
1
Lectures In the
Internal medicine
LEC(4&5&6)
Dr.Dhaher JS Al-habbo
FRCP London UK
Assistant Professor in Medicine
DEPARTMENT OF MEDICINE

2
4th stage
باطنية
Lec-4
د.ظاهر
8/11/2015
Tuberculosis (TB)
Tuberculosis (TB) is caused by infection with Mycobacterium tuberculosis
(MTB), which is part of a complex of organisms including M. bovis (reservoir
cattle) and M. africanum (reservoir human).
With1.5 million deaths attributable to TB.
One-third of the world's population has latent TB.
The majority of cases occur in the world's poorest nations.
The resurgence of TB has been largely driven in Africa by HIV disease, and
in the former Soviet Union and Baltic states by lack of appropriate health
care exacerbated by social and political upheaval.
Tuberculosis (TB)
• Tuberculosis (TB) is caused by infection with Mycobacterium tuberculosis
(MTB), M. tuberculosis is spread by the inhalation of
aerosolised droplet nuclei from other infected
patients.
• Once inhaled, the organisms lodge in the alveoli and initiate the
recruitment of macrophages and lymphocytes.
• Macrophages undergo transformation into epithelioid and Langhans cells
which aggregate with the lymphocytes to form the classical tuberculous
granuloma .
• M. bovis infection arises from drinking non-sterilised milk from infected
cows.

3
Tuberculosis (TB)
Numerous granulomas aggregate to form a primary lesion or 'Ghon focus' ,which
is characteristically situated in the periphery of the lung.
Also spread of organisms to the hilar lymph nodes is followed by 'Ghon focus
reaction;
Combination of a primary lesion and regional lymph nodes is referred to as the
'primary complex of Ranke'.
Reparative processes encase the primary complex in a fibrous capsule limiting the
spread of bacilli: so-called latent TB.
If no further complications ensue, this lesion eventually calcifies and is clearly
seen on a chest X-ray
Factors increasing the risk of TB
Patient-related
Age (children > young adults < elderly) ,Close contacts of patients with
smear-positive pulmonary TB ,Overcrowding,
Chest radiographic evidence of self-healed TB , Smoking: cigarettes
Primary infection < 1 year previously
Associated diseases
Immunosuppression: HIV, anti-TNF therapy, high-dose corticosteroids,
cytotoxic agents
Malignancy (especially lymphoma and leukaemia)
Type 1 diabetes mellitus ,Chronic renal failure ,Silicosis
Gastrectomy, jejuno-ileal bypass, cancer of the pancreas, malabsorption.

4
Deficiency of vitamin D or A
• Recent measles: increases risk of child contracting TB
Timetable of TB
Time from
infection
Primary complex, positive tuberculin skin test
3-8 weeks
Meningeal, miliary and pleural disease
3-6 months
Gastrointestinal, bone and joint, and lymph
node disease
Up to 3 years
Renal tract disease
Around 8 years
Post-primary disease due to reactivation or
reinfection
From 3 years
onwards
Features of primary TB infection(4-8weeks)
• Influenza-like illness.
• Skin-test conversion.
• Primary complex.
Hypersensitivity .
Erythema nodosum.
Phlyctenular conjunctivitis .
Dactylitis

5
Primary TB
Primary TB refers to the infection of a previously uninfected (tuberculin-negative)
individual.
A few patients develop a self-limiting febrile illness but clinical disease only occurs
if there is a hypersensitivity reaction or progressive infection .
Progressive primary disease may appear during the course of the initial illness or
after a latent period of weeks or months.
Miliary TB
• Blood-borne dissemination gives rise to miliary TB, which may present
acutely but more frequently is characterised by 2-3 weeks of fever, night
sweats, anorexia, weight loss and a dry cough.
• Hepatosplenomegaly may develop and the presence of a headache may
indicate coexistent tuberculous meningitis.
• Auscultation of the chest is frequently normal, although with more
advanced disease widespread crackles are evident. Fundoscopy may show
choroidal tubercles.
• The classical appearances on chest X-ray are of fine 1-2 mm lesions ('millet
seed') distributed throughout the lung fields, although occasionally the
appearances are coarser.
• Anaemia and leucopenia reflect bone marrow involvement. 'Cryptic'
miliary TB is an unusual presentation sometimes seen in old age
Post-primary pulmonary TB
Cryptic M. TB
• Age over 60 years
• Intermittent low-grade pyrexia of unknown origin

6
• Unexplained weight loss, general debility (hepatosplenomegaly in 25-50%)
• Normal chest X-ray
• Blood dyscrasias; leukaemoid reaction, pancytopenia
• Negative tuberculin skin test
• Confirmation by biopsy (granulomas and/or acid-fast bacilli demonstrated)
of liver or bone marrow
Post-primary pulmonary TB
Clinical presentations of pulmonary TB
• Chronic cough, often with haemoptysis
• Pyrexia of unknown origin
• Unresolved pneumonia
• Exudative pleural effusion
• Asymptomatic (diagnosis on chest X-ray)
• Weight loss, general debility
• Spontaneous pneumothorax
Post-primary pulmonary TB
• Post-primary disease refers to exogenous ('new' infection) or endogenous
(reactivation of a dormant primary lesion) infection in a person who has
been sensitised by earlier exposure.
• It is most frequently pulmonary and characteristically occurs in the apex of
an upper lobe where the oxygen tension favours survival of the strictly
aerobic organism.
• The onset is usually insidious, developing slowly over several weeks.

