
DR. J abar Etaby Lecture 1
Ascaris lumbricoides and Ascaris suum)
(intestinal roundworms of humans and pigs
)
Introduction:
Ascaris lumbricoides is one of the largest and most common
parasites found in humans. The adult females of this speciescan
measure up to 18 inches long (males are generally shorter), and
it is estimated that 25% of the world's population is infected
with this nematode. The adult worms live in the small
Intestine and eggs are passed in the feces.
Habitat:-
The adult worm lives in the small intestine of man
Morphology :-
The adult worm is the largest round worm parasitizing the human
intestinal tract. It is elongated, cylindrical, and tapers both anteriorly
&posteriorly to relatively blunt conical ends. The head is
provided
with three fleshy lips the digestive &reproductive organs float
inside the body cavity which contain an irritating allergic fluid
.The irritant action is due to the presence of atoxin called a
scarone or a scarase which is probably of the nature of primary
albomenoses.
A single female can produce up to 200,000 eggs each day! About two
weeks after passage in the feces the eggs contain an infective larval eggs
Eggs:
The fertilized egg of Ascaris lumbricoides at the time of oviposition is
spherical or sub-spherical,measures 65-75µmby35-50 µm &consists
of the following observable structures
1-A coarsely granular
,spherical ovum that usually does not completely fill the shell.
2-A thin innermost membrane that is highly impermeable.
3-A relatively thick,colorless middle layer that is smooth on both
inner &outersurfaces .
4-An outermost ,coarsely mammilated
Female worms without males produce infertile eggs that are
markedly subspherical (88 µm by38-44 µm),internally they contain
a mass of disorganized granules that completely fill the shell
Life cycle, The humans are infected when they ingest such
infective eggs. The eggs hatch in the small intestine, the
juvenile
penetrates the small intestine and enters the circulatory
system, and eventually the juvenile worm enters the lungs.
In the lungs the juvenile worm leaves the circulatory system and
enters the air passages of the lungs. The juvenileworm then
migrates up the air passages into the pharynx where it is
swallowed, and once in the small intestine the juvenilegrows
into an adult worm. Why Ascaris undergoes such a migration
through the body to only end up where it started is unknown.
Such a migration is not unique to Ascaris, as its close relatives
undergo a similar migration in the bodies of

Pathology and symptoms
Ascaris infections in humans can cause significant pathology.
The migration of the larvae through the lungs causes the blood
vessels of the lungs to hemorrhage, and there is an inflammatory
response accompanied by edema. The resulting accumulation of
fluids in the lungs results in "ascaris pneumonia," and this can
be fatal. The large size of the adult worms also presents
problems, especially if the worms physically block the
gastrointestinal tract. Ascaris is not orious for it reputation to
migrate within the small intestine, and when large worm begins
to migrate there is not much that can stop it.
Instances have been reported in which Ascaris havemigratedinto
and blocked the bile or pancreatic duct or in which the worms
have penetrated the small intestine resulting in acute (and fatal)
peritonitis. Ascaris seems to be especiallysensitive to
anesthetics, and numerous cases have been documented where
patients in surgical recovery rooms have had worms migrate
from the small intestine, through the stomach, and out the
patient's nose or mouth. Ascaris
suum is found in pigs. Its life
cycle is identical to that of A. lumbricoides. If a human ingests
eggs of A. suum the larvae will migrate to the lungs and die.
This can cause a particularly serious form of "ascaris
pneumonia." Adult worms of this species do not develop in the
human's intestine. (Some parasitologists believe that there is but
one species of Ascaris that infects both pigs and humans, but
any commentary on this issue is beyond the scope of this web
site.)
Diagnosis
Infections of Ascaris are diagnosed by finding characteristic eggs in
the feces of the infected host
.

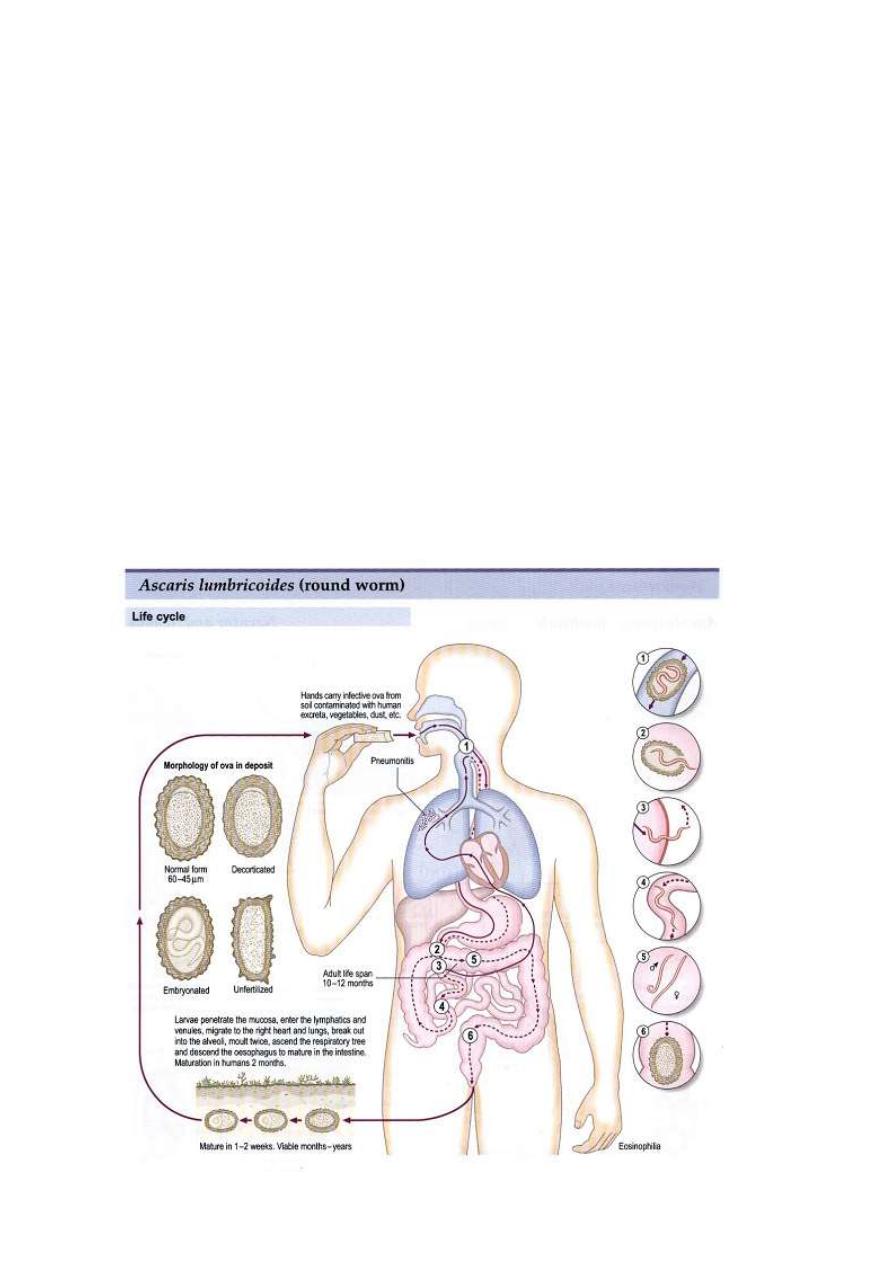
Note the presence of three large lips, a characteristic of
ascarids.
A scanning electron micrograph of the anterior end of Ascaris
showing the three prominent "lips
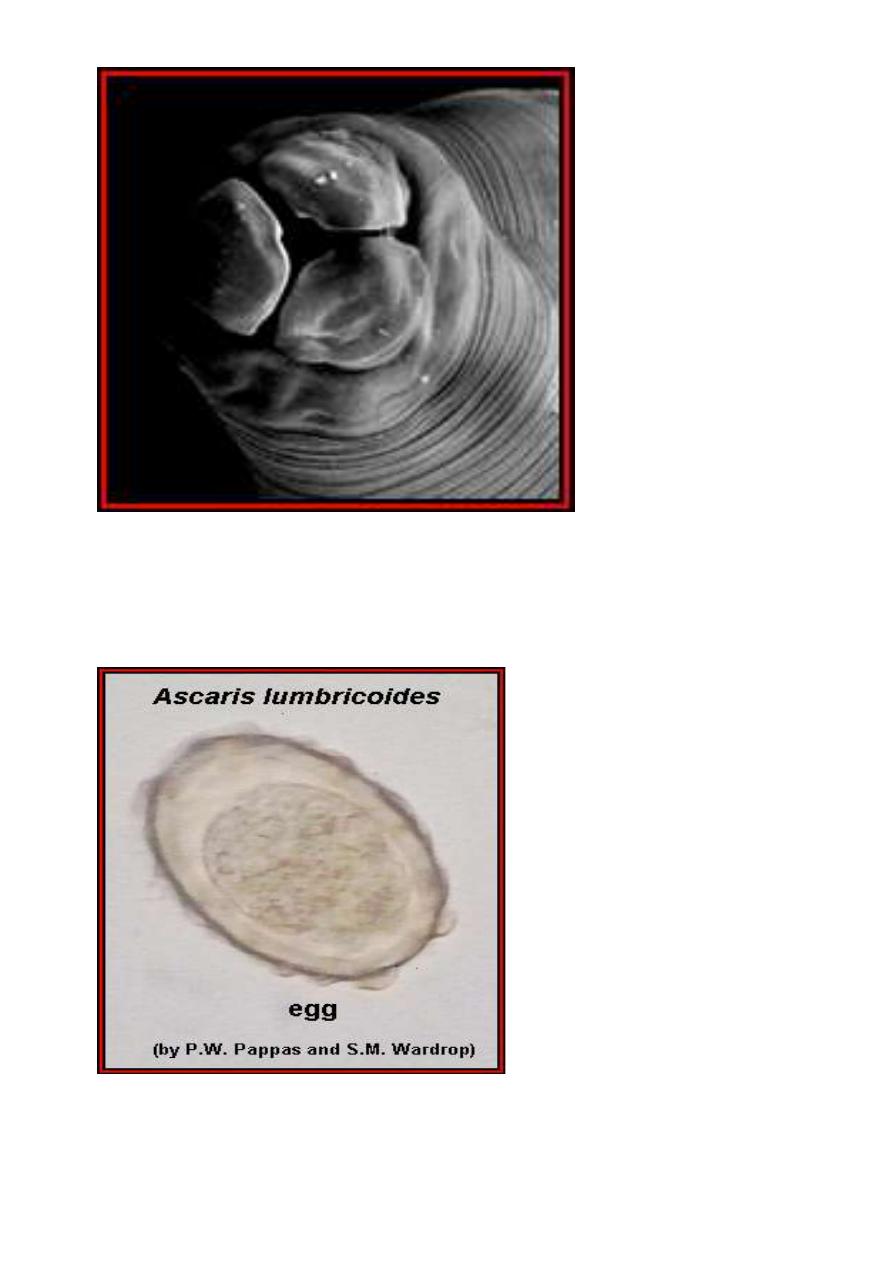
Ascaris lumbricoides, fertilized egg. Note that the egg is
covered with a thick shell that appears lumpy (bumpy) or

