Lab-3 practical pharmacology 2010-2011
Clinical parameters in drug pharmacokinetics:-pharmacokinetics:
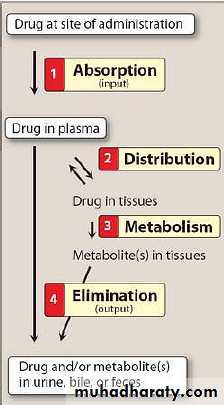
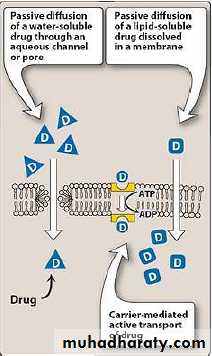
The processes to which the drug undergone during its movement through the body. ( i.e. how the body handles the drug)Absorption:
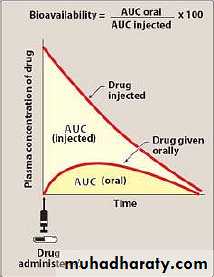
1-Bioavailability
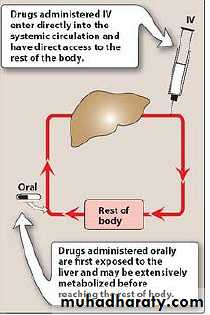
Bioavailability is the fraction of administered drug that reaches the systemic circulation in a chemically unchanged formClinical importance
Important to decide which rout is suitable to administer a given drugArea under the curve(for IV) (Auc(IV)):-
AUC (iv)= C0 / KeC0= concentration at zero time
Ke=elimination rate constant
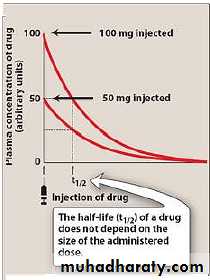
2-The half-life
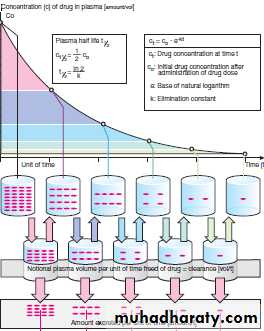
is the time required for the drug concentration to change by fifty percent. the t1/2 of a drug depends upon 2 important factors:-a-Apparent volume of distribution(AVd).
b-Clearance of drug(CL).
percent
Clinical importance
important to be Known for drugs in which drug conc. Is closely related to the pharmacological effects e.g: phenytoin.So used to predict the maximal effects both on initiation and on termination of therapy.
*It is of less value in drugs of which the effects are poorly related to plasma conc. e.g: diazepam
t1/2= (o.693*avd)/ cl
-AVd is apparent volume of distributionof a drug
-CL the drug clearance from the body.( see interactive pharm.)
3-Volume of Distribution
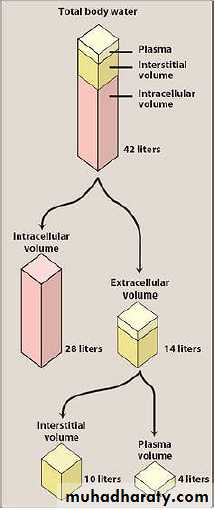
The volume of distribution is a hypothetical volume of fluid into which a drug is dispersed. Although the volume of distribution has no physiologic or physical basis, it is sometimes useful to compare the distribution of a drug with the volumes of the water compartments in the body.-The apparent volume of distribution assumes that the drug distributes uniformly, in a single compartment. However, most drugs distribute unevenly, in several compartments, and the volume of distribution does not describe a real, physical volume, but rather, reflects the ratio of drug in the extraplasmic spaces relative to the plasma space.
Avd= amount of drug (dose)/ Co
Co = Auc*Ke4-Elimination rate constant (Ke):
is the amount of drug lost per unit time.Ke= o.693/t1/2
T1/2=o693/Ke
Clinical application
(t1/2) and is related to ke by the equation t1/2 = 0.693/Ke. The two parameters, together with
the initial concentration co, describe a
first-order (exponential) rate process.
The constancy of the process permits
calculation of the plasma volume
that would be cleared of drug,
5-Clearance(cl):-
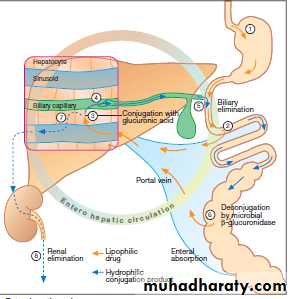
The total body (systemic) clearance, CLtotal or CLt, is the sum of the clearances from the various drug-metabolizing and drug eliminating organs.The kidney is often the major organ of excretion; however, the liver also contributes to drug loss through metabolism and/or excretion into the bile. A patient in renal failure may sometimes benefit from a drug that is excreted by
this pathway, into the intestine and feces, rather than through the kidney. Some drugs may also be reabsorbed through the enterohepatic circulation, thus prolonging their half-life.(see interactive pharma.)
CL total= CL hepatic + CL renal+ CL pulmonary+CL others.
It is not possible to measure and sum these individual clearances. How-ever, total clearance can be derived from the steadystate equation:CL total= rate of elimination/ Css
Css is conc.at steady state.
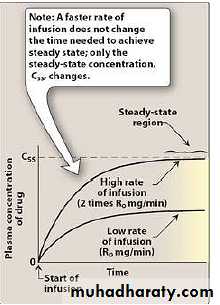
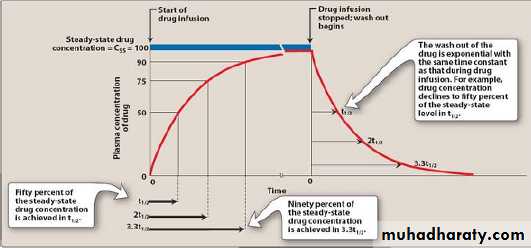
6-Steady state
Steady-state drug levels in blood: Following the initiation of an IV infusion, the plasma concentration of drug rises until the rate of drug eliminated from the body precisely balances the input rate. Thus, a steady-state is achieved in which the plasma concentration of drug remains constant.*it means that dosing rate= eliminating rate
(DR=ER).* 5 t1/2 needed to achieve Css and 5 t1/2 to eliminate drug from steady state.(see interactive pharma).
7-Amount of drug present in body at any time(at certain conc.):-
Amount of drug at any time= drug conc * AVd
Dose at any time= Css * AVd.
summery
1-T1/2= 0.693*avd/ clT1/2=0.693/Ke
2-bioavailability=AUC(oral)/AUC(IV)
3-AUC= Co/Ke
4-Ke=o.693/t1/2
5-avd=dose/Co
6-Cltotal=avd*Ke
7- amount of drug at any time=drug conc*avd
Or Css*avd