
BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
1
Objectives
state the types of bone marrow
Identify the major sites of hematopoiesis in the fetus and normal
adult.
outline the compartments of red bone marrow
describe the types and distinctive characteristic of stem cells
know the differences between stem cells, progenitor cells, blast cells
and mature cells
Review the stem cell theory of hematopoiesis.
Discuss growth factors involved in the stimulation and regulation of
hematopoietic activity.
Review maturation stages of each cell lineage, including changes that
occur as cells mature.
Bone marrow : Is a soft connective tissue occupies the medullary cavity of
long bones and all the spaces between the trabeculae of spongy bone. It
accounts for approximately 5% of the body weight in humans
Two varieties of marrow are recognized
Red marrow
Yellow marrow
Red marrow
is the
only site
for genesis of blood cells in adults and it Is the
only type
found in fetal and young bones but in adult it is restricted to the
vertebrae , sternum , ribs , cranial bones and epiphysis of long bones
Childhood- red marrow is
100%
of bone marrow and present in
virtually every bone.
Adults- red marrow is
50%
of bone marrow presenting in sternum,
ribs, pelvis and skull.
70 years- red marrow reduced to
30%
of the bone marrow.
Consists in main of fat cells which have gradually replaced the other
marrow elements.
With an adequate stimulus, yellow marrow may resume the character of
red marrow and play an active role in the process of blood development .

BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
2
has
A vascular compartment and
An extravascular compartment
Vascular compartment
Is supplied by a nutrient artery which branches into central longitudinal
arteries which send out radial branches that eventually open into sinuses.
These sinuses converge into a central vein that carries the blood out of the
bone marrow into the general circulation
The marrow does not have lymphatic drainage
Extravascular compartment
Is composed of :
1. Stroma of reticular connective tissue
reticular tissue ( reticular cells, .Reticular fibers )
macrophages and adipose cells
Matrix
2. hematopoietic cords
3. sinusoidal capillaries
Reticular cells
Stellate in shape and are in contact with each other along extended cellular
processes.
Function of reticular cells:
Act as a meshwork to support and protect the haemopoietic cells
Formation of reticular fibers
Phagocytosis
They transfer to adipocytes by accumalating lipid in their contents
Matrix Contain:
collagen type I , III
laminin ,
fibronectin and
proteoglycan
Laminin, fibronectin, and another cell-binding substance, hemonectin,
interact with cell receptors to bind cells to the stroma.
The sinusoids are formed by a discontinuous layer of endothelial cells.

BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
3
Sinusoidal capillaries
An external discontinuous layer of reticular cells and a loose net of reticular
fibers reinforce the sinusoidal capillaries.
Bone marrow barrier:
The blood vessels of the bone marrow constitute a
barrier, inhibiting immature blood cells from leaving the marrow. Only
mature blood cells contain the membrane proteins required to attach to
and pass the blood vessel endothelium. Hematopoietic stem cells may also
cross the bone marrow barrier, and may thus be harvested from blood.
are pluripotential cells capable of self-renewal. Some of their daughter
cells form specific, irreversibly differentiated cell types, and other daughter
cells remain stem cells.
They
retain the ability to renew themselves through mitotic cell division
can differentiate into a diverse range of specialized cell types.
found in all multi-cellular organism .
Two broad types of mammalian stem cells
1. Embryonic stem cells that are found in blastocysts
2. Adult stem cells that are found in adult tissues
Embryonic Stem Cells
are derived from the inner cell mass of the embryo. Because these cells
are pluripotent and can virtually form any cell or tissue type, they have the
potential for curing a variety of diseases, including diabetes, Alzheimer and
Parkinson diseases, anemias, spinal cord injuries, and many others.
ES cells may be obtained
1. reproductive cloning.
ES cells may be obtained from embryos after in vitro fertilization.
This approach has the disadvantage that
the cells may cause immune rejection, since they would not be
genetically identical to their hosts.
Another issue with this approach is based on ethical considerations,
since the cells are derived from fertilized viable embryos.
2. therapeutic cloning
(somatic nuclear transfer)
take nuclei from adult cells (e.g., skin) and introduce them into enucleated
oocytes.

BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
4
Oocytes are stimulated to differentiate into blastocysts, and ES cells are
harvested.
Since the cells are derived from the host, they are compatible genetically
and since fertilization is not involved, the technique is less controversial
Adult Stem Cells
Adult tissues contain stem cells that also may prove valuable in treating
diseases.
These cells are restricted in their ability to form different cell types and,
therefore, are multipotent, not pluripotent.
Adult stem cells isolated from rat brains have been used to cure Parkinson
disease in rats, suggesting that the approach has promise.
Disadvantages of the approach include
the slow rates of cell division characteristic of these cells and
their scarcity, which makes them difficult to isolate in sufficient
numbers for experiments.
Hematopoiesis
takes place in the extravascular compartment
The currently accepted theory on how this process works is called the
monophyletic theory which simply means that a single type of stem cells
gives rise to all mature blood cells in the body.
This stem cells is called the pluripotential stem cells.
It is believed that all blood cells arise from a single type of stem cell in the
bone marrow . It is called a pleuripotential stem cell because it can porduce
all blood cell types .
Pleuripotential stem cell proliferate and form
1. lymphoid multipotential cells: one cell lineage that will become
lymphocytes .
2. myeloid multipotential cells : another lineage that will form the myeloid
cells ( granulocytes, monocytes, erythrocytes and megakaryocytes)
BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
5
Site of hematopoiesis
yolk sac then liver
Embryo
Spleen
3
rd
- 7
th
months
marrow cavity - esp. granulocytes and platelets
4
th
& 5
th
months
marrow cavity
– erythrocytes
7
th
month
mostly bone marrow; spleen and liver when
needed
Birth
number of active sites in bone marrow
decreases but retain ability for hematopoiesis
Birth to maturity
bone marrow of skull, ribs, sternum, vertebral
column, pelvis, proximal ends of femurs
Adullts
Hematopoiesis depends on
favorable microenvironmental conditions and
the presence of growth factors.
The microenvironmental conditions are furnished by cells of the stroma of
hematopoietic organs, which produce an adequate extracellular matrix.
A general view of hematopoiesis shows that both the potential for
differentiation and the self-renewing capacity of the initial cells gradually
decrease.
the mitotic response to growth factors gradually increases, attaining its
maximum in the middle of the process.
From that point on, mitotic activity decreases, morphological
characteristics and functional activity develop, and mature cells are
formed
stem cells:
Progenitor cells :
This cell can produce all blood cell
types
Could be unipotential or bipotential
Low mitotic activity
High mitotic activity
Self renewing
Self renewing
Scarce in the bone marrow
Common in marrow and lymphoid
organs
Cannot be morphologically
distinguished ( resemble large
lymphocyte )
Cannot be morphologically
distinguished ( resemble large
lymphocyte )
Age

BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
6
Precursor cells( blast ):
Mature cells :
Monopotential cells
High mitotic activity
No mitotic activity
Not self renewing
Common in marrow and lymphoid
organs
Abundant in the blood and
haematopoietic organs
Beginning of morphologic
differentiation
Clear morphologic differentiation
Hematopoiesis is a compartmentalized process within the hematopoietic tissue
erythropoiesis taking place in distinct anatomical units (erythroblastic
islands);
granulopoiesis occurs in less distinct foci
megakaryopoiesis occurs adjacent to the sinus endothelium.
Upon maturation, the hematopoietic cells, regulated by the reticular cells,
traverse the wall of the venous sinuses to enter the bloodstream
o Leukocytes, after the action of releasing substances, cross the wall of the
sinusoid by their own activity.
o Because erythrocytes (unlike leukocytes) do not have sufficient motility to
cross the wall of the sinusoid, they are believed to enter the sinusoid by a
pressure gradient that exists across its wall.
o Megakaryocytes form thin processes (proplatelet processes) that cross
the wall of the sinusoid and fragment at their tips, liberating the platelets.
MATURATION OF ERYTHROCYTES
Mainly regulated by erythropoietin released by thekidneys;
also influenced by androgens
) : large cell , rounded nucleus coarse
chromatin , visible nucleoli, intense basophilia of the cytoplasm
Maturation of erythrocytes
(basophilic normoblasts) : condensed nucleus , no visible nucleoli , strongly
basophilic cytoplasm because of free ribosomes and polyribosomes.

BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
7
:
mixed color cytoplasm purplish blue to grey
: the amount of haemoglobin is the
same as that of erythrocyte . Nucleus with dense and compact chromatin -------
--pyknotic --------- extruded from the cell with a thin rim of cytoplasm and
plasma membrane
: youngest erythrocyte containing a delicate reticulum
the clumped ribosomes responsible for the distinctive staining of the
reticulocytes are
degraded within 24 hours
9.
anucleated and biconcave in peripheral blood
Several major changes take place during maturation of erythrocyte
1. cell volume decreases
2. nucleoli diminish in size until they become invisible
3. nuclear diameter decrease and chromatin increase until the nucleus become
pyknotic and extruded from the cell
4. gradual decrease in the number of polyribosomes ( basophilia )with a
simultaneous increase in the amount of haemoglobin( acidophilic protein )
.5mitochondria and other organelles gradually disappear
GRANULOPIOSIS
Regulated by GM-CSF
1. pluripotential stem cells
2. myeloid multipotential stem cells
3. granulocyte colony forming cell
4. Myeloblast:
ovoid nucleus with 2 or more nucleoli , basophilic cytoplasm (abundance of
RER and ribosomes )
5. Promyelocyte:
round or oval nucleus , occasionally indented , basophilic cytoplasm containing
azurophilic granules ( lysosomal enzyme and myeloperoxidase )
6. Myelocyte: appearance of specific granules and the developing myelocytes
can be distinguished into 3 types :
Neutrophilicmyelocyte
Acidophilic myelocyte
Basophilic myelocyte
BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
8
7. Metamyelocyte :
nuclei irregular in shape known as band form , cytoplasm with increasing free
ribosomes , mitochondria and RER .
Neutrophilicmetamyelocyte
Acidophilic metamyelocyte
Basophilic metamyelocyte
8. Mature granulocyte :
: horse
–shoe or S- shaped nucleus
Acidophil : nucleus with 2 lobes , common in connective tissue of certain
organs ( intestine epithelium )
B a s o p h i l : nucleus is large , irregular
AZUROPHILIC GRANULES
SPECIFIC GRANULES
1st appear in promyelocyte
1
st
appear in myelocyte
Derived from the inner cisternae of
Golgi complex
Derived from an outer cisternae of
Golgi complex
Decrease in number with frequent
division and maturation
Increase in number with maturation
They
are lysosomes , it’s
histochemical structure
myeloperoxidase and acid
phosphatase
neutrophil contain alkaline
phosphatase and antibacterial
lysozyme
Acidophil contain sulphatase ,
peroxidase and histaminase
Basophil contain heparin and
histamine
MEDICAL APPLICATION
The appearance of large numbers of immature neutrophils (band cells) in the
blood is called a shift to the left and is clinically significant, usually indicating
bacterial infection.
MONOPOIESIS
Maturation of monocytes is regulated by GM-CSF
Maturation of monocytes

BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
9
:
large cell 18 micrometer in diameter , slightly indented nucleus with lacy
chromatin and evident nucleoli , basophilic cytoplasm
6. Monocyte :
indented nucleus
cytoplasm contain large amount of RER, extensive Golgi complex and granule
condensation. These granules are primary lysosomes, which are observed as
fine azurophilic granules in blood monocytes.
Monocytes migrate into the circulation where they remain for about 8 hours
before migrating into the connective tissue
in the connective tissue they increase in size, acquire multiple lysosomes and
becomeactive in phagocytosis (macrophages)
life span of macrophages in different tissues may be up to several months
LYMPHOPOIESIS
Maturation of lymphocyte
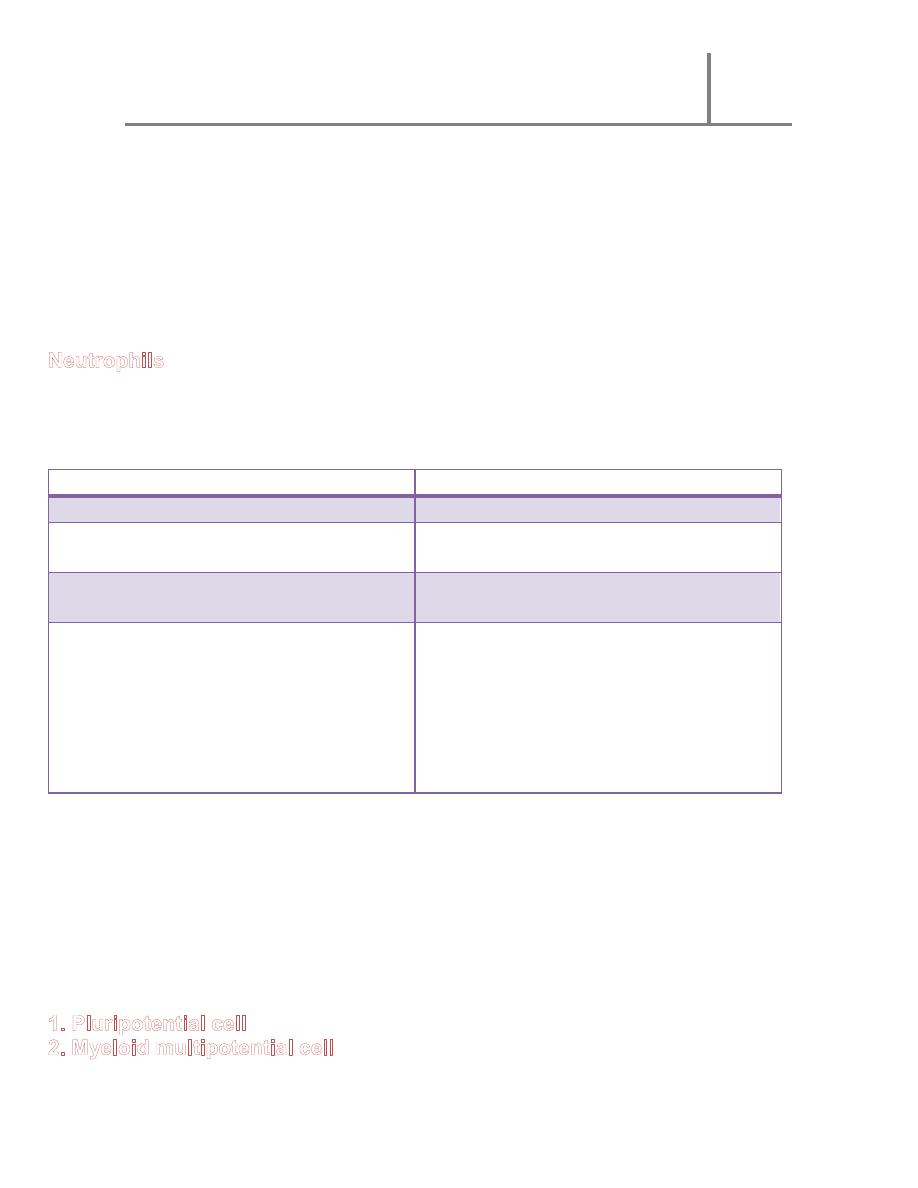
1. Pluripotential cell
2. Lymphoid multipotential cells : migrate to lymphoid organs
3. Lymphocyte colony forming cell
4. Lymphoblast :
large cells , large nucleus with prominent nucleoli ,
basophilic cytoplasm
capable of incorporating [3H]thymidine and dividing two or three times to form
prolymphocytes
5. Prolymphocyte
:
smaller with condensed chromatin ,nucleoli less obvious ,
few azurophilic granules appear in the cytoplasm ,
no cell surface receptor that mark them as T or B lymphocyte
6. B and T lymphocytes :
in the thymus or bone marrow , these cells synthesize cell surface receptors
but they are not recognized as distinct cell types using routine histological
procedure .
They can be recognized by immunohistochemistry
Embryo

BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
10
MEDICAL APPLICATION:
Leukemias are malignant clones of leukocyte precursors.
They occur in
lymphoid tissue (lymphocytic leukemias)
bone marrow (myelogenous and monocyticleukemias).
In these diseases, there is usually a release of large numbers of immature
cells into the blood.
The symptoms of leukemias are a consequence of this shift in cell proliferation,
with a lack of some cell types and excessive production of others (which are
often abnormal in function). The patient is usually anemic and prone to
infection.
THROMBOPOIESIS
Maturation of platelets is regulated by thrombopoietin (TPO). Mainly
produced by the liver
:
large cell ( 15-50 micrometer ) , large ovoid or kidney shaped nucleus with
numerous nucleoli ( DNA 30X as much as a normal cell ) , cytoplasm is
homogenous and basophilic
giant cell ( 35-150 micrometer), irregular lobulated nucleus , coarse chromatin ,
no visible nucleoli ,
Cytoplasm contain numerous mitochondria RER, extensive Golgi complex ,
conspicuous granules contain biologically active substances such as platelet
derived growth factor , fibroblast growth factor
The demarkation membranes arise from numerous invaginations of the plasma
membrane through out the cytoplasm
Electron micrographs indicate that megakaryocytic cytoplasm can fragment
through the formation of tiny channels arising from rows of vesicle.
The vesicles fuse with their neighbors and establish continuity with the cell
membrane, producing an extensive system of tubular platelet demarcation
channels that subdivide the cytoplasm into hundreds of platelets each with its
covering
Medical application
: In certain forms of thrombocytopenic purpura, a disease

BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
11
in which the number of blood platelets is reduced, the platelets appear to be
bound to the cytoplasm of the megakaryocytes, indicating a defect in the
liberation mechanism of these corpuscles. The life span of platelets is
approximately 10 days
.
CLINICAL EVALUATION
Tests of bone marrow function
Bone marrow aspiration- cytology and maturation
Bone marrow biopsy- cellularity and architecture
Bone marrow transplantion (or hematopoietic stem cell transplantation)
is a very complicated and risky process, and therefore applied only to patients
with life-threatening diseases (who are resistant to chemotherapy).
Mostly patients with congenital neutropenia, aplastic anemia, sickle-cell
disease, neuroblastoma, lymphoma and many other diseases are treated by
stem cell / bone marrow transplantation.
Autologous hematopoietic stem cell transplant
Autologous HSCT requires the extraction of haematopoietic stem cells (HSC)
from the patient and storage of the harvested cells in a freezer.
The patient is then treated with high-dose chemotherapy with or without
radiotherapy with the intention of eradicating the patient's malignant cell
population at the cost of partial or complete bone marrow ablation (destruction
of patient's bone marrow function to grow new blood cells).
The patient's own stored stem cells are then returned to his/her body, where
they replace destroyed tissue and resume the patient's normal blood cell
production
Allogeneic hematopoietic stem cell transplan
t
Allogeneic HSCT involves two people: the (healthy) donor and the (patient)
recipient. Allogeneic HSC donors must have a tissue HLA
– human leukocyte
antigene - type that matches the recipient.
Sources of hematopoietic stem cells
: in the case of a bone marrow transplant, the HSC are
removed from a large bone of the donor, typically the pelvis, through a
large needle that reaches the center of the bone. The technique is
performed under general anesthesia.
are
now the most common source of
stem cells for allogeneic HSCT. They are collected from the blood

BONE MARROW
PROF. DR. MALAK A. AL-YAWER
2012
12
through a process known as apheresis. The donor's blood is
withdrawn through a sterile needle in one arm and passed through a
machine that removes white blood cells. The red blood cells are
returned to the donor. The peripheral stem cell yield is boosted with
daily subcutaneous injections of Granulocyte-colony stimulating factor,
serving to mobilize stem cells from the donor's bone marrow into the
peripheral circulation.
: It is also possible to extract hematopoietic stem cells
from amniotic fluid for both autologous or heterologous use at the time of
childbirth.
: Umbilical cord blood is obtained when a mother
donates her infant's Umbilical Cord and Placenta after birth. Cord blood
has a higher concentration of HSC than is normally found in adult blood.
