Dept.of Microbiology/Virology Assist.prof. Shatha F. Abdullah
HEPATITIS VIRUSES
Medically important hepatitis v. (liver)are:
1.
HAV
2.
HBV
3.
HCV
4.
HDV
5.
HEV
6.
HGV
Other causes (not exclusively hepatitis v.)also called
sporadic
hepatitis:
1.EBV
2.CMV
3.Yellow fever v.
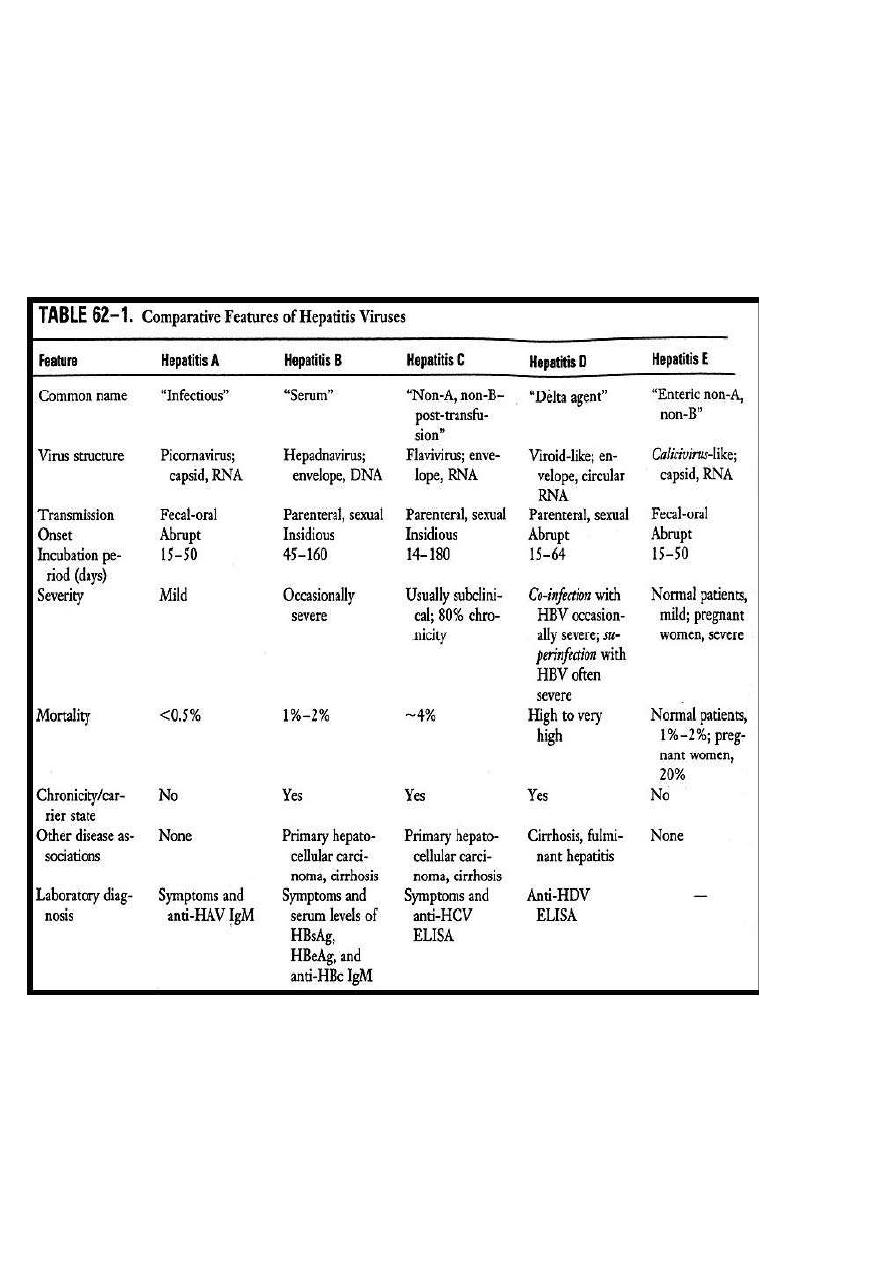
HEPATITIS A
(infectious hepatitis)
BASICS structure:
• enterovirus 72, picornoviridae family, Naked ,genome SS RNA with
Icosahedral nucleocapsid
•Replication occurs in the cytoplasm of the cell
• Single serotype
• Worldwide distribution. Humans are the only reservoir
TRANSMISSION
• Fecal-oral
• Contaminated raw seafood- e.g oysters
• Day-care center outbreaks
•Rarely transmitted via blood because the level of viremia is low.

