
AMINOGLYCOSIDES

Among the many antibiotics isolated from that
actinomycetes genus Streptomyces, several are
compounds closely related in structure to
streptomycin
All aminoglycoside antibiotics are absorbed very
poorly (less than 1% under normal circumstances)
following oral administration, and some of them
(kanamycin, neomycin, and paromomycin) are
administered by that route for the treatment of GI
infections

Because of their potent broadspectrum antimicrobial
activity, they are also used for the treatment of
systemic infections.
Their undesirable side effects, particularly
ototoxicity and nephrotoxicity, have restricted their
systemic use to serious infections or infections
caused by bacterial strains resistant to other agents

Chemistry
Aminoglycosides are so named because their
structures consist of amino sugars linked
glycosidically
All have at least one aminohexose, and some have a
pentose lacking an amino group (e.g., streptomycin,
neomycin, and paromomycin).
Additionally, each of the clinically useful
aminoglycosides contains a highly substituted 1,3-
diaminocyclohexane central ring; in kanamycin,
neomycin, gentamicin, and tobramycin

Microbial Resistance against
Aminoglycosides
Resistant strains have emerged against
streptomycin, kanamycin and gentamycin in
clinic.
R factor is resposible for the production of
aminoglycoside deactivating enzymes:
1)
Acetyl transferases (AAC)
2)
Phosphotransferases (APH),
3)
Nucleotidyl transferases (ANT)
These enzymes transfer to hydroxyl and
amino groups of the drug.
5
REVOLUTIOPHARMD.COM

They distribute well into most body fluids but not
into the central nervous system, bone, or fatty or
connective tissues.
They tend to concentrate in the kidneys and are
excreted by glomerular filtration.
Aminoglycosides are apparently not metabolized in
vivo

Streptomycin is the most effective of the group for the
chemotherapy of TB, brucellosis, tularemia, and Yersinia
infections.
Paromomycin is used primarily in the chemotherapy of
amebic dysentery
Under certain circumstances, aminoglycoside and B-
lactam antibiotics exert a synergistic action in vivo
against some bacterial strains when the two are
administered jointly
Damage to the cell wall caused by the B -lactam
antibiotic is believed to increase penetration of the
aminoglycoside into the bacterial cell.
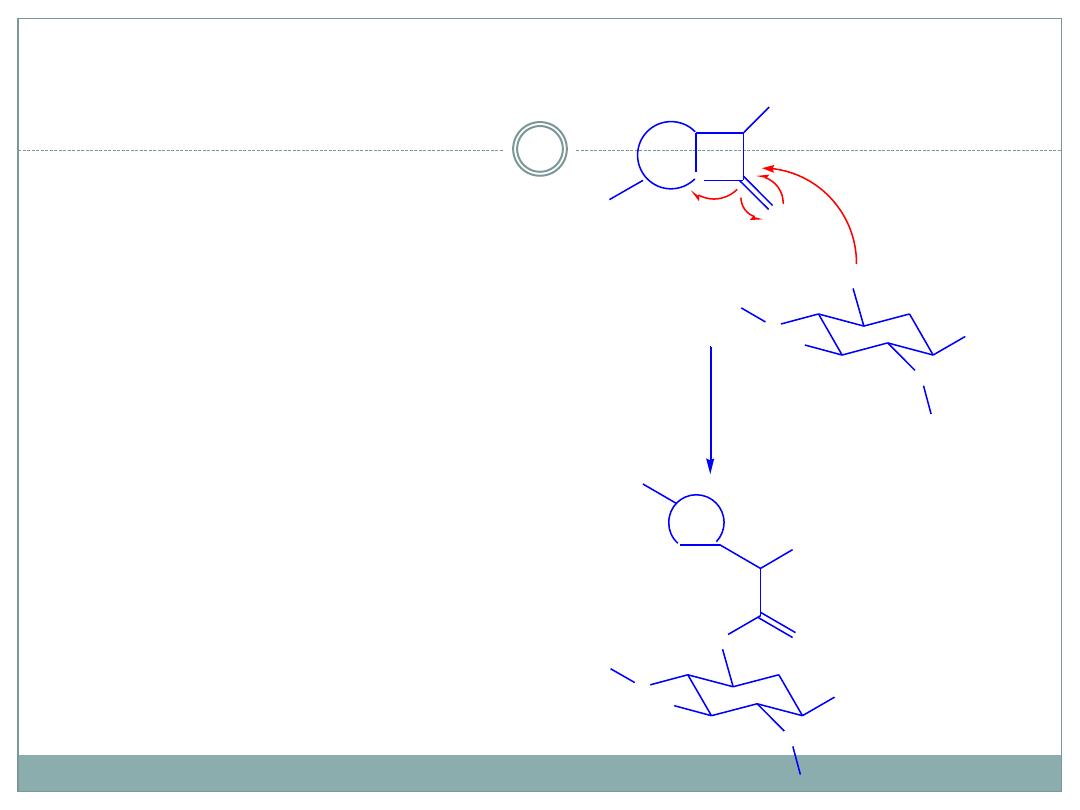
Mechanism of Chemical incompatility of
Aminoglycosides with β-lactams
Acylation of
aminocyclitol
portion by the β-
lactam molecule.
Begins with
nucleophilic addition
of the amino group
to the carbonyl
group of β-lactam
ring.
N
O
NHCOR
HOOC
O
NHCOR
HN
HO
O
O
SUGAR
NH
2
N
SUGAR
HOOC
H
2
N
HO
O
O
SUGAR
NH
2
SUGAR

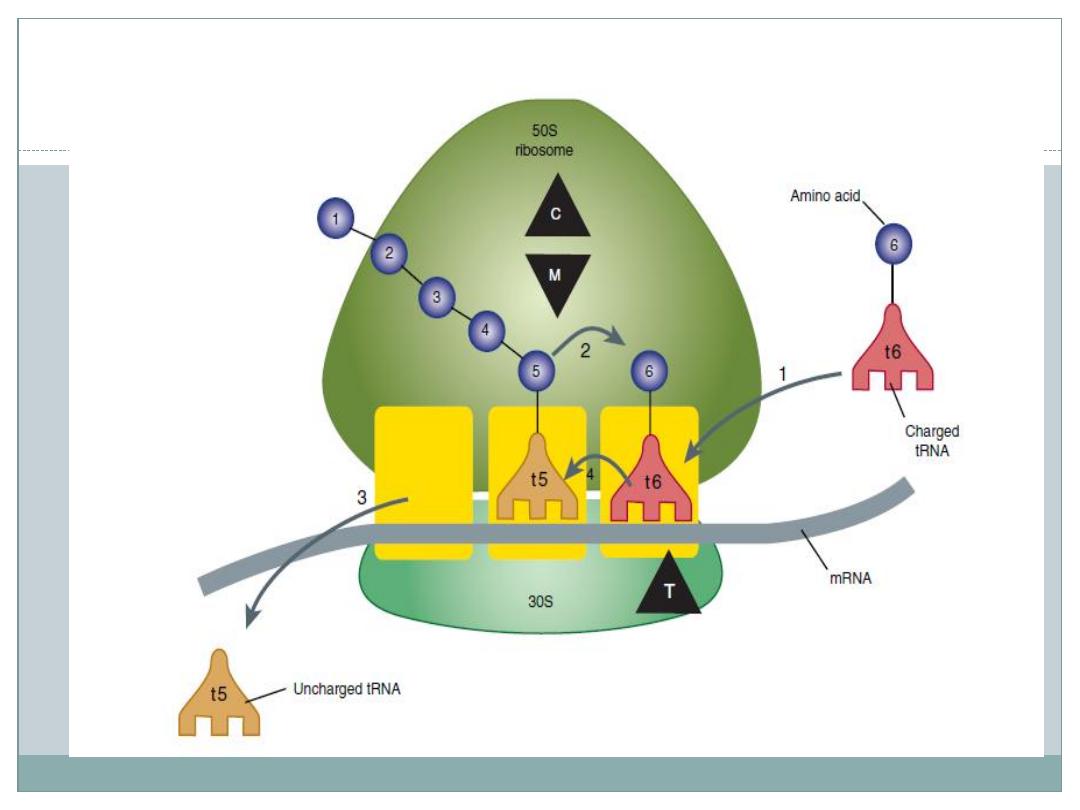
The aminoglycosides act directly on the bacterial
ribosome to inhibit the initiation of protein synthesis
and to interfere with the fidelity of translation of the
genetic message.
They bind to the 30S ribosomal subunit to form a
complex that cannot initiate proper amino acid
polymerization
All of the commercially available aminoglycoside
antibiotics are bactericidal, except spectinomycin.
The mechanism for the bactericidal action of the
aminoglycosides is not known.