7
• Systemic symptoms include fever, night sweats, malaise, and loss of
appetite and weight, and are accompanied by progressive pulmonary
symptoms .
• Very occasionally, this form of TB may present with one of the
complications of TB.
Post-primary pulmonary TB
Radiological changes include ill-defined opacification in one or both of the upper
lobes, and as progression occurs, consolidation, collapse and cavitation develop
to varying degrees .
It is often difficult to distinguish active from quiescent disease on radiological
criteria alone, but the presence of a miliary pattern or cavitation favours active
disease.
In extensive disease, collapse may be marked and result in significant
displacement of the trachea and mediastinum.
Occasionally, a caseous lymph node may drain into an adjoining bronchus
resulting in tuberculous pneumonia.
Clinical features: extrapulmonary disease
Pulmonary
• Massive haemoptysis
• Cor pulmonale
• Fibrosis/emphysema
• Atypical mycobacterial infection
• Aspergilloma
• Lung/pleural calcification
• Obstructive airways disease

8
• Bronchiectasis
• Bronchopleural fistula
Clinical features: extrapulmonary disease
Non-pulmonary
• Empyema necessitans
• Laryngitis
• Enteritis(From swallowed sputum).
• Anorectal disease(From swallowed sputum).
• Amyloidosis
• Poncet's polyarthritis
TB Gastrointestinal disease
Upper gastrointestinal tract involvement is rare and is usually an
unexpected histological finding in an endoscopic or laparotomy specimen.
Ileocaecal disease accounts for approximately half of abdominal TB cases.
Fever, night sweats, anorexia and weight loss are usually prominent and a
right iliac fossa mass may be palpable.
Up to 30% of cases present with an acute abdomen.
Ultrasound or CT may reveal thickened bowel wall, abdominal
lymphadenopathy, mesenteric thickening or ascites.
TB Gastrointestinal disease .
Pericardial effusion and Constrictive pericarditis.
TB of the Central nervous system disease .

9
TB of Bone and Join Disease.
TB of Genitourinary disease
Contrast enhanced abdominal CT of a 21 year-old female patient demonstrates
multiple mesenteric lymphadenopathy forming a conglomerate mass (arrows) with 6
cm in major axis. Most enlarged nodes have central hypoenhancing areas due to
necrosis.
Diagnosis of Tuberculosis
Specimens required:
• Sputum* (induced with nebulised hypertonic saline if not expectorating) At
least 2 but preferably 3, including an early morning sample
• Bronchoscopy with washings or BAL
• Gastric washing* (mainly used for children) At least 2 but preferably 3,
including an early morning sample
Extrapulmonary
• Fluid examination (cerebrospinal, ascitic, pleural, pericardial, joint): yield
classically very low
• Tissue biopsy (from affected site); also bone marrow/liver may be
diagnostic in patients with disseminated disease
10
Diagnostic Tests
• Circumstantial (ESR, CRP, anaemia etc.)
• Tuberculin skin test (low sensitivity/specificity; useful only in primary or
deep-seated infection)
• Stain
• Ziehl-Neelsen
• Auramine fluorescence
• Nucleic acid amplification
• Culture
• Solid media (Löwenstein-Jensen, Middlebrook)
• Liquid media (e.g. BACTEC or MGIT) mycobacteria growth indicator
tube
• Response to empirical antituberculous drugs (usually seen after 5-10 days)
TB-Diagnostic tests :
The presence of an otherwise unexplained cough for more than 2-3 weeks,
particularly in an area where TB is highly prevalent, or typical chest X-ray
changes should prompt further investigation .
Direct microscopy of sputum is the most important first step.
The probability of detecting acid-fast bacilli is proportional to the bacillary
burden in the sputum (typically positive when 5000-10 000 organisms are
present).
By virtue of their substantial lipid-rich wall, tuberculous bacilli are difficult
to stain.

11
The most effective techniques are the Ziehl-Neelsen and rhodamine-
auramine stains.
The latter causes the tuberculous bacilli to fluoresce against a dark
background and is easier to use when numerous specimens need to be
examined;
However, it is more complex and expensive, limiting applicability in
resource-poor regions.
A positive smear is sufficient for the presumptive diagnosis of TB but
definitive diagnosis requires culture.
Smear-negative sputum should also be cultured, as only 10-100 viable
organisms are required for sputum to be culture-positive.
A diagnosis of smear-negative TB may be made in advance of culture if the
chest X-ray appearances are typical of TB and there is no response to a
broad-spectrum antibiotic.
MTB grows slowly and may take between 4 and 6 weeks to appear on solid
medium such as Löwenstein-Jensen or Middlebrook.
Faster growth (1-3 weeks) occurs in liquid media such as the radioactive
BACTEC system or the non-radiometric mycobacteria growth indicator tube
(MGIT).
The BACTEC method is commonly used in developed nations and detects
mycobacterial growth by measuring the liberation of
14
CO
2
, following
metabolism of
14
C-labelled substrate present in the medium.

12
New strategies for the rapid confirmation of TB at low cost are being developed;
These include the nucleic acid amplification test (NAT), designed to amplify
nucleic acid regions specific to MTB such as IS6110, and the MPB64 skin
patch test, in which immunogenic antigen detects active but not latent TB,
and has the potential to provide a simple, non-invasive test which does not
require a laboratory or highly skilled personnel.
Drug sensitivity testing is particularly important in those with a previous
history of TB, treatment failure or chronic disease, those who are resident
in or have visited an area of high prevalence of resistance, or those who are
HIV-positive.
The detection of rifampicin resistance, using molecular tools to test for the
presence of the rpo gene currently associated with around 95% of
rifampicin-resistant cases, is important as the drug forms the cornerstone
of 6-month chemotherapy..
• If a cluster of cases suggests a common source, confirmation may be sought
by fingerprinting of isolates with restriction-fragment length polymorphism
(RFLP) or DNA amplification.
• The diagnosis of extrapulmonary TB can be more challenging.
• There are generally fewer organisms (particularly in meningeal or pleural
fluid), so culture or histopathological examination of tissue is more
important.
• In the presence of HIV, however, examination of sputum may still be useful,
as subclinical pulmonary disease is common
Skin testing in TB:
Tests using purified protein derivative (PPD)

13
Heaf test
• Read at 3-7 days
• Multipuncture method
• Grade 1: 4-6 papules
• Grade 2: Confluent papules forming ring
• Grade 3: Central induration
• Grade 4: > 10 mm induration
Mantoux test
• Read at 2-4 days
• Using 10 tuberculin units
• Positive when induration 5-14 mm (equivalent to Heaf grade 2) and >
15 mm (Heaf grade 3-4)
Skin
False negatives
• Severe TB (25% of cases negative)
• Newborn and elderly
• HIV (if CD4 count < 200 cells/mL)
• Malnutrition
• Recent infection (e.g. measles) or immunisation
• Immunosuppressive drugs
• Malignancy
• Sarcoidosis