EPIDEMIOLOGY
• Over 100,000 infections/ year in US
• Much higher prevalence in 3rd world
• Children are the most frequently infected group.
PATHOGENESIS
• Incubation 4 weeks (2-6wk range)
• Oral cavity---replicate in the GI tract, then spread to liver via blood.
• Virus in stool 2 weeks after infection, usually shed in stool prior to
symptoms
• Symptoms related to immune response and not direct cytopathic
effect of virus
•
No
chronicity,
no
carrier state,
nor
hepatocellular ca.
SIGNS & SYMPTOMS
Adults will have signs and symptoms more often than children. 70-80%
of adults develop symptoms, <10% of kids (<6 years) develop symptoms
•
fever
•
loss of appetite
•
fatigue
•
nausea, vomiting
•
diarrhea
•
abdominal pain
•
jaundice,dark urine &pale feces.
•
Elevated ALT/AST
•
Complete
recovery in 99%
within2-4wks.
LONG-TERM EFFECTS
• There is no chronic (long-term) infection.
• Lifelong immunity after infection- i.e no repeats infection
DIAGNOSIS
• Hep A IgM antibody: usually present when symptoms occur
• four fold rise in IgG indicate current infection
• IgG: suggests prior infection (followed by 1-3 wks) or vaccination
• Virus culture & Isolation: not used
TREATMENT
• Supportive- no antiviral therapy
PREVENTION
• Hepatitis A vaccine (
formalin inactivated
) is the best protection.
•
Two doses( Vaccine dosed at 0,followed by a booster 6-12months later.
• Protection begins 4 weeks post vaccine
• Protection probably at least 20 years (likely lifelong)-no need for
repeating
• Twinrix vaccine (for both HAV/HBV)
• Short-term protection against hepatitis A is available from
immune
globulin
. It can be given before and within 2 weeks after coming in
contact with HAV.
• Good hygiene- hand washing, etc
VACCINE RECOMMENDATIONS
Vaccine is recommended for the following persons 2 years of age and
older:
• Travelers to areas with increased rates of hepatitis A
• Men who have sex with men
• Injecting and non-injecting drug users
• Persons with clotting-factor disorders (e.g. hemophilia)
• Persons with chronic liver disease

• Children living in areas with increased rates of hepatitis A
HEPATITIS B
(serum hepatitis)
STRUCTURE
HBV is a member of the Hepadnavirus family,42-nm Enveloped virion,
with Icosahedral nucleocapsid core containing a partially DS circular DNA
genome.
May exist in multiple forms:
- 42 nm virions( Dane particle),few in patient serum.
- 22nm spheres and long filaments 22nm width which do not contain
DNA; only HBsAg (not infectious)
Humans are the only reservoir
Genome
contains 4 genes:
A- Surface protein (HBsAg) Austerlia Ag=envelope protein
B- core (nucleoprot) HBcAg, HBeAg
C- DNA polymerase (RNA dp RT) & (DNA dp activity)
D- X-protein, activator of viral RNA transcription

TRANSMISSION
• Blood-borne (almost never through transfusion)
• Sexual
• Perinatal (from mother to newborn)
Persons at risk for HBV infection might also be at risk for infection with
hepatitis C virus (HCV) or HIV.