mammillated; approximate size = 65 μm in length. Another
example
Another example of a fertilized Ascaris lumbricoides egg.
(Original image from:
Atlas of Medical Parasitology
.)
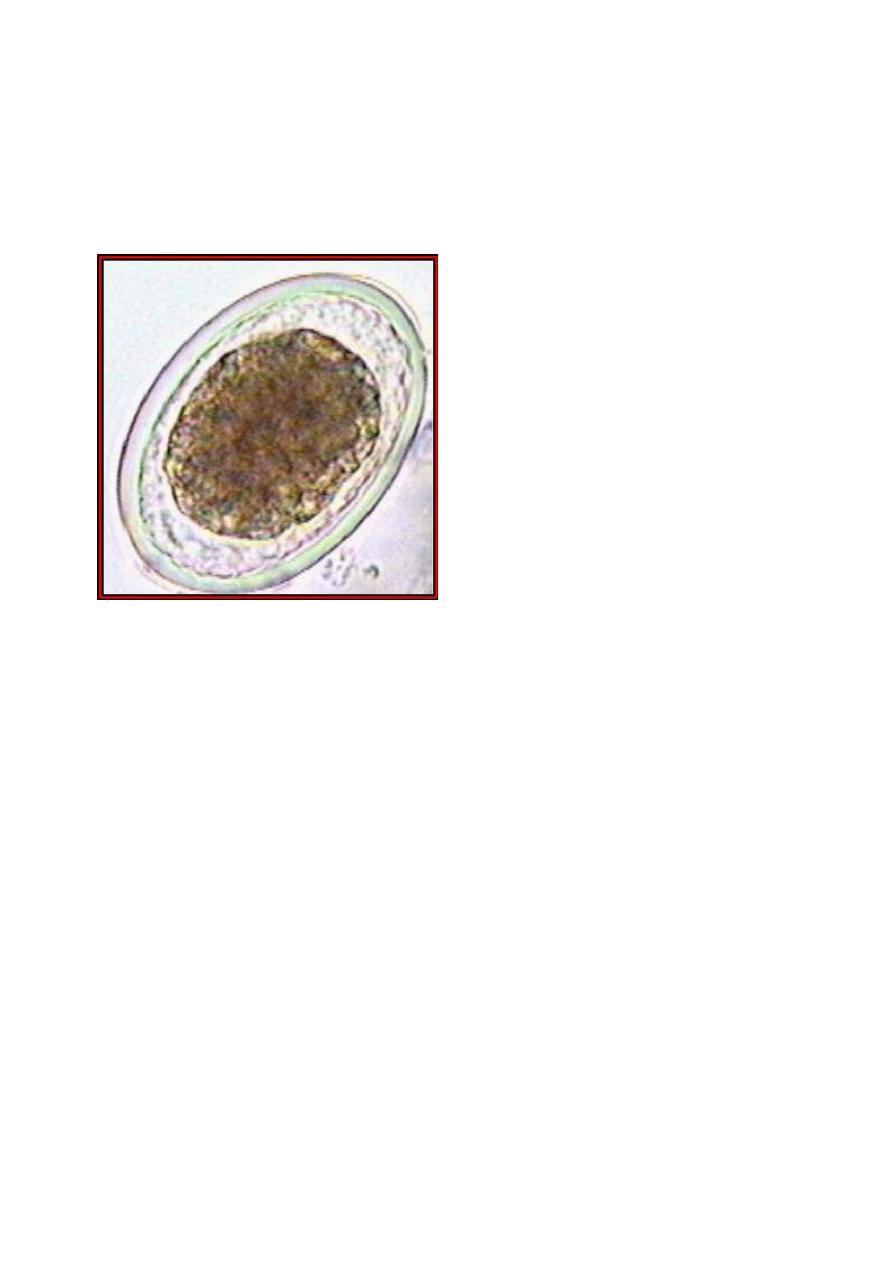
An example of an unfertilized A. lumbricoides egg. (Original
image from:
Atlas of Medical Parasitology
.)
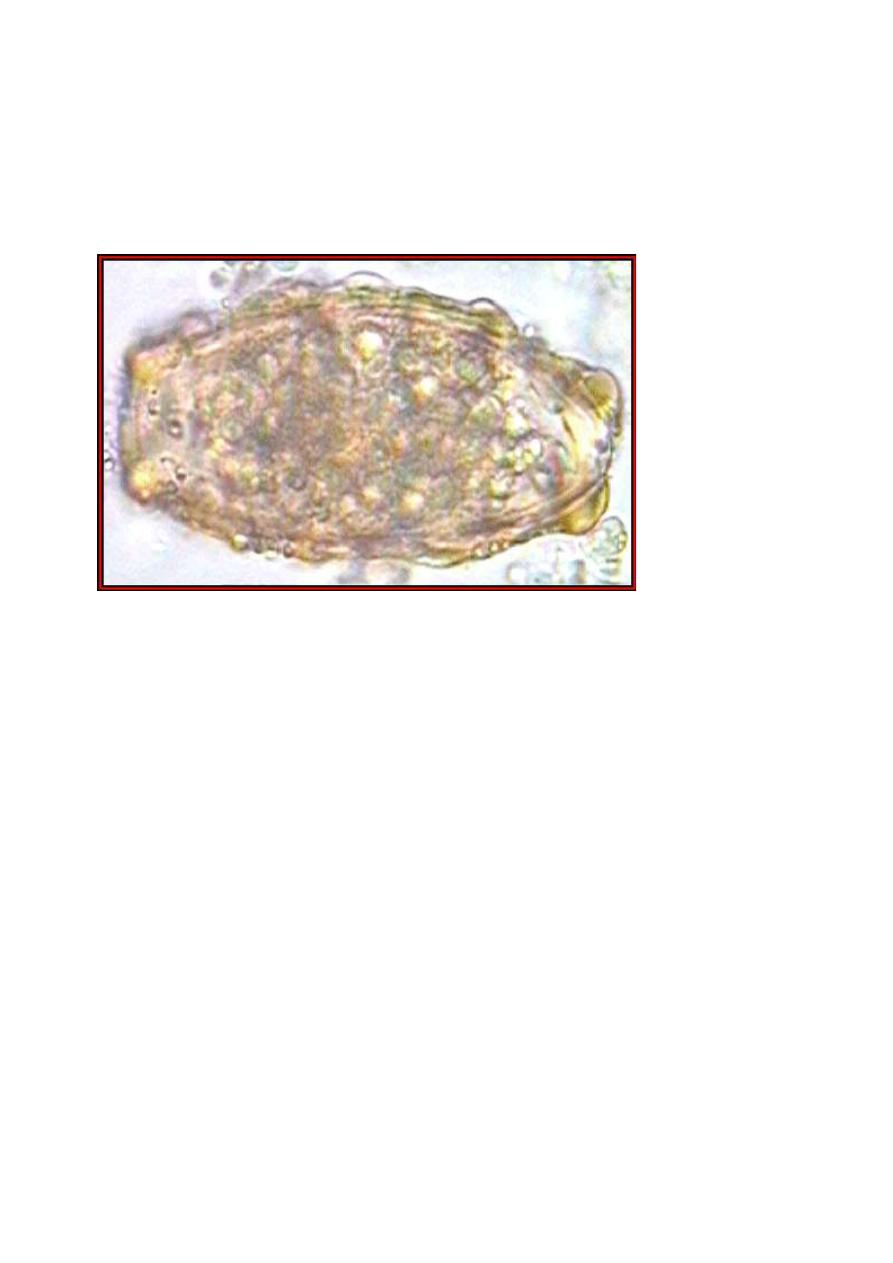
A "decorticated," fertilized, Ascaris lumbricoides. (Original
image from:
Atlas of Medical Parasitology
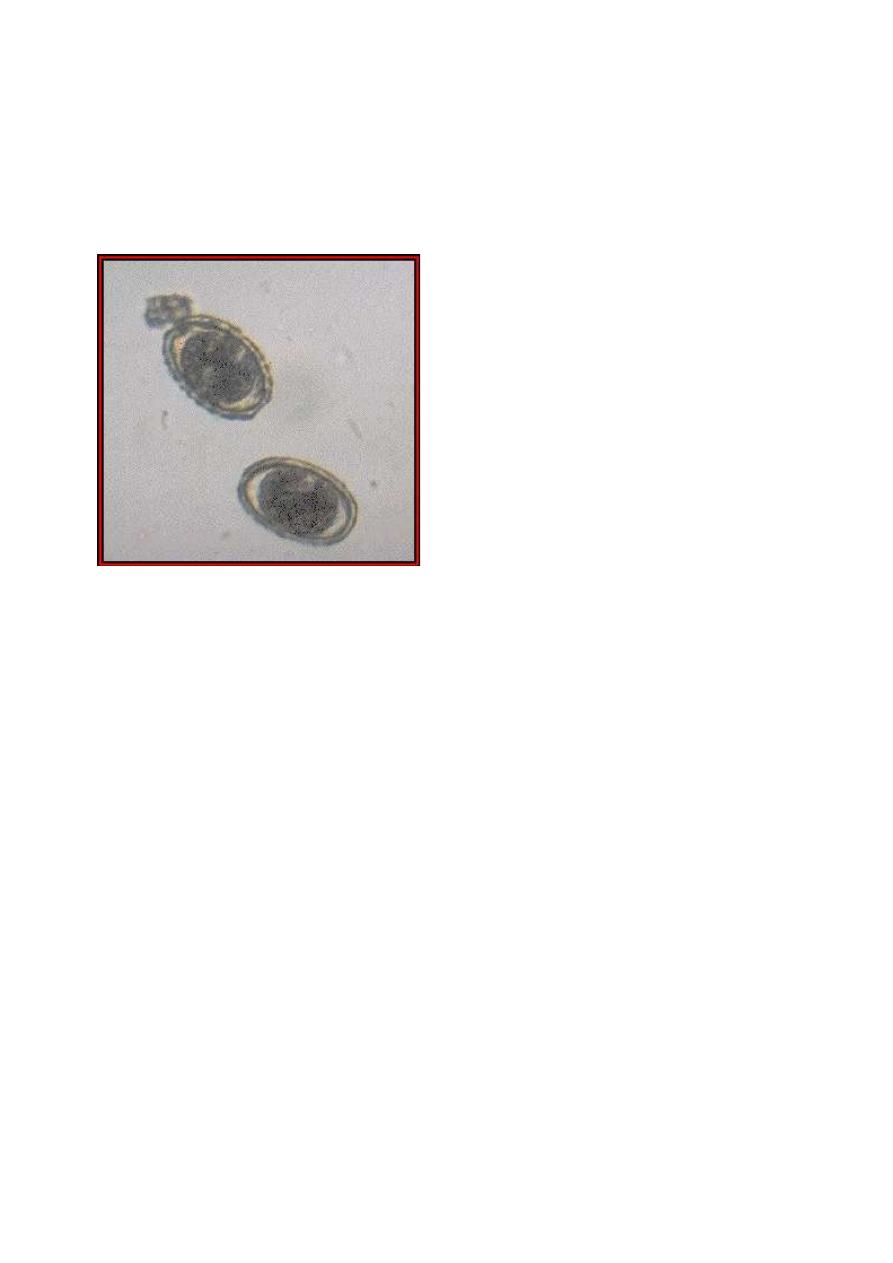
Eggs of Ascaris suum. A. suum is a common parasite of pigs.
The eggs are virtually indistinguishable from those of A.
lumbricoides. (Original image from
Oklahoma State University,
College of Veterinary Medicine
.)

