior synechia and YAG
laser PI with topical medications alone cannot control IOP.
4- Chronic angle closure glaucoma:
Types:
a- Creeping synechial obstruction of the angle, gradual and
progressive (narrow angle is closed by gradual formation of
synechiae).
b- Synechial obstruction of the angle as a result of intermittent
(sub-acute) attacks.
c- Mixed combination of POAG + narrow angles usually
associated with long use of miotics (pilocarpine).
Treatment:
a- PI (YAG laser) for both eyes.
b- If IOP is not controlled with PI alone, the trabeculectomy is
indicated to one or both eyes.

11
Congenital glaucomas
They are uncommon (not rare), sever and potentially blinding disease.
* in general, the most common type of glaucoma is POAG 65%,
followed by secondary glaucomas 20%, then comes PACG 12%
(especially the acute one) then and the least common ones are the
congenital glaucomas 3%.
Types:
1- True congenital glaucoma: represents (40%) of all congenital
glaucoma, the IOP is elevated during intra-uterine life.
2- Infantile glaucoma: represents (55%) of congenital glaucomas, the
disease is manifested before 3 years age, but the patient was born with
normal IOP.
3- Juvenile glaucoma: represents (5%) of congenital glaucomas, least
common, present after 3 years but before reaching 16 years (>16 →
adult glaucoma).
In general, congenital glaucoma:
- Occurs 1 in 10,000 births.
- Most cases are sporadic, some are inherited as autosomal recessive
trait.
- 65% in boys and 35% in girls.
- Bilateral in 75% and 25% unilateral.
Aetiology:
Anterior segment dysgenesis, defect in cleavage during fetal
development, so we have abnormal angle and abnormal development of
anterior chamber, so there will be no trabecular meshwork and the angle
is completely obstructed.
Clinical Features:
The first sign noticed by parents is the corneal haze:
1- Corneal haze (or opacity): caused by epithelial oedema and corneal
clouding secondary to elevated IOP.
2- Photophobia, lacrimation and blepharospasm: due to damage of
corneal endothelium producing corneal oedema.
3- Buphthalmus: a large eye due to elevated IOP prior to the age of 3
years, so we should expect elevated IOP in every child with large eye
below age of 3 years.
- Sclera also enlarges becoming stretched and takes blue appearance
due to the enhanced visualization of underlying uvea.
- Corneal enlargement leading to deep AC (Anterior chamber), zonule
becomes stretched, rarely lens becomes subluxated.
- Ocular enlargement leads to axial myopia.
4- Breaks in descemet's membrane (due to stretching).

11
5- Optic disc cupping, C/D >0.3, it is not a reliable sign, as stretching of
sclera will cause enlargement of scleral canal and cupping due to
separation of nerve fibers, and as soon as we control IOP, C/D will
return to normal.
6- Visual field: cannot be done as majority of cases are under 3 years
and the diagnosis is straight forward by clinical signs.
* The intraocular pressure should be check under general anesthesia by
using Perkins hand-held applanation tonometer or Schiøtz tonometer.
Management: is always surgical, and no role for medical treatment, and
surgery should be done as early as possible. (On day of delivery)
Diagnosis:
1- Signs.
2- Checking IOP.
3- Corneal diameter: normally, corneal diameter at delivery is ≤ 10 mm,
if corneal diameter was > 12mm at age of 1 year, or 13mm at any age,
then it is viewed with suspicion. In children, corneal diameter is
regarded as visual field assessment in adults to follow patients with
glaucoma.
Surgery:
1- Goniotomy. We incised the trabecular meshwork to create a direct
communication between the anterior chamber and schlemm's canal
bypassing the trabecular meshwork, this operation needs clean cornea to
use the instruments and visualized the structures of the angle of the AC.
successful rate is 80%.
2- Trabeculotomy. From the limbus, we introduce a probe inside the
schlemm's canal and then we open the inner wall of it toward the AC.
Also here there is a direct communication between the anterior chamber
and schlemm's canal bypassing the trabecular meshwork
3- Trabeculectomy: same operation discussed in previous lecture, but
there is high failure rate due to very high activity of fibroblast cells.
The motto is: "Operate as soon as possible even a one day old baby".