14
Skin testing in TB
Tuberculin skin testing may be associated with false-positive reactions in
those who have had a BCG vaccination and in areas where exposure to non-
tuberculous mycobacteria is high.
These limitations may be overcome by employing interferon-gamma
release assays (IGRAs).
These tests measure the release of IFN-γ from sensitised T cells in response
to antigens such as early secreted antigenic target (ESAT)-6 or culture
filtrate protein (CFP)-10 that are encoded by genes specific to the MTB and
are not shared with BCG or opportunistic mycobacteria.
The greater specificity of these tests, combined with the logistical
convenience of one blood test, as opposed to two visits for skin testing,
suggests that IGRAs will replace the tuberculin skin test in low-incidence,
high-income countries.
Managements and Chemotherapy
:
They are based on the principle of an initial intensive phase (which rapidly
reduces the bacterial population), followed by a continuation phase to
destroy any remaining bacteria.
Treatment should be commenced immediately in any patient who is smear-
positive, or who is smear-negative but with typical chest X-ray changes and
no response to standard antibiotics.
15
Continuation
phase
Initial phase*
Category of TB
4 months H
3
R
3
2 months H
3
R
3
Z
3
E
3
or 2 months
H
3
R
3
Z
3
S
3
(
i.e
3=week)
New cases of smear-positive
pulmonary TB
1
4 months HR
2 months HRZE or 2 months HRZS
Severe extra pulmonary TB
6 months HE
†
Severe smear-negative
pulmonary TB
Severe concomitant HIV disease
5 months
H
3
R
3
E
3
2 months H
3
R
3
Z
3
E
3
or 1 month
H
3
R
3
Z
3
E
Previously treated smear-
positive pulmonary TB
2
5 months HRE
2 months HRZES or 1 month
HRZE
Relapse
Treatment failure
Treatment after default
4 months H
3
R
3
2 months H
3
R
3
Z
3
E
3
New cases of smear-negative
pulmonary TB
3
4 months HR
2 months HRZE
Less severe extrapulmonary
TB
6 months HE
†
H= isoniazid;; E =ethambutol; R = rifampicin; Z = pyrazinamide
S = streptomycin
Ethambutol
Streptomycin
Pyrazinamide
Rifampicin
Isoniazid
Cell wall
synthesis
Protein
synthesis
Unknown
DNA
transcription
Cell wall
synthesis
Mode of
action
Retrobulbar
neuritis
3
Arthralgia
8th nerve
damage
Rash
Hepatitis
Gastrointesti
nal
disturbance
Hyperuricae
mia
Febrile
reactions
Hepatitis
Rash
Gastrointesti
nal
disturbance
Peripheral
neuropathy
1
Hepatitis
2
Rash
Major
adverse
reactions
Peripheral
neuropathy
Rash
Nephrotoxici
ty
Agranulocyt
osis
Rash
Photosensiti
sation
Gout
Interstitial
nephritis
Thrombocyt
openia
Haemolytic
anaemia
Lupoid
reactions
Seizures
Psychoses
Less
common
adverse
reactions

16
Quadruple therapy has become standard in the UK, although Ethambutol
may be omitted under certain circumstances.
Fixed-dose tablets combining two or three drugs are generally favoured: for
example, Rifater (rifampicin, isoniazid and pyrazinamide) daily for 2
months, followed by 4 months of Rifinah (rifampicin and isoniazid).
Streptomycin is rarely used in the UK, but is an important component of
short-course treatment regimens in developing nations.
Six months of therapy is appropriate for all patients with new-onset,
uncomplicated pulmonary disease.
However, 9-12 months of therapy should be considered if the patient is
HIV-positive, or if drug intolerance occurs and a second-line agent is
substituted.
Meningitis should be treated for a minimum of 12 months.
Pyridoxine should be prescribed in pregnant women and malnourished
patients to reduce the risk of peripheral neuropathy with isoniazid.
Where drug resistance is not anticipated, patients can be assumed to be
non-infectious after 2 weeks of appropriate therapy.
Most patients can be treated at home. .
Admission to a hospital unit with appropriate isolation facilities should be
considered where there is uncertainty about the diagnosis, intolerance of
medication, questionable compliance, adverse social conditions or a
significant risk of multidrug-resistant TB (MDR-TB:

17
Culture-positive after 2 months on treatment, or contact with known MDR-
TB).
In choosing a suitable drug regimen, underlying comorbidity (renal and
hepatic dysfunction, eye disease, peripheral neuropathy and HIV status), as
well as the potential for drug interactions, must be considered.
• Baseline liver function and regular monitoring are important for patients
treated with standard therapy including rifampicin, isoniazid and
pyrazinamide, as all of these agents are potentially hepatotoxic.
• Mild asymptomatic increases in transaminases are common but serious
liver damage is rare.
• Women taking the oral contraceptive pill must be warned that its efficacy
will be reduced and alternative contraception may be necessary.
• Ethambutol should be used with caution in patients with renal failure, with
appropriate dose reduction and monitoring of drug levels.
• Adverse drug reactions occur in about 10% of patients, but are significantly
more common in the presence of HIV co-infection .
Corticosteroids reduce inflammation and limit tissue damage, and are
currently recommended when treating pericardial or meningeal disease,
and in children with endobronchial disease.
They may confer benefit in TB of the ureter, pleural effusions and extensive
pulmonary disease, and can suppress hypersensitivity drug reactions.
Surgery is still occasionally required (e.g. for massive haemoptysis,
loculated empyema, constrictive pericarditis, lymph node suppuration,
spinal disease with cord compression), but usually only after a full course of
antituberculosis treatment

