
Prof.Dr. Hedef Dhafir El-Yassin 2012
1
Biochemistry of Hormones
A case oriented approach
1. Lecture 1: Introduction to the Biochemistry of hormones and their mechanism of actions.
Thursday
16/2
2. Lecture 2: Introduction to the Biochemistry of hormones and their mechanism of actions.
Sunday
19/2
3. Lecture 3: Biochemistry and disorders of hormones of the hypothalamus and pituitary gland
(hypothalamus- pituitary axis).
Thursday
23/2
4. Lecture 4: Biochemistry and disorders of hormones of the hypothalamus and pituitary gland
(hypothalamus- pituitary axis).
Sunday
26/2
5. Lecture 5: Biochemistry and disorders of hormones of the thyroid and parathyroid gland
Sunday
4/3
6. Lecture 6: Biochemistry and disorders of hormones of the pancreas
Thursday
8/3
7. Lecture 7: Biochemistry and disorders of hormones of the adrenal gland
Sunday
11/3
8. Lecture 8: Biochemistry and disorders of hormones of the adipose tissues, heart and kidney
Sunday
18/3
Aim and objective of the above eight lectures is to understand:
1. The functions of hormones and the mechanisms involved in the regulation of their
secretion.
2. The types of receptor-hormone interactions and the specific effect that each type can
be produced in the cell.
3. the hypothalamus- pituitary axis.
4. the biochemistry and disorders of hormones secretion from different glands.
5. and to learn the major causes of endocrine disorders by discussing clinical cases.
References:
1. "Biochemistry" by Lubert Stryer
(textbook)
2. "Textbook of Biochemistry with Clinical Correlations" by T.M.Devlin
(additional reading)
3. "Lippincott's Illustrated Reviews in Biochemistry" by P.C.Champe, R.A.Harvey and
D.R.Ferrier
(additional reading)
4. "Harper's Biochemistry" by R.K.Murray, D.K.Granner, P.A. Mayes and V.W.Rodwell.
(additional reading)
5. "Clinical Laboratory Science Review" By Robert R. Harr
(additional reading)
Prof.Dr. Hedef Dhafir El-Yassin 2012
2
INTRODUCTION
TO THE
BIOCHEMISTRY
OF HORMONES
AND THEIR
MECHANISM
OF ACTIONS
2012
Prof. Dr. Hedef Dhafir El-Yassin

Prof.Dr. Hedef Dhafir El-Yassin 2012
3
Introduction to the Biochemistry of
Hormones and their Mechanism of Actions
Lecture 1
Thursday 16/2/2012
Lecture 2
Sunday 19/2/2012
The endocrine system is one of the two coordinating and integrating systems of the
body. It acts through chemical messengers - hormones –carried in the circulation.
Two systems control all physiologic processes:
•
The nervous system
exerts point-to-point control through nerves, similar to
sending messages by conventional telephone. Nervous control is electrical in
nature and fast.
•
The endocrine system
broadcasts its hormonal messages to essentially all cells
by secretion into blood and extracellular fluid. Like a radio broadcast, it
requires a receiver to get the message - in the case of endocrine messages, cells
must bear a receptor for the hormone being broadcast in order to respond.
Endocrinology is the study of hormones, their receptors and the intracellular
signaling pathways they invoke. Distinct endocrine organs are scattered throughout
the body. These are organs that are largely or at least famously devoted to secretion
of hormones. In addition to the classical endocrine organs, many other cells in the
body secrete hormones. Myocytes in the atria of the heart and scattered epithelial
cells in the stomach and small intestine are examples of what is sometimes called the
"diffuse" endocrine system. If the term hormone is defined broadly to include all
secreted chemical messengers, then virtually all cells can be considered part of the
endocrine system.
•
All pathophysiologic events are influenced by the endocrine milieu: There are
no cell types, organs or processes that are not influenced - often profoundly -
by hormone signaling.
•
All "large" physiologic effects a re mediated by multiple hormones acting in
concert: Normal growth from birth to adulthood, for example, is surely
dependent on growth hormone, but thyroid hormones, insulin-like growth
Prof.Dr. Hedef Dhafir El-Yassin 2012
4
factor-1, glucocorticoids and several other hormones are also critically
involved in this process.
•
There are many hormones known and little doubt that others remain to be
discovered.
Hormones, Receptors and Target Cells
1. Classes of Hormones
Knowing the basic structure of a hormone gives a considerable knowledge
about its receptor and mechanism of action.
Like all molecules, hormones are synthesized, exist in a biologically active
state for a time, and then degrade or are destroyed. Having an appreciation
for the "half-life" and mode of elimination of a hormone aids in understanding
its role in physiology and is critical when using hormones as drugs.
Most commonly, hormones are categorized into four structural groups,
with members of each group having many properties in common:
•
Peptides and proteins
•
Amino acid derivatives
•
Steroids
•
Fatty acid derivatives - Eicosanoids
1.
Peptides and Proteins
Peptide and protein hormones are products of translation. They vary
considerably in size and post-translational modifications, ranging from
peptides as short as three amino acids to large, multisubunit glycoproteins.
Peptide hormones are synthesized in endoplasmic reticulum, transferred to
the Golgi and packaged into secretory vesicles for export.

Prof.Dr. Hedef Dhafir El-Yassin 2012
5
They can be secreted by one of two pathways:
•
Regulated secretion: The cell stores hormone in secretory granules
and releases them in "bursts" when stimulated. This is the most
commonly used pathway and allows cells to secrete a large amount of
hormone over a short period of time.
•
Constitutive secretion: The cell does not store hormone, but secretes
it from secretory vesicles as it is synthesized.
Most peptide hormones circulate unbound to other proteins, but exceptions exist; for
example, insulin-like growth factor-1 binds to one of several binding proteins. In general,
the half-life of circulating peptide hormones is only a few minutes.
Several important peptide hormones are secreted from the pituitary gland.
The anterior pituitary secretes:
•
Luteinizing hormone and follicle stimulating hormone, which act on the
gonads.
•
prolactin, which acts on the mammary gland,
•
adrenocortiotrpic hormone (ACTH), which acts on the adrenal cortex to
regulate the secretion of glucocorticoids, and
•
growth hormone, which acts on bone, muscle and liver.
The posterior pituitary gland secretes:
•
antidiuretic hormone, also called vasopressin, and
•
oxytocin.
Peptide hormones are produced by many different organs and tissues,
however, including:
•
the heart (atrial-natriuretic peptide (ANP) or atrial natriuretic factor
(ANF))
•
pancreas (insulin and somatostatin),
•
the gastrointestinal tract cholecystokinin, gastrin, and
•
fat stores (leptin)
Prof.Dr. Hedef Dhafir El-Yassin 2012
6
Many neurotransmitters are secreted and released in a similar fashion to peptide
hormones, and some 'neuropeptides' may be used as neurotransmitters in the nervous
system in addition to acting as hormones when released into the blood. When a peptide
hormone binds to receptors on the surface of the cell, a second messenger appears in the
cytoplasm.
2.
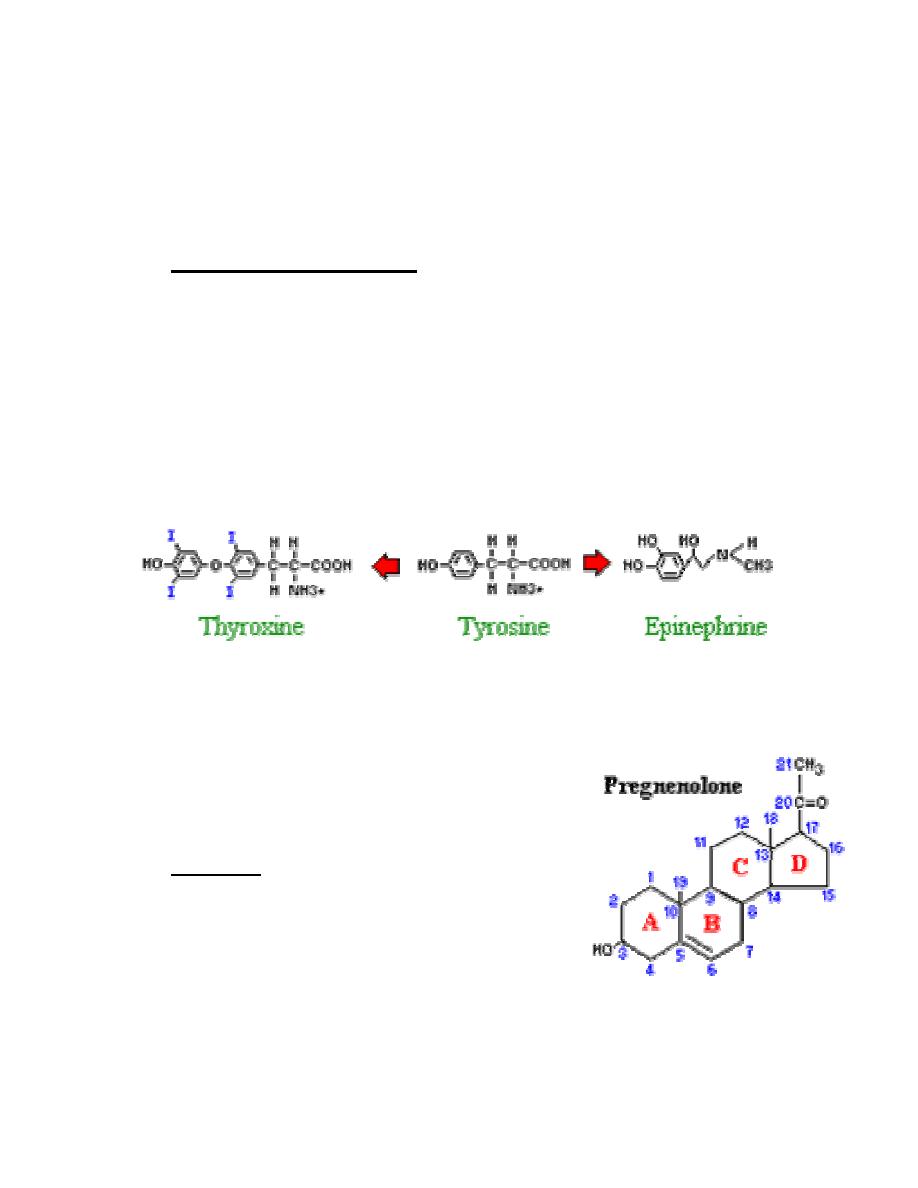
Amino acid derivatives
There are two groups of hormones derived from the amino acid
tyrosine:
•
Thyroid hormones are basically a "double" tyrosine with the critical
incorporation of 3 or 4 iodine atoms.
•
Catecholamines include epinephrine and norepinephrine, which are
used as both hormones and neurotransmitters.
The circulating half-life of thyroid hormones is on the order of a few days.
Two other amino acids are used for synthesis of hormones:
•
Tryptophan is the precursor to serotonin and
the pineal hormone melatonin
•
Glutamic acid is converted to histamine
3.
Steroids
Steroids are lipids and, more specifically,
derivatives of cholesterol. Examples include the
sex steroids such as testosterone and adrenal
steroids such as cortisol. The first and rate-limiting step in the synthesis of all
steroid hormones is conversion of cholesterol to pregnenolone, which
Prof.Dr. Hedef Dhafir El-Yassin 2012
7
demonstrate the system of numbering rings and carbons for identification of
different steroid hormones.
Pregnenolone is formed on the inner membrane of mitochondria then shuttled
back and forth between mitochondrion and the endoplasmic reticulum for
further enzymatic transformations involved in synthesis of derivative steroid
hormones. Newly synthesized steroid hormones are rapidly secreted from the
cell, with little if any storage. Increases in secretion reflect accelerated rates
of synthesis. Following secretion, all steroids bind to some extent to plasma
proteins.
Steroid hormones are typically eliminated by inactivating metabolic
transformations and excretion in urine or bile.
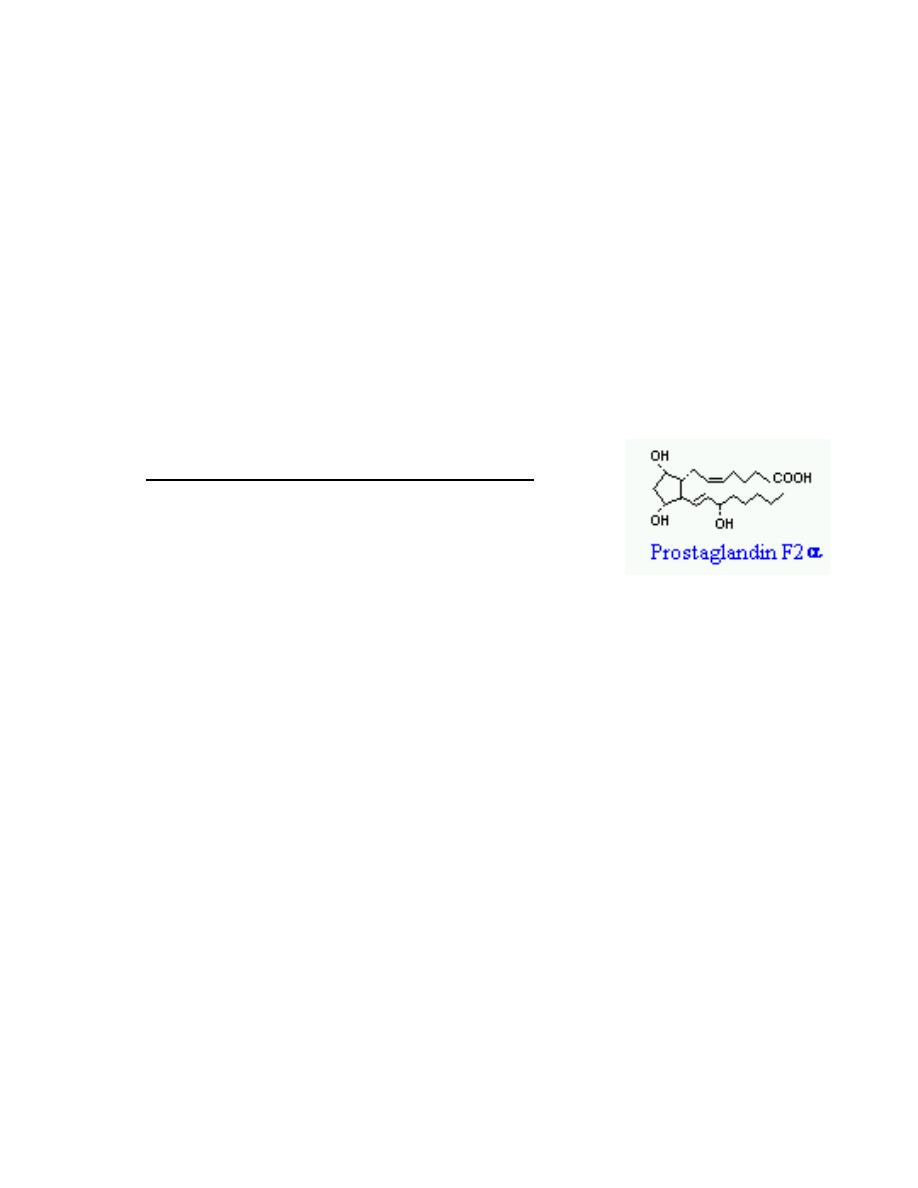
4. Fatty Acid Derivatives - Eicosanoids
Eicosanoids are a large group of molecules derived from
polyunsaturated fatty acids. The principal groups of
hormones of this class are prostaglandins, prostacyclins,
leukotrienes and thromboxanes.
Arachadonic acid is the most abundant precursor for these hormones. Stores
of arachadonic acid are present in membrane lipids and released through the
action of various lipases.
A great variety of cells produce prostaglandins , including those of the liver,
kidneys, heart, lungs, thymus gland, pancreas, brain, and reproductive
organs. In contrast to hormones, prostaglandins usually act locally, affecting
only adjacent cells or the very cell that secreted it.
Prostaglandins are potent and are presented in very small quantities. They
are not stored in cells but rather are synthesized just before release. These
hormones are rapidly inactivated by being metabolized, and are typically
active for only a few seconds
Q
How are prostaglandins different from hormones?
A
They do not use the blood for transportation, they act locally and are synthesized in
cell membrane just before release
Prof.Dr. Hedef Dhafir El-Yassin 2012
8
2.
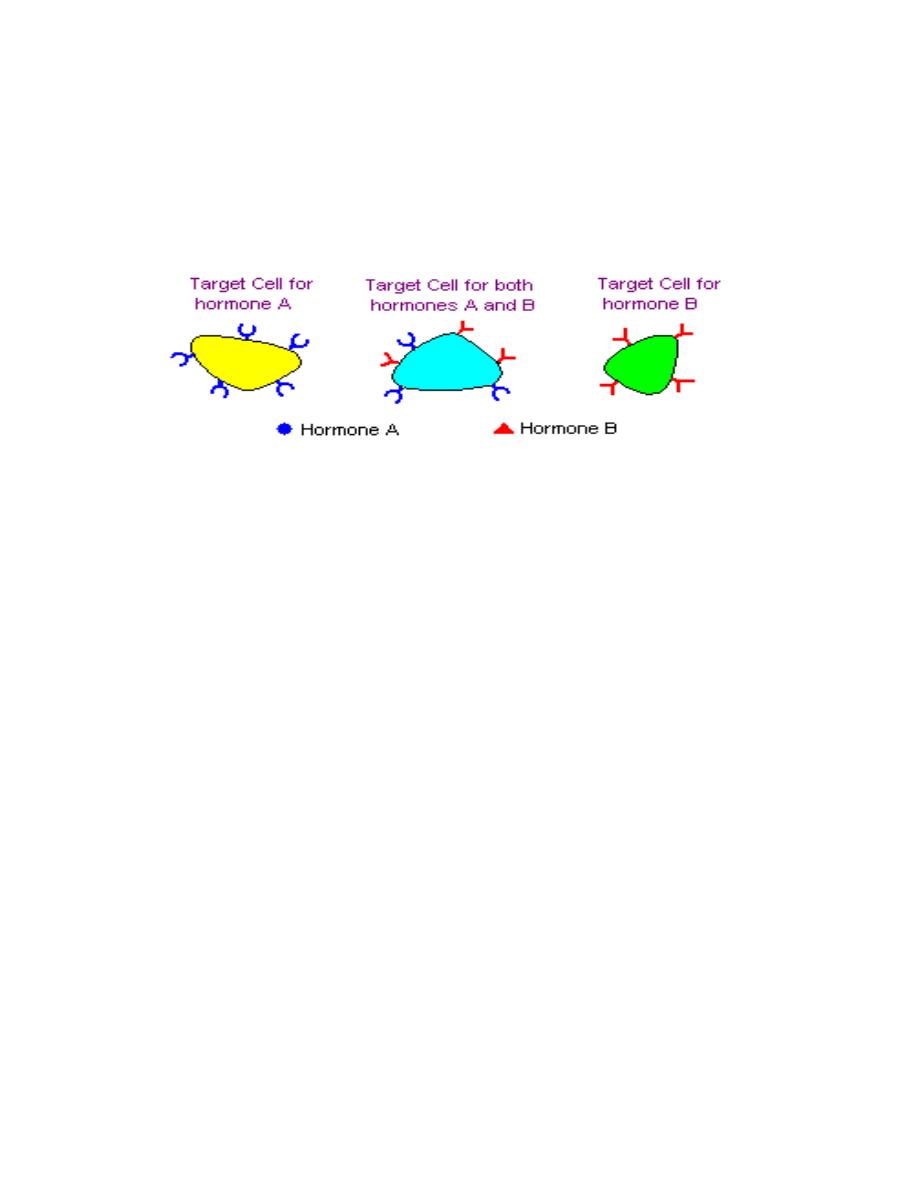
Receptors and Target Cells
A given hormone usually affects only a limited number of cells, which
are called target cells. A target cell responds to a hormone because it
bears receptors for the hormone.
Hormone receptors are found either exposed on the surface of the cell or
within the cell, depending on the type of hormone. In very basic terms,
binding of hormone to receptor triggers a cascade of reactions within the cell
that affects function.
Hormone receptors have two essential qualities:
1. The receptor must be able to recognize a unique binding site within the
hormone in order to discriminate between the hormone and all other
proteins.
2. The receptor must be able to transmit the information gained from
binding to the hormone into a cellular response.
Hormones may be secreted into blood and affect cells at distant sites. Some
hormones known to act and affect neighboring cells or even have effects on
the same cells that secreted the hormone. . Three actions are defined:
•
Endocrine action
: the hormone is distributed in blood and binds to distant
target cells.
•
Paracrine action
: the hormone acts locally by diffusing from its source to
target cells in the neighborhood.
•
Autocrine action
: the hormone acts on the same cell that produced it.
Prof.Dr. Hedef Dhafir El-Yassin 2012
9
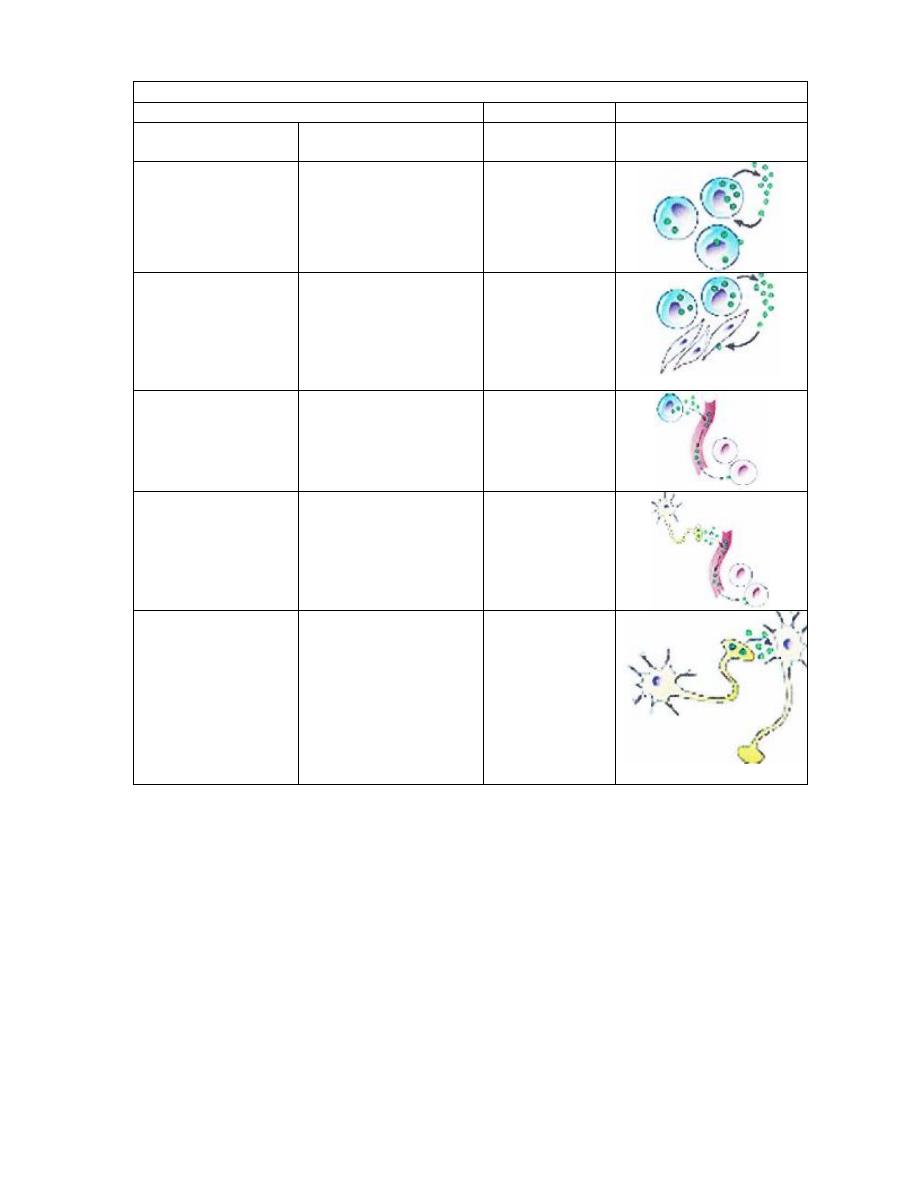
Types of hormones
Chemical messengers
Example
Description
Intracellular
chemical signal
Prostaglandins
Secreted by cells in a
local area and
influences the activity
of the same cell from
which it was secreted
Autocrine
Histamine,
Prostaglandins
Produced by a wide
variety of tissues and
secreted into tissue
spaces; has a
localized effect on
adjacent cells
Paracrine
Thyroxine,
Insulin
Secreted into the
blood by specialized
cells; travels by the
blood to target tissues
Hormone
Oxytocin,
Antidiuretic
hormone
Produced by neurons
and functions like
hormones
Neurohormone
Acetylcholine,
norepinephrine
Produced by neurones
and secreted into
extracellular spaces
by nerve terminals;
travels short
distances, influences
postsynaptic cells or
effector cells.
Neurotransmitter
Two important terms are used to refer to molecules that bind to the
hormone-binding sites of receptors:
•
Agonists
are molecules that bind the receptor and induce all the post-
receptor events that lead to a biologic effect. In other words, they act
like the "normal" hormone, although perhaps more or less potently.
Antagonists
are molecules that bind the receptor and block binding of
the agonist, but fail to trigger intracellular signaling events. Hormone
antagonists are widely used as drugs.
Prof.Dr. Hedef Dhafir El-Yassin 2012
10
Mechanism of Action of Hormone:
Understanding mechanism of action is not only of great interest to basic
science, but critical to understanding and treating diseases of the endocrine
system, and in using hormones as drugs.
There are two fundamental mechanisms by which a hormone can change its
target cell. These mechanisms are:
•
Activation of enzymes and other dynamic molecules: Most
enzymes fluctuate between conformational states that are catalytically
active versus inactive. Many hormones affect their target cells by
inducing such transitions, usually causing an activation of one of more
enzymes. Because enzymes are catalysts and often serve to activate
additional enzymes, a seemingly small change induced by hormone-
receptor binding can lead to widespread consequences within the cell.
•
Modulation of gene expression: Stimulating transcription of a group of
genes clearly can alter a cell's phenotype by leading to a burst of
synthesis of new proteins. Similarly, if transcription of a group of
previously active genes is shut off, the corresponding proteins will soon
disappear from the cell.
More specifically, when a receptor becomes bound to a hormone, it
undergoes a conformational change which allows it to interact
productively with other components of the cells, leading ultimately to an
alteration in the physiologic state of the cell.
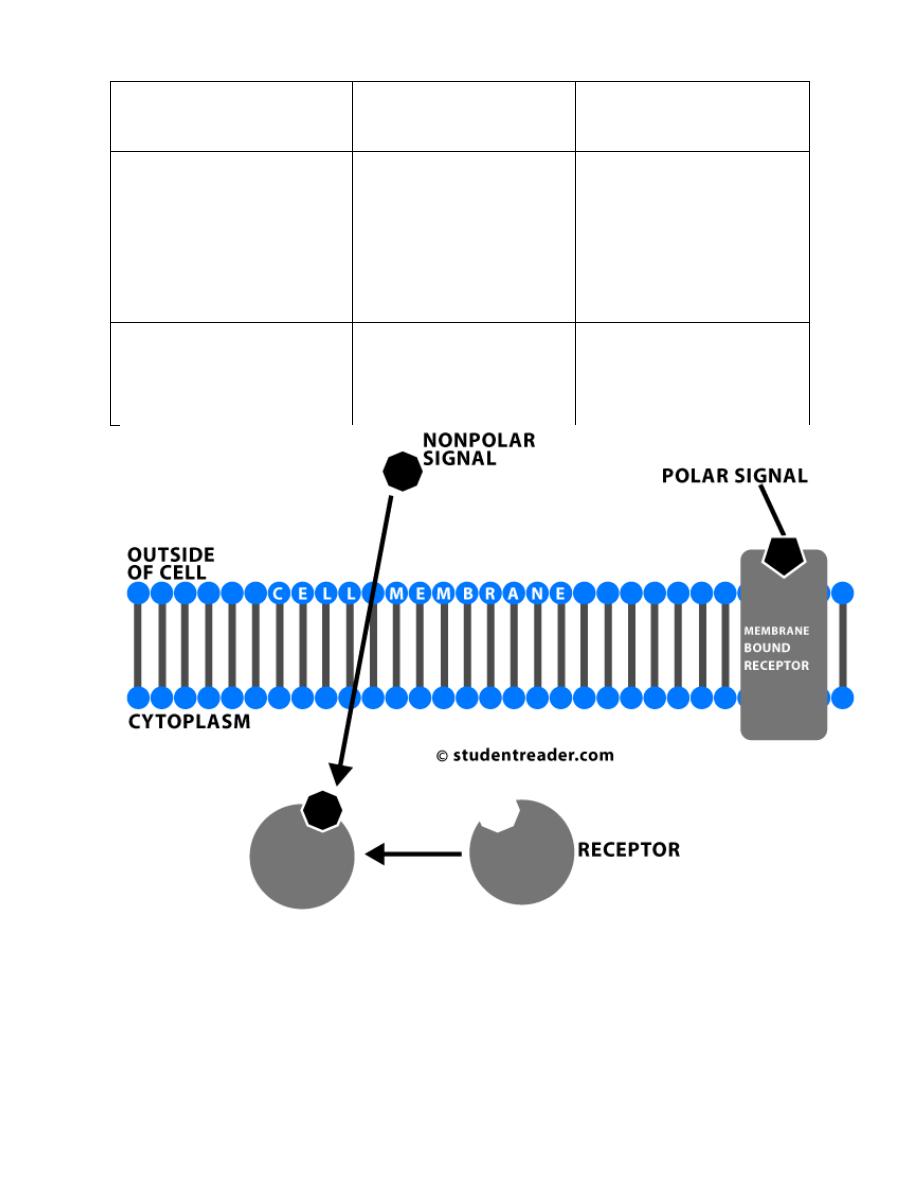
Despite the molecular diversity of hormones, all hormone receptors can be
categorized into one of two types, based on their location within the cell:
Prof.Dr. Hedef Dhafir El-Yassin 2012
11
Location of Receptor
Classes of Hormones
Principle Mechanism
of Action
Cell surface receptors
(plasma membrane)
Proteins and peptides,
catecholamines and
eicosanoids
(water soluble)
Generation of second
messengers which alter
the activity of other
molecules - usually
enzymes - within the cell
Intracellular receptors
(cytoplasm and/or
nucleus)
Steroids and thyroid
hormones
(lipid soluble)
Alter transcriptional
activity of responsive
genes
Prof.Dr. Hedef Dhafir El-Yassin 2012
12
1. Hormones with Cell Surface Receptors
Protein and peptide hormones, catecholamines like epinephrine, and
eicosanoids such as prostaglandins find their receptors decorating the
plasma membrane of target cells. Binding of hormone to receptor initiates a
series of events which leads to generation of so-called second messengers
within the cell (the hormone is the first messenger). The second messengers
then trigger a series of molecular interactions that alter the physiologic state
of the cell. Another term used to describe this entire process is signal
transduction.
Structure of Cell Surface Receptors
Cell surface receptors are integral membrane proteins and, as such, have
regions that contribute to three basic domains:
•
Extracellular domains: Some of the residues exposed to the outside
of the cell interact with and bind the hormone - another term for these
regions is the ligand-binding domain.
•
Transmembrane domains: Hydrophobic stretches of amino acids are
"comfortable" in the lipid bilayer and serve to anchor the receptor in the
membrane.
•
Cytoplasmic or intracellular domains: Tails or loops of the receptor
that are within the cytoplasm react to hormone binding by interacting in
some way with other molecules, leading to generation of second
messengers.
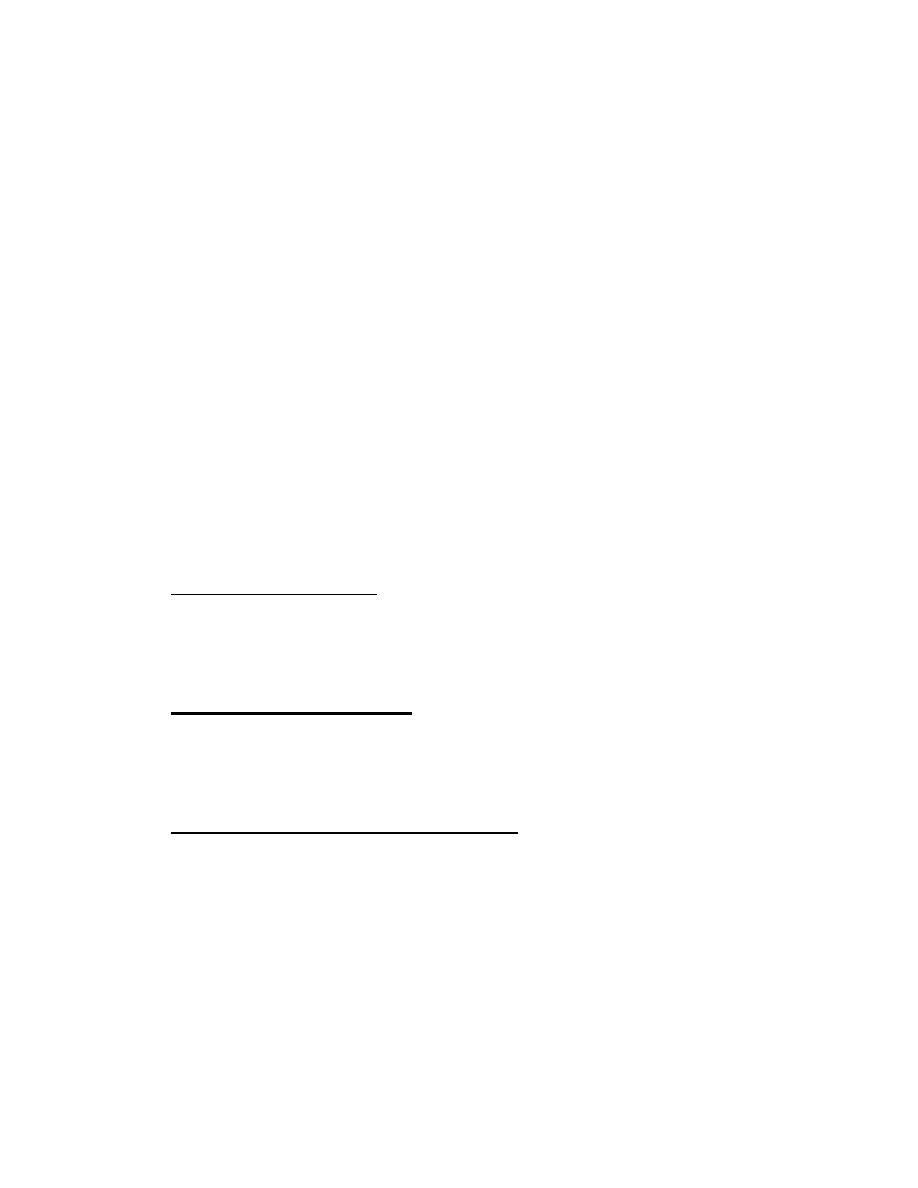
As shown below, some receptors are simple, single-pass proteins; many
growth factor receptors take this form. Others, such as the receptor for
insulin, have more than one subunit. Another class, which includes the beta-
adrenergic receptor, is threaded through the membrane seven times.
Prof.Dr. Hedef Dhafir El-Yassin 2012
13
Interaction of the hormone-bound receptor with other membrane or
cytoplasmic proteins is the key to generation of second messengers and
transduction of the hormonal signal.
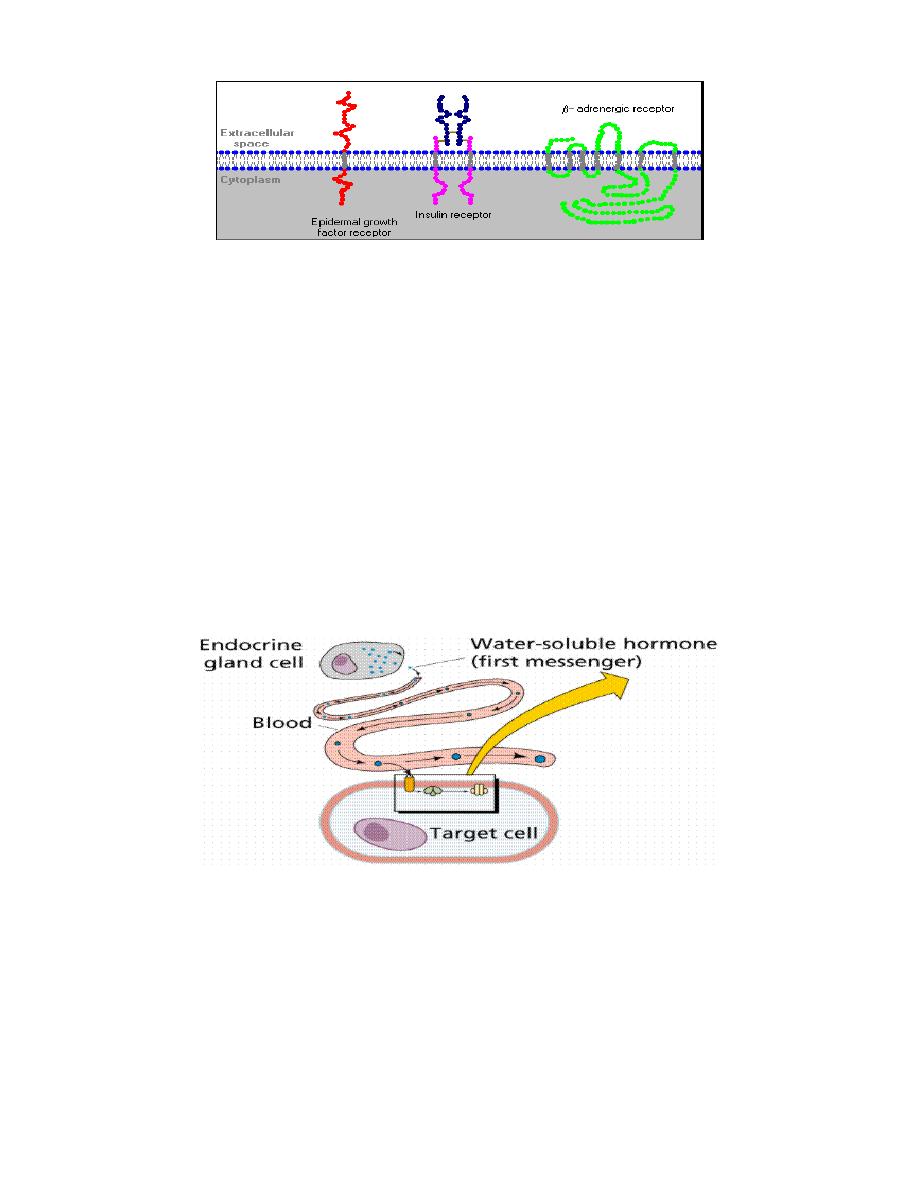
Second Messenger Systems
Nonsteroid hormones (water soluble) do not enter the cell but bind to plasma
membrane receptors, generating a chemical signal (second messenger)
inside the target cell. Second messengers activate other intracellular
chemicals to produce the target cell response.
Prof.Dr. Hedef Dhafir El-Yassin 2012
14
The action of nonsteroid hormones. Images from Purves et al., Life: The Science of Biology, 4th Edition, by
Sinauer Associates
Currently, four second messenger systems are recognized in cells, as
summarized in the table below. Note that not only do multiple hormones
utilize the same second messenger system, but a single hormone can utilize
more than one system.