EPIDEMIOLOGY
• 100,000 infections/ year
• Higher seroprevalence among Asian-Americans
• World-wide- high rates in SE Asia, Alaska, Africa
• Estimated 1.25 million chronically infected Americans, of whom 20-
30% acquired their infection in childhood.
PATHOGENESIS
• Illness is immune mediated
• 5% chronic carriers (in adults)
• Higher rate of hepatocellular ca in chronic carriers- especially “e”
antigen positive
• Surface ab likely confers lifelong immunity
• Antibody to “e” antigen indicates low transmissibility
• Incubation 60-90 d (range 45-180d)
SIGNS & SYMPTOMS
About
1/3
of persons have
no
signs or symptoms. Signs and symptoms
are less common in children than adults.
Chronic
infection
more
common when infected
at younger age
and more likely in
asymptomatic
infection.
I.P.
= 10-12wks
• fatigue
• abdominal pain
• loss of appetite
• nausea, vomiting
• joint pain
LONG-TERM EFFECTS WITHOUT VACCINATION
Chronic infection occurs in:
• 90% of infants infected at birth
• 30% of children infected at age 1 - 5 years
• 6% of persons infected after age 5 years
•Death from chronic liver disease occurs in 15-25% of chronically
infected persons
RISK GROUPS
• Persons with multiple sex partners or diagnosis of a sexually
transmitted disease
• Men who have sex with men
• Sex contacts of infected persons
• Injection drug users
• Household contacts of chronically infected persons

Interpretation of the Hepatitis B Panel
Interpretation
Results
Tests
susceptible
negative
negative
negative
HBsAg
anti-HBc
anti-HBs
immune due to natural
infection
negative
positive
positive
HBsAg
anti-HBc
anti-HBs
immune due to hepatitis B
vaccination
negative
negative
positive
HBsAg
anti-HBc
anti-HBs
acutely infected
positive
positive
positive
negative
HBsAg
anti-HBc
IgM anti-HBc
anti-HBs
chronically infected
positive
positive
negative
negative
HBsAg
anti-HBc
IgM anti-HB c
anti-HBs
four interpretations
possible *
Window phase
negative
positive
negative
HBsAg
anti-HBc
anti-HBs
Four interpretations are possible if
*
HBsAg negative
anti-HBc positive
anti-HBs negative
1. May be recovering from acute HBV infection.
2. May be distantly immune and test not sensitive enough to detect very
low level of anti-HBs in serum.
3. May be susceptible with a false positive anti-HBc.
4. May be undetectable level of HBsAg present in the serum and the
person is actually a carrier
Hbe Ag: arise during IP , Ab against eAg= low transmission
Viral DNA detection in serum =acute infection
VACCINE RECOMMENDATIONS
• Hepatitis B vaccine available since 1982.The initial vaccine was
prepared by purifying HBsAg associated with the 22-nm particles from
healthy HBsAg-positive carriers and treating the particles with virus-
inactivating agents. Although plasma-derived vaccines are still in use in
certain countries, they have been replaced in the United States by
recombinant DNA-derived vaccines. These vaccines consist of HBsAg
produced by a recombinant DNA in yeast cells or in continuous
mammalian cell lines.
• HBV vaccine is recommended:
1-
for all children as part of their regular
immunization schedule(Routine vaccination of 0-18 year olds)
2-
Vaccination of risk groups of all ages (commercial name: Engerix-B
,Recombivax HB)
PASSIVE IMMUNIZATION
• Hep B immune globulin (high titer of HBsAb) should be given in
addition to vaccine in exposures to known HepB
* infected patients/sources
**Newborne whose mother is HBsAg+ve.
• Give immune globulin preferably within (24 hours of exposure)
TREATMENT & MEDICAL MANAGEMENT
• Adefovir dipivoxil, alpha interferon, and lamivudine are three drugs
licensed for the treatment of persons with chronic active hepatitis B.
• These drugs should not be used by pregnant women.