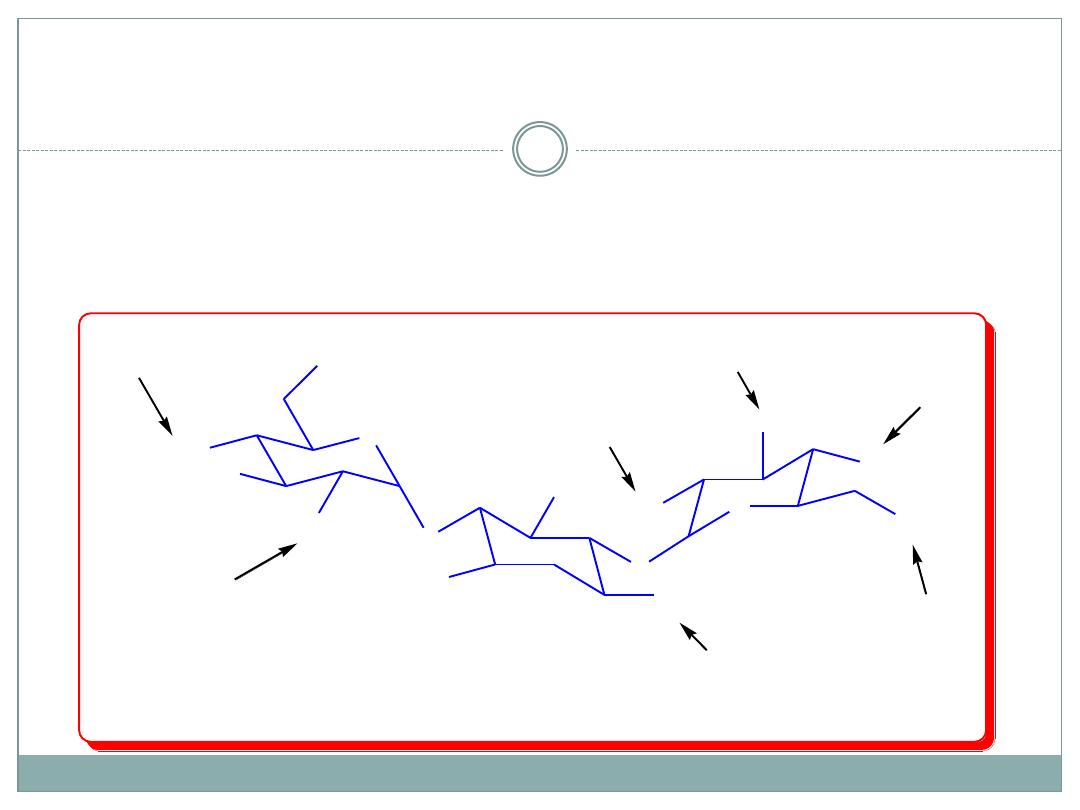
Aminoglycoside-inactivating enzymes include
(a) aminoacetyltransferases (designated AAC), which
acetylate the 6-NH2 of ring I, the 3-NH2 of ring II,
or the 2-NH2 of ring I;
(b) phosphotransferases (designated APH), which
phosphorylate the 3-OH of ring I or the 2-OH of ring
III; and
(c) nucleotidyltransferases (ANT), which adenylate
the 2-OH of ring III, the 4-OH of ring I, or the 4-OH
of ring III.
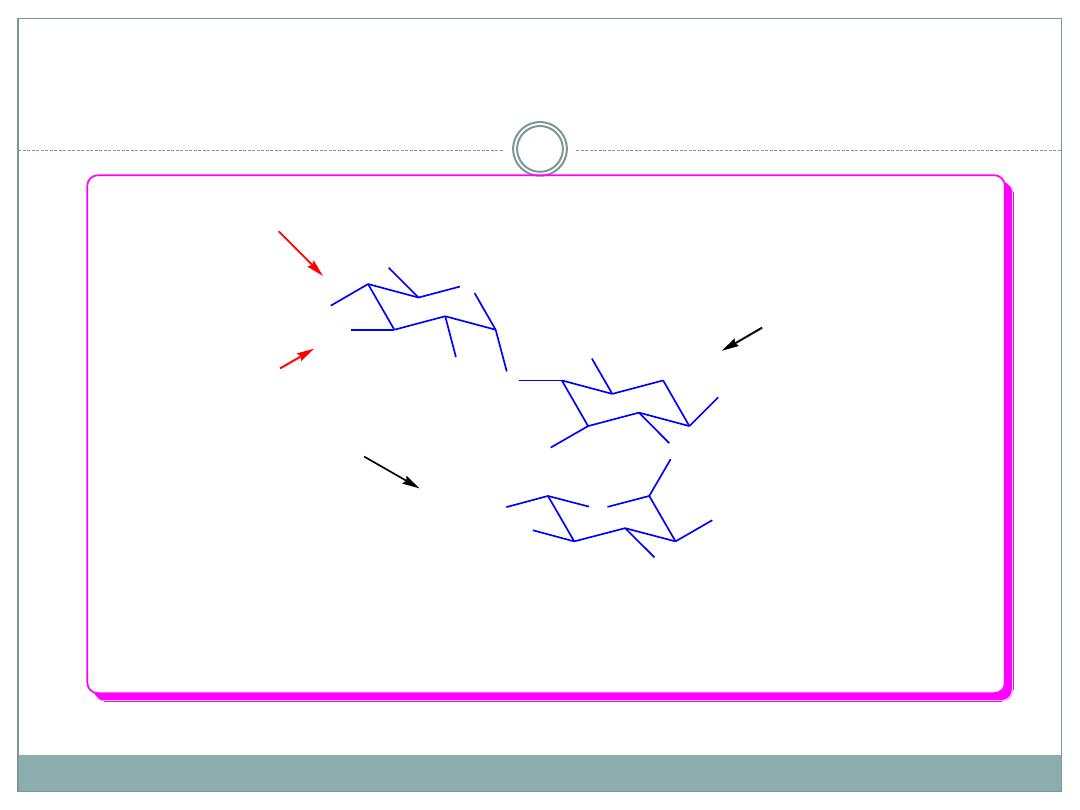
Aminoglycoside Deactivating Enzymes
AAC acetylates 3-NH
2
of the ring II, and 2`, 6`- NH
2
of the ring I.
APH phosphorylates 3`-OH
of the ring I and 2``-OH of the ring III.
ANT adenylates 2``,4``-OH of the ring III and 4`-OH of the ring I.
O
O
O
O
OH
NH
2
OH
H
2
N
OH
NH
2
H
2
N
HO
H
2
N
HO
OH
1
2
3
4
5
6
1
'
2
'
4'
1''
3'
2''
5'
3''
5''
4''
6''
6'
I
II
III
ANT-4
'
ANT-4
''
ANT-2
''
, APH-2
''
AAC- 6
'
AAC- 2
'
APH- 3
'
AAC- 3
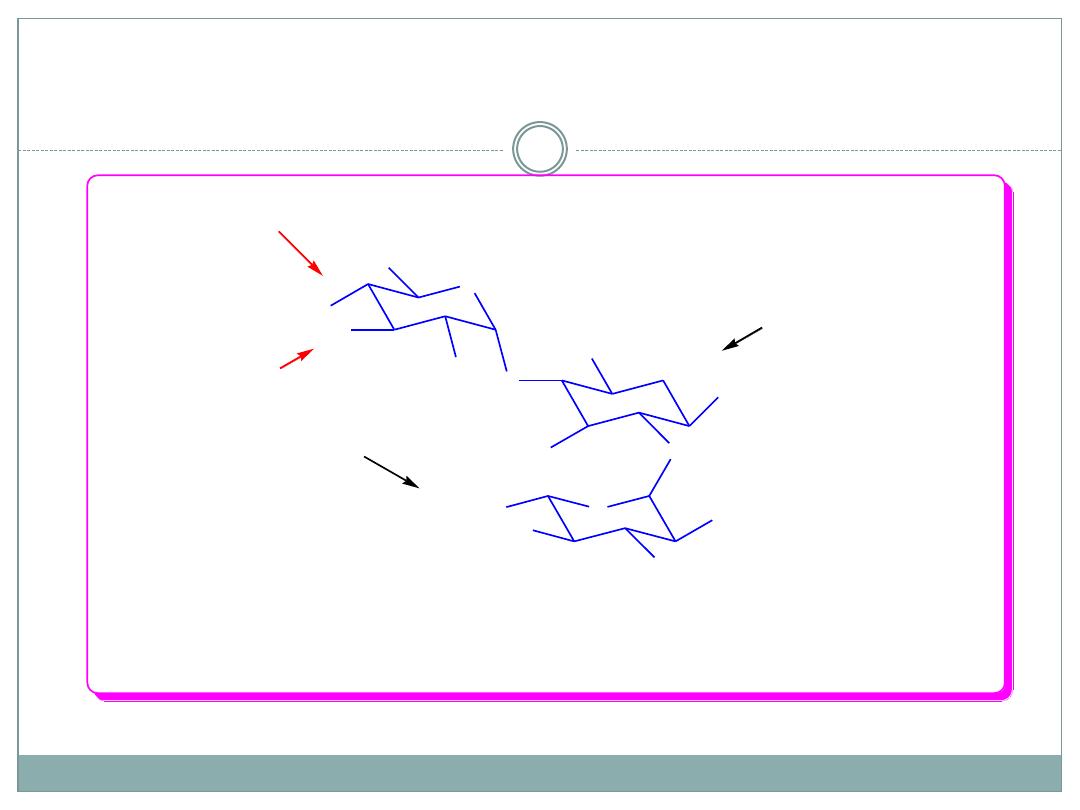
Kanamycin B
12
Kanamycin and Deactivatig Enzymes
O
O
O
O
R
1
H
2
C
HO
HO
R
2
H
2
N
OH
NH
2
HOH
2
C
HO
NH
2
HO
1''
2''
3''
4''
5''
6''
1
'
2
'
3'
4'
6'
5'
1