Prof.Dr. Hedef Dhafir El-Yassin 2012
15
Second Messenger
Examples of Hormones Which Utilize This
System
Cyclic AMP
Epinephrine and norepinephrine, glucagon,
luteinizing hormone, follicle stimulating
hormone, thyroid-stimulating hormone,
calcitonin, parathyroid hormone, antidiuretic
hormone
Protein kinase activity
Insulin, growth hormone, prolactin, oxytocin,
erythropoietin, several growth factors
Calcium and/or
phosphoinositides
Epinephrine and norepinephrine, antidiuretic
hormone, gonadotropin-releasing hormone,
thyroid-releasing hormone.
Cyclic GMP
Atrial naturetic hormone, nitric oxide
In all cases, the seemingly small signal generated by hormone binding
its receptor is amplified within the cell into a cascade of actions that
changes the cell's physiologic state. Presented below are two examples of
second messenger systems commonly used by hormones.
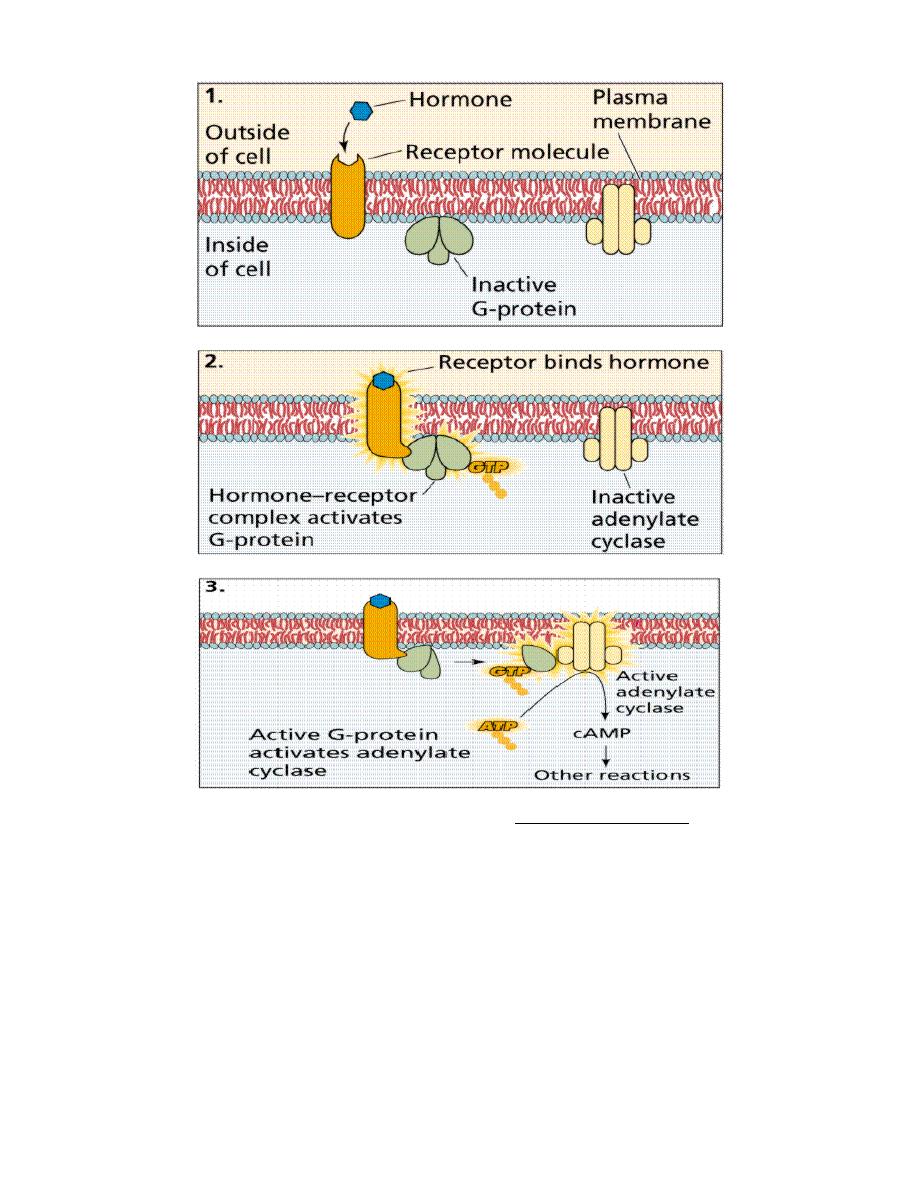
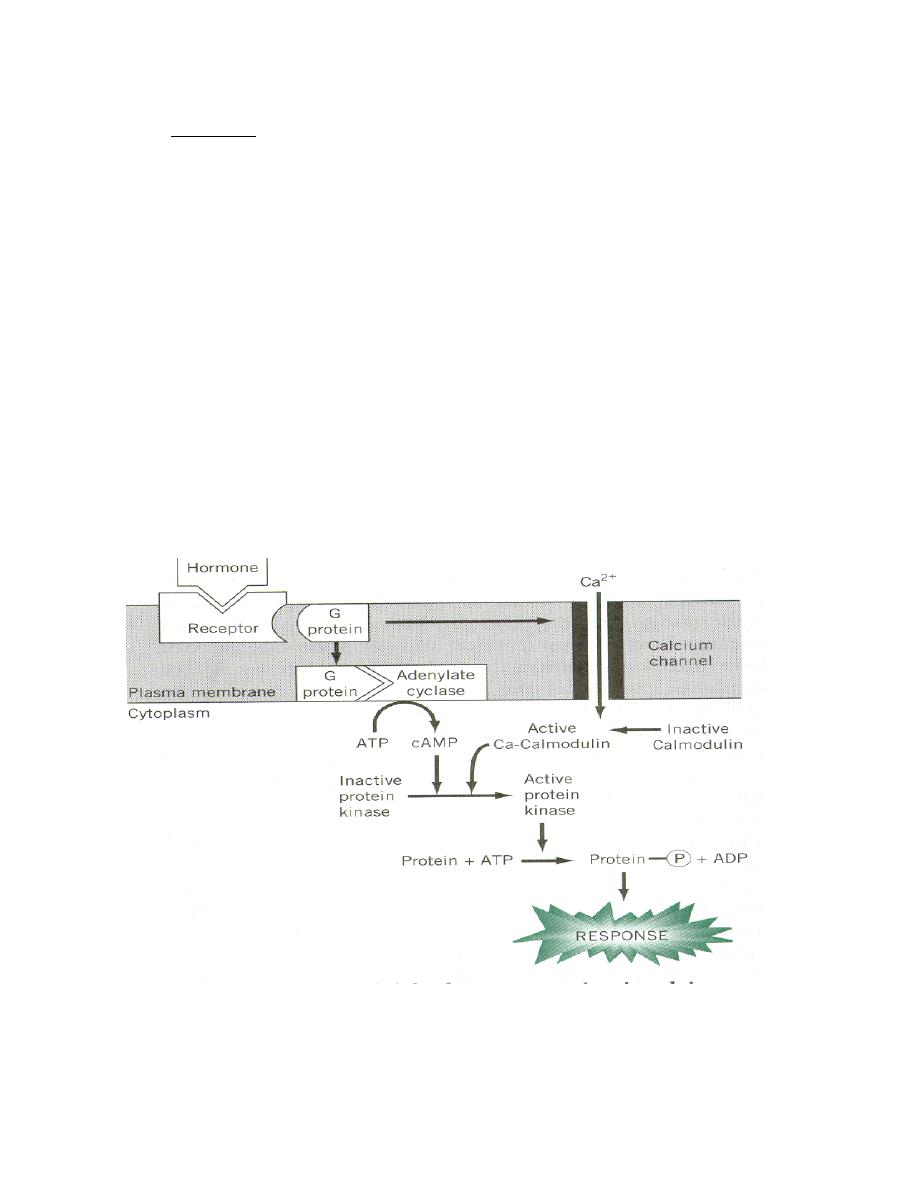
1. Cyclic AMP Second Messenger Systems
Cyclic adenosine monophosphate (cAMP) is a nucleotide generated from
ATP through the action of the enzyme adenylate cyclase. The intracellular
concentration of cAMP is increased or decreased by a variety of hormones
and such fluctuations affect a variety of cellular processes. One prominent
and important effect of elevated concentrations of cAMP is activation of a
cAMP-dependent protein kinase called protein kinase A.
Protein kinase A is nominally in a catalytically-inactive state, but becomes
active when it binds cAMP. Upon activation, protein kinase A phosphorylates
a number of other proteins, many of which are themselves enzymes that are
either activated or suppressed by being phosphorylated. Such changes in
enzymatic activity within the cell clearly alter its state.
Prof.Dr. Hedef Dhafir El-Yassin 2012
16
Simple Example: mechanism of action of glucagon:
•
Glucagon binds its receptor in the plasma membrane of target cells
(e.g. hepatocytes).
•
Bound receptor interacts with and, through a set of G proteins, turns on
adenylate cyclase, which is also an integral membrane protein.
•
Activated adenylate cyclase begins to convert ATP to cyclic AMP,
resulting in an elevated intracellular concentration of cAMP.
•
High levels of cAMP in the cytosol make it probable that protein kinase
A will be bound by cAMP and therefore catalytically active.
•
Active protein kinase A "runs around the cell" adding phosphates to
other enzymes, thereby changing their conformation and modulating
their catalytic activity .
•
Levels of cAMP decrease due to destruction by cAMP-
phosphodiesterase and the inactivation of adenylate cyclase.
In the above example, the hormone's action was to modify the activity of pre-
existing components in the cell. Elevations in cAMP also have important
effects on transcription of certain genes.

Prof.Dr. Hedef Dhafir El-Yassin 2012
17
2. Tyrosine Kinase Second Messenger Systems
The receptors for several protein hormones are themselves protein
kinases which are switched on by binding of hormone. The kinase
activity associated with such receptors results in phosphorylation of tyrosine
residues on other proteins. Insulin is an example of a hormone whose
receptor is a tyrosine kinase.
The hormone binds to domains exposed on the cell's surface, resulting in a
conformational change that activates kinase domains located in the
cytoplasmic regions of the receptor. In many cases, the receptor
phosphorylates itself as part of the kinase activation process. The activated
receptor phosphorylates a variety of intracellular targets, many of which are
enzymes that become activated or are inactivated upon phosphorylation.
As seen with cAMP second messenger systems, activation of receptor
tyrosine kinases leads to rapid modulation in a number of target proteins
within the cell. Some of the targets of receptor kinases are protein
phosphatases which, upon activation by receptor tyrosine kinase, become
competent to remove phosphates from other proteins and alter their activity.
Again, a seemingly small change due to hormone binding is amplified into a
multitude of effects within the cell.
2.
Hormones with Intracellular Receptors
Receptors for steroid and thyroid hormones are located inside target cells, in
the cytoplasm or nucleus, and function as ligand-dependent transcription
factors. The hormone-receptor complex binds to promoter regions of
responsive genes and stimulate or sometimes inhibit transcription from those
genes. Thus, the mechanism of action of these hormones is to modulate
gene expression in target cells. By selectively affecting transcription from a
battery of genes, the concentration of those respective proteins are altered,
which clearly can change the phenotype of the cell.

Prof.Dr. Hedef Dhafir El-Yassin 2012
18
Structure of Intracellular Receptors
Steroid and thyroid hormone receptors are members of a large group of
transcription factors. All of these receptors are composed of a single
polypeptide chain that has, three distinct domains:
•
The amino-terminus
: In most cases, this region is involved in
activating or stimulating transcription by interacting with other
components of the transcriptional machinery. The sequence is highly
variable among different receptors.
•
DNA binding domain
: Amino acids in this region are responsible for
binding of the receptor to specific sequences of DNA.
•
The carboxy-terminus or ligand-binding domain
: This is the region
that binds hormone.
Prof.Dr. Hedef Dhafir El-Yassin 2012
19
In addition to these three core domains, two other important regions of the
receptor protein are a nuclear localization sequence, which targets the protein
to nucleus, and a dimerization domain, which is responsible for latching two
receptors together in a form capable of binding DNA.

Prof.Dr. Hedef Dhafir El-Yassin 2012
20
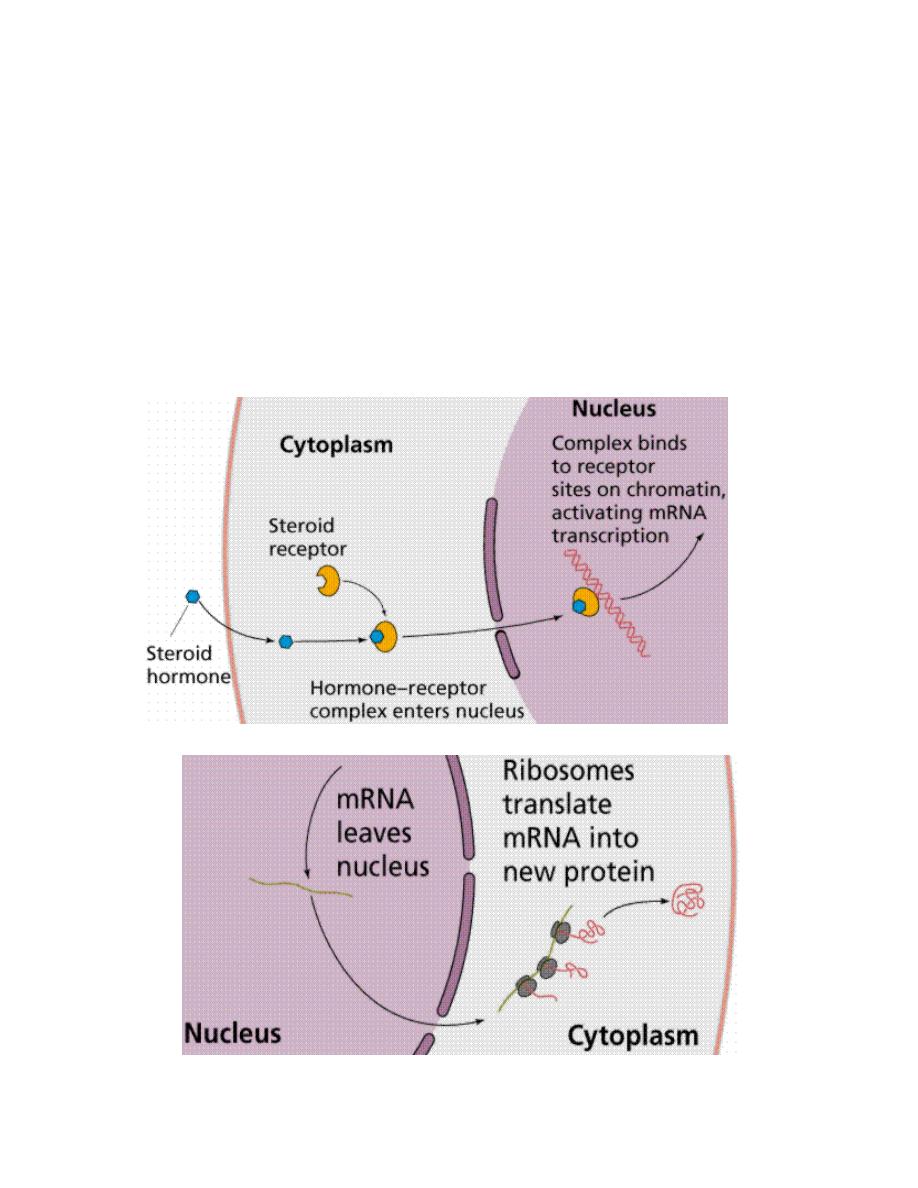
Hormone-Receptor Binding and Interactions with DNA
Being lipids, steroid hormones enter the cell by simple diffusion across the
plasma membrane. Thyroid hormones enter the cell by facilitated diffusion.
The receptors exist either in the cytoplasm or nucleus, which is where they
meet the hormone. When hormone binds to receptor, a characteristic
series of events occurs:
•
Receptor activation
is the term used to describe conformational
changes in the receptor induced by binding hormone. The major
consequence of activation is that the receptor becomes competent to
bind DNA.
•
Activated receptors bind to hormone response elements
, which are
short specific sequences of DNA which are located in promoters of
hormone-responsive genes.
•
Transcription from those genes to which the receptor is bound is
affected.
Most commonly, receptor binding stimulates transcription.
The hormone-receptor complex thus functions as a transcription factor.
Prof.Dr. Hedef Dhafir El-Yassin 2012
21
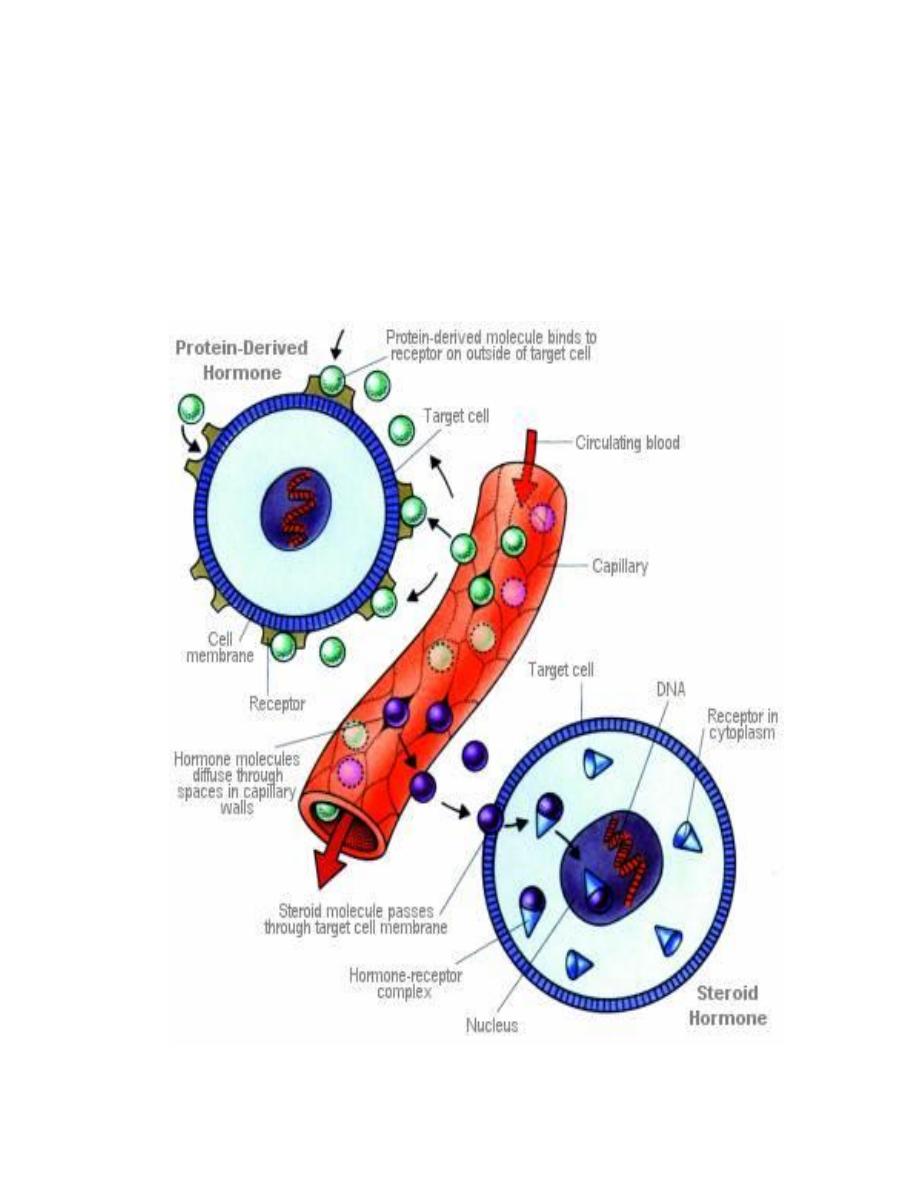
Steroid Hormones
The second mechanism involves steroid hormones, which pass through the
plasma membrane and act in a two step process. Steroid hormones bind,
once inside the cell, to the nuclear membrane receptors, producing an
activated hormone-receptor complex. The activated hormone-receptor
complex binds to DNA and activates specific genes,
increasing production of
proteins.

Prof.Dr. Hedef Dhafir El-Yassin 2012
22
Control of Endocrine Activity
The physiologic effects of hormones depend largely on their concentration in blood and
extracellular fluid.
The concentration of hormone as seen by target cells is determined by three factors:
1. Rate of production
:
Synthesis and secretion of hormones are the most highly
regulated aspect of endocrine control. Such control is mediated by positive and
negative feedback circuits.
2. Rate of delivery
:
An example of this effect is blood flow to a target organ or group
of target cells:
TRANSPORT OF HORMONES: hormones must be transported at least some distance to
their target organs. The primary transport medium is the plasma, although the lymphatic
system and the cerebrospinal fluid are also important. Since delivery of the hormone to its
target tissue is required before a hormone can exert its effects, the presence or absence of
specific transport mechanisms play a major role in mediating hormonal action.
A) The water-soluble hormones (peptide hormones, catecholamines) are transported
in plasma in solution and require no specific transport mechanism. Because of this,
the water-soluble hormones are generally short-lived. These properties allow for
rapid shifts in circulating hormone concentrations, which is necessary with the
pulsatile tropic hormones or the catecholamines. This is consistent with the
rapid onset of action of the water-soluble hormones.
B) The lipid-soluble hormones (thyroid hormone, steroids) circulate in the plasma
bound to specific carrier proteins. Many of the proteins have a high affinity for
specific hormone, such as thyroxine-binding globulin (TBG), sex hormone-
binding globulin (SHBG), and cortisol-binding globulin (CBG). Non-specific, low-
affinity] binding of these hormones to albumin also occurs. Carrier proteins
act as reservoirs of hormone. Since it is generally believed that only the free hor-
mone can enter cells, a dynamic equilibrium must exist between the bound and
free hormone. Thus, alterations in the amount of binding protein available, or in
the affinity of the hormone for the binding protein, can markedly alter the total
circulating pool of hormone without affecting the free concentration of hormone.
Carrier proteins act as buffers to both blunt sudden increases in hormone
concentration and to diminish degradation of the hormone once it is secreted. Thus, the
half-life of hormones that utilize carrier proteins is longer than those that are not protein-
Prof.Dr. Hedef Dhafir El-Yassin 2012
23
bound. Indeed, carrier proteins have a profound effect on the clearance rate of hormones;
the greater the capacity for high affinity binding of the hormone in the plasma, the slower
the clearance rate. Also, the carrier proteins allow slow, tonic delivery of the hormone to
its target tissue. This is consistent with the slower onset of action of the lipid-soluble
hormones.
3. Rate of degradation and elimination
:
Hormones, like all biomolecules, have
characteristic rates of decay, and are metabolized and excreted from the body
through several routes.
HORMONE METABOLISM: Clearance of hormones from the circulation plays a critical role
in the modulation of hormone levels in response to varied physiologic and pathologic
processes. The time required to reach a new steady-state concentration in response to
changes in hormone release is dependent upon the half-life of the hormone in the serum.
Thus, an increase in hormone release or administration will have a much more marked effect
if the hormone is cleared rapidly from the circulation as opposed to one that is cleared more
slowly.
Most peptide hormones have a plasma half-life measured in minutes, consistent with the
rapid actions and pulsatile nature of the secretion of these hormones. This rapid clearance
is achieved by the lack of protein binding in the plasma, degradation or internalization of the
hormone at its site of action, and ready clearance of the hormone by the kidney. Binding to
serum proteins markedly decreases hormone clearance, as is observed with the steroid
hormones and the iodothyronines. Metabolism of the steroid hormones occurs primarily in
the liver by reductions, conjugations, oxidations, and hydroxylations, which serve to
inactivate the hormone and increase their water-solubility, facilitating their excretion in the
urine and the bile. Metabolic transformation also may serve to activate an inactive hormone
precursor, such as the deiodination of thyroxine to form T
3
. Hormone metabolism is not as
tightly regulated as is hormone synthesis and release. However, alterations in the metabolic
pathways may be clinically important.
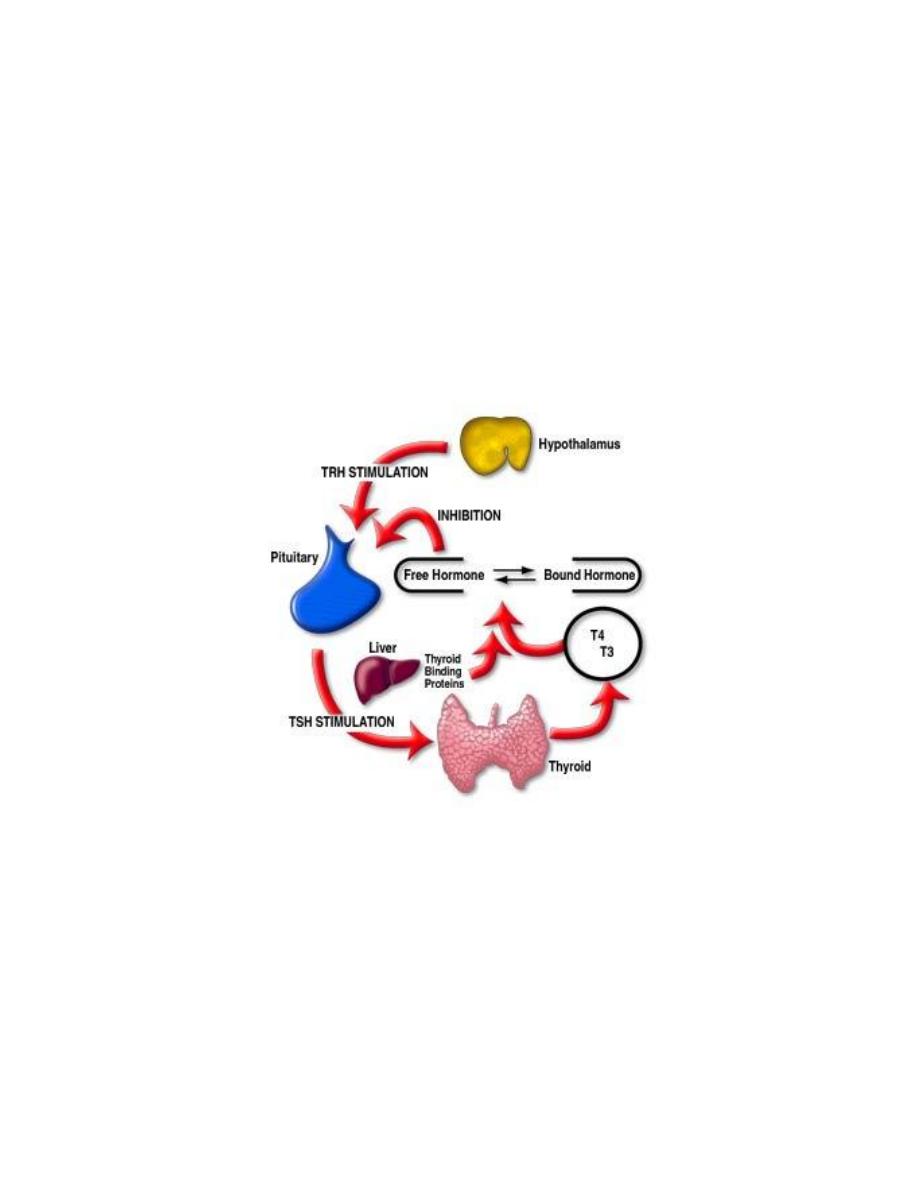
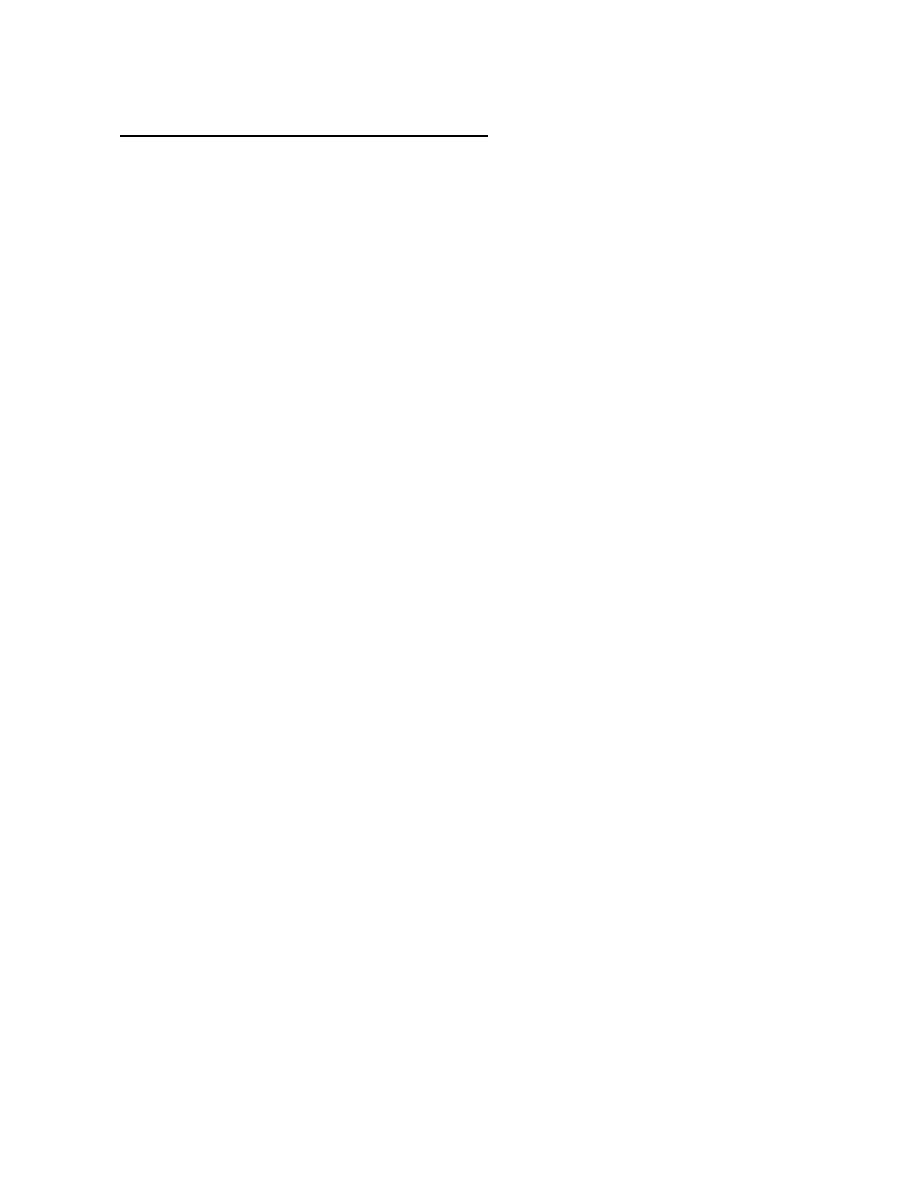
Feedback Control of Hormone Production
Feedback circuits are at the root of most control mechanisms in physiology, and are
particularly prominent(obvious) in the endocrine system. Instances of positive feedback
certainly occur, but negative feedback is much more common.
Feedback loops are used extensively to regulate secretion of hormones in the
hypothalamic-pituitary axis. An important example of a negative feedback loop is seen in
control of thyroid hormone secretion. The thyroid hormones thyroxine and triiodothyronine
Prof.Dr. Hedef Dhafir El-Yassin 2012
24
("T4 and T3") are synthesized and secreted by thyroid glands and affect metabolism
throughout the body. The basic mechanisms for control in this system are:
•
Neurons in the hypothalamus secrete thyroid releasing hormone (TRH), which
stimulates cells in the anterior pituitary to secrete thyroid-stimulating hormone
(TSH).
•
TSH binds to receptors on epithelial cells in the thyroid gland, stimulating synthesis
and secretion of thyroid hormones, which affect probably all cells in the body.
•
When blood concentrations of thyroid hormones increase above a certain threshold,
TRH-secreting neurons in the hypothalamus are inhibited and stop secreting TRH.
This is an example of "negative feedback".
Inhibition of TRH secretion leads to shut-off of TSH secretion, which leads to shut-off of
thyroid hormone secretion. As thyroid hormone levels decay below the threshold, negative
feedback is relieved, TRH secretion starts again, leading to TSH secretion...