female Ascaris lumbricoides. Females of this species can
measure over 16 inches long. This specimen was passed by a
young girl in Florida. (Original image from
DPDx
[Identification and Diagnosis of Parasites of Public Health
Concern]
.)
Female
Female and male Ascaris lumbricoides; the female measures
approximately 16 inches (40 cm) in length.

A large mass of Ascaris lumbricoides that was passed from the
intestinal tract. The ruler at the bottom of the image is 4
cm (about 1.5 inches) in length
.
Conclusion
the infective stage is larvated eggs
to cause pneumonia in the lung during circulation& the adult will be bloked
the small intestinal tract
-
up stream movement this movement for A. lumbericoides through mouth
or nose
some times the infected man may die due to this irritation action after
changing to anaphylatic or HSR

Lecture 2 DR. Jabar Etaby
Introduction
Patients with hookworm infection often are
asymptomatic; however, chronic hookworm infection is
a common cause of moderate and severe hypochromic,
microcytic anemia in people living in tropical
developing countries, and heavy infection can cause
hypoproteinemia with edema.
EPIDEMIOLOGY
Humans are the only reservoir. Hookworms are
prominent in rural, tropical, and subtropical areas where
soil contamination with human feces is common.
Although the prevalence of both hookworm species is
equal in many areas, A duodenale is the predominant
species in the Mediterranean region, northern Asia, and

selected foci of South America. N americanus is
predominant in the Western hemisphere, sub-Saharan
Africa, Southeast Asia, and a number of Pacific islands.
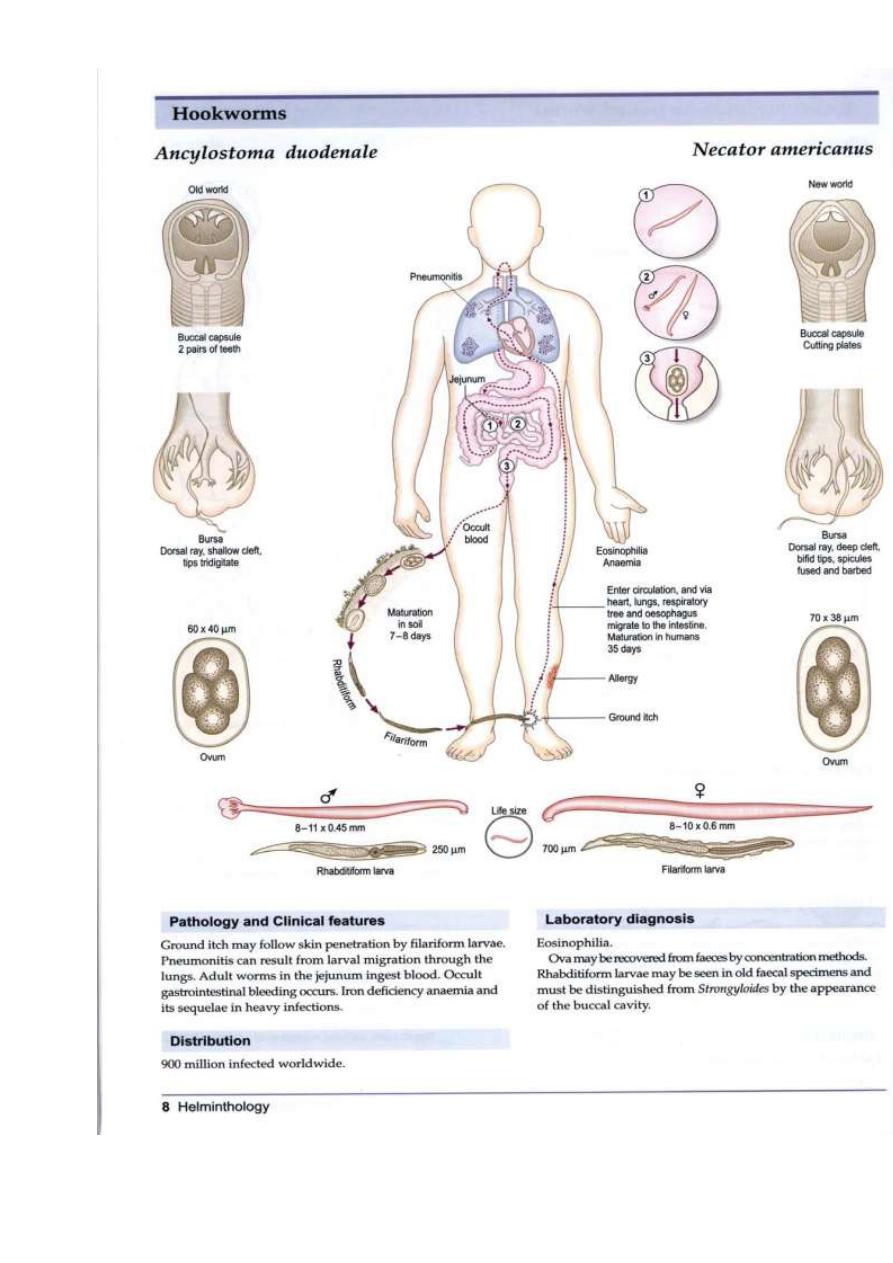
LIFE CYCLE
Larvae and eggs survive in loose, sandy, moist, shady,
well-aerated, warm soil (optimal temperature 23°C–
33°C [73°F–91°F]). Hookworm eggs from stool hatch in
soil in 1 to 2 days as rhabditiform larvae. These larvae
develop into infective filariform larvae in soil within 5 to
7 days and can persist for weeks to months.
Percutaneous infection occurs after exposure to
infectious larvae. A duodenale transmission can occur by
oral ingestion and possibly through human milk.
Untreated infected patients can harbor worms for 5
years or longer. The time from exposure to development
of noncutaneous symptoms is 4 to 12 weeks.
Clinical signs
Patients with hookworm infection often are
asymptomatic; however, chronic hookworm infection is a
common cause of moderate and severe hypochromic,
microcytic anemia in people living in tropical developing
countries, and heavy infection can cause
hypoproteinemia with edema. Chronic hookworm
infection in children may lead to physical growth delay,
deficits in cognition, and developmental delay. After
contact with contaminated soil, initial skin penetration of
larvae, often involving the feet, can cause a stinging or
burning sensation followed by pruritus and a
papulovesicular rash that may persist for 1 to 2 weeks.
Pneumonitis associated with migrating larvae is