18
Control and prevention of TB:
The effectiveness of therapy for pulmonary TB may be judged by a further
sputum smear at 2 months and at 5 months.
A positive sputum smear at 5 months defines treatment failure.
Extrapulmonary TB must be assessed clinically or radiographically as
appropriate.
The WHO is committed to reducing the incidence of TB by 2015.
Important components of this goal include supporting the development of
laboratory and health-care services to improve detection and treatment of
active and latent TB.
Detection of latent TB
• It has the potential to identify the probable index case, other cases infected
by the same index patient (with or without evidence of disease), and close
contacts who should receive BCG vaccination (see below) or chemotherapy.
• Approximately 10-20% of close contacts of patients with smear-positive
pulmonary TB and 2-5% of those with smear-negative, culture-positive
disease have evidence of TB infection.
• Cases are commonly identified using the tuberculin skin test (Box 19.63 and
Fig. 19.39).
• An otherwise asymptomatic contact with a positive tuberculin skin test but
a normal chest X-ray may be treated with chemoprophylaxis to prevent
infection progressing to clinical disease
• .Chemoprophylaxis is also recommended for children aged less than 16
years identified during contact tracing to have a strongly positive tuberculin
test, children aged less than 2 years in close contact with smear-positive

19
pulmonary disease, those in whom recent tuberculin conversion has been
confirmed, and babies of mothers with pulmonary TB.
• It should also be considered for HIV-infected close contacts of a patient
with smear-positive disease.
• Rifampicin plus isoniazid for 3 months or isoniazid for 6 months is effective
Directly observed therapy (DOT) :
• Poor adherence to therapy is a major factor in prolonged infectious illness,
risk of relapse and the emergence of drug resistance.
• DOT involves the supervised administration of therapy thrice weekly and
improves adherence.
• It has become an important control strategy in resource-poor nations.
• In the UK, it is currently only recommended for patients thought unlikely to
be adherent to therapy:
• Those who are homeless, alcohol or drug users, drifters, those with serious
mental illness and those with a history of non-compliance

20
4th stage
باطنية
Lec-5
د.ظاهر
8/11/2015
Bronchiectasis
Bronchiectasis means abnormal dilatation of the bronchi.
Chronic suppurative airway infection with sputum production, progressive
scarring and lung damage are present, whatever the cause.
Aetiology and pathogenesis Bronchiectasis
Congenital defect affecting airway ion transport or ciliary function, such as
cystic fibrosis .
Acquired secondary to damage to the airways by a destructive infection,
inhaled toxin or foreign body. The result is chronic inflammation
,tuberculosis is the most common world-wide.
Localised bronchiectasis may occur due to the accumulation of pus beyond
an obstructing bronchial lesion, such as enlarged tuberculous hilar lymph
nodes, a bronchial tumour or an inhaled foreign body (e.g. an aspirated
peanut).
Pathology
The bronchiectatic cavities may be lined by granulation tissue, squamous
epithelium or normal ciliated epithelium.
Chronic inflammatory and fibrotic changes are usually found in the
surrounding lung tissue, resulting in progressive destruction of the normal
lung architecture in advanced case

21
Clinical features and symptoms of bronchiectasis(table)
Physical signs in the chest may be unilateral or bilateral. If the
bronchiectatic airways do not contain secretions and there is no associated
lobar collapse, there are no abnormal physical signs.
When there are large amounts of sputum in the bronchiectatic spaces,
numerous coarse crackles may be heard over the affected areas.
Acute haemoptysis is an important complication of bronchiectasis;
management is covered on page
Investigations Bacteriological and mycological examination of sputum
In addition to common respiratory pathogens, sputum culture may reveal
Pseudomonas aeruginosa, fungi such as Aspergillus and various
mycobacteria..
This time should not exceed 20 minutes but is greatly prolonged in patients
with ciliary dysfunction.
Ciliary beat frequency may also be assessed using biopsies taken from the
nose. Structural abnormalities of cilia can be detected by electron
microscopy.
Management
In airflow obstruction, inhaled bronchodilators and corticosteroids should
be used to enhance airway patency.
Physiotherapy Patients should be instructed on how to perform regular
daily physiotherapy to assist the drainage of excess bronchial secretions.
Patients should adopt a position in which the lobe to be drained is
uppermost.

22
Deep breathing followed by forced expiratory (the 'active cycle of
breathing' technique) is of help in moving secretions in the dilated bronchi
towards the trachea, from which they can be cleared by vigorous coughing.
'Percussion' of the chest wall with cupped hands may help to dislodge
sputum, but does not suit all patients. .
The optimum duration and frequency of physiotherapy depend on the
amount of sputum, but 5-10 minutes once or twice daily is a minimum for
most patients.
Antibiotic therapy in general, require larger doses and longer courses .
When secondary infection occurs with staphylococci and Gram-negative
bacilli, in particular Pseudomonas species, antibiotic therapy becomes more
challenging and should be guided by the microbiological sensitivities.
For Pseudomonas, oral ciprofloxacin (250-750 mg 12-hourly) or ceftazidime
by intravenous injection or infusion (1-2 g 8-hourly) may be required.
Haemoptysis in bronchiectasis often responds to treating the underlying
infection, although in severe cases percutaneous embolisation of the
bronchial circulation by an interventional radiologist may be necessary.
Surgical treatment Excision of bronchiectatic areas is only indicated in a
small proportion of cases.
Cystic fibrosis:
The most common fatal genetic disease in Caucasians, with autosomal recessive
inheritance.
CF is the result of mutations affecting a gene on the long arm of chromosome 7
which codes for a chloride channel known as cystic fibrosis transmembrane
conductance regulator (CFTR), that influences salt and water movement across
epithelial cell membranes.

23
This lead to increased sodium and chloride content in sweat and increased
resorption of sodium and water from respiratory epithelium.
Relative dehydration of the airway epithelium is thought to predispose to chronic
bacterial infection and ciliary dysfunction, leading to bronchiectasis.
The gene defect also causes disorders in the gut epithelium, pancreas, liver and
reproductive tract .
Clinical features
The lungs are most commonly infected with Staphylococcus aureus; however,
many patients become colonised with Pseudomonas aeruginosa by the time they
reach adulthood.
The upper lobes but subsequently throughout both lungs, cause progressive lung
damage resulting ultimately in death from respiratory failure.
Most men with CF are infertile due to failure of development of the vas deferens,
but microsurgical sperm aspiration and in vitro fertilisation are now possible.
Complications of cystic fibrosis
Respiratory :
Infective exacerbations of bronchiectasis,Spontaneous
pneumothorax,Haemoptysis,Nasal polyps,Respiratory failure,Cor
pulmonale,Lobar collapse due to secretions .
Gastrointestinal: Malabsorption and steatorrhoea,Distal intestinal obstruction
syndrome ,Biliary cirrhosis and portal hypertension,Gallstones
Others :Diabetes (25% of adults),Delayed puberty,Male infertility,Stress
incontinence due to repeated forced cough ,Psychosocial
problems,Osteoporosis,Arthropathy,Cutaneous vasculitis.