Prof.Dr. Hedef Dhafir El-Yassin 2012
25
Biochemistry and
Disorders of
Hormones of the
Hypothalamic and
pituitary gland
(hypothalamus
and pituitary axis)
1. Hormones of the hypothalamus
Prof. Dr. Hedef Dhafir El-Yassin 2012
Prof.Dr. Hedef Dhafir El-Yassin 2012
26
Hypothalamic and Pituitary Hormones
Lecture 3
Thursday 23/2
1. Hormones of the hypothalamus
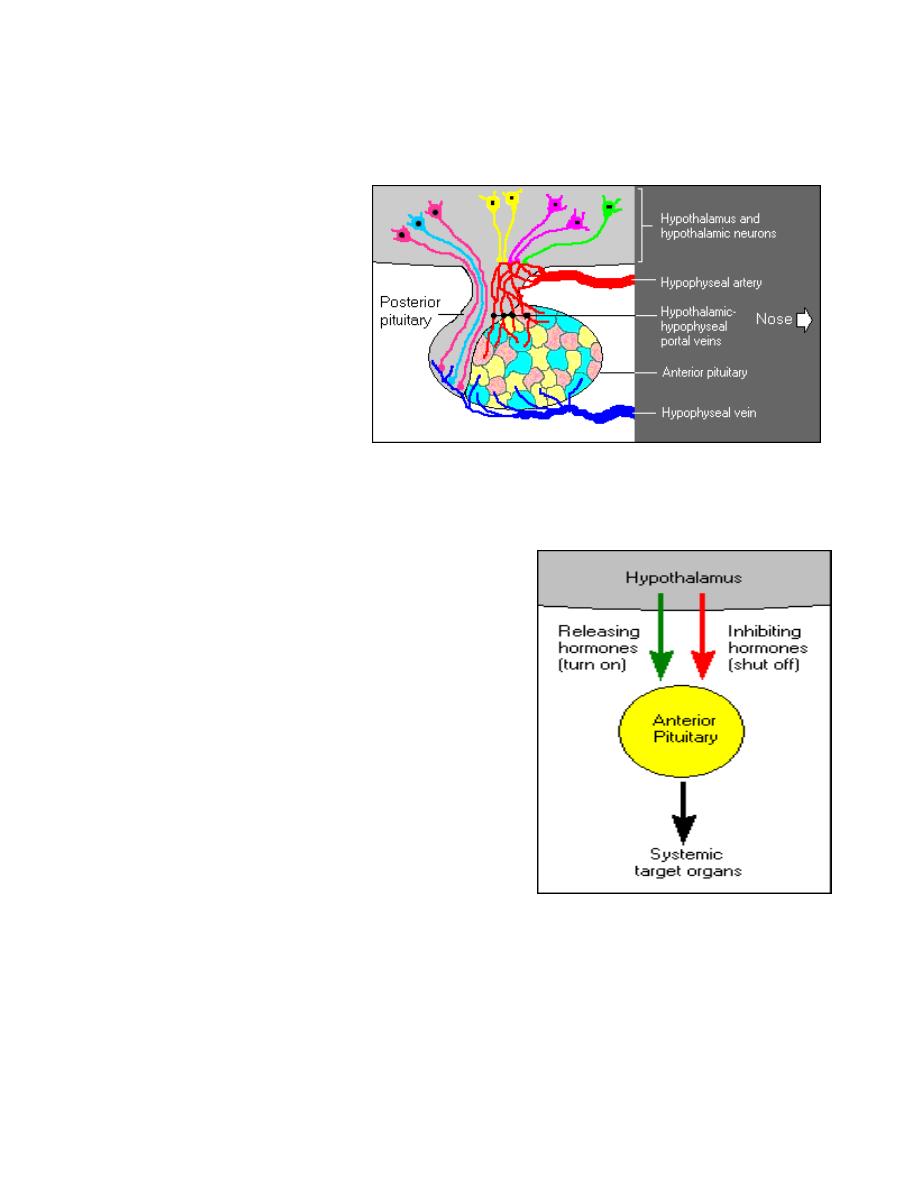
The hypothalamus is an integral part of the substance of the brain. A small cone-shaped
structure, it projects downward, ending in the pituitary stalk, a tubular connection to the
pituitary gland, which is a double lobed structure that produces the endocrine secretions
when stimulated by the hypothalamus.
Regulation of hormone secretion
The hypothalamus regulates homeostasis
.
It links the {nervous_system} to the {endocrine
system}. In addition to secreting neurotransmitters and neuromodulators, the
hypothalamus synthesizes and secretes a number of neurohormones. The neurons
secreting neurohormones are true endocrine cells.
Prof.Dr. Hedef Dhafir El-Yassin 2012
27
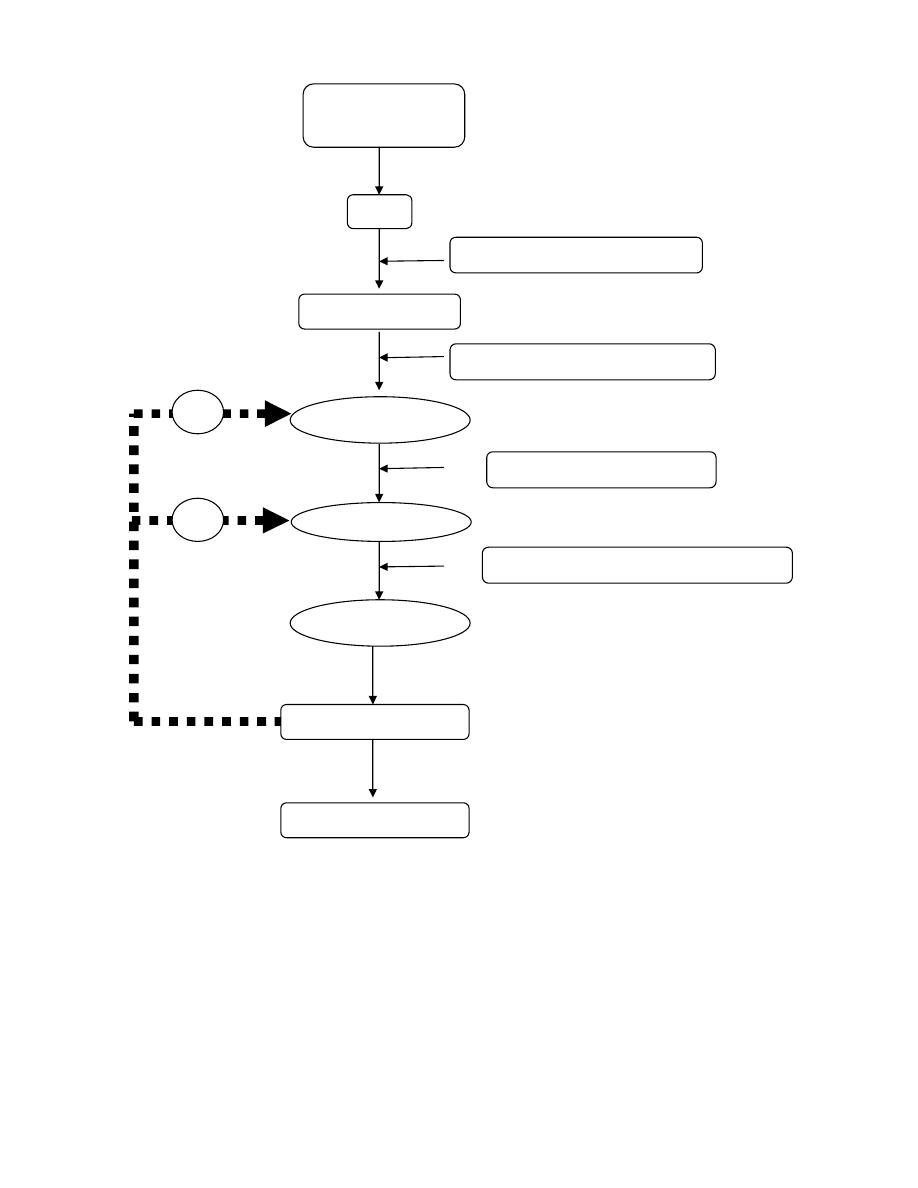
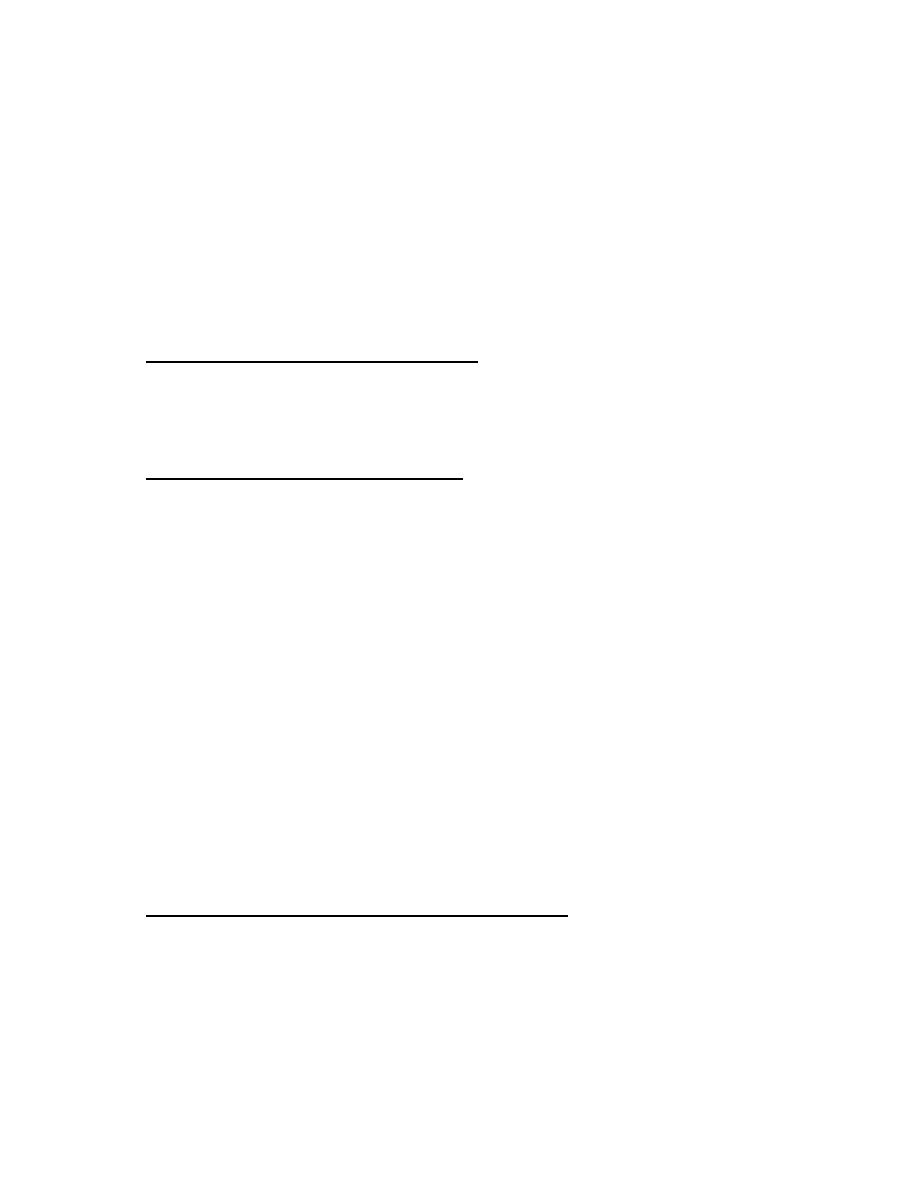
Hormonal cascade of signals from CNS to ultimate hormone.
The target "gland" is the last hormone-producing tissue in the cascade, which is stimulated
by an appropriate anterior pituitary hormone. Examples are thyroid gland, adrenal cortex,
ovary and testes. Ultimate hormone feedback negatively on sites producing intermediate
hormones in the cascade. Amounts (nanogram (ng), microgram (µg), and milligram (mg)
represent approximate quantities of hormone released.
Environmental or
internal signal
CNS
Limbic system
Electrical –chemical signal
Hypothalamus
Electrical –chemical signal
Anterior
Target "gland"
Releasing hormones (ng)
Anterior pituitary tropic hormone (µg)
Ultimate Hormone (mg)
Systemic effects
N
e
g
a
ti
v
e
f
e
e
d
b
a
c
k
i
n
h
ib
it
io
n
-
ve
-
ve
Prof.Dr. Hedef Dhafir El-Yassin 2012
28
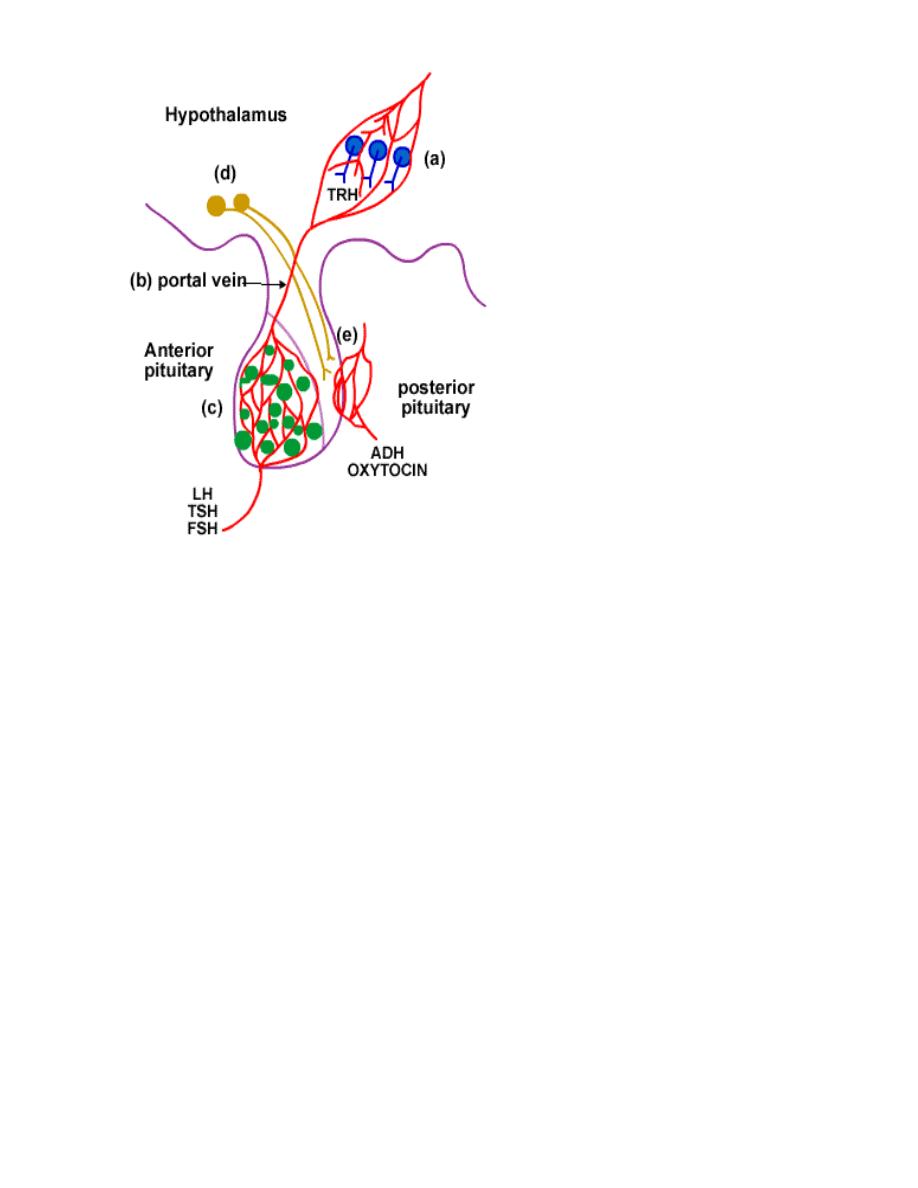
The hypothalamus controls each lobe
of the pituitary slightly differently.
1. control of Anterior lobe
a. The hypothalamus acts
as an endocrine gland.
b. Hormones are sent
from the hypothalamus
to the anterior pituitary
via a blood vessel
called the portal vein.
c. The target tissue is the
anterior lobe of the
pituitary e.g. LH, TSH,
and FSH.
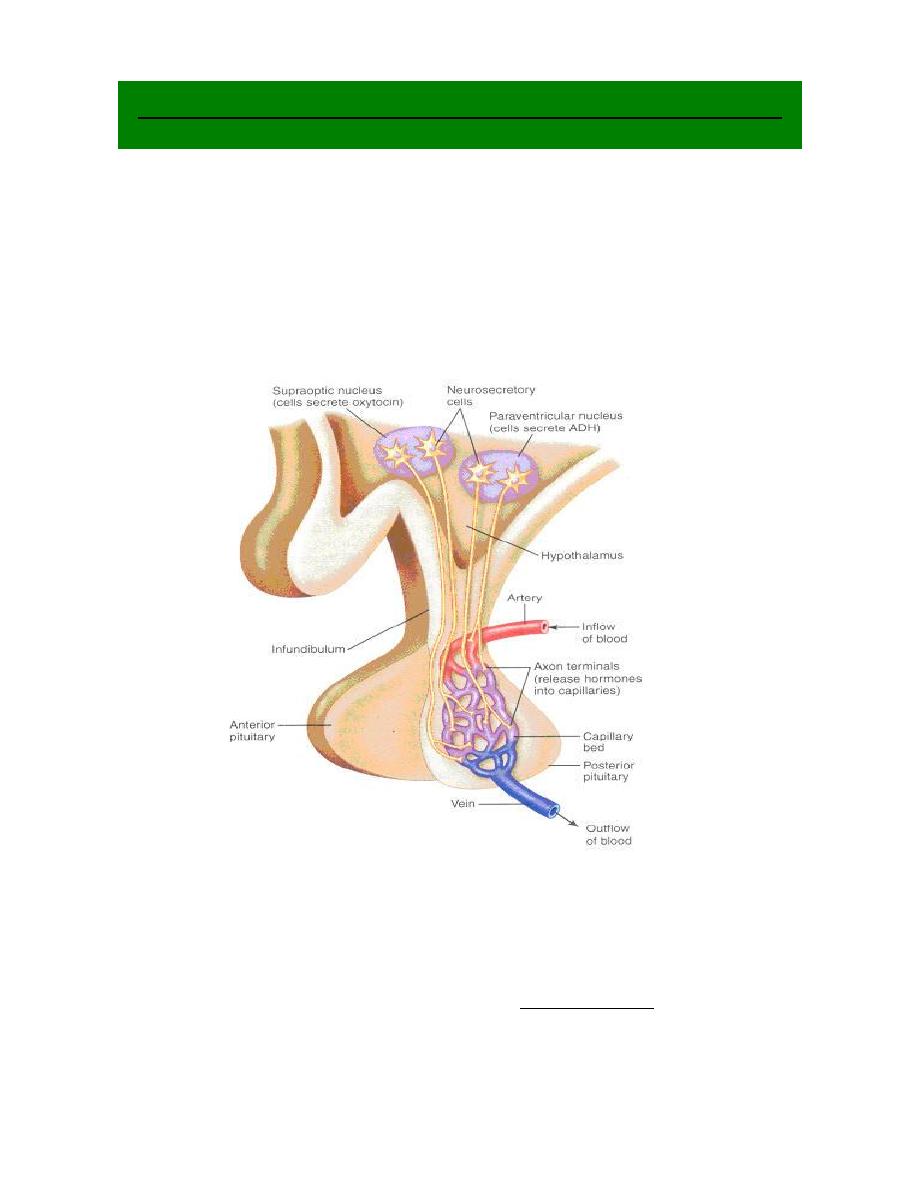
2. control of the Posterior lobe
d. Neuro-hormones are synthesized in the hypothalamus neurons. They are
transported and stored in vesicles in the axon ending located in the posterior
pituitary.
e. Nerve impulses travel down the axon into the posterior pituitary. This causes
the release of the vesicles of hormones into the blood stream at the
posterior pituitary e.g. oxytocin , and ADH.
Prof.Dr. Hedef Dhafir El-Yassin 2012
29
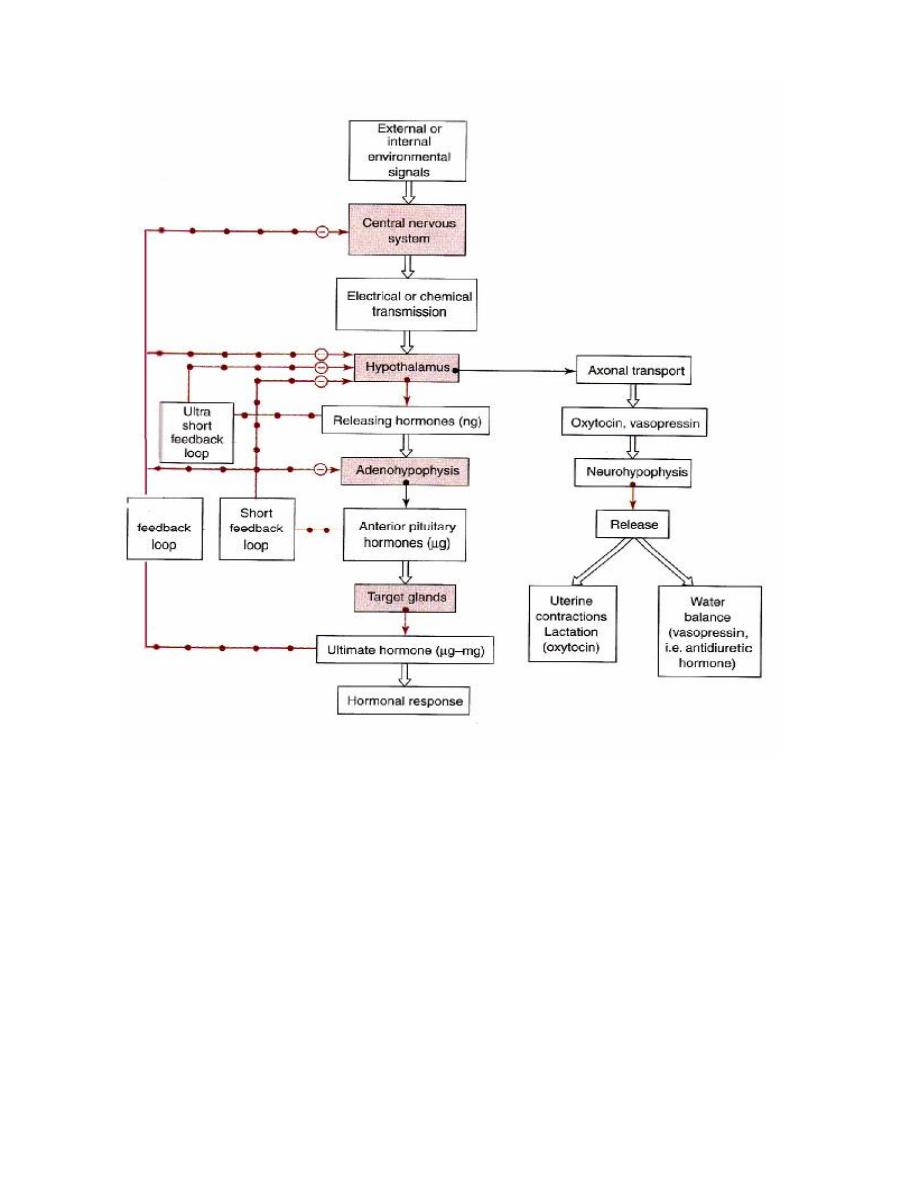
Many hormonal systems involve hypothalamus.
Cascade of hormonal responses starting with an external or internal signal. This signal is
transmitted first to the CNS and may involve the limbic system, including the hippocampus
and amygdala. These components innervate the hypothalamus in a specific region, which
responds by secreting (nanogram amounts) a specific releasing hormone. Releasing
hormones are transported down a closed portal system to the anterior pituitary, where they
cause secretion of microgram amounts of specific anterior pituitary hormones. These
access the general circulation through fenestrated local capillaries and trigger release of
an ultimate hormone in microgram to milligram daily amounts, which generates its
response by binding to receptors in target tissues. Overall, this system is an amplifying
cascade. Consequently, the organism is in intimate association with the external
environment. Solid arrows indicate a secretory process. Long arrows studded with open or
closed circles indicate negative feedback pathways (ultra-short, short, and long feedback
loops).
Prof.Dr. Hedef Dhafir El-Yassin 2012
30
1) Thyrotropin-releasing hormone(TRH)
Is the simplest of the hypothalamic neuropeptides. It consists essentially of three amino
acids. Its basic sequence is glutamic acid-histidine-proline, although both ends of the
peptide are modified. The simplicity of this structure is deceiving for TRH is involved in an
extraordinary array of functions. Some of which are:
a. It stimulates the secretion of thyroid-stimulating hormone from the pituitary.
b. It also affects the secretion of prolactin from the pituitary.
The TRH-secreting cells are subject to stimulatory and inhibitory influences from higher
centers in the brain and they also are inhibited by circulating thyroid hormone.
2) Gonadotropin-releasing hormone (GnRH)
Also known as luteinizing hormone-releasing hormone (LHRH), is a peptide chain of 10
amino acids. It stimulates the synthesis and release of the two pituitary gonadotropins,
luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
3) Corticotropin-releasing hormone (CRH)
Is a large peptide consisting of a single chain of 41 amino acids. It stimulates not only
secretion of corticotropin in the pituitary gland but also the synthesis of corticotropin in the
corticotropin-producing cells (corticotrophs) of the anterior lobe of the pituitary gland. Many
factors, both neurogenic and hormonal, regulate the secretion of CRH. Among the
hormones that play an important role in modulating the influence of CRH is cortisol, the
major hormone secreted by the adrenal cortex, which, as part of the negative feedback
mechanism. Vasopressin, the major regulator of the body's excretion of water, has an
additional ancillary role in stimulating the secretion of CRH.
Excessive secretion of CRH leads to an increase in the size and number of corticotrophs
in the pituitary gland, often resulting in a pituitary tumor. This, in turn, leads to excessive
stimulation of the adrenal cortex, resulting in high circulating levels of adrenocortical
hormones, the clinical manifestations of which are known as Cushing's syndrome.
Conversely, a deficiency of CRH-producing cells can, by a lack of stimulation of the
pituitary and adrenal cortical cells, result in adrenocortical deficiency.
4) Growth hormone-releasing hormone (GHRH or GRH)
Like CRH, growth hormone-releasing hormone (GHRH) is a large peptide. A number of
forms have been described that differ from one another only in minor details and in the
number of amino acids (varying from 37 to 49). It is stimulated by stresses, including
physical exercise, and secretion is blocked by a powerful inhibitor called somatostatin.
Negative feedback control of GHRH secretion is mediated largely through compounds
called somatomedins, growth-promoting hormones that are generated when tissues are
Prof.Dr. Hedef Dhafir El-Yassin 2012
31
exposed to growth hormone itself.
Isolated deficiency of GHRH (in which there is normal functioning of the hypothalamus
except for this deficiency) may be the cause of one form of dwarfism, a general term
applied to all individuals with abnormally small stature.
5) Prolactin release factor (PRF):
Appears to be released from the hypothalamus in a pulsatile fashion and it is the
fluctuation in PRF that regulates the circulating level of prolactin.
6) Somatostatin (Growth hormone release-inhibiting hormone; somatotropin
release-inhibiting hormone ( GHRIH or SRIH)
Somatostatin refers to a number of polypeptides consisting of chains of 14 to 28 amino
acids. Somatostatin is also a powerful inhibitor of pituitary TSH secretion. Somatostatin,
like TRH, is widely distributed in the central nervous system and in other tissues. It serves
an important paracrine function in the islets of Langerhans, by blocking the secretion of
both insulin and glucagon from adjacent cells. Somatostatin has emerged not only as a
powerful blocker of the secretion of GH, insulin, glucagon, and other hormones but also as
a potent inhibitor of many functions of the gastrointestinal tract, including the secretion of
stomach acid, the secretion of pancreatic enzymes, and the process of intestinal
absorption.
7) Prolactin release-inhibiting hormones (Dopamine and GAP)
GAP= GnRH-associated peptide
The hypothalamic regulation of prolactin secretion from the pituitary is different from the
hypothalamic regulation of other pituitary hormones in two respects:
1. First, the hypothalamus primarily inhibits rather than stimulates the release of
prolactin from the pituitary.
2. Second, this major inhibiting factor is not a neuropeptide, but rather the
neurotransmitter dopamine. Prolactin deficiency is known to occur, but only rarely.
Excessive prolactin production (hyperprolactinemia) is a common endocrine
abnormality.
Prof.Dr. Hedef Dhafir El-Yassin 2012
32
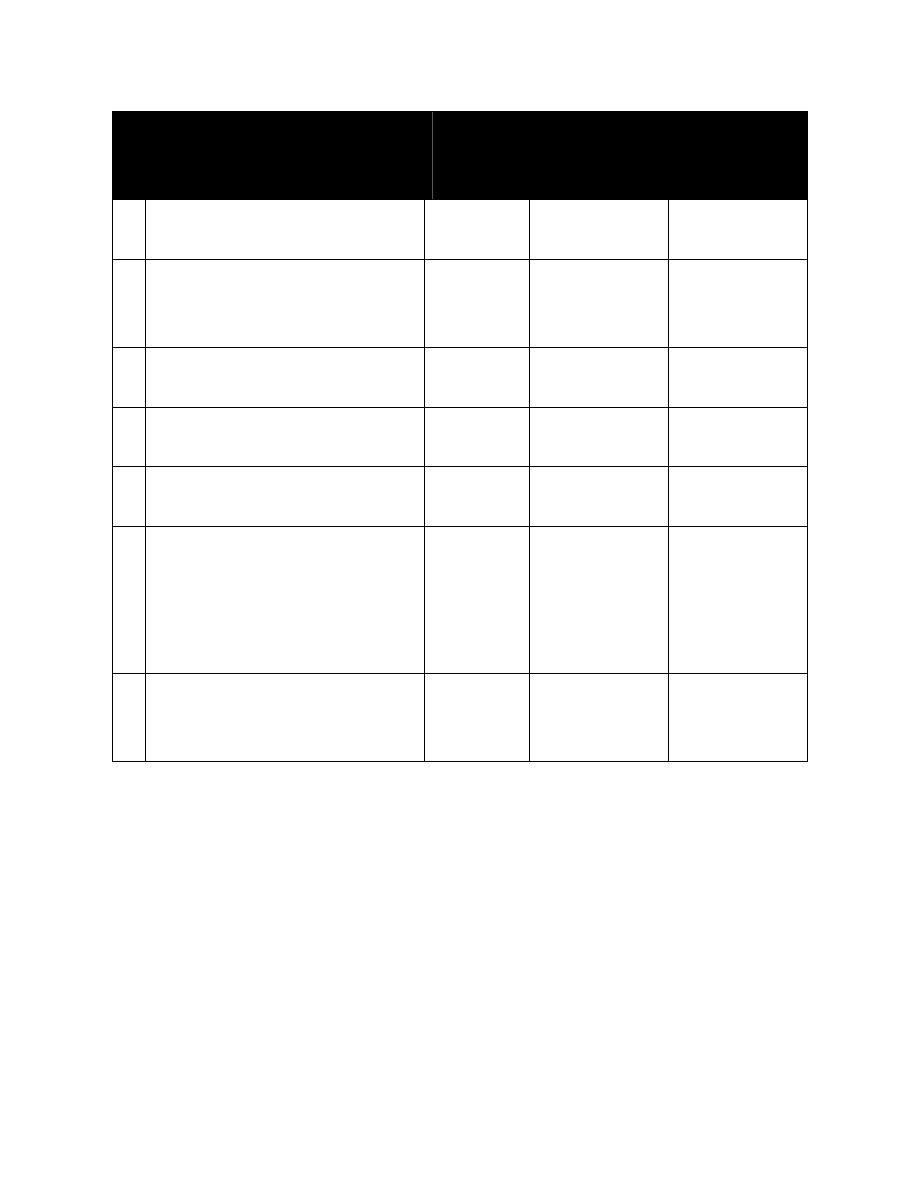
Table: Hypothalamic hypophysial-target gland hormones form integrated feedback loops
Hypothalamic hormones
No. of A.A
in
structure
Pituitary
Hormone
Affected
1
Target Gland
Hormone
Affected
1
Thyrotropin-releasing hormone
(TRH)
3
TSH (PRL)
T
3
, T
4
2
Gonadotropin-releasing
hormone (GnRH)
10
LH, FSH
Androgens,
estrogens,
progestins
3
Corticotropin-releasing
hormone (CRH)
41
ACTH
Cortisol
4
Growth hormone-releasing
hormone(GHRH or GRH)
49
GH
IGF-1
5
Prolactin release factor
Not
established
PRL
neurohormones
6
Somatostatin (Growth hormone
release-inhibiting hormone;
somatotropin release-inhibiting
hormone (GHRIH or SRIH)
14
GH (TSH, FSH,
ACTH)
IGK-1; T
3
andT
4
7
Prolactin- release-inhibiting
hormones (Dopamine and GAP)
(PRIH or PIH)
PRL
neurohormones
1
The hypothalamic hormone has a secondary or lesser effect on the hormones in parentheses.

Prof.Dr. Hedef Dhafir El-Yassin 2012
33
Biochemistry and
Disorders of
Hormones of the
Hypothalamic and
pituitary gland
(hypothalamus
and pituitary axis
2. Hormones of the pituitary gland
Prof. Dr. Hedef Dhafir El-Yassin 2012
Prof.Dr. Hedef Dhafir El-Yassin 2012
34
Lecture 4
Sunday 26/2
2. Hormones of the Pituitary gland
The pituitary gland, also known
as the hypophysis, is a roundish
organ that lies immediately
beneath the hypothalamus.
Careful examination of the
pituitary gland reveals that it
composed of two distinctive
parts:
•
The
anterior pituitary
(adenohypophysis) is a
classical gland composed predominantly of cells that secrete protein hormones.
•
The
posterior pituitary
(neurohypophysis) is not really an organ, but an extension
of the hypothalamus. It is composed largely of the axons of hypothalamic neurons
which extend downward as a large bundle
behind the anterior pituitary.
The target cells for most of the hormones produced in
these tissues are themselves endocrine cells.
The pituitary gland is often called the "master
gland" of the body. The anterior and posterior pituitary
secretes a number of hormones that collectively
influence all cells and affect virtually all physiologic
processes.