2
3
4
5
6
Kanosamine
2-Deoxystreptamine
Kanamycin A: R
1
= NH
2
; R
2
= OH
Kanamycin B: R
1
= NH
2
; R
2
= NH
2
Kanamycin C: R
1
= OH; R
2
= NH
2
III
II
I
3`-deoxy derivative
APH Resistant
4`-deoxy derivative
ANT Resistant
13
REVOLUTIOPHARMD.COM
Kanamycin and Deactivatig Enzymes
O
O
O
O
R
1
H
2
C
HO
HO
R
2
H
2
N
OH
NH
2
HOH
2
C
HO
NH
2
HO
1''
2''
3''
4''
5''
6''
1
'
2
'
3'
4'
6'
5'
1
2
3
4
5
6
Kanosamine
2-Deoxystreptamine
Kanamycin A: R
1
= NH
2
; R
2
= OH
Kanamycin B: R
1
= NH
2
; R
2
= NH
2
Kanamycin C: R
1
= OH; R
2
= NH
2
III
II
I
3`-deoxy derivative
APH Resistant
4`-deoxy derivative
ANT Resistant
14
REVOLUTIOPHARMD.COM
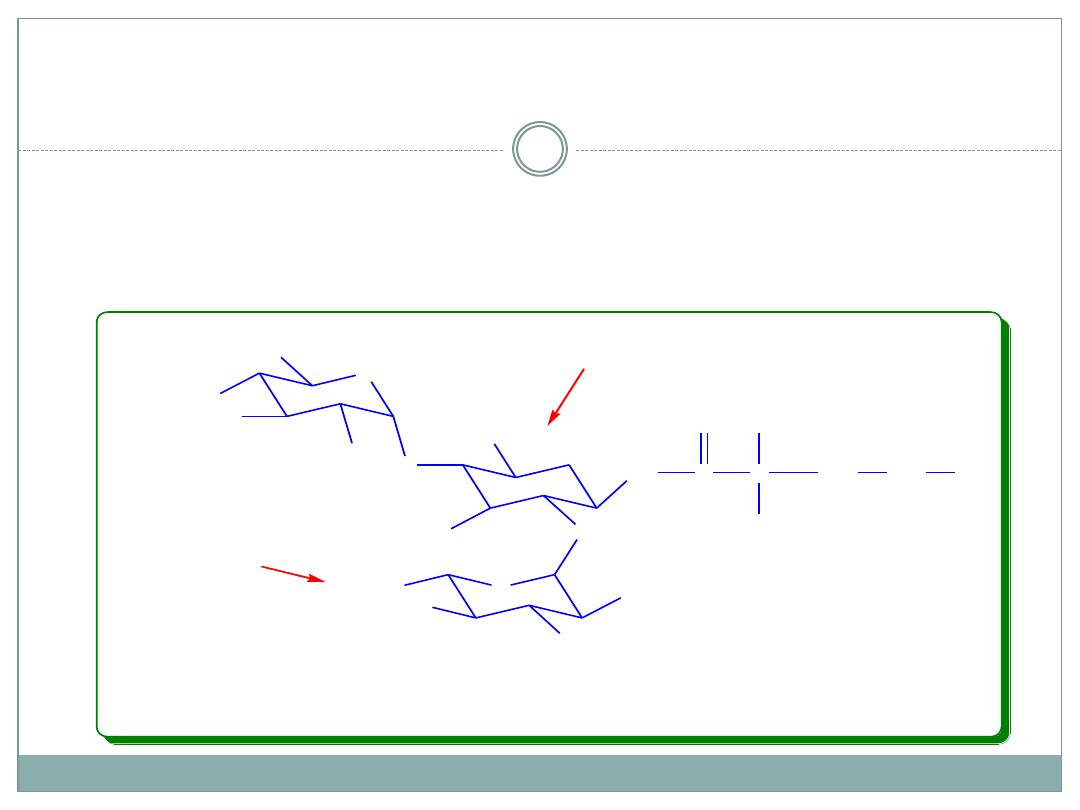
Amikacin and Dactivating Enzymes
1-N-L-(-)-amino-α-hydroxybutyric acid derivative of
kanamycin A. Susceptible only against the action of
AAC 6`-amino and ANT 4`-OH, resistant against all
other deactivating enzymes.
O
O
O
O
H
2
NH
2
C
HO
HO
OH
H
2
N
OH
NH
2
HOH
2
C
HO
NH
HO
1''
2''
3''
4''
5''
6''
1
'
2
'
3'
4'
6'
5'
1
2
3
4
5
6
Amikacin, L-AHBA derivative of
Kanamycin A
C
C
O
CH
2
CH
2
NH
2
OH
H
2
2-Deoxystreptamine
Kanosamine
15

