ANTI-EPILEPTIC DRUGS
Dr.Nasser A. H. Al-Harchan
Dr.Nasser A. H. Al-Harchan
Asst. Prof. of Pharmacology
College of Medicine
Baghdad University

introduction
Seizure
Seizure
• Seizures
are
sudden,
transitory,
and
uncontrolled episodes of brain dysfunction
resulting from abnormal discharge of neuronal
cells with associated motor, sensory or
behavioral changes.
Epilepsy
• A group of chronic CNS disorders
characterized by recurrent seizures.

Classification of Epileptic Seizures
I. Partial (focal) Seizures
I. Partial (focal) Seizures
A. Simple Partial Seizures
B. Complex Partial Seizures
C. Partial with secondary generalized tonic
classic seizure
II. Generalized Seizures
A. Generalized Tonic-Clonic Seizures
B. Absence Seizures
C. Myoclonic Seizures
Pathological Basis
o
Abnormal electrical discharge in the brain
o
Coordinated activity among neurons depends on a
controlled balance between excitation and inhibition
o
Any local imbalance will lead to a seizure
o
Imbalances occur between glutamate-mediated
excitatory neurotransmission and gamma-
aminobutyric acid (GABA) mediated inhibitory
neurotransmission
o
Generalised epilepsy is characterised by disruption
of large scale neuro-networks in the higher centres.
Strategies in Treatment
• Stabilize membrane and prevent
depolarization by action on ion channels
• Increase GABAergic transmission
• Decrease EAA (Excitatory Amino Acid)
Transmission
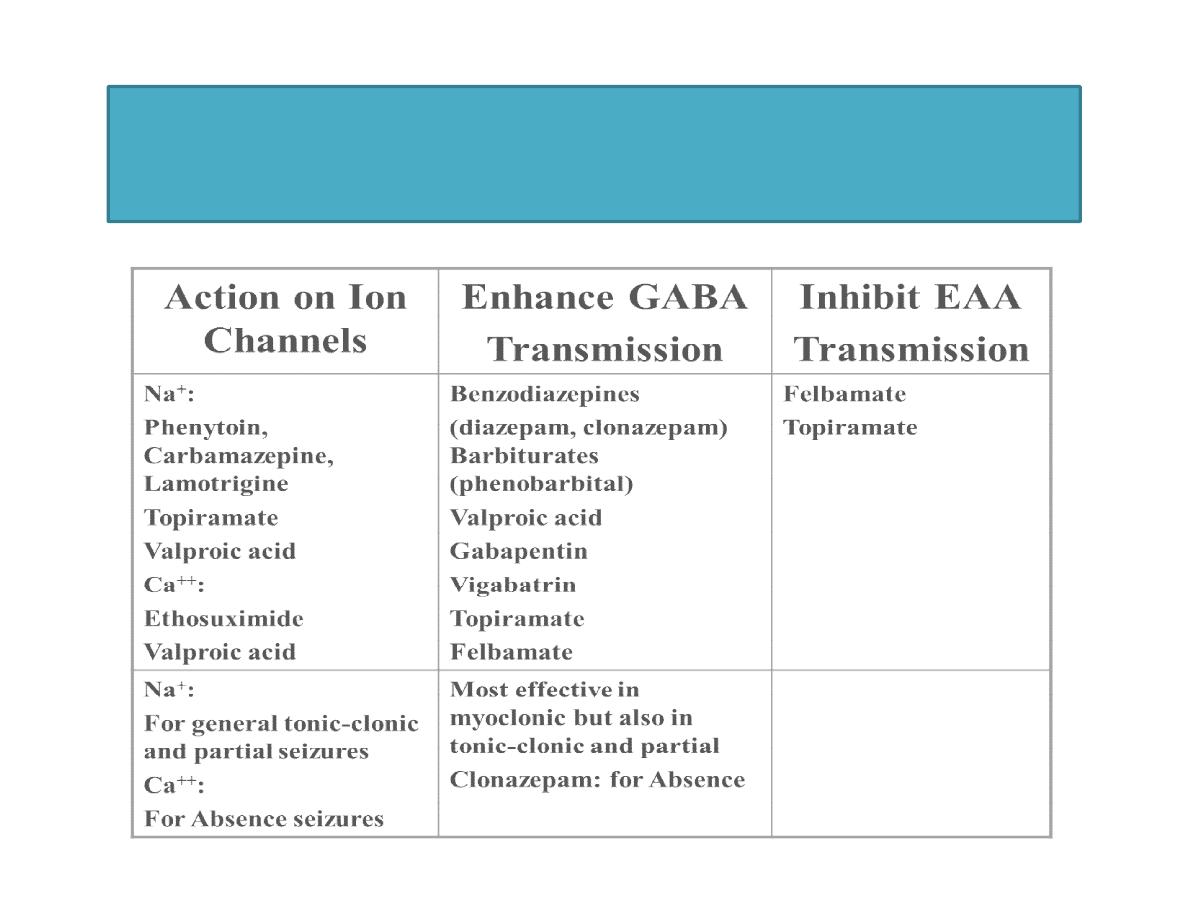
Classification of Anticonvulsants
Classification of Anticonvulsants
Classical
• Phenytoin
• Phenobarbital
• Primidone
• Carbamazepine
• Ethosuximide
• Valproic Acid
• Trimethadione
Newer
• Lamotrigine
• Felbamate
• Topiramate
• Gabapentin
• Tiagabine
• Vigabatrin
• Oxycarbazepine
• Levetiracetam
• Fosphenytoin
• Others
Phenytoin
• Limited water solubility – not given i.m.