Prof.Dr. Hedef Dhafir El-Yassin 2012
35
Table: The major hormones synthesized and secreted by the pituitary gland, along
with summary statements about their major target organs and physiologic effects.
Hormone
Major target
organ(s)
Major Physiologic Effects
Growth hormone
Liver, adipose
tissue
Promotes growth (indirectly),
control of protein, lipid and
carbohydrate metabolism
Thyroid-stimulating h.
Thyroid gland
Stimulates secretion of thyroid
hormones
Adrenocorticotropic h. Adrenal gland
(cortex)
Stimulates secretion of
glucocorticoids
Prolactin
Mammary gland
Milk production
Luteinizing hormone
Ovary and testis
Control of reproductive function
A
n
te
ri
o
r
P
it
u
it
a
ry
Follicle-stimulating h.
Ovary and testis
Control of reproductive function
Antidiuretic hormone
Kidney
Conservation of body water
P
o
s
te
ri
o
r
P
it
u
it
a
ry
Oxytocin
Ovary and testis
Stimulates milk ejection and uterine
contractions
As seen in the table above, the anterior pituitary synthesizes and secreted six major
hormones. Individual cells within the anterior pituitary secrete a single hormone (or
possibly two in some cases). Thus, the anterior pituitary contains at least six distinctive
endocrinocytes
The cells that secrete thyroid-stimulating hormone do not also secrete growth hormone,
and they have receptors for thyroid-releasing hormone, not growth hormone-releasing
hormone.
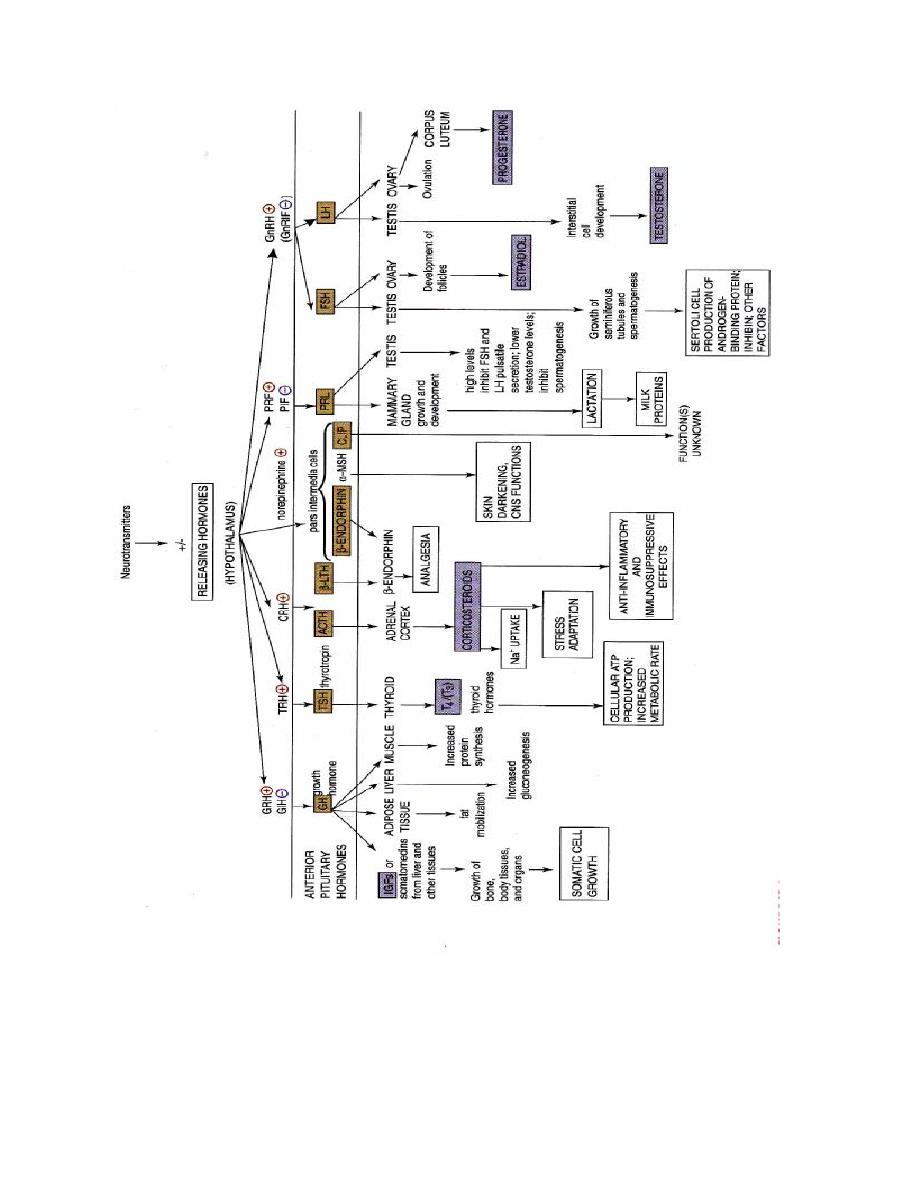
Prof.Dr. Hedef Dhafir El-Yassin 2012
36
Overview of anterior pituitary hormones with hypothalamic releasing
hormones and their actions
Prof.Dr. Hedef Dhafir El-Yassin 2012
37
Anterior Pituitary Hormones
1. Growth Hormone
Growth hormone, also known as somatotropin, is a
protein hormone of about 190 amino acids that is
synthesized and secreted by cells called somatotrophs in
the anterior pituitary. It is a major participant in control of
several complex physiologic processes, including growth
and metabolism. Growth hormone is also of considerable
interest as a drug used in both humans and animals.
Physiologic Effects of Growth Hormone
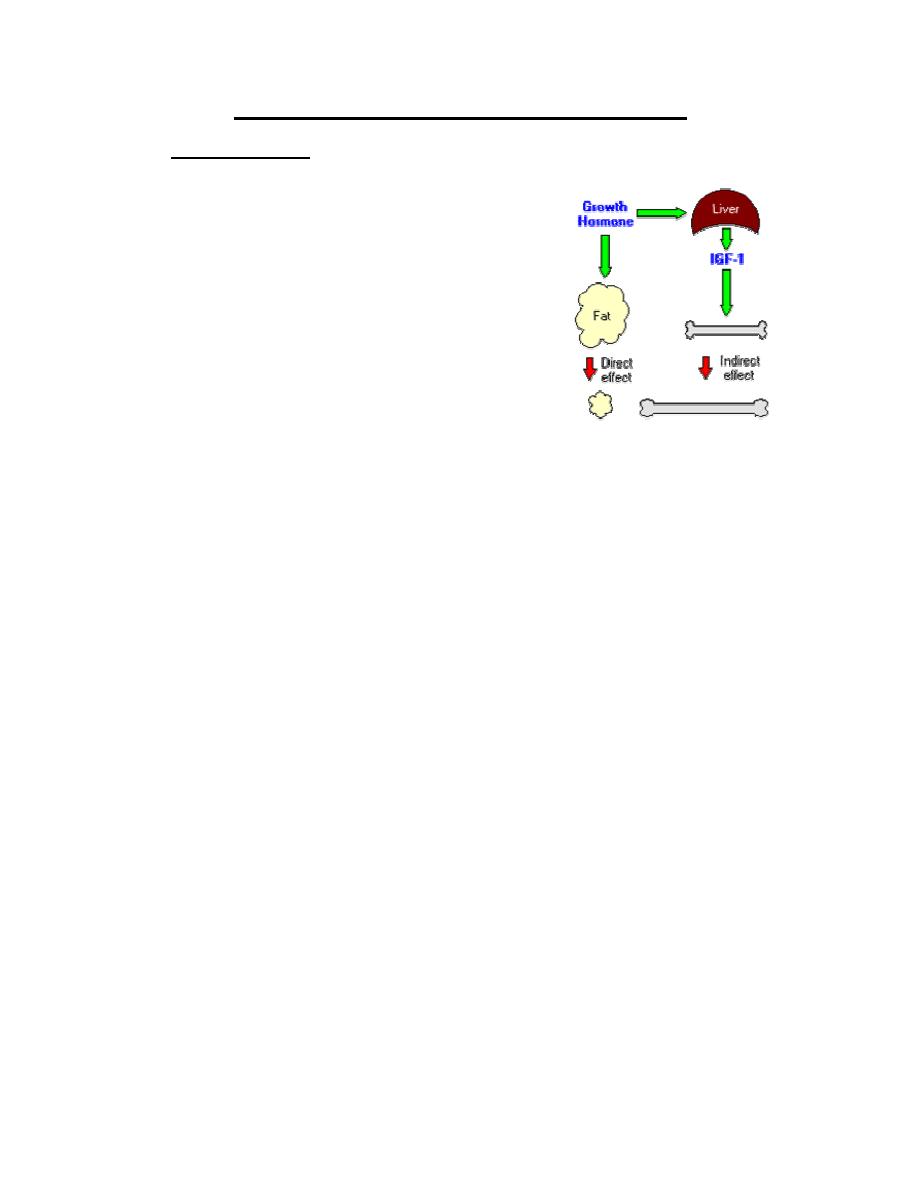
A critical concept in understanding growth hormone
activity is that it has two distinct types of effects:
•
Direct effects
are the result of growth hormone binding its receptor on target cells.
Fat cells (adipocytes), for example, have growth hormone receptors, and growth
hormone stimulates them to break down triglyceride and suppresses their ability to
take up and accumulate circulating lipids.
Indirect effects
are mediated primarily by a insulin-like growth factor-1 (IGF-1), a
hormone that is secreted from the liver and other tissues in response to growth hormone.
A majority of the growth promoting effects of growth hormone is actually due to IGF-1
acting on its target cells. IGF-1 also appears to be the key player in muscle growth. It
stimulates amino acid uptake and protein synthesis in muscle and other tissues.
Metabolic Effects
•
Protein metabolism: In general, growth hormone stimulates protein anabolism in
many tissues. This effect reflects increased amino acid uptake, increased protein
synthesis and decreased oxidation of proteins.
•
Fat metabolism: Growth hormone enhances the utilization of fat by stimulating
triglyceride breakdown and oxidation in adipocytes.
•
Carbohydrate metabolism: Growth hormone is one of a battery of hormones that
serves to maintain blood glucose within a normal range. Growth hormone is often
said to have anti-insulin activity, because it suppresses the abilities of insulin to
stimulate uptake of glucose in peripheral tissues and enhance glucose synthesis in
the liver.
•
Mineral metabolism: promotes a positive calcium, magnesium and phosphate
balance and causes the retention of Na
+
, K
+
and Cl
-
.
Prof.Dr. Hedef Dhafir El-Yassin 2012
38
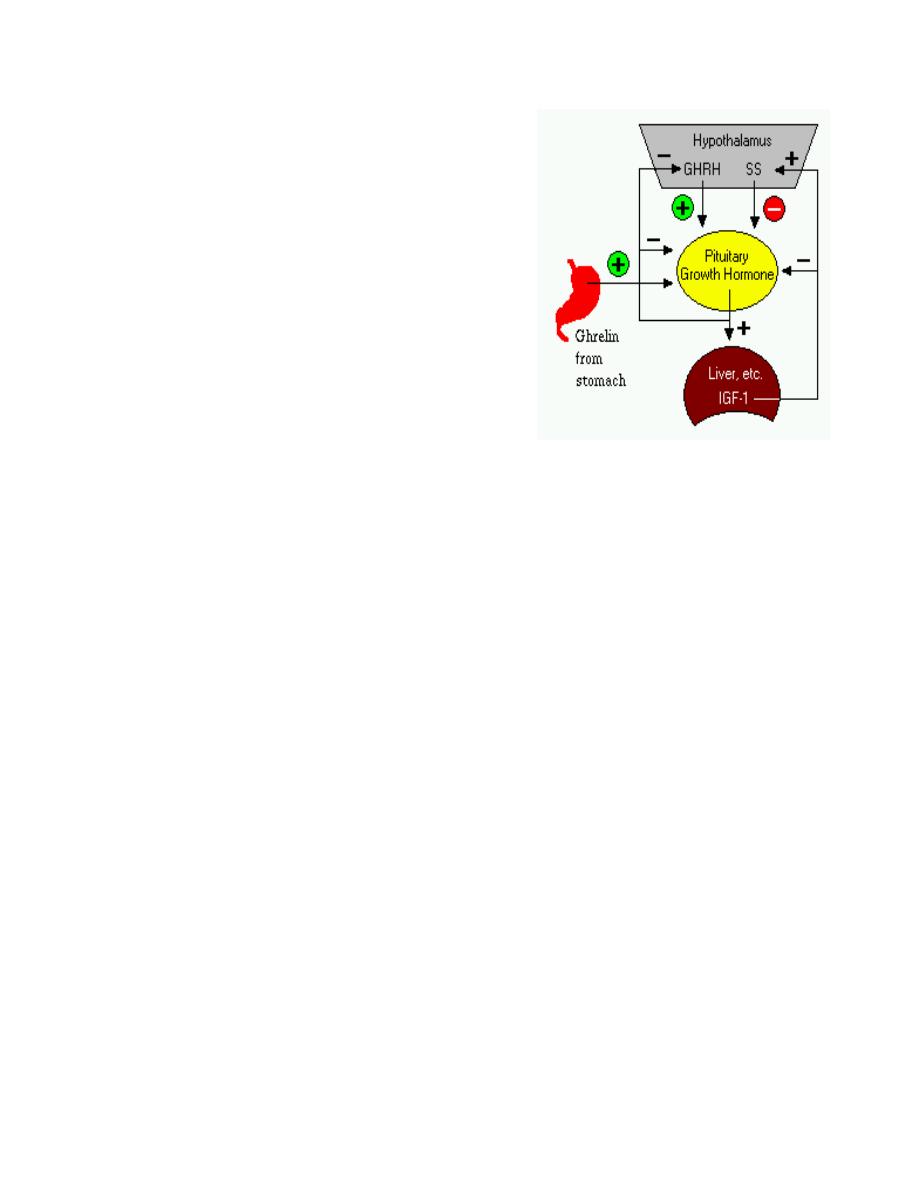
Control of Growth Hormone Secretion
Production of growth hormone is modulated by many
factors, including stress, exercise, nutrition, sleep and
growth hormone itself. However, its primary controllers
are two hypothalamic hormones and one hormone from
the stomach:
•
Growth hormone-releasing hormone (GHRH)
is a hypothalamic peptide that stimulates both
the synthesis and secretion of growth hormone.
•
Somatostatin (SS) is a peptide produced by
several tissues in the body, including the
hypothalamus. Somatostatin inhibits growth
hormone release in response to GHRH and to
other stimulatory factors such as low blood glucose concentration.
•
Ghrelin is a peptide hormone secreted from the stomach. Ghrelin binds to
receptors on somatotrophs and potently stimulates secretion of growth hormone.
Growth hormone secretion is also part of a negative feedback loop involving IGF-1.
High blood levels of IGF-1 lead to decreased secretion of growth hormone not only by
directly suppressing the somatotroph, but by stimulating release of somatostatin from the
hypothalamus.
Growth hormone also feeds back to inhibit GHRH secretion and probably has a direct
(autocrine) inhibitory effect on secretion from the somatotroph.
Integration of all the factors that affect growth hormone synthesis and secretion lead to a
pulsatile pattern of release. In children and young adults, the most intense period of growth
hormone release is shortly after the onset of deep sleep.
Disease States
A deficiency state can result not only from a deficiency in production of the hormone, but in
the target cell's response to the hormone.
Clinically, deficiency in growth hormone or receptor defects are known as growth
retardation or dwarfism. The manifestation of growth hormone deficiency depends upon
the age of onset of the disorder and can result from either heritable or acquired disease.
The effect of excessive secretion of growth hormone is also very dependent on the age of
onset and is seen as two distinctive disorders:
Prof.Dr. Hedef Dhafir El-Yassin 2012
39
•
Gigantism is the result of excessive growth hormone secretion that begins in young
children or adolescents. It is a very rare disorder, usually resulting from a tumor of
somatotropes.
•
Acromegaly results from excessive secretion of growth hormone in adults. The
excessive growth hormone and IGF-1 also lead to metabolic derangements,
including glucose intolerance.
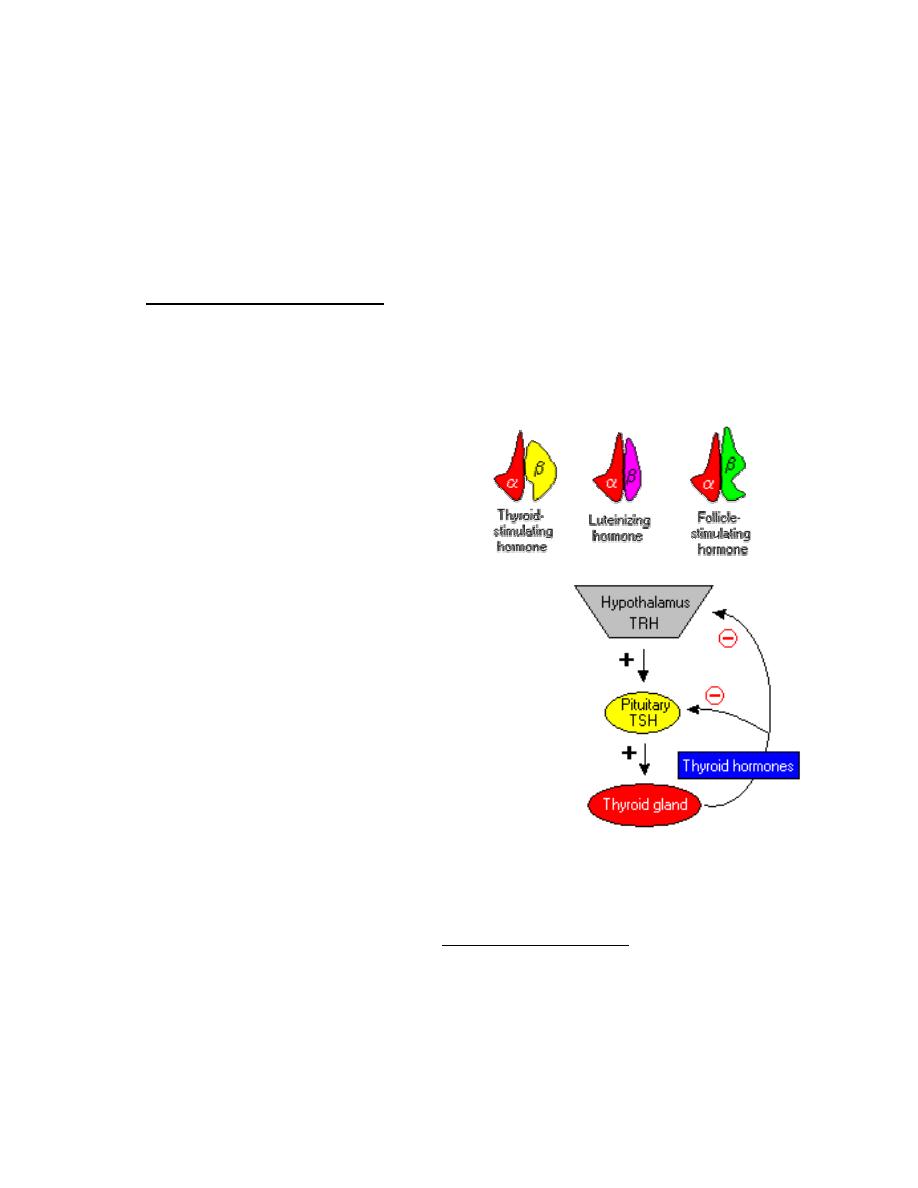
2. Thyroid Stimulating Hormone
Thyroid-stimulating hormone, also known as thyrotropin, is secreted from cells in
the anterior pituitary called thyrotrophs, finds its receptors on epithelial cells in the
thyroid gland, and stimulates that gland to synthesize and release thyroid
hormones.
TSH is a glycoprotein hormone composed of
two subunits, which are non-covalently bound
to one another. The alpha subunit of TSH is
also present in two other pituitary glycoprotein
hormones, follicle-stimulating hormone and
luteinizing hormone. In other words, TSH is composed of
alpha subunit bound to the TSH beta subunit, and TSH
associates only with its own receptor. Free alpha and beta
subunits have essentially no biological activity.
TSH has several acute effects on thyroid function. These
occur in minutes and involve increases of all phases of T
3
and T
4
biosynthesis. TSH also has several chronic effects
on the thyroid. These require several days and include
increases in the synthesis of proteins, phospholipids, and
nucleic acids and in the size of number of thyroid cells.
The most important controller of TSH secretion is thyroid-releasing hormone.
Secretion of thyroid-releasing hormone, and hence, TSH, is inhibited by high blood
levels of thyroid hormones in a classical negative feedback loop.
Prof.Dr. Hedef Dhafir El-Yassin 2012
40
3. Adrenocorticotropic Hormone
Adrenocorticotropic hormone, stimulates the adrenal cortex by enhancing the
conversion of cholesterol to pregnenolone. More specifically, it stimulates secretion
of glucocorticoids such as cortisol, and has little control over secretion of aldosterone,
the other major steroid hormone from the adrenal cortex. Another name for ACTH is
corticotropin.
ACTH is secreted from the anterior pituitary in response to corticotropin-releasing
hormone from the hypothalamus. Corticotropin-releasing hormone
is secreted in response to many types of stress, which makes sense
in view of the "stress management" functions of glucocorticoids.
Corticotropin-releasing hormone itself is inhibited by
glucocorticoids, making it part of a classical negative feedback
loop.
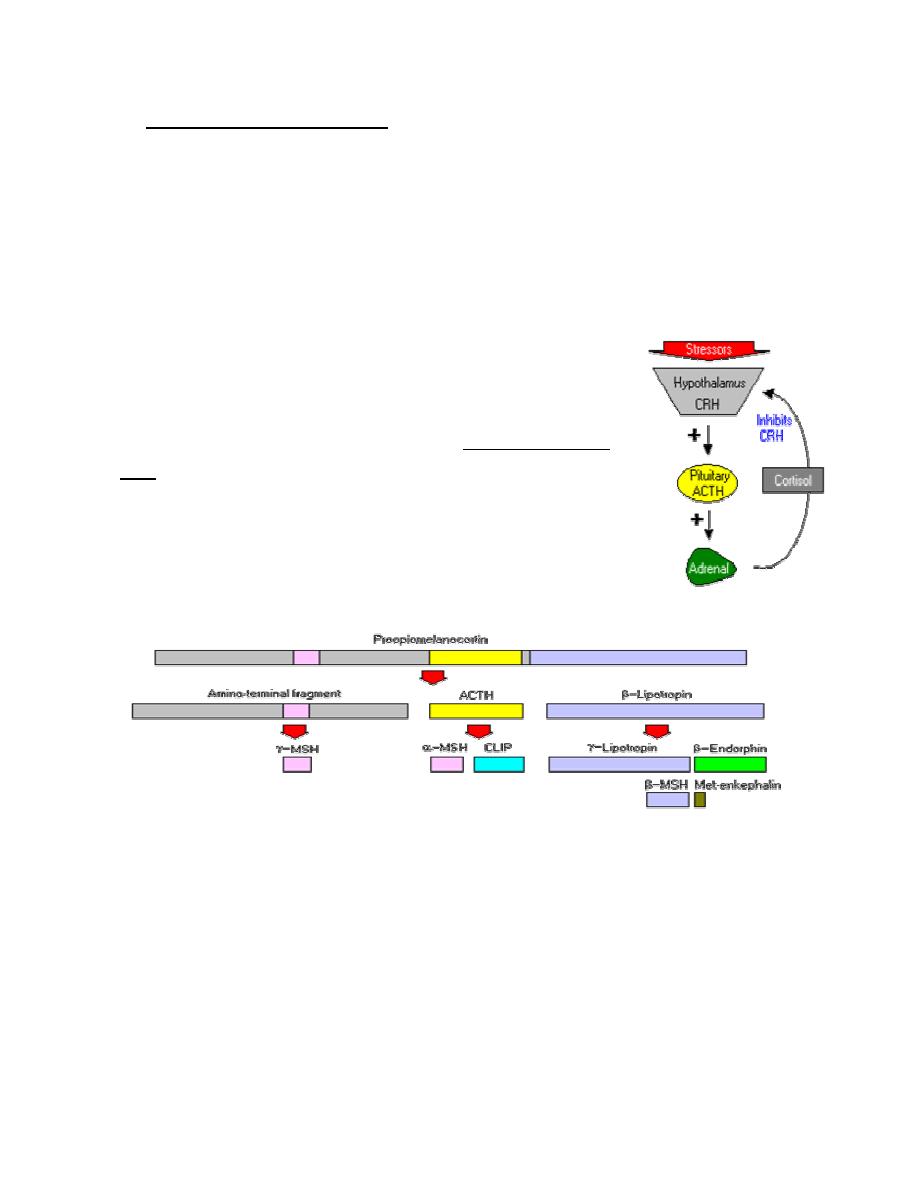
Within the pituitary gland, ACTH is produced in a process that
also generates several other hormones. A large precursor protein
named proopiomelanocortin (POMC) is synthesized and
proteolytically chopped into several fragments as depicted below.
The major attributes of the hormones other than ACTH that are produced in this process
are summarized as follows:
•
Lipotropin: Originally described as having weak lipolytic effects, its major
importance is as the precursor to beta-endorphin.
•
Beta-endorphin and Met-enkephalin: Opioid peptides with pain-alleviation and
euphoric effects.
•
Melanocyte-stimulating hormone (MSH): Known to control melanin pigmentation
in the skin of most vertebrates.
Prof.Dr. Hedef Dhafir El-Yassin 2012
41
4. Prolactin
Prolactin is a single-chain protein hormone closely related to growth hormone. It is
secreted by so-called lactotrophs in the anterior pituitary. It is also synthesized and
secreted by a broad range of other cells in the body.
Prolactin is synthesized as a prohormone. Following cleavage of the signal peptide, the
length of the mature hormone is between 194 and 199 amino acids, depending on
species. Hormone structure is stabilized by three intramolecular disulfide bonds.
Overall, several hundred different actions have been reported for prolactin in various
species. Some of its major effects are:
1. Mammary Gland Development, Milk Production and Reproduction
2. Effects on Immune Function
The prolactin receptor is widely expressed by immune cells, and some types of
lymphocytes synthesize and secrete prolactin. These observations suggest that prolactin
may act as an autocrine or paracrine modulator of immune activity..
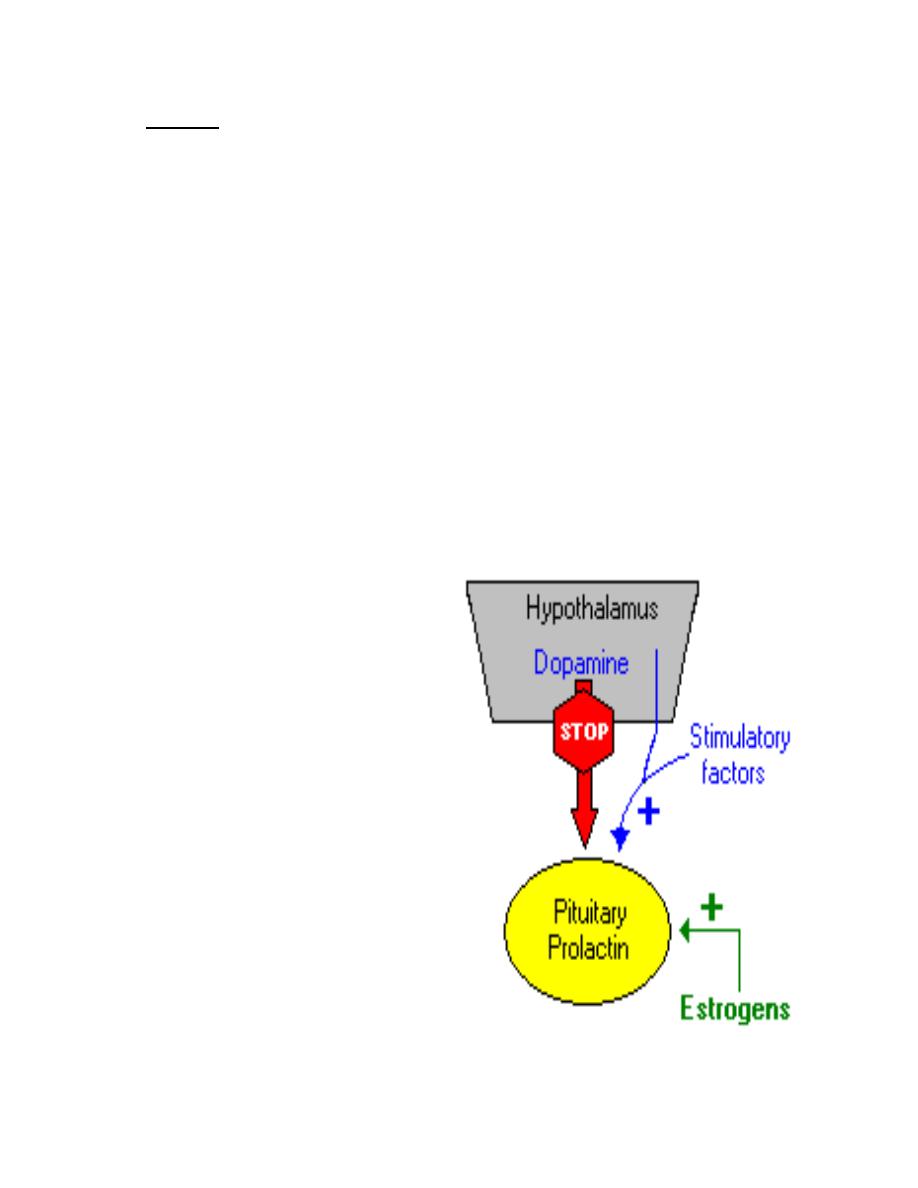
Control of Prolactin Secretion
In contrast to what is seen with all the
other pituitary hormones, the
hypothalamus suppresses prolactin
secretion from the pituitary.
Dopamine serves as the major prolactin-
inhibiting factor or brake on prolactin
secretion. In addition to inhibition by
dopamine, prolactin secretion is positively
regulated by several hormones, including
thyroid-releasing hormone, gonadotropin-
releasing hormone and vasoactive
intestinal polypeptide.
Estrogens provide a well-studied
positive control over prolactin synthesis
and secretion.
Prof.Dr. Hedef Dhafir El-Yassin 2012
42
5. Gonadotropins: Luteinizing and Follicle Stimulating Hormones
Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are called
gonadotropins because stimulate the gonads - in males, the testes, and in females,
the ovaries. As described for thyroid-simulating hormone, LH and FSH are large
glycoproteins composed of alpha and beta subunits. The alpha subunit is identical in all
three of these anterior pituitary hormones, while the beta subunit is unique for each
hormone with the ability to bind its own receptor.
a.
Luteinizing Hormone
In both sexes, LH stimulates secretion of sex steroids from the gonads. In the testes,
it stimulates the synthesis and secretion of testosterone. The ovary respond to LH
stimulation by secretion of testosterone, which is converted into estrogen by adjacent
granulosa cells.
LH is required for continued development and function of corpora lutea. The name
luteinizing hormone derives from this effect of inducing luteinization of ovarian follicles.
b.
Follicle-Stimulating Hormone
As its name implies, FSH stimulates the maturation of ovarian follicles. FSH is also
critical for sperm production. It supports the function of Sertoli cells, which in turn
support many aspects of sperm cell maturation.
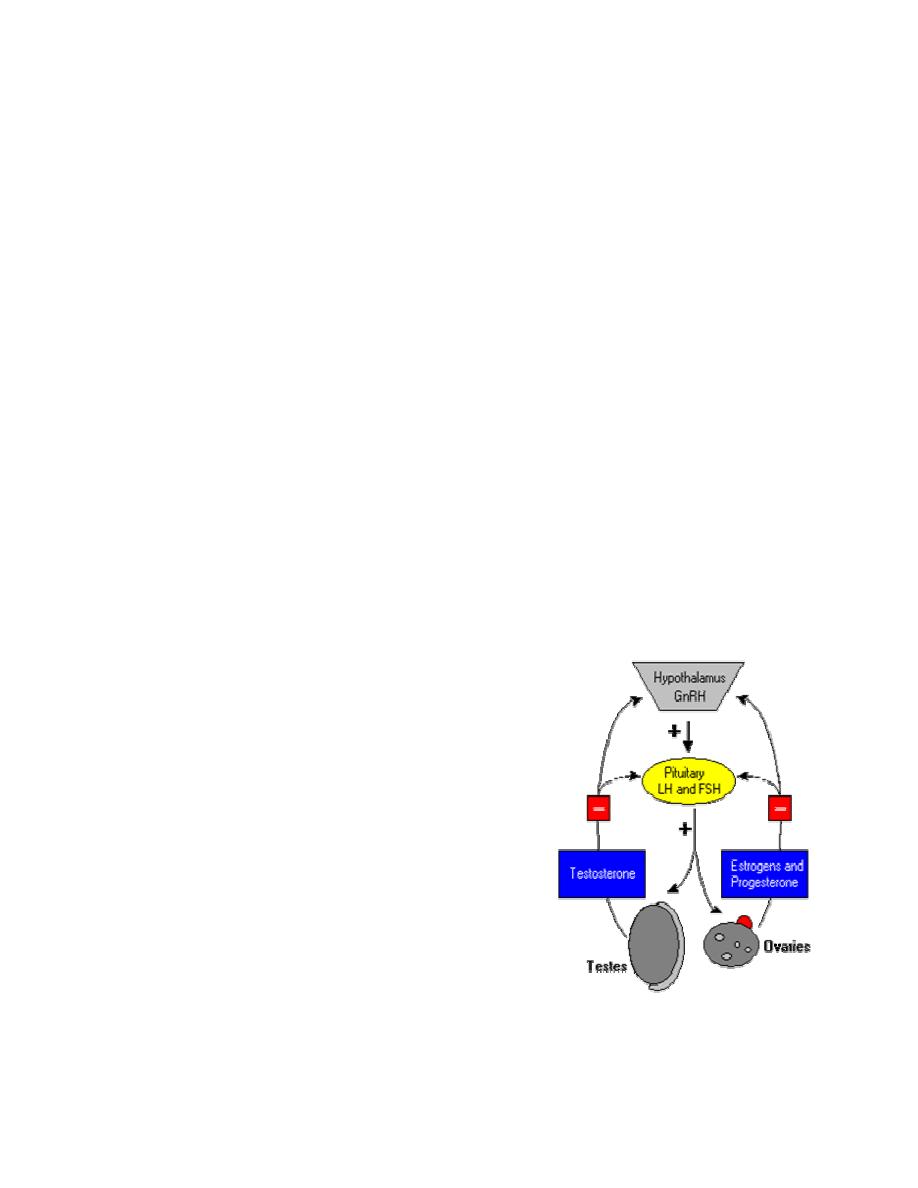
Control of Gonadotropin Secretion
The principle regulator of LH and FSH secretion is
gonadotropin-releasing hormone or GnRH (also
known as LH-releasing hormone). In a classical
negative feedback loop, sex steroids inhibit secretion of
GnRH and also appear to have direct negative effects
on gonadotrophs.
This regulatory loop leads to pulsatile secretion of LH
and, to a much lesser extent, FSH. Numerous
hormones influence GnRH secretion, and positive and
negative control over GnRH and gonadotropin secretion
is actually considerably more complex than described in
the figure. For example, the gonads secrete at least two additional hormones - inhibin and
activin , which selectively inhibit and activate FSH secretion from the pituitary.
Prof.Dr. Hedef Dhafir El-Yassin 2012
43
Posterior Pituitary Hormones
1. Antidiuretic Hormone (Vasopressin)
Roughly, 60% of the mass of the body is water, and despite wide
variation in the amount of water taken in each day, body water
content remains incredibly stable. Such precise control of body
water and solute concentrations is a function of several hormones
acting on both the kidneys and vascular system, but there is no doubt
that antidiuretic hormone is a key player in this process.
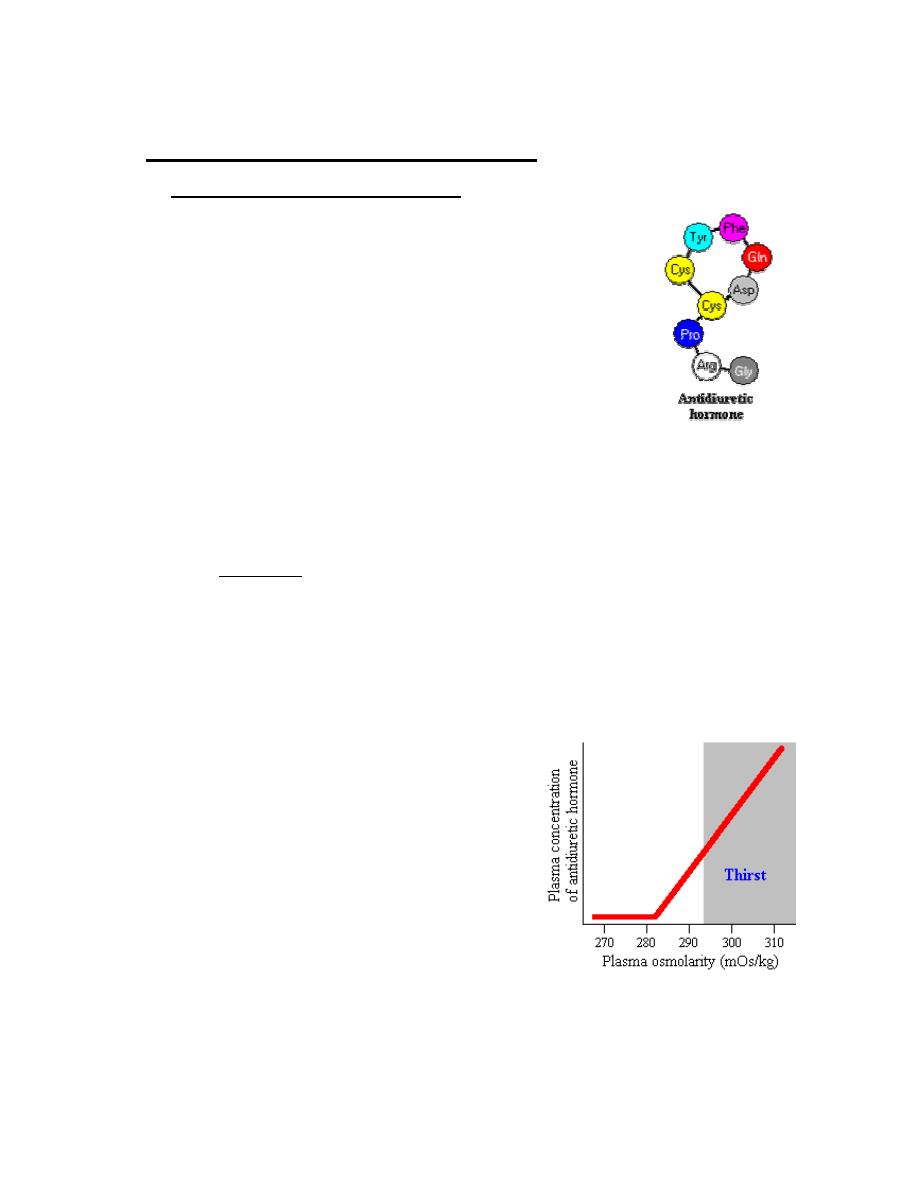
Antidiuretic hormone, also known as vasopressin, is a nine
amino acid peptide secreted from the posterior pituitary.
Physiologic Effects of Antidiuretic Hormone
The single most important effect of antidiuretic hormone is to conserve body water by
reducing the output of urine.
Antidiuretic hormone stimulates water reabsorbtion by stimulating insertion of "water
channels" or aquaporins into the membranes of kidney tubules. These channels transport
solute-free water through tubular cells and back into blood, leading to a decrease in
plasma osmolarity and an increase osmolarity of urine.
Control of Antidiuretic Hormone Secretion
1. The most important variable regulating antidiuretic hormone secretion is
plasma osmolarity, or the concentration of solutes in blood. When plasma
osmolarity is below a certain threshold, the
osmoreceptors are not activated and antidiuretic
hormone secretion is suppressed. When
osmolarity increases above the threshold, the
ever-alert osmoreceptors recognize this and
stimulate the neurons that secrete antidiuretic
hormone. As seen the figure, antidiuretic
hormone concentrations rise steeply and linearly
with increasing plasma osmolarity.
2. Secretion of antidiuretic hormone is also simulated by decreases in blood
pressure and volume, conditions sensed by stretch receptors in the heart and
large arteries. Changes in blood pressure and volume are not nearly as sensitive a
stimulator as increased osmolarity, but are nonetheless potent in severe conditions.