The most significant breakthrough yet achieved in the
search for aminoglycosides resistant to bacterial enzymes
has been the development of amikacin, the 1-N-L-(-)-
amino-alfa-hydroxybutyric acid (L-AHBA) derivative of
kanamycin A.
This remarkable compound retains most of the intrinsic
potency of kanamycin A and is resistant to virtually all
aminoglycoside-inactivating enzymes known, except the
aminoacetyltransferase that acetylates the 6-amino
group and the nucleotidyltransferase that adenylylates
the 4- hydroxyl group of ring I.
one study prove that amikacin was effective against
91% of the isolates that resistance to other
aminoglycosides

The Minor Mechanism for
Microbial Resistance
Decreased uptake of the drug in some strains of
p. aeroginosa in hospital infections because of
blockade in the active transport of aminoglycosides.
Aminoglycoside molecules attach through their
cationic groups to anionic portions of membrane
phospholipids of bacteria. Upon this attachment
the the ATP-dependent uptake occurs.
Bivalent cations such as Ca
2+
and Mg
2+
compete
with the drug in this process and antagonise them.
Anaerobic bacteria lack the ATP-dependent uptake
process, so they are resistant to aminoglycosides.
18
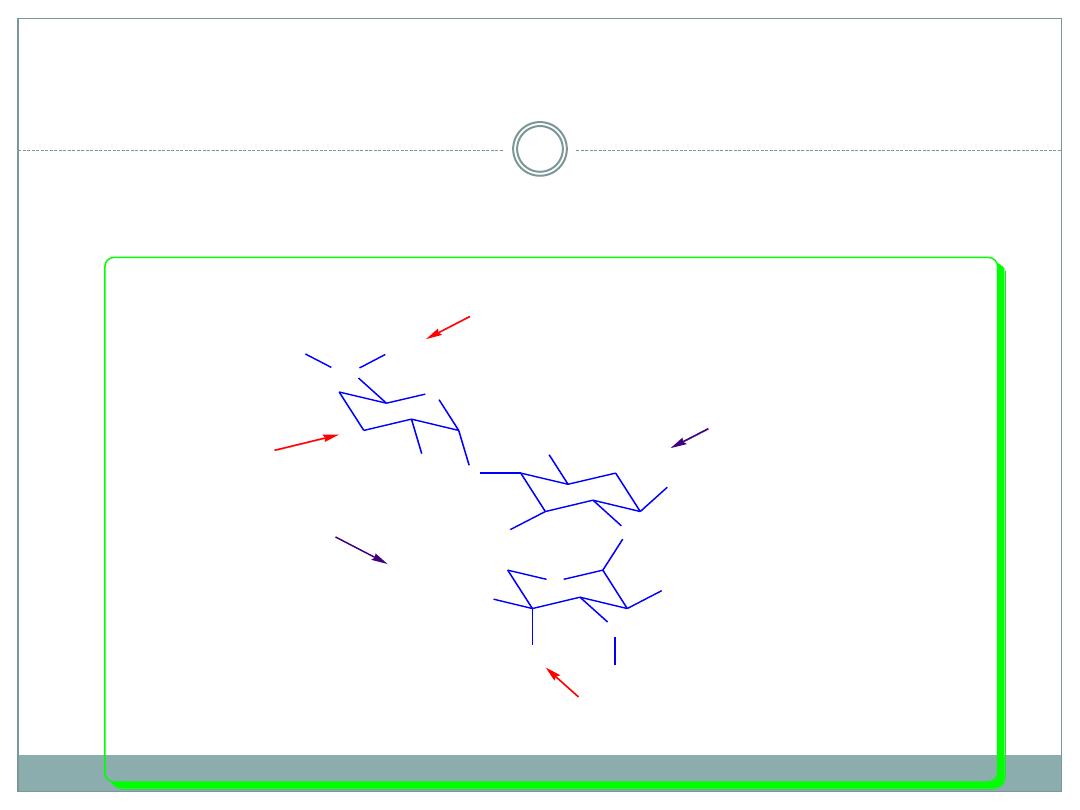
SAR of ring I continued
Methylation of C-6` or 6`- NH
2
doesn’t alter
the antibacterial activity, but increases the
resistance against AAC.
O