uncommon and usually mild, except in heavy infections.
Colicky abdominal pain, nausea, and/or diarrhea and
marked eosinophilia can develop 4 to 6 weeks after
exposure. Blood loss secondary to hookworm infection
develops 10 to 12 weeks after initial infection and
symptoms related to serious iron-deficiency anemia can
develop in long-standing moderate or heavy hookworm
infections. After oral ingestion of infectious Ancylostoma
duodenale larvae, disease can manifest with pharyngeal
itching, hoarseness, nausea, and vomiting shortly after
ingestion.
ETIOLOGY
Necator americanus is the major cause of hookworm
infection worldwide, although A duodenale also is an
important hookworm in some regions. Mixed infections
are common. Both are roundworms (nematodes) with
similar life cycles
.
Ancylostoma spp. and Necator spp. (hookworms)
There are many species of hookworms that infect mammals.
The most important, at least from the human standpoint, are
the human hookworms, Ancylostoma duodenale and Necator
americanus, which infect an estimated 800,000,000 persons,
and the dog and cat hookworms, A. caninum and A. braziliense,
respectively. Hookworms average about 10 mm in length
and live in the small intestine of the host. The males and
females mate, and the female produces eggs that are passed in
the feces. Depending on the species, female hookworms can
produce 10,000-25,000 eggs per day. About two days after
passage the hookworm egg hatches, and the juvenile worm (or
larva) develops into an infective stage in about five days.
The next host is infected when an infective larva penetrates the
host's skin. The juvenile worm migrates through the host's
body and finally ends up in the host's small intestine where it

grows to sexual maturity. The presence of hookworms can be
demonstrated by finding the characteristic eggs in the feces; the
eggs can not, however, be differentiated to species
Juveniles (larvae) of the dog and cat hookworms can infect
humans, but the juvenile worms will not mature into adult
worms. Rather, the juveniles remain in the skin where they
continue to migrate for weeks (or even months in some
instances).
This results in a condition known as
"cutaneous" or
"dermal larval migrans"
or "creeping eruption." Hence the
importance of not allowing dogs and cats to defecate
indiscriminately. The following image provides an excellent
example of how hookworms are attached to and embedded in
the epithelium of the host's gastrointestinal tract.
DIAGNOSTIC TESTS
Microscopic demonstration of hookworm eggs in feces is
diagnostic. Adult worms or larvae rarely are seen.
Approximately 5 to 8 weeks are required after infection
for eggs to appear in feces. Adirect stool smear with
saline solution or potassium iodide saturated with iodine
is adequate for diagnosis of heavy hookworm infection;
light infections require concentration techniques.
Quantification techniques (eg, Kato-Katz, Beaver direct
smear, or Stoll egg-counting techniques) to determine
the clinical significance of infection and the response to
treatment may be available from state or reference
laboratories
.
CONTROL MEASURES
Sanitary disposal of feces to prevent contamination of
soil is necessary in areas with endemic infection.
Treatment of all known infected people and screening of
high-risk groups (ie, children and agricultural workers) in
areas with endemic infection can help decrease
environmental contamination. Wearing shoes may not be
fully protective, because cutaneous exposure to
hookworm larvae over the entire body surface of children
could result in infection. Despite relatively rapid
reinfection, periodic deworming treatments targeting
preschool-aged and school-aged children have been
advocated to prevent morbidity associated with heavy
intestinal helminth infections
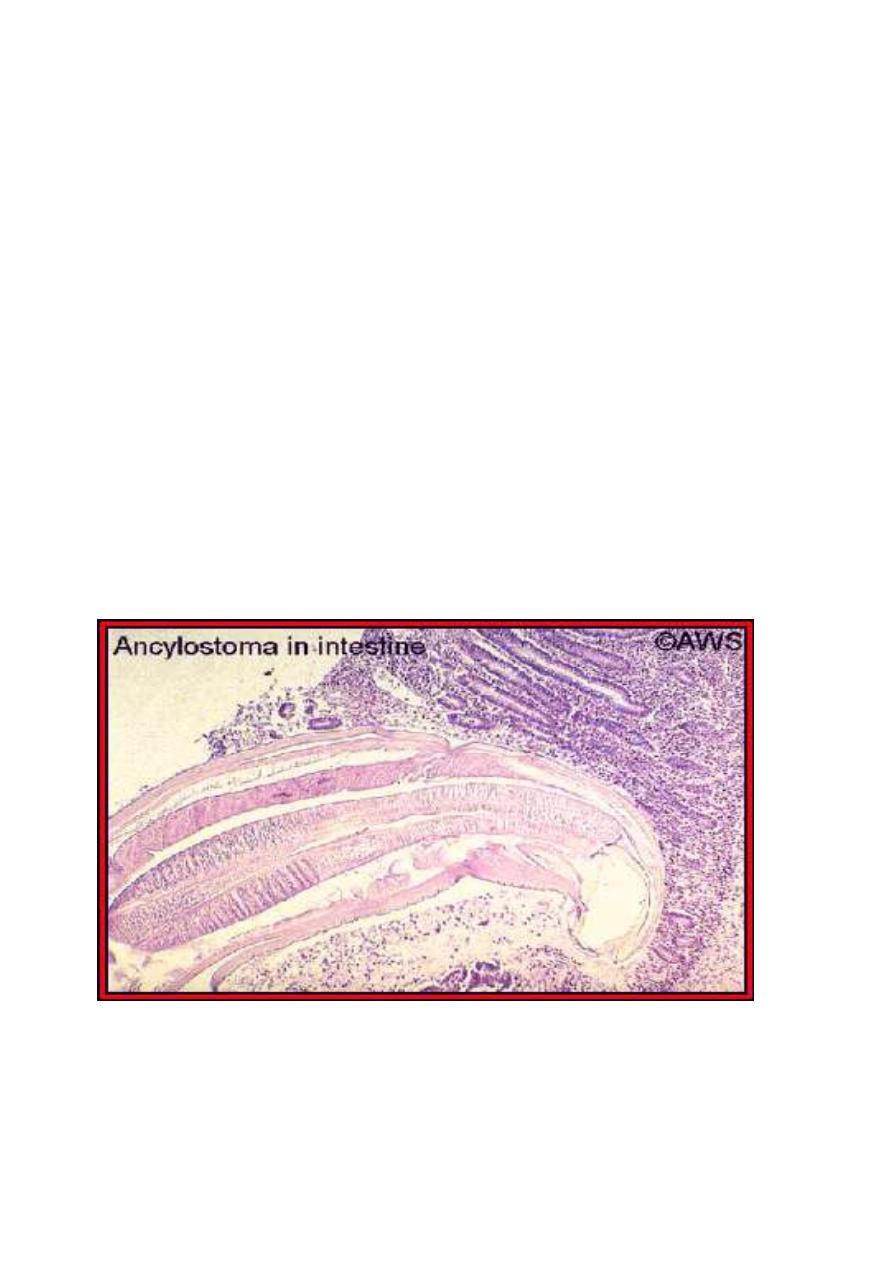
A histological section of a hookworm in the host's small
intestine. Original image copyrighted and provided by Dr. A.W.
Shostak, and used with permission

Lecture 3 DR. Jabar Etaby
ABSTRACT
Strongyloides stercoralis is an intestinal nematode of humans
that infects tens of millions of people worldwide. S. stercoralis is
unique among intestinal nematodes in its ability to complete its
life cycle within the host through an asexual autoinfective cycle,
allowing the infection to persist in the host indefinitely. Under
some conditions associated with immunocompromise, this
autoinfective cycle can become amplified into a potentially fatal
hyperinfection syndrome, characterized by increased numbers of
infective filariform larvae in stool and sputum and clinical
manifestations of the increased parasite burden and migration,
such as gastrointestinal bleeding and respiratory distress. S.
stercoralis hyperinfection is often accompanied by sepsis or
meningitis with enteric organisms. Glucocorticoid treatment and
human T-lymphotropic virus type 1 infection are the two
conditions most specifically associated with triggering
hyperinfection, but cases have been reported in association with
hematologic malignancy, malnutrition, and AIDS. Anthelmintic
agents such as ivermectin have been used successfully in treating
the hyperinfection syndrome as well as for primary and secondary
prevention of hyperinfection in patients whose exposure history
and underlying condition put them at increased risk.