Prof.Dr. Hedef Dhafir El-Yassin 2012
44
For example, Loss of 15 or 20% of blood volume by hemorrhage results in massive
secretion of antidiuretic hormone.
Another potent stimulus of antidiuretic hormone is nausea and vomiting.
Disease States
The most common disease of man and animals related to antidiuretic hormone is diabetes
insipidus. This condition can arise from either of two situations:
•
Hypothalamic ("central") diabetes insipidus
results from a deficiency in
secretion of antidiuretic hormone from the posterior pituitary. Causes of this disease
include head trauma, and infections or tumors involving the hypothalamus.
•
Nephrogenic diabetes insipidus
occurs when the kidney is unable to respond to
antidiuretic hormone. Most commonly, this results from some type of renal disease,
but mutations in the ADH receptor gene or in the gene encoding aquaporin-2 have
also been demonstrated in affected humans.
The major sign of either type of diabetes insipidus is excessive urine production.
Some human patients produce as much as 16 liters of urine per day! If adequate water is
available for consumption, the disease is rarely life-threatening, but withholding water can
be very dangerous.
2. Oxytocin
Oxytocin in a nine amino acid peptide that is synthesized in hypothalamic neurons and
transported down axons of the posterior pituitary for secretion into
blood. Oxytocin differs from antidiuretic hormone in two of the nine
amino acids. Both hormones are packaged into granules and
secreted along with carrier proteins called neurophysins.
Control of Oxytocin Secretion
A number of factors can inhibit oxytocin release, among them acute
stress. For example, oxytocin neurons are repressed by
catecholamines, which are released from the adrenal gland in response to many types of
stress, including fright.

Prof.Dr. Hedef Dhafir El-Yassin 2012
45
Clinical correlation
Testing Activity of the Anterior Pituitary
Releasing hormones and chemical analogs, particularly of the smaller peptides, are now
routinely synthesized. The gonadotropin-releasing hormone, a decapeptide, is available for
use in assessing the function of the anterior pituitary. This is of importance when a disease
situation may involve either the hypothalamus, the anterior pituitary, or the end organ.
Infertility is an example of such a situation. What needs to be assessed in which organ is
at fault in the hormonal cascade. Initially, the end organ, in this case the gonads, must be
considered. This can be accomplished by injecting the anterior pituitary hormone LH or
FSH. If sex hormone secretion is elicited, then the ultimate gland would appear to be
functioning properly. Next, the anterior pituitary would need to be analyzed. This can be
done by i.v. administration of synthetic GnRH; by this route, GnRH can gain access to the
gonadotropic cells of the anterior pituitary and elicit secretion of LH and FSH. Routinely,
LH levels are measured in the blood as a function of time after the injection. These levels
are measured by radioimmunoassay (RIA) in which radioactive LH or hCG is displaced
from binding to an LH-binding protein by LH in the serum sample. The extent of the
competition is proportional to the amount of LH in the serum. In this way a progress of
response is measured that will be within normal limits or clearly deficient. If the response is
deficient, the anterior pituitary cells are not functioning normally and are the cause of the
syndrome. On the other hand, normal pituitary response to GnRH would indicate that the
hypothalamus was non-functional. Such a finding would prompt examination of the
hypothalamus for conditions leading to insufficient availability/production of releasing
hormones. Obviously, the knowledge of hormone structure and the ability to synthesize
specific hormones permits the diagnosis of these disease states.
Source: Marshall, J. C. and Barkan, A. L., "Disorders of the hypothalamus and anterior
pituitary. In: W. N. Kelley (Ed.), Internal Medicine." New York: Lippincott, 1989, p. 2759.
Conn, P. M. "The molecular basis of gonadotropin-releasing hormone action". Endocr.
Rev. 7:3, 1986.

Prof.Dr. Hedef Dhafir El-Yassin 2012
46
Clinical correlation
Hypopituitarism
The hypothalamus is connected to the anterior pituitary by a delicate stalk that contains
the portal system through which releasing hormones, secreted from the hypothalamus,
gain access to the anterior pituitary cells. In the cell membranes of these cells are specific
receptors for releasing hormones. In most cases, different cells express deferent releasing
hormone receptors. The connection between the hypothalamus and anterior pituitary can
be disrupted by trauma or tumors. Trauma can occur in automobile accidents or other local
damaging events that may result in severing of the stalk, thus preventing the releasing
hormones from reaching their target anterior pituitary cells. When this happens, the
anterior pituitary cells no longer have their signaling mechanism for the release of anterior
pituitary hormones. In the case of tumors of the pituitary gland, all of the anterior pituitary
hormones may not be shut off to the same degree or the secretion of some may disappear
sooner than others.
In any case, if the hypopituitarism occurs, this condition may result in a life-threatening
situation in which the clinician must determine the extent of loss of pituitary hormones,
especially ACTH. Posterior pituitary hormones (Oxytocin and vasopressin) may also be
lost, precipitating a problem of excessive urination (vasopressin deficiency) that must be
addressed. The usual therapy involves administration of the end-organ hormones, such
as, thyroid hormone, cortisol, sex hormones, and progestin; with female patients it is also
necessary to maintain the ovarian cycle. These hormones can be easily administered in
the oral form. Growth hormone deficiency is not a problem in the adult but would be an
important problem in a growing child.
The patient must learn to anticipate needed increases of cortisol in the face of stressful
situations. Fortunately, these patients are usually maintained in reasonably good condition.
Source: Marshall, J. C. and Barkan, A. L., "Disorders of the hypothalamus and anterior
pituitary. In: W. N. Kelley (Ed.), Internal Medicine." New York: Lippincott, 1989, p.
2159.Robinson,A.G."Disorders of the posterior pituitary". In: W. N. Kelley(Ed.), Internal
Medicine. New York:

Prof.Dr. Hedef Dhafir El-Yassin 2012
47
Question : Hypopituitarism may result from trauma, such as an automobile accident
severing the stalk connecting the hypothalamus and anterior pituitary, or from tumors of
the pituitary gland. In trauma, usually all of the releasing hormones from hypothalamus fail
to reach the anterior pituitary. With a tumor of the gland, some or all of the pituitary
hormones may be shut off. Posterior pituitary hormones may also be lost. Hypopituitarism
can be life threatening. Usual therapy is administration of end-organ hormones in oral
form.
1) If the stalk between the hypothalamus and anterior pituitary is severed, the pituitary
would fail to cause the ultimate release of all of the following hormones except :
a) ACTH.
b) estradiol.
c) oxytocin.
d) testosterone.
e) thyroxine.
Answers:
1) C Oxytocin is released from posterior pituitary. A, B, D, and E all require releasing
hormones from hypothalamus for anterior pituitary to release them.

Prof.Dr. Hedef Dhafir El-Yassin 2012
48
Lecture 5
Sunday 4/3
Biochemistry and Disorder of Hormones of the
Thyroid and Parathyroid Glands
1.
The Thyroid Gland
The thyroid gland (Greek thyros “shield”) is shaped like a shield and lies just below
the Adam’s apple in the front of the neck.
The thyroid gland secretes thyroxine and smaller amounts of triiodothyronine (T3),
which stimulate oxidative respiration in most cells in the body and, in so doing, help
set the body’s basal metabolic rate. In children, these thyroid hormones also promote
growth and stimulate maturation of the central nervous system. Children with
underactive thyroid glands are therefore stunted in their growth and suffer severe
mental retardation, a condition called cretinism. This differs from pituitary dwarfism,
which results from inadequate GH and is not associated with abnormal intellectual
development.
People who are hypothyroid (whose secretion of thyroxine is too low) can take
thyroxine orally, as pills. Only thyroxine and the steroid hormones (as in
contraceptive pills), can be taken orally because they are nonpolar and can pass
through the plasma membranes of intestinal epithelial cells without being digested.
The thyroid gland also secretes calcitonin, a peptide hormone that plays a role in
maintaining proper levels of calcium (Ca++) in the blood. When the blood Ca++
concentration rises too high, calcitonin stimulates the uptake of Ca++ into bones, thus
lowering its level in the blood.
Prof.Dr. Hedef Dhafir El-Yassin 2012
49
Thyroid Hormones
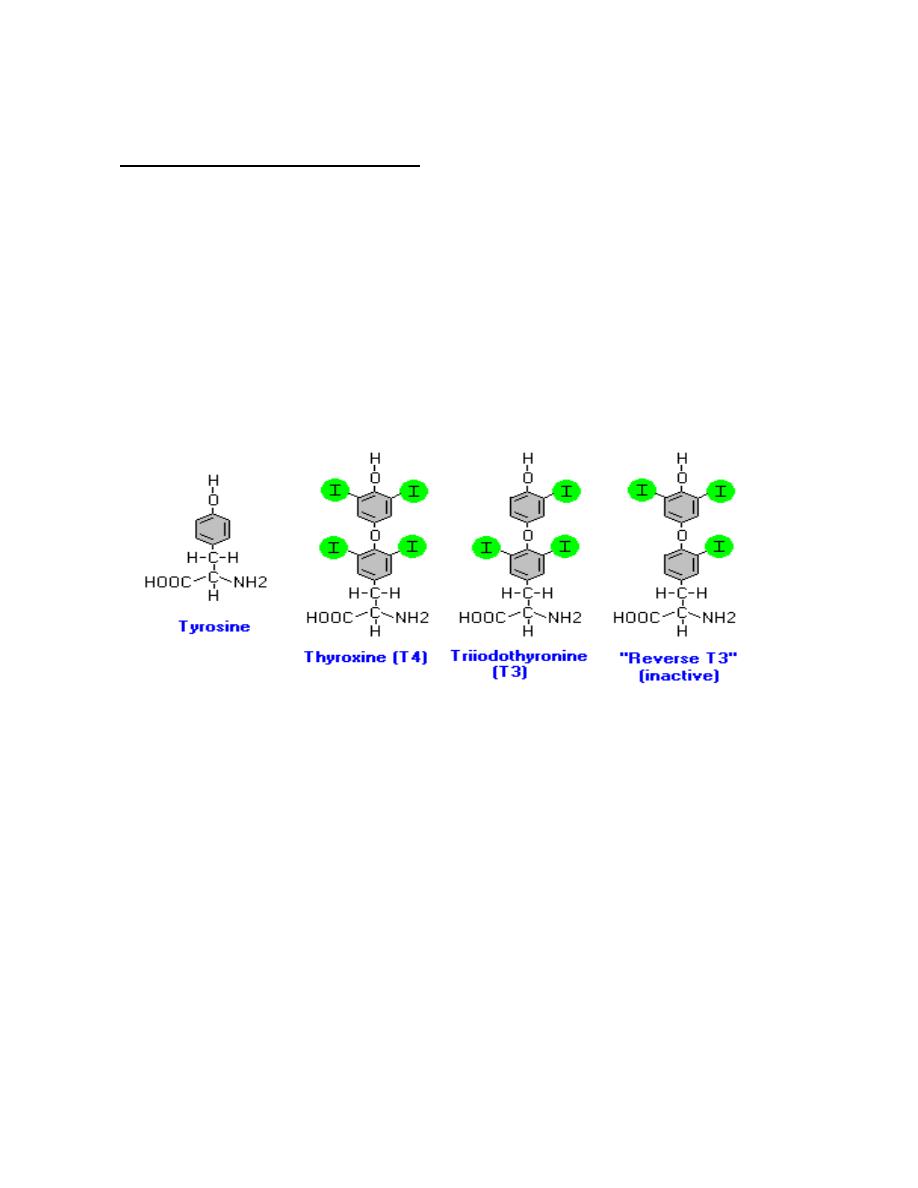
1. Thyroxin (T4) and triiodotyonine T3
Biochemistry of Thyroid Hormones
Thyroid hormones are derivatives of the amino acid tyrosine bound covalently to
iodine. The two principal thyroid hormones are:
•
thyroxine (T
4
or L-3,5,3',5'-tetraiodothyronine)
•
triiodotyronine (T
3
or L-3,5,3'-triiodothyronine).
Thyroid hormones are basically two tyrosines linked together with the critical
addition of iodine at three or four positions on the aromatic rings. The number
and position of the iodines is important. Several other iodinated molecules are
generated that have little or no biological activity; so called "reverse T
3
"
A large majority of the thyroid hormone secreted from the thyroid gland is T
4
,
but T
3
is the considerably more active hormone. Although some T
3
is also
secreted, the bulk of the T
3
is derived by deiodination of T
4
in peripheral tissues,
especially liver and kidney. Deiodination of T
4
also yields reverse T
3
, a molecule
with no known metabolic activity.
Thyroid hormones are poorly soluble in water, and more than 99% of the T
3
and T
4
circulating in blood is bound to carrier proteins. The principle carrier of thyroid
hormones is thyroxine-binding globulin, a glycoprotein synthesized in the liver.
Another carrier is albumin.
Prof.Dr. Hedef Dhafir El-Yassin 2012
50
Synthesis and Secretion of Thyroid Hormones
The entire synthetic process occurs in three major steps:
•
Production and accumulation of the raw
materials
•
Fabrication or synthesis of the hormones on a
backbone or scaffold of precursor
•
Release of the free hormones from the
scaffold and secretion into blood
Raw materials:
•
Tyrosines are provided from a large
glycoprotein scaffold called thyroglobulin,.
A molecule of thyroglobulin contains 134 tyrosines, although only a handful of
these are actually used to synthesize T
4
and T
3
.
•
Iodine, or more accurately iodide (I
-
), is taken up from blood by thyroid epithelial
cells, which have on their outer plasma membrane an "iodine trap". Once inside
the cell, iodide is transported into the lumen of the follicle along with
thyroglobulin.
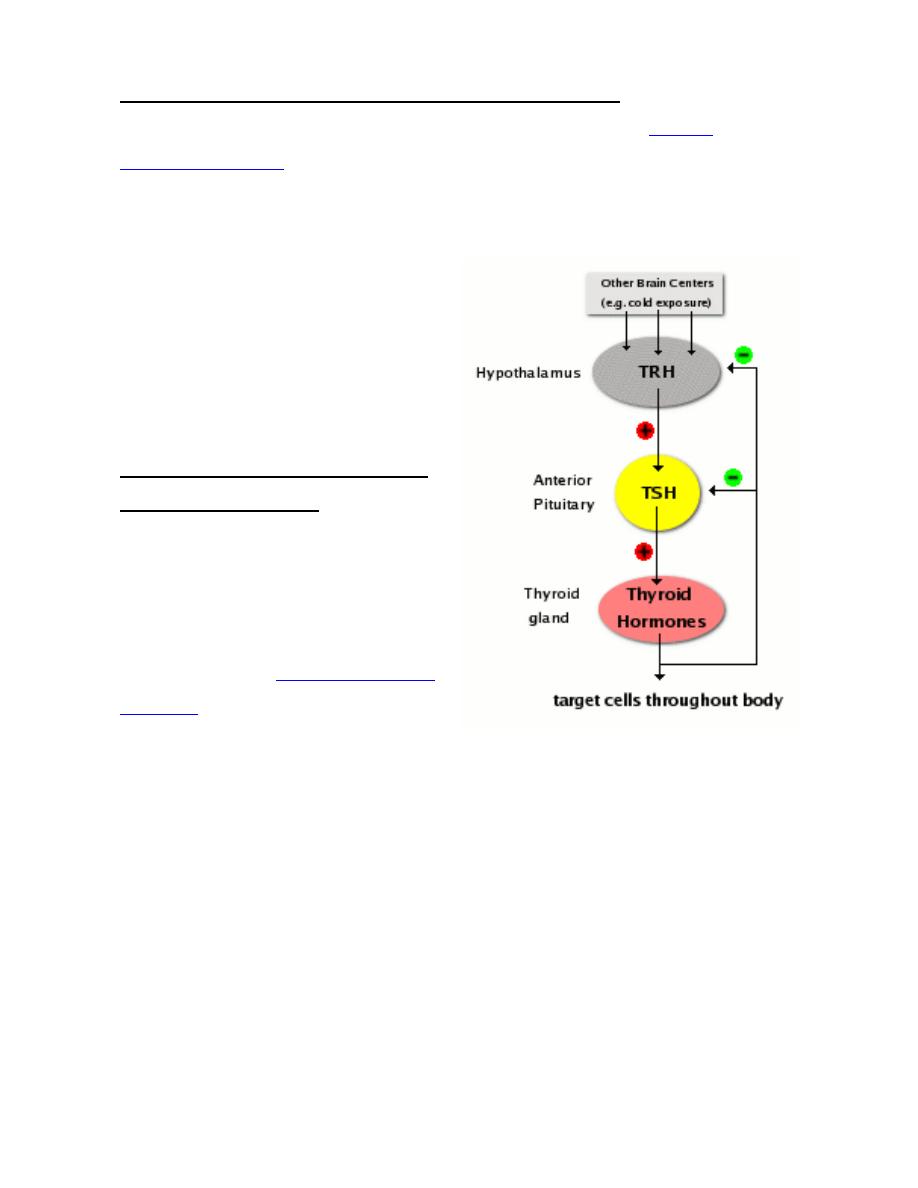
Fabrication of thyroid hormones is conducted by the enzyme thyroid peroxidase,
Thyroid peroxidase catalyzes two sequential reactions:
1. Iodination of tyrosines on thyroglobulin (also known as "organification of
iodide").
2. Synthesis of thyroxine or triiodothyronine from two iodotyrosines.
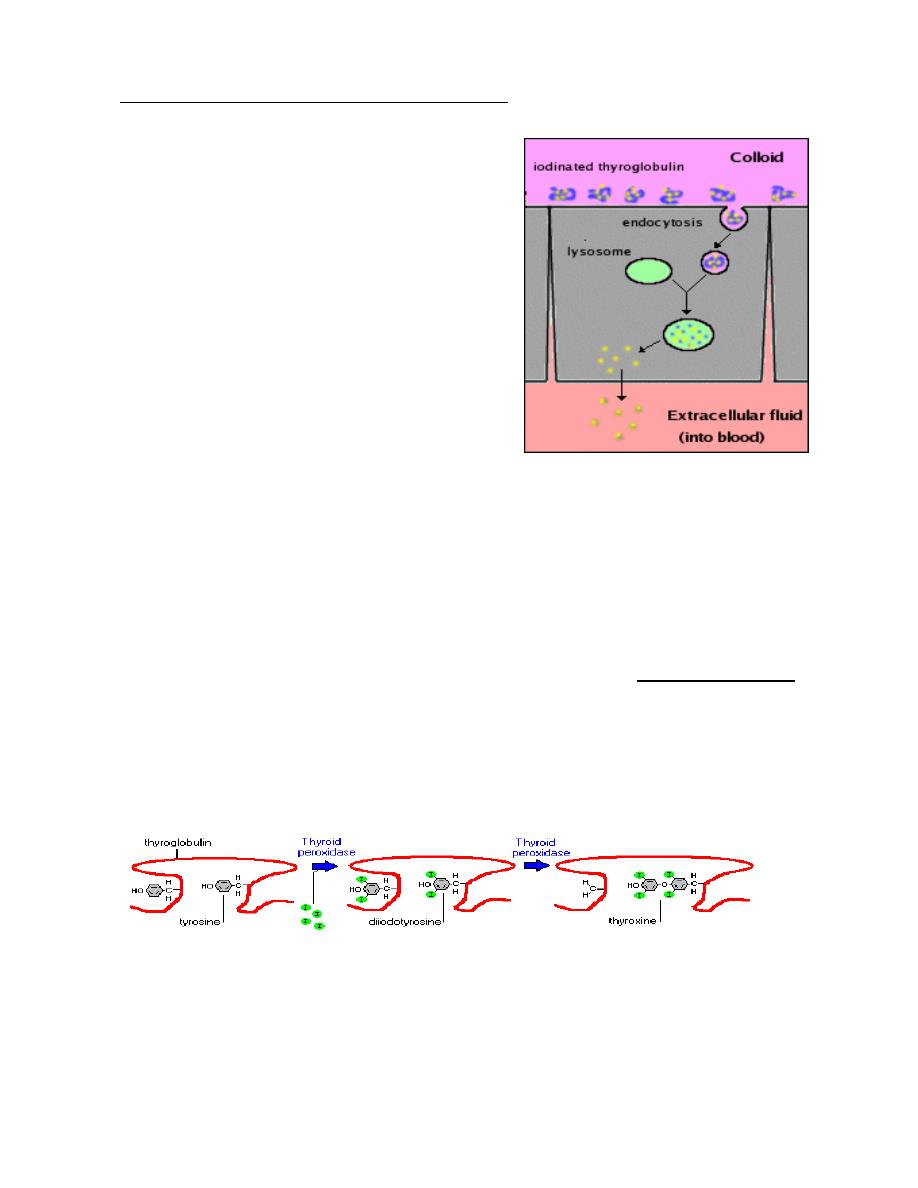
Thyroid hormones are excised from their thyroglobulin scaffold by digestion in
lysosomes of thyroid epithelial cells. Free thyroid hormones apparently diffuse out
of lysosomes, through the basal plasma membrane of the cell, and into blood where
they quickly bind to carrier proteins for transport to target cells.
Prof.Dr. Hedef Dhafir El-Yassin 2012
51
Control of Thyroid Hormone Synthesis and Secretion
Each of the processes described above appears to be stimulated by
thyroid-
stimulating hormone
from the anterior pituitary gland. Binding of TSH to its
receptors on thyroid epithelial cells stimulates synthesis of the iodine transporter,
thyroid peroxidase and thyroglobulin. When TSH levels are low, rates of thyroid
hormone synthesis and release diminish.
The thyroid gland is part of the
hypothalamic-pituitary-thyroid axis, and
control of thyroid hormone secretion is
exerted by classical negative feedback.
Thyroid Hormone Receptors and
Mechanism of Action
Receptors for thyroid hormones are
intracellular DNA-binding proteins that
function as hormone-responsive
transcription factors, very similar
conceptually to the
receptors for steroid
hormones
.
Thyroid hormones enter cells through
membrane transporter proteins. A number of plasma membrane transporters have
been identified, some of which require ATP hydrolysis; the relative importance of
different carrier systems is not yet clear and may differ among tissues. Once inside
the nucleus, the hormone binds its receptor, and the hormone-receptor complex
interacts with specific sequences of DNA in the promoters of responsive genes.
The effect of receptor binding to DNA is to modulate gene expression, either by
stimulating or inhibiting transcription of specific genes.
Prof.Dr. Hedef Dhafir El-Yassin 2012
52
Metabolic Effects of Thyroid Hormones
Thyroid hormones have profound effects on many physiologic processes, such as
development, growth and metabolism. They stimulate diverse metabolic activities
most tissues, leading to an increase in basal metabolic rate. One consequence of
this activity is to increase body heat production, which seems to result, at least in
part, from increased oxygen consumption and rates of ATP hydrolysis. A few
examples of specific metabolic effects of thyroid hormones include:
•
Lipid metabolism: Increased thyroid hormone levels stimulate fat
mobilization, leading to increased concentrations of fatty acids in plasma. They
also enhance oxidation of fatty acids in many tissues. Finally, plasma
concentrations of cholesterol and triglycerides are inversely correlated with
thyroid hormone levels - one diagnostic indication of hypothyroidism is
increased blood cholesterol concentration.
•
Carbohydrate metabolism: Thyroid hormones stimulate almost all aspects of
carbohydrate metabolism, including enhancement of insulin-dependent entry of
glucose into cells and increased gluconeogenesis and glycogenolysis to
generate free glucose.
Other Effects: A few additional, effects of thyroid hormones include:
•
On muscle: T3 increases glucose uptake by muscle cells it also stimulate
protein synthesis and therefore growth of muscle through its stimulatory
actions on gene expression. Thyroid hormone sensitizes the muscle cell to the
glycogenolytic actions of epinephrine. Glycolysis in muscle is increased by
this action of T
3
.
•
On the pancreas: thyroid hormone increases the sensitivity of the
β
cells of
the pancreas to those stimuli that normally promote insulin release and is
required for optimal insulin secretion
•
On Cardiovascular system: Thyroid hormones increases heart rate, cardiac
contractility and cardiac output. They also promote vasodilation, which leads
to enhanced blood flow to many organs.

Prof.Dr. Hedef Dhafir El-Yassin 2012
53
•
On Central nervous system: Both decreased and increased concentrations of
thyroid hormones lead to alterations in mental state. Too little thyroid
hormone, and the individual tends to feel mentally sluggish, while too much
induces anxiety and nervousness.
•
On Reproductive system: Normal reproductive behavior and physiology is
dependent on having essentially normal levels of thyroid hormone.
Hypothyroidism in particular is commonly associated with infertility.
Thyroid Disease States
1. Hypothyroidism
2. Hyperthyroidism
a. . Graves's disease.
2. Calcitonin
Calcitonin is a hormone secreted from the parafolicular of C cells in the thyroid
gland, known to participate in calcium and phosphorus metabolism.
Calcitonin is a 32 amino acid peptide cleaved from a larger prohormone. It contains a
single disulfide bond, which causes the amino terminus to assume the shape of a ring.
Physiologic Effects of Calcitonin
Calcitonin plays a role in calcium and phosphorus metabolism. In particular,
calcitonin has the ability to decrease blood calcium levels at least in part by
effects on two well-studied target organs:
•
Bone: Calcitonin suppresses resorption of bone by inhibiting the activity of
osteoclasts, a cell type that "digests" bone matrix, releasing calcium and
phosphorus into blood.
•
Kidney: Calcium and phosphorus are prevented from being lost in urine by
reabsorption in the kidney tubules. Calcitonin inhibits tubular reabsorption of
these two ions, leading to increased rates of their loss in urine.

Prof.Dr. Hedef Dhafir El-Yassin 2012
54
Control of Calcitonin Secretion
The most prominent factor controlling calcitonin secretion is the extracellular
concentration of ionized calcium. Elevated blood calcium levels strongly stimulate
calcitonin secretion, and secretion is suppressed when calcium concentration falls
below normal.
Disease States
A large number of diseases are associated with abnormally increased or decreased
levels of calcitonin, but pathologic effects of abnormal calcitonin secretion per se are
not generally recognized.
2.
The Parathyroid Glands and Calcium Homeostasis
The parathyroid glands are four small glands attached to the thyroid. Because of their
size, researchers ignored them until well into this century.
The hormone produced by the parathyroid glands is a peptide called parathyroid
hormone (PTH). It is one of only two hormones in humans that are absolutely
essential for survival (the other is aldosterone). PTH is synthesized and released in
response to falling levels of Ca++ in the blood.
Hormones that regulate calcium metabolism
Calcium ions regulate a number of important physiological and biochemical process.
These include:
1. neuromuscular excitability
2. blood coagulation
3. secretory processes
4. membrane integrity and plasma membrane transport
5. enzyme reactions
6. the release of hormones and neurotransmitters
7. and the intracellular action of a number of hormones.
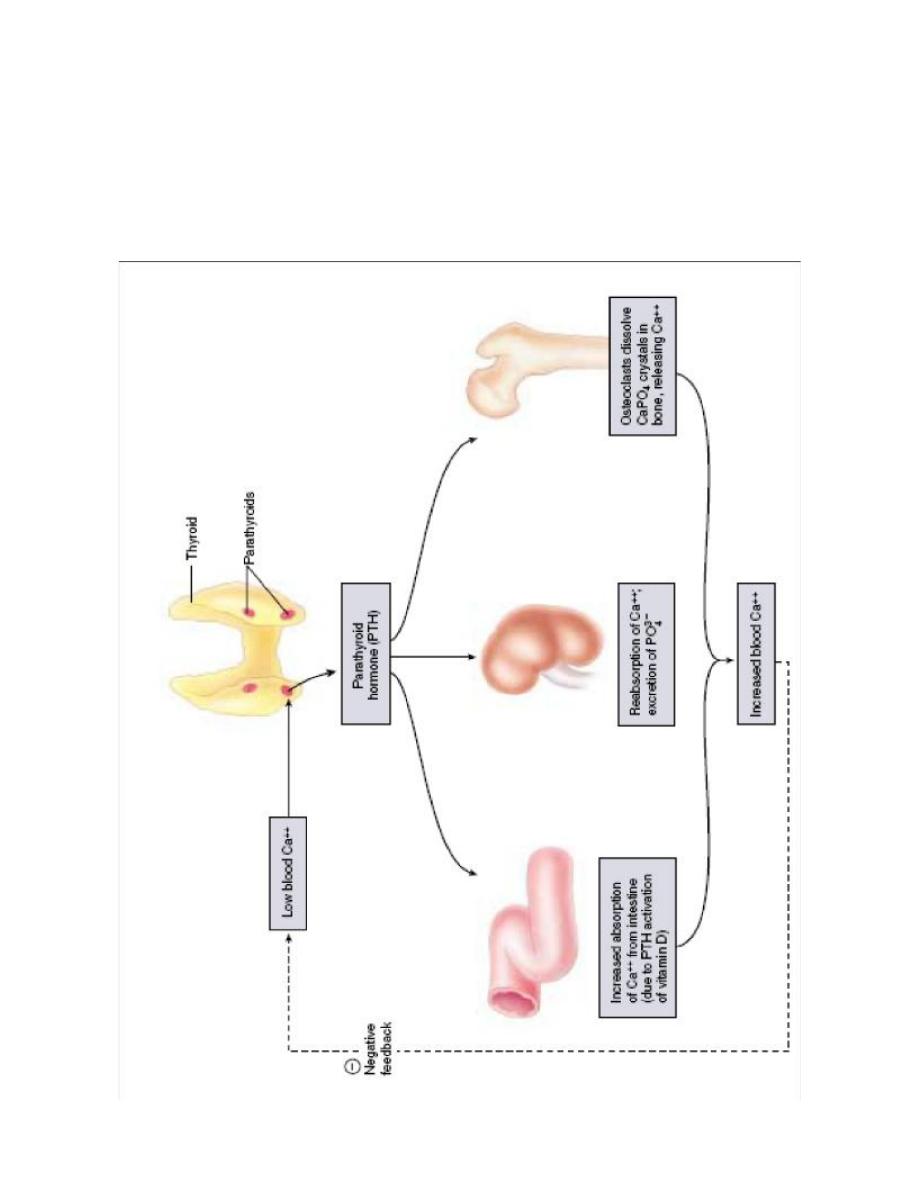
Prof.Dr. Hedef Dhafir El-Yassin 2012
55
In addition the proper extracellular fluid and periosteal concentration of Ca
+2
and
PO
4
-3
are required for bone mineralization.