O
O
O
HC
NH
2
H
2
N
OH
NH
H
3
C
NH
2
HO
1''
2''
3''
4''
5''
1
'
2
'
3'
4'
6'
5'
1
2
3
4
5
6
Garosamine
2-Deoxystreptamine
Gentamicin C
1
: R
1
=R
2
= CH
3
Gentamicin C
2
: R
1
= CH
3
; R
2
= H
Gentamicin C
1a
: R
1
=R
2
= H
OH
CH
3
NHR
2
R
1
Lack 3`-OH
APH Resistant
Axial and tertiary 4``-OH instead of
equatorial secondary 4``-OH in Kanamycin
ANT Resistant
Secondary amino group at 6`-NH
2
in
Gentamycin C1, spacial hynderance
AAC Resisistant
I
II
III
19
REVOLUTIOPHARMD.COM
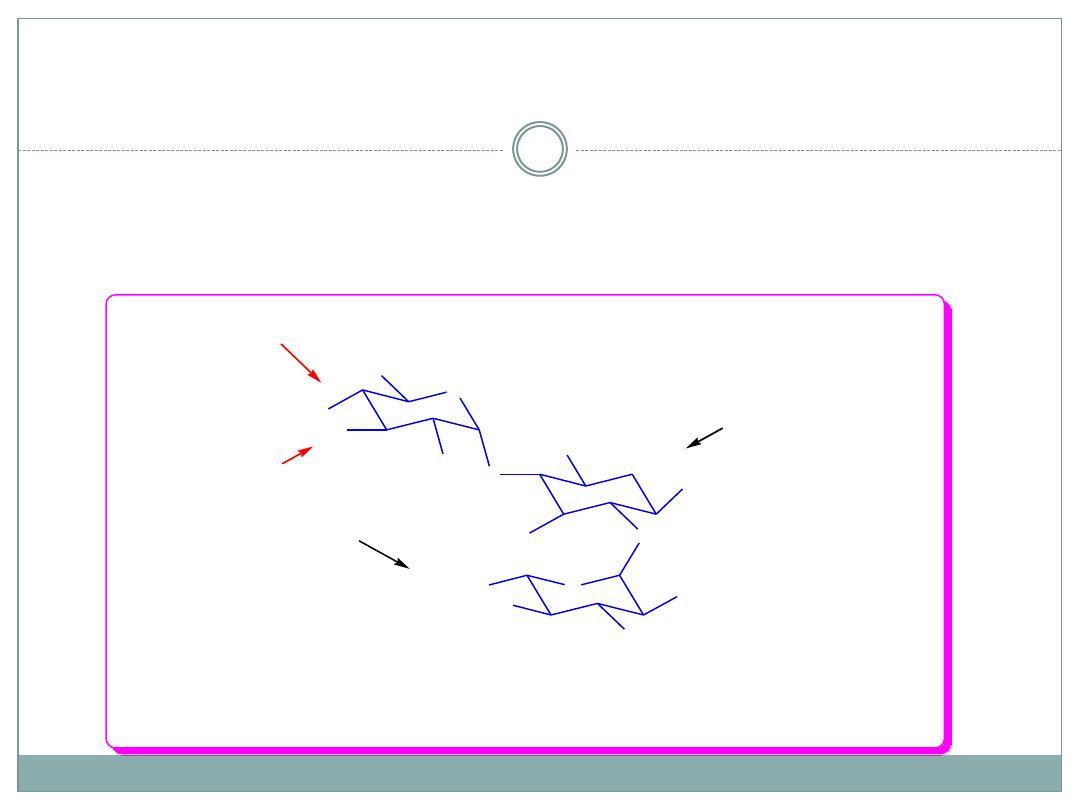
SAR of ring I continued
Omitting the 3`-OH and/or 4`-OH in kanamycin
doesn’t decrease the antibacterial activity but increases
the resistance against AAC: 3`,4`-dideoxykanamycin
B: Dibekacin.
The same is true for gentamicin.
O
O
O
O
R
1
H
2
C
HO
HO
R
2
H
2
N
OH
NH
2
HOH
2
C
HO
NH
2
HO
1''
2''
3''
4''
5''
6''
1
'
2
'
3'
4'
6'
5'
1
2
3
4
5
6
Kanosamine
2-Deoxystreptamine
Kanamycin A: R
1
= NH
2
; R
2
= OH
Kanamycin B: R
1
= NH
2
; R
2
= NH
2
Kanamycin C: R
1
= OH; R
2
= NH
2
III
II
I
3`-deoxy derivative
APH Resistant
4`-deoxy derivative
ANT Resistant
20
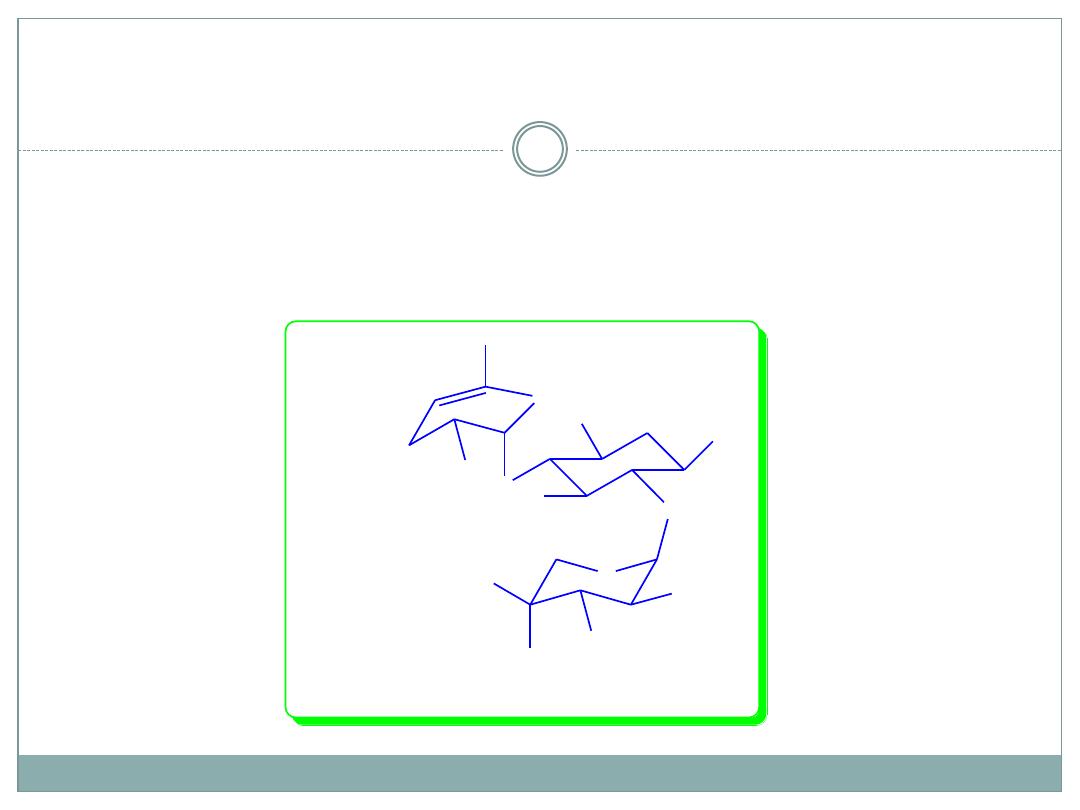
SAR of ring I continued
O
OH
H
3
C
NHCH
3
OH
O
NHR
HO
O
O
NH
2
CH
2
OH
H
2
N
1
6
2
4
3
5
1
'
2
'
3'
4'
1
''
2
''
3''
5''
4''
5'
Sisomicin: R=H
Netilmicin: R=C
2
H
5
Omitting the 3`-OH and 4`-OH and the
addition of a double bond between C-4` and
C-5`has the same effect.
21
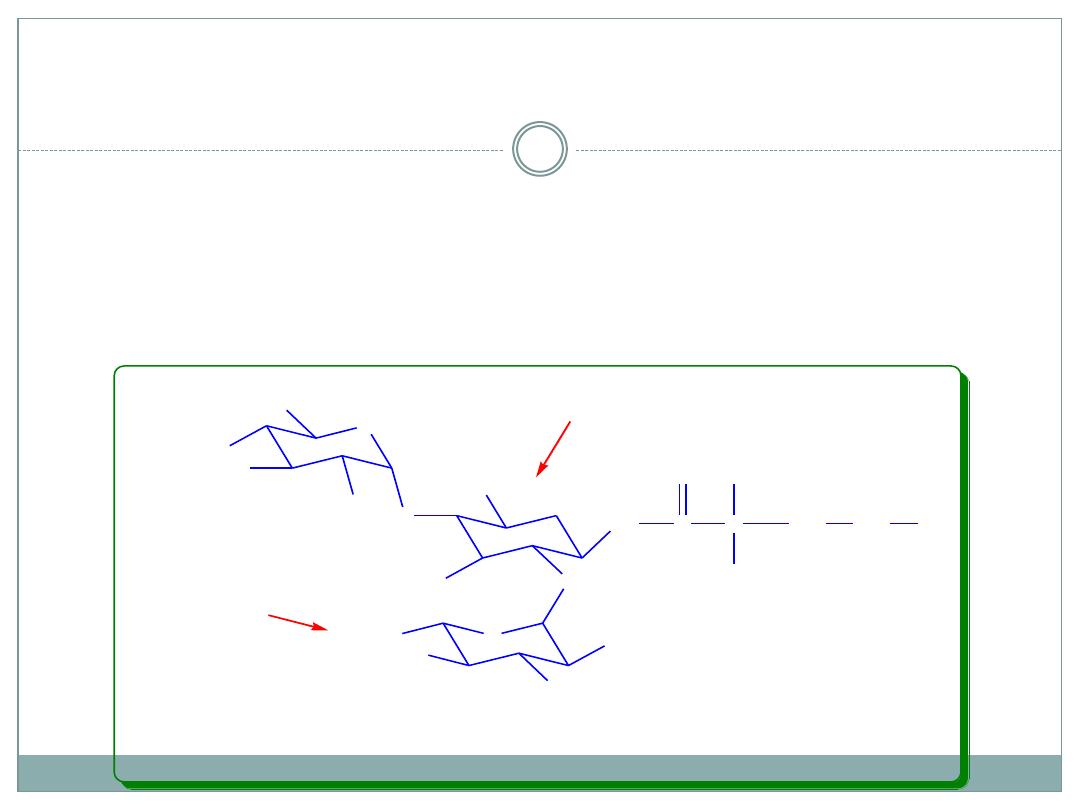