24
Management
Treatment of CF lung disease :
The management of CF lung disease is that of severe bronchiectasis. All patients
with CF who produce sputum should perform regular chest physiotherapy, and
should do so more frequently during exacerbations. While infections with Staph.
aureus can often be managed with oral antibiotics, intravenous treatment (often
self-administered at home through a subcutaneous vascular port) is usually
needed for Pseudomonas species. Regular nebulised antibiotic therapy
(colomycin or tobramycin) is used between exacerbations in an attempt to
suppress chronic Pseudomonas infection.
Treatments that may reduce chest exacerbations and/or improve lung function in
CF
1) Nebulised recombinant human DNase 2.5 mg daily used in patient Age ≥ 5, FVC
> 40% predicted
2) Nebulised tobramycin 300 mg 12-hourly, given in alternate months used in
Patients colonised with pseudomonas aeruginosa
3)Regular oral azithromycin 500 mg three times/week used in Patients colonised
with Pseudomonas aeruginosa .
Bronchi of many CF patients eventually become colonised with pathogens which
are resistant to most antibiotics. Resistant strains of P. aeruginosa,
Stenotrophomonas maltophilia and Burkholderia cepacia are the main culprits,
and may require prolonged treatment with unusual combinations of antibiotics.
Aspergillus and 'atypical mycobacteria' are also frequently found in the sputum of
CF patients, but in most cases these behave as benign 'colonisers' of the
bronchiectatic airways and do not require specific therapy.

25
Some patients have coexistent asthma, which is treated with inhaled
bronchodilators and corticosteroids; allergic bronchopulmonary aspergillosis also
occurs occasionally in CF.
For advanced CF lung disease, home oxygen and NIV may be necessary to treat
respiratory failure.
Ultimately, lung transplantation can produce dramatic improvements but is
limited by donor organ availability.
Treatment of non-respiratory manifestations of CF
Malabsorption is treated with oral pancreatic enzyme supplements and vitamins.
The increased calorie requirements of CF patients are met by supplemental
feeding, including nasogastric or gastrostomy tube feeding if required.
Diabetes eventually appears in over 25% of patients and often requires insulin
therapy. Osteoporosis secondary to malabsorption and chronic ill health should
be sought and treated.
Somatic gene therapy :
The discovery of the CF gene and the fact that the lethal defect is located in the
respiratory epithelium (which is accessible by inhaled therapy) presents an
exciting opportunity for gene therapy. Manufactured normal CF gene can be
'packaged' within a viral or liposome vector and delivered to the respiratory
epithelium to correct the genetic defect.

26
4th stage
باطنية
Lec-6
د.ظاهر
8/11/2015
TUMOURS OF THE BRONCHUS AND LUNG
The burden of lung cancer
• Cigarette smoking is by far the most important cause of lung cancer., for at
least 90 being proportional to the amount smoked and to the tar content of
cigarettes.
• The death rate from the disease in heavy smokers is 40 times that in non-
smokers. Risk falls slowly after smoking cessation, but remains above that
in non-smokers for many years
• Exposure to naturally occurring radon is another risk.
• The incidence of lung cancer is slightly higher in urban than in rural
dwellers.which may reflect differences in atmospheric .
Bronchial carcinoma:
• Bronchial carcinomas arise from the bronchial epithelium or mucous
glands.With Lymphatic spread &or Blood-borne metastases .
• The common cell types are:
• Squamous30-35%,
• Adenocarcinoma40-30%,
• Small-cell20%, spread very fast
• and Large-cell 15%.
• Tumour occurs in a large bronchus, symptoms arise early, but tumours
originating in a peripheral bronchus can grow very large without producing
symptoms, resulting in delayed diagnosis.
• Peripheral squamous tumours may undergo central necrosis and cavitation,
and may resemble a lung abscess on X-ray .