Prof.Dr. Hedef Dhafir El-Yassin 2012
56
To ensure that these processes operate normally, the plasma Ca
+2
concentration is
maintained within very narrow limits by the actions of the following hormones:
1. Parathyroid Hormone (PTH)
Parathyroid hormone is the most important endocrine regulator of calcium and
phosphorus concentration in extracellular fluid. This hormone is secreted from
cells of the parathyroid glands and finds its major target cells in bone and kidney.
Like most other protein hormones, parathyroid hormone is synthesized as a
preprohormone. After intracellular processing, the mature hormone is packaged
within the Golgi into secretory vesicles, then secreted into blood by exocytosis.
Parathyroid hormone is secreted as a linear protein of 84 amino acids.
Physiologic Effects of Parathyroid Hormone
If calcium ion concentrations in extracellular fluid fall below normal, PTH
brings them back within the normal range. In conjunction with increasing calcium
concentration, the concentration of phosphate ion in blood is reduced. Parathyroid
hormone accomplishes its job by stimulating at least three processes:
•
Mobilization of calcium from bone: Although the mechanisms remain obscure, a
well-documented effect of parathyroid hormone is to stimulate osteoclasts to
reabsorb bone mineral, liberating calcium into blood.
•
Enhancing absorption of calcium from the small intestine: Facilitating calcium
absorption from the small intestine would clearly serve to elevate blood levels of
calcium. Parathyroid hormone stimulates this process, but indirectly by
stimulating production of the active form of vitamin D in the kidney. Vitamin D
induces synthesis of a calcium-binding protein in intestinal epithelial cells that
facilitates efficient absorption of calcium into blood.
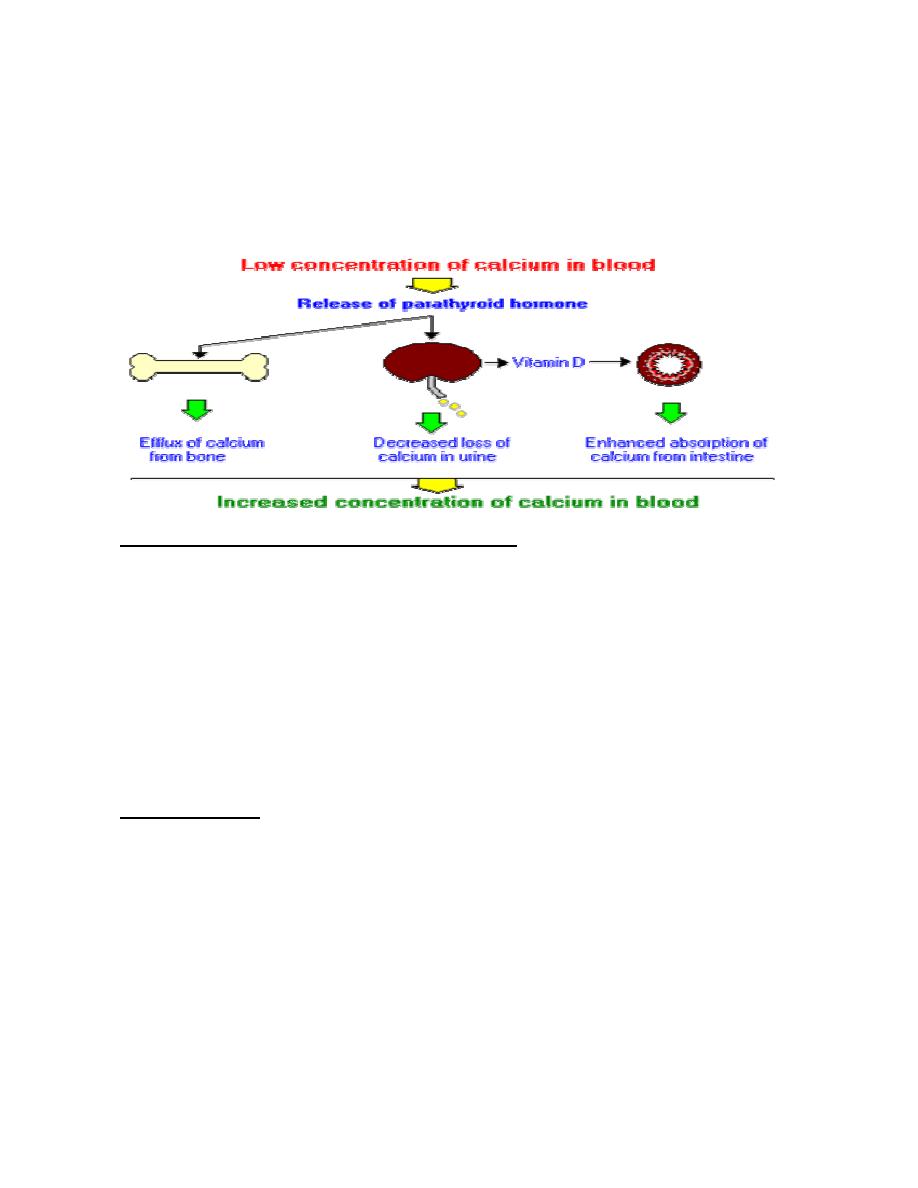
Prof.Dr. Hedef Dhafir El-Yassin 2012
57
•
Suppression of calcium loss in urine: In addition to stimulating fluxes of
calcium into blood from bone and intestine, parathyroid hormone puts a brake on
excretion of calcium in urine, thus conserving calcium in blood.
This effect is mediated by stimulating tubular reabsorption of calcium. Another effect
of parathyroid hormone on the kidney is to stimulate loss of phosphate ions in urine.
Control of Parathyroid Hormone Secretion
Parathyroid hormone is released in response to low extracellular concentrations
of free calcium. Changes in blood phosphate concentration can be associated with
changes in parathyroid hormone secretion, but this appears to be an indirect effect
and phosphate per se is not a significant regulator of this hormone.
When calcium concentrations fall below the normal range, there is a steep increase in
secretion of parathyroid hormone. Low levels of the hormone are secreted even when
blood calcium levels are high.
Disease States
Excessive secretion of parathyroid hormone is seen in two forms:
•
Primary hyperparathyroidism is the result of parathyroid gland disease.
•
Secondary hyperparathyroidism is the situation where disease outside of the
parathyroid gland leads to excessive secretion of parathyroid hormone. A
common cause of this disorder is kidney disease. It can also result from
inadequate nutrition - for example, diets that are deficient in calcium or vitamin
D, or which contain excessive phosphorus.
Prof.Dr. Hedef Dhafir El-Yassin 2012
58
Inadequate production of parathyroid hormone - hypoparathyroidism -
typically results in decreased concentrations of calcium and increased
concentrations of phosphorus in blood. Common causes of this disorder include
surgical removal of the parathyroid glands and disease processes that lead to
destruction of parathyroid glands.
2. Vitamin D
3
Vitamin D is a fat-soluble steroid hormone precursor that contributes to the
maintenance of normal levels of calcium and phosphorus in the bloodstream. It is
also known as calciferol.
Vitamin D
3
is produced in the skin by conversion of 7-dehydrocholesterol by UV.
Calciferol travels in the blood to the liver where it is converted into 25[OH] Vitamin
D
3
. This compound travels to the kidney where it is converted into Calcitriol
(1,25[OH]
2
Vitamin D
3
). This final step is promoted by the PTH.
Although called a vitamin, calciferol and its products fully qualify as hormones
because they are:
•
Made in certain cells
•
Carried in the blood
•
Affect gene transcription in target cells
Diseases
Vitamin D deficiency is known to cause several bone diseases, due to insufficient
calcium or phosphate in the bones:
§
Rickets: a childhood disease characterized by failure of growth and deformity
of long bones.
§
Osteoporosis: a condition characterized by fragile bones.
§
Osteomalacia: a bone-thinning disorder in adults that is characterised by
proximal muscle weakness and bone fragility. Osteomalacia can only occur in
a mature skeleton.
3. Calcitonin (discussed earlier)
The following table summarizes body responses to conditions that would otherwise
lead to serious imbalances in calcium and phosphate levels in blood
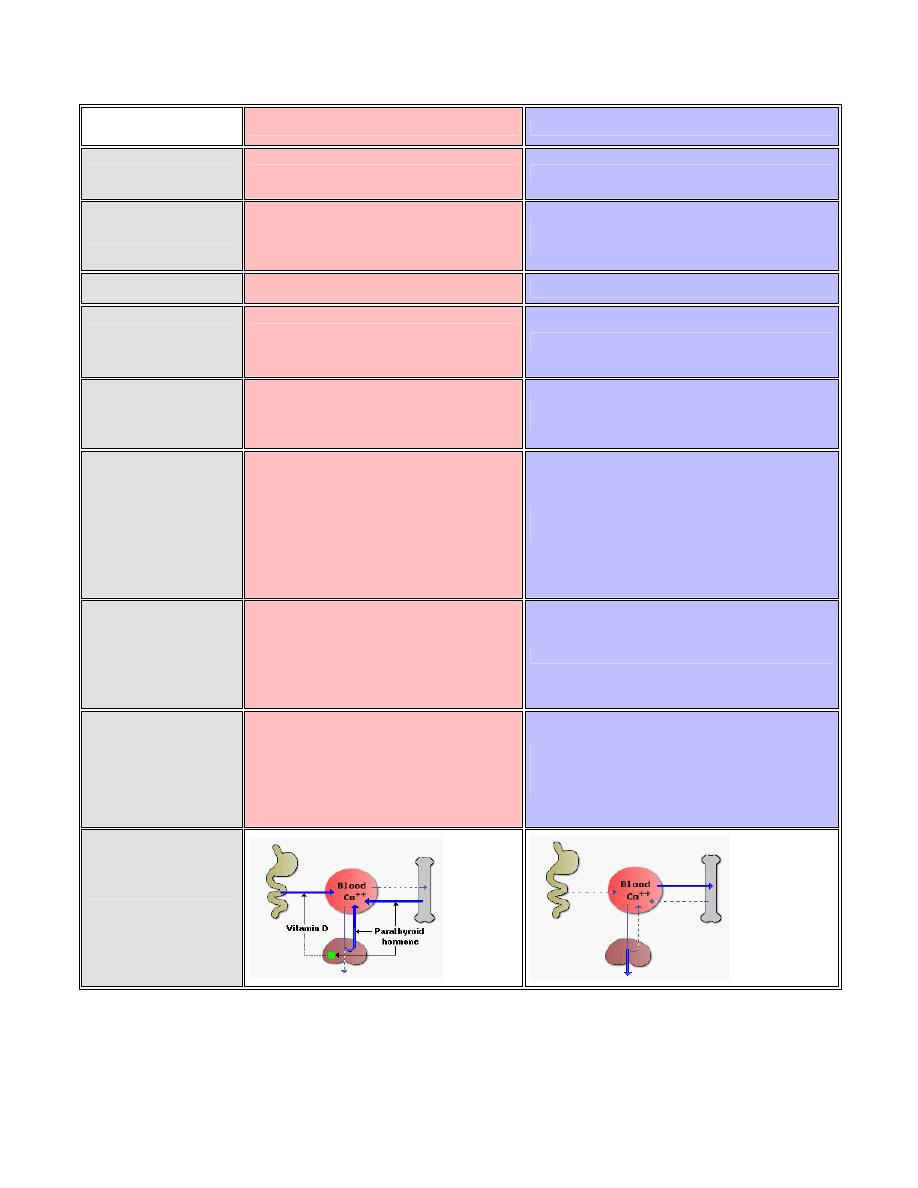
Prof.Dr. Hedef Dhafir El-Yassin 2012
59
Calcium Deprivation
Calcium Loading
Parathyroid hormone
Secretion stimulated
Secretion inhibited
Vitamin D
Production stimulated by increased
parathyroid hormone secretion
Synthesis suppressed due to low parathyroid
hormone secretion
Calcitonin
Very low level secretion
Secretion stimulated high blood calcium
Intestinal absorption
of calcium
Enhanced due to activity of vitamin D on
intestinal epithelial cells
Low basal uptake
Release of calcium
and phosphate from
bone
Stimulated by increased parathyroid
hormone and vitamin D
Decreased due to low parathyroid hormone and
vitamin D
Renal excretion of
calcium
Decreased due to enhanced tubular
reabsorption stimulated by elevated
parathyroid hormone and vitamin D;
hypocalcemia also activates calcium
sensors in loop of Henle to directly
facilitate calcium reabsorption
Elevated due to decreased parathyroid hormone-
stimulated reabsorption.
Renal excretion of
phosphate
Strongly stimulated by parathyroid
hormone; this phosphaturic activity
prevents adverse effects of elevated
phosphate from bone resorption
Decreased due to hypoparathyroidism
General Response
Typically seen near normal serum
concentrations of calcium and phosphate
due to compensatory mechanisms. Long
term deprivation leads to bone thining
(osteopenia).
Low intestinal absorption and enhanced renal
excretion guard against development of
hypercalcemia.
Summary

Prof.Dr. Hedef Dhafir El-Yassin 2012
60
Clinical Correlations
A 48-year-old woman was admitted to the hospital because of weight loss, palpitation,
weakness, and exophthalmos. She stated that a goiter, which had been present for years,
had recently begun to enlarge. She was extremely irritable, could not tolerate heat and
was short of breath. Physical examination revealed bilateral eyelid lag. The thyroid gland
was diffusely enlarged, and a bruit was audible over the right lobe. Her heart was
enlarged, a prominent apical thrust was noted, and there was a soft systolic heart murmur
along the left sternal border. Laboratory examination revealed that the hemoglobin was 1.8
mmol/L and that the hematocrit was 38%. The basal metabolic rate was 145% of normal.
plasma T4 and T3 were grossly elevated, and the
131
I uptake by the thyroid gland was
very high (18% in 4 hr). A diagnosis of hyperthyroidism was made.
Biochemical questions:
1. What are T3 and T4, and how are they related to the thyroid gland?
2. How are TRH and TSH involved in the regulation of thyroid hormone production and
secretion?
3. What is the mechanism of increased
131
I uptake in hyperthyroidism?
T3 and T4, are the thyroid hormones, triiodothyronine and thyroxine, respectively. Both are
tyrosine derivatives. Although lesser amounts of T3 are released by the thyroid gland, it
has a more potent effect than T4 in producing the hyper metabolic effects of the thyroid
hormones. T4 is converted to T3 in the target cells, and it is likely that T3, actually is the
metabolically active form of the thyroid hormone. In this sense, T4 may be considered as a
prohormone.
TSH is released from-the anterior pituitary and stimulates T3 and T4 production and
release. In turn, TSH release is stimulated by TRH, which is made in the hypothalamus.
When the plasma T3 and T4 concentrations are elevated, TRH production and release are
inhibited. This leads to decreased T3 and T4 release from the thyroid gland.
T3 and T4 contain iodine atoms attached to their phenolic rings. Thyroglobulin, the protein
precursor of these hormones that is contained in the thyroid cells, has many iodinated
tyrosine residues. The iodine is obtained from iodide ions in the blood plasma, and the
thyroid-cells have the capacity to take up and concentrate iodide ions.

Prof.Dr. Hedef Dhafir El-Yassin 2012
61
In hyperthyroidism the thyroid gland is rnore active than normal. It synthesizes more
thyroglobulin, T3, and T4 and takes up much larger amounts of iodine than in the euthyroid
(normal) state. Therefore, when
131
I is administered to a hyperthyroid patient, a larger
fraction of the dose is concentrated within the thyroid gland than in a euthyroid subject.
This is useful clinically in two ways.
1. Small quantities of
131
I can be administered as a diagnostic test of thyroid function.
After administration, the radiation emanating from the thyroid grand can be
measured at various times by placing a scanning device over the neck. Greater
than normal uptakes occur in hyper thyroidism, and less than normal uptakes occur
if the thyroid gland is hypo functioning (hypothyroidism).
2. The enhanced iodine uptake can be used to treat hyper thyroidism. If larger
amounts of
131
I are administered, enough
131
I will concentrate in the thyroid to
provide intense but localized radiation to the glandular cells. This will destroy many
of the T3- and T4- producing cell, reducing the excessive function of the thyroid and
correcting the hyperthyroidism.
As compared with the thyroid, other tissues take up very little iodine. Consequently, most
of the
131
I that is not taken up by the thyroid is rapidly excreted in the urine, and there is
comparatively little radiation exposure in other tissues. In some respects this is a safer
form of treatment than surgical removal of a large portion of he hyperactive grand. It is not
without some danger, however, for
131
I treatment can lean in some cases to either
hypothyroidism or even thyroid cancer.
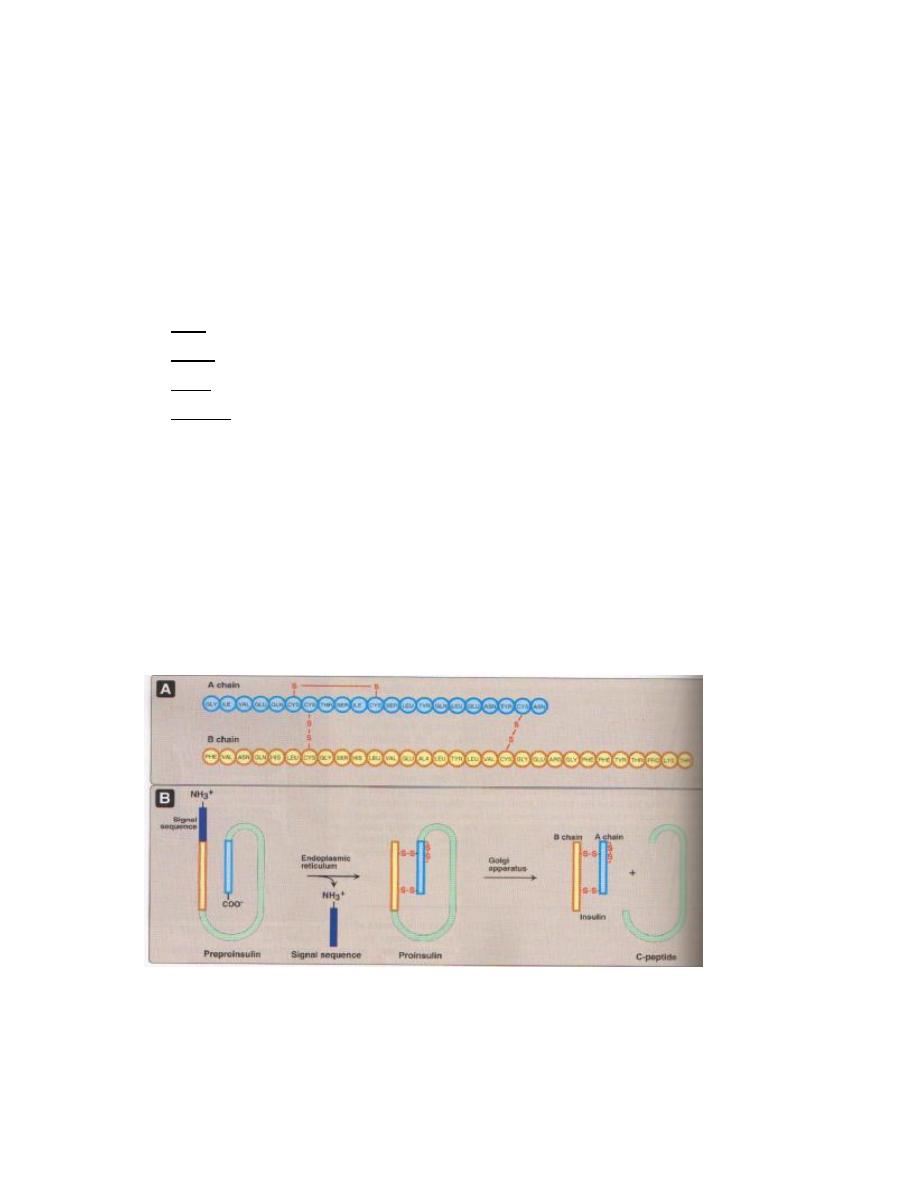
Prof.Dr. Hedef Dhafir El-Yassin 2012
62
Lecture
6
Thursday
8
/
3
/2012
Biochemistry and Disorders of Hormones of the Pancreas
The bulk of the pancreas is an exocrine gland secreting pancreatic fluid into the duodenum
after a meal.
However, scattered through the pancreas are several hundred thousand clusters of cells
called islets of Langerhans. The islets are endocrine tissue containing four types of cells.
In order of abundance, they are the:
1.
beta cells, which secrete insulin and amylin.
2.
alpha cells, which secrete glucagon;
3.
delta cells, which secrete somatostatin, and
4.
gamma cells, which secrete pancreatic polypeptide (PP).
Beta Cells
Insulin is a small protein consisting of
•
an alpha chain of 21 amino acids linked by two disulfide (S
—S) bridges to a
•
beta chain of 30 amino acids.
Beta cells have channels in their plasma membrane that serve as glucose detectors. Beta
cells secrete insulin in response to a rising level of circulating glucose ("blood sugar").
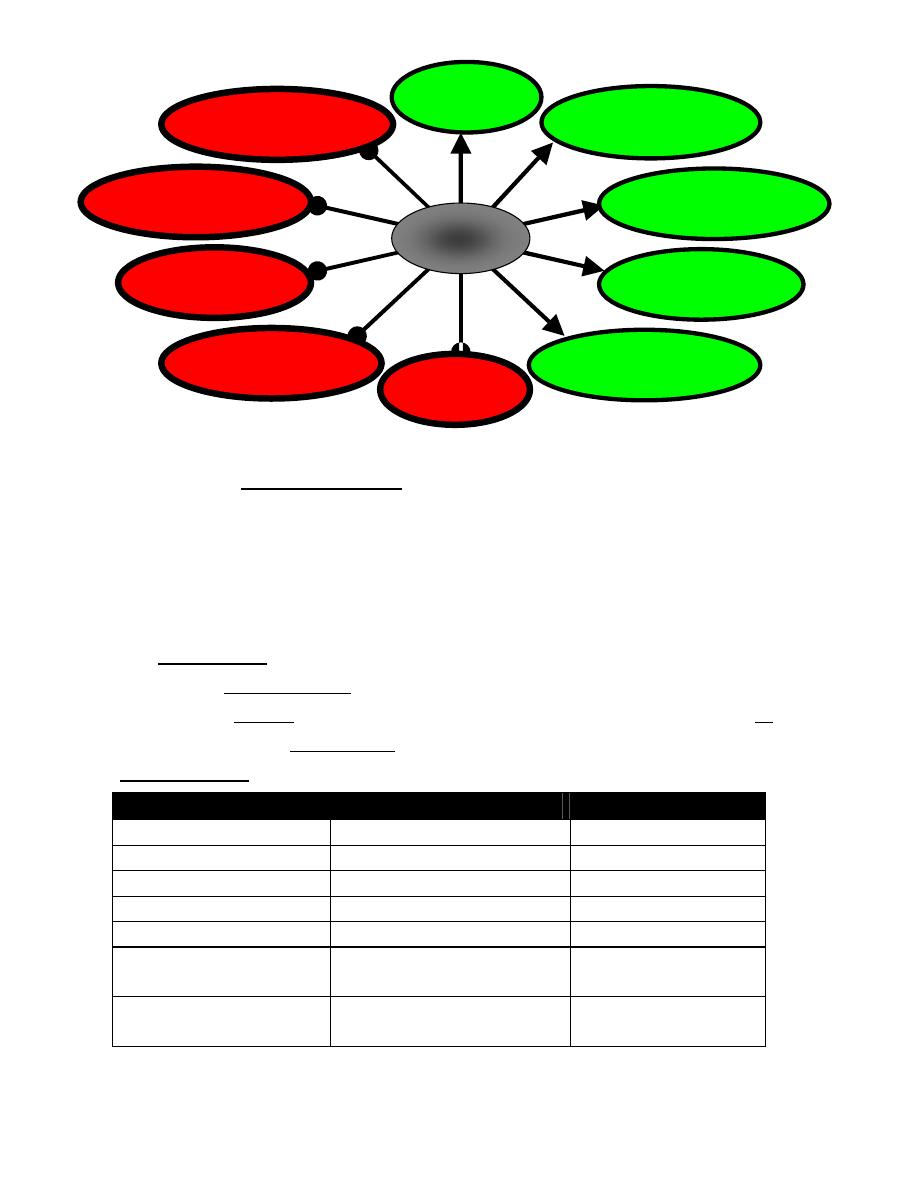
Prof.Dr. Hedef Dhafir El-Yassin 2012
63
Gluconeogenesis
Glucogenolysis
Lipolysis
Ketogenesis
Proteolysis
Uptake of ions (especially
K
+
and PO
4
-3
Protein synthesis
Glycogen synthesis
Glycolysis
Glucose uptake in muscle
and adipose tissue
Insulin
Insulin affects many organs.
1.
It stimulates skeletal muscle fibers to
•
take up glucose and convert it into glycogen;
•
Take up amino acids from the blood and convert them into protein.
2.
acts on liver cells
•
stimulating them to take up glucose from the blood and convert it into glycogen
•
inhibiting production of the enzymes involved in breaking glycogen back down
("glycogenolysis") and
•
inhibiting "gluconeogenesis"; that is, the conversion of fats and proteins into glucose.
3.
acts on fat (adipose) cells to stimulate the uptake of glucose and the synthesis of fat.
4.
acts on cells in the hypothalamus to reduce appetite.
Actions of Insulin
Metabolic process
Reaction
consequence
glycogenesis
Glucose to Glycogen
(-) Blood glucose
glycogenolysis
Glycogen to Glucose
(+) Blood glucose
gluconeogenesis
Amino acids to Glucose
(+) Blood glucose
Protein synthesis
Amino acids to protein
(-) Blood amino acids
Protein degradation
Protein to Amino acids
(+) Blood amino acids
Fat synthesis (lipogenesis or
triglyceride synthesis
Fatty acids and glycerol to
triglycerides
(-) Blood fatty acids
Fat breakdown (lipolysis or
triglycerides degradation
Triglycerides to Fatty acids and
glycerol
(+) Blood fatty acids
(-) decrease in
(+) increase in

Prof.Dr. Hedef Dhafir El-Yassin 2012
64
Amylin
Amylin is a peptide of 37 amino acids, which is also secreted by the beta cells of the
pancreas.
Some of its actions:
•
inhibits the secretion of glucagon;
•
slows the emptying of the stomach;
•
sends a satiety signal to the brain.
Amylin (IAPP) was identified independently by two groups as the major component of
diabetes-associated islet amyloid deposits in 1987
Alpha Cells
The alpha cells of the islets secrete glucagon, a polypeptide of 29 amino acids.
Glucagon acts principally on the liver where it stimulates the conversion of
•
glycogen into glucose ("glycogenolysis") and
•
fat and protein into intermediate metabolites that are ultimately converted into
glucose ("gluconeogenesis")
In both cases, the glucose is deposited in the blood.
Glucagon secretion is
•
stimulated by low levels of glucose in the blood;
•
inhibited by high levels of glucose in the blood, and
•
inhibited by amylin.
The physiological significance of this is that glucagon functions to maintain a steady level
of blood sugar level between meals.
Delta Cells
The delta cells secrete somatostatin. This consists of two polypeptides, one of 14 amino
acids and one of 28.
Somatostatin has a variety of functions. Taken together, they work to reduce the rate at
which food is absorbed from the contents of the intestine.
Somatostatin is also secreted by the hypothalamus and by the intestine.
Gamma Cells
The gamma cells of the islets secrete a 36-amino-acid pancreatic polypeptide. Its function
is to self regulate the pancreas secretion activities . it also has effects on hepatic glycogen
levels and gastrointestinal secretions.
Its secretion human is increased after a protein meal, fasting, exercise and acute
hypoglycemia and is decreased by somatostatin.,.
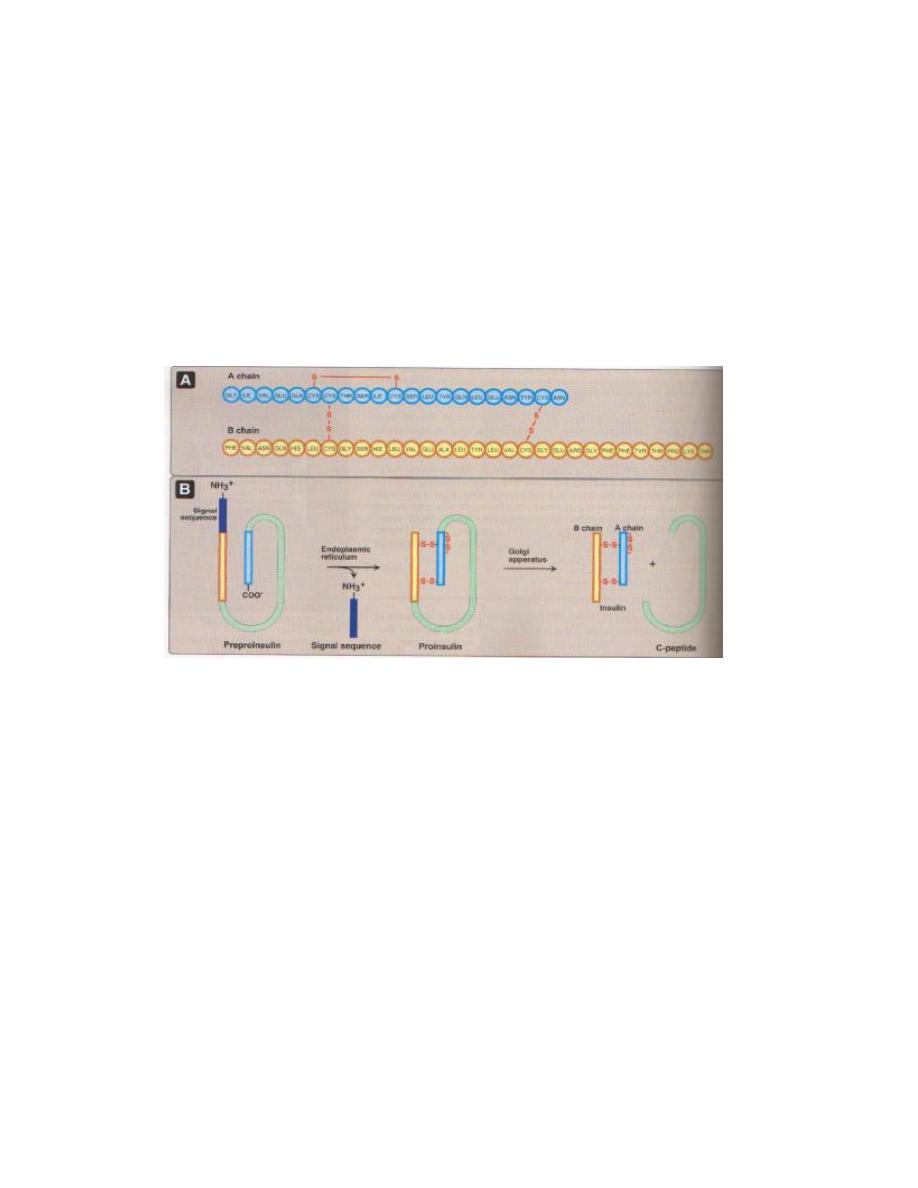
Prof.Dr. Hedef Dhafir El-Yassin 2012
65
Synthesis and release of insulin and glucagon
Insulin and glucagon are synthesized in different cell types of the endocrine pancreas, which
consists of microscopic clusters of small glands (the islets of Langerhans) . The
α cells secrete
glucagon, and the
β cells secrete insulin into the hepatic portal vein via the pancreatic veins.
•
Synthesis and secretion of Insulin
Insulin is a polypeptide hormone. The active form of ins ulin is composed of two polypeptide
chains (the A-chain and the B-chain) linked by two interchain disulfide bonds. The A-chain has
an additional intrachain disulfide bond.
Insulin, like many other polypeptide hormones, is synthesized as a preprohormone that is
converted in the rough endoplasmic reticulum (RER) to proinsulin. The "pre" sequence, a short
hydrophobic signal sequence at the N-terminal end, is cleaved as it enters the lumen of the
RER. Proinsulin folds into the proper conformation and disulfide bonds are formed between
the cysteine residues. It is then transported in microvesicles to the Golgi complex. It leaves
the Golgi complex in storage vesicles, where a protease removes the C-peptide (a fragment
with no hormonal activity) and a few small remnants, resulting in the formation of biologically
active insulin. Zinc ions are also transported in these storage vesicles. Cleavage of the C-
peptide decreases the solubility of the resulting insulin, which then coprecipitates with zinc.
Exocytosis of the insulin storage vesicles from the cytosol of the
β cell into the blood is
stimulated by rising levels of glucose in the blood bathing the
β cells.
Glucose enters the
β cell via specific glucose transporter proteins known as GLUT2.
Glucose is phosphorylated through the action of glucokinase to form glucose 6-phosphate,
which is metabolized through glycolysis, the TCA cycle, and oxidative phosphorylation.
Prof.Dr. Hedef Dhafir El-Yassin 2012
66
These reactions result in an increase
in ATP levels within the
β cell. As the β
cell [ATP|/ [ADP] ratio increases, the
activity of a membrane-bound, ATP-
dependent K
+
channel is inhibited (i.e.,
the channel is closed). The closing of
this channel leads to a membrane
depolarization, which activates a
voltage-gated
Ca
2+
channel
that
allows Ca
+
to enter the
β cell such that
intracellular Ca
2+
levels increase
significantly. The increase in intra-
cellular Ca
2+
stimulates the fusion of
insulin containing exocytotic vesicles with the plasma membrane, resulting in insulin
secretion. Thus, an increase in glucose levels within the
β cells initiates insulin release.
•
Stimulation and inhibition of insulin release
The release of insulin occurs within minutes after the pancreas is exposed to a high glucose
concentration. The threshold for insulin release is approximately 80 mg/glucose /dL. Above
80 mg/dL, the rate of insulin release is not an all-or-nothing -response but is proportional to
the glucose concentration up to approximately 300 mg/dL glucose. As insulin is secreted,
the synthesis of new insulin molecules is stimulated, so that secretion is maintained until
blood glucose levele fall. Insulin is rapidly removed from the circulation and degraded by the
liver and to a lesser extent by kidney and skeletal muscle) so that blood insulin levels decrease
rapidly
A number of factors other than the blood glucose concentration can modulate insulin such
as:
1. neural signals
2. certain amino acids
3. gastric inhibitory polypeptide (GIP, a gut hormone released after the ingestion of
food)
4. epinephrine secreted in response to fasting, stress, trauma and vigorous exercise
decrease the release of insulin
Prof.Dr. Hedef Dhafir El-Yassin 2012
67
•
Synthesis and secretion of Glucagon
Glucagon a polypeptide hormone, is synthesized in the
α cells of the pancreas by cleavage
of the much larger preproglucagon, a 160-amino acid peptide. Like insulin preproglucagon
is produced on the rough endoplasmic reticulum and is converted to proglucagon as it enters
the ER lumen. Proteolytic cleavage at various sites produce the mature 29-amino acid
glucagon and larger glucagon-containing fragments (named glucagon-like peptides I and
2). Glucagon is rapidly metabolized, primarily in the liver and kidneys. Its plasma half-life is only
about 3 to 5 minutes.
Insulin
Glucagon Epinephrine
Glucagon secretion is regulated principally by circulating levels of glucose and insulin.
Increasing levels of each inhibit glucagon release. Glucose probably has both a direct
suppressive effect on secretion of glucagon from the
α cell as well as an indirect effect, the latter
being mediated by its ability to stimulate the release of insulin.
Certain hormones stimulate glucagon secretion. :
1) catecholamines (epinephrine)
2) cortisol
3) gut hormones
4) Many amino acids also stimulate glucagon release.
Metabolic effects of glucagon
1. Effects on carbohydrate metabolism: The intravenous administration of glucagon leads to an immediate
rise in blood glucose. This results from an increase in the breakdown of liver (not muscle) glycogen and
an increase in gluconeogenesis.
2. Effects on lipid metabolism: Glucagon favors hepatic oxidation
OF
fatty acids and the
subsequent formation of ketone bodies acetyl CoA. The lipolytic effect of glucagon in adipose
tissue is minimal in humans.
Glycogenolysis
Gluconeogenesis
Glycogenolysis
Gluconeogenesis

Prof.Dr. Hedef Dhafir El-Yassin 2012
68
3. Effects on protein metabolism: Glucagon increases uptake of amino acids by the liver,
resulting in increased availability of carbon skeletons for gluconeogenesis. As a
consequence plasma levels of amino acids are decreased.
DIABETES MELLITUS:
This is a metabolic disorder occurring as a result of an insulin lack or a surplus of insulin
antagonists leading to a relative insulin lack. It is characterized by hyperglycemia and
glucosuria. Three types of the disease are described-
1. Juvenile type (type 1)
in children and is due to an absolute deficiency of insulin.
2. Maturity onset type
: It is usually associated with obesity. A form of diabetes known as
MODY (maturity onset diabetes of the young) results from mutations in either
pancreatic glucokinase or specific nuclear transcription factors. Thus, although these
patients can release insulin, they do so at higher than normal glucose levels, and are
therefore almost always in a hyperglycemic state. Interestingly, however, these
patients are somewhat resistant to the long-term complications of chronic
hyperglycemia.
3. Secondary diabetes:
There is hyperfunction of one or other of insulin anatgonists leading to
a relative insufficiency of insulin eg: acromegaly (excess of growth hormone); Cushing's
disease (excess of glucocorticoids); hyperthyroidism etc.
Glucagon is one of the contributory factors in the etiology of diabetes mellitus. Its blood levels
are elevated in severe diabetes with ketoacidosis. The
α cells seem to be insensitive to the high
blood glucose levels in diabetes and continue to secrete large amounts of glucagon.
Somatostatin, a hypothalamic factor inhibiting the release of growth hormone, also inhibits the
release of glucagon and is in experimental use as an adjunct to insulin in the control of
severe diabetes mellitus.
4. Type 2 diabetes:
a combination of insulin resistance and dysfunctional
β
cells.
Insulin resistance:
Is the decreased ability of target tissues, such as liver adipose and
muscle to respond properly to normal circulating concentrations of insulin. For example ,
insulin resistance is characterized by uncontrolled hepatic glucose production and decreased
glucose uptake by muscle and adipose tissue.
In a moderately severe early diabetes mellitus the following features are present-
1. Hyperglycemia.
2. Glycosuria.
3. Loss of weight due to increased breakdown of fat and tissue protein.
4. Increased production of ketone bodies by the liver and their incomplete utilization by
tissues leading to their accumulation in blood (ketosis) and elimination in urine (ketonuria).