INTRODUCTION
Strongyloides stercoralis is an intestinal nematode of humans.
It is estimated that tens of millions of persons are infected
worldwide, although no precise estimate is available. Although
most infected individuals are asymptomatic , S. stercoralis is
capable of transforming into a fulminant fatal illness under
certain conditions associated with a compromise of host
immunity. Such conditions have commonly been summarized as
“defects in cell-mediated immunity,” although the specific
circumstances under which S. stercoralis hyperinfection develops
are not always predictable.
Given the increasing numbers of immunocompromised
individuals throughout the world, a closer examination of the
conditions under which S. stercoralis infection becomes
dangerous is warranted. Better approaches to identifying,
screening, and treating those at risk will likely decrease the
morbidity and mortality associated with S. stercoralis infection.
Etiology :
Strongyloides stercoralis
is the Nematodes of small
intestinal tract
Life cycle of Strongyloides stercoralis is an unusual "parasite"
in that it has both free-living and parasitic life cycles. In the
parasitic life -cycle, female worms are found in the superficial
tissues of the human small intestine; there are apparently no
parasitic males. The female worms produce larvae
parthenogenically (without fertilization), and the larvae are
passed in the host's feces. The presence of nematode larvae in a
fecal sample is characteristic of strongylodiasis. Once passed in
the feces, some of the larvae develop into "free-living" larvae,
while others develop into "parasitic" larvae. The "free-living"
larvae will complete their development in the soil and mature
into free-living males and females. The free-living males and
females mate, produce more larvae, and (as above) some of
these larvae will develop into "free-living" larvae, while other
will develop into "parasitic larvae." As one might imagine, this
free-living life cycle constitutes an important reservoir for
human infections. The "parasitic" larvae infect the human host
by penetrating the skin (like the
hookworms
). The larvae
migrate to the lungs, via the circulatory system, penetrate the
alveoli into the small bronchioles, and they are "coughed up"
and swallowed. Once they return to the small intestine, the
larvae mature into parasitic females. S. stercoralis also infects
humans via a mechanism called "autoinfection." Under some
circumstances, such as chronic constipation, larvae produced by
the parasitic females will remain in the intestinal tract long
enough to develop into infective stages. Such larvae will
penetrate the tissues of the intestinal tract and develop as if they
had penetrated the skin. Autoinfection can also occur when
larvae remain on and penetrate the perianal skin. Autoinfection
often leads to very high worm burdens in humans (
view
diagram of the life cycle
).
EPIDEMIOLOGY
Although S. stercoralis is often considered a disease of tropical
and subtropical areas, endemic foci are also seen in temperate
regions . Low socioeconomic status , alcoholism , white race , and
male gender have been associated with higher prevalences of S.
stercoralis stool positivity. Clusters of cases in institutionalized
individuals with mental retardation others suggest that
nosocomial transmission can occur. Occupations that increase
contact with soil contaminated with human waste, which may
include farming and coal mining depending on local practices,
increase the risk of infection. Swimming in or drinking
contaminated water has not been proven to be a significant
source of transmission, perhaps because larvae do not thrive
when immersed in water . Different prevalences among ethnic
groups may simply reflect behavioral or socioeconomic factors,
but some have suggested that different skin types may be more or
less resistant to larval penetration .
CLINICAL SYNDROMES

As the clinical syndromes of S. stercoralis encompass a spectrum
and terms are used variably, it is necessary to set forth some
definitions before proceeding further.
Acute Strongyloidiasis
From experimental human infections, it is known that a local
reaction at the site of larval entry can occur almost immediately
and may last up to several weeks
.
Pulmonary symptoms such as a
cough and tracheal irritation, mimicking bronchitis, occur as
larvae migrate through the lungs several days later.
Gastrointestinal symptoms (diarrhea, constipation, anorexia,
abdominal pain) begin about 2 weeks after infection, with larvae
detectable in the stool after 3 to 4 weeks. Experimental human
infections on which this description is based were initiated with
many hundreds of larvae and most likely overestimate the
severity and perhaps the tempo of naturally acquired infections.
Chronic Strongyloidiasis
Chronic infection with S. stercoralis is most often asymptomatic .
There are a number of signs and symptoms attributable to
chronic strongyloidiasis that are unrelated to accelerated
autoinfection. Chronic gastrointestinal manifestations, such as
intermittent vomiting, diarrhea, constipation, and borborygmus,
are common complaints. Pruritus ani and dermatologic
manifestations such as urticaria and larva currens rashes are also
common . Recurrent asthma and nephrotic syndrome have also
been associated with chronic strongyloidiasis infection.
Complications such as intestinal obstruction , ileus
hemodynamically significant gastrointestinal bleeding, and acute
worsening of chronic intestinal manifestations have occurred in
the context of an increased larval burden. Even in the absence of
pulmonary symptoms, such presentations could be considered a
manifestation of hyperinfection
Hyperinfection
Hyperinfection describes the syndrome of accelerated
autoinfection, generally — although not always the result of an
alteration in immune status. Parasitologically, the distinction
between autoinfection and hyperinfection is quantitative and not

strictly defined. Therefore, the diagnosis of hyperinfection
syndrome implies the presence of signs and symptoms
attributable to increased larval migration. Development or
exacerbation of gastrointestinal and pulmonary symptoms is
seen, and the detection of increased numbers of larvae in stool
and/or sputum is the hallmark of hyperinfection. Larvae in
nondisseminated hyperinfection are increased in numbers but
confined to the organs normally involved in the pulmonary
autoinfective cycle (i.e., gastrointestinal tract, peritoneum, lungs),
although enteric bacteria, which can be carried by the filariform
larvae or gain systemic access through intestinal ulcers, may
affect any organ system
.
Disseminated Infection
The term disseminated infection is often used to refer to
migration of larvae to organs beyond the range of the pulmonary
autoinfective cycle. This does not necessarily imply a greater
severity of disease. Extrapulmonary migration of larvae has been
shown to occur routinely during the course of experimental
chronic S. stercoralis infections in dogs and has been reported to
cause symptoms in humans without other manifestations of
hyperinfection syndrome Similarly, many cases of hyperinfection
are fatal without larvae being detected outside the pulmonary
autoinfective route.
As documenting disseminated infection may be more a matter of
vigilance than a fundamental difference in disease mechanisms,
the term hyperinfection will be used here to include cases with
evidence of larval migration beyond the pulmonary autoinfective
route.

CONCLUSIONS
Our understanding of S. stercoralis infections in normal and
immunocompromised hosts continues to evolve. Relatively
recently, it was thought that any defect in cellular immunity could
tip the equilibrium of chronic strongyloidiasis toward
hyperinfection. Although various immunocompromising
conditions have been associated with hyperinfection,