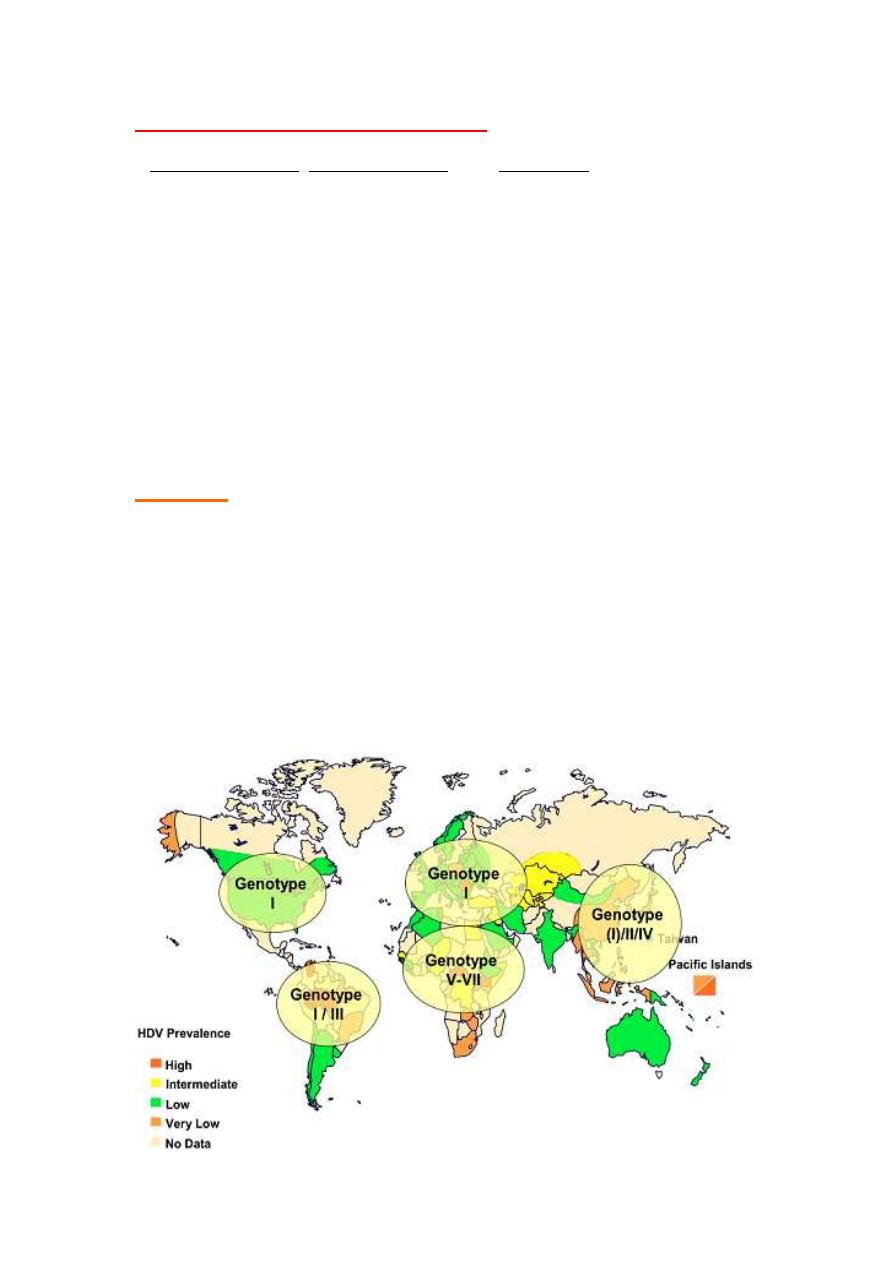
HEPATITIS D
Structure
• Caused by delta agent- cannot infect without HepB
•Unusual virus (defective virus), it can't replicate by itself because it
does not have the gene for its envelope protein.
•Enveloped ssRNA,-ve polarity. The RNA genome of HDV encodes only
one internal core protein called delta antigen.