SAR of Aminoglycosides continued
O
O
O
O
H
2
NH
2
C
HO
HO
OH
H
2
N
OH
NH
2
HOH
2
C
HO
NH
HO
1''
2''
3''
4''
5''
6''
1
'
2
'
3'
4'
6'
5'
1
2
3
4
5
6
Amikacin, L-AHBA derivative of
Kanamycin A
C
C
O
CH
2
CH
2
NH
2
OH
H
2
2-Deoxystreptamine
Kanosamine
Ring II
is flexible toward changes. 1-NH
2
in
kanamycin can be acylated and the antibacterial
activity remains almost unchanged , but resistance
against deactivating
enzymes
increases: Amikacin
22
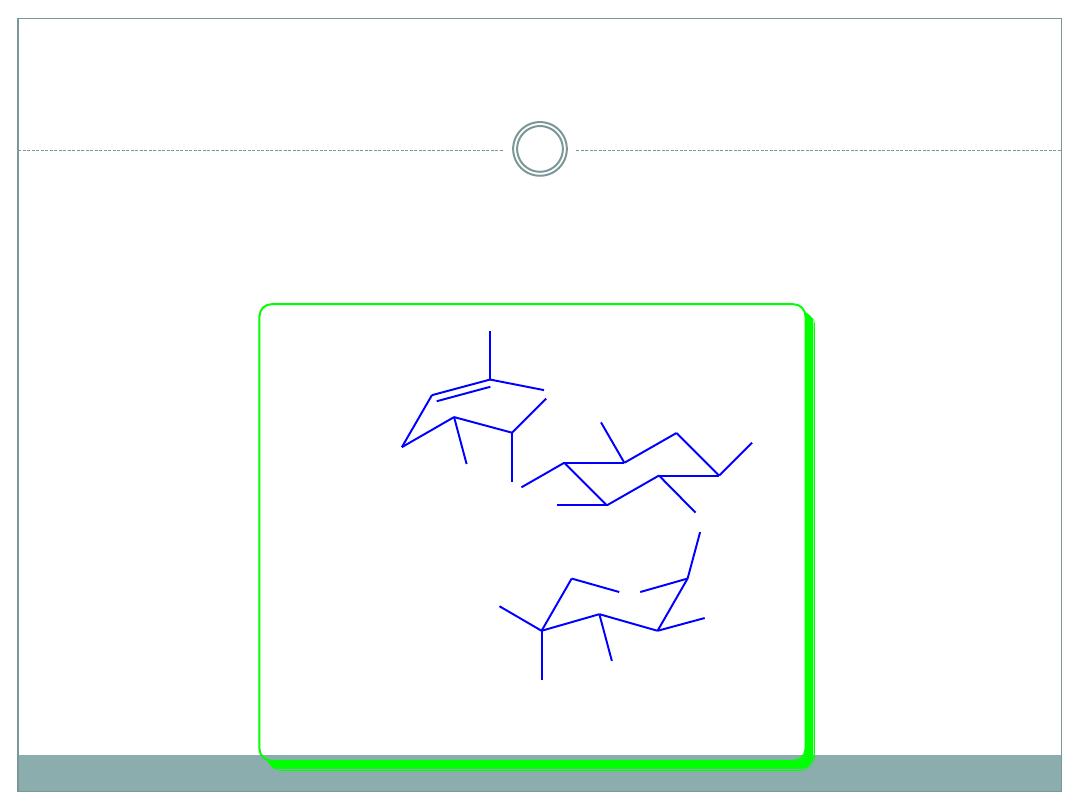
SAR of ring II continued
1-NH
2
ethylation of sisomycin saves the
antibacterial activity and increases the
enzymatic resistance: Netilmycin
O
OH
H
3
C
NHCH
3
OH
O
NHR
HO
O
O
NH
2
CH
2
OH
H
2
N
1
6
2
4
3
5
1
'
2
'
3'
4'
1
''
2
''
3''
5''
4''
5'
Sisomicin: R=H
Netilmicin: R=C
2
H
5
23
REVOLUTIOPHARMD.COM
SAR of Aminoglycosides continued
O
O
O
O
HC
NH
2
H
2
N
OH
NH
H
3
C
NH
2
HO
1''
2''
3''
4''
5''
1
'
2
'
3'
4'
6'
5'
1
2
3
4
5
6
Garosamine
2-Deoxystreptamine
Gentamicin C
1
: R
1
=R
2
= CH
3
Gentamicin C
2
: R
1
= CH
3
; R
2
= H
Gentamicin C
1a
: R
1
=R
2
= H
OH
CH
3
NHR
2
R
1
I
II
III
Ring III
functional groups are less sensitive to modifications:
2``-deoxy gentamicins are less active than 2``-OH ones, but 2``-
NH
2
derivative (seldomycin) are very active.
3``- NH
2
can be primary or secondary.
4``-OH can be axial or equatorial, the former is resistant against
the deactivating enzymes (ANT).