Lecture 4 DR. Jabar Eaby
Cutaneous (dermal) larval migrans
There are several examples of parasites that are normally found in pets but can be
transmitted to humans. For example, acommon tapeworm of dogs,
Dipylidium
caninum
, can be transmitted to humans. Immature forms of the common
roundworm of dogs,
Toxocara canis
can also be found in humans, causing a
disease known as
visceral larval migrans
.
Immature forms of both cat and dog hookworms can also infect humans, and this
results in a disease called cutaneous or
dermal larval migrans (CLM or DLM).
The eggs of dog and cat hookworms hatch after being passed in the host's feces,
and the next host is infected when these
larvae penetrate the host's skin. Unfortunately, these larvae can not tell the skin of
one animal from another, so they will
penetrate human skin if they come in contact with it. However, a human is an
unnatural host, so the larvae do not enter the blood stream as they would in a dog
or cat. Rather, they remain in the skin for extended periods of time (weeks or
months in some instances) and finally die. As the larvae migrate through the skin
and finally die, there is an inflammatory response, and the progress of the larvae
through the skin can actually be followed since they leave a tortuous "track" of
inflammed tissue just under the surface of the skin. Treatment of such infections
requires surgical removal of the migrating larvae. Considering the location of
larvae, just under the skin, in light infections this can be done under local
anesthesia and is a relatively simple procedure. Infections involving large numbers