• Slow, incomplete and variable absorption.
• Extensive binding to plasma protein.
• Metabolized by hepatic ER by hydroxylation.
Chance for drug interactions.
• Therapeutic plasma concentration: 10-20 µg/ml
• Shift from first to zero order elimination within
therapeutic concentration range.
Phenytoin – Toxicity and
Adverse Events
Acute Toxicity
• High i.v. rate: cardiac arrhythmias ±
hypotension; CNS depression.
• Acute oral overdose: cerebellar and
vestibular symptoms and signs:
nystagmus, ataxia, diplopia vertigo.
Chronic Toxicity
• Dose related vestibular/cerebellar effects
• Behavioral changes
• Gingival Hyperplasia
• GI Disturbances
• Sexual-Endocrine Effects:
– Osteomalacia
– Hirsutism
– Hyperglycemia
Phenytoin – Toxicity
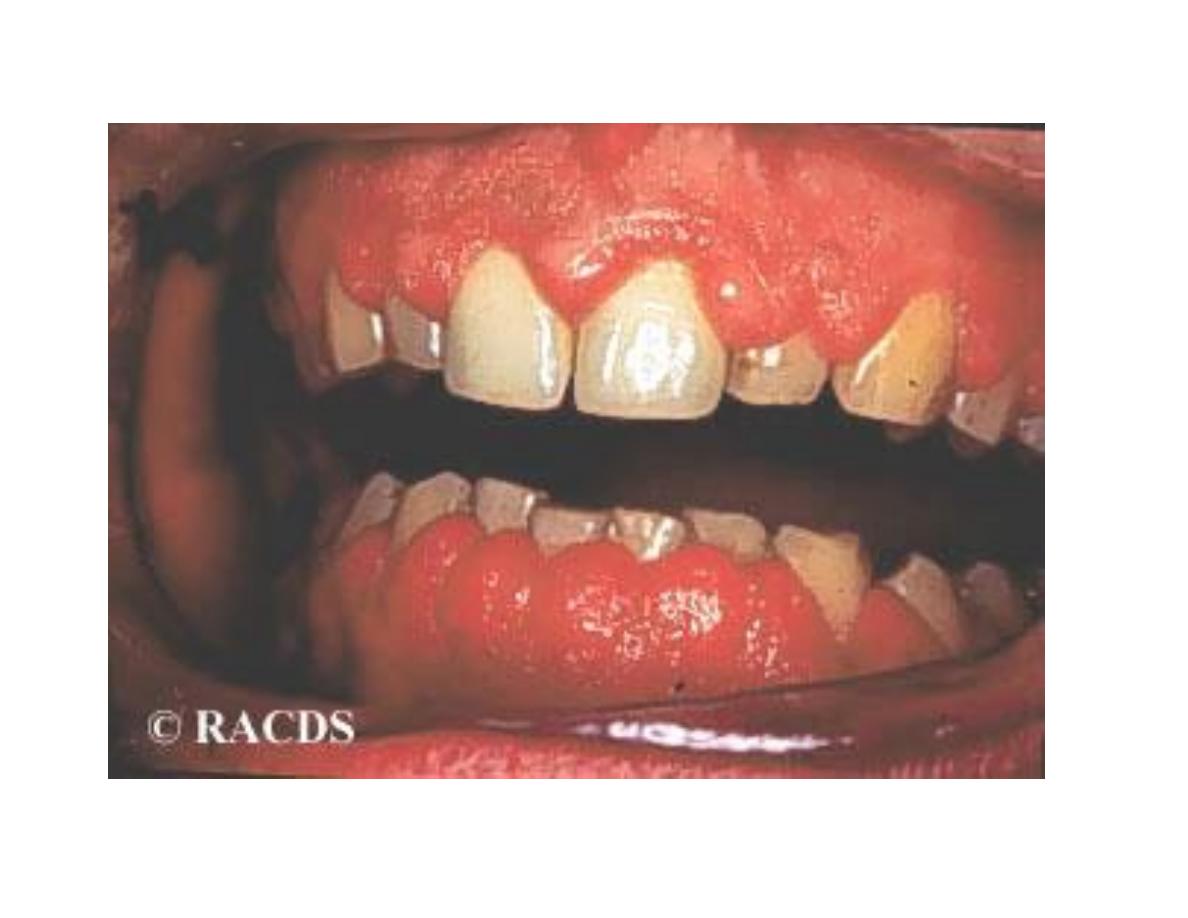
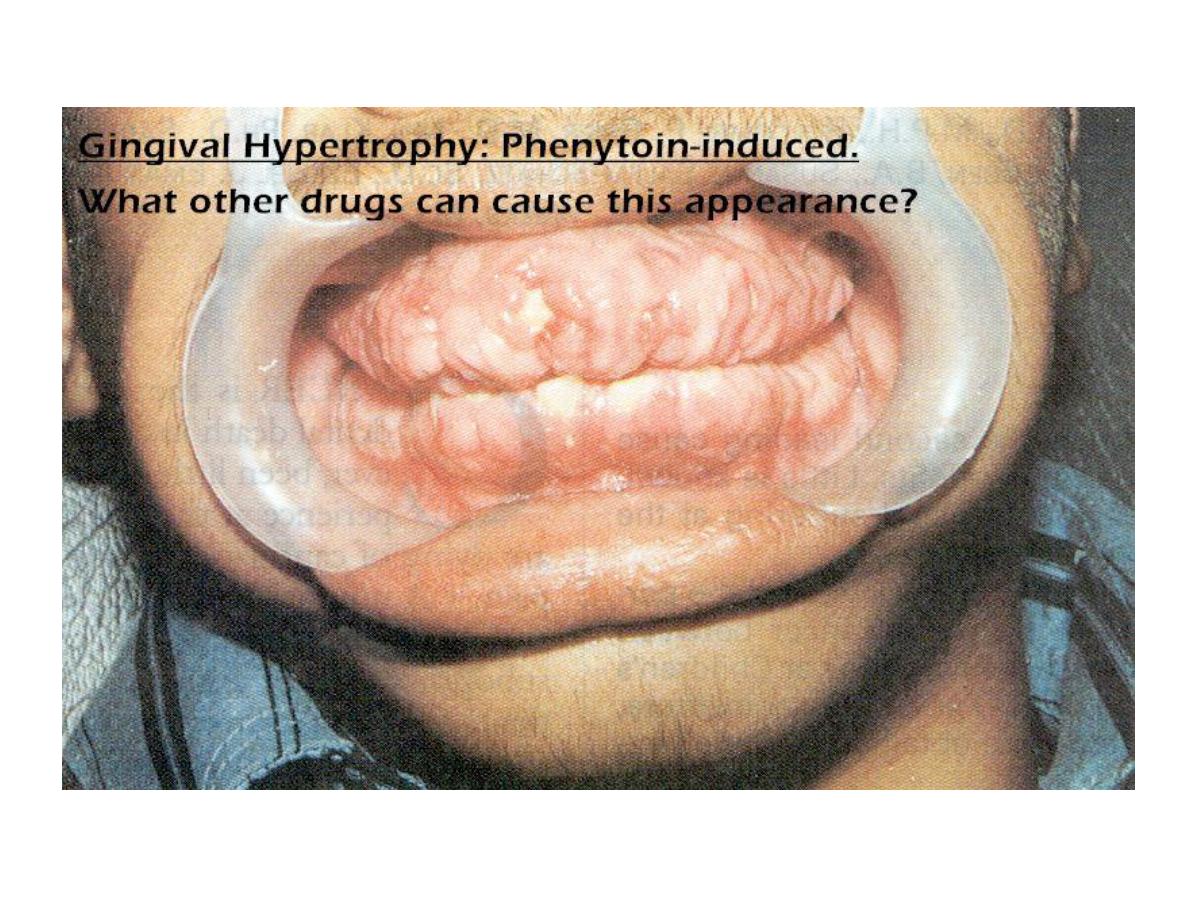
Chronic Toxicity
• Folate Deficiency - megaloblastic anemia
• Hypoprothrombinemia and hemorrhage in newborns
• Hypersenstivity Reactions – could be severe. SLE,
fatal hepatic necrosis, Stevens-Johnson syndrome.
• Pseudolymphoma syndrome
• Teratogenic
• Drug Interactions: decrease (cimetidine, isoniazid) or
increase (phenobarbital, other AED’s) rate of
metabolism; competition for protein binding sites.