Prof.Dr. Hedef Dhafir El-Yassin 2012
69
5. Lowering of the pH of blood due to circulating keto acids (acidosis).
6. Dehydration due to elimination of large amounts of water with glucose in urine.
7. Negative nitrogen balance due to conversion of more amino acids into glucose
(increased gluconeogensis).
8. Increased levels of lipid, fatty, acid and cholesterol in blood (lipemia).
9. Increased tendency to develop cataract in the eye and arteriosclerotic lesions of blood
vessels.
Hyperinsulinism; This may occur on account of islet-cell tumors involving the
β-cells. There is
overproduction of insulin resulting in spontaneous attacks of hypoglycemia associated with
sweating, and fainting attacks which are relieved by ingestion of glucose or a lump of sugar.
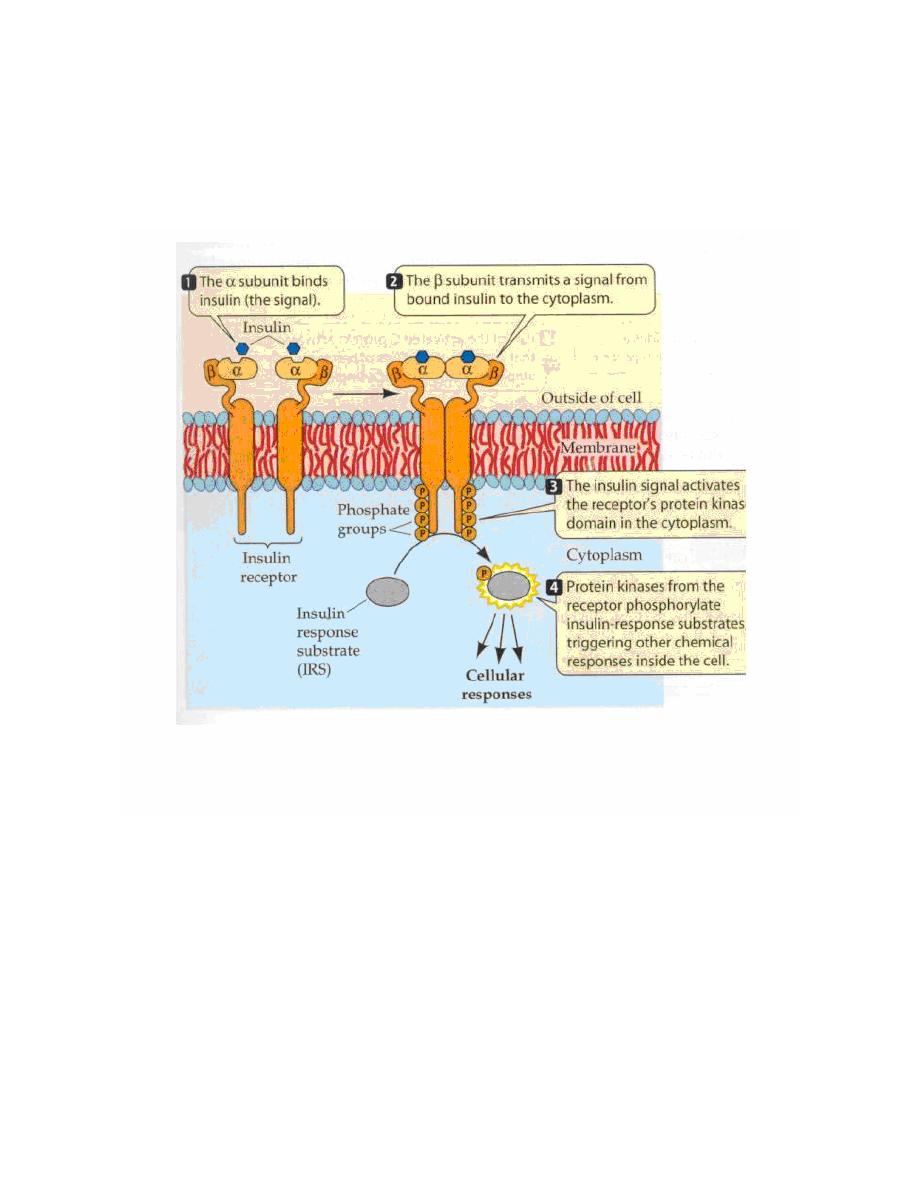
Prof.Dr. Hedef Dhafir El-Yassin 2012
70
Insulin and Glucagon receptors
1. Insulin Receptor
Insulin Binding to it's Receptor Followed by Activation of the Receptor:
1.
Insulin binds switching ON the receptor. Once the receptor is ON (catalytically active)
insulin may disaccociate and be degraded.
2.
The Tyr Kinase domain is phosphorylated.
3.
A "cascade" of events takes place (see below).
4.
Biochemical / Physiological Responses:
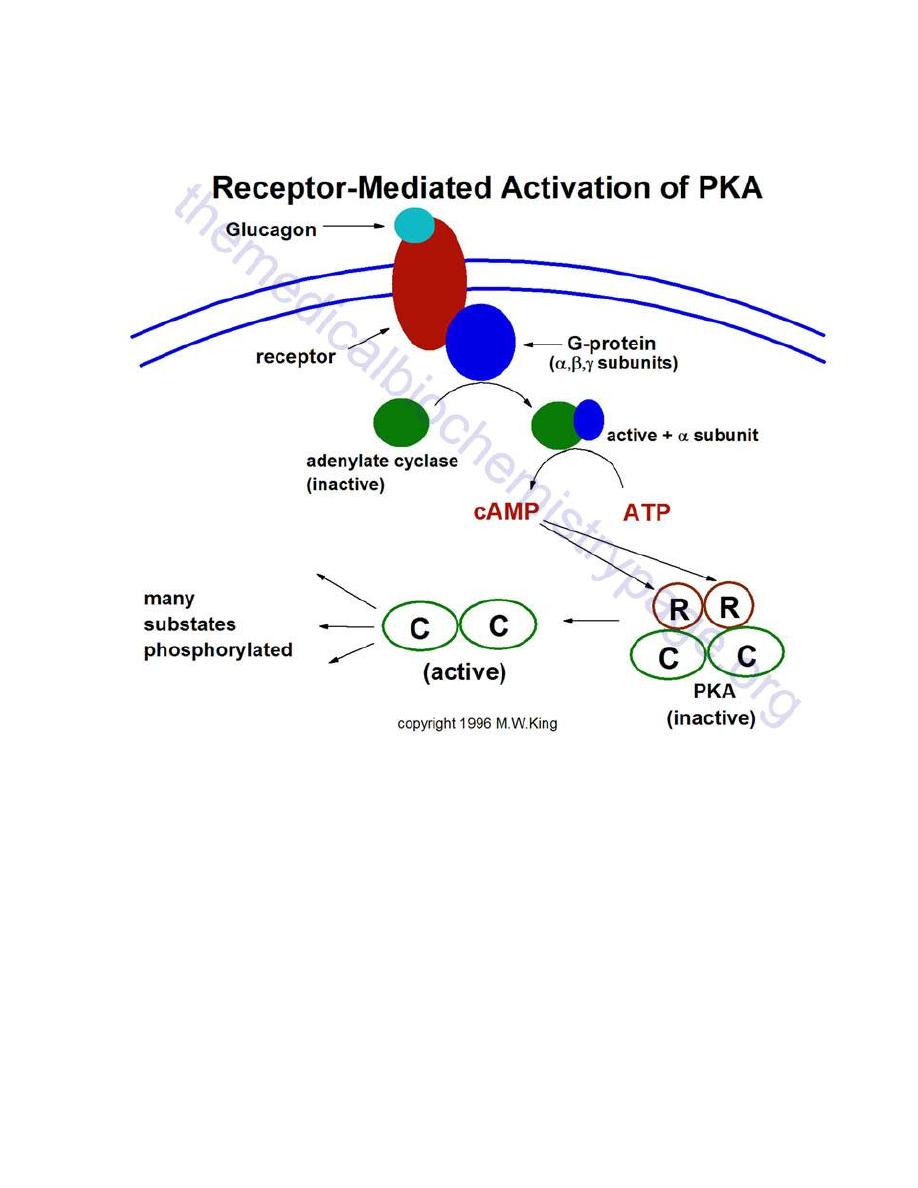
Prof.Dr. Hedef Dhafir El-Yassin 2012
71
2. Glucagon Receptor
Representative pathway for the activation of cAMP-dependent protein kinase, PKA. In this
example glucagon binds to its' cell-surface receptor, thereby activating the receptor.
Activation of the receptor is coupled to the activation of a receptor-coupled G-protein
(GTP-binding and hydrolyzing protein) composed of 3 subunits. Upon activation the
α-
subunit dissociates and binds to and activates adenylate cyclase. Adenylate cylcase then
converts ATP to cyclic-AMP (cAMP). The cAMP thus produced then binds to the
regulatory subunits of PKA leading to dissociation of the associated catalytic subunits. The
catalytic subunits are inactive until dissociated from the regulatory subunits. Once released
the catalytic subunits of PKA phosphorylate numerous substrate using ATP as the
phosphate donor

Prof.Dr. Hedef Dhafir El-Yassin 2012
72
Clinical cases and correlations
Insulinoma
A 36-year-old woman was referred to a university hospital for evaluation of spells of
dizziness and weakness. These spells typically lasted for 10 min and were occurring with
increasing frequency. The spells usually came on after a large meal and could be
terminated by her eating candy or drinking fruit juice. After each episode the patient was
hungry and tired, and her memory was blurred. The patient's physical examination was
within normal limits except for mild obesity. She claimed to have gained 20 kg during the
preceding 2 yr. After a 13-hr fast her blood glucose concentration was 2.1 mmollL. After a
5-hr glucose tolerance test, her blood glucose was 2.6 mmol/L.
Celiac angiography revealed an abnormality in the body and tail of the pancreas. The
patient developed one of her spells while a medical student was in her room, and he was
able to obtain a blood sample during the episode. This sample contained 1.1mmol/L of
glucose. The patient was transferred to the surgical service, and an insulin-secreting
pancreatic adenoma (tumor) was removed, requiring resection of 90% of the pancreas.
Biochemical questions
1. An insulinoma is an insulin-secreting tumor. How did the presence of such a tumor
explain the patient's symptoms?
2. Proinsulin was found in large quantities in this patient's plasma. What is the
relationship of proinsulin to insulin? What is preproinsulin?
3. What effects of increased insulin secretion might have predisposed this woman to
obesity?
4. What digestive problems might result from excision of 90% of the pancreas?
The insulinoma was producing insulin. Because of the excessive amount of insulin-
secreting tissue, too much insulin was released after dietary carbohydrate intake. This
caused hypoglycemia during the 5-hr glucose tolerance test and after meals, producing
the spells of weakness and dizziness. In addition, to this normal insulin release when
carbohydrate was ingested, the tumor also was secreting some insulin continuously.
This inappropriate insulin release caused the low blood glucose concentration during
prolonged fasting.
Proinsulin is the prohormone form of insulin that is made in the
β
-cells of the pancreatic
islets. It has no insulin-like action. After synthesis on the ribosomes, the initial precursor,
preproinsulin, penetrates through the endoplasmic reticulum into the lumen of this

Prof.Dr. Hedef Dhafir El-Yassin 2012
73
organelle. The leader sequence is removed in this process, forming proinsulin, which is
transported to the Golgi apparatus and stored in granules.
Proinsulin is converted to insulin in these granules by proteolytic cleavage, but the
conversion is incomplete. When insulin is discharged from the
β
-cell, some proinsulin that
remains in the granule also is released. Likewise, C-peptide that is split out in the
conversion of proinsulin to insulin is released during insulin secretion, but it too, has no
insulin-like activity.
Insulin acts on adipocytes, enhancing fatty acid storage as triglyceride. It binds to specific
receptors on the cell surface and facilitates glucose entry into the adipocyte, increasing the
availability of the triose backbone, glycerol 3-phosphate, needed for triglyceride synthesis.
This also provide glucose carbon atoms for fatty acid syntesis.
In addition, it increases the content of lipoprotein lipase in the adipose tissue. This enzyme
catalyzes the hydrolysis of chyromicron and VLDL triglycerides, a step that is required to
transfer their fatty acids into the adipocytes for resynthesis into triglyceride. Much of the
fatty acid stored in the adipose tissue is delivered to the adipocytes in the form of
lipoprotein triglycerides, either VLDL from the liver or chylomicrons from the intestine.
Therefore the elevated lipoprotein lipase activity also favors triglyceride formation in the
adipose tissue. Those adipose tissue effects that were mediated by the excessive insulin
production could have contributed to he recent weight gain noted by this patient.
In addition to polypeptide hormones, the pancreas making many digestive enzymes.
These include amylase for dietary starches, lipase for triglycerides, chymotrypsin and
trypsin for proteins, as well as several others. Since 90%, of the pancrease was excised,
the remaining 10% may not produce sufficient amounts of these enzymes to adequately
digest large meals. This might lead to malnutrition and weight toss in spite of an adequate
diet. Because of this possibility, six or more smaller meals rather than three regular meals
each day might be recommended.
Question: Binding of insulin to its receptor:
a) Occurs on the
ß-subunit.
b) Induces autophosphorylation.
c) Reduces binding of cytosolic substrate proteins.
d) Leads only to phosphorylation of proteins.
e) Does not lead to release of a second messenger.
Answer:
B This occurs on tyrosine residues of the
ß-subunit. A: Binding is to the α-
subunit. C: Autophosphorylation facilitates binding. D: Some proteins are
dephosphorylated. E: A second messenger may account for short-term metabolic effects.
Prof.Dr. Hedef Dhafir El-Yassin 2012
74
Hormones of the
adrenal gland
[Prof. Dr. H.D.El-Yassin 2012
1.
Hormones of the adrenal cortex
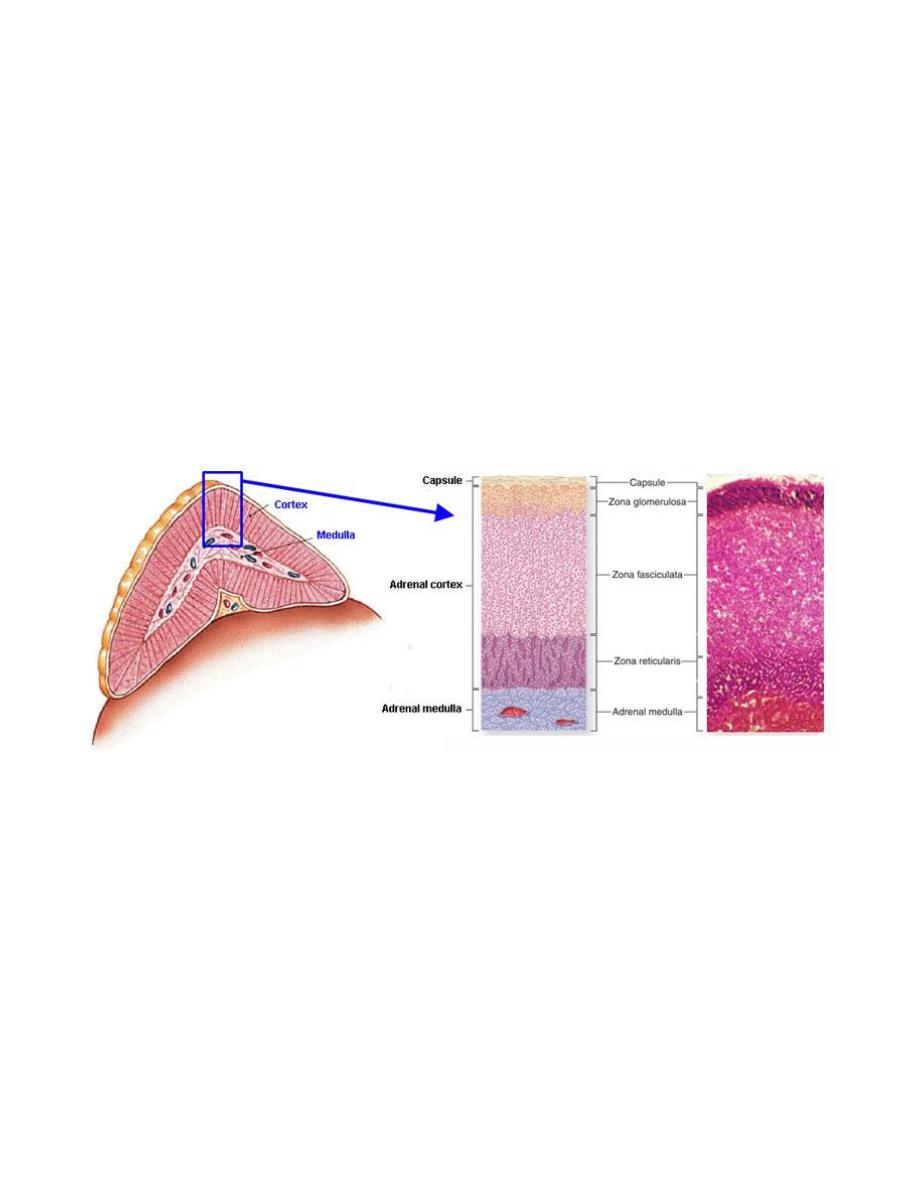
Prof.Dr. Hedef Dhafir El-Yassin 2012
75
Lecture 7
Sunday 11/3/2012
Hormones of the adrenal gland
The structure of the adrenal gland
The two adrenal glands (also called the suprarenal glands) are situated in the abdomen,
on either side of the vertebral column, above the kidneys and below the diaphragm
.
When cut in half each gland consists of
1.
An outer cortex, yellow in color and
2.
An inner medulla, which is dark red, or grey
.
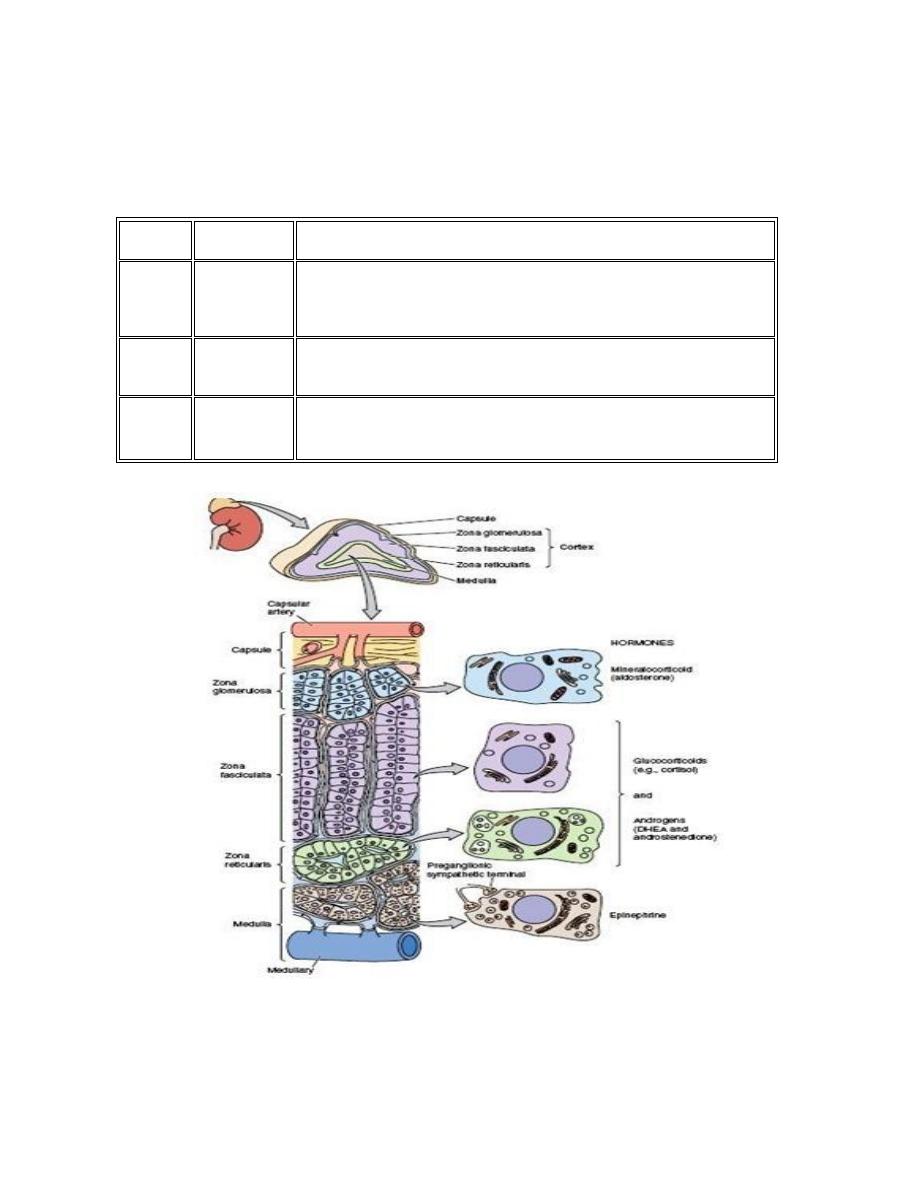
Prof.Dr. Hedef Dhafir El-Yassin 2012
76
The cortex consists of three distinct zones
1.
Zona glomerulosa
2.
Zona fasciculata
3.
Zona reticularis
Each zone has a characteristic histology and secretes different types of hormones
Layer
Name
Primary product
Most
superficial
cortical
layer
Zona
glomerulosa
mineralocorticoid (aldosterone) which is responsible for the
regulation of salt and water balance in the body
Middle
cortical
layer
Zona
fasciculata
glucocorticoid (cortisol) which regulates the level of
carbohydrate in the body
Deepest
cortical
layer
Zona
reticularis
sex hormones (progesterone, oestrogen precursors and
androgens) which have a role in the development of sexual
characteristics

Prof.Dr. Hedef Dhafir El-Yassin 2012
77
The medulla consists of many large columnar cells called "chromaffin cells". These
synthesize and secrete catecholamines
when stimulated by the sympathetic nervous
system.
Hormone synthesis
All adrenocortical hormones are synthesized from cholesterol. Cholesterol is transported
into the adrenal gland. Subsequent steps to generate aldosterone and cortisol, primarily
occur in the adrenal cortex:
•
Progesterone
→ (hydroxylation at C21) → 11-Deoxycorticosterone → (two further
hydroxylations at C11 and C18)
→ Aldosterone
•
Progesterone → (hydroxylation at C17) → 17-alpha-hydroxyprogesterone
→
(hydroxylation at C21)
→ 11-Deoxycortisol → (hydroxylation at C11) → Cortisol
Cholesterol is converted to pregnenolone (P5) by cytochrome P450 cholesterol side
chain cleavage . P5 is the precursor of all the other steroids and stands at the first
branch point in the adrenal steroidogenic network
Steroidogenic defects can cause congenital adrenal hyperplasia (CAH). This condition
may cause symptoms ranging from mild acne to salt wasting, depending on the nature of
the genetic mutation.
In hypoandrenocorticism (Addison's disease) and CAH the error involves the two
enzymes cytochrome P450 17a-hydroxylase/17-20 lyase and cytochrome P450 21-
hydroxylase respectively.
Because of a lack of the glucocorticoids and mineralocorticoids the brain signals the
adrenal gland with adrenocorticotrophic hormone (ACTH) to produce more of the deficient
steroids. Consequently, there is an over production of P5 that in turn leads to an over
production of DHEA, which is converted to androgens and estrogens outside of the
adrenal gland due to the DHEA in circulation diffusing into other steroidogenic tissues with
the appropriate activities.
Hormones secreted by the Adrenal Cortex
1. Mineralocorticoids
The primary mineralocorticoids aldosterone is
aldosterone
. Its
secretion is regulated by the
oligopeptide
angiotensin II
(angiotensin II is regulated by
angiotensin I
, which in turn is
regulated by
renin
). Aldosterone is secreted in response to high
extracellular
potassium
levels, low extracellular
sodium
levels,
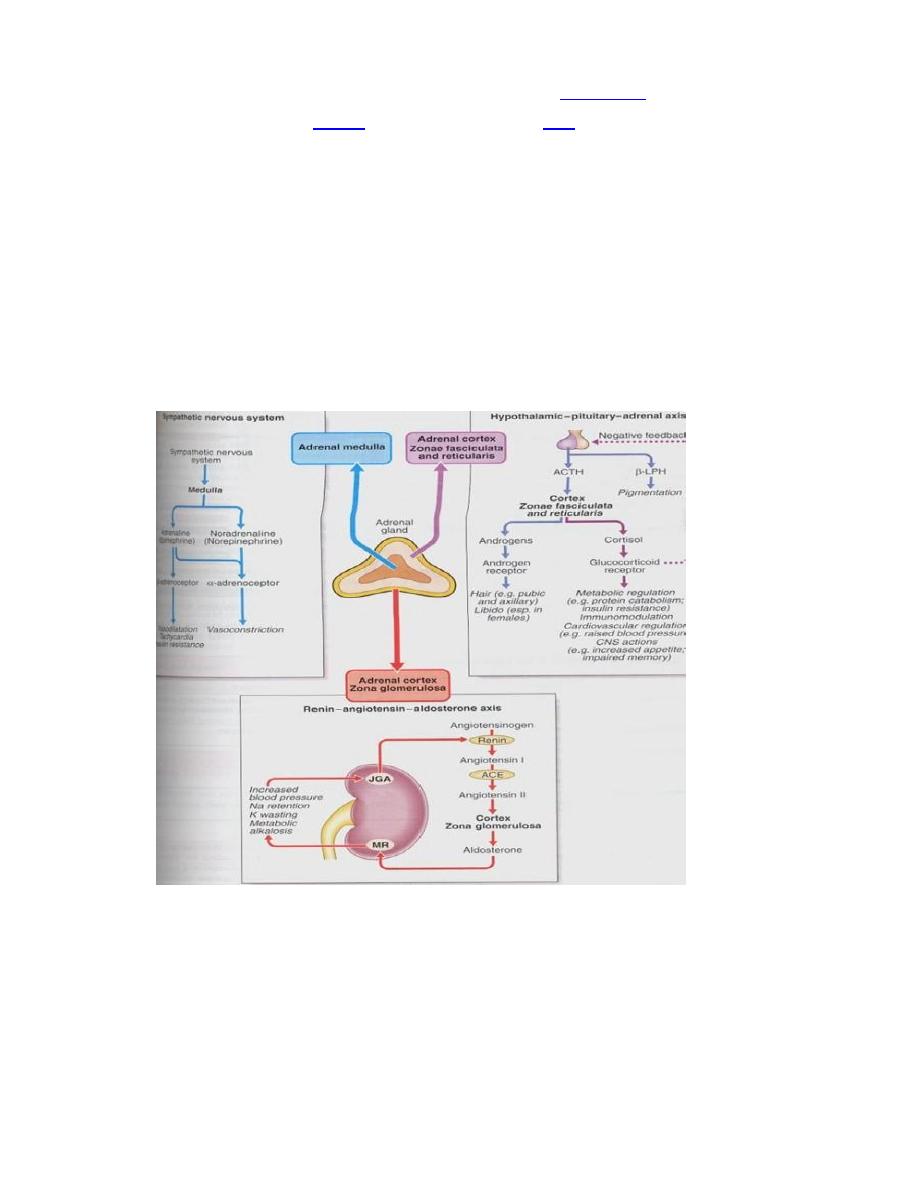
Prof.Dr. Hedef Dhafir El-Yassin 2012
78
and low fluid levels and blood volume. Aldosterone affects
metabolism
in different ways:
a. It increases
urinary
excretion of potassium
ions
b. It increases interstitial levels of sodium ions
c. It increases water retention and blood volume
Removal of the adrenal glands leads to death within just a few days. Due to:
1.
the concentration of potassium in extracelluar fluid becomes dramatically elevated
2.
urinary excretion of sodium is high and the concentration of sodium in extracellular
fluid decreases significantly
3.
volume of extracellular fluid and blood decrease
4.
the heart begins to function poorly, cardiac output declines and shock ensues
Clearly mineralocorticoids are acutely critical for maintenance of life!

Prof.Dr. Hedef Dhafir El-Yassin 2012
79
Aldosterone and Mineralocorticoid
Receptors
Cortisol, have "weak mineralocorticoid
activity", which is of some importance
because cortisol is secreted very much
more abundantly than aldosterone. i.e. a
small fraction of the mineralocorticoid response in the body is due to cortisol rather than
aldosterone.
The mineralocorticoid receptor binds both aldosterone and cortisol with equal affinity.
Moreover, the same DNA sequence serves as a
hormone response element
for the
activated (steroid-bound) forms of both mineralocorticoid and glucocorticoid receptors.
Q:
How can aldosterone stimulate specific biological effects in this kind of
system, particularly when blood concentrations of cortisol are something like 2000-
fold higher than aldosterone?
A:
In aldosterone-responsive cells, cortisol is effectively destroyed, allowing
aldosterone to bind its receptor without competition. Target cells for aldosterone express
the enzyme 11-beta-hydroxysteroid dehydrogenase, which has no effect on aldosterone,
but converts cortisol to cortisone, which has only a very weak affinity for the
mineralocorticoid receptor. In essence, this enzyme "protects" the cell from cortisol and
allows aldosterone to act appropriately.
An interesting demonstration of this enzyme protection system is seen in chronic licorice
intoxication. The major components of licorice are glycyrrhizic acid and its hydrolytic
product, glycyrrhetinic acid (GE). GE is a potent inhibitor of 11
ß-hydroxysteroid
dehydrogenase. By blocking the activity of this inactivating enzyme. GE facilitates the
binding of cortisol to renal mineralocorticoid receptors and hence induces AME syndrome
(Apparent Mineralocorticoid Excess Syndrome).
Control of Aldosterone Secretion
The two most significant regulators of aldosterone secretion are:
•
Concentration of potassium ions in extracellular fluid: Small increases in blood
levels of potassium strongly stimulate aldosterone secretion.
•
Angiotensin II: Activation of the renin-angiotensin system as a result of decreased
renal blood flow (usually due to decreased vascular volume) results in release of
angiotensin II, which stimulates aldosterone secretion.
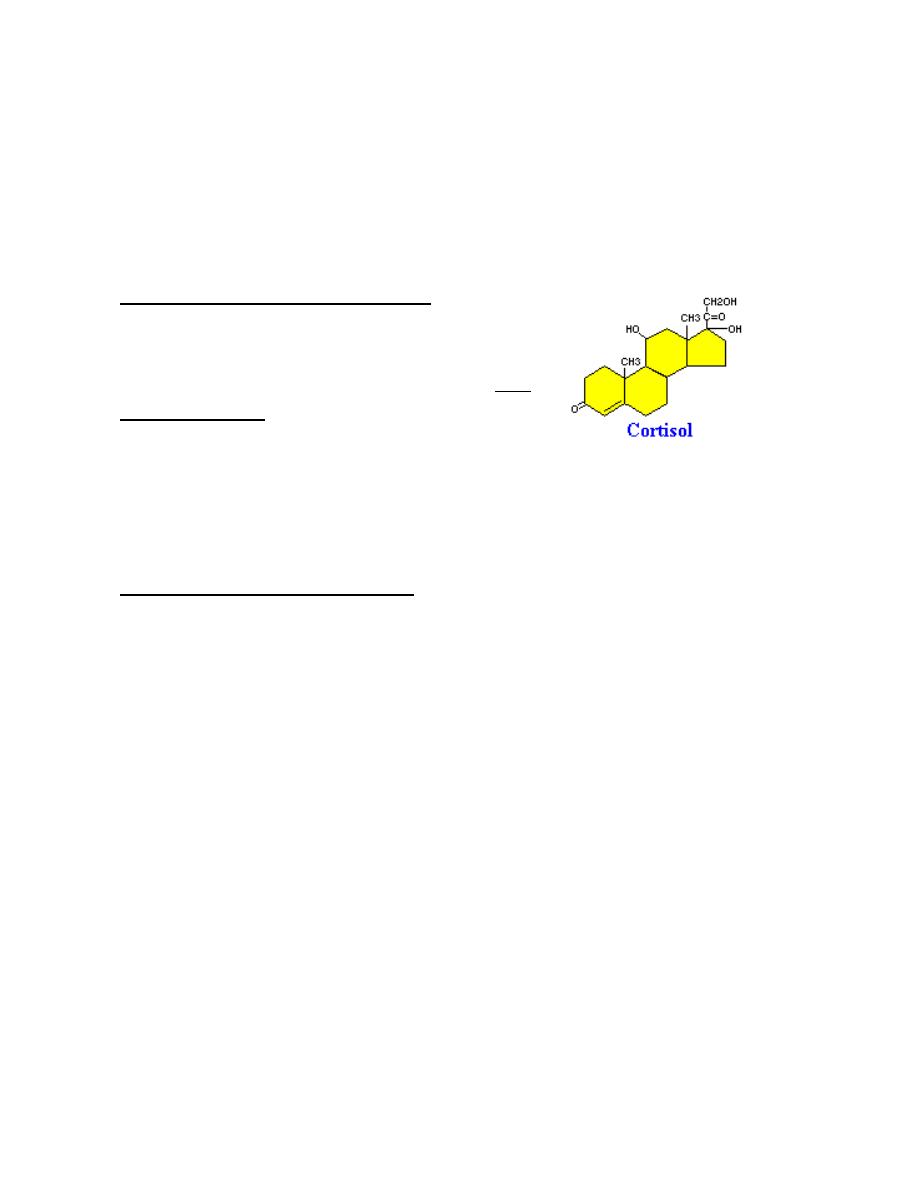
Prof.Dr. Hedef Dhafir El-Yassin 2012
80
Factors which suppress aldosterone secretion include atrial naturetic hormone, high
sodium concentration and potassium deficiency.
Disease States: A deficiency in aldosterone can occur by itself or, more commonly, in
conjunction with a glucocorticoid deficiency, and is known as hypoadrenocorticism or
Addison's disease
2. Glucocorticoids
Cortisol and Glucocorticoid Receptors
Cortisol binds to the glucocorticoid receptor in the
cytoplasm and the hormone-receptor complex is then
translocated into the nucleus, where it binds to its DNA
response element and modulates transcription from a
battery of genes, leading to changes in the cell's
phenotype.
Only about 10% of circulating cortisol is free. The remaining majority circulates bound to
plasma proteins, particularly corticosteroid-binding globulin (transcortin).
Metaboilc Effects of Glucocorticoids
There seem to be no cells that lack glucocorticoid receptors and as a consequence, these
steroid hormones have a huge number of effects on physiologic systems.
The name glucocorticoid derives from early observations that these hormones were
involved in glucose metabolism.
Cortisol stimulates several processes that collectively serve to increase and maintain
normal concentrations of glucose in blood. These effects include:
•
Stimulation of gluconeogenesis, particularly in the liver: This pathway results in
the synthesis of glucose from non-hexose substrates such as amino acids and
lipids . Enhancing the expression of enzymes involved in gluconeogenesis is
probably the best known metabolic function of glucocorticoids.
•
Mobilization of amino acids from extrahepatic tissues: These serve as
substrates for gluconeogenesis.
•
Inhibition of glucose uptake in muscle and adipose tissue: A mechanism to
conserve glucose.
•
Stimulation of fat breakdown in adipose tissue: The fatty acids released by
lipolysis are used for production of energy in tissues like muscle, and the released
glycerol provide another substrate for gluconeogenesis.
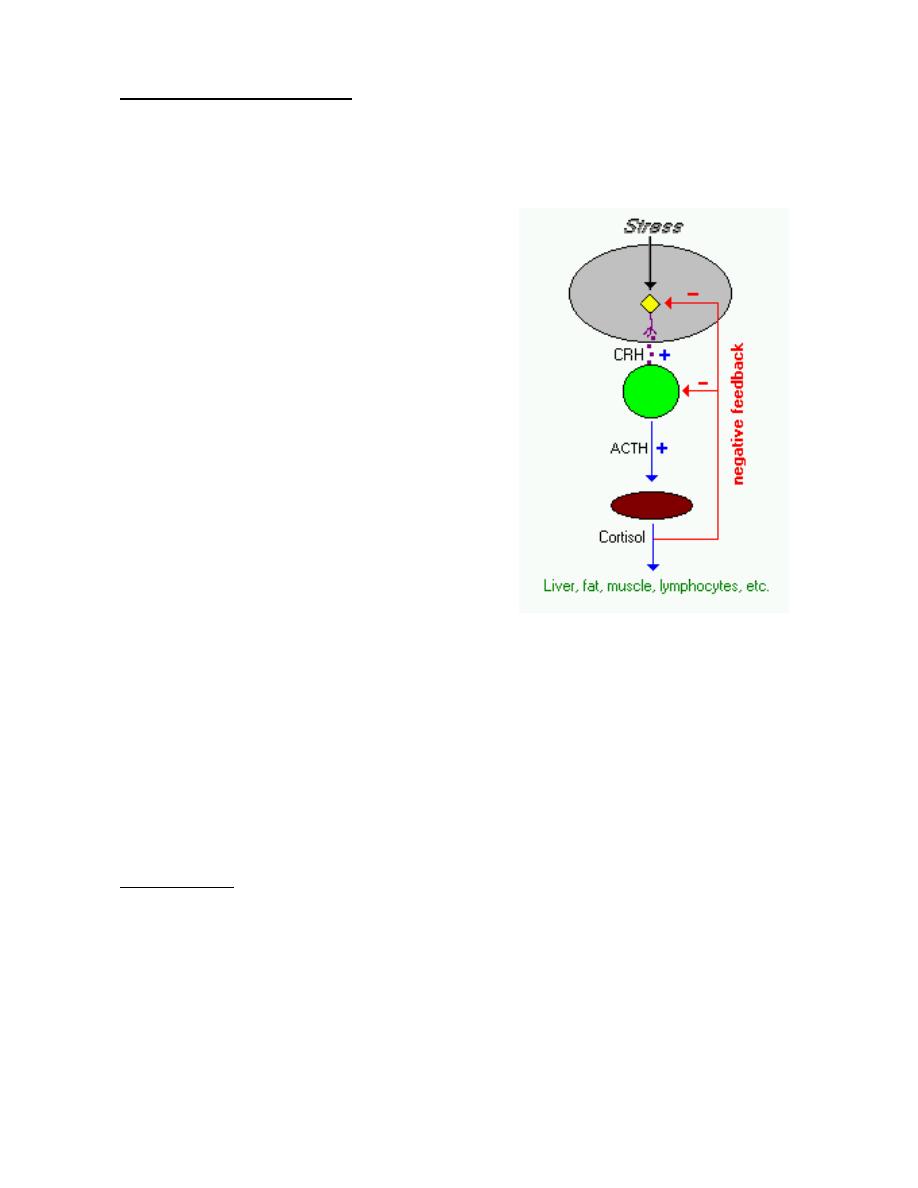
Prof.Dr. Hedef Dhafir El-Yassin 2012
81
Control of Cortisol Secretion
Cortisol and other glucocorticoids are secreted in response to a single stimulator:
adrenocorticotropic hormone (ACTH) from the anterior pituitary. ACTH is itself
secreted under control of the hypothalamic peptide corticotropin-releasing hormone
(CRH).
Virtually any type of physical or mental stress
results in elevation of cortisol concentrations in
blood due to enhanced secretion of CRH in the
hypothalamus. This fact sometimes makes it very
difficult to assess glucocorticoid levels, especially
being restrained for blood sampling, is enough
stress to artificially elevate cortisol levels several
fold!
Cortisol secretion is suppressed by classical
negative feedback loops. When blood
concentrations rise above a certain threshold,
cortisol inhibits CRH secretion from the
hypothalamus, which turns off ACTH secretion,
which leads to a turning off of cortisol secretion from
the adrenal. The combination of positive and negative control on CRH secretion results in
pulsatile secretion of cortisol. Typically, pulse amplitude and frequency are highest in the
morning and lowest at night.
ACTH, also known as corticotropin, binds to receptors in the plasma membrane of cells in
the adrenal. Hormone-receptor engagement activates adenyl cyclase, leading to elevated
intracellular levels of cyclic AMP which leads ultimately to activation of the enzyme
systems involved in biosynthesis of cortisol from cholesterol.
Disease States
1. Cushings disease or hyperadrenocorticism.
2. Insufficient production of cortisol, often accompanied by an aldosterone deficiency,
is called Addison's disease or hypoadrenocorticism.