27
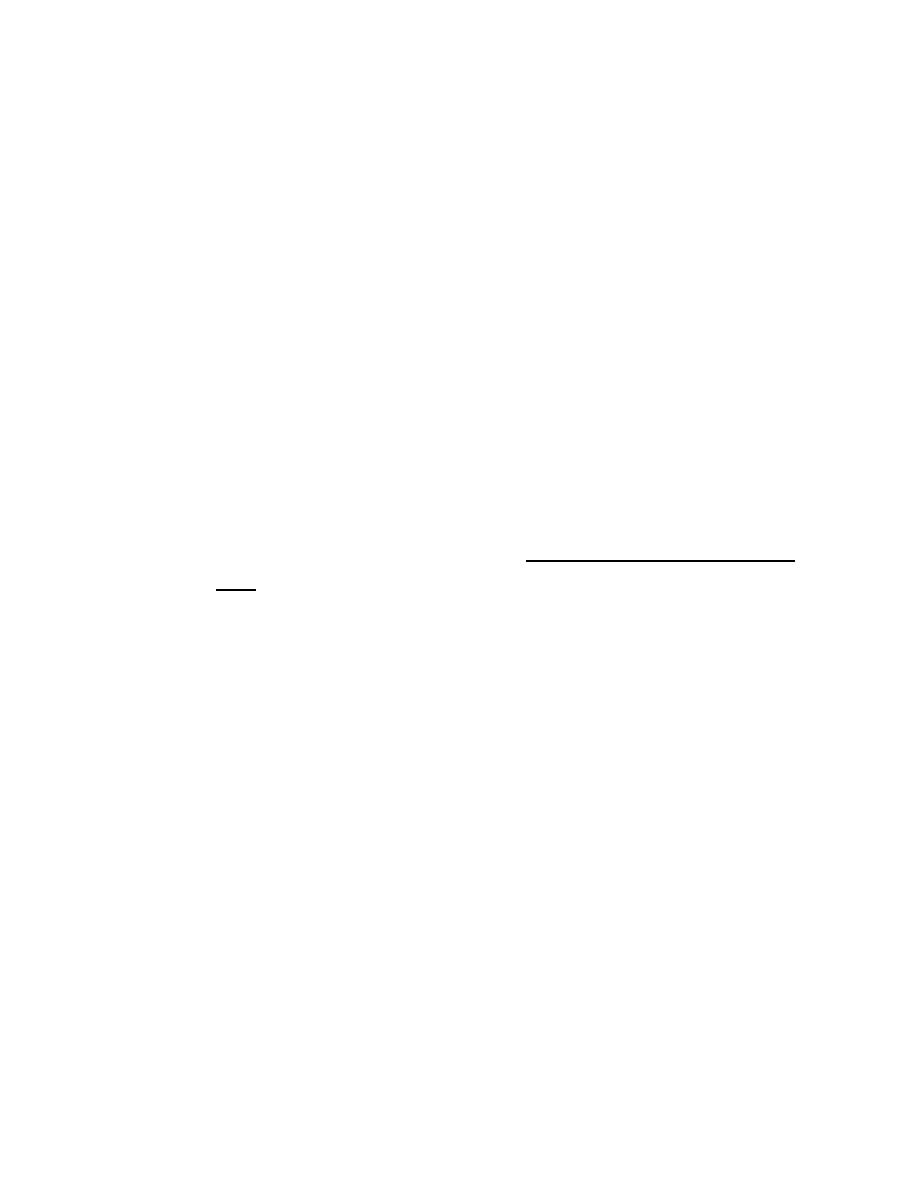
Clinical Presentation of Bronchial carcinoma
1. Cough.
2. Haemoptysis. Occasionally, central tumours invade large vessels, causing
sudden massive haemoptysis which may be fatal.
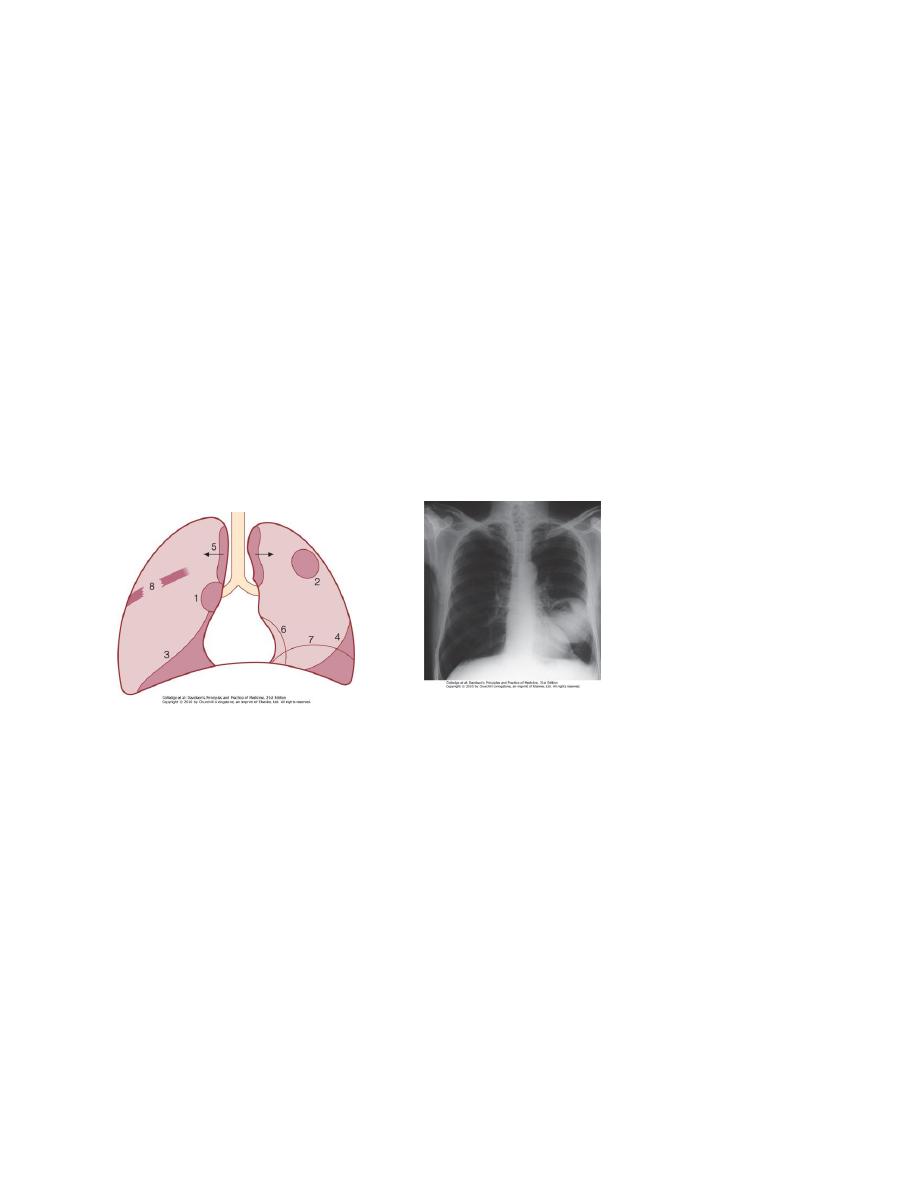
3. Bronchial obstruction.Complete obstruction causes collapse of a lobe or
lung, Partial bronchial obstruction may cause a monophonic, unilateral
wheeze that fails to clear with cough.
4. Pneumonia that recurs at the same site.
5. Stridor (a harsh inspiratory noise) occurs when the lower trachea,.
6. Breathlessness. This may be caused by collapse or pneumonia, or by
tumour causing a large pleural effusion or compressing a phrenic nerve
causing diaphragmatic paralysis.
7. Pain and nerve entrapment. Pleural pain usually indicates malignant pleural
invasion.
8. Carcinoma in the lung apex may cause Horner's syndrome (ipsilateral
partial ptosis, enophthalmos, miosis and hypohidrosis of the face due to
involvement of the sympathetic chain at or above the stellate ganglion.
9. Pancoast's syndrome, malignant destruction of the T1 and C8 roots.
Mediastinal spread. Involvement of the oesophagus may cause dysphagia.
10. If the pericardium, arrhythmia or pericardial effusion ..
11. Superior vena cava obstruction,left recurrent laryngeal nerve
12. Supraclavicular lymph nodes ,Digital clubbing& Hypertrophic pulmonary
osteoarthropathy (HPOA),
13. Non-metastatic extrapulmonary effects .Syndrome of inappropriate
antidiuretic hormone secretion (SIADH) and ectopic adrenocorticotrophic
hormone secretion are usually associated with small-cell lung cancer
14. Hypercalcaemia is usually caused by squamous cell carcinoma.
15. Associated neurological syndromes may occur with any type of bronchial
carcinoma