Phenytoin – Toxicity and
Adverse Events

Carbamazepine (Tegretol)
• Second - most commonly prescribed
anticonvulsant
• Structurally related to tricyclic
antidepressants
• Uses:
– partial and tonic-clonic seizures
– neuropathic pain management
– Schizophrenia, bipolar disorder
• May be used in combination with Dilantin
or Phenobarbital
Ø all types except absence seizures; particularly useful for
generalized tonic-clonic, simple and complex partial
Ø inhibition of voltage-gated Na
+
channels
Ø oral, slow and erratic, 75% plasma protein bound; t
1/2
=36 hrs
initially, decreasing to 20 hrs following continuous therapy
(autoinduction), active metabolite excreted
Ø diplopia and ataxia, GI upset, hypersensitivity, serious toxicity
including aplastic anemia, agranulocytosis
Ø drug interactions are many, related to the hepatic enzyme
inducing properties of carbamazepine
Carbamazepine (Tegretol)
Carbamazepine (Tegretol)
• Toxicity similar to phenytoin
• Adverse effects
– CNS:
• Restlessness, irritability, agitation
• Dizziness, confusion, ataxia, encephalopathy
– Renal
• Renal failure, urinary frequency
• Water retention (stimulates ADH)
– Visual changes
Carbamazepine (Tegretol)
• Agranulocytosis
• Lupus
• Arrhythmia & cardiac conduction
abnormalities
• Toxicity: bone marrow depression, hepatic
dysfunction, visual changes
Carbamazepine (Tegretol)
• Numerous drug interactions
• Erratically absorbed, better absorption on
full stomach
Phenobarbital
• The only barbiturate with selective anticonvulsant effect.
• Bind at allosteric site on GABA receptor and ↑ duration
of opening of Cl channel.
• ↓
Ca-dependent release of neurotransmitters at high doses.
• Inducer of microsomal enzymes – drug interactions.
• Toxic effects: sedation (early; tolerance develops); nystagmus &
ataxia at higher dose; osteomalacia, folate deficiency and vit. K
deficiency.
• In children: paradoxical irritability, hyperactivity and behavioral
changes.
• Deoxybarbiturates: primidone: active but also converted to
phenobarbital. Some serious additional ADR’s: leukopenia,
SLE-like.
Valproic Acid
• Effective in multiple seizure types.
• Blocks Na and Ca channels. Inhibits GABA
transaminase. Increases GABA synthesis.
• Toxicity: most serious: fulminant hepatitis. More
common if antiepileptic polytherapy in children <
2 years old. (?) Toxic metabolites involved.
• Drug interactions: inhibits phenobarbital and
phenytoin metabolism.
Ethosuximide
• Second - most commonly prescribed Drug of
choice for Absence. Blocks Ca++ currents (T-
currents) in the thalamus.
• Not effective in other seizure types
• GI complaints most common
• CNS effects: drowsiness lethargy).
• Has dopamine antagonist activity (? In seizure
control) but causes Parkinsonian like symptoms.
• Potentially fatal bone marrow toxicity and skin
reactions (both rare)
Benzodiazepines
• Diazepam (Valium) IV, IM
• Lorazepam (Ativan) IV
– Used to terminate status epilepticus
– Close medical supervision & resuscitative
equipment should be available
qpotentiates GABAA receptor function via a distinct
allosteric binding site on the protein termed the
benzodiazepine receptor. ↑ frequency of opening of Cl
channel.
Benzodiazepines
Ø diazepam (i.v.)- drug of choice for the treatment of status
epilepticus
Ø clonazepam and clorazepate- long-term treatment of absence,
myoclonic,
akinetic
and
atonic
seizures;
tolerance
to
anticonvulsive
action
limits
clinical
usefulness
of
benzodiazepines
Ø potentiates GABA
A
receptor function via a distinct allosteric
binding site on the protein termed the benzodiazepine receptor
Ø oral, t
1/2
: clonazepam=1 day; clorazepate=2 hrs; i.v. diazepam=1-
2 days); very high plasma protein binding, N-desmethyldiazepam
(t
1/2
=3 days) is active metabolite of diazepam and clorazepate,
clonazepam is primarily reduced to inactive metabolites
Ø sedation, ataxia; hyperactivity and irritability in children; high
therapeutic index, low incidence of toxicity
Ø additive or synergistic effects with other sedative hypnotics
Enhancers of GABA Transmission
• Gabapentin: Developed as GABA analogue.
Mechanism: Increases release of GABA by
unknown mechanism.
• Vigabatrin: Irreversible inhibitor of GABA
transaminase. Potential to cause psychiatric
disorders (depression and psychosis).
• Tiagabine: decreases GABA uptake by
neuronal and extraneuronal tissues.

٥٢
THANK YOU
THANK YOU