•One serotype because HBsAg has only one serotype.
At least seven
HDV genotypes (1 most common)
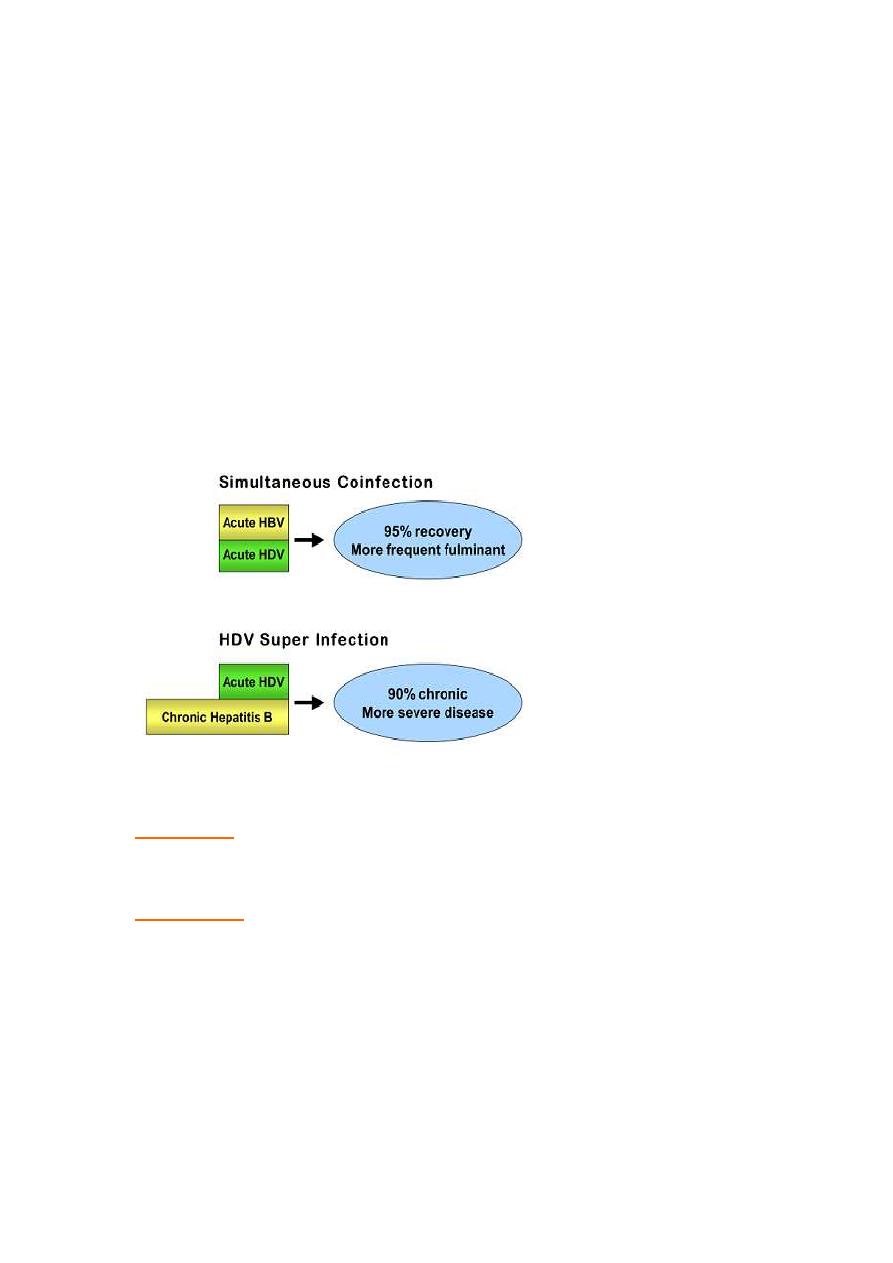
•
Transmission risks
same as Hep B,
occurs only in HBsAg-positive
individuals
either as
• Co-infection:
acquire infection at same time as Hep B- usually
Hepatitis is more severe than those infected by HBV alone.