TETRACYCLINES
Among the most important broad-spectrum
antibiotics are members of the tetracycline family.
Nine such compounds—tetracycline, rolitetracycline,
oxytetracycline, chlortetracycline, demeclocycline,
meclocycline, methacycline,doxycycline, and
minocyclinehave been introduced into medical use
The tetracyclines are obtained by fermentation
procedures from Streptomyces spp.

The group name is derived from this tetracyclic
system.
The antibiotic spectra and chemical properties of
these compounds are very similar but not identical
The stereochemistry of the tetracyclines is very
complex.
Carbon atoms 4, 4a, 5, 5a, 6, and 12a are potentially
chiral, depending on substitution.

Oxytetracycline and doxycycline, each with a 5-
hydroxyl substituent, have six asymmetric centers;
the others, lacking chirality at C-5,have only five
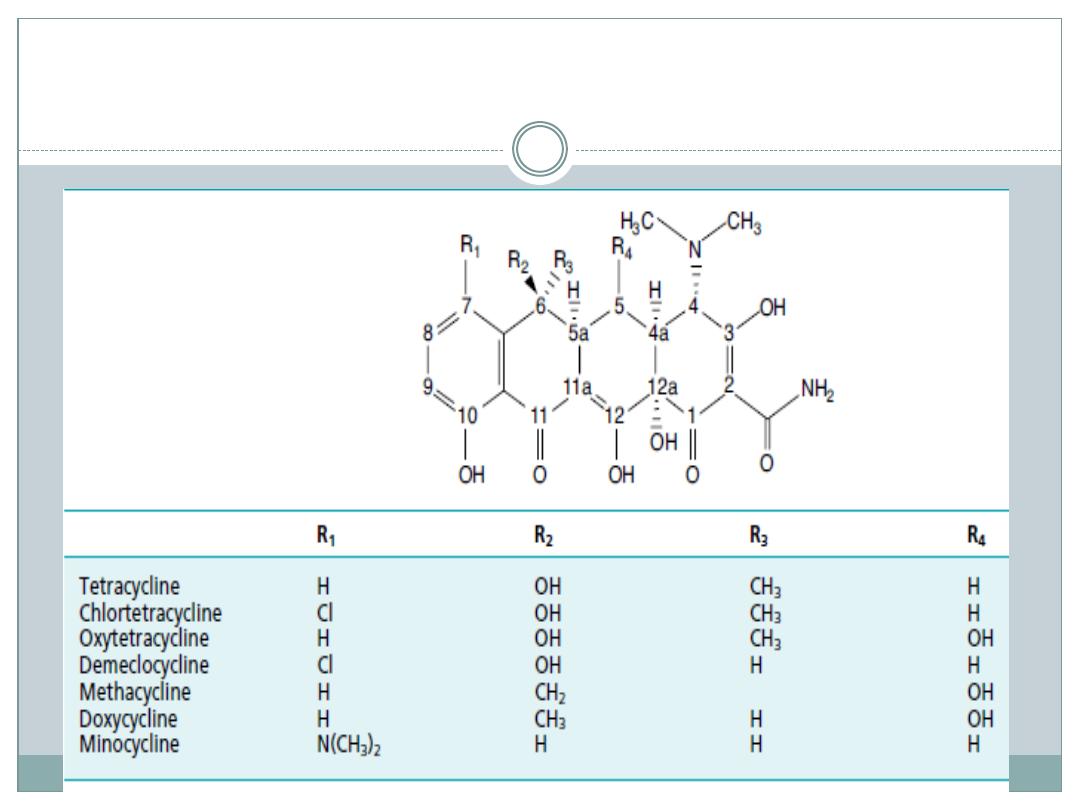
Structure of the Tetracyclines

The tetracyclines are amphoteric compounds,
forming salts with either acids or bases
In neutral solutions, these substances exist mainly as
zwitterions
The hydrochloride salts are used most commonly for
oral administration and usually are encapsulated
because they are bitter
Water-insoluble salts are formed with divalent and
polyvalent metals

An interesting property of the tetracyclines is their
ability to undergo epimerization at C-4 in solutions
of intermediate pH range
These isomers are called epitetracyclines
Under acidic conditions, an equilibrium is
established in about 1 day and consists of
approximately equal amounts of the isomers
The 4-epitetracyclinesexhibit much less activity than
the ―natural‖ isomers, thus accounting for the
decreased therapeutic value of aged solutions
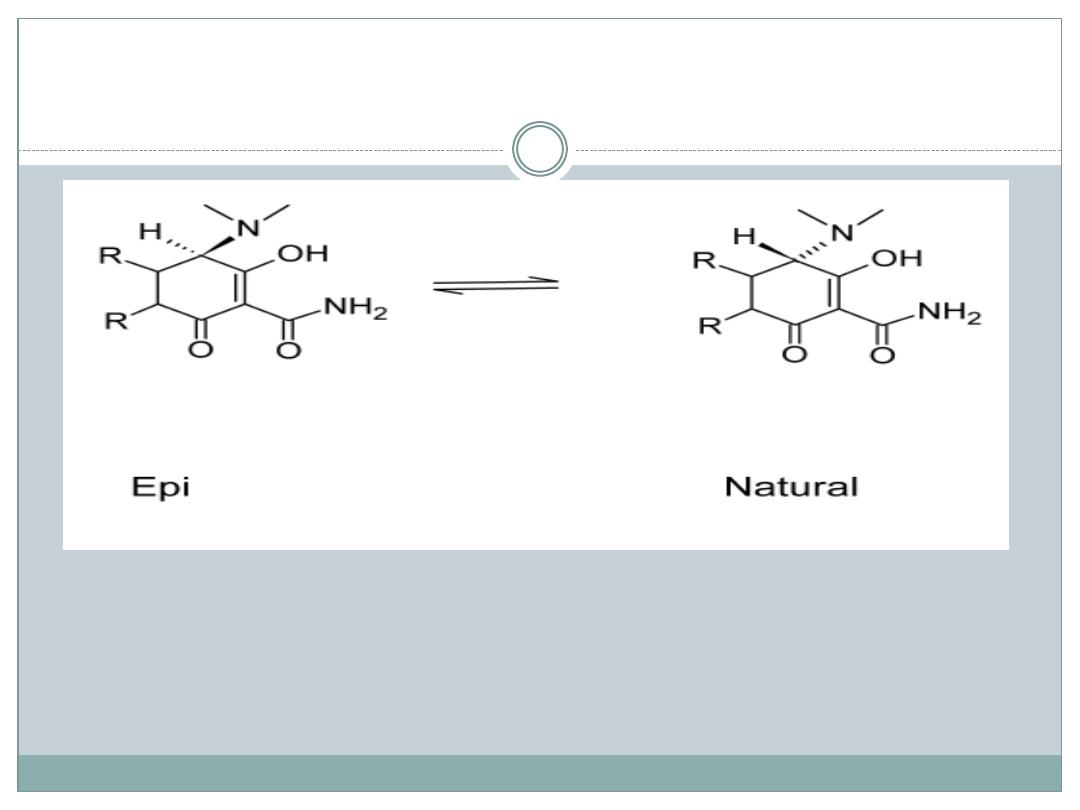