28
Non-metastatic extrapulmonary manifestations of bronchial carcinoma
Endocrine
• Inappropriate antidiuretic hormone secretion causing hyponatraemia
• Ectopic adrenocorticotrophic hormone secretion
• Hypercalcaemia due to secretion of parathyroid hormone-related peptides
• Carcinoid syndrome
• Gynaecomastia
• Non-metastatic extrapulmonary manifestations of bronchial carcinoma
Neurological
• Polyneuropathy
• Myelopathy
• Cerebellar degeneration
• Myasthenia (Lambert-Eaton syndrome).
Other
• Digital clubbing
• Hypertrophic pulmonary osteoarthropathy
• Nephrotic syndrome
• Polymyositis and dermatomyositis
• Eosinophilia
29
Investigations for Bronchial carcinoma
• Plain X-rays
• CT is usually performed for :
localization,operability, metastatic spread
and for accessible or not by bronchoscopy .
Bronchoscopy ;three-quarters of primary lung tumours can be visualised and
sampled directly by biopsy and brushing using a flexible bronchoscope.
Percutaneous needle biopsy under CT or ultrasound guidance; more reliable way
to obtain a histological diagnosis for tumours which are too peripheral
Large cavitated bronchial carcinoma in left lower
lobe
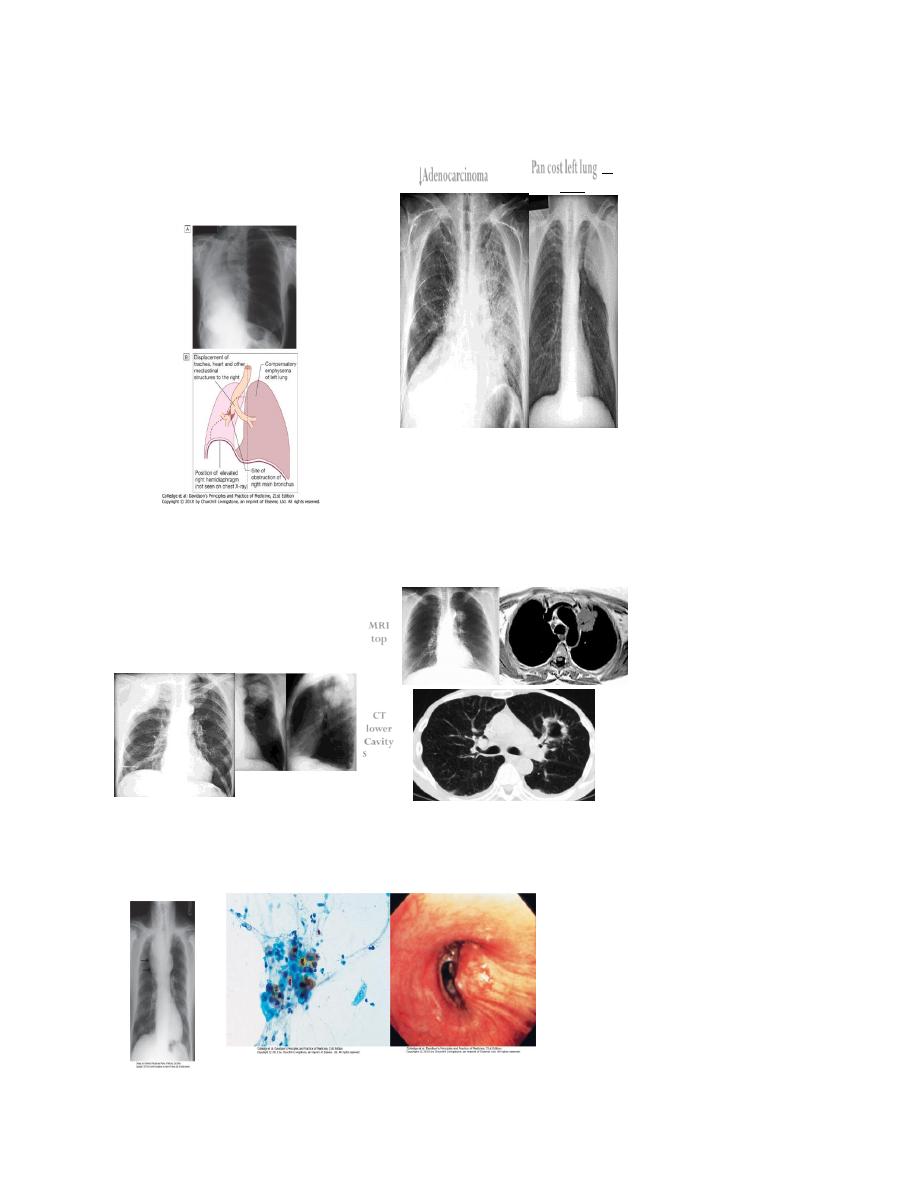
30
↓Adenocarcinoma
Pan cost left lung↓
with
Rib eroson
↓Pancost Right lung
Pancostleft lung
↓
MRI
top
CT
lower
Cavity
Squ.carc
31
Management
• Surgical resection carries the best hope of long-term survival; however,
some patients treated with radical radiotherapy and chemotherapy also
achieve prolonged remission or cure.
• Unfortunately, in over 75% of cases, treatment with curative intent is not
possible, or is inappropriate due to extensive spread or comorbidity. Such
patients can only be offered palliative therapy and best supportive care.
• Radiotherapy, and in some cases chemotherapy, can relieve distressing
symptoms
Treatment and Staging NSCLC
Stage
Description
Treatment Options
Stage I a/ b
Tumor of any size is found only in
the lung
Surgery
Stage II a/ b Tumor has spread to lymph nodes
associated with the lung
Surgery
Stage III a
Tumor has spread to the lymph
nodes in the tracheal area,
including chest wall and
diaphragm
Chemotherapy
followed by radiation
or surgery
Stage III b
Tumor has spread to the lymph
nodes on the opposite lung or in
the neck
Combination of
chemotherapy and
radiation
Stage IV
Tumor has spread beyond the chest Chemotherapy and/ or
palliative
(maintenance) care