11
Secondary glaucomas
1- Lens-related glaucoma:
a- Phacolytic glaucoma (lens protein glaucoma):
Is occur in association with hypermature cataract (leakage of lens
materials and shrinkage of lens). Leaked lens material is engulfed by
microphages. The trabecular obstruction is caused by high molecular
weight lens proteins which have leaked through the intact capsule into
the aqueous humour or by microphages laden with these proteins.
Treatment: control IOP medically, then surgery (cataract extraction).
b- Phacomorphic (Intumescent) glaucoma:
Is acute secondary angle-closure glaucoma precipitated by an
intumescent cataractous lens. Swelling of lens pushes the lens-iris
diaphragm forward to occlude the angle of the anterior chamber.
Presentation is the same like that of PACG but with cataract.
Treatment: surgery (cataract extraction).
c- Phacoanaphylactic (phacoantigenic) glaucoma:
Is an autoimmune reaction to lens proteins occurring in an eye with a
traumatic ruptured anterior capsule (large lens matter passing through a
rupture in capsule, this matter is regarded as foreign body and
antibodies against it is produced). Therefore, there will be occlusion of
the pores of trabecular meshwork by immune complexes and cells.
Treatment: cataract extraction.
2- Neovascular glaucoma (NVG):
Is a relatively common and serious condition, which occurs as a result
of iris neovascularization (Rubeosis iridis).
The common aetiopathogenic factor is severe, diffuse and chronic
retinal ischaemia.
Retinal ischaemia leads to hypoxic retina which tend to produces
vasoproliferative growth factors in an attempt to revascularize hypoxic
areas (form new blood vessels to compensate for hypoxia), but
unfortunately these blood vessels are very fragile, so they may rupture
suddenly and patient get sudden blindness due to retinal or vitreous
hemorrhage. These vessels may rupture spontaneously or during
valsalva manoeuvre. However, these factors also diffuse in to the
anterior segment and initiate Rubeosis iridis (neovascularization of iris)
and neovascularization in the angle of the anterior chamber (leading to
occlusion of trabecular meshwork).
* If you see rubeosis iridis in any person, you can say without any
doubt that there is retinal ischaemia.

11
Causes of retinal ischaemia (causes of NVG):
a- Central retinal vein occlusion. (commonest cause)
b- Diabetes mellitus (proliferative diabetic retinopathy).
c- miscellaneous, e.g.: - Carotid obstructive disease.
- Central retinal artery occlusion.
- Intraocular tumours.
- Long standing retinal detachment.
- Chronic intraocular inflammation.
Treatment:
1- Medical treatment is initially with topical beta-blockers and
acetazolamide. Topical atropine and steroids may decrease
inflammation and make the eye more comfortable and less
congested, even if IOP remains high.
2- Panretinal photocoagulation with laser: We destruct retinal tissue
in order to decrease O2 consumption and control hypoxia. We
photcoagulate the mid periphery and the periphery of retina and we
preserve the macula only.
3- Filteration surgery may be consider if still there is useful vision
either by trabeculectomy with anti metabolite or by use artificial
filtering shunts.
4- Cyclodestruction by trans scleral diode laser or trans scleral
cyclocryotherapy which lead to destructs part of Ciliary body and
decrease aqueous production.
5- Retrobulbar alcohol injection is useful in relieving pain by
destruction of sensory enervation.
6- Enucleation (evacuation of all intraocular contents and we leave
just the sclera) may be considered if all else fails.
3- Inflammatory glaucomas
Glaucomas secondary to intraocular inflammation frequently present a
considerable diagnostic and therapeutic challenge. Although in some
cases the elevation of IOP is transient and innocuous, often it is persistent
and severely damaging.
In acute anterior uveitis, the IOP is usually normal or subnormal as a
result of concomitant ciliary shutdown. Occasionally, however, a
trabecular-block open angle glaucoma develops secondary to obstruction
of aqueous outflow, most commonly just as the acute inflammation is and
ciliary body function returning to normal. The block may be caused by

11
either inflammatory cells and debris or acute trabeculitis. The IOP usually
returns to normal once the inflammation has subsided.
Secondary angle closure is caused by 360° iridolenticular adhesions
(seclusio pupillae), The pupil block obstructs the passage of aqueous
humour from the posterior to the anterior chamber, and the increased
pressure in the posterior chamber causes an anterior bowing of the
peripheral iris (iris bombé), If severe, iris bombé is associated with a
shallowing of the anterior chamber, and apposition of the peripheral iris
to the trabeculum and peripheral cornea. If this occurs in an eye with
active inflammation, the iris sticks to the trabeculum and the iridocorneal
contact becomes permanent with the development of peripheral anterior
synechiae (PAS).
Slit lamp examination shows seclusio pupillae, iris bombé and a shallow
anterior chamber.
Gonioscopy shows angle closure from iridotrabecular contact.
Treatment involves the following measures:
1. Prevention of synechial angle closure can be effective by a
reduction in the 'stickiness' of the peripheral iris using a
combination of intensive topical steroids and anterior sub-Tenon's
injection of a long-acting depot steroid preparation.
2. Lowering
of
IOP
with
topical
ß-blockers
and/or
sympathomimetics may be effective in relatively mild cases where
the IOP is <30 mmHg. Carbonic anhydrase inhibitors are usually
required if the IOP is >30mmHg.
3. Nd-YAG laser iridotomy may be required if medical therapy
fails, It will only eliminate the element of pupil block and will
therefore only be effective in lowering IOP, if at least 25% of the
angle is still open.
The End