Strong acids and strong bases attack tetracyclines
with a hydroxyl group on C-6, causing a loss in
activity through modification of the C ring
Strong acids produce dehydration through a reaction
involving the 6-hydroxyl group and the 5a-hydrogen
The double bond thus formed between positions 5a
and 6 induces a shift in the position of the double
bond between C-11a and C-12 to a position between
C-11 and C-11a, forming the more energetically
favored resonant system of the naphthalene group
found in the inactive anhydrotetracyclines

Bases promote a reaction between the 6 -hydroxyl
group and the ketone group at the 11-
position,causing the bond between the 11 and 11a
atoms to cleave , forming the lactone ring found in
the inactive isotetracycline
These two unfavorable reactions stimulated research
that led to the development of the more stable and
longer acting compounds 6-deoxytetracycline,
methacycline, doxycycline, and minocycline

Mechanism of Action and Resistance
The strong binding properties of the tetracyclines with
metal ions
The strong binding properties of the tetracyclines with
metal ions caused to suggest that their antibacterial
properties may be because of an ability to remove
essential metal ions as chelated compounds.
Tetracyclines are bind to the 30S ribosomal subunit and,
thereby, prevent the binding of aminoacyl tRNA to the
mRNA–ribosome complex. Both the binding of
aminoacyl tRNA and the binding of tetracyclines at the
ribosomal binding site require magnesium ions

Tetracyclines enter bacterial cells by two processes:
passive diffusion and active transport
Three biochemically distinct mechanisms of
resistance to tetracyclines have been described in
bacteria: (a) efflux mediated by transmembrane-
spanning, active-transport proteins that reduces the
intracellular tetracycline concentration;
(b) ribosomal protection, in which the bacterial
protein synthesis apparatus is rendered resistant to
the action of tetracyclines by an inducible
cytoplasmic protein; and (c) enzymatic oxidation.

Structure–Activity Relationships
All derivatives containing fewer than four rings are inactive or
nearly inactive
The simplest tetracycline derivative that retains the characteristic
broad-spectrum activity associated with this antibiotic class is 6-
demethyl-6-deoxytetracycline
A-ring substituents can be modified only slightly without dramatic
loss of antibacterial potency.
The enolized tricarbonylmethane system at C-1 to C-3 must be
intact for good activity.
Replacement of the amide at C-2 with other functions (e.g.,
aldehyde or nitrile) reduces or abolishes activity.
Monoalkylation of the amide nitrogen reduces activity
proportionately to the size of the alkyl group.
Additional glycylaminosubstitution at9 position lead to new class of
antibiotics called glycyclines e.g Tigecycline
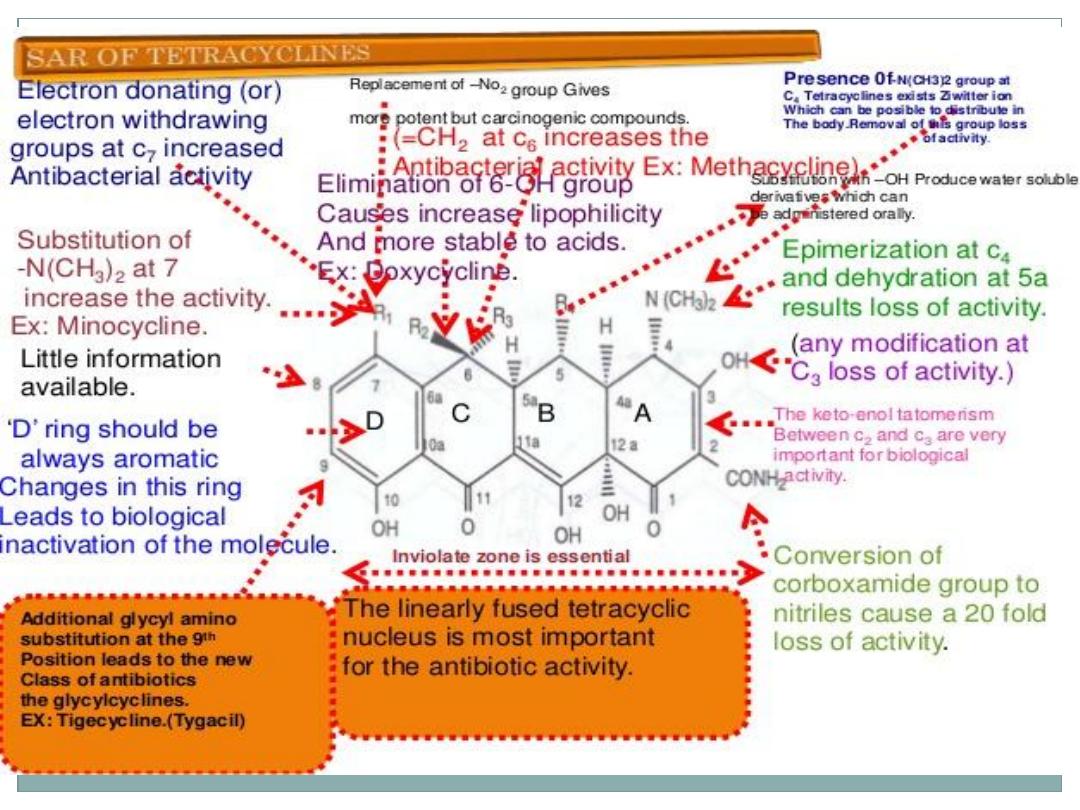

The dimethylamino group at the 4-position must
have the natural orientation: 4-epitetracyclines are
very much less active than the natural isomers
Removal of the 4-dimethylamino group reduces
activity even further.
Activity is largely retained in the primary and N-
methyl secondary amines but rapidly diminishes in
the higher alkylamines

Esters of the C-12a hydroxyl group are inactive, with
the exception of the formyl ester, which readily
hydrolyzes in aqueous solutions
The most fruitful site for semisynthetic modification
of the tetracyclines has been the 6-position.
Neither the 6-methyl nor the 6-hydroxyl group is
essential for antibacterial activity.
In fact, doxycycline and methacycline are more
active in vitro than their parent oxytetracycline
against most bacterial strains.

More successful from a clinical stand point, however
, is 6-demethyl-6-deoxy-7-
dimethylaminotetracycline (minocycline) because of
its activity against tetracycline resistant bacterial
strains
6-Deoxytetracyclines possess important chemical
and pharmacokinetic advantages over their 6-oxy
counter parts because its stable in acidic and basic
conditions

Polar substituents (i.e., hydroxyl groups) at C-5 and C-6
decrease lipid versus water solubility of the tetracyclines.
The 6-position is, however, considerably more sensitive
than the 5-position to this effect. Thus, doxycycline (6-
deoxy-5-oxytetracycline) has a much higher partition
coefficient mthan either tetracycline or oxytetracycline.
The tetracyclines can form stable chelate complexes with
metal ions such as calcium and magnesium, which retard
absorption from the GI tract