of larvae can be very uncomfortable, and treatment (removal) might require
general anesthesia and supportive treatment with anti-inflammatory drugs.
How do humans come in contact with the larvae of dog and cat hookworms? A
common source of infection in developed countries is probably sandboxes. If you
have a sandbox in your backyard, it is almost certain that cats in the neighborhood
are using it as a large litter box. Moreover, the sand provides a nearly ideal
environment for the hookworm eggs to develop and hatch and for the larvae to
survive. Thus, keeping sandboxes covered to prevent cats from defecating in them
is a worthwhile "ounce of prevention." Other places where cats might defecate are
also possible sources of infection, including flower beds and vegetable gardens.
Dogs are much less fastidious about where they defecate, so it is more difficult to
control dog feces as a possible source of infection. If you own a dog two measures
that you should take are (1) keep you dog free of hookworms and (2) make sure
that you clean up the dog's feces on a regular basis. Also, if you "walk" your dog
in a park or playground, and in particular in my front yard, make sure that you
pick up and dispose of any fecal material the dog might leave behind.
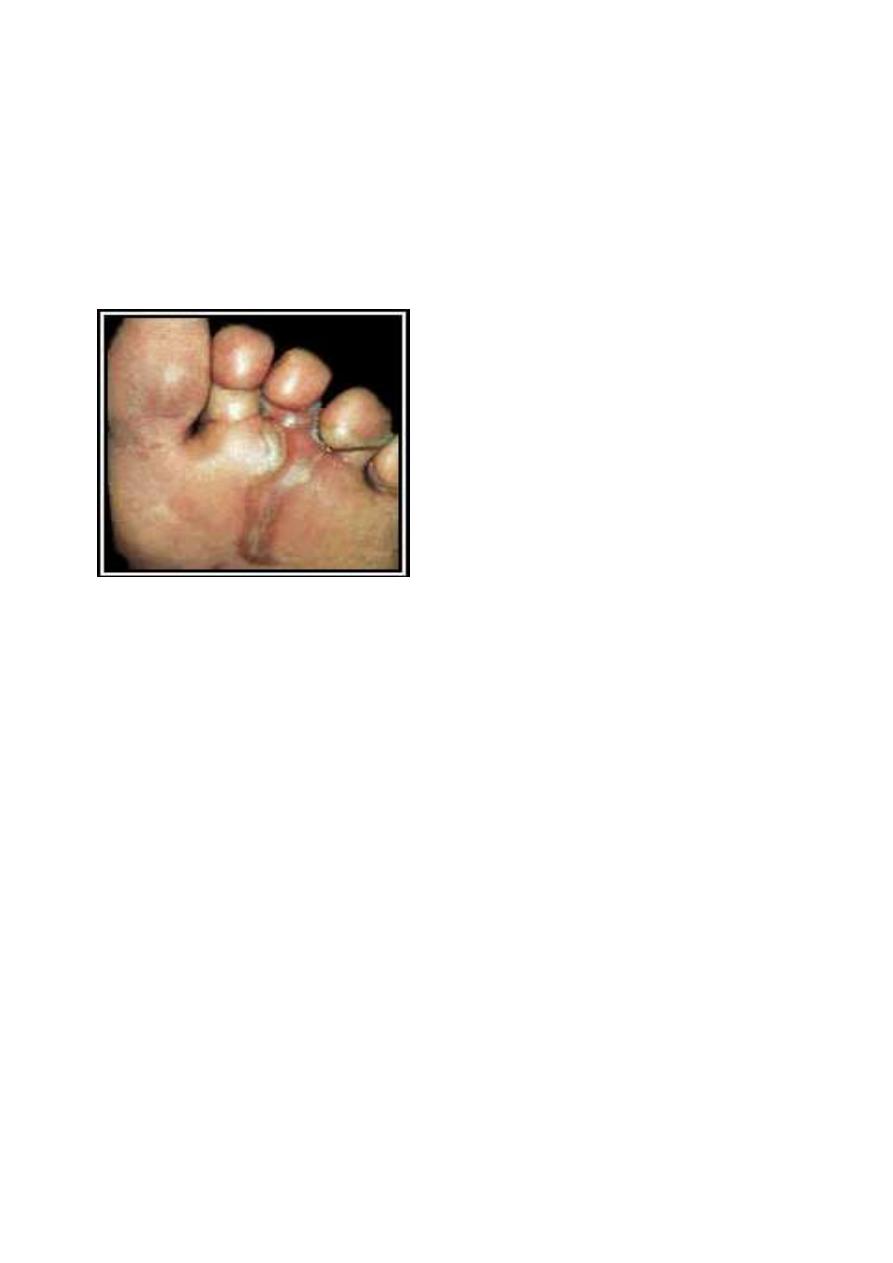
CLM of the foot.
(Original image from:
Companion Animal Surgery
.")
CLM of the foot.
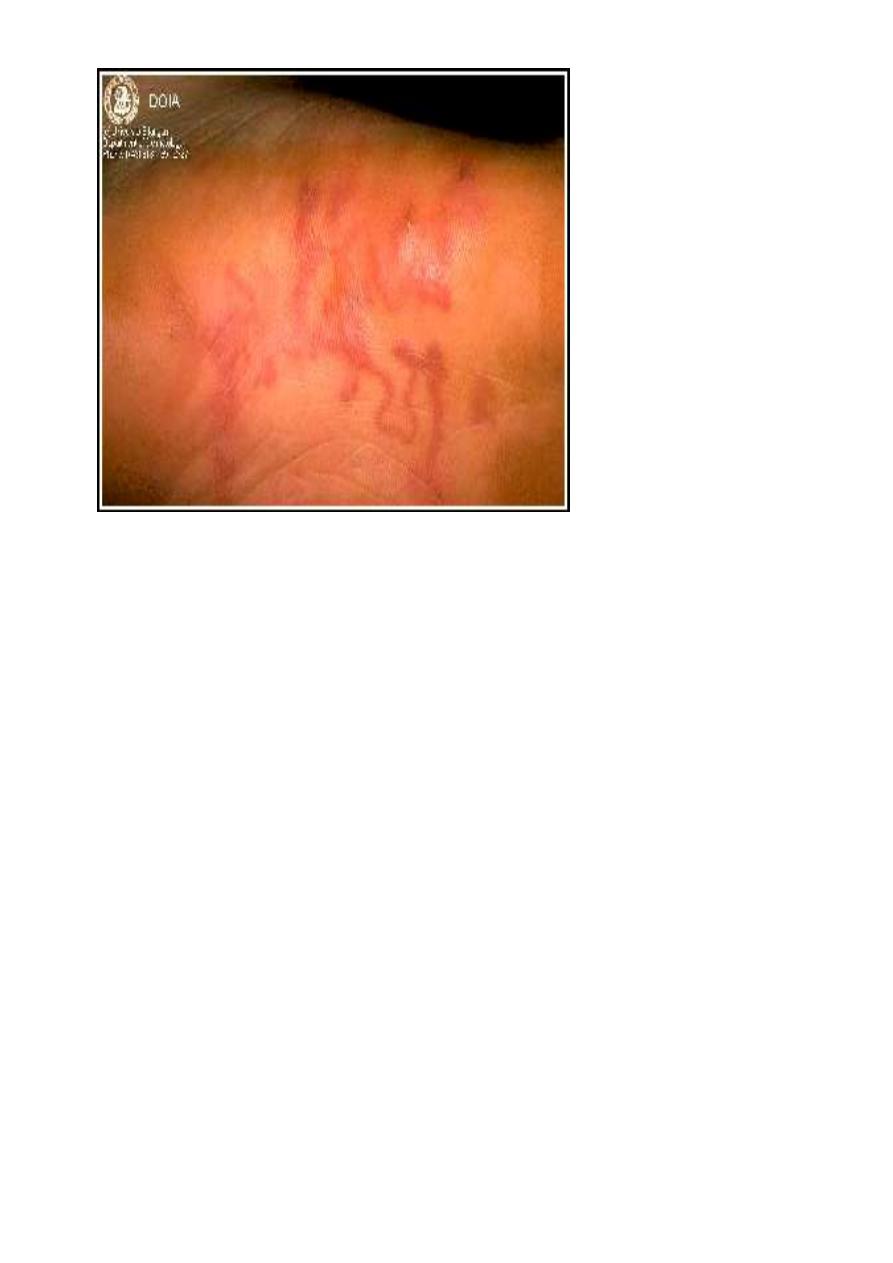
CLM (Original image from and copyrighted by
Dermatology Internet Service,
Department of Dermatology, University of
Erlangen