32
Surgical treatment :
• Accurate pre-operative staging, coupled with improvements in surgical and
post-operative care, now offers 5-year survival rates of over 75% in stage I
disease (N0, tumour confined within visceral pleura) and 55% in stage II
disease, which includes resection in patients with ipsilateral peribronchial
or hilar node involvement.
Contraindications to surgical resection in bronchial carcinoma
• Distant metastasis (M1)
• Invasion of central mediastinal structures including heart, great vessels,
trachea and oesophagus (T4)
• Malignant pleural effusion (T4)
• Contralateral mediastinal nodes (N3)
• FEV
1
< 0.8 L
• Severe or unstable cardiac or other medical condition
Radiotherapy :
• While much less effective than surgery, radical radiotherapy can offer long-
term survival in selected patients with localised disease in whom
comorbidity precludes surgery.
• Continuous hyper-fractionated accelerated radiotherapy (CHART), in which
a similar total dose is given in smaller but more frequent fractions, may
offer better survival prospects than conventional schedules.
• The greatest value of radiotherapy, however, is in the palliation of
distressing complications such as superior vena cava obstruction, recurrent
haemoptysis, and pain caused by chest wall invasion or by skeletal
metastatic deposits

33
• . Obstruction of the trachea and main bronchi can also be relieved
temporarily.
• can be used in conjunction with chemotherapy in the treatment of small-
cell carcinoma, and is particularly efficient at preventing the development
of brain metastases in patients who have had a complete response to
chemotherapy
Chemotherapy
• The treatment of small-cell carcinoma with combinations of cytotoxic
drugs, sometimes in combination with radiotherapy, can increase the
median survival from 3 months to well over a year.
• Combination chemotherapy leads to better outcomes than single-agent
treatment.
• In particular, oral etoposide leads to more toxicity and worse survival than
standard combination chemotherapy.
• Regular cycles of therapy, including combinations of i.v. cyclophosphamide,
doxorubicin and vincristine or i.v. cisplatin and etoposide, are commonly
used.
• Nausea and vomiting are common side-effects and are best treated with 5-
HT
3
receptor antagonists
• The use of combinations of chemotherapeutic drugs requires considerable
skill and should be overseen by teams of expert clinicians and nurses.
• In general, chemotherapy is less effective in non-small-cell bronchial
cancers.
• However, studies in such patients using platinum-based chemotherapy
regimens have shown a 30% response rate associated with a small increase
in survival.

34
Neoadjuvant and adjuvant chemotherapy:
• In non-small-cell carcinoma, there is some evidence that chemotherapy
given before surgery may increase survival and can effectively 'down-stage'
disease with limited nodal spread.
• Post-operative chemotherapy is now proven to improve survival rates
when operative samples show nodal involvement by tumour.
Laser therapy and stenting :
• Palliation of symptoms caused by major airway obstruction can be achieved
in selected patients using bronchoscopic laser treatment to clear tumour
tissue and allow re-aeration of collapsed lung.
• The best results are achieved in tumours of the main bronchi.
Endobronchial stents can be used to maintain airway patency in the face of
extrinsic compression by malignant nodes
General aspects of management :
• The best outcomes are obtained when lung cancer is managed in specialist
centres by multidisciplinary teams including oncologists, thoracic surgeons,
respiratory physicians and specialist nurses; effective communication, pain
relief and attention to diet are important.
• Lung tumours can cause clinically significant depression and anxiety, and
these may need specific therapy. The management of non-metastatic
endocrine manifestations is described in
. When a malignant
pleural effusion is present, an attempt should be made to drain the pleural
cavity using an intercostal drain; provided the lung fully re-expands,
pleurodesis with a sclerosing agent such as talc should be performed

35
Prognosis:
• The overall prognosis in bronchial carcinoma is very poor, with around 70%
of patients dying within a year of diagnosis and only 6-8% of patients
surviving 5 years after diagnosis.
• The best prognosis is with well-differentiated squamous cell tumours that
have not metastasised and are amenable to surgical resection.
Secondary tumours of the lung:
• Blood-borne metastatic deposits in the lungs may be derived from many
primary tumours, in particular the breast, kidney, uterus, ovary, testes and
thyroid.
• The secondary deposits are usually multiple and bilateral. Often there are
no respiratory symptoms and the diagnosis is made on radiological
examination.
• Breathlessness may occur if a considerable amount of lung tissue has been
replaced by metastatic tumour. Endobronchial deposits are uncommon but
can cause haemoptysis and lobar collapse
36
Rare types of lung tumour
Tumour
Status
Histology
Typical presentation
Prognosis
Adenosquamous carcinoma
Malignant
Tumours with areas of
unequivocal squamous and
adeno-differentiation
Peripheral or central lung mass
Stage-dependent
Carcinoid tumour
Low-grade malignant
Neuroendocrine differentiation
Bronchial obstruction, cough
95% 5-year survival with
resection
Bronchial gland adenoma
Benign
Salivary gland differentiation
Tracheobronchial
irritation/obstruction
Local resection curative
Bronchial gland carcinoma
Low-grade malignant
Salivary gland differentiation
Tracheobronchial
irritation/obstruction
Local recurrence occurs
Hamartoma
Benign
Mesenchymal cells, cartilage
Peripheral lung nodule
Local resection curative
Bronchoalveolar carcinoma
Malignant
Tumour cells line alveolar
spaces
Alveolar shadowing, productive
cough
Variable, worse if multifocal