• Superinfection:
infection of HDV in chronic Hep B. Hepatitis in chronic
carriers of HBV who become superinfected with HDV is much more
severe ,and the incidence of fulminant ,life threatening hepatitis, chronic
hepatitis and liver failure is higher.
DIAGNOSIS
• Detection of either delta Ag or IgM ab to HDV in patient serum.
TREATMENT
•No antiviral therapy, No vaccine,but a personimmunized against HBV
will not be infected by HDV.
•Alpha interferon
HEPATITIS C (NON-A,NON-B)- post transfusion hepatitis
CLASSIFICATION
Flavivirus
Enveloped SS RNA virus
6 serotypes (genotypes) and multiple subtypes
Humans (and chimps) only reservoir
TRANSMISSION
Recommendations for testing based on risk for HCV infection
• Occurs when blood or body fluids from an infected person enters the
body of a person who is not infected.
• Persons at risk for HCV infection might also be at risk for infection with
hepatitis B virus (HBV) or HIV.
PERSONS AT RISK OF INFECTION
High
Injecting drug users
Recipients of clotting factors made before 1987
Intermediate
Hemodialysis patients
Recipients of blood and/or solid organs before 1992
People with undiagnosed liver problems
Infants born to infected mothers
Low
(
Healthcare/public safety workers (Only after known exposure
People having sex with multiple partners
People having sex with an infected steady partner
EPIDEMIOLOGY
• Number of new infections per year has declined from an average of
240,000 in the 1980s to about 25,000 in 2001.
• Most infections are due to illegal injection drug use.
• Transfusion-associated cases occurred prior to blood donor screening;
now occurs in less than one per million transfused unit of blood.
PATHOGENESIS
• Damage and illness immune mediated
• Can lead to HCC
• Alcoholism is greatly enhances the rate of HCC.
SIGNS & SYMPTOMS
I.P.
= 8wks
80% of persons have no signs or symptoms. Those who do may develop:
• jaundice
• fatigue
• dark urine
• abdominal pain
• loss of appetite
• nausea
LONG-TERM EFFECTS
• Chronic infection: 75-85% of infected persons (is much higher than in
HBV infection)
• Chronic liver disease: 70% of chronically infected persons
• Deaths from chronic liver disease: 1-5%
• Leading indication for liver transplant
• Extrahepatic manifestations may also be present and are due to
immune complex deposition.auto immune reactions
(vasculitis,arthralgias,purpura..etc..)
TREATMENT & MEDICAL MANAGEMENT
• HCV positive persons should be evaluated by their doctor for liver
disease.
• Interferon and ribavirin are two drugs licensed for the treatment of
persons with chronic hepatitis C.
• Interferon can be taken alone or in combination with ribavirin.
Combination therapy, using pegylated interferon and ribavirin, is
currently the treatment of choice.(note: pegylated interferon=alpha
Interferon conjugated to polyethylene glycol)
• Combination therapy can get rid of the virus in up to 50% of genotype
1 and in up to 80% for genotype 2 and 3
DIAGNOSIS
• Hepatitis C ELISA or EIA 99% sensitive and specific (high false +ve
cases)
• Usually positive 2-5 months after infection
• RIBA and PCR used to confirm diagnosis (RIBA=recombinant
immunoblot assay ,should be performed )
Hepatitis E
• RNA virus- calicivirus
• Similar syndrome to Hep A
• Fecal-oral transmission
• Higher mortality in pregnant women
•
No
chronicity,
no
carrier cases. Diagnosis made by excluding HAV and
other causes
•
NO
antiviral drug,
no
vaccine.
Hepatitis
G
•
Member of flavivirus flamily; as HCV.
•
Transmitted by blood &sexual.
•
Unlike
HCV,
not
cause acute &chronic hepatitis, or HCC.
• Patient infected with HIV &HGV have lower mortality rate than those
infected with HIV alone, it might interfere with the replication of HIV.