.)
CLM of the foot.
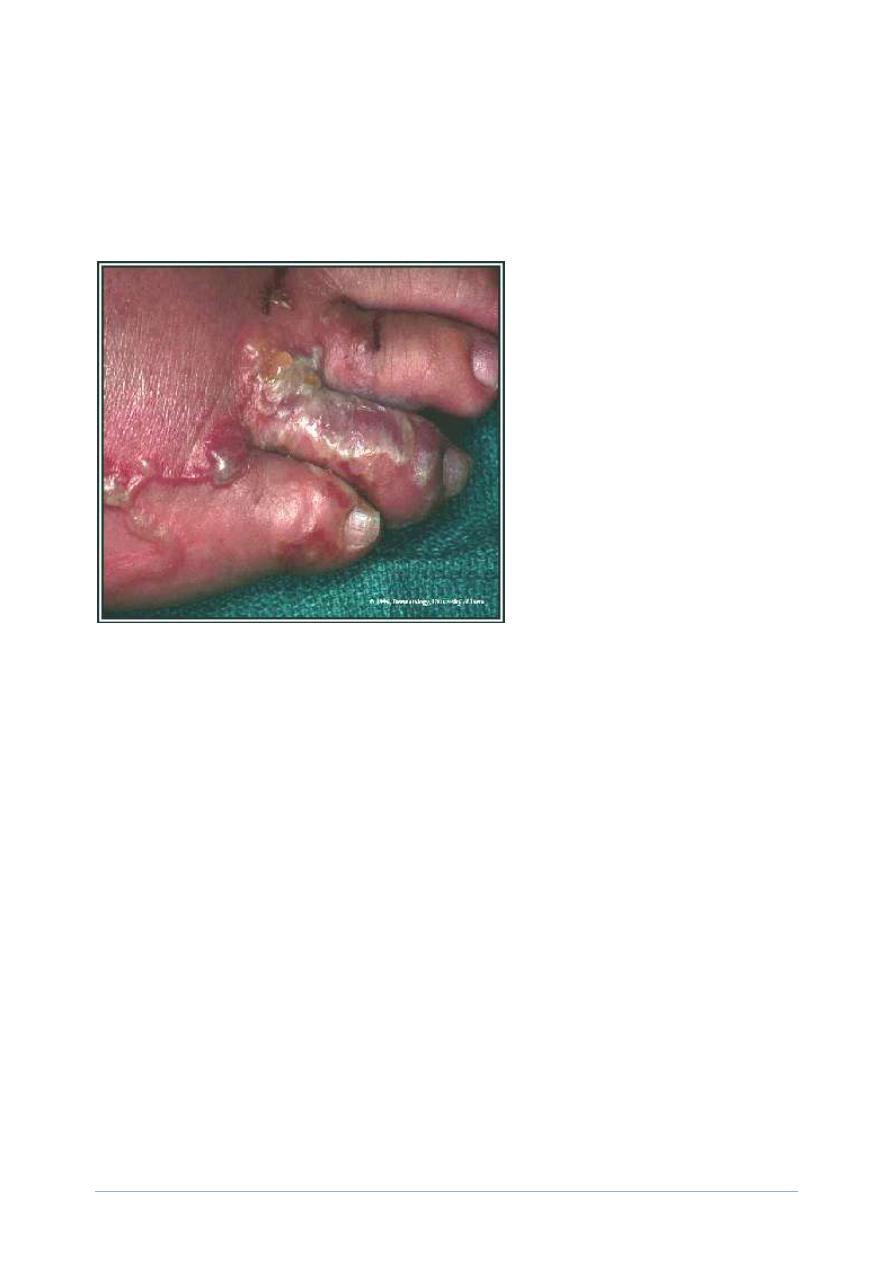
(Original image from and copyrighted by
Dermatologic Image Database,
Department of Dermatology, University of Iowa
College of Medicine
).
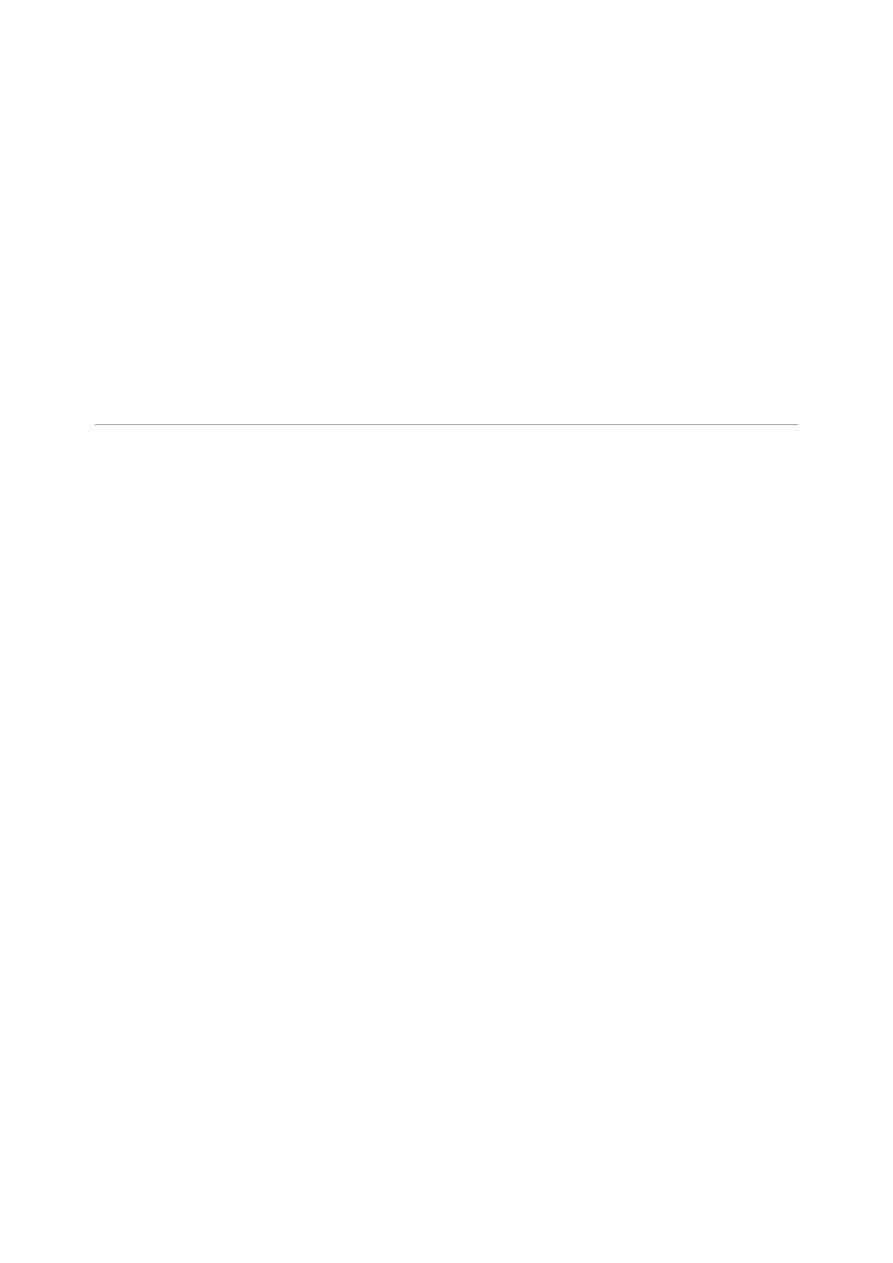
Strongyloides stercoralis in the wall of the small intestine; numerous
adults are visible in this section, as is the abnormal appearance of the
intestinal mucosa.
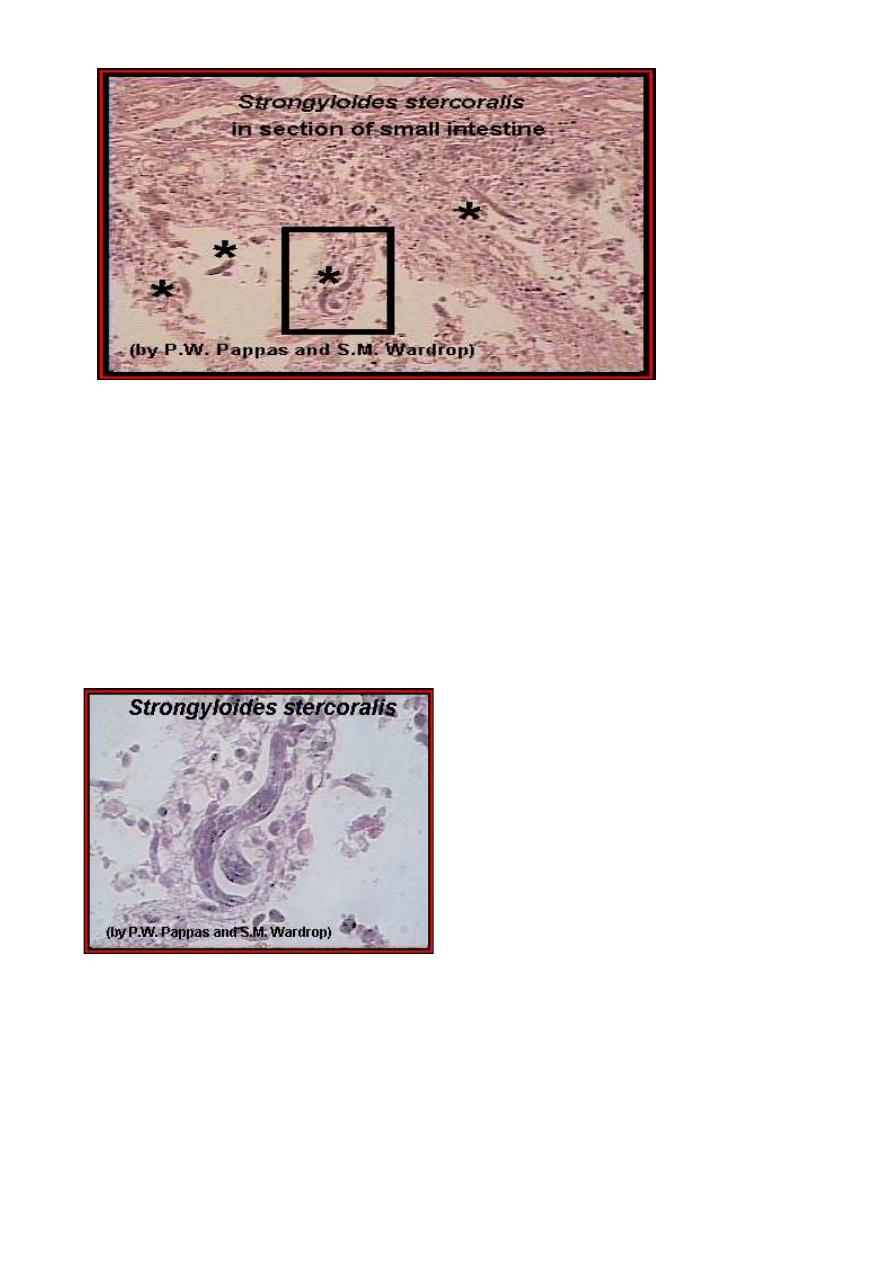
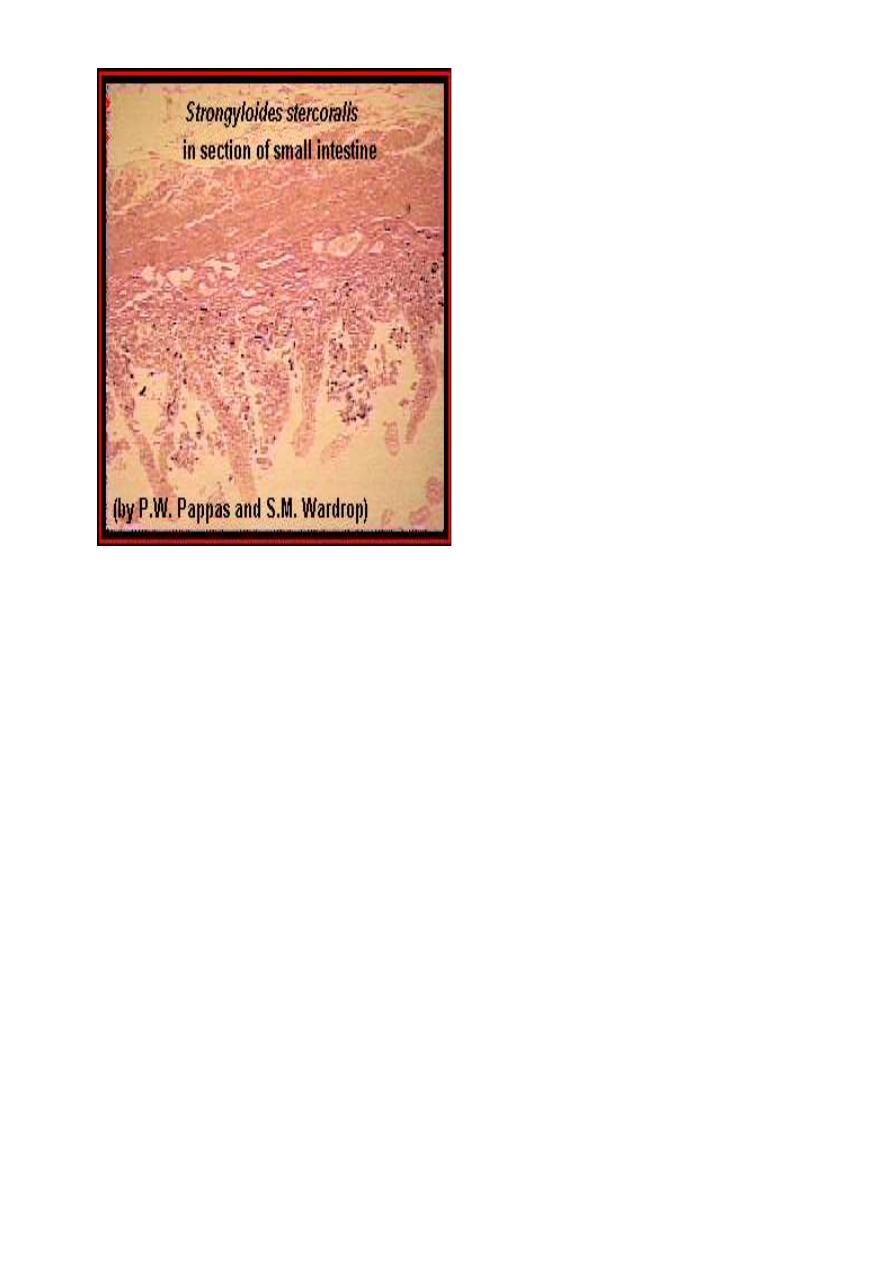
A higher power magnification of the above image; the adult worms are
labeled (*), and a higher power magnification of the enclosed area is
shown in the following image. An enlargement
An enlargement of the enclosed area in the above image
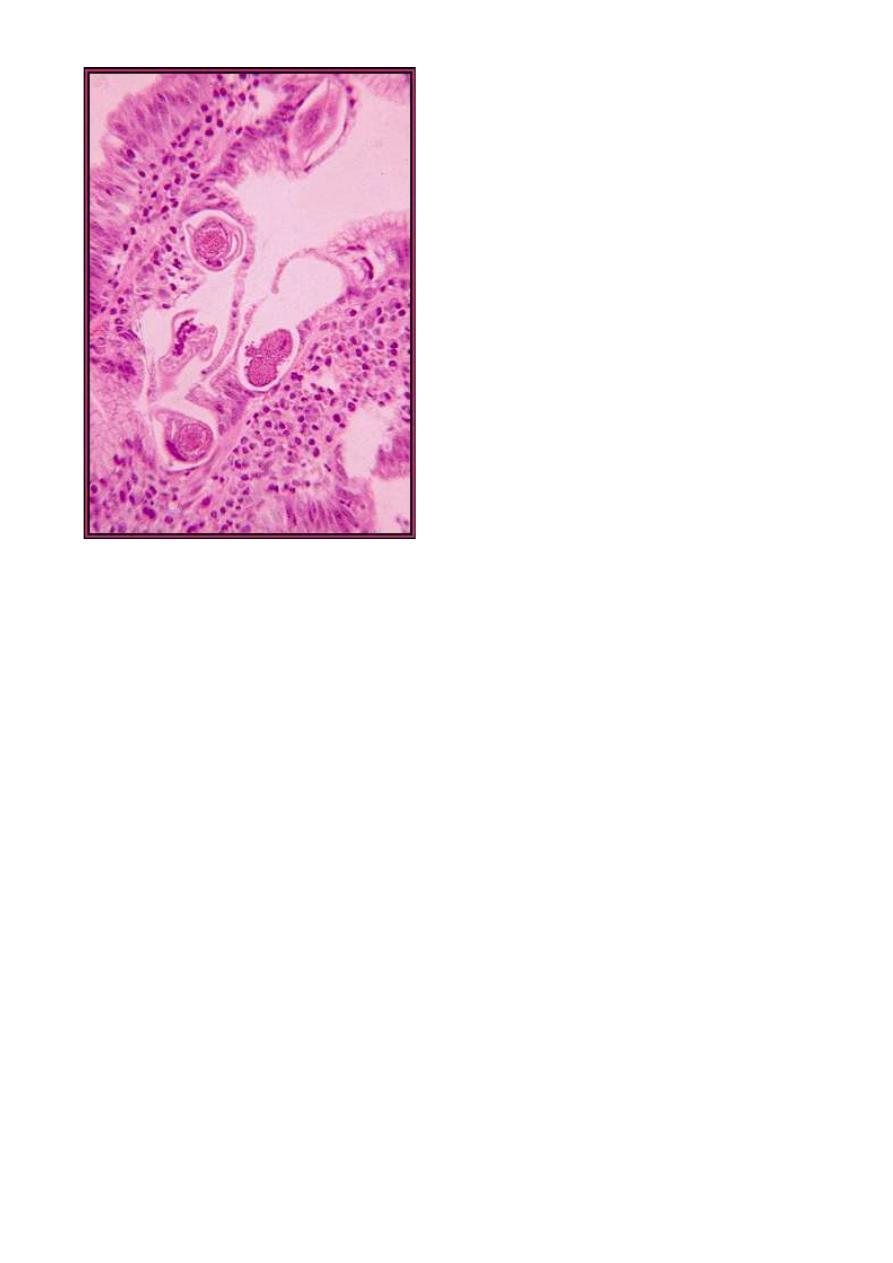
Strongyloides stercoralis adults in the small intestine. (From "Parasite of the
Month.")
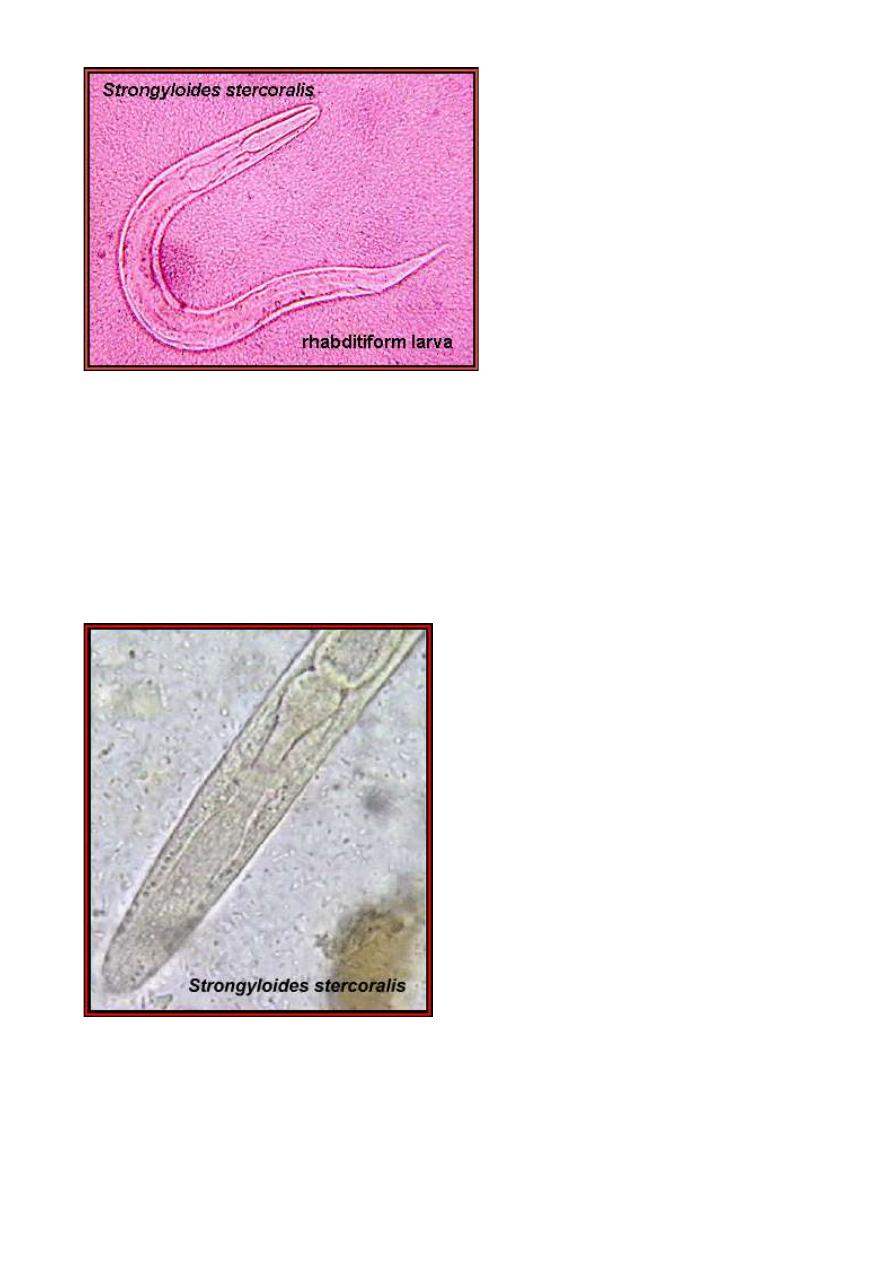
Strongyloides stercoralis larva as it would appear in a fecal sample.
Note the rhabditiform esophagus. (From "Parasite of the Month
Another example of the larva in which the rhabditiform esophagus
shows up clearly. (Original image from "Atlas ofMedical Parasitology
.")

CONCLUSIONS
Our understanding of S. stercoralis infections in normal and
immunocompromised hosts continues to evolve. Relatively recently, it
was thought that any defect in cellular immunity could tip the
equilibrium of chronic strongyloidiasis toward hyperinfection.
Although various immunocompromising conditions have been
associated with hyperinfection, steroids and HTLV-1 infection are the
most consistent