Prof.Dr. Hedef Dhafir El-Yassin 2012
82
3. Androgens
The most important androgens include:
1. Testosterone: a hormone with a wide variety of effects, ranging from enhancing
muscle mass and stimulation of cell growth to the development of the secondary
sex characteristics.
2. Dihydrotestosterone (DHT): a metabolite of testosterone, and a more potent
androgen than testosterone in that it binds more strongly to androgen receptors.
3. Androstenedione (Andro): an androgenic steroid produced by the testes, adrenal
cortex, and ovaries. While androstenediones are converted metabolically to
testosterone and other androgens, they are also the parent structure of estrone.
4. Dehydroepiandrosterone (DHEA): It is the primary precursor of natural estrogens.
DHEA is also called dehydroisoandrosterone or dehydroandrosterone.
Prof.Dr. Hedef Dhafir El-Yassin 2012
83
[Hormones of the
Adrenal Gland]
[Prof.Dr.H.D.El-Yassin]
2. Hormones of the adrenal Medulla
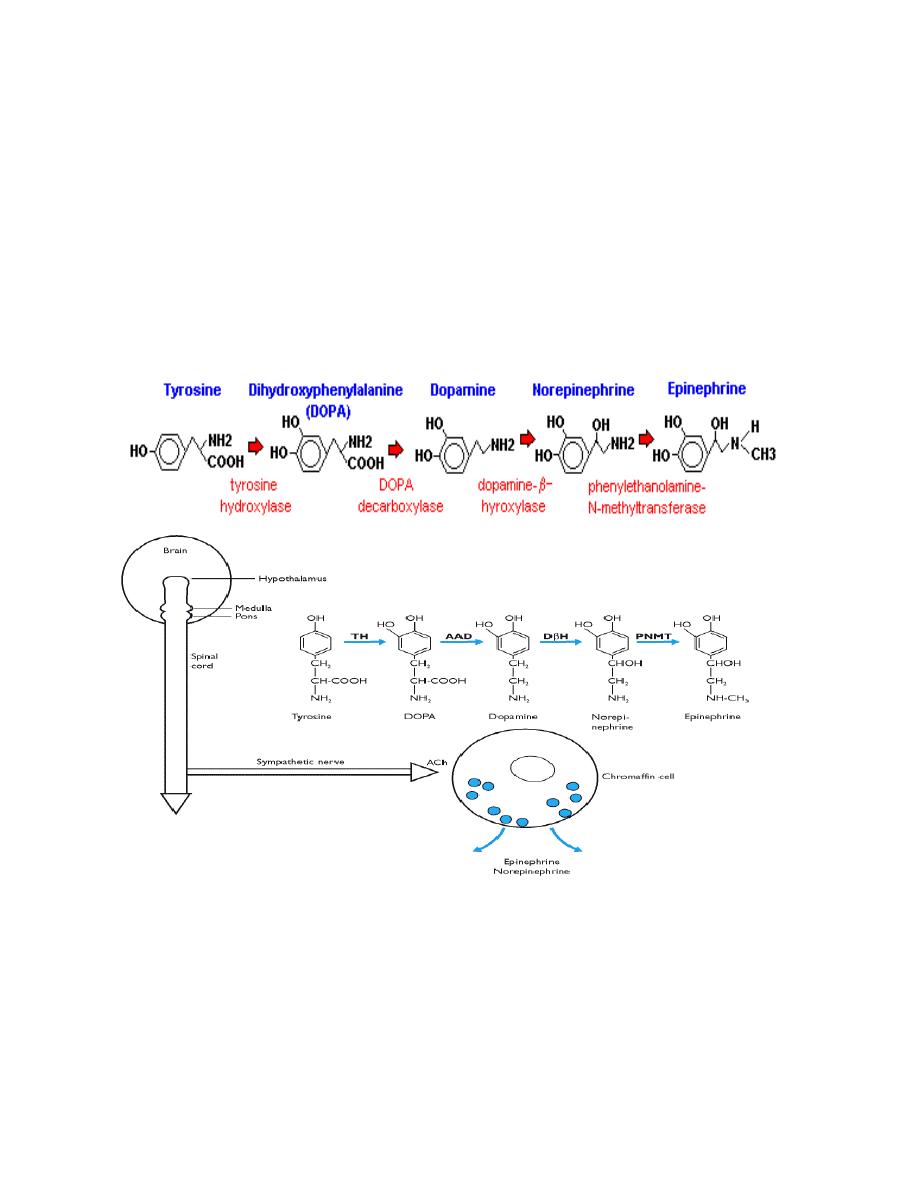
Prof.Dr. Hedef Dhafir El-Yassin 2012
84
Hormones secreted by the Adrenal Medulla
Cells in the adrenal medulla synthesize and secrete epinephrine and norepinephrine.
Following release into blood, these hormones bind adrenergic receptors on target cells,
where they induce essentially the same effects as direct sympathetic nervous stimulation.
Synthesis Catecholamines
Synthesis of catecholamines begins with the amino acid tyrosine, which is taken up
by chromaffin cells in the medulla and converted to norepinephrine and epinephrine
through the following steps:
Norepinephine and epinephrine are stored in electron-dense granules which also contain
ATP and several neuropeptides. Secretion of these hormones is stimulated by
acetylcholine release. Many types of "stresses" stimulate such secretion, including
exercise, hypoglycemia and trauma. Following secretion into blood, the catecholamines
bind loosely to and are carried in the circulation (50%) by albumin and other serum
proteins. Once secreted their half life in the circulation is short (approximately 12 min) but
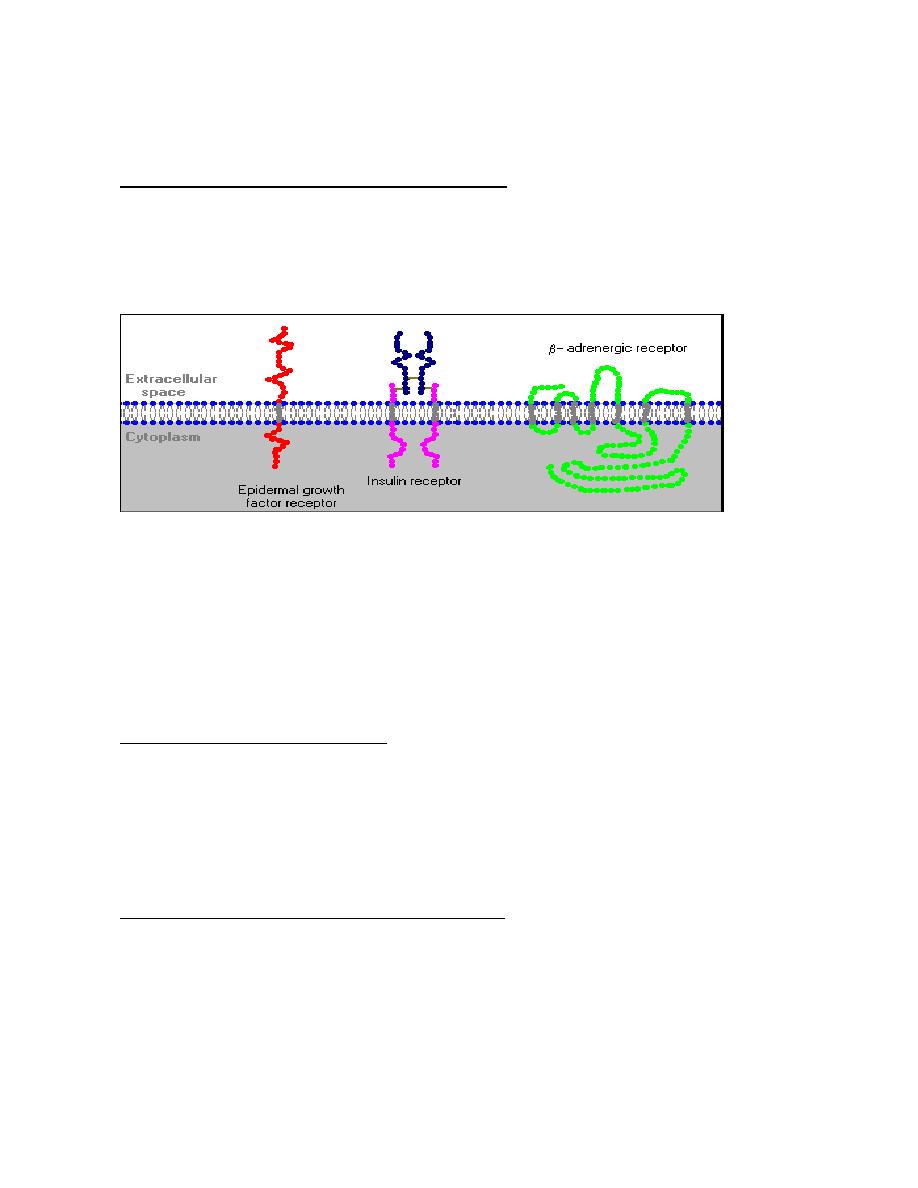
Prof.Dr. Hedef Dhafir El-Yassin 2012
85
they have a large effect on heart, vessels, metabolism, brain, muscles etc. all as part of
stress responses.
Adrenergic Receptors and Mechanism of Action
The physiologic effects of epinephrine and norepinephrine are initiated by their binding to
adrenergic receptors on the surface of target cells. These receptors are prototypical
examples of seven-pass transmembrane proteins that are coupled to G proteins which
stimulate or inhibit intracellular signaling pathways.
There are two major classes of adrenergic receptors these are:
1.
α adrenergic receptor (epinephrine and norepinephrine)
a.
α
1
b.
α
2
2.
β adrenergic receptor (epinephrine )
a.
β
1
b.
β
2
Control of catecholamine release
The release of the catecholinamines is controlled from nerve cells within the posterior
hypothalamus which can ultimately stimulate acetylcholine release from nerve terminals of
the sympathetic nerves. This induces depolarization of the chromaffin cells and exocytosis
of the catecholamine containing granules following a rise in intracellular calcium
concentration.
Metabolic Effects of catecholamines Hormones
•
In general, circulating epinephrine and norepinephrine released from the
adrenal medulla have the same effects on target organs as direct stimulation
by sympathetic nerves, although their effect is longer lasting.
•
glycogenolysis to provide extra sources of glucose
•
Stimulation of lipolysis in fat cells to provided fatty acids for energy production in
many tissues and aids in conservation of dwindling reserves of blood glucose.
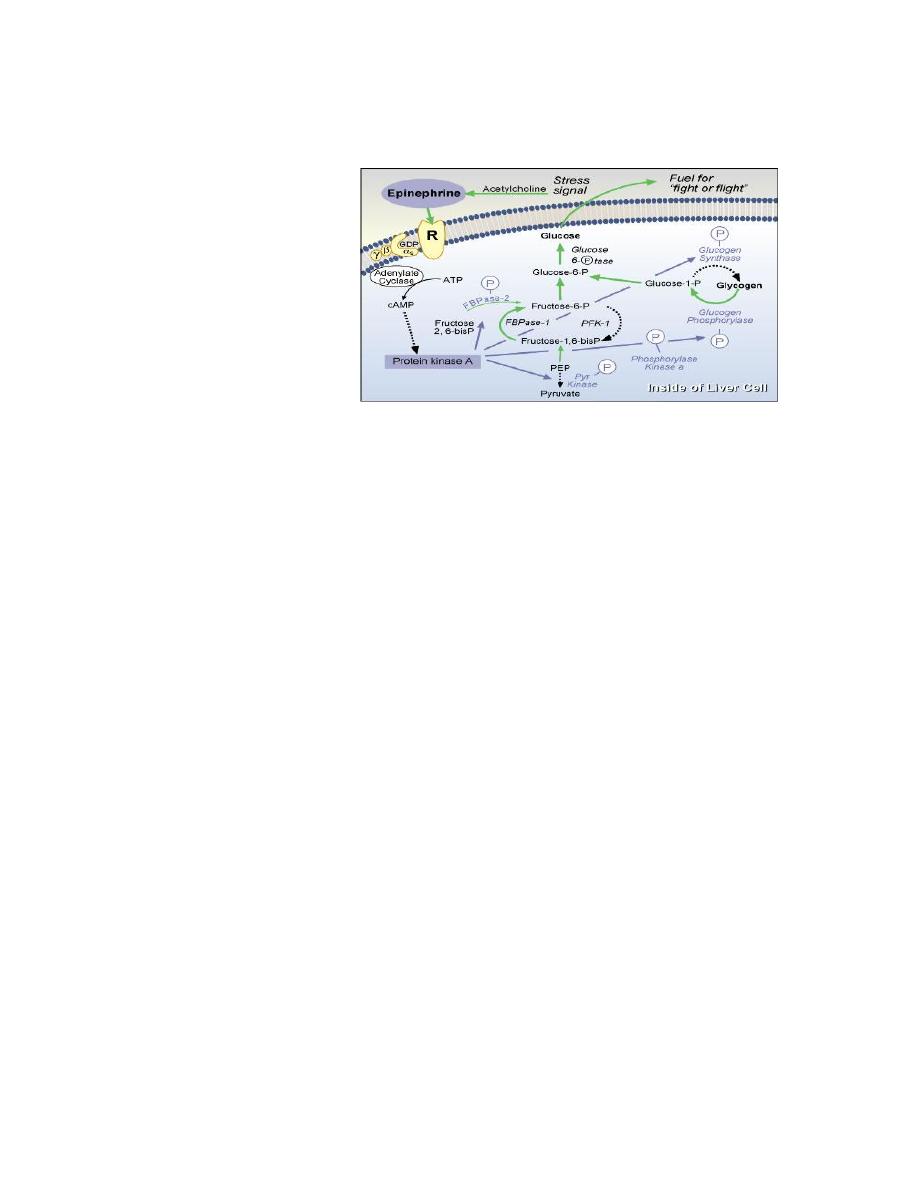
Prof.Dr. Hedef Dhafir El-Yassin 2012
86
•
Increased metabolic rate due to increased oxygen consumption and heat
production increase throughout the body in response to epinephrine binding beta
receptors.
•
Increased breakdown
of glycogen in skeletal
muscle to provide
glucose for energy
production.
Water and electrolytes
metabolism
•
Decreased sodium
excretion and glomerular
filtration due to direct effects on the kidney
•
effects on renin secretion leads to increased aldosterone production with effects on
distal sodium handling
•
Serum potassium may be increased
Catecholamine Degradation
All catecholamines are rapidly eliminated from target cells and the circulation by three
mechanisms:
1. reuptake into secretory vesicles
2. uptake in non-neural cells (mostly liver)
3. degradation.
Degradation relies on two enzymes:
1. catechol O-methyltransferase (COMT) in non-neuronal tissues
2. and monoamine oxidase (MAO) within neurons.
to produce metabolites (metanephrines and vanillylmandelic acid (VMA)) from free
catecholamines.
Metabolites and free catecholamine are eliminated by direct filtration into the urine and
excreted as:
1) free norepinephrine,
2) conjugated norepinephrine
3) metanephrines and
4) VMA
Urine epinephrine (50%) is converted from norepinephrine by renal (not adrenal)
phenylethanolamine N-methyltransferase (PNMT) before excretion.

Prof.Dr. Hedef Dhafir El-Yassin 2012
87
Clinical cases and Correlations:
Cushing's syndrome
A 35-year-old man was admitted to the hospital because of irritability and emotional lability
together with muscle weakness and easy fatigability. Physical examination revealed that
his trunk was obese but his arms and legs were quite lean. He had a rounded facial
appearance and a small, nontender hump at the junction of his neck and back. Fullness
was noted in the supraclavicular regions, and purple striae were present in the subaxillary
areas. Laboratory examination revealed that the plasma cortisol concentration was
elevated (0.55 mmol/L), the fasting blood glucose was 8.3 mmol/L, and the urinary
excretion of 11-hydroxyandrosterone and 11-hydroxyetiocholanolone were greatly
increased. A diagnosis of Cushing's syndrome was made.
Biochemical questions
1. From what substance is cortisol synthesized?
2. How is cortisol synthesis regulated?
3. How is cortisol transported in the blood plasma?
4. What are the metabolic effects of cortisol in humans?
5. How does cortisol function at the molecular level?
6. How are 11-hydroxyandrosterone and 11-hydroxyetiocholanolone related to cortisol
and why were they excreted in increased amounts in this patient's urine?
7. Would you expect that excessive quantities of vanillylmandelic acid (VMA) also
would be excreted in this patient's urine?
Case discussion:
Cortisol, the main glucocorticoid hormone in humans, is synthesized in the zona
fasciculata of the adrenal cortex. Cholesterol is the precursor of cortisol as well as of the
approximately 50 other steroids that can be isolated from the adrenal cortex. Most of the
cholesterol used for steroid hormone synthesis is taken up from plasma lipoproteins and
stored in the adrenal cortical cells as cholesteryl esters. Progesterone is the common
intermediate in steroid hormone synthesis.
Regulation of synthesis cortisol synthesis is regulated by ACTH, a polypeptide hormone
secreted by the anterior pituitary gland. ACTH release, in turn, is stimulated by CRH,
produced in the hypothalamus. Cortisol production is decreased by high concentrations of
plasma cortisol or ACTH, both of which inhibit CRH release. ACTH stimulates cortisol
production by activating the hydrolysis of cholesteryl esters through a cAMp-dependent
mechanism.
Prof.Dr. Hedef Dhafir El-Yassin 2012
88
Transport Like the other steroid hormones, cortisol is transported in the blood by a
specific carrier protein, transcortin. This protein is a plasma globulin hat is synthesized in
the liver. It also transports corticosterone, the other major glucocoricoiod produce by the
adrenal cortex.
Metabolic effects One of the main metabolic effects of the glucocorticoid hormones is to
raise the blood glucose concentration by stimulating gluconeogenesis. Body proteins are
catabolized to provide the amino acid substrates for gluconeogenesis, leading to
decreases in muscle mass and weakness. Glucocorticoids operate through a
transcriptional mechanism. This involves binding of the hormone to cytoplasmic protein
receptors and transport of the steroid-receptor complex into the cell nucleus.
Once inside the nucleus, it activates the transcription of mRNA, which is specific for the
synthesis of these amino acid catabolic enzymes. The glucocorticoids have additional
actions, such as indirectly facilitating the stimulation of lipolysis, and destruction of
lymphocytes.
Catabolism and excretion Cortisol is metabolized and the l7-keto metabolites of cortisol
are 1l-hydroxyandrosterone and 11-hydroxyetiocholanolone. Almost all the cortisol
metabolites are excreted in the urine. Since cortisol is overproduced in Cushing's
syndrome, it is understandable that these two cortisol metabolites are excreted in
excessive amounts. .
VMA is the main urinary excretion product of the catecholamines. It is elevated in diseases
associated with excessive catecholamine production, such as pheochromcytoma. If a
tumor of the adrenal medulla or other chromaffin tissue is present, VMA excretion would
be increased. Cushing's disease results from overproduction of glucocorticoids by the
adrenal cortex and, of itself, does not involve the adrenal medulla or other catecholamine-
producing tissues. Thus there is no reason to suspect overproduction of either epinephrine
or norepinephrine in this case, and one would not expect VMA excretion to be affected if
the diagnosis of Cushing's syndrome is correct.

Prof.Dr. Hedef Dhafir El-Yassin 2012
89
Primary aldosteronism
A 44-year-old man who complained of headaches was discovered to be hypertensive.
Hypokalemia was noted; the plasma potassium concentration remained between 2.5 and 3
mmol/L during 3 wk of observation. Peripheral vein renin concentrations were consistently
less than 1 µlL, and they remained low even when the patient was placed on a low-sodium
diet. Urinary aldosterone excretion was consistently high, even when a high-sodium diet
was given. A venogram showed a questionable lesion in the left adrenal gland. A left
adrenalectomy was performed. On follow-up examination 2 months later, the patient was
found to be normotensive and normokalemic.
Biochemical questions
1. Explain the hypokalemia in this case. Would you expect any abnormality in total
body sodium content?
Apparent Mineralocorticoid Excess Syndrome
Some patients (usually children) exhibit the hypertension, hypokalemia, and suppression
of the renin-angiotensin-aldosterone system that would be expected if they were hyper-
secreting aldosterone. Since assays of plasma and urine may fail to identify, excess
mineralocorticoids, these patients are said to have the apparent mineralocorticoid excess
(AME) syndrome. This syndrome results from the failure of inactivation of cortisol by 11
ß-
hydroxysteroid dehydrogenase. Since the plasma levels of cortisol are about 100 times
higher than the levels for aldosterone, cortisol saturates the renal mineralocorticoid
receptor, causing sodium retention and suppression of the renin-angiotensin-aldosterone
axis.
Although this syndrome can result from a congenital defect in renal 11
ß-hydroxysteroid
dehydrogenase isoform, it can also be caused by ingesting excessive amounts of licorice.

Prof.Dr. Hedef Dhafir El-Yassin 2012
90
Questions
2) Glucocorticoid receptors are in the cytoplasm. AII of the following statements about the
process by which the hormone influences transcription arc correct except:
a) The hormone must be in the free state to cross the cell membrane.
b) Cytoplasmic receptors may be associated with heat shock proteins.
c) The receptor-hormone complex is not activated/transformed until it is
translocated to the nucleus.
d) In the nucleus, the activated/transformed receptor-hormone complex searches for
specific sequences on DNA called HREs (hormone receptor elements).
e) The activated receptor-hormone complex may either activate or repress
transcription of specific genes (only one activity per gene).
C
Dissociation of the heat shock protein from the receptor- hormone complex in the
cytosol activates the complex. A: Steroid hormones travel bound to plasma proteins, but
some is always free. D: These are consensus sequences in DNA. E: Activation is more
common, but glucocorticoids repress transcription of the proopiomelanocortin gene.
3) All of the following are normal events leading to secretion of aldosterone from the
adrenal gland except:
a) Renin is released by the kidney in hypovolemia.
b) Angiotensinogen binds to membrane receptors.
c) Ca
2+
levels in the cell rise.
d) aldosterone is secreted into the blood.
B
Angiotensinogen is cleaved by renin to angiotensin I, which must further be cleaved
by converting enzyme to active angiotensin II. A: This is a major signal. C: This lead to
increased Ca
2+
and activation of protein kinase C.

Prof.Dr. Hedef Dhafir El-Yassin 2012
91
Biochemistry and Disorders of Hormones of the
Kidney, Heart and Adipose tissue
Lecture 8
Sunday 8/3/2012
The human kidney secretes two hormones
•
Erythropoietin
•
Calcitriol (1,25[OH]
2
Vitamin D
3
)
•
Erythropoietin
(EPO) is a glycoprotein hormone that is a growth factor for erythrocyte (red blood cell)
precursors in the bone marrow.
•
In adults primarily by peritubular cells in the kidneys, where its production is stimulated
by low oxygen levels in the blood.
•
Some EPO is also produced by the liver, which is the primary source in the fetus.
Actions
EPO acts by binding to a specific erythropoietin receptor (EpoR) on the surface of red cell
precursors in the bone marrow, stimulating them to transform into mature red blood cells.
As a result the oxygen level in blood reaching the kidney rises and the amount of EPO
produced decreases.
People with failing kidneys can be kept alive by dialysis. But dialysis only cleanses the
blood from wastes. Without a source of EPO, these patients suffer from anemia.
In response to a rise in blood pressure, the heart releases two peptides
•
A-type Natriuretic Peptide (BNP)
This hormone of 28 amino acids is released from stretched atria (hence the "A").
•
B-type Natriuretic Peptide (BNP)
This hormone (29 amino acids) is released from the ventricles. (It was first discovered in
brain tissue; hence the "B")
Both hormones lower blood pressure by:
•
Relaxing arterioles
•
Inhibiting the secretion of renin and aldosterone
•
Inhibiting the reabsorption of sodium ion by the kidneys.
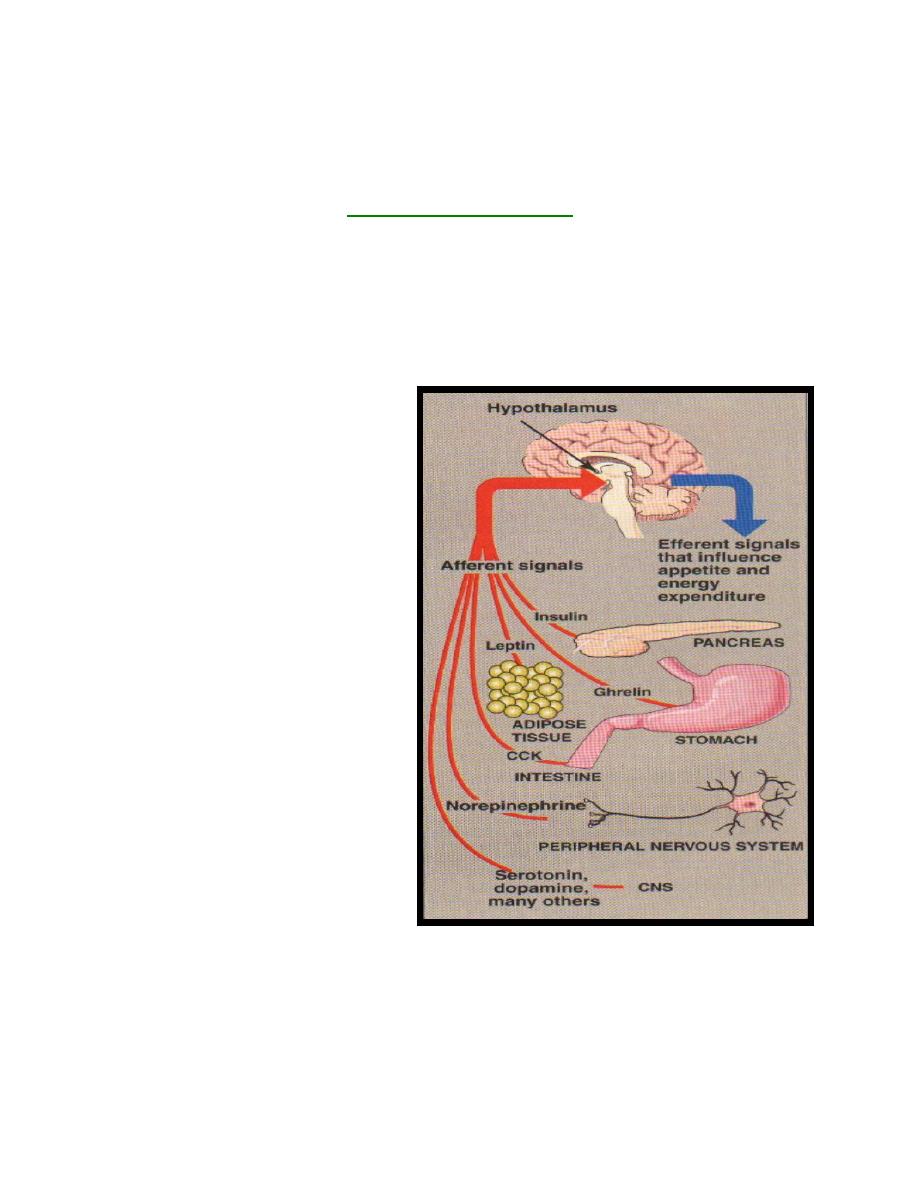
Prof.Dr. Hedef Dhafir El-Yassin 2012
92
The latter two effects reduce the reabsorption of water by the kidneys. So the volume of
urine increases as does the amount of sodium excreted in it. Te net effect of these actions
is to reduce blood pressure by reducing the volume of blood in the circulating system.
These effect give ANP and BNP their name (natrium= sodium; uresis= urinate)
Hormones of adipose tissue
Although the adipocyte's primary role is to store fat, it also functions as an endocrine cell that
releases numerous regulatory molecules, such as leptin, adiponectin, and resistin
1. Leptin: Studies of the molecular genetics of mouse obesity have led to the isolation of at
least six genes associated with obesity. The most well-known mouse gene, named Ob (for
obesity), leads to severe hereditary obesity in mice. It has been identified and cloned. In
one strain of fat mice, the gene
was completely absent, indicating
that the gene's protein product is
required to keep the animals'
weight under control. The
product of the Ob gene is a
hormone called leptin.
Leptin is produced proportionally to the
adipose mass and, thus, informs the
brain of the fat store level. It is secreted
by fat cells, and acts on the
hypothalamus of the brain to regulate
the amount of body fat through the
control of appetite and energy
expenditure. Leptin's secretion is
suppressed by depletion of fat stores
(starvation) and enhanced by expansion
of fat stores (well-fed state). Daily
injection of leptin causes overweight mice to lose weight and maintain weight loss. The protein also
causes weight loss in mice that are not obese. In humans, leptin increases the metabolic rate and
decreases appetite.
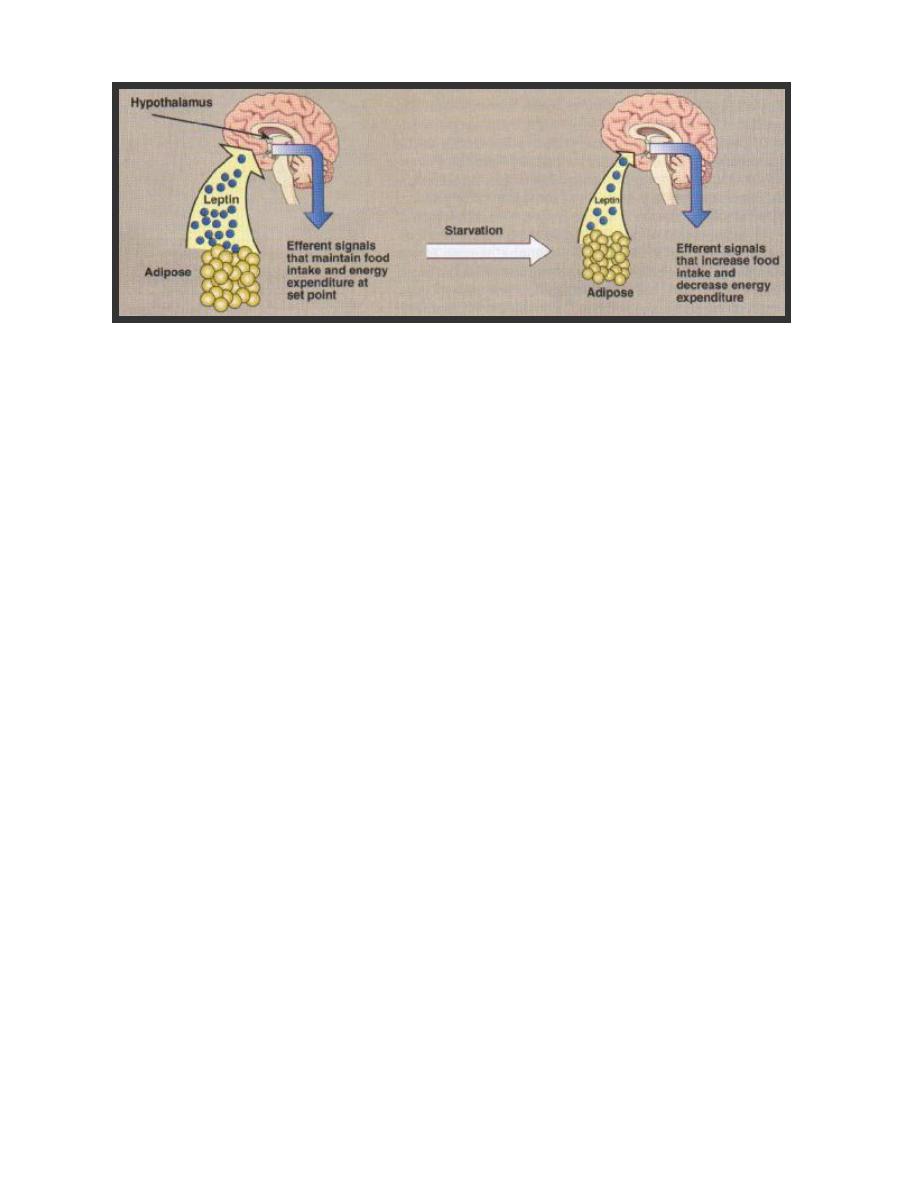
Prof.Dr. Hedef Dhafir El-Yassin 2012
93
However, plasma leptin in obese humans is usually normal for their fat mass, suggesting that
resistance to leptin, rather than its deficiency, occurs in human obesity. Other hormones
released by adipose tissue, such as adiponectin and resistin, may mediate insulin resistance
observed in obesity
2. resistin
The hormone resistin is one amongst a novel family of three proteins , known as resistin-
like molecules (RELMs). They are cysteine-rich secreted proteins associated with
pulmonary inflammation (also known as FIZZ3, found in inflammatory zone). It has 11
cysteine-residues synthesized as a propeptide of 108 amino acids and secreted as a
dimmer, build by a disulfide bridge of cysteine residues. Beside this intermolecular
disulfide bridge, 5 additional intramolecular ones exist.
Source of reistin
In humans, resistin expression in adiposite can be detected at a low level. it is higher in
abdominal fat stores than in thigh adipose tissue, this suggest a potential role in linking
central obesity to type 2 diabetes and/or cardiovascular disease. Human resistin is
expressed mainly in pancreatic islet, preadiposites, macrophages and bone marrow. So
resistin is of relevance for inflammation processes as well as for lipid metabolism.
In mice a correlation between adiposity, insulin resistance and resistin expression was
found empirically. In humans respective studies are not clear. Several show an association
of resistin serum concentration and adiposity or insulin resistance.

Prof.Dr. Hedef Dhafir El-Yassin 2012
94
Resistin putative role(s):
Relevance of resistin in physiological processes other than energy metabolism was
investigated. Experiments with endothelial cells gave interesting results, in which resistin
shown to be potentially able to influence endothelial inflammation and thereby
atherosclerosis.
Resistin shares some qualities with another protein secreted by fat cells and associated
with obesity, the hormone leptin. This hormone, discovered in 1995, seems to regulate
food intake.
There is still much to learn about resistin. But with each new piece fitted into the diabetes
puzzle, new possibilities arise.
There are two putative roles of resistin:
a. To directly cause insulin resistance
b. To block adipocyte differentiation
The latter might lead to ectopic fat storage (increased amounts of fat in skeletal
muscle and liver.
Future work….
Future research in this area aims to establish the role of resistin in human disease.
Measurement of resistin in a simple blood test might then be useful in detecting insulin
resistance and prediabetic conditions. Looking forward, counteracting resistin's affects on
the body might be a new approach to preventing and treating diabetes.